DNA Damage-Induced Cell Cycle Regulation and Function of Novel Chk2 Phosphoresidues (original) (raw)
Abstract
Chk2 kinase is activated by DNA damage to regulate cell cycle arrest, DNA repair, and apoptosis. Phosphorylation of Chk2 in vivo by ataxia telangiectasia-mutated (ATM) on threonine 68 (T68) initiates a phosphorylation cascade that promotes the full activity of Chk2. We identified three serine residues (S19, S33, and S35) on Chk2 that became phosphorylated in vivo rapidly and exclusively in response to ionizing radiation (IR)-induced DNA double-strand breaks in an ATM- and Nbs1-dependent but ataxia telangiectasia- and Rad3-related-independent manner. Phosphorylation of these residues, restricted to the G1 phase of the cell cycle, was induced by a higher dose of IR (>1 Gy) than that required for phosphorylation of T68 (0.25 Gy) and declined by 45 to 90 min, concomitant with a rise in Chk2 autophosphorylation. Compared to the wild-type form, Chk2 with alanine substitutions at S19, S33, and S35 (Chk2S3A) showed impaired dimerization, defective auto- and _trans_-phosphorylation activities, and reduced ability to promote degradation of Hdmx, a phosphorylation target of Chk2 and regulator of p53 activity. Besides, Chk2S3A failed to inhibit cell growth and, in response to IR, to arrest G1/S progression. These findings underscore the critical roles of S19, S33, and S35 and argue that these phosphoresidues may serve to fine-tune the ATM-dependent response of Chk2 to increasing amounts of DNA damage.
Activation of the DNA damage response plays a key role in safeguarding genomic DNA against errors that may occur during replication or that are induced by mutagenic intracellular metabolites or environmental agents. In eukaryotic cells, this response involves a coordinated network of events that sense DNA lesions and transduce this information to proteins that effect DNA repair, cell cycle arrest at multiple checkpoints, and eventually apoptosis (37). Defects in the DNA damage response system are associated with several inherited human disorders (40, 48, 52) and cancer (29, 30).
Signal transduction upon DNA damage involves the sequential activation of protein kinases and dynamic association with other interactors or adaptors, together amplifying the signal elicited by even a single DNA lesion. In response to DNA double-strand breaks (DSBs), ataxia telangiectasia-mutated (ATM), the protein kinase defective in ataxia telangiectasia (38), plays a fundamental role as the first activator of the damage response (31) by phosphorylating a wide range of target proteins on a consensus sequence characterized by serine or threonine residue followed by a glutamine (SQ/TQ sites). For an effective activity on substrates, ATM requires Nbs1, which recruits and dramatically enhances the activity of ATM (14, 21, 22, 33).
Human Chk2, the homolog of Saccharomyces cerevisiae Rad53 and Schizosaccharomyces pombe Cds1, is a kinase directly activated by phosphorylation on threonine 68 (T68) by ATM following DNA damage (5). Activated Chk2 propagates the damage signal through the phosphorylation of several targets involved in cell cycle phase progression or apoptosis (8). By phosphorylating Cdc25A and Cdc25C and targeting these proteins for degradation and sequestration in the cytoplasm, respectively (23, 35), Chk2 induces arrest at G1, S, and G2/M phases. Chk2 can also phosphorylate E2F-1, regulating its stability and transcriptional activity (50) and consequently apoptosis (45). Chk2 has been reported to phosphorylate p53, thereby enhancing the transcriptional activity of p53-responsive genes (51), although this event has been questioned in later investigations (28). The functional link between Chk2 and p53 in the DNA damage has been further substantiated in recent studies showing that Hdmx, a negative regulator of p53, is directly phosphorylated by Chk2 and this event accelerates Hdmx degradation (17, 32). Other known Chk2 substrates are Brca1 and PML (57, 58), implicated in DNA repair and apoptosis. Last, Chk2 has been shown to be involved in the replicative senescence signaling pathway in response to telomere erosion (24). The importance of Chk2 in the DNA damage response in cancer is underscored by the finding of CHK2 somatic mutations in various human tumors (reviewed in reference 8).
Chk2 shows three evolutionarily conserved domains: an N-terminal SQ/TQ cluster domain (SCD) (amino acids [aa] 19 to 69), which contains multiple consensus SQ/TQ phosphoresidues; a forkhead-associated (FHA) domain (aa 112 to 175); and a C-terminal catalytic domain (aa 220 to 486). ATM phosphorylates Chk2 primarily on T68 (4), which promotes Chk2 oligomerization through phospho-SCD/FHA interactions. The autophosphorylation step within the activation loop of the kinase domain (T383 and T387) then promotes the full activity of Chk2 (47). This multistep process allows the tightly controlled amplification of the DNA damage signal response.
In this study, we describe the phosphorylation of S19 and S33/S35 residues in vivo in response to DNA damage and their regulatory roles in Chk2 activation and function.
MATERIALS AND METHODS
Cell lines and treatments.
Lymphoblastoid cell lines (LBCs) were established by Epstein-Barr virus immortalization of blood from healthy (normal) individuals (LBC-N), from an AT patient (AT52RM) (kind gift of Luciana Chessa, University of Roma La Sapienza, Roma, Italy) and from two Nijmegen breakage syndrome (NBS) patients (GM07078 and 1548). The ataxia telangiectasia- and Rad3-related (ATR)-defective Seckel LBC DK0064 cell line (6) was a kind gift of Penny Jeggo, University of Sussex, Brighton, United Kingdom. LBCs were cultured in RPMI 1640 medium (BioWhittaker, Walkersville, MD) supplemented with 15% heat-inactivated fetal calf serum; MCF-7 breast adenocarcinoma, HCT15 colon cancer, and U2OS osteosarcoma cell lines were cultured in Dulbecco modified Eagle medium (BioWhittaker) plus 10% fetal calf serum. Culture media contained penicillin (100 U/ml) and streptomycin (100 μg/ml). Irradiations were performed with an IBL437CO instrument (Oris Industries, France) equipped with a 137Cs source providing 675 cGy/min. In some experiments, 4-nitroquinoline 1-oxide (4-NQO) (0.2 or 2 μM) was added to exponentially growing LBCs for 1 h and removed by washing, and incubation was continued for 30 min to allow cells to recover before harvesting. Treatments with 1 mM hydroxyurea (HU) were for 18 h, and an aliquot of the harvested samples was analyzed by flow cytofluorimetry to confirm S-phase arrest. The proteasome inhibitor _N_-acetyl-leucinal-leucinal-norleucinal (ALLN) (Sigma-Aldrich, St. Louis, MO) was added (50 μM) to cells 2 h before irradiation. Caffeine (Sigma-Aldrich, St. Louis, MO) was added (3 mM) 30 min before 4-NQO or HU treatment.
Plasmids and transfections.
The full-length wild-type Chk2 cDNA was cloned in the EcoRI/NotI sites of pCDNA3-HA and pCDNA3-FLAG vectors. Alanine substitutions on T68, S33/35, S33, and S35 were introduced with the QuikChange II site-directed mutagenesis kit (Stratagene, La Jolla, CA), while Ser19-to-Ala and Lys249-to-Arg (K249R to produce a kinase-dead Chk2 [Chk2-KD]) substitutions were introduced with the Gene Editor kit (Promega, Madison, WI). The forward primers used were as follows: 5′-GCTCCTTAGAGACAGTGTCCgCTCAGGAACTCTATTCTATTC-3′ for the T68A mutation, 5′-TCCCAAGGCTCCTCCgCACAGgCCCAGGGCATATC-3′ for S33A and S35A mutations, 5′-CCCAAGGCTCCTCCgCACAGTCCCAGGGC-3′ for S33A, and 5′-GGCTCCTCCTCACAGgCCCAGGGCATATC for S35A. Primers to obtain S19A and Chk2-KD mutants, respectively, were as follows: 5′-CAGTGCCTGTgCACAGCCCCATG-3′ and 5′-AAAGTAGCCATAAgaATCATCAGCAAAAG-3′.Lowercase letters indicate mutated bases. To obtain the S19A S33A S35A mutant (S3A), the HA-Chk2 S19A coding vector was subsequently mutagenized using the QuikChange II kit and the same primer used to introduce S33A and S35A mutations in the HA-Chk2 Ser33A/S35A construct. To obtain the HA-Chk2-KD-FHAΔ mutant, two PCR fragment (encoding aa 1 to 115 and 175 to 543) were cloned in the pCDNA3-HA vector. For stable suppression of Chk2 by short hairpin RNA interference, MCF7 cells were transfected with the pRetro-SUPER vector (kindly provided by Reuven Agami, The Netherlands Cancer Institute, Amsterdam, The Netherlands) carrying Chk2 (pSUPER-Chk2 [ATCTTTATAAGACAGTCCTCTT]) or green fluorescent protein (GFP) (pSUPER-GFP [5′-CAAGCUGACCCUGAAGUUC-3′]) interference sequences (the latter used as a negative control). Following selection with 1 μg/ml puromycin, single clones were expanded and characterized for Chk2 protein expression. For complementation experiments in MCF7 cells in which endogenous Chk2 was silenced by short hairpin RNA (shRNA), the pCDNA3-HA-Chk2wt and pCDNA3-HA-Chk2S33A/S35A constructs were engineered with two silent mutations inserted within the region targeted by the Chk2 shRNA (the forward primer used was 5′-CTCAAGAAGAGGACTGTCTcATtAAGATTACTGATTTTGGGC-3′). In this way, the shChk2 RNA, while interfering with the endogenous Chk2 mRNA, did not interfere with the ectopic Chk2 cDNAs. The constructs were transfected in the shChk2-MCF7 cells, and selection was carried out with 400 μg/ml G418. The full-length wild-type Chk2 cDNA and the triple mutant S3A were then introduced in the pEGFP and pdsRed-2 vectors to produce GFP and Red2 fusion proteins, respectively. These constructs were transfected into MCF7 and HCT15 cell lines, and when indicated, positive cells were purified by fluorescence-activated cell sorting (FACS). Exponentially growing cells were transiently transfected with the constructs using TransFast (Promega) according to the manufacturer's instructions. Stable transfectants of MCF7 and HCT15 cells were obtained by selection for 2 weeks in G418 (600 μg/ml and 1,200 μg/ml, respectively). ATR silencing was achieved by transfection of U2OS cells with an ON-TARGET plus SMART pool (Dharmacon Research, Lafayette, CO).
Immunoblot analyses.
Immunoblotting was performed as described previously (11). Mouse monoclonal anti-Chk2 antibody 44D4/21 was generated in-house (12), while rabbit antibodies specific for Chk2 phosphoresidues T68, S19, S33/35, and T387 were purchased from Cell Signaling Technology (Beverly, MA) and used to detect T387 (1:400), S19 (1:800), and S33/35 and T68 (1:1,000). The specificity of these phospho-specific antibodies was confirmed on immunoblots of cells ectopically expressing Ser-to-Ala mutants of Chk2 at residues 19, 33, and 35 (see Fig. S1 in the supplemental material). Note that anti-phospho-S33/S35 reacted with both S33 and S35 phosphoresidues, since the binding to single Ser-to-Ala substitutions was not abolished. The specificity and sensitivity of each phospho-specific antibody were also evaluated by indirect enzyme-linked immunosorbent assays on phosphorylated peptides adsorbed on microtiter plates and/or by enhanced chemiluminescence (ECL) on a nitrocellulose membrane spotted with constitutively phosphorylated bacterial recombinant glutathione _S_-transferase-Chk2 protein (see Fig. S1 in the supplemental material). High-performance liquid chromatography (HPLC)-purified peptides with phosphorylated Ser19 or Thr68 were synthesized by Primm (Milan, Italy), whereas the peptide with phosphorylated Ser33 and Ser35 residues was purchased from Cell Signaling Technology. Monoclonal antibodies against FLAG tag (clone M2; Sigma, St. Louis, MO), hemagglutinin (HA) tag (clone 12CA5; Roche, Mannheim, Germany), β-actin (Sigma), Hdmx (Bethyl Laboratories, Montgomery, TX), and cyclin B1 (clone GNS-1; BD Pharmingen, San Jose, CA) and rabbit antibodies against ATR (ABR Inc., Golden, CO), pS317 Chk1 (Cell Signaling Technology, Beverly, MA), and GFP (Santa Cruz BioTechnology, Santa Cruz, CA) were used. Antibody binding to polyvinylidene difluoride membranes was revealed by ECL Super Signal (Pierce, Rockford, IL) on autoradiographic films. Densitometric analyses of scanned bands were performed with the ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
FACS.
Staining and sorting of viable G1, S, and G2-M fractions were performed as described previously (18). Briefly, LBCs were irradiated, incubated for 25 min, treated with 10 mg/ml of the DNA-specific permeable dye Hoechst 33342 (Calbiochem, La Jolla, CA) and harvested 20 min later. Cells were separated according to cell cycle phase with a FACS-Vantage sorter (BD Biosciences) equipped with a UV argon ion laser and a sample refrigeration system.
Immunoprecipitations and in vitro kinase reactions.
Cells were lysed on ice for 30 min in buffer containing 50 mM Tris-HCl, pH 7.4, 0.2% Triton X-100, 0.3% NP-40, 150 mM NaCl, and 1 mM EDTA plus protease and phosphatase inhibitors (1 mM phenylmethylsulfonyl fluoride [PMSF], 1 μg/ml pepstatin, 2 μg/ml leupeptin, 2 μg/ml aprotinin, 25 mM NaF, 1 mM Na3VO4). Lysates were clarified by centrifugation, precleared for 1 h with 10 μl Sepharose-protein G (Sigma), and immunoprecipitated at 4°C for 2 h with 5 μg of the anti-Chk2 monoclonal antibody 44D4/21 using 10 μl of Sepharose-protein G. Kinase reactions were carried out at 30°C for 30 min in 20-μl volume containing 50 mM HEPES, pH 8.0, 10 mM MgCl2, 2.5 mM EDTA, 1 mM dithiothreitol, 10 μM β-glycerophosphate, 1 mM NaF, 0.1 mM Na3VO4, 0.1 mM PMSF, 10 μM ATP, 20 μCi [γ-32P]ATP, and 1 μg of glutathione _S_-transferase-Cdc25C fragment as a substrate (53). Reaction products were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, autoradiographed, and immunoblotted for Chk2 to verify the amount of immunoprecipitated protein per sample.
Gel filtration and dimerization analysis.
Whole-cell extracts were prepared from untreated or IR-treated LBCs or HCT15 cells with NETN buffer (150 mM NaCl, 20 mM Tris-HCl, pH 8.0, 1 mM EDTA, and 0.1% NP-40). Extracts (250 μl) were loaded on a Superdex 200 HR 10/30 gel filtration column (Amersham Biosciences, United Kingdom) equilibrated with NETN buffer and run in the same buffer at a flow rate of 0.4 to 0.5 ml/min (55). Twenty fractions of 400 to 500 μl in the 30- to 600-kDa range were collected. The column was calibrated using 100 μl of gel filtration molecular weight markers (Bio-Rad, Hercules, CA). To evaluate the ability of Chk2 to homodimerize, HCT15 cells were cotransfected with HA-tagged wild-type Chk2 (HA-Chk2wt) and FLAG-Chk2wt or alternatively with HA-Chk2S3A and FLAG-Chk2S3A. After 24 h, cells were exposed to 0 or 10 Gy of IR and 1 h later lysed with NETN buffer. Lysates were precleared for 1 h with Sepharose-protein G and immunoprecipitated with anti-HA antibody for 3 h at 4°C. Immunoprecipitated complexes were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted with an anti-FLAG antibody.
G1/S transition analysis.
HCT15 cells were transfected with the GFP fusion constructs described above and 24 h later treated for 30 min with 10 μM bromodeoxyuridine (BrdU) and 200 ng/ml nocodazole (2), then harvested (untreated sample) or irradiated with 10 Gy, and collected 6 and 24 h later. Cells were labeled by double-color immunofluorescence with a polyclonal anti-GFP and monoclonal anti-BrdU antibodies. Three hundred GFP-positive cells were evaluated for BrdU staining.
RESULTS
S19 and S33/S35 are phosphorylated in vivo in an ATM- and Nbs1-dependent way in response to IR.
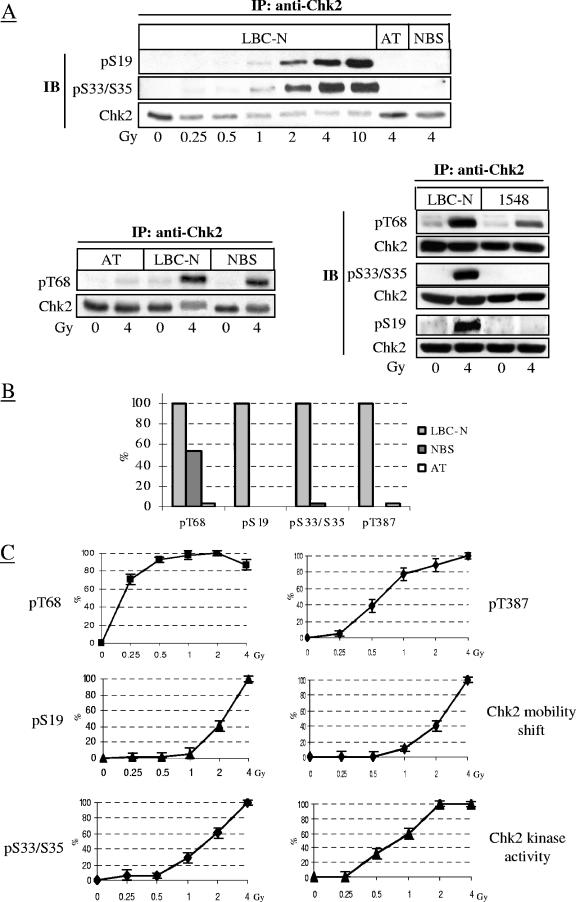
Seven SQ/TQ residues located in the N-terminal SQ/TQ-rich region of Chk2 provide a consensus motif for phosphorylation by ATM and other phosphatidylinositol 3-kinase family members (1, 39). IR dose- and time-dependent analysis of endogenous Chk2 immunoprecipitated from normal LBC-N cells and Western blotted with antibodies phospho specific for Chk2 Ser19 (S19) and Ser33/35 (S33/S35) (the specificity and sensitivity of these antibodies are described in Materials and Methods and data shown in Fig. S1 in the supplemental material) revealed a marked phosphorylation of S19 and S33/S35, but only in response to IR doses of ≥1 Gy (Fig. 1A). Similar analyses were performed on cells from an AT patient (AT52RM, carrying heterozygous ATM mutations 7327C and T/8365delA, null for ATM protein) (19) and two NBS patients (GM07078 and 1548, homozygous for the NBS1 mutation 657del5 and 835del4, respectively). These cell lines, characterized by impaired Chk2 activation (11, 13, 15), revealed virtually no detectable phosphorylation of S19 and S33/S35 in response to 4 Gy of IR, whereas phosphorylation of T68 was very faint in the cell line from the AT patient and about 50% lower in both NBS cell lines (Fig. 1A; results summarized in Fig. 1B).
FIG. 1.
IR-induced in vivo phosphorylation of S19 and S33/S35 and dependence on ATM and Nbs1. (A) Chk2 immunoprecipitated from extracts of LBC-N, AT cells, and two NBS cell lines (GM07078 [denoted as NBS] and 1548) collected 1 h after exposure to increasing doses of IR were immunoblotted with antibodies detecting specific phosphoresidues or total Chk2. Abbreviations: IP, immunoprecipitation; IB, immunoblotting; pS19, phospho-S19. (B) The graph shows the densitometric levels of Chk2 phosphorylation at T68, S19, S33/S35, and T387 at 1 h after IR, assessed on immunoblots of immunoprecipitated Chk2. Values were normalized to those of irradiated LBC-N cells. (C) IR dose-response results refer to LBC-N and were derived from the densitometry analysis of the experiment shown in panels A and B. Chk2 electrophoretic mobility shift was visualized on immunoblots (not shown) of total cell lysates run under conditions that allowed optimal separation of the phosphorylated Chk2 forms. Activity of immunoprecipitated Chk2 was measured in in vitro kinase reactions containing a Cdc25C substrate. Signals were quantified by optical densitometry, and results are the means (± standard deviations [error bars]) of three independent experiments. For each phosphoresidue, values were normalized to those of LBC-N cells irradiated with 4 Gy of IR.
In LBC-N cells, the induced phosphorylation of S19 and S33/S35 correlated with slower electrophoretic mobility, autophosphorylation on T387, and in vitro catalytic activity of Chk2, all observed in response to >1 Gy of IR (Fig. 1C). Conversely, phosphorylation of T68 was induced by doses of IR as low as 0.25 Gy, which had no effect on the electrophoretic mobility or catalytic activity of Chk2 (Fig. 1C) (12), indicating that albeit indispensable, phosphorylation of T68 alone does not confer full activity to Chk2.
These data demonstrate that at least four residues within the SCD of Chk2 are responsive in vivo to IR and that in contrast to phosphorylation of T68, which primes Chk2 for activation (4), phosphorylation of S19 and S33/S35 enhances Chk2 activity. Moreover, like ATM, the full-length Nbs1 (which is absent in NBS cells), is required for the phosphorylation of S19 and S33/S35, but unlike ATM, it is relatively dispensable for T68 phosphorylation, underscoring the functional requirement of Nbs1 for ATM activity, essential for full activation of Chk2.
S19, S33/S35, and T68 phosphorylations are not interdependent nor influenced by the catalytic integrity of Chk2.
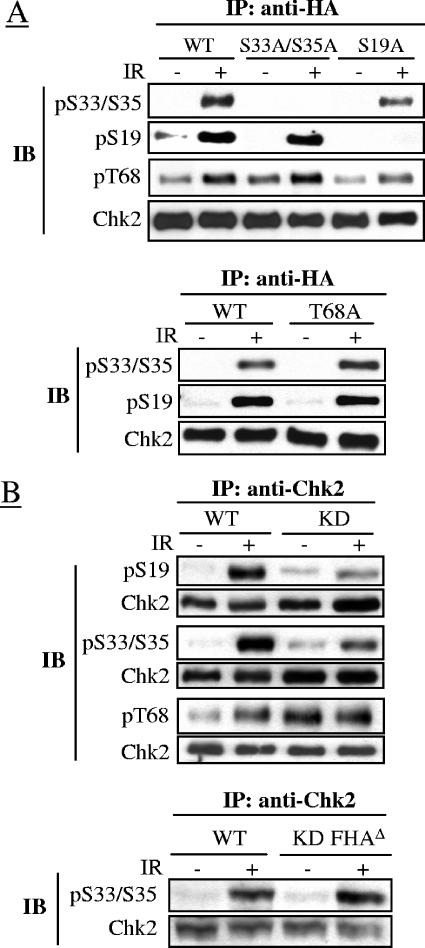
The roles of these phosphoresidues in relation to Chk2 activation were evaluated in the HCT15 cells, which carry a mutation in the CHEK2 gene and express limiting amounts of Chk2 with undetectable kinase activity (9, 55). In these cells stably transfected with expression constructs encoding Chk2 with alanine substitutions at S19, S33/S35, and T68, we found an apparently normal IR-induced phosphorylation of the Chk2-T68A mutant on S19 and S33/S35, like the phosphorylation of Chk2-S33A/S35A and Chk2-S19A on T68 (Fig. 2A), thus excluding an interdependence of these phosphorylations in vivo. On the contrary, a kinase-dead Chk2 ectopically expressed in HCT15 cells (see Fig. S2 in the supplemental material) exhibited reduced phosphorylation of these residues after IR (Fig. 2B). This result could be explained by the requirement of the autocatalytic activity for optimal phosphorylation of S19 and S33/S35 but could also be explained by an extensive dimerization of Chk2-KD through an SCD/FHA interaction, as previously found even in the absence of DNA damage (47), that might hinder the phosphorylation of these residues. To test this possibility, Chk2-KD deleted of its FHA domain (Chk2-KD-FHAΔ), thus unable to dimerize, was ectopically expressed in HCT15 cells and examined for S33/S35 phosphorylation, but compared to Chk2wt, Chk2-KD-FHAΔ showed the same level of S33/S35 phosphorylation (Fig. 2B). These results suggest that the defective phosphorylation of S33/S35 in Chk2-KD might arise from a steric hindrance effect as a result of its dimerization.
FIG. 2.
Interdependence of phosphorylation events in wild-type Chk2 and kinase-dead forms. (A) HA-tagged S19A, T68A, S33A, S35A, and S33A/S35A mutant forms of Chk2 transiently expressed in HCT15 cells were immunoprecipitated 45 min after no IR (−) or 10 Gy of IR (+) and immunoblotted with antibodies detecting the indicated phosphoresidues or total Chk2. Abbreviations: IP, immunoprecipitation; WT, wild-type Chk2; IB, immunoblotting; pS33/S35, phospho-S33/S35. (B) HA-tagged wild-type Chk2 and KD and KD-FHAΔ forms of Chk2 transiently expressed in HCT15 cells were immunoprecipitated 45 min after no IR (−) or 10 Gy of IR (+) and immunoblotted with antibodies detecting the indicated phosphoresidues or total Chk2.
S19 and S33/S35 residues are phosphorylated only in response to DSBs.
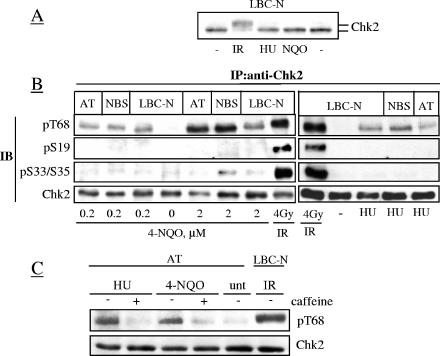
The role of Chk2 in response to DSBs is firmly established (12, 27) but less defined in response to other types of DNA lesions (10, 25, 36). To further assess the selectivity of Chk2 for DNA lesions, LBC-N were treated with the UV-mimetic 4-NQO and with the replication fork blocker hydroxyurea. Low doses of these agents (0.2 to 2 μM 4-NQO, 1 mM HU) and limited exposure times were used to avoid the indirect formation of DSBs (see Fig. S3 in the supplemental material), and their respective genotoxic effects were confirmed by the accumulation of p53 (determined by immunoblot analysis [data not shown]) and S-phase-arrested cells (>80% after 18 h, as detected by flow cytometry [data not shown]). Unlike IR, neither 4-NQO nor HU caused any appreciable electrophoretic mobility shifts of Chk2 (Fig. 3A). However, neither 4-NQO nor HU induced the phosphorylation of S33/S35 and S19, although each stimulated to some extent the phosphorylation of T68 independent of ATM and Nbs1 (Fig. 3B), as reported previously (36). Since the ATM/ATR inhibitor caffeine (46) suppressed this T68 phosphorylation in AT cells (Fig. 3C), most likely this event reflected the activity of ATR. Thus, while T68 is a signaling target of different types of DNA lesions in an ATM- or ATR-dependent way, S19 and S33/S35 are signaling targets of DSBs only.
FIG. 3.
4-NQO and HU do not induce Chk2 phosphorylation on S19 or S33/35 residues. Cells were treated with the UV-mimetic 4-NQO for 1 h, washed, and incubated for another hour before harvesting. The HU treatment was 1 mM HU for 18 h. (A) Electrophoretic mobility shift of Chk2 from total cell lysates. (B) Chk2 immunoprecipitates from treated cells were Western blotted with antibodies for the indicated phosphoresidues or total Chk2. Abbreviations: IP:anti-Chk2, immunoprecipitation with anti-Chk2 antibody; IB, immunoblotting; pT68, phospho-T68. (C) T68 phosphorylation was evaluated in AT cells pretreated with 3 mM caffeine (+) 30 min before the addition of HU or 4-NQO as described above. unt, untreated.
S19 and S33/S35 differ in dephosphorylation kinetics after IR.
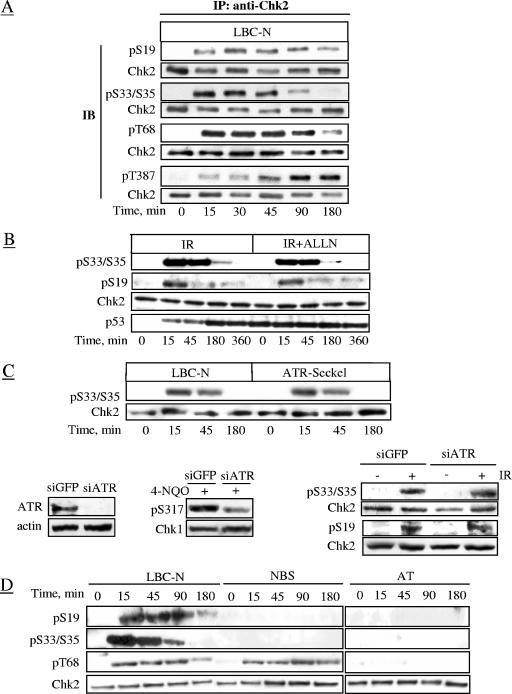
Time course analyses of Chk2 phosphorylation in LBC-N cells revealed marked levels of S19, S33/S35, and T68 phosphorylation within 15 min of IR, increasing thereafter in the case of S19 and S33/S35 and reaching a plateau at 30 to 45 min (Fig. 4A). Levels of S19 and T68 phosphorylation began to decline at 180 min, whereas S33/S35 phosphorylation markedly declined at 90 min, coinciding with a rise in levels of T387 autophosphorylation (Fig. 4A). Thus, Chk2 activation is slower than that of ATM (7). Similar kinetics of S19 and S33/S35 phosphorylation/dephosphorylation were seen in the U2OS osteosarcoma cell line (data not shown). Together, these results indicate that phosphorylation of the SCD is necessary to promote but not to sustain Chk2 activation. Moreover, LBC-N cells treated with the proteasome inhibitor ALLN before IR exhibited the time-dependent decrease in phospho-S19 and phospho-S33/S35 (Fig. 4B), suggesting that this decrease reflects an active dephosphorylation rather than a proteolytic process. The kinetics of IR-induced phosphorylation of S33/S35 (Fig. 4C) and of S19 (data not shown) were unaffected by ATR, as seen in the ATR-defective Seckel cell line. This was further supported by the results obtained in U2OS cells in which ablation of ATR expression by small interfering RNA (siRNA) caused a marked decrease in Chk1 phosphorylation in response to HU exposure (thus confirming the impairment of ATR function) but left unaffected the phosphorylation of S19 and S33/S35 in response to IR (Fig. 4C).
FIG. 4.
Time course analysis of phosphorylative events. (A) Chk2 immunoprecipitates from LBC-N cells collected at various time points after 4 Gy of IR were immunoblotted with the indicated antibodies. Abbreviations: IP, immunoprecipitation; IB, immunoblotting; pS19, phospho-S19. (B) LBC-N cells were preincubated for 2 h with 50 μM ALLN proteasome inhibitor, then treated with 4 Gy of IR, and collected at various time points thereafter, and tested by immunoblot analysis. (C) ATR-Seckel cells were analyzed at the indicated time points after treatment with 4 Gy of IR. In the bottom panels, U2OS cells were transfected with ATR siRNA or GFP siRNA as a control. ATR protein levels were evaluated 72 h later. Chk1-S317 phosphorylation was analyzed 18 h after 4-NQO exposure, while S33/35 and S19 phosphorylation was analyzed 45 min after 4 Gy of IR. (D) Total extracts from LBC-N, AT, and NBS cells collected at various time points after 4 Gy of IR and examined by immunoblot analysis.
In AT and NBS cells, S19 and S33/S35 remained unphosphorylated at any time point post-IR (Fig. 4D), indicating that phosphorylation of these residues in cells with ATM or Nbs1 deficiency is simply absent. On the other hand, attenuated and delayed phosphorylation of T68, undetectable in AT cells, was observed in NBS cells (Fig. 4D). Thus, Nbs1 is dispensable to some extent for the phosphorylation of T68, a high-affinity substrate for ATM in vitro (39) but indispensable for phosphorylation of S19 and S33/S35 residues.
S19 and S33/S35 are phosphorylated predominantly in G1 phase in response to IR.
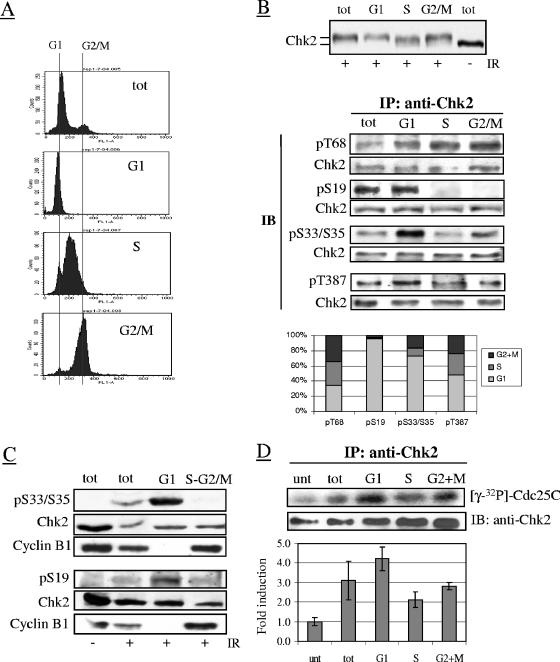
Because Chk2 regulates multiple cell cycle transitions, we examined its phosphorylation in relation to cell cycle phases in samples sorted by FACS according to DNA content (Fig. 5A). No differences in the overall level or electrophoretic mobility of Chk2 were observed between cell cycle phases before IR (data not shown), but Chk2 from irradiated cells migrated more slowly in G1 and G2-M phase cells than in S-phase cells (Fig. 5B). These differently migrating forms of Chk2 could not be clearly detected in unfractionated samples, probably because of the preponderance of G1 cells (70 to 80% of the total). Analysis of S33/S35, S19, and T68 in Chk2 immunoprecipitated from unirradiated sorted samples revealed no phosphorylation of these residues in any of the cell cycle phases (data not shown), but in samples sorted 45 min after IR treatment, S33/S35, S19 and, to a lesser extent, T387 became phosphorylated in the G1 phase only, while T68 became phosphorylated in all phases (Fig. 5B). The G1-phase-restricted phosphorylation of S19 and S33/S35 was also detectable 10 min after IR (Fig. 5C) and even in the presence of the protein phosphatase inhibitor calyculin A (data not shown), the latter arguing against a role for a phosphatase in dephosphorylating S19 and S33/S35 residues in S and G2-M phases. Notably, besides lymphoblastoid cells, the G1-phase-restricted phosphorylation of S19 and S33/S35 was also seen in hTERT-immortalized ovarian epithelial cells (data not shown). To establish a role for these phosphorylations, in vitro Chk2 kinase assays were performed on irradiated samples separated according to the cell cycle phases. The results showed that the IR-induced catalytic activity of Chk2, though maximal in G1, was also detectable in G2/M phase cells and to a lower extent in S-phase cells (Fig. 5D). Together, these data demonstrate that Chk2 undergoes a differential phosphorylation in G1 and S-G2/M after DNA damage, but this affects the catalytic activity of Chk2 through the cell cycle phases only partly.
FIG. 5.
Cell cycle phase-related phosphorylation of Chk2 S19 and S33/S35 and kinase activity after IR. LBC-N cells were collected 45 or 90 min after exposure to 4 Gy of IR and separated by FACS according to DNA content. (A) FACS analysis verifying the purity of the sorted G1, S, and G2/M fractions. Abbreviation: tot, total unsorted cells. (B) (Top) Chk2 electrophoretic mobility shift in relation to cell cycle phases detected by immunoblot analysis 45 min after 4 Gy of IR. (Middle) Chk2 immunoprecipitated from each fraction was immunoblotted with the indicated antibodies. Abbreviations: IP, immunoprecipitation; IB, immunoblotting; pT68, phospho-T68. (Bottom) Graph summarizing the results of different experiments, obtained by the densitometric analysis of the bands relative to each Chk2 phosphoresidue, after normalization of each lane for the total amount of Chk2. For each phosphoresidue, the amount of signal (as a percentage) distributed along the cell cycle phases is shown. Note that T387 phosphorylation was examined 90 min post-IR. (C) Cells were separated by FACS 10 min after 4 Gy of IR in G1 and S-G2/M and analyzed for phospho-S33/S35 (pS33/S35), phospho-S19, and cyclin B1, the latter as a control for the cell phase specificity of the purified fractions. (D) Chk2 immunoprecipitated from separated LBC-N cells 1 h after treatment with 4 Gy of IR (or no treatment for a control) was examined by in vitro kinase assays using Cdc25C substrate. The graph below shows the changes in induction (means ± standard deviations [error bars]) of Chk2 kinase activity relative to untreated cells, determined by densitometry analysis of the radiolabeled Cdc25C substrate from three independent experiments, and normalization for the levels of immunoprecipitated Chk2 per lane. Abbreviation: unt, untreated.
S19 and S33/S35 phosphorylations play a role in Chk2 kinase activity and complex formation.
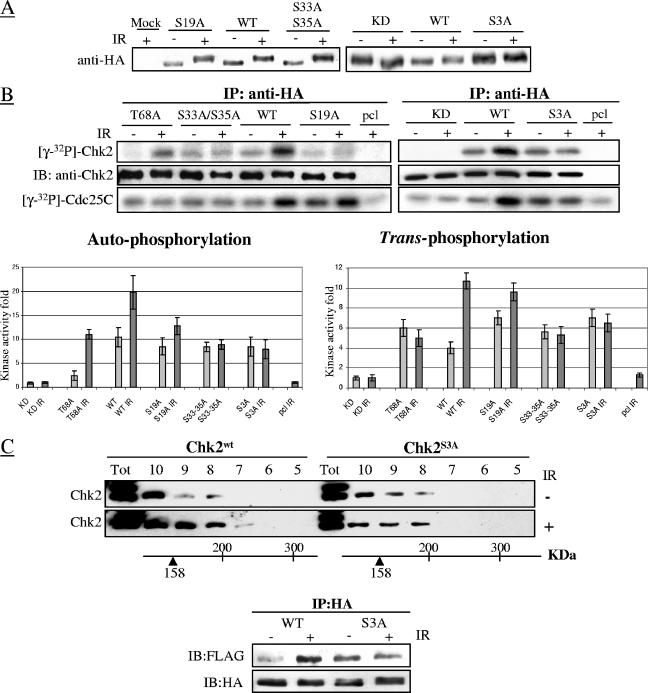
To further evaluate the roles of S19 and S33/S35 phosphoresidues, in vitro kinase reactions were performed on extracts from HCT15 cells expressing Ser-to-Ala substitutions on these residues, all of which showed an electrophoretic mobility delay comparable to that of wild-type Chk2 after IR (Fig. 6A). In contrast to wild-type Chk2, Chk2 with T68A, S33A-35A, and S19A S33A S35A (triple mutant denoted Chk2S3A) had defective auto- and _trans_-phosphorylation activities, whereas the S19A mutant had only impaired autophosphorylation activity (Fig. 6B).
FIG. 6.
SQ/TQ mutants show different defects in Chk2 kinase activity. (A) HCT15 cells were stably transfected with HA-tagged wild-type (WT), S19A, S33A/S35A, T68A, kinase-dead (KD), and S3A (S19A S33A S35A) mutant forms of Chk2. Expression of ectopic Chk2 was tested before (−) and 1 h after exposure to 20 Gy of IR (+). (B) Ectopic Chk2 was immunoprecipitated from HCT15 cells collected before or 1 h after treatment with 20 Gy of IR and assayed in in vitro reactions for autophosphorylation and _trans_-phosphorylation kinase activities. Bar graphs of data obtained from three independent experiments show changes in induction (means ± standard deviations [error bars]), normalized to the activity of the Chk2-KD mutant. Abbreviations: IP, immunoprecipitation; IB, immunoblotting; pcl, precleared lysate. (C) (Top) HPLC size fractionation was performed on extracts of HCT15 cells stably transfected with wild-type or S3A triple-mutant forms of Chk2, harvested before (−) or 1 h after 20 Gy of IR (+). Twenty fractions ranging from 30 to 600 kDa were collected, and fractions 5 to 10 were tested by immunoblotting. Abbreviation: Tot, total unsorted cells. (Bottom) HCT15 cells were cotransfected with HA-Chk2wt and FLAG-Chk2wt or alternatively with HA-Chk2S3A and FLAG-Chk2S3A, treated with 0 or 20 Gy of IR for 24 h, and harvested 1 h later. Complexes were immunoprecipitated with an anti-HA antibody and tested on immunoblots with an anti-FLAG antibody to evaluate Chk2 dimerization and with an anti-HA antibody to verify the amount of immunoprecipitated Chk2.
Homodimerization of Chk2, involving the interaction of the phospho-SCD of one molecule with the FHA domain of another molecule, is required for its catalytic activity, particularly autophosphorylation (5). To determine whether the catalytic defects described above might reflect impairment of Chk2 homodimerization, the ability of mutant molecules to engage in homo- and/or heterointeractions was tested in size-fractionated lysates from HCT15 cells stably transfected with Chk2wt or Chk2S3A. After IR, the complex-forming ability of Chk2wt increased, as demonstrated by the appearance of Chk2 in the higher-molecular-weight fractions (Fig. 6C) and consistent with dimer formation (3). This ability was defective in the case of Chk2S3A, based on the molecular size distribution of the molecule, which did not change after IR (Fig. 6C). Thus, these phosphoresidues may enhance the dimerization and in turn the catalytic activity of Chk2. To further evaluate the ability of mutant Chk2 to homodimerize, HCT15 cells were cotransfected with HA- and FLAG-tagged Chk2wt or Chk2S3A and ectopically expressed proteins immunoprecipitated with an anti-HA antibody followed by immunoblotting with an anti-FLAG antibody. In unstressed cells, Chk2S3A dimers were slightly more abundant than Chk2wt dimers, but after irradiation the amount of Chk2S3A dimers remained unchanged, in contrast to Chk2wt dimers which increased substantially (Fig. 6C bottom), consistent with the results of fractionation analysis.
S19 and S33/S35 phosphoresidues and stability of Hdmx.
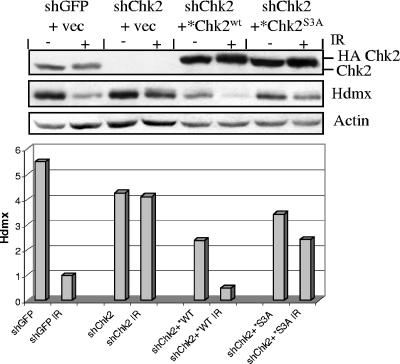
It was recently reported that Chk2 phosphorylates Hdmx protein and targets it for degradation after DNA damage (17, 41, 44). We therefore evaluated the effects of S19 and S33/S35 on Hdmx in vivo on MCF7 cells in which the endogenous Chk2 was silenced with shRNA (MCF7-shChk2) and that were complemented with constructs encoding Chk2wt or Chk2S3A (two silent mutations introduced in these vectors within the shRNA-Chk2 binding sequence prevented reinduction of endogenous Chk2 [see Materials and Methods for details]). Whereas MCF7-shChk2 cells were quite unable to degrade Hdmx after IR (Fig. 7) (>1.1-fold reduction compared to >5-fold reduction of the mock-silenced cells), complementation with Chk2wt restored the capacity to degrade Hdmx (>4.7-fold reduction [Fig. 7]). On the other hand, the radiation-induced proteolysis of Hdmx was only partially restored by Chk2S3A (<1.5-fold reduction [Fig. 7]).
FIG. 7.
Defective capacity of Chk2 phosphoresidue mutants to induce degradation of Hdmx. MCF7 cells were stably transfected with short hairpin RNA interference constructs to silence endogenous Chk2 (shChk2). shGFP was used as a control. These cells were then complemented with expression vectors (vec) encoding HA-Chk2wt or HA-Chk2S3A (or an empty vector as a control). It should be noticed that these Chk2 cDNAs carried two silent mutations within the sequence targeted by the Chk2 shRNA to avoid reexpression of the endogenously silenced Chk2 (see Materials and Methods). The cells were harvested before (−) or 4 h after 10 Gy of IR (+), and Hdmx protein was evaluated by Western blotting. The relative levels of Hdmx after normalization for β-actin, as determined by densitometry analysis of the bands, are shown in the histogram.
The Chk2S3A mutant lacks the antiproliferative effects of wild-type Chk2.
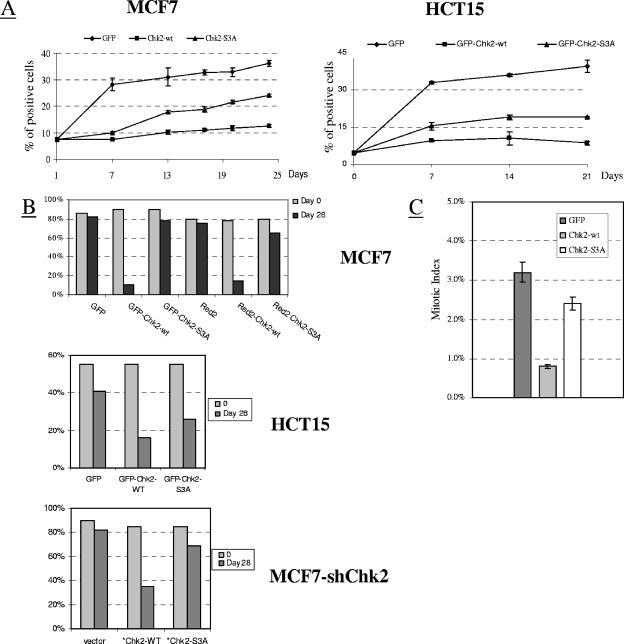
In the absence of DNA damage in cancer cell lines bearing the wild-type CHEK2 gene, the overexpression of Chk2 causes growth arrest and apoptosis (16). We therefore examined in MCF7 and HCT15 cells (the former expressing wild-type Chk2, the latter null for Chk2) the effects of Chk2 mutants after transfection with GFP-tagged constructs carrying the neomycin selection marker. In MCF7 cells, 24 days after selection, the fluorescent cells showed a sixfold enrichment upon transfection with GFP alone (control), whereas upon transfection with GFP-Chk2wt or GFP-Chk2S3A, the enrichment was twofold and fourfold, respectively (Fig. 8A). Comparable results were seen in HCT15 cells transfected with similar constructs (Fig. 8A). Moreover, MCF7 and HCT15 cell samples transfected with constructs tagged with GFP or Red2 (the latter has been claimed to be less toxic than GFP), sorted 48 h later by FACS to almost 100% purity (on the basis of green or red fluorescence), and cultured for 28 days thereafter revealed in MCF7 cells no indication of growth arrest or death induced by Chk2S3A, unlike Chk2wt (Fig. 8B). Similar data were obtained in HCT15 cells (Fig. 8B) and in MCF7-shChk2 cells transfected with the Chk2wt or Chk2S3A construct (that contained two silent mutations within the shRNA-Chk2 binding sequence to prevent reinduction of endogenous Chk2, as described in Materials and Methods) (Fig. 8B). Analysis of the number of mitotic cells within the GFP-positive fractions in MCF7 cells 48 h after transfection with GFP-tagged Chk2 constructs showed a greatly reduced number of mitotic events in cells expressing GFP-Chk2wt compared to cells transfected with GFP-Chk2S3A or with GFP alone (Fig. 8C). Thus, whereas wild-type Chk2 overexpression retards or arrests cell cycle progression (irrespective of the endogenous expression of Chk2), Chk2S3A loses this capacity.
FIG. 8.
Defects in Chk2 phosphorylation impair Chk2 function. (A) MCF7 or HCT15 cells transfected with constructs encoding GFP, GFP-Chk2wt, and GFP-Chk2S3A (S19A S33A S35A) and grown in G418 selection medium, enumerated by fluorescence microscopy at the indicated intervals of time (data from three independent experiments; mean ± standard deviations [error bars]). The percentage of fluorescent cells is shown on the vertical axis. (B) MCF7 and HCT15 cells transfected with the GFP-Chk2 or Red2-Chk2 construct (only for MCF7 cells), purified 48 h later by FACS on the basis of GFP or Red2 fluorescence staining (>95% purity), cultured for 28 days in G418 selection medium, and assessed by fluorescence microscopy. MCF7-shChk2 cells were transfected with vector alone. HA-Chk2wt or HA-Chk2S3A constructs and positive cells were evaluated 28 days later by immunofluorescence staining with an anti-HA antibody. The percentage of fluorescent cells is shown on the vertical axis for all graphs. (C) MCF7 cells were examined 48 h after transfection with the indicated GFP-tagged constructs for the presence of mitotic cells in 4′,6′-diamidino-2-phenylindole (DAPI)-stained cytospin preparations. More than 2,000 nuclei were scored.
Chk2S3A mutant is unable to arrest G1/S progression in response to DNA damage.
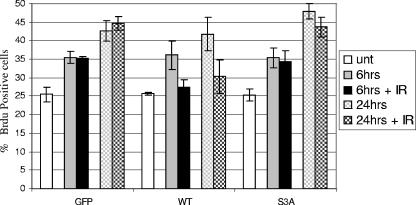
Chk2 protein has been implicated in DNA damage-induced G1/S and S-phase arrest (23). This evidence and the predominant phosphorylation of S19 and S33/S35 residues in G1 phase prompted us to evaluate the G1/S progression in response to IR. For this, HCT15 cells, which show a Chk2-dependent G1/S checkpoint defect (23), were transfected with GFP-tagged Chk2 constructs and examined after BrdU labeling to determine the fraction of cells entering S phase. The experiments were conducted in the presence of a mitotic trap to prevent cells from reentering G1 after mitosis. HCT15 expressing GFP alone failed to arrest BrdU incorporation and therefore replication 6 and 24 h after IR exposure (Fig. 9), in accordance with previous results (23). However, HCT15 expressing GFP-Chk2wt showed a significant decrease in BrdU-positive cells, but this was not observed upon expression of GFP-Chk2S3A (Fig. 9). These data demonstrate that mutations in S19 and S33/S35 disable Chk2 to activate cell cycle checkpoint arrest.
FIG. 9.
Defects in Chk2 phosphorylation impair G1/S phase arrest in response to IR. HCT15 cells were transfected with GFP, GFP-Chk2wt (WT), and GFP-Chk2S3A (S3A) constructs and 24 h later incubated for 30 min with 10 μM BrdU and 200 ng/ml nocodazole (nocodazole was used as a trap to prevent mitotic cells from reentering G1). Afterwards, an aliquot of the cells was collected (untreated [unt] sample), and the remaining cells were irradiated with 0 or 10 Gy of IR and harvested 6 and 24 h later. Samples were labeled by double-color immunofluorescence for GFP and BrdU, and 300 GFP-positive cells were scored for BrdU staining.
DISCUSSION
The checkpoint kinase Chk2 is an important component of the DNA damage machinery, mediating cell cycle arrests at multiple checkpoints and apoptosis (8). After DNA damage, ATM phosphorylates Chk2 on T68 (39), promoting Chk2 oligomerization through the interaction of the amino-terminal SCD of one molecule with the FHA domain of another molecule (3) as well as the autophosphorylation at T383 and T387 in the activation loop (5), for full activation of Chk2. Phosphorylation of T68, a necessary but not sufficient step for Chk2 activation, is not required for Chk2 autophosphorylation (47) (Fig. 6B), and amino acid substitutions at T68 do not abolish the kinase activity (56) or the DNA damage-induced association with chromatin (34).
We have identified three serine phosphoresidues (S19, S33, and S35) located in the amino-terminal SQ/TQ-rich region of Chk2 that provides a consensus motif for phosphorylation by ATM. In vivo phosphorylation of these serine residues in normal cells was rapidly induced in response to IR doses of >1 Gy (generating >19 DSBs/cell), in contrast to the phosphorylation of T68 occurring in response to much lower doses of IR (∼0.25 Gy, estimated to generate <8 DSBs/cell). Interestingly, Chk2 autophosphorylation at T387 occurred at a later time point (45 to 90 min), when phosphorylation levels of T68, S19, and S33/S35 decreased markedly, suggesting that these residues serve as a trigger for Chk2 activation. Given that the proteasome inhibitor ALLN did not prevent the decline in T68, S19, and S33/S35 phosphorylation levels, our results are consistent with dephosphorylation rather than proteolytic degradation to account for this phenomenon. In addition, the SCD appears to have a dual function, i.e., to initially promote Chk2 activation through SCD phosphorylation and to limit the amplification of the number of activated Chk2 molecules through its dephosphorylation.
Phosphorylation of S19 and S33/S35 was normal in an T68A mutant, indicating the independence of these events. Conversely, phosphorylation of S19 and S33/S35 was markedly attenuated in Chk2-KD, possibly reflecting a hindering effect played by Chk2 oligomerization rather than catalytic loss, as demonstrated by the efficient phosphorylation of these residues in Chk2-KD-FHAΔ, a mutant form of Chk2 that is unable to oligomerize because of the deletion of the FHA domain (5).
The rapidity of S19 and S33/S35 phosphorylation is consistent with a direct activity of ATM on these residues. Indeed, the absence of S19 and S33/S35 phosphorylation in two NBS cell lines lacking full-length Nbs1 protein expression, together with the impaired activity of ATM towards its substrates (14, 26, 54), further supports this contention. The partial phosphorylation of T68 in NBS cells might thus be explained by the differential affinity of ATM, much greater for T68 than for S19 and S33/S35 (39). Therefore, the ability of Nbs1 to increase ATM affinity for its substrate (43) should be dispensable for T68 phosphorylation and indispensable for S19 and S33/S35 phosphorylation. Such a model is also supported by the lack of S19 and S33/S35 phosphorylation in response to IR doses of <1 Gy which nevertheless vigorously activate ATM (7). The role of ATM in S19 and S33/S35 phosphorylation was also highlighted by the fact that neither 4-NQO nor HU treatment affected these residues. Conversely, T68 was phosphorylated by 4-NQO and HU, although in an ATM-independent and ATR-dependent manner, again differentiating between these phosphorylation events. Our results with an ATR-defective Seckel cell line and ATR-silenced cells excluded an involvement of ATR in the IR-induced phosphorylation of S19 and S33/S35.
We evaluated the molecular roles of S19 and S33/S35 in MCF7 cells, characterized by a normal ATM-Chk2- and p53-dependent DNA damage response, and in HCT15 cells, which lack endogenous Chk2, stably transfected with single (S19A), double (S33A S35A) or triple (S19A S33A S35A) Chk2 mutants. In these cells, the overall nuclear distribution of Chk2 and relocalization of phospho-T68 in IR-induced foci was unaffected by Ser-to-Ala mutations in S19/S33/S35 (see Fig. S4 in the supplemental material). In vitro kinase assays revealed deficient autophosphorylation and trans phosphorylation catalytic activities by Chk2S19A, Chk2S33A/S35A, and Chk2S3A, thus implicating these serine residues in the activation of Chk2. Size fractionation analysis of cell extracts from transiently transfected HCT15 cells revealed a marked decrease in the amount of mutant Chk2 in the fractions corresponding to 161 to 238 kDa, suggesting that the Ser-to-Ala substitutions impair Chk2 dimerizations. This observation was confirmed by a direct analysis of the dimerization ability of Chk2, obtained by using two different tagged forms of the protein, and is in accordance with the model proposed for its activation recently supported by crystallographic analysis (42).
Mdmx, an important regulator of p53, was recently shown to be phosphorylated by Chk2 on Ser367 after DNA damage, causing ubiquitination and degradation of Mdmx and activation of p53 (17, 32, 41, 44). We have shown that in cells with stably silenced endogenous Chk2, ectopic Chk2wt restored the IR-induced degradation of Hdmx, whereas Chk2S3A had a partial effect, suggesting a role for S19 and S33/S35 in the Chk2/Hdmx pathway, leading to p53 accumulation and apoptosis in response to a high level of DNA damage.
Analysis of Chk2 in relation to the cell cycle revealed that in response to radiation, T68 became phosphorylated in G1, S, and G2/M, whereas S19 and S33/S35, and to a lower extent T387, became phosphorylated in G1 phase only. This event was observed not only in lymphoblastoid cells but also in hTERT-immortalized epithelial cells. One mechanism to account for this cell cycle-restricted phosphorylation might depend on the activity of a kinase that specifically targets (in an ATM-dependent manner) these residues in the G1 phase, as in the case of other target substrates of phosphatidylinositol 3-kinase in yeast (49) and mammalian cells (20). Alternatively, a phosphatase(s) might selectively dephosphorylate S19 and S33/S35 in S and G2/M, assuming that DNA damage induces phosphorylation of these residues in all phases of the cell cycle. However, preliminary results with phosphatase inhibitors, e.g., calyculin A or orthovanadate (data not shown), would argue against the latter possibility.
Whatever the underlying mechanism, the biological significance of the G1 phase-restricted phosphorylation of S19 and S33/S35 remains unclear, given that the radiation-induced Chk2 kinase activity, though maximal in G1 phase cells (4.3-fold), was also seen in G2/M phase cells (2.6-fold) and to a lower extent in S-phase (2.1-fold) cells. This would be compatible with Chk2 playing a major role in G1/S checkpoint arrest in the presence of a high level of DNA damage and with the restoration of the G1/S checkpoint in Chk2 null cells (Fig. 9) (23) by the wild-type Chk2, but not by the S3A mutant Chk2. It is possible that this phosphorylated form of Chk2, occurring in response to >18 DSBs/cell, might provide a more effective and sustained G1 arrest to prevent replication of cells with damaged DNA that reenter G1 after escaping mitotic checkpoint arrest.
Finally, we showed that ectopic expression of wild-type Chk2 in the absence of de novo DNA damage has an antiproliferative effect, possibly reflecting a constitutive trans autophosphorylation and activation of Chk2 when overexpressed (47) and, in turn, an increased apoptotic cell death and senescence, as recently reported (16). Conversely, the Chk2S3A mutant lacked the antiproliferative activity of Chk2wt, underscoring the biological role of S19 and S33/S35 phosphoresidues in Chk2 activity.
Together, our data shed light on several aspects of the intricate and tightly coordinated phosphorylation events leading to the ATM-dependent functional activation of Chk2 kinase. Finally, the differential phosphorylation of Chk2 at multiple residues as a function of the IR dose (T68 is responsive to even 0.25 Gy, estimated to generate <8 DSBs/cell, whereas S19 and S33/S35 are responsive to >1 Gy, estimated to generate >19 DSBs/cell [12]) might represent a mechanism by which ATM fine-tunes Chk2's pleiotropic responses to increasing numbers of genotoxic lesions.
Supplementary Material
[Supplemental material]
Acknowledgments
We thank Elena Luison, Istituto Nazionale Tumori, Milano, Italy, for skilled technical assistance with HPLC.
This work was supported by grants from the Italian Association for Cancer Research (AIRC), Italian Ministry of Health (Ricerca Finalizzata), Consiglio Nazionale Ricerche (CNR), and Telethon (grant GGP05226B).
Footnotes
▿
Published ahead of print on 28 August 2006.
REFERENCES
- 1.Abraham, R. T. 2001. Cell cycle checkpoint signalling through the ATM and ATR kinases. Genes Dev. 15**:**2177-2196. [DOI] [PubMed] [Google Scholar]
- 2.Agami, R., and R. Bernards. 2000. Distinct initiation and maintenance mechanisms cooperate to induce G1 cell cycle arrest in response to DNA damage. Cell 102**:**55-66. [DOI] [PubMed] [Google Scholar]
- 3.Ahn, J., and C. Prives. 2002. Checkpoint kinase 2 (Chk2) monomers or dimers phosphorylate Cdc25C after DNA damage regardless of threonine 68 phosphorylation. J. Biol. Chem. 277**:**48418-48426. [DOI] [PubMed] [Google Scholar]
- 4.Ahn, J. Y., J. K. Schwarz, H. Piwnica-Worms, and C. E. Canman. 2000. Threonine 68 phosphorylation by ataxia telangiectasia mutated is required for efficient activation of Chk2 in response to ionizing radiation. Cancer Res. 60**:**5934-5936. [PubMed] [Google Scholar]
- 5.Ahn, J. Y., X. Li, H. L. Davis, and C. E. Canman. 2002. Phosphorylation of threonine 68 promotes oligomerization and autophosphorylation of the Chk2 protein kinase via the forkhead-associated domain. J. Biol. Chem. 277**:**19389-19395. [DOI] [PubMed] [Google Scholar]
- 6.Alderton, G. K., H. Joenje, R. Varon, A. D. Borglum, P. A. Jeggo, and M. O'Driscoll. 2004. Seckel syndrome exhibits cellular features demonstrating defects in the ATR-signalling pathway. Hum. Mol. Genet. 13**:**3127-3138. [DOI] [PubMed] [Google Scholar]
- 7.Bakkenist, C. J., and M. B. Kastan. 2003. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421**:**499-506. [DOI] [PubMed] [Google Scholar]
- 8.Bartek, J., and J. Lukas. 2003. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 3**:**421-429. [DOI] [PubMed] [Google Scholar]
- 9.Bell, D. W., J. M. Varley, T. E. Szydlo, D. H. Kang, D. C. Wahrer, K. E. Shannon, M. Lubratovich, S. J. Verselis, K. J. Isselbacher, J. F. Fraumeni, J. M. Birch, F. P. Li, J. E. Garber, and D. A. Haber. 1999. Heterozygous germ line hCHK2 mutations in Li-Fraumeni syndrome. Science 286**:**2528-2531. [DOI] [PubMed] [Google Scholar]
- 10.Brown, A. L., C. H. Lee, J. K. Schwarz, N. Mitiku, H. Piwnica-Worms, and J. H. Chung. 1999. A human Cds1-related kinase that functions downstream of ATM protein in the cellular response to DNA damage. Proc. Natl. Acad. Sci. USA 96**:**3745-3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buscemi, G., C. Savio, L. Zannini, F. Micciche, D. Masnada, M. Nakanishi, H. Tauchi, K. Komatsu, S. Mizutani, K. Khanna, J. Chen, P. Concannon, L. Chessa, and D. Delia. 2001. Chk2 activation dependence on Nbs1 after DNA damage. Mol. Cell. Biol. 21**:**5214-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buscemi, G., P. Perego, N. Carenini, M. Nakanishi, L. Chessa, J. Chen, K. Khanna, and D. Delia. 2004. Activation of ATM and Chk2 kinases in relation to the amount of DNA strand breaks. Oncogene 23**:**7691-7700. [DOI] [PubMed] [Google Scholar]
- 13.Cerosaletti, K., and P. Concannon. 2004. Independent roles for nibrin and Mre11-Rad50 in the activation and function of Atm. J. Biol. Chem. 279**:**38813-38819. [DOI] [PubMed] [Google Scholar]
- 14.Cerosaletti, K., J. Wright, and P. Concannon. 2006. Active role for nibrin in the kinetics of ATM activation. Mol. Cell. Biol. 26**:**1691-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaturvedi, P., W. K. Eng, Y. Zhu, M. R. Mattern, R. Mishra, M. R. Hurle, X. Zhang, R. S. Annan, Q. Lu, L. F. Faucette, G. F. Scott, X. Li, S. A. Carr, R. K. Johnson, J. D. Winkler, and B. B. Zhou. 1999. Mammalian Chk2 is a downstream effector of the ATM-dependent DNA damage checkpoint pathway. Oncogene 18**:**4047-4054. [DOI] [PubMed] [Google Scholar]
- 16.Chen, C. R., W. Wang, H. A. Rogoff, X. Li, W. Mang, and C. J. Li. 2005. Dual induction of apoptosis and senescence in cancer cells by Chk2 activation: checkpoint activation as a strategy against cancer. Cancer Res. 65**:**6017-6021. [DOI] [PubMed] [Google Scholar]
- 17.Chen, L., D. M. Gilkes, Y. Pan, W. S. Lane, and J. Chen. 2005. ATM and Chk2-dependent phosphorylation of MDMX contribute to p53 activation after DNA damage. EMBO J. 24**:**3411-3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delia, D., K. Goi, S. Mizutani, T. Yamada, A. Aiello, E. Fontanella, G. Lamorte, S. Iwata, C. Ishioka, S. Krajewski, J. C. Reed, and M. A. Pierotti. 1997. Dissociation between cell cycle arrest and apoptosis can occur in Li-Fraumeni cells heterozygous for p53 gene mutations. Oncogene 14**:**2137-2147. [DOI] [PubMed] [Google Scholar]
- 19.Delia, D., S. Mizutani, S. Panigone, E. Tagliabue, E. Fontanella, M. Asada, T. Yamada, Y. Taya, S. Prudente, S. Saviozzi, L. Frati, M. A. Pierotti, and L. Chessa. 2000. ATM protein and p53-serine 15 phosphorylation in ataxia-telangiectasia (AT) patients and AT heterozygotes. Br. J. Cancer 82**:**1938-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delia, D., E. Fontanella, C. Ferrario, L. Chessa, and S. Mizutani. 2003. DNA damage-induced cell-cycle phase regulation of p53 and p21waf1 in normal and ATM-defective cells. Oncogene 22**:**7866-7869. [DOI] [PubMed] [Google Scholar]
- 21.Difilippantonio, S., A. Celeste, O. Fernandez-Capetillo, H. T. Chen, B. Reina San Martin, F. Van Laethem, Y. P. Yang, G. V. Petukhova, M. Eckhaus, L. Feigenbaum, K. Manova, M. Kruhlak, R. D. Camerini-Otero, S. Sharan, M. Nussenzweig, and A. Nussenzweig. 2005. Role of Nbs1 in the activation of the Atm kinase revealed in humanized mouse models. Nat. Cell Biol. 7**:**675-685. [DOI] [PubMed] [Google Scholar]
- 22.Dupre, A., L. Boyer-Chatenet, and J. Gautier. 2006. Two-step activation of ATM by DNA and the Mre11-Rad50-Nbs1 complex. Nat. Struct. Mol. Biol. 13**:**451-457. [DOI] [PubMed] [Google Scholar]
- 23.Falck, J., N. Mailand, R. G. Syljuasen, J. Bartek, and J. Lukas. 2001. The ATM-Chk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature 410**:**842-847. [DOI] [PubMed] [Google Scholar]
- 24.Gire, V., P. Roux, D. Wynford-Thomas, J. M. Brondello, and V. Dulic. 2004. DNA damage checkpoint kinase Chk2 triggers replicative senescence. EMBO J. 23**:**2554-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo, Z., A. Kumagai, S. X. Wang, and W. G. Dunphy. 2000. Requirement for Atr in phosphorylation of Chk1 and cell cycle regulation in response to DNA replication blocks and UV-damaged DNA in Xenopus egg extracts. Genes Dev. 14**:**2745-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horejsi, Z., J. Falck, C. J. Bakkenist, M. B. Kastan, J. Lukas, and J. Bartek. 2004. Distinct functional domains of Nbs1 modulate the timing and magnitude of ATM activation after low doses of ionizing radiation. Oncogene 23**:**3122-3127. [DOI] [PubMed] [Google Scholar]
- 27.Ismail, I. H., S. Nystrom, J. Nygren, and O. Hammarsten. 2005. Activation of ataxia telangiectasia mutated by DNA strand break-inducing agents correlates closely with the number of DNA double strand breaks. J. Biol. Chem. 280**:**4649-4655. [DOI] [PubMed] [Google Scholar]
- 28.Jallepalli, P. V., C. Lengauer, B. Vogelstein, and F. Bunz. 2003. The Chk2 tumor suppressor is not required for p53 responses in human cancer cells. J. Biol. Chem. 278**:**20475-20479. [DOI] [PubMed] [Google Scholar]
- 29.Kastan, M. B., and J. Bartek. 2004. Cell-cycle checkpoints and cancer. Nature 432**:**316-323. [DOI] [PubMed] [Google Scholar]
- 30.Khanna, K. K., and S. P. Jackson. 2001. DNA double-strand breaks: signalling, repair and the cancer connection. Nat. Genet. 27**:**247-254. [DOI] [PubMed] [Google Scholar]
- 31.Lavin, M. F., G. Birrell, P. Chen, S. Kozlov, S. Scott, and N. Gueven. 2005. ATM signaling and genomic stability in response to DNA damage. Mutat. Res. 569**:**123-132. [DOI] [PubMed] [Google Scholar]
- 32.LeBron, C., L. Chen, D. M. Gilkes, and J. Chen. 2006. Regulation of MDMX nuclear import and degradation by Chk2 and 14-3-3. EMBO J. 25**:**1196-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee, J. H., and T. T. Paull. 2005. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science 308**:**551-554. [DOI] [PubMed] [Google Scholar]
- 34.Li, J., and D. F. Stern. 2005. DNA damage regulates CHK2 association with chromatin. J. Biol. Chem. 280**:**37948-37956. [DOI] [PubMed] [Google Scholar]
- 35.Matsuoka, S., M. Huang, and S. J. Elledge. 1998. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science 282**:**1893-1897. [DOI] [PubMed] [Google Scholar]
- 36.Matsuoka, S., G. Rotman, A. Ogawa, Y. Shiloh, K. Tamai, and S. J. Elledge. 2000. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc. Natl. Acad. Sci. USA 97**:**10389-10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGowan, C. H., and P. Russell. 2004. The DNA damage response: sensing and signaling. Curr. Opin. Cell Biol. 16**:**629-633. [DOI] [PubMed] [Google Scholar]
- 38.McKinnon, P. J. 2004. ATM and ataxia telangiectasia. EMBO Rep. 5**:**772-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melchionna, R., X. B. Chen, A. Blasina, and C. H. McGowan. 2000. Threonine 68 is required for radiation-induced phosphorylation and activation of Cds1. Nat. Cell Biol. 2**:**762-765. [DOI] [PubMed] [Google Scholar]
- 40.O'Driscoll, M., A. R. Gennery, J. Seidel, P. Concannon, and P. A. Jeggo. 2004. An overview of three new disorders associated with genetic instability: LIG4 syndrome, RS-SCID and ATR-Seckel syndrome. DNA Repair 3**:**1227-1235. [DOI] [PubMed] [Google Scholar]
- 41.Okamoto, K., K. Kashima, Y. Pereg, M. Ishida, S. Yamazaki, A. Nota, A. Teunisse, D. Migliorini, L. Kitabayashi, J. C. Marine, C. Prives, Y. Shiloh, A. G. Jochemsen, and Y. Taya. 2005. DNA damage-induced phosphorylation of MdmX at serine 367 activates p53 by targeting MdmX for Mdm2-dependent degradation. Mol. Cell. Biol. 25**:**9608-9620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oliver, A. W., A. Paul, K. J. Boxall, S. E. Barrie, G. W. Aherne, M. D. Garrett, S. Mittnacht, and L. H. Pearl. 2006. Trans-activation of the DNA-damage signalling protein kinase Chk2 by T-loop exchange. EMBO J. 25**:**3179-3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paull, T. T., and J. H. Lee. 2005. The Mre11/Rad50/Nbs1 complex and its role as a DNA double-strand break sensor for ATM. Cell Cycle 4**:**737-740. [DOI] [PubMed] [Google Scholar]
- 44.Pereg, Y., D. Shkedy, P. de Graaf, E. Meulmeester, M. Edelson-Averbukh, M. Salek, S. Biton, A. F. Teunisse, W. D. Lehmann, A. G. Jochemsen, and Y. Shiloh. 2005. Phosphorylation of Hdmx mediates its Hdm2- and ATM-dependent degradation in response to DNA damage. Proc. Natl. Acad. Sci. USA 102**:**5056-5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rogoff, H. A., M. T. Pickering, F. M. Frame, M. E. Debatis, Y. Sanchez, S. Jones, and T. F. Kowalik. 2004. Apoptosis associated with deregulated E2F activity is dependent on E2F1 and Atm/Nbs1/Chk2. Mol. Cell. Biol. 24**:**2968-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarkaria, J. N., E. C. Busby, R. S. Tibbetts, P. Roos, Y. Taya, L. M. Karnitz, and R. T. Abraham. 1999. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 59**:**4375-4382. [PubMed] [Google Scholar]
- 47.Schwarz, J. K., C. M. Lovly, and H. Piwnica-Worms. 2003. Regulation of the Chk2 protein kinase by oligomerization-mediated cis- and trans-phosphorylation. Mol. Cancer Res. 1**:**598-609. [PubMed] [Google Scholar]
- 48.Shiloh, Y. 2003. ATM and related protein kinases: safeguarding genome integrity. Nat. Rev. Cancer 3**:**155-168. [DOI] [PubMed] [Google Scholar]
- 49.Shroff, R., A. Arbel-Eden, D. Pilch, G. Ira, W. M. Bonner, J. H. Petrini, J. E. Haber, and M. Lichten. 2004. Distribution and dynamics of chromatin modification induced by a defined DNA double-strand break. Curr. Biol. 14**:**1703-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stevens, C., L. Smith, and N. B. La Thangue. 2003. Chk2 activates E2F-1 in response to DNA damage. Nat. Cell Biol. 5**:**401-409. [DOI] [PubMed] [Google Scholar]
- 51.Takai, H., K. Naka, Y. Okada, M. Watanabe, N. Harada, S. Saito, C. W. Anderson, E. Appella, M. Nakanishi, H. Suzuki, K. Nagashima, H. Sawa, K. Ikeda, and N. Motoyama. 2002. Chk2-deficient mice exhibit radioresistance and defective p53-mediated transcription. EMBO J. 21**:**5195-5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor, A. M., A. Groom, and P. J. Byrd. 2004. Ataxia-telangiectasia-like disorder (ATLD)—its clinical presentation and molecular basis. DNA Repair 3**:**1219-1225. [DOI] [PubMed] [Google Scholar]
- 53.Tominaga, K., H. Morisaki, Y. Kaneko, A. Fujimoto, T. Tanaka, M.Ohtsubo, M. Hirai, H. Okayama, K. Ikeda, and M. Nakanishi. 1999. Role of human Cds1 (Chk2) kinase in DNA damage checkpoint and its regulation by p53. J. Biol. Chem. 274**:**31463-31467. [DOI] [PubMed] [Google Scholar]
- 54.Uziel, T., Y. Lerenthal, L. Moyal, Y. Andegeko, L. Mittelman, and Y. Shiloh. 2003. Requirement of the MRN complex for ATM activation by DNA damage. EMBO J. 22**:**5612-5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu, X., S. R. Webster, and J. Chen. 2001. Characterization of tumor-associated Chk2 mutations. J. Biol. Chem. 276**:**2971-2974. [DOI] [PubMed] [Google Scholar]
- 56.Xu, X., L. M. Tsvetkov, and D. F. Stern. 2002. Chk2 activation and phosphorylation-dependent oligomerization. Mol. Cell. Biol. 22**:**4419-4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang, S., C. Kuo, J. E. Bisi, and M. K. Kim. 2002. PML-dependent apoptosis after DNA damage is regulated by the checkpoint kinase hCds1/Chk2. Nat. Cell Biol. 4**:**865-870. [DOI] [PubMed] [Google Scholar]
- 58.Zhang, J., H. Willers, Z. Feng, J. C. Ghosh, S. Kim, D. T. Weaver, J. H. Chung, S. N. Powell, and F. Xia. 2004. Chk2 phosphorylation of BRCA1 regulates DNA double-strand break repair. Mol. Cell. Biol. 24**:**708-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental material]