Type I Interferon Production by Tertiary Lymphoid Tissue Developing in Response to 2,6,10,14-Tetramethyl-Pentadecane (Pristane) (original) (raw)
Abstract
Lymphoid neogenesis is associated with antibody-mediated autoimmune diseases such as Sjogren’s syndrome and rheumatoid arthritis. Although systemic lupus erythematosus is the prototypical B-cell-mediated autoimmune disease, the role of lymphoid neogenesis in its pathogenesis is unknown. Intraperitoneal injection of 2,6,10,14-tetramethyl-pentadecane (TMPD, pristane) or mineral oil causes lipogranuloma formation in mice, but only TMPD-treated mice develop lupus. We report that lipogranulomas are a form of lymphoid neogenesis. Immunoperoxidase staining of lipogranulomas revealed B cells, CD4+ T cells, and dendritic cells and in some cases organization into T- and B-cell zones. Lipogranulomas also expressed the lymphoid chemokines CCL21, CCL19, CXCL13, CXCL12, and CCL22. Expression of the type I interferon (IFN-I)-inducible genes Mx1, IRF7, IP-10, and ISG-15 was greatly increased in TMPD- versus mineral oil-induced lipogranulomas. Dendritic cells from TMPD lipogranulomas underwent activation/maturation with high CD86 and interleukin-12 expression. Magnetic bead depletion of dendritic cells markedly diminished IFN-inducible gene (Mx1) expression. We conclude that TMPD-induced lupus is associated with the formation of ectopic lymphoid tissue containing activated dendritic cells producing IFN-I and interleukin-12. In view of the increased IFN-I production in systemic lupus erythematosus, these studies suggest that IFN-I from ectopic lymphoid tissue could play a role in the pathogenesis of experimental lupus in mice.
Chronic inflammation can lead to the formation of ectopic lymphoid tissue, a process known as “lymphoid neogenesis.”1 The development of this tertiary lymphoid tissue partially recapitulates normal lymph node development, which depends in part on lymphotoxin α as well as B-cell (CXCL13) and T-cell (CCL21 and CCL19) attractant chemokines.2–5 Lymphoid neogenesis is associated with humoral autoimmunity in a variety of situations6 : the thyroid gland in Hashimoto’s thyroiditis, thymus in myasthenia gravis, nervous system in multiple sclerosis, salivary glands in Sjogren’s syndrome, and synovium in rheumatoid arthritis.7–10 Precisely how the formation of tertiary lymphoid tissue leads to autoimmunity and autoantibody production is not clear. We have shown previously that chronic inflammation induced by the hydrocarbon oil 2,6,10,14-tetramethyl-pentadecane (TMPD) causes autoantibodies and clinical manifestations closely resembling systemic lupus erythematosus (SLE) in most nonautoimmune-prone mouse strains.11,12 BALB/c mice injected intraperitoneally with TMPD develop high levels of the lupus-related autoantibodies against double-stranded DNA, Sm, RNP, and Su11 along with immune complex-mediated glomerulonephritis12 and arthritis.13–15 In contrast, medicinal mineral oil (a complex hydrocarbon mixture) does not induce these autoantibodies nor does it cause renal or joint disease.16 In the present study, we show that TMPD-induced chronic inflammation leads to lymphoid neogenesis. Dendritic cells present in the tertiary lymphoid tissue produced high levels of type I interferons (IFN-I), which are implicated in the pathogenesis of SLE.17,18 In contrast, there was little IFN-I production in inflammatory tissues elicited by mineral oil injection. This study provides evidence for a link between chronic inflammation, lymphoid neogenesis, and the pathogenesis of SLE.
Materials and Methods
Mice
Four-week-old female BALB/c mice were purchased from Jackson Laboratory (Bar Harbor, ME) and housed in a conventional facility in barrier cages. At 3 months of age, they received a single intraperitoneal injection (0.5 ml) of either TMPD (Sigma-Aldrich, St. Louis, MO) or medicinal mineral oil (Harris Teeter, Mathews, NC). Peritoneal cells, granulomas, spleen, and blood were harvested 12 to 20 weeks later. These studies were approved by the Institutional Animal Care and Use Committee.
Harvesting of Peritoneal Cells and Dendritic Cell Depletion
The peritoneal cavity was lavaged with 5 ml of Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, 10 mmol/L HEPES, glutamine, and penicillin/streptomycin (complete Dulbecco’s modified Eagle’s medium) plus 10 U/ml heparin. Cells were collected by centrifugation, and supernatants were frozen at −80°C until analysis. After lysing erythrocytes in 0.15 mol/L NH4Cl, 10 mmol/L KHCO3, and 2 mmol/L ethylenediaminetetraacetic acid, the peritoneal cells were resuspended in complete medium.
Peritoneal CD11c+ dendritic cells, CD11b+ macrophages/monocytes, CD19+ B cells, and CD3+ T cells were positively selected using magnetic beads (Miltenyi Biotech, Auburn, CA) following the manufacturer’s instructions. Cells were usually >95% positive for each population by flow cytometry. In some experiments, CD11c+ cells were depleted using anti-CD11c microbeads. Untreated and CD11c-depleted peritoneal cells were cultured in 12-well culture dishes either without stimulation or with lipopolysaccharide (LPS, from Salmonella minnesota, 100 ng or 10 μg/ml; Sigma-Aldrich), polyinosinic-polycytidylic acid (poly IC, 50 μg/ml; Sigma-Aldrich), or CpG oligodeoxyribonucleotide (ODN) no. 1826 (10 μg/ml; Coley Pharmaceutical Group Inc., Wellesley, MA). RNA was extracted 4 hours later using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA).
Lipogranulomas
Individual and pooled lipogranulomas were picked from the peritoneal lining of mice treated with TMPD or mineral oil 12 to 20 weeks earlier, and RNA was extracted using TRIzol reagent. Single cell suspensions for flow cytometry were prepared by digesting pooled lipogranulomas with collagenase (2 mg/ml) and dispase (1 mg/ml) (Invitrogen Life Technologies) and shaking at 200 rpm at 37°C for 30 minutes. The cells were washed three times with phosphate-buffered saline (PBS) and 2 mmol/L ethylenediaminetetraacetic acid, filtered through a 70-μm pore-size cell strainer, and resuspended in flow cytometry buffer.
Gene Expression in RAW264.7 Cells
In some experiments, RAW 264.7 cells (mouse monocyte-macrophage cell line; American Type Culture Collection, Bethesda, MD) were incubated in vitro with cytokines. Cells were grown in complete Dulbecco’s modified Eagle’s medium and incubated at 37°C in a 5% CO2 atmosphere, resuspended at 2 × 106/ml in complete Dulbecco’s modified Eagle’s medium and plated overnight in 12-well culture dishes. The cells were cultured an additional 24 hours either without stimulation or with LPS (1 ng/ml, 10 ng/ml, 100 ng/ml, 1 μg/ml, or 10 μg/ml), poly(I:C) (50 μg/ml), with IFN-α (500 to 1000 U/ml) ± anti-IFN-α neutralizing antibody (1 to 2 μg/ml), or IFN-β (500 to 1000 U/ml) (all from PBL Biomedical Laboratories, Piscataway, NJ), or with interleukin (IL)-12 (10 to 20 ng/ml), tumor necrosis factor (TNF)-α (20 ng/ml), or IL-6 (5 ng/ml) (all from BD Biosciences, San Jose, CA). Cells were harvested at specified time points for RNA extraction using TRIzol.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total RNA was precipitated with isopropanol and the pellet washed with cold 75% (v/v) ethanol and resuspended in diethyl pyrocarbonate-treated water. One μg of RNA was treated with DNase I (Invitrogen) to remove genomic DNA and reverse transcribed to cDNA using Superscript first-strand synthesis system for RT-PCR (Invitrogen). One μl of cDNA was added to the PCR mixture containing 1× PCR buffer (2.5 mmol/L MgCl2, 400 μmol/L dNTPs), 0.025 U of _Taq_DNA polymerase (Invitrogen), 1 μmol/L each of forward and reverse primers, and diethyl pyrocarbonate-water in a 20-μl volume. Amplification was performed for 5 minutes at 94°C, followed by 35 cycles of denaturation at 94°C for 1 minute, annealing at 60°C for 1 minute, extension at 72°C for 1 minute, and a final extension of 72°C for 10 minutes in a PTC-100 programmable thermal controller (MJ Research, Inc., Waltham, MA). PCR primers were synthesized by Invitrogen (Table 1).
Table 1.
Primers Used for RT-PCR
| Gene | Forward | Reverse | bp |
|---|---|---|---|
| β_-Actin_ | 5′-TGGAATCCTGTGGCATCCTGAAAC-3′ | 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′ | 348 |
| Mx1* | 5′-GATCCGACTTCACTTCCAGATGG-3′ | 5′-CATCTCAGTGGTAGTCAACCC-3′ | 181 |
| IRF-7* | 5′-TGCTGTTTGGAGACTGGCTAT-3′ | 5′-TCCAAGCTCCCGGCTAAGT-3′ | 359 |
| IP-10* | 5′-ATCATCCCTGCGAGCCTAT-3′ | 5′-ATTCTTGCTTCGGCAGTTAC-3′ | 361 |
| ISG15* | 5′-CAGAAGCAGACTCCTTAATTC-3′ | 5′-AGACCTCATATATGTTGCTGTG-3′ | 339 |
| CCL21 | 5′-ATGATGACTCTGAGCCTCC-3′ | 5′-GAGCCCTTTCCTTTCTTTCC-3′ | 346 |
| CCL19 | 5′-GCCTCAGATTATCTGCCAT-3′ | 5′-AGACACAGGGCTCCTTCTGGT-3′ | 340 |
| CCR7 | 5′-GAGAGACAAGAACCAAAAGCAC-3′ | 5′-GGGAAGTAATTAGGAGGAAAAGG-3′ | 394 |
| CXCL13 | 5′-ATGAGGCTCAGCACAGCAAC-3′ | 5′-CCATTTGGCACGAGGATTCAC-3′ | 245 |
| CXCR5 | 5′-ACTACCCACTAACCCTGGAC-3′ | 5′-AGGTGATGTGGATGGAGAGGAG-3′ | 409 |
| IL-12p40 | 5′-GAGTGGACTGGACTCCCGA-3′ | 5′-CAAGTTCTTGGGCGGGTCTG-3′ | 618 |
| IL-6 | 5′-CTGGTGACAACCACGGCCTTCCCT-3′ | 5′-ATGCTTAGGCATAACGCACTAGGTT-3′ | 600 |
| IL-4 | 5′-CGAAGAACACCACAGAGAGTGAGCT-3′ | 5′-GACTCATTCATGGTGCAGCTTATCG-3′ | 181 |
| _IFN-_γ | 5′-AGCGGCTGACTGAACTCAGATTGTA-3′ | 5′-GTCACAGTTTTCAGCTGTATAGGG-3′ | 247 |
| 18S | 5′-CGGCTACCACATCCAAGGAA-3′ | 5′-GCTGGAATTACCGCGGCT-3′ | 198 |
| CCL22 | 5′-CCAGGACTACATCCGTCACC-3′ | 5′-TGGCAGAAGAATAGGGCTTG-3′ | 239 |
| CXCL12 | 5′-TGCTCTCTGCTTGCCTCCA-3′ | 5′-GGTCCGTCAGGCTACAGAGGT-3′ | 69 |
Quantitative PCR
Gene expression was quantified by real-time PCR. One μl of cDNA was added to a mixture containing 3.75 mmol/L MgCl2, 1.25 mmol/L dNTP mixture, 0.025 U of Amplitaq Gold, SYBR Green dye (Applied Biosystems, Foster City, CA), optimized concentrations of specific forward and reverse primers, and diethyl pyrocarbonate-treated water to a final volume of 20 μl. Amplification conditions were 95°C (10 minutes), followed by 45 cycles of 94°C (15 seconds), 60°C (25 seconds), 72°C (25 seconds), and a final extension at 72°C for 8 minutes using a DNA Engine Opticon 2 continuous fluorescence detector (MJ Research). Primers were synthesized by Invitrogen (Table 2). Transcripts were quantified using the comparative (2−ΔΔCt) method.
Table 2.
Primers Used for Real-Time PCR
| Gene | Forward | Reverse |
|---|---|---|
| Mx1* | 5′-GATCCGACTTCACTTCCAGATGG-3′ | 5′-CATCTCAGTGGTAGTCAACCC-3′ |
| ISG15* | 5′-AGCGGAACAAGTCACGAAGAC-3′ | 5′-TGGGGCTTTAGGCCATACTC-3′ |
| IP-10* | 5′-CCTGCAGGATGATGGTCAAG-3′ | 5′-GAATTCTTGCTTCGGCAGTT-3′ |
| IRF-7* | 5′-TGCTGTTTGGAGACTGGCTAT-3′ | 5′-TCCAAGCTCCCGGCTAAGT-3′ |
| TLR 3 | 5′-TCCGCCCTCTTCGTAACT TG-3′ | 5′-TTGGCGGCTGGTAATCTTCT-3′ |
| TLR 4 | 5′-GAGGCAGCAGGTGGAATT GT-3′ | 5′-TGCTCAGGATTCGAGGCTTT-3′ |
| _TNF_α | 5′-GGCAGGTCTACTTTGGAGTCATTGC-3′ | 5′-ACATTCGAGGCTGCTCCAGTGAATTCGG-3′ |
| IL-4 | 5′-TCAACCCCCAGCTAGTTGTC-3′ | 5′-TGTTCTTCGTTGCTGTGAGG-3′ |
| MyD88 | 5′-ACTGGCGGCCTGAGCAACTAGGA-3′ | 5′-TGTCCCAAAGGAAACACACA-3′ |
| TRIF | 5′-CCTGTCAGCACGTTTTCTGT-3′ | 5′-TCCGGACATGCTCTTTCTCT-3′ |
| TRAM | 5′-TGCAAACCATCAATGCCTTA-3′ | 5′-TCAAATACAGACTCCCGGAAA-3′ |
| β_-Actin_ | 5′-CCCACACTGTGCCCATCTAC-3′ | 5′-CGCTCGGTCAGGATCTTCAT-3′ |
Flow Cytometry
The following labeled antibodies were used: anti-CD19-FITC or PE-Cy7; anti-CD8-PE; anti-CD4-PE-Cy5; anti-CD3-APC; anti-CD5-PE; anti-CD45R (B220)-PE-Cy5 or PerCP Cy5.5; anti-CD11b-APC; anti-I-Ad-FITC or PE; anti-CD11c-PE or APC; anti-CD86-biotin; and anti-CD40-biotin (all from BD Biosciences, San Jose, CA), anti-CD-11b-Pacific Blue (Caltag Laboratories, Burlingame, CA), and anti-PDCA-1-FITC (Miltenyi Biotec, Auburn, CA). For biotinylated reagents, avidin-PE-Cy5 was used (BD Biosciences). Biotinylated isotype controls were used to evaluate background fluorescence. Isolated peritoneal cells were washed in staining buffer (PBS supplemented with 0.1% NaN3 and 1% bovine serum albumin). After aliquotting 106 cells/well into 96-well round-bottom polystyrene plates (Costar, Corning, NY), the cells were preincubated for 10 minutes with 1 μg of anti-CD16 in 10 μl of staining buffer to block Fc binding. Primary antibodies were then added at pretitrated amounts and incubated for 20 minutes, followed by washing in staining buffer. After the addition of avidin-PE-Cy5 to appropriate wells, the cells were washed and resuspended in PBS supplemented with 1% paraformaldehyde. Data were acquired on a FACSCalibur (BD Biosciences) and analyzed with FCS Express Version 2 (DeNovo Software, Thornhill, ON, Canada). At least 20,000 events per sample were acquired and analyzed using size gating to exclude dead cells.
Immunohistochemistry
Lipogranulomas were picked from the peritoneal wall and snap-frozen in OCT compound (Tissue-Tek; Sakura Fine-tek USA, Inc., Torrance, CA). Cryostat sections (5 μm) were collected on gelatin/chromium potassium sulfate-treated slides, fixed in cold acetone, and stored at −80°C. For staining, sections were air-dried at room temperature then quenched with 0.3% H2O2 for 10 minutes and blocked with 10% normal goat serum (Vector Laboratories, Burlingame, CA) for 30 minutes. Sections were then incubated with biotinylated anti-B220, -CD4, -CD11c, -CD11b (BD Biosciences), or peanut agglutinin (Vector) for 1 hour followed by incubation with horseradish peroxidase-streptavidin (Vector) for 30 minutes and aminoethylcarbazole. For peripheral lymph node addressin staining, sections were incubated with purified rat anti-mouse PNAd (MECA-79, BD Biosciences), and then with biotinylated mouse anti-rat IgM (BD Biosciences), followed by horseradish peroxidase-streptavidin (Vector). Sections were counterstained with Mayer’s hematoxylin and dilute ammonia.
Cytokine Enzyme-Linked Immunosorbent Assay (ELISA)
Unfractionated peritoneal exudate cells, peritoneal cells depleted of CD11c+ cells, and the CD11c+ fraction were plated in 96-well plates (105 cells/well) and treated with LPS (10 μg/ml), CpG ODN no. 1826 (10 μg/ml), or poly (I:C) (50 μg/ml). Culture supernatants were collected 24 hours later and TNF-α and IL-12 ELISAs were performed using hamster monoclonal and rabbit polyclonal antibodies (TNF-α) or rat monoclonal antibody pairs (IL-12) from BD Biosciences. After incubation with biotinylated cytokine-specific antibodies, streptavidin-alkaline phosphatase (1:1000 dilution; Southern Biotechnology Associates, Birmingham, AL) was added for 30 minutes at 22°C, and the reaction was developed. Peritoneal washings obtained by lavage were assayed for IFN-β (antibodies from PBL Biomedical Laboratories, Piscataway, NJ), IFN-γ, IL-6, and IL-12 (antibodies from BD Biosciences) in the same manner. Optical density was converted to concentration based on standard curves produced using recombinant cytokines using a four-parameter logistic equation (Softmax Pro 3.1 software; Molecular Devices Corporation, Sunnyvale, CA).
Results
Peritoneal Inflammatory Responses to TMPD and Mineral Oil
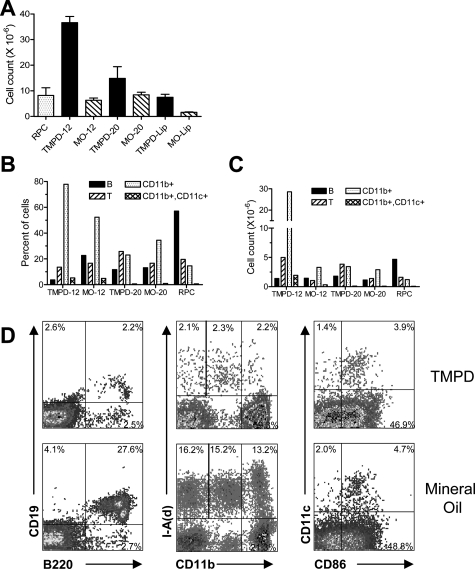
Both TMPD and mineral oil induce chronic inflammation when injected into the peritoneal cavity. Because the onset of IgG autoantibody production in TMPD-treated mice is between 12 and 20 weeks after intraperitoneal injection of the oil, we compared the peritoneal inflammatory responses to TMPD and mineral oil (a mixture of hydrocarbons that does not induce lupus in mice) at these time points (Figure 1, A–C). At 12 weeks, the inflammatory response to TMPD (and to a lesser extent mineral oil) was predominated by a striking influx of CD11b+ cells that did not bear B-cell (CD19, B220) or dendritic cell (CD11c) markers, and thus were probably monocytes or monocytes/macrophages (Figure 1, A–C; Table 3). At the same time, the number of B cells decreased in comparison with resident peritoneal cells from untreated mice (Figure 1, B and C). At 12 weeks, there were marked differences in CD19+/B220+ B cells (2.2% in TMPD-treated versus 27.6% in mineral oil-treated mice) and the number of I-Ad+ cells (6.6% TMPD-treated versus 32.4% mineral oil-treated mice). Representative flow cytometry data are shown in Figure 1D. Although the percentage of B1/macrophages by scatter was significantly higher in TMPD-treated mice, very few of these were B1 cells, which would be reflected in the I-Ad+ and CD11bint population (Figure 1D). Additional staining with CD19, CD5, and B220 confirmed that B1 cells were markedly decreased in TMPD-treated mice (data not shown). Dendritic cells (CD11c+) represented ∼5% of the peritoneal exudate cells both in TMPD- and in mineral oil-treated mice. Because of the higher peritoneal cell counts, the absolute numbers of peritoneal dendritic cells were greater in TMPD- versus mineral oil-treated mice at 12 weeks (Figure 1C). Normal resident peritoneal cells contained very few dendritic cells. The expression of CD86 on peritoneal DCs from TMPD- versus mineral oil-treated mice was comparable (Figure 1D).
Figure 1.
Flow cytometry of peritoneal exudate cells. BALB/c mice were treated intraperitoneally with TMPD or mineral oil (MO). Peritoneal exudate cells were harvested by lavage 12 or 20 weeks later (TMPD-12, MO-12, TMPD-20, and MO-20, respectively). Resident peritoneal cells (RPCs) from three untreated BALB/c mice were analyzed for comparison. Pooled lipogranulomas from individual mice were harvested at 20 weeks after TMPD or mineral oil injection (TMPD-Lip and MO-Lip, respectively) and treated with collagenase and dispase. A: Total peritoneal cell counts. Total cell counts were determined using a hemocytometer (mean and SE are shown). B and C: Cellular composition of peritoneal exudates by flow cytometry. Peritoneal cells were analyzed by flow cytometry using anti-B220 and CD19 (B cells), anti-CD3 (T cells), anti-CD11b (monocytes and some B cells), and CD11c (dendritic cells). The percent (B) and absolute numbers (C) of B and lymphocytes, CD11b+ cells, and CD11b+, CD11c+ monocyte-derived dendritic cells are shown. D: Representative examples of the staining for mineral oil- versus pristane-treated mice. Peritoneal cells were stained with anti-B220 and anti-CD19 mAbs (left), anti-CD11b and anti-I-Ad mAbs (middle), or anti-CD11c and anti-CD86 mAbs (right).
Table 3.
Absolute Cell Counts in Lipogranulomas and Peritoneal Exudate at 20 Weeks
| Lipogranulomas | Peritoneal exudate | |||
|---|---|---|---|---|
| Absolute cell count (×10−6) | TMPD | Mineral oil | TMPD | Mineral oil |
| Total cells | 7.5 ± 1.5 | 1.6 ± 0.2 | 15 ± 4.6 | 8.5 ± 0.1 |
| B cells | ||||
| CD19+, CD5− | 0.5 ± 0.1 (6.4%) | 0.08 ± 0.03 (4.5%) | 1.8 ± 0.8 (11.8%) | 1.2 ± 0.3 (13.2%) |
| CD19+, CD5+ | 0.01 ± 0.003 (0.1%) | 0.001 ± 0.0001 (0.07%) | 0.1 ± 0.006 (0.06%) | 0.01 ± 0.002 (0.1%) |
| T cells | ||||
| CD3+ | 1.4 ± 0.2 (19%) | 0.2 ± 0.1 (16%) | 3.9 ± 1.3 (26%) | 1.5 ± 0.8 (17%) |
| CD3+, CD4+ | 0.6 ± 0.08 (8%) | 0.1 ± 0.04 (8%) | 2.6 ± 0.8 (17%) | 1.0 ± 0.6 (11%) |
| CD3+, CD4−* | 0.8 (11%) | 0.1 (8%) | 1.3 (8%) | 0.5 (6%) |
| Antigen-presenting cells | ||||
| CD11c+, CD11b− | 0.04 ± 0.005 (0.6%) | 0.02 ± 0.006 (1%) | 0.03 ± 0.01 (0.3%) | 0.04 ± 0.006 (0.6%) |
| CD11c+, CD11b+ | 0.4 ± 0.09 (5%) | 0.2 ± 0.06 (9%) | 0.09 ± 0.02 (0.6%) | 0.1 ± 0.01 (1%) |
| CD11c−, CD11b+ | 1.9 ± 0.5 (24%) | 0.6 ± 0.3 (20%) | 3.8 ± 1.8 (23%) | 2.9 ± 0.3 (34%) |
Between 12 and 20 weeks after injection of TMPD or mineral oil, the numbers of total B cells increased somewhat in both TMPD- and mineral oil-treated mice (Figure 1C, Table 3). T cell counts were greatly increased in TMPD- versus mineral oil-treated mice at 12 weeks, but did not increase further at 20 weeks (Figure 1C, Table 3). The absolute number of T cells in the peritoneal exudate of TMPD-treated mice was approximately twice that in mineral oil-treated mice; in both cases the ratio of CD4+/CD8+ cells was ∼2:1. Between 12 and 20 weeks, both the percentage and the absolute number of CD11c+ dendritic cells in the peritoneal exudate decreased in both TMPD- and mineral oil-treated mice, concomitant with the appearance of relatively large numbers of dendritic cells in the lipogranulomas (Figure 1, B and C; Table 3). These data suggest that intraperitoneal administration of TMPD stimulated an early influx of monocytes, dendritic cells, and T cells followed by an influx of B lymphocytes. TMPD was relatively more potent than mineral oil in attracting T lymphocytes into the peritoneal exudate and promoting the formation of lipogranulomas with large numbers of monocytes, dendritic cells, and T and B lymphocytes at 20 weeks (Table 3).
TMPD Granulomas Are Ectopic Lymphoid Tissue
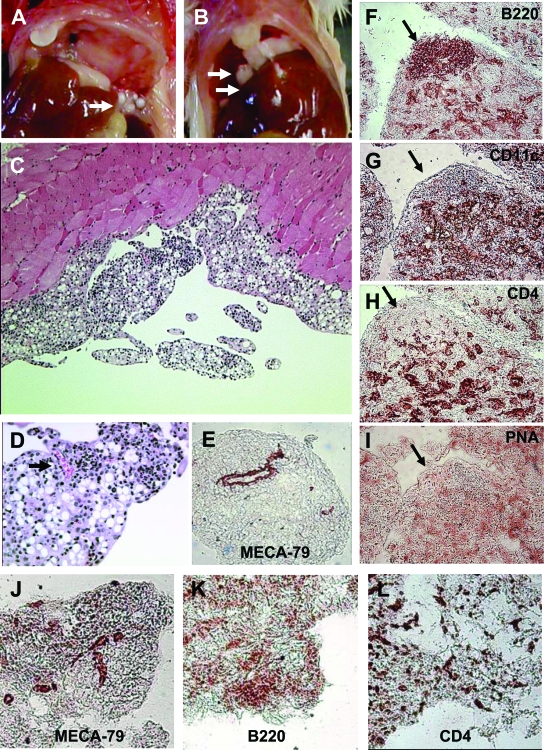
Chronic inflammation induced by either TMPD or mineral oil causes the formation of lipogranulomas adherent to the mesothelial surfaces of the peritoneal lining.19–22 However, relatively little is known about the organization of these structures. Lipogranulomas induced by TMPD (Figure 2A) were more compact and nodular than those induced by mineral oil (Figure 2B). Hematoxylin and eosin (H&E) staining of TMPD-induced granulomas revealed a predominantly mononuclear cell infiltrate surrounding numerous oil droplets (Figure 2C). Neovascularization of TMPD-induced lipogranulomas was apparent by H&E staining (Figure 2D). The blood vessels were strongly positive for peripheral lymph node addressin (MECA-79 staining), a marker for high endothelial venules (Figure 2E).
Figure 2.
Pathology of lipogranulomas. A: Lipogranulomas adherent to the mesothelial surface of a mouse treated 12 weeks earlier with TMPD (arrow). B: Lipogranulomas adherent to the mesothelial surface of the diaphragm of a mouse treated 12 weeks earlier with mineral oil (arrows). C: H&E staining of lipogranulomas from a TMPD-treated mouse. Note numerous oil droplets and lymphocytic infiltration. D and E: Neovascularization of TMPD-induced lipogranulomas. D: H&E staining of a peritoneal lipogranuloma from a mouse treated 12 weeks earlier with TMPD showing a blood vessel filled with erythrocytes (arrow). E: Immunohistochemistry of frozen tissue (5-μm sections, immunoperoxidase technique) from a peritoneal lipogranuloma from a mouse treated 12 weeks earlier with TMPD and stained with MECA-79 antibody (anti-peripheral lymph node addressin, a marker for high endothelial venules). F–I: Immunohistochemistry of serial sections through a TMPD lipogranuloma. A snap-frozen lipogranuloma was sectioned at 5 μm and stained by the immunoperoxidase technique as follows: biotinylated anti-mouse CD45R/B220 (F), biotinylated anti-mouse CD11c (G), biotinylated anti-mouse CD4 (H), and biotinylated peanut agglutinin (I). Arrow indicates the location of a collection of B cells in the serial sections. J–L: Immunohistochemistry of mineral oil lipogranulomas. Snap-frozen lipogranulomas from mineral oil-treated mice were sectioned (5 μm) and stained by the immunoperoxidase technique using biotinylated MECA-70 (anti-PNAd) (J), B220 (B cells) (K), or CD4 (T cells) (L).
Serial sections through individual TMPD-induced lipogranulomas revealed the presence of B220+ B cells, CD4+ T cells, and CD11c+ dendritic cells (Figure 2, F–H). In some cases, there was organization into discrete areas consisting of B cells, or T cells plus dendritic cells (Figure 2, F–H). However, the B cells did not stain with a germinal center marker, peanut agglutinin (Figure 2I). The lipogranulomas also contained large numbers of CD11b+ cells, which were probably mainly monocytes, macrophages, and dendritic cells (not shown). Thus, the overall structure of TMPD-induced peritoneal lipogranulomas resembled that of secondary lymphoid tissue, although with some differences, such as absence of peanut agglutinin staining and FDC-M1+ follicular dendritic cells (not shown). Immunohistochemistry of mineral oil-induced lipogranulomas also revealed the presence of MECA-79+ high endothelial venules (Figure 2J), B220+ B cells, and CD4+ T cells (Figure 2, K and L), but there was no evidence of organization into discrete T-cell/dendritic cell and B-cell zones as seen in some TMPD lipogranulomas.
When the lipogranulomas were dissociated with collagenase/dispase and analyzed by flow cytometry, it was apparent that lipogranulomas from TMPD-treated mice were considerably more cellular than those from mineral oil-treated mice (sevenfold higher total cells) (Table 3). This was reflected in the absolute numbers of B cells (sixfold increased), T cells (sixfold increased), monocytes (threefold increased), and both CD11c+/CD11b+ and CD11c+/CD11b− dendritic cells (twofold increased). As a percentage of total cells, dendritic cells were more abundant in lipogranulomas than in peritoneal exudate (6 to 10% in lipogranulomas from TMPD- or mineral oil-treated mice versus 1 to 2% in peritoneal exudate), suggesting that they might home specifically to the lipogranulomas.
Expression of Lymphoid Chemokines in Lipogranulomas
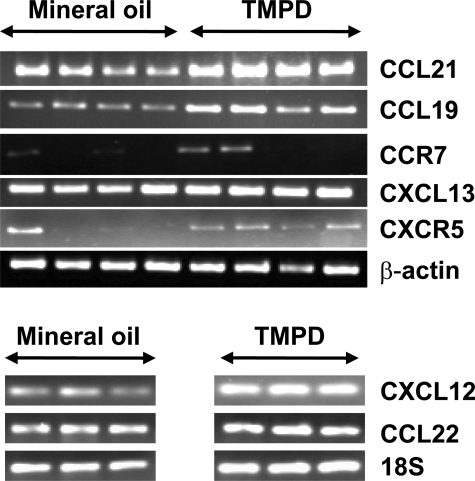
As high endothelial venules are a source of the T-cell/dendritic cell attractant chemokines SLC (CCL21) and ELC (CCL19),23,24 we looked for expression of CCL21/CCL19 as well as the B-cell attractant chemokine BLC (CXCL13) in TMPD and mineral oil lipogranulomas (Figure 3). Both TMPD and mineral oil-induced lipogranulomas expressed mRNA for CCL21 and CCL19, ligands for the CC-chemokine receptor CCR7, but levels appeared higher in TMPD versus mineral oil lipogranulomas. Expression of CCR7, the receptor on T cells and DCs for CCL21/CCL19, also was detected. In addition, TMPD and mineral oil lipogranulomas expressed the CXC-chemokine receptor CXCR5 and its ligand CXCL13 at high levels. Two other chemokines implicated in the organization of secondary lymphoid tissue, CXCL12 (SDF-1) and CCL22 (MDC), also were detected in both TMPD and mineral oil lipogranulomas (Figure 3, bottom). The data suggest that locally produced chemokines might mediate the accumulation of dendritic cells, T cells, and B cells within the lipogranulomas and their organization into distinct zones.
Figure 3.
Expression of chemokines and chemokine receptors in lipogranulomas. RNA isolated from pooled lipogranulomas (12 weeks) from four TMPD-treated and four mineral oil-treated mice was used to synthesize cDNA. Expression of SLC (CCL21), ELC (CCL19), CCR7, BLC (CXCL13), and CXCR5 (top), as well as SDF-1 (CXCL12) and MDC (CCL22) (bottom) was analyzed by RT-PCR, normalized to β-actin (top) or 18S ribosomal RNA (bottom).
Activation of Dendritic Cells in TMPD-Induced Lymphoid Tissue
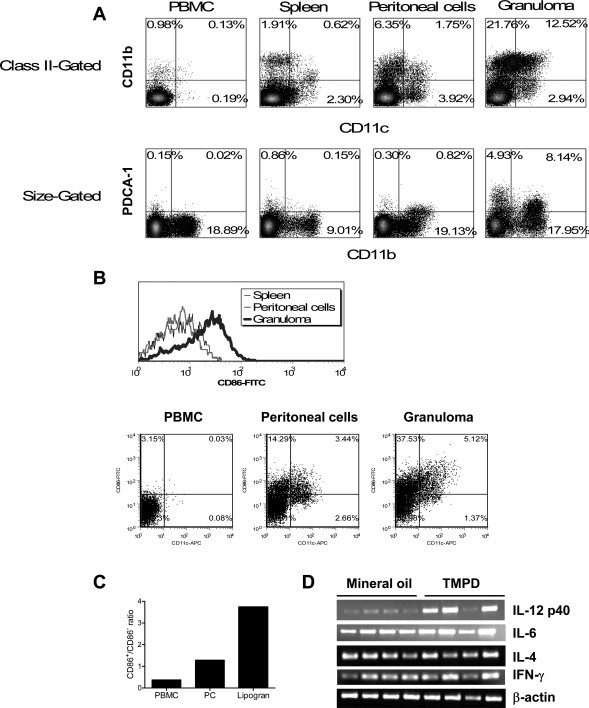
The resident peritoneal cells of normal mice contain few dendritic cells, whereas there were substantial numbers of CD11c+ cells in the peritoneal exudate (Figure 1) and lipogranulomas (Figure 2) of TMPD-treated mice. The activation state and maturation of these cells was evaluated by determining expression of the co-stimulatory molecule CD86 and cytokine production (Figure 4). As shown by flow cytometry of cells from a representative mouse, a progressive enrichment of CD11c+ cells from peripheral blood mononuclear cells (0.13% CD11c+/CD11b+ and 0.19% CD11c+/CD11b−) to spleen (0.62% and 2.30%) to peritoneal exudate cells (1.75% versus 3.92%, respectively) to dissociated lipogranuloma cells (12.52% versus 2.94%, respectively) was seen (Figure 4A, top). There was a striking enrichment of PDCA-1+ plasmacytoid dendritic cells in the lipogranulomas (Figure 4A, bottom). Percentages of PDCA-1+, CD11b− cells increased from 0.15% in PBMCs to 4.93% in the lipogranulomas. There also were large numbers of cells that were PDCA-1+, CD11b+, or CD11c+, CD11b+ in lipogranulomas (12.52% and 8.14%, respectively) but they were rare in PBMCs).
Figure 4.
TMPD lipogranulomas contain activated dendritic cells. A: Accumulation of dendritic cells in the lipogranulomas. Peripheral blood mononuclear cells (PBMCs), spleen, peritoneal exudate cells, and pooled lipogranuloma cells from a representative BALB/c mouse 20 weeks after intraperitoneal TMPD injection were analyzed by flow cytometry. Top: Percentages of CD11c−/CD11b+ monocytes/macrophages, CD11c+/CD11b+ monocyte-derived (?) dendritic cells, and CD11c+/CD11b− dendritic cells in each location (PBMCs, spleen, peritoneal exudate, and lipogranulomas) are indicated. Bottom: Percentages of PDCA-1+ dendritic cells in each location (peripheral blood, spleen, peritoneal exudate, and lipogranulomas) are shown. B: Lipogranuloma dendritic cells express high levels of CD86. Expression of CD86 by CD11c+ cells in the peripheral blood, spleen, peritoneal exudate, and lipogranulomas from representative TMPD-treated mice was determined by flow cytometry. Top: Mean fluorescence intensity of CD86 in CD11c-gated cells from spleen, peritoneum, and lipogranulomas. Bottom: CD11c and CD86 staining of PBMCs, peritoneal cells, and isolated lipogranuloma cells. C: Dendritic cells in lipogranulomas preferentially express CD86. Shown is the ratio of CD86+ to CD86− dendritic cells (CD11c+) in peripheral blood mononuclear cells (PBMCs), peritoneal cells (PCs), and TMPD-treated mouse lipogranulomas. D: Increased expression of IL-12 p40 in TMPD lipogranulomas. RT-PCR was performed using cDNA from lipogranulomas from four mineral oil-treated mice and four TMPD-treated mice. Sequences of the IL-12 p40, IL-6, IL-4, IFN-γ, and β-actin primers used are shown in Table 1.
Strikingly, lipogranuloma dendritic cells expressed high levels of CD86 (Figure 4B) and there was a progressive increase in the ratio of CD11c+/CD86+ cells to CD11c+/CD86− cells from blood to peritoneal exudate to lipogranulomas (Figure 4C), suggesting that homing to the lipogranulomas was associated with increased dendritic cell activation/maturation. The mean fluorescence intensity of CD86 staining on CD11c+ cells was considerably higher in the lipogranulomas than in either spleen or peritoneal exudate cells (Figure 4B). Expression of IL-12p40 mRNA also was higher in TMPD granulomas than in mineral oil granulomas (Figure 4D), consistent with increased activation of myeloid dendritic cells. In contrast, IL-6, IL-4, and IFN-γ expression was similar in TMPD and mineral oil granulomas, indicating that the increased expression of IL-12 was specific to TMPD treatment and not because of a generalized, nonspecific enhancement of cytokine expression.
Inflammatory Response to TMPD Is Associated with High Levels of Type I IFN
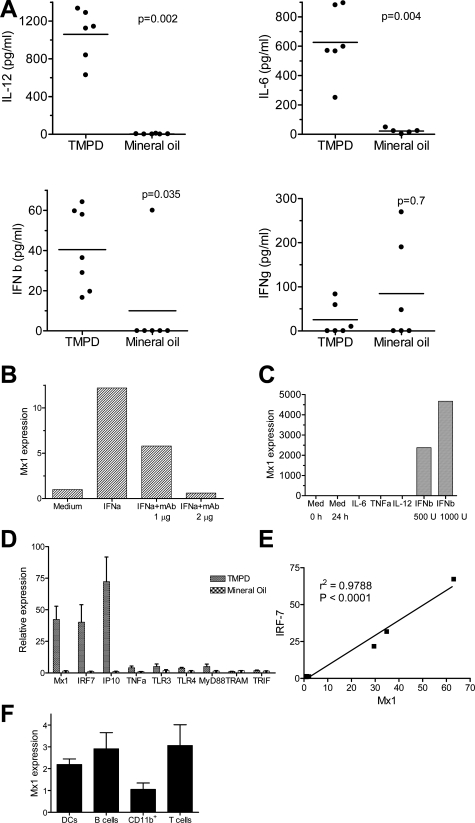
Although both TMPD and mineral oil cause peritoneal inflammation, TMPD induces lupus-like disease (autoantibodies and glomerulonephritis) but mineral oil does not. In light of the proposed role of IFN-α in SLE, it was of interest to see if TMPD stimulates IFN-I production. Peritoneal lavage was performed in mice treated 12 weeks earlier with TMPD or mineral oil and the levels of IL-12, IL-6, IFN-β, and IFN-γ in the washings were measured by ELISA (Figure 5A). IL-12 (P = 0.002), IL-6 (P = 0.004), and IFN-β (P = 0.035) (all Mann-Whitney test) were present at greatly increased levels in the peritoneal washings from TMPD-treated mice versus mineral oil-treated controls. The levels of IFN-γ in the peritoneal lavage were not statistically different between TMPD and mineral oil-treated mice (P = 0.7, Mann-Whitney). Similar results were obtained comparing PBS-treated controls with TMPD-treated mice (data not shown). Because all IFN-I isoforms (IFN-α, -β, -ω) signal via the same receptor, we explored the feasibility of measuring of IFN-I-inducible gene expression as a bioassay for the production of any of the IFN-I isoforms. Treatment of RAW264.7 cells with IFN-α increased expression of the IFN-I-inducible gene Mx1 and this could be blocked in a dose-dependent manner using a neutralizing antibody against IFN-α (Figure 5B). Mx1 was specific for IFN-I because IFN-β also enhanced its expression; in contrast there was no enhancement by TNF-α, IL-6, or IL-12 (Figure 5C). Peritoneal cells from TMPD-treated mice expressed markedly higher levels of Mx1 as well as other IFN-I-inducible genes (IRF-7 and IP-10) than cells from mineral oil-treated mice, indicating that TMPD strongly induced IFN-I production (Figure 5D). In contrast, differences in _TNF-_α, TLR3, TLR4, MyD88, TRAM, and TRIF expression in TMPD- versus mineral oil-treated mice were much less dramatic. Mx1 expression levels closely correlated with expression of IRF-7, another IFN-inducible gene (P < 0.0001, linear regression) (Figure 5E), consistent with coordinate expression of multiple IFN-regulated genes.17,18 Because peritoneal exudates contain a mixture of lymphocytes (T and B cells) and APCs (monocytes/macrophages and dendritic cells) in different proportions, it was important to exclude the possibility that one or more of these cell types might show a dispro-portionate response to IFN-I, potentially complicating interpretation of the data. B cells, T cells, monocytes/macrophages, and dendritic cells from TMPD-induced peritoneal exudate were purified using anti-CD19, -CD3, -CD11b, and -CD11c magnetic beads, respectively, and Mx1 expression was determined by real-time PCR. As shown in Figure 5F, there was not a significant difference between cell types in the level of Mx1 expression, consistent with the fact that essentially all cell types express type I IFN receptors. These data suggest that measurement of the expression of IFN-I-inducible gene expression provides a reasonable estimate of IFN-I production that is primarily independent of the cell type. We next used this assay to assess IFN-I production in the lipogranulomas that form in response to TMPD or mineral oil.
Figure 5.
IFN-I-inducible gene expression in peritoneal exudate cells. A: Cytokine expression in peritoneal washings. Peritoneal lavage was performed in mice treated with TMPD or mineral oil and levels of IL-12, IL-6, IFN-β, and IFN-γ in the washings were measured by sandwich ELISAs. Levels were compared using the Mann-Whitney test. B: Stimulation of Mx1 gene expression by IFN-α. RAW 264.7 cells were treated for 24 hours with IFN-α (1000 U/ml) in the absence or presence of anti-IFN-α neutralizing antibodies (1 or 2 μg/ml). Mx1 expression (normalized to β_-actin_) was measured from cDNA by real-time PCR. C: Specificity of Mx1 expression for IFN-I. RAW 264.7 cells were treated with IFN-β (1000 or 500 U/ml), IL-6 (5 ng/ml), TNF-α (20 ng/ml), or IL-12 (10 or 20 ng/ml) or with medium (Med) alone. After 24 hours, cells were harvested and RNA extracted for cDNA synthesis. Mx1 expression was determined by real-time PCR, normalized to β_-actin_. D: Increased expression in TMPD- versus mineral oil-elicited peritoneal cells. RNA was extracted and cDNA was synthesized from freshly collected peritoneal exudate cells of mice treated with TMPD or mineral oil. Expression of mRNAs for the IFN-inducible genes Mx1, IRF-7, and IP-10 as well as Toll-like receptors (TLR3, TLR4) and adapter proteins MyD88, TRAM, and TRIF was measured by real-time PCR normalized to β_-actin_. E: Correlation of Mx1 and IRF-7 expression. Expression of Mx1 and IRF-7 was measured (real-time PCR) in RNA isolated from either unstimulated peritoneal cells or LPS-stimulated peritoneal cells from the same group of mice (seven measurements). Expression of the two IFN-inducible genes correlated highly (r_2 = 0.9788, P < 0.0001). F: Mx1 expression by individual cell types. Dendritic cells (CD11c+), B cells (CD19+), monocytes/macrophages (CD11b+), and T cells (CD3+) were isolated from peritoneal exudates of TMPD-treated mice using magnetic beads and RNA was extracted. Mx1 expression by each cell type was determined by real-time PCR normalized to β_-actin.
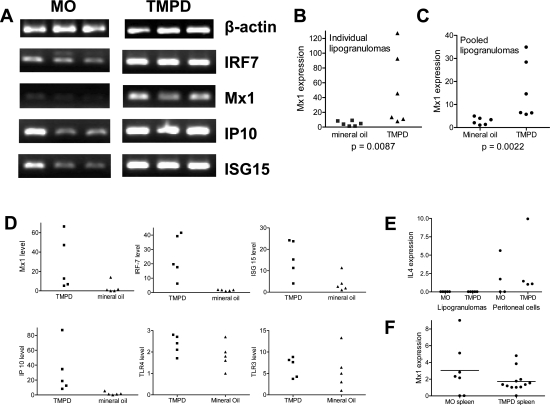
Expression of IFN-I in Lipogranulomas
Increased expression of IRF-7, Mx1, IP-10, and ISG15 was apparent in TMPD versus mineral oil lipogranulomas by RT-PCR (Figure 6A). This was confirmed using quantitative (real-time) PCR, which showed increased Mx1 expression in individual lipogranulomas of TMPD versus mineral oil-treated mice (Figure 6B). There was considerable variability among individual lipogranulomas from the same mouse, possibly reflecting differences in cellularity (data not shown). Pooled lipogranulomas from mice treated with TMPD (n = 6) or mineral oil (n = 6) again demonstrated increased Mx1 expression in the TMPD group (Figure 6C). Mx1, IRF-7, ISG-15, and IP-10 mRNA all were increased in individual lipogranulomas from TMPD versus mineral oil-treated mice, whereas TLR4 and TLR3 expression was comparable (Figure 6D). This was not because of the induction of a nonspecific increase in cytokine production by TMPD, because neither TMPD nor mineral oil lipogranulomas contained substantial amounts of IL-4. Peritoneal cells from TMPD-treated mice expressed higher levels of IL-4 than TMPD lipogranuloma cells (Figure 6E) and the peritoneal cells from mineral oil lipogranulomas expressed IL-4 at levels approximately comparable to TMPD peritoneal cells, further supporting the conclusion that TMPD specifically increased Mx1 expression. Moreover, spleen cells from TMPD and mineral oil-treated mice expressed comparable levels of Mx1, indicating that the enhanced production of IFN-I was localized to the sites of inflammation (lipogranulomas and peritoneal exudate) and was not a systemic response (Figure 6F). Taken together, the data shown in Figures 4C and 6 indicate that the inflammatory response to TMPD is distinguished from the response to mineral oil by localized production of IFN-I and IL-12.
Figure 6.
IFN-I-inducible gene expression in lipogranulomas. BALB/c mice were treated with TMPD or mineral oil and RNA was isolated from lipogranulomas adherent to the peritoneal lining or diaphragm at 12 weeks and used to synthesize cDNA. A: Expression of IFN-inducible genes. RT-PCR was performed for β_-actin_, IRF-7, Mx1, IP-10, and ISG-15 using lipogranuloma cDNA from mineral oil (MO)- or TMPD-treated mice. B: Mx1 expression, individual lipogranulomas. Expression of Mx1 normalized to β_-actin_ was measured in individual lipogranulomas (n = 6) from mice treated with either TMPD or mineral oil by real-time PCR. Mx1 expression was significantly higher in TMPD versus mineral oil lipogranulomas (P = 0.0087, Mann-Whitney test). C: Mx1 expression, pooled lipogranulomas. Expression of Mx1 normalized to β_-actin_ was measured in pooled lipogranulomas from six mice treated with TMPD and six mice treated with mineral oil. Mx1 expression (real-time PCR) was significantly higher in TMPD versus mineral oil lipogranulomas (P = 0.0022, Mann-Whitney test). D: Expression of other IFN-I-inducible genes, individual lipogranulomas. Gene expression in individual lipogranulomas from representative TMPD or mineral oil mice (five lipogranulomas per mouse) was measured by real-time PCR, normalized to β_-actin_. Expression of IFN-inducible genes Mx1, IRF-7, ISG-15, and IP-10 as well as TLR3 and TLR4 expression is shown. E: IL-4 expression in pooled lipogranulomas and peritoneal exudate. Expression of IL-4 normalized to β_-actin_ (real-time PCR) was measured in pooled lipogranulomas and peritoneal exudate cells from four mice treated with TMPD and four mice treated with mineral oil (MO). F: Mx1 expression in the spleen. Mx1 expression normalized to β_-actin_ (real-time PCR) was measured in spleen cells from mice treated with mineral oil (MO, n = 7) or TMPD (n = 12).
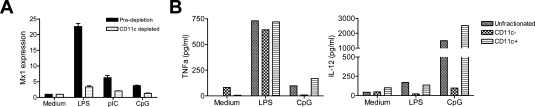
Dendritic Cells from TMPD-Treated Mice Are the Source of IFN-I
To identify the cell type(s) producing IFN-I in TMPD-treated mice, CD11c+ cells were depleted from the peritoneal exudate using magnetic beads. Nearly 98% of the CD11c+ cells were removed (7.29% versus 0.2% before and after fractionation). Peritoneal cells before and after depletion were then stimulated for 4 hours in vitro with TLR3, -4, or -9 ligands. As expected, all induced IFN-I production in unfractionated cells. After depleting the CD11c+ cells, expression of the IFN-I-inducible gene Mx1 was markedly reduced, indicating that the IFN-I came mostly from CD11c+ dendritic cells (Figure 7A). In contrast, LPS-stimulated _TNF-_α production (ELISA) was only slightly reduced by depleting CD11c+ cells, suggesting that it originated from nondendritic cells such as monocytes (Figure 7B). CpG ODN-stimulated _TNF-_α production was abrogated by depleting CD11c+ cells as was LPS- or CpG DNA-stimulated IL-12 production (Figure 7B). These data confirm that dendritic cells, presumably myeloid dendritic cells, were the primary source of IL-12 in TMPD-treated mice and suggest that IFN-I also was produced mainly by dendritic cells.
Figure 7.
IFN-I and IL-12 production by CD11c+ peritoneal cells. A: Reduced TLR ligand stimulated Mx1 expression after depleting CD11c+ cells. Unfractionated peritoneal exudate cells and peritoneal exudate cells depleted of CD11c+ cells (magnetic beads) were treated with LPS (10 μg/ml), CpG ODN no. 1826 (10 μg/ml), or poly (I:C) (50 μg/ml) for 4 hours before extracting RNA. Mx1 expression was quantified by real-time PCR (normalized to β_-actin_). B: Reduced TLR ligand stimulated IL-12 production after depleting CD11c+ cells. CD11c+ cells from peritoneal exudate of a mouse treated 5 months previously with TMPD were depleted with magnetic beads, and _TNF-_α (left) and IL-12 (right) production in culture supernatants in response to LPS (10 μg/ml) or CpG DNA (10 μg/ml ODN no. 1826) were determined 24 hours later by ELISA.
Discussion
TMPD, squalene, and other adjuvant hydrocarbons cause intense peritoneal inflammation and lipogranuloma formation19–22 leading to autoantibody production and autoimmune disease in nonautoimmune prone mice such as BALB/c.11,12,14 Although medicinal mineral oil also causes chronic peritoneal inflammation, it does not induce either lupus-specific autoantibodies or disease.16 This study provides evidence that intraperitoneal exposure to TMPD initiates a chronic inflammatory response that progresses to lymphoid neogenesis.1 As in other instances of lymphoid neogenesis,7–10 autoimmunity, in this case SLE, can follow.11,12 To our knowledge, TMPD-induced lupus provides the first evidence that lymphoid neogenesis can be involved in the pathogenesis of SLE. In view of the association of IFN-I production with SLE,17,18 it may be significant that the lupus-inducing hydrocarbon TMPD strongly stimulated IFN-I whereas mineral oil did not.
There is considerable evidence that the inflammatory response to TMPD is a form of lymphoid neogenesis. TMPD-induced lipogranulomas expressed SLC (CCL21) and ELC (CCL19), which attract T cells and dendritic cells expressing CCR7.25 By analogy with secondary lymphoid tissues,23,24 CCL21 may have been produced by high endothelial venules in the lipogranulomas (Figure 2), whereas it is more likely that the CCL19 was derived from dendritic cells or stromal cells.26,27 However, further studies are needed to verify this point. Consistent with the high levels of CCL21 and CCL19 expression, CD4+ T cells were found in TMPD lipogranulomas along with CD11c+ dendritic cells (Figure 2, Table 3). The lipogranulomas also contained CD8+ T cells, but they were difficult to enumerate because the CD8 molecule was partially degraded during collagenase/dispase treatment. The lipogranulomas also expressed large amounts of BLC (CXCL13) and by 20 weeks after TMPD treatment, large numbers of B lymphocytes were found in them (Figures 1 and 2, Table 3). At 12 weeks, the numbers of B cells in the peritoneal exudates of TMPD- or mineral oil-treated mice were substantially lower than the usual numbers of resident peritoneal B cells, suggesting that the peritoneal B cells may home to the lipogranulomas or perhaps undergo apoptosis. In some TMPD (but not mineral oil) lipogranulomas discrete zones containing T cells/dendritic cells and B cells were seen, reminiscent of the characteristic architecture of secondary lymphoid tissue. Finally, we have found evidence of somatic hypermutation of immunoglobulin variable region genes and clonal expansion of B cells in TMPD-induced lipogranulomas (D.C. Nacionales et al, manuscript in preparation).
Lymphoid neogenesis is associated with humoral autoimmunity in a variety of human diseases, although so far it has not been implicated in SLE.6,9 In idiopathic autoimmune diseases, such as autoimmune thyroiditis, myasthenia gravis, multiple sclerosis, Sjogren’s syndrome, and rheumatoid arthritis,7–10 the stimulus initiating development of ectopic lymphoid tissue is unknown. In the case of ectopic lymphoid tissue in chronic hepatitis C infection or Helicobacter pylori infection, the stimulus for lymphoid neogenesis and autoimmunity is chronic microbial infection.28–31 The characteristics of ectopic lymphoid tissue at different locations can vary. Follicular dendritic cells are prominent in the ectopic lymphoid tissue developing in response to H. pylori infection but there is little expression of CCL21,29 in contrast to the situation in TMPD-induced lymphoid neogenesis. A similar pattern occurs in rheumatoid synovium,10,32,33 although CCL21 is expressed when ectopic germinal centers develop,32 as in TMPD-induced tertiary lymphoid tissue. The expression of CXCL13 (BLC) is nearly ubiquitous in all forms of lymphoid neogenesis. It has been proposed that B-cell maturation (clonal expansion and immunoglobulin somatic hypermutation) is not necessarily restricted to germinal centers, and may occur wherever B cells are stimulated to undergo a substantial number of cell cycles under conditions in which antigen- and T cell-derived signals are present.34 Clonal expansion and somatic hypermutation have been reported in ectopic lymphoid tissues associated with autoimmune disease9,10 and is also seen in TMPD-induced lymphoid tissues (D.C. Nacionales et al, unpublished data). The expression of CCL21 and/or CCL19 and the recruitment of T cells and dendritic cells may be one of the factors determining whether or not autoimmunity develops. In the case of intraperitoneal mineral oil injection, CCL21/CCL19 expression was lower, few T cells were present in the ectopic lymphoid tissue, and an abortive form of autoimmunity develops.16 The high expression of CCL21/CCL19 in tertiary lymphoid tissue induced by TMPD and the relatively large numbers of T cells present (Figures 1 and 2, Table 3) may help convert preautoimmunity (mineral oil) to autoantibody production with full-blown autoimmune disease (TMPD).
Ectopic lymphoid tissue induced by TMPD contained numerous activated (CD86+) dendritic cells, including both myeloid dendritic cells producing IL-12 and PDCA-1+ plasmacytoid dendritic cells (Figure 4). Local production of IFN-I (Figure 6) is likely to have played a role in the maturation of myeloid dendritic cells in TMPD-induced lymphoid tissue.35 IL-12, a key product of myeloid dendritic cells,36 is greatly overproduced in the peritoneal cavities of mice treated with TMPD and other lupus-inducing hydrocarbons in comparison with mineral oil-treated mice.16 The severity of lupus induced by TMPD is diminished in IL-12-deficient mice,37 suggesting that one of the mechanisms by which TMPD causes lupus is by promoting the IFN-I-mediated maturation of myeloid dendritic cells.
This study provides the first evidence that certain types of ectopic lymphoid tissues can produce high levels of IFN-I, as also seen in inflamed lymph nodes.38 IFN-I production can be difficult to assess because there are 14 or more different IFN-I isoforms. The specificity of ELISAs for IFN-I depends on the detection antibodies.39 Measurement of serum IFN-I levels by ELISA is unreliable in lupus because of the presence of circulating rheumatoid factor in 20 to 30% or more of SLE sera.40 Rheumatoid factor can cross-link the two antibodies used in sandwich-type assays leading to a high frequency of false-positives.41 Moreover, it is technically difficult to measure IFN-I production by small numbers of dissociated lipogranuloma cells without stimulation (eg, by LPS). For all of these reasons, we used expression of the IFN-I-inducible gene Mx1 as a bioassay for signaling mediated by the binding of any of the IFN-I isoforms to the type I interferon receptor. This approach has been shown previously to accurately reflect IFN-I production.42 We confirmed that Mx1 gene expression was dependent on and specific for IFN-I (Figure 5, B and C) and that the expression levels of Mx1 and IRF-7 (a second IFN-inducible gene) were highly correlated (Figure 5E). Finally, we showed that Mx1 was expressed by all of the major cell types present in the peritoneal exudate and lipogranulomas (Figure 5F). This result was not unexpected because all of these cell types express type I IFN receptors. Thus, measurement of the expression of IFN-I-inducible genes, such as Mx1, appears to provide a reasonable estimate of IFN-I production.
Along with high Mx1 expression, other IFN-I-inducible genes were expressed by the peritoneal exudate cells and lipogranulomas from TMPD-treated mice at levels 50- to 100-fold higher than seen in mineral oil-treated mice (Figures 5 and 6) and peritoneal lavage fluid from TMPD-treated mice contained high levels of IFN-β by ELISA (Figure 5A). Intraperitoneal IL-6 and IL-12 production also were greatly increased in TMPD versus mineral oil-treated control mice (Figure 5A). We previously showed that IL-12 is produced at substantially higher levels in the peritoneal cavity in TMPD versus mineral oil-treated mice.16 Thus, like IL-12 (Figure 4D), IFN-I appears to be a specific feature of the local inflammatory response to TMPD (Figures 5 and 6). Despite the extraordinarily high expression of Mx1 and other IFN-I-inducible genes in TMPD lipogranulomas, there was very little IL-4. IL-4 and _IFN-_γ were expressed at comparable levels in peritoneal exudate and lipogranulomas from TMPD versus mineral oil-treated mice (Figures 4 and 6E), indicating that TMPD does not stimulate cytokine production nonspecifically. Interestingly, although the expression of IL-6 mRNA in TMPD versus mineral oil lipogranulomas was similar (Figure 4D), protein expression was higher in TMPD peritoneal washings (Figure 5), suggesting that TMPD may stimulate IL-6 production outside the lipogranulomas.
Because TMPD promotes the development of an IL-6- and IL-12-dependent lupus-like autoimmune syndrome in mice,11,12,37,43,44 whereas medicinal mineral oil does not,16 it is of interest to speculate on how IFN-I also might promote lupus. The gene expression profile in TMPD-elicited peritoneal cells and lipogranulomas (Figures 5 and 6) is consistent with the IFN signature reported recently in SLE.17,18 In humans, IFN-α treatment can induce antinuclear antibodies or even lupus45,46 and IFN-I expression is increased in lupus skin lesions.47 In NZB mice, autoimmune hemolytic anemia is milder in the absence of IFN-I signaling48 and (NZB × NZW)F1 mice develop a lupus-like syndrome that is accelerated by IFN-α.49 We have shown previously that TMPD exposure greatly accelerates the onset of lupus and disease severity in NZB/W mice.50 In light of the exacerbation of disease in NZB/W mice treated with IFN-α,49 it is likely that the chronic IFN-I production stimulated by TMPD also can accelerate lupus. However, despite the evidence that IFN-I production is linked with SLE, not all patients treated with IFN-α develop lupus and treatment of BALB/c mice with IFN-α does not induce lupus.49 Thus, other factors are likely to act in concert with IFN-I to cause lupus. We propose that local IFN-I production within ectopic lymphoid tissue (and outside of authentic germinal centers) may be important, perhaps by enhancing the activation, or inhibiting the censoring, of autoreactive B cells arising as part of a germinal center-like reaction taking place within ectopic lymphoid tissue.34
IFN-I enhances dendritic cell maturation and mediates the action of oil adjuvants (eg, incomplete Freund’s adjuvant) by enhancing the development of immature (tolerogenic) dendritic cells into mature (immunostimulatory) dendritic cells expressing high levels of CD86.51–55 It also has important effects on B-cell differentiation into plasma cells,56 isotype switching,51,57 and the survival of antigen-activated T cells.58 Switching to IgG2a, the predominant isotype of autoantibodies in murine lupus, is enhanced by IFN-α57 as is the establishment of B-cell memory.51 IFN-I production in lipogranulomas also could promote lupus by increasing B-cell expression of BLyS and APRIL, leading to CD40-independent immunoglobulin class switching and plasma cell differentiation.51,59 Thus, IFN-I may promote autoimmunity by several mechanisms. The TMPD lupus model affords a means of examining the interrelationships between lymphoid neogenesis, chronic inflammation, and IFN-I production in the pathogenesis of SLE and other autoimmune diseases. Inflammatory lesions closely resembling those seen in the peritoneal cavity of TMPD- or mineral oil-treated mice develop in humans who ingest or inhale mineral oil.21,22,60,61 However, it remains to be determined whether ingestion or aspiration of TMPD can trigger lupus in either humans or mice or whether the development of lupus is linked specifically to peritoneal exposure. Studies to address this question are in progress.
Acknowledgments
We thank Mr. Ed Butfiloski for assistance with flow cytometry, Dr. Sonali Narain for statistical expertise, and Ms. Gina Tonogbanua for assistance in preparation of the manuscript.
Footnotes
Address reprint requests to Westley H. Reeves, Division of Rheumatology and Clinical Immunology, University of Florida, PO Box 100221, Gainesville, FL 32610-0221. E-mail: whreeves@ufl.edu.
Supported by the US Public Health Service (research grants R01-AR44731 and R21-AR050661), Lupus Link, Inc. (Daytona Beach, FL), Mr. Lewis M. Schott (to the University of Florida Center for Autoimmune Disease), and the Malcolm Randall Veterans Administration Medical Center, Gainesville, FL. D.C.N. is the recipient of an Arthritis Foundation Postdoctoral Fellowship.
References
- Kratz A, Campos-Neto A, Hanson MS, Ruddle NH. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J Exp Med. 1996;183:1461–1472. doi: 10.1084/jem.183.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayton DL, Ying X, Lee J, Lesslauer W, Ruddle NH. Ectopic LT alpha beta directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase. J Exp Med. 2003;197:1153–1163. doi: 10.1084/jem.20021761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther SA, Lopez T, Bai W, Hanahan D, Cyster JG. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity. 2000;12:471–481. doi: 10.1016/s1074-7613(00)80199-5. [DOI] [PubMed] [Google Scholar]
- Yoneyama H, Matsuno K, Zhang Y, Murai M, Itakura M, Ishikawa S, Hasegawa G, Naito M, Asakura H, Matsushima K. Regulation by chemokines of circulating dendritic cell precursors, and the formation of portal tract-associated lymphoid tissue, in a granulomatous liver disease. J Exp Med. 2001;193:35–49. doi: 10.1084/jem.193.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmstrom P, Fjell J, Nakagawa T, Sacca R, Cuff CA, Ruddle NH. Lymphoid tissue homing chemokines are expressed in chronic inflammation. Am J Pathol. 2000;156:1133–1138. doi: 10.1016/S0002-9440(10)64981-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmstrom P. Lymphoid neogenesis: de novo formation of lymphoid tissue in chronic inflammation through expression of homing chemokines. J Leukoc Biol. 2001;69:331–339. [PubMed] [Google Scholar]
- Stott DI, Hiepe F, Hummel M, Steinhauser G, Berek C. Antigen-driven clonal proliferation of B cells within the target tissue of an autoimmune disease. The salivary glands of patients with Sjogren’s syndrome. J Clin Invest. 1998;102:938–946. doi: 10.1172/JCI3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xanthou G, Polihronis M, Tzioufas AG, Paikos S, Sideras P, Moutsopoulos HM. “Lymphoid” chemokine messenger RNA expression by epithelial cells in the chronic inflammatory lesion of the salivary glands of Sjogren’s syndrome patients: possible participation in lymphoid structure formation. Arthritis Rheum. 2001;44:408–418. doi: 10.1002/1529-0131(200102)44:2<408::AID-ANR60>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Schroder AE, Greiner A, Seyfert C, Berek C. Differentiation of B cells in the nonlymphoid tissue of the synovial membrane of patients with rheumatoid arthritis. Proc Natl Acad Sci USA. 1996;93:221–225. doi: 10.1073/pnas.93.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randen I, Mellbye OJ, Forre O, Natvig JB. The identification of germinal centres and follicular dendritic cell networks in rheumatoid synovial tissue. Scand J Immunol. 1995;41:481–486. doi: 10.1111/j.1365-3083.1995.tb03596.x. [DOI] [PubMed] [Google Scholar]
- Satoh M, Reeves WH. Induction of lupus-associated autoantibodies in BALB/c mice by intraperitoneal injection of pristane. J Exp Med. 1994;180:2341–2346. doi: 10.1084/jem.180.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh M, Kumar A, Kanwar YS, Reeves WH. Antinuclear antibody production and immune complex glomerulonephritis in BALB/c mice treated with pristane. Proc Natl Acad Sci USA. 1995;92:10934–10938. doi: 10.1073/pnas.92.24.10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter M, Wax JS. Genetics of susceptibility to pristane-induced plasmacytomas in BALB/cAn: reduced susceptibility in BALB/cJ with a brief description of pristane-induced arthritis. J Immunol. 1981;127:1591–1595. [PubMed] [Google Scholar]
- Wooley PH, Seibold JR, Whalen JD, Chapdelaine JM. Pristane-induced arthritis. The immunologic and genetic features of an experimental murine model of autoimmune disease. Arthritis Rheum. 1989;32:1022–1030. doi: 10.1002/anr.1780320812. [DOI] [PubMed] [Google Scholar]
- Bigazzi PE. Murine lupus induced by tetramethylpentadecane: an animal model of systemic human autoimmunity. Clin Immunol. 2005;114:97–99. doi: 10.1016/j.clim.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Satoh M, Kuroda Y, Yoshida H, Behney KM, Mizutani A, Akaogi J, Nacionales DC, Lorenson TD, Rosenbauer RJ, Reeves WH. Induction of lupus autoantibodies by adjuvants. J Autoimmun. 2003;21:1–9. doi: 10.1016/s0896-8411(03)00083-0. [DOI] [PubMed] [Google Scholar]
- Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, Shark KB, Grande WJ, Hughes KM, Kapur V, Gregersen PK, Behrens TW. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci USA. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, Pascual V. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter M, MacCardle RC. Histology of developing plasma cell neoplasia induced by mineral oil in BALB/c mice. J Natl Cancer Inst. 1964;33:497–515. [PubMed] [Google Scholar]
- Leak LV, Potter M, Mayfield WJ. Response of the peritoneal mesothelium to the mineral oil, pristane. Curr Top Microbiol Immunol. 1985;122:221–233. doi: 10.1007/978-3-642-70740-7_32. [DOI] [PubMed] [Google Scholar]
- Cruickshank B, Thomas MJ. Mineral oil (follicular) lipidosis: II. histologic studies of spleen, liver, lymph nodes, and bone marrow. Hum Pathol. 1984;15:731–737. doi: 10.1016/s0046-8177(84)80163-x. [DOI] [PubMed] [Google Scholar]
- Dincsoy HP, Weesner RE, MacGee J. Lipogranulomas in non-fatty human livers: a mineral oil induced environmental disease. Am J Clin Pathol. 1982;78:35–41. doi: 10.1093/ajcp/78.1.35. [DOI] [PubMed] [Google Scholar]
- Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci USA. 1998;95:258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekkevold ES, Yamanaka T, Palframan RT, Carlsen HS, Reinholt FP, von Andrian UH, Brandtzaeg P, Haraldsen G. The CCR7 ligand elc (CCL19) is transcytosed in high endothelial venules and mediates T cell recruitment. J Exp Med. 2001;193:1105–1112. doi: 10.1084/jem.193.9.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001;31:3388–3393. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Ngo VN, Tang HL, Cyster JG. Epstein-Barr virus-induced molecule 1 ligand chemokine is expressed by dendritic cells in lymphoid tissues and strongly attracts naive T cells and activated B cells. J Exp Med. 1998;188:181–191. doi: 10.1084/jem.188.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci USA. 2000;97:12694–12699. doi: 10.1073/pnas.97.23.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami J, Shimizu Y, Kashii Y, Kato T, Minemura M, Okada K, Nambu S, Takahara T, Higuchi K, Maeda Y, Kumada T, Watanabe A. Functional B-cell response in intrahepatic lymphoid follicles in chronic hepatitis C. Hepatology. 1999;30:143–150. doi: 10.1002/hep.510300107. [DOI] [PubMed] [Google Scholar]
- Mazzucchelli L, Blaser A, Kappeler A, Scharli P, Laissue JA, Baggiolini M, Uguccioni M. BCA-1 is highly expressed in Helicobacter pylori-induced mucosa-associated lymphoid tissue and gastric lymphoma. J Clin Invest. 1999;104:R49–R54. doi: 10.1172/JCI7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray RW, Elbourne K. Hepatitis C virus infection and autoimmunity. Semin Arthritis Rheum. 1997;26:689–701. doi: 10.1016/s0049-0172(97)80005-4. [DOI] [PubMed] [Google Scholar]
- Claeys D, Faller G, Appelmelk BJ, Negrini R, Kirchner T. The gastric H+,K+-ATPase is a major autoantigen in chronic Helicobacter pylori gastritis with body mucosa atrophy. Gastroenterology. 1998;115:340–347. doi: 10.1016/s0016-5085(98)70200-8. [DOI] [PubMed] [Google Scholar]
- Weyand CM, Goronzy JJ. Ectopic germinal center formation in rheumatoid synovitis. Ann NY Acad Sci. 2003;987:140–149. doi: 10.1111/j.1749-6632.2003.tb06042.x. [DOI] [PubMed] [Google Scholar]
- Page G, Miossec P. Paired synovium and lymph nodes from rheumatoid arthritis patients differ in dendritic cell and chemokine expression. J Pathol. 2004;204:28–38. doi: 10.1002/path.1607. [DOI] [PubMed] [Google Scholar]
- William J, Euler C, Christensen S, Shlomchik MJ. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science (Wash DC) 2002;297:2066–2070. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- Luft T, Luetjens P, Hochrein H, Toy T, Masterman KA, Rizkalla M, Maliszewski C, Shortman K, Cebon J, Maraskovsky E. IFN-alpha enhances CD40 ligand-mediated activation of immature monocyte-derived dendritic cells. Int Immunol. 2002;14:367–380. doi: 10.1093/intimm/14.4.367. [DOI] [PubMed] [Google Scholar]
- Reis e Sousa C, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain RN, Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvani N, Satoh M, Croker BP, Reeves WH, Richards HB. Nephritogenic autoantibodies but absence of nephritis in IL-12p35-deficient mice with pristane-induced lupus. Kidney Int. 2003;64:897–905. doi: 10.1046/j.1523-1755.2003.00178.x. [DOI] [PubMed] [Google Scholar]
- Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- Hornung V, Schlender J, Guenthner-Biller M, Rothenfusser S, Endres S, Conzelmann KK, Hartmann G. Replication-dependent potent IFN-alpha induction in human plasmacytoid dendritic cells by a single-stranded RNA virus. J Immunol. 2004;173:5935–5943. doi: 10.4049/jimmunol.173.10.5935. [DOI] [PubMed] [Google Scholar]
- Witte T, Hartung K, Sachse C, Matthias T, Fricke M, Kalden JR, Lakomek HJ, Peter HH, Schmidt RE. Rheumatoid factors in systemic lupus erythematosus: association with clinical and laboratory parameters. SLE study group. Rheumatol Int. 2000;19:107–111. doi: 10.1007/s002960050112. [DOI] [PubMed] [Google Scholar]
- Zhuang H, Narain S, Sobel E, Nacionales DC, Kelly KM, Richards HB, Segal M, Stewart C, Satoh M, Reeves WH. Association of anti-nucleoprotein autoantibodies with type I interferon production and dendritic cell maturation in systemic lupus erythematosus. Clin Immunol. 2005;117:238–250. doi: 10.1016/j.clim.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Gilli F, Sala A, Bancone C, Salacone P, Gallo M, Gaia E, Bertolotto A. Evaluation of IFNalpha bioavailability by MxA mRNA in HCV patients. J Immunol Methods. 2002;262:187–190. doi: 10.1016/s0022-1759(02)00008-x. [DOI] [PubMed] [Google Scholar]
- Richards HB, Satoh M, Shaw M, Libert C, Poli V, Reeves WH. IL-6 dependence of anti-DNA antibody production: evidence for two pathways of autoantibody formation in pristane-induced lupus. J Exp Med. 1998;188:985–990. doi: 10.1084/jem.188.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards HB, Satoh M, Jennette JC, Croker BP, Reeves WH. Interferon-gamma promotes lupus nephritis in mice treated with the hydrocarbon oil pristane. Kidney Int. 2001;60:2173–2180. doi: 10.1046/j.1523-1755.2001.00045.x. [DOI] [PubMed] [Google Scholar]
- Ioannou Y, Isenberg DA. Current evidence for the induction of autoimmune rheumatic manifestations by cytokine therapy. Arthritis Rheum. 2000;43:1431–1442. doi: 10.1002/1529-0131(200007)43:7<1431::AID-ANR3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Ronnblom LE, Alm GV, Oberg KE. Autoimmunity after alpha-interferon therapy for malignant carcinoid tumors. Ann Intern Med. 1991;115:178–183. doi: 10.7326/0003-4819-115-3-178. [DOI] [PubMed] [Google Scholar]
- Farkas L, Beiske K, Lund-Johansen F, Brandtzaeg P, Jahnsen FL. Plasmacytoid dendritic cells (natural interferon-alpha/beta-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am J Pathol. 2001;159:237–243. doi: 10.1016/s0002-9440(10)61689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Raber ML, Baccala R, Haraldsson KM, Choubey D, Stewart TA, Kono DH, Theofilopoulos AN. Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. J Exp Med. 2003;197:777–788. doi: 10.1084/jem.20021996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathian A, Weinberg A, Gallegos M, Banchereau J, Koutouzov S. IFN-alpha induces early lethal lupus in preautoimmune (New Zealand Black x New Zealand White) F1 but not in BALB/c mice. J Immunol. 2005;174:2499–2506. doi: 10.4049/jimmunol.174.5.2499. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Satoh M, Behney KM, Lee CG, Richards HB, Shaheen VM, Yang JQ, Singh RR, Reeves WH. Effect of an exogenous trigger on the pathogenesis of lupus in NZB X NZW (F1) mice. Arthritis Rheum. 2002;46:2235–2244. doi: 10.1002/art.10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bon A, Schiavoni G, D’Agostino G, Gresser I, Belardelli F, Tough DF. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14:461–470. doi: 10.1016/s1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature (Lond) 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebe K, Janssen EM, Kim SO, Alexopoulou L, Flavell RA, Han J, Beutler B. Upregulation of costimulatory molecules induced by lipopolysaccharide and double-stranded RNA occurs by Trif-dependent and Trif-independent pathways. Nat Immunol. 2003;4:1223–1229. doi: 10.1038/ni1010. [DOI] [PubMed] [Google Scholar]
- Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- Finkelman FD, Svetic A, Gresser I, Snapper C, Holmes J, Trotta PP, Katona IM, Gause WC. Regulation by interferon alpha of immunoglobulin isotype selection and lymphokine production in mice. J Exp Med. 1991;174:1179–1188. doi: 10.1084/jem.174.5.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189:521–529. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanless IR, Geddie WR. Mineral oil lipogranulomata in liver and spleen: a study of 465 autopsies. Arch Pathol Lab Med. 1985;109:283–286. [PubMed] [Google Scholar]
- Spickard A. Exogenous lipoid pneumonia. Arch Intern Med. 1994;154:686–692. [PubMed] [Google Scholar]