Myeloid lineage switch of Pax5 mutant but not wild-type B cell progenitors by C/EBPα and GATA factors (original) (raw)
Abstract
The developmental potential of hematopoietic progenitors is restricted early on to either the erythromyeloid or lymphoid lineages. The broad developmental potential of _Pax5_–/– pro-B cells is in apparent conflict with such a strict separation, although these progenitors realize the myeloid and erythroid potential with lower efficiency compared to the lymphoid cell fates. Here we demonstrate that ectopic expression of the transcription factors C/EBPα, GATA1, GATA2 and GATA3 strongly promoted in vitro macrophage differentiation and myeloid colony formation of _Pax5_–/– pro-B cells. GATA2 and GATA3 expression also resulted in efficient engraftment and myeloid development of _Pax5_–/– pro-B cells in vivo. The myeloid transdifferentiation of _Pax5_–/– pro-B cells was accompanied by the rapid activation of myeloid genes and concomitant repression of B-lymphoid genes by C/EBPα and GATA factors. These data identify the _Pax5_–/– pro-B cells as lymphoid progenitors with a latent myeloid potential that can be efficiently activated by myeloid transcription factors. The same regulators were unable to induce a myeloid lineage switch in Pax5+/+ pro-B cells, indicating that Pax5 dominates over myeloid transcription factors in B-lymphocytes.
Keywords: C/EBPα/GATA factors/Pax5 mutantpro-B cells/myeloid lineage switch
Introduction
The pluripotent hematopoietic stem cell (HSC) continuously regenerates all blood cell types by differentiating to multipotent progenitors, which undergo commitment to one of several pathways and then differentiate into mature cells of the selected lineage. All non-lymphoid lineages develop from a common myeloid progenitor, which later gives rise to more restricted progenitors known as granulocyte/monocyte and megakaryocyte/erythrocyte (MEP) progenitors (Akashi et al., 2000). The lymphoid lineages are instead generated from the common lymphoid progenitor (CLP; Kondo et al., 1997) or related lymphoid progenitors (Igarashi et al., 2002; Allman et al., 2003).
Early B cell development depends on the transcription factors E2A, EBF and Pax5 (BSAP). E2A and EBF act upstream of Pax5 to activate the B-lymphoid gene expression program, whereas Pax5 is essential for the commitment of lymphoid progenitors to the B cell pathway (reviewed by Schebesta et al., 2002). B cell development is arrested at an early pro-B cell stage in the bone marrow of _Pax5_–/– mice (Urbánek et al., 1994). _Pax5_–/– pro-B cells, which can be cultured ex vivo in the presence of IL-7 and stromal cells (Nutt et al., 1997), retain a broad lymphomyeloid potential characteristic of uncommitted progenitors (Nutt et al., 1999; Rolink et al., 1999). Upon IL-7 withdrawal and appropriate cytokine stimulation, the _Pax5_–/– pro-B cells are able to differentiate in vitro into functional natural killer (NK) cells, dendritic cells, macrophages, osteoclasts and granulocytes (Nutt et al., 1999). This multilineage potential of _Pax5_–/– pro-B cells is, however, suppressed by retroviral restoration of Pax5 expression, which rescues development to the mature B cell stage (Nutt et al., 1999). Hence, Pax5 is the critical B-lineage commitment factor that restricts the developmental options of early progenitors to the B cell pathway.
After injection into sublethally irradiated mice, the _Pax5_–/– pro-B cells home to the bone marrow, where they undergo self-renewal and develop into functional cells of all major hematopoietic lineages including T cells, NK cells, dendritic cells, osteoclasts, macrophages, neutrophils and erythrocytes (Nutt et al., 1999; Rolink et al., 1999; Schaniel et al., 2002a). Although the _Pax5_–/– pro-B cells share the characteristic features of long-term reconstitution, self-renewal and multipotency with the HSC (Schaniel et al., 2002a,b), they differ from pluri potent stem cells, as they fail to reconstitute the hematopoietic system of lethally irradiated mice. This inability is caused by the different efficiencies, with which _Pax5_–/– pro-B cells give rise to the distinct blood cell types in vivo. _Pax5_–/– pro-B cells efficiently develop into T-lymphocytes within 1 week after cell transfer (Rolink et al., 1999), whereas macrophages and granulocytes require 2–3 months and erythrocytes even 4–6 months for their generation (Schaniel et al., 2002a). It appears therefore that the _Pax5_–/– pro-B cells are able to realize their lymphoid options more effectively than their myeloid potential.
Here we have investigated whether the developmental potential of _Pax5_–/– pro-B cells can be efficiently redirected to the myeloid lineages by ectopic expression of C/EBPα and GATA transcription factors. C/EBPα is the founding member of the CCAAT/enhancer-binding protein family that contains a conserved leucine-zipper dimerization motif adjacent to a basic DNA-binding domain (Landschulz et al., 1988). Within the hematopoietic system, _C/EBP_α is exclusively expressed in early myeloid progenitors (Akashi et al., 2000), where it is up-regulated during granulocyte development and down-regulated along the monocytic pathway (Scott et al., 1992; Radomska et al., 1998). The loss of C/EBPα in gene-targeted mice results in the complete absence of mature granulocytes due to arrest at an early myeloblast stage (Zhang et al., 1997). Hematopoietic development also depends on three zinc-finger transcription factors of the GATA protein family. GATA1 is predominantly expressed in MEP progenitors and in erythroid, megakaryocytic and eosinophilic precursors (Tsai et al., 1989; Martin et al., 1990; Akashi et al., 2000), where it controls cell maturation, survival and proliferation (Pevny et al., 1991; Fujiwara et al., 1996; Shivdasani et al., 1997; Hirasawa et al., 2002). GATA2 is essential for the expansion of multipotent hematopoietic progenitors and the formation of mast cells (Tsai et al., 1994; Tsai and Orkin, 1997), consistent with its broad expression in the HSC, myeloid progenitors, mast cells, erythroblasts and megakaryocytes (Leonard et al., 1993; Mouthon et al., 1993; Akashi et al., 2000). GATA3 is expressed in the T-lymphoid lineage and is required for early T cell development (Ting et al., 1996), although its expression is also detected in hematopoietic precursors of the embryonic aorta-gonad-mesonephros (AGM) region (Manaia et al., 2000) as well as in adult HSCs and CLPs (Akashi et al., 2000).
The _C/EBP_α and GATA1/2/3 genes are transcribed only at very low levels, if at all, in _Pax5_–/– pro-B cells (Nutt et al., 1999, and this study). Here we show that ectopic expression of C/EBPα or one of the three GATA factors is able to induce a myeloid lineage switch in _Pax5_–/– pro-B cells. GATA2- and GATA3-expressing _Pax5_–/– pro-B cells efficiently engrafted in vivo in the bone marrow and differentiated into granulocytes and monocytes within 12 days after injection into _RAG2_–/– mice. All four transcription factors activated the myeloid colony-forming potential of _Pax5_–/– pro-B cells and efficiently promoted in vitro macrophage differentiation under lymphoid growth conditions. Myeloid genes were rapidly induced and B-lymphoid genes were repressed by the C/EBPα and GATA factors, thus providing molecular evidence for the myeloid cell fate conversion of infected _Pax5_–/– pro-B cells. Together, these experiments identified the _Pax5_–/– pro-B cells as lymphoid progenitors with a latent myeloid potential that can be efficiently activated by myeloid transcription factors. The same regulators were, however, unable to induce myeloid differentiation in wild-type pro-B cells, indicating that the B-lineage commitment function of Pax5 is dominant over the myeloid-inducing activity of the C/EBPα and GATA factors.
Results
Efficient in vivo engraftment and myeloid development of Pax5–/– pro-B cells upon expression of GATA2 and GATA3
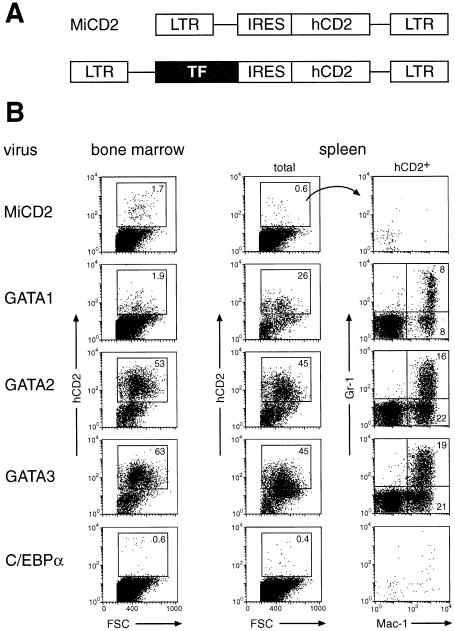
As _Pax5_–/– pro-B cells develop into macrophages and granulocytes only within 2–3 months after transplantation into sublethally irradiated mice (Schaniel et al., 2002a), we tested the possibility that the expression of myeloid transcription factors may enhance the myeloid potential of _Pax5_–/– pro-B cells. To this end, we expressed the GATA1, GATA2 and C/EBPα proteins from the bicistronic retrovirus MiCD2, which codes for a human CD2 protein, facilitating the detection of infected cells (Figure 1A). We also included the T cell-specific GATA3 protein in our analysis, as this transcription factor can partially rescue the loss of GATA1 in erythroid cells (Tsai et al., 1998). _Pax5_–/– pro-B cells were infected by co-culture with retrovirus-producing packaging cells. Three days later the infected cells were isolated by FACS sorting and intravenously injected into sublethally irradiated _RAG2_–/– mice. The bone marrow, spleen and thymus of the transplanted mice was analyzed by flow cytometry 12 days (Figure 1B) or 3 weeks (Figure 2) after cell transfer.
Fig. 1. GATA factors rapidly induce myeloid differentiation of _Pax5_–/– pro-B cells in vivo. (A) Retroviral vectors. Full-length cDNA coding for C/EBPα or GATA transcription factors (TF) was inserted into the parental vector MiCD2, which uses an internal ribosomal entry sequence (IRES) to express a human (h) CD2 protein lacking its intracellular domain. (B) _Pax5_–/– pro-B cells were infected with the indicated retroviruses, and sorted hCD2+ cells were injected into sublethally irradiated _RAG2_–/– mice. Twelve days after transfer, the donor cell contribution was investigated by flow cytometric analysis of the bone marrow and spleen. The expression of Gr-1 and Mac-1 is displayed for the hCD2+ cells of donor origin, and the percentages of cells in each gate or quadrant are indicated. Similar results were obtained for each retrovirus by analyzing four mice, which were generated in two separate transplantation experiments.
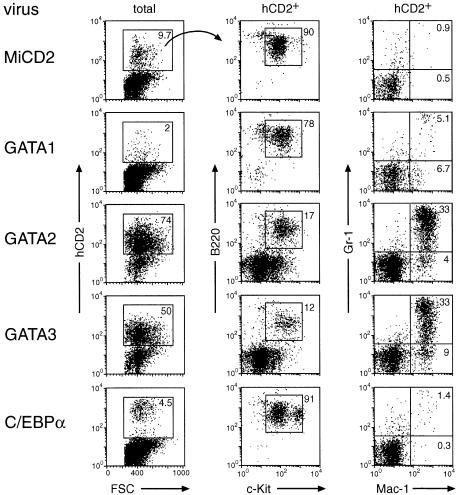
Fig. 2. GATA2 and GATA3 promote efficient engraftment and myeloid development of _Pax5_–/– pro-B cells in the bone marrow. Following infection with the indicated retroviruses, hCD2+ _Pax5_–/– pro-B cells were injected into sublethally irradiated _RAG2_–/– mice. Twenty-one days after transfer, the bone marrow was analyzed by flow cytometry, and the expression of B220/c-Kit and Gr-1/Mac-1 is shown for the hCD2+ cells together with the percentages of the different cell populations. The relative ratio of hCD2+c-Kit+B220+ pro-B cells was determined by multiplying the percentage of hCD2+ cells with the percentage of c-Kit+B220+ cells (MiCD2, 8.7%; GATA1, 1.6%; GATA2, 12.6%; GATA3, 6%; C/EBPα, 4.1%). Similar results were obtained for each retrovirus by analyzing 5–9 mice, which were generated in 2–4 independent transplantation experiments. The absence of myeloid differentiation of GATA1 or C/EBPα virus-infected cells may indicate a strong selection for mutation of the GATA1 and C/EBPα proteins, which otherwise seem to interfere with the engraftment of _Pax5_–/– pro-B cells in the bone marrow.
Ectopic expression of GATA2 or GATA3 resulted in rapid and efficient engraftment of the infected _Pax5_–/– pro-B cells in the bone marrow and spleen of transplanted mice. Already at 12 days after injection, 50–60% of all bone marrow cells and 45% of the splenocytes expressed hCD2 and were thus of donor origin in contrast to the low chimerism of control mice that were transplanted with MiCD2-infected _Pax5_–/– pro-B cells (Figure 1B). The engraftment of the GATA2- or GATA3-expressing cells was further increased with time, reaching 80% in the bone marrow 5 weeks after transplantation (Figure 2; data not shown). The GATA2- and GATA3-expressing cells efficiently differentiated into Gr-1+Mac-1+ granulocytes and Gr-1–Mac-1+ monocytes in the bone marrow and spleen (Figures 1B and 2), whereas Ter119+ erythroblasts of donor origin could not be detected (data not shown). Consistent with a myeloid lineage switch, most of the hCD2+ bone marrow cells lost their pro-B cell phenotype, as they down-regulated c-Kit and B220 expression at 3 weeks (Figure 2). However, the majority of the c-Kit–B220– cells did not yet up-regulate Mac-1 or Gr-1 (Figure 2), but instead expressed the early markers CD43 and PECAM-1 (CD31; data not shown), suggesting that they correspond to early myeloid progenitors. A small proportion of the GATA2- and GATA3-expressing cells did, however, not undergo a myeloid lineage switch, but instead maintained their c-Kit+B220+ pro-B cell phenotype in vivo (Figure 2). Interestingly, these hCD2+ pro-B cells were present at similar ratios (6–12%) in the bone marrow of experimental (GATA2 or GATA3) and control (MiCD2) mice (Figure 2; see legend for calculation of percentages). The c-Kit+B220+ _Pax5_–/– pro-B cells of the bone marrow were previously shown to seed the thymus and to reconstitute T cell development in _RAG2_–/– mice (Rolink et al., 1999). Consistent with this finding, the thymic cellularity and T cell development were restored to the same degree in _RAG2_–/– mice that were transplanted with GATA2, GATA3 or MiCD2 virus-infected pro-B cells (data not shown).
Retroviral expression of GATA1 or C/EBPα led to low engraftment and little myeloid differentiation of _Pax5_–/– pro-B cells in the bone marrow at all times after transplantation (Figure 1B and 2). At day 12, GATA1-expressing cells were, however, present in the spleen where they contributed to a chimerism of 26% and differentiated into Gr-1+Mac-1+ and Gr-1–Mac-1+ myeloid cells (Figure 1B). These GATA1-expressing splenocytes were no longer detected at 3 weeks (data not shown), indicating that ectopic GATA1 expression only gave rise to a transient wave of myeloid differentiation in the spleen. C/EBPα-expressing cells could, however, not be found even at early time points in the spleen (Figure 1). In summary, these results indicate that the transcription factors GATA2 and GATA3, in contrast to GATA1 and C/EBPα, can efficiently promote long-lasting in vivo engraftment and rapid myeloid differentiation of _Pax5_–/– pro-B cells in the bone marrow.
Activation of the myeloid colony-forming potential of Pax5–/– pro-B cells by C/EBPα and GATA factors
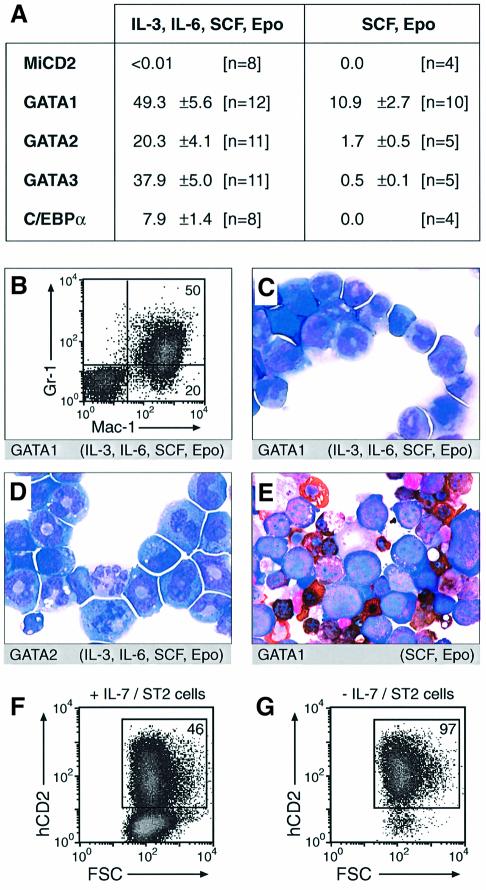
Previously we have shown that the _Pax5_–/– pro-B cells are able to differentiate in vitro into macrophages, granulocytes and osteoclasts within 14 days after withdrawal of IL-7 and stimulation with appropriate myeloid cytokines (Nutt et al., 1999). Differentiation to these myeloid cell types was, however, relatively inefficient and only seen in liquid culture (Nutt et al., 1999). We therefore used semisolid methylcellulose conditions to investigate the myeloid colony-forming activity of _Pax5_–/– pro-B cells expressing C/EBPα or GATA proteins (Figure 3A). All four transcription factors activated the myeloid colony-forming potential of _Pax5_–/– pro-B cells in the presence of erythropoietin and the multilineage cytokines IL-3, IL-6 and stem cell factor (SCF; Figure 3A). GATA1 expression allowed every second _Pax5_–/– pro-B cells to grow into a colony (49.3%), whereas C/EBPα gave rise to the lowest plating efficiency (7.9%) among the four transcription factors analyzed. Moreover, the C/EBPα-expressing cells grew only into small colonies and ceased proliferation after 5 days of in vitro culture in marked contrast to the GATA factor-expressing cells (data not shown). The majority of the cells growing in methylcellulose expressed the myeloid markers Gr-1 and Mac-1 (Figure 3B) and had the characteristic morphology of immature myelocytes with ring-shaped nuclei (Figure 3C and D). More mature granulocytes with segmented nuclei as well as vacuolar macrophages were detected only at low frequency, possibly due to the short in vitro culture period which did not allow for terminal differentiation of myeloid cells starting from pro-B cells (Figure 3C and D; data not shown). Importantly, we could not detect a single colony with MiCD2-infected _Pax5_–/– pro-B cells even if 10 000 cells were seeded in methylcellulose. Hence, the cloning efficiency of _Pax5_–/– pro-B cells is <0.01% and is thus increased >1000-fold by expression of the C/EBPα or GATA proteins.
Fig. 3. Myeloid colony formation of _Pax5_–/– pro-B cells upon expression of C/EBPα and GATA factors. (A) Plating efficiency in methyl cellulose. Infected hCD2+ _Pax5_–/– pro-B cells were seeded in methylcellulose medium containing IL-3, IL-6, SCF and erythropoietin (Epo) or only SCF and Epo. The colonies were counted after 4–5 or 8 days, respectively, and are indicated as percentage of the input cells. The number (n) of independent experiments and the corresponding standard deviation are shown. (B) Gr-1/Mac-1 expression. _Pax5_–/– cells infected with the GATA1 virus were incubated for 8 days in methylcellulose with the indicated cytokines and then collected from the entire Petri dish prior to flow cytometric analysis. (C–E) Cytospin preparation of May–Grünwald Giemsa-stained cells. _Pax5_–/– cells infected with the GATA1 (C and E) or GATA2 (D) retrovirus were cultured in methylcellulose under the indicated cytokine conditions for 8 (C and D) or 10 (E) days followed by May–Grünwald Giemsa and benzidine staining. Erythroblasts (E) were identified as benzidine-stained cells (brown). (F and G) Increased cell survival upon GATA3 expression. _Pax5_–/– pro-B cells were infected for 3 days with the GATA3 virus and then incubated for 2 days in the presence (F) or absence (G) of stromal ST2 cells and IL-7 prior to flow cytometric analysis.
The erythroid GATA1 protein efficiently induced colony formation of _Pax5_–/– pro-B cells (10.9%) even in methylcellulose containing erythropoietin and SCF as the only cytokines (Figure 3A). The GATA1-expressing colonies consisted to a large part of nucleated erythroblasts that were identified as benzidine-stained cells due to their hemoglobin synthesis (Figure 3E). GATA2 and GATA3 were less effective in inducing erythroid colonies, whereas C/EBPα failed to do so altogether. Hence, GATA1 expression is able to activate a latent erythroid potential of _Pax5_–/– pro-B cells, which could, however, be realized only under in vitro conditions in contrast to the in vivo situation observed in transplanted mice.
The proliferation and survival of _Pax5_–/– pro-B cells depends on signaling from the IL-7 and c-Kit receptors, which are activated by IL-7 and ligands expressed on stromal cells (Nutt et al., 1997). Uninfected hCD2+ _Pax5_–/– pro-B cells were indeed lost within 2 days after withdrawal of IL-7 and stromal ST2 cells, whereas GATA3 expression prevented the loss of the infected hCD2+ cells (Figure 3F and G). These data suggest therefore that the control of cell survival by GATA factors may contribute to the colony formation of _Pax5_–/– pro-B cells in the methylcellulose assay, which was also performed in the absence of IL-7 and ST2 cells.
Macrophage differentiation of Pax5–/– pro-B cells under lymphoid growth conditions
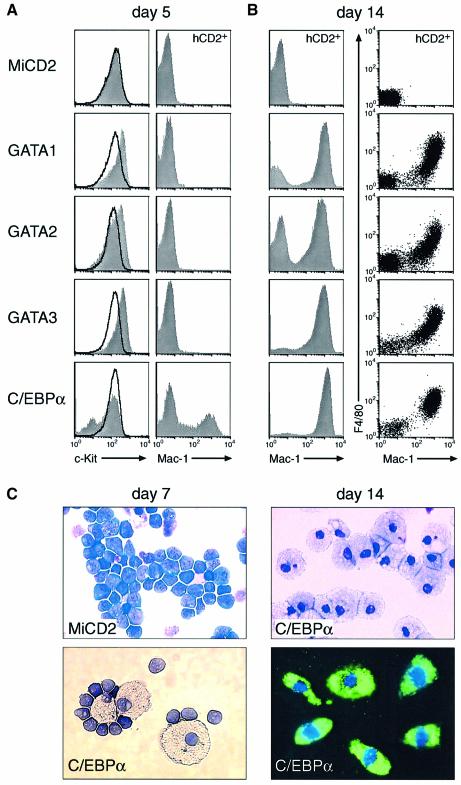
Stromal ST2 cells together with the lymphoid cytokine IL-7 define growth conditions, which allow _Pax5_–/– pro-B cells to undergo extensive self-renewal without any sign of differentiation (Nutt et al., 1997; Schaniel et al., 2002b). We therefore tested the possibility that C/EBPα or the GATA factors may induce myeloid differentiation of _Pax5_–/– pro-B cells even in the presence of these lymphoid conditions. Within 5 days after the start of retroviral infection, the three GATA factors rapidly down-regulated the expression of Sca1 and further increased the transcription of the early marker c-Kit in all infected cells, while expression of the myeloid Mac-1 and F4/80 proteins could not be observed at this time point (Figure 4A; and supplementary figure 1 available at The EMBO Journal Online). The majority of the GATA-expressing cells thereafter lost c-Kit expression, increased their size, became adherent and expressed Mac-1 as well as F4/80 by day 14 (Figure 4B; data not shown). The vacuolar morphology and phagocytic activity further identified these Mac-1+F4/80+ cells as mature macrophages (data not shown). Hence, all three GATA factors were able to instruct _Pax5_–/– pro-B cells to undergo macrophage differentiation even under lymphoid culture conditions.
Fig. 4. C/EBPα and GATA factors induce macrophage differentiation of _Pax5_–/– pro-B cells under lymphoid culture conditions. (A and B) In vitro macrophage differentiation. The infection of _Pax5_–/– pro-B cells was initiated at day 0 by co-culture with virus-producing GP+E-86 cells in IL-7 medium (for 3 days) followed by the subsequent propagation on stromal ST2 cells in the presence of IL-7. At day 5, expression of c-Kit was analyzed on infected hCD2+ (gray surface) and non-infected hCD2– (black line) cells by flow cytometry, while the Mac-1 and F4/80 expression profiles at day 5 and 14 were displayed only for the infected hCD2+ cells. (C) Morphology and phagocytosis of in vitro differentiated cells. The morphological appearance and phagocytic activity of _Pax5_–/– cells was revealed at day 7 and 14 after MiCD2 or C/EBPα virus infection by May–Grünwald Giemsa staining or incubation with FITC-labeled E.coli, respectively.
In contrast to the GATA factors, expression of C/EBPα resulted in c-Kit down-regulation and Mac-1 expression already at day 5 (Figure 4A) and in the appearance of macrophages at day 7 after retroviral infection of _Pax5_–/– pro-B cells (Figure 4C). By day 14, almost all of the infected hCD2+ cells co-expressed Mac-1 and F4/80 (Figure 4B), had the morphology of vacuolar macrophages and efficiently phagocytosed fluorescently labeled Escherichia coli (Figure 4C). We conclude therefore that C/EBPα can induce rapid transdifferentiation of _Pax5_–/– pro-B cells into macrophages even under lymphoid growth conditions.
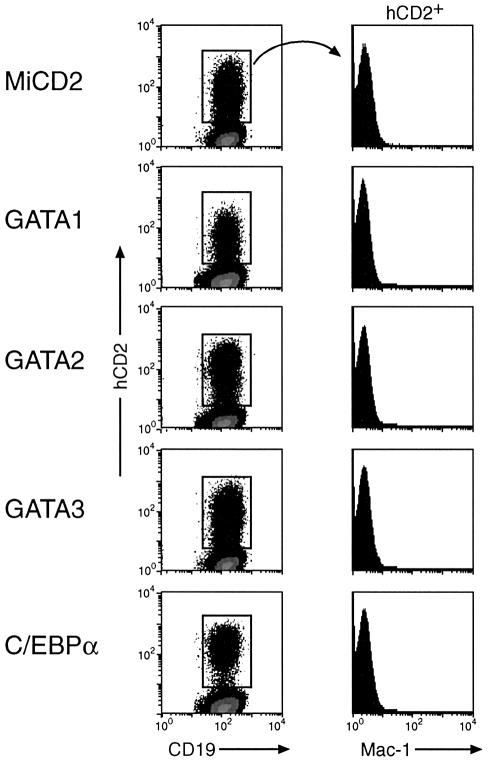
Committed pro-B cells are resistant to the myeloid-inducing activity of C/EBPα and GATA factors
Wild-type pro-B cells can also be propagated under the same lymphoid conditions (Rolink et al., 1991), but are committed to the B-lymphoid lineage due to expression of the transcription factor Pax5 (Nutt et al., 1999). As the gene coding for the B cell surface protein CD19 is a direct target of Pax5 (Nutt et al., 1998), its expression in B-lymphocytes serves as an indicator of both Pax5 activity and B cell commitment (Nutt et al., 1999). Wild-type pro-B cells maintained their CD19 expression level, did not alter their cell size and failed to activate Mac-1 expression even 14 days after infection with C/EBPα- or GATA-expressing retroviruses (Figure 5). Hence, the C/EBPα and GATA factors were unable to interfere with Pax5 expression and to induce myeloid differentiation in wild-type pro-B cells in agreement with the fact that commitment to the B-lymphoid lineage is only lost upon inactivation of Pax5 expression (Mikkola et al., 2002). This conclusion was further supported by the observation that retroviral C/EBPα expression was able to induce myeloid differentiation in _Pax5_F/F pro-B cells (Horcher et al., 2001) only after deletion of the floxed (F) Pax5 alleles by Cre recombinase (supplementary figure 2). Together these data indicate, therefore, that the B-lineage commitment function of Pax5 is dominant over the myeloid-inducing activity of C/EBPα and GATA factors in wild-type pro-B cells.
Fig. 5. C/EBPα and GATA factors fail to induce myeloid differentiation in wild-type pro-B cells. Bone marrow pro-B cells from wild-type mice were infected with the indicated retroviruses and cultured in the presence of IL-7 and stromal cells for 14 days prior to flow cytometric analysis.
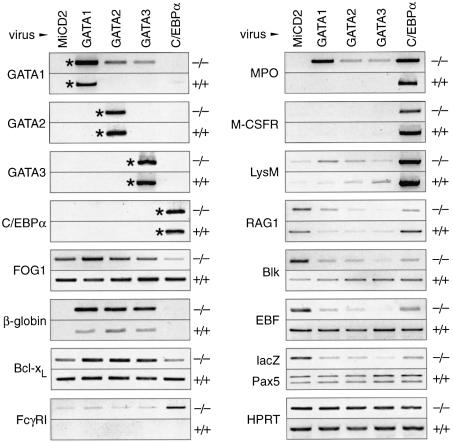
C/EBPα and GATA factors induce a rapid switch from lymphoid to myeloid gene expression in Pax5–/– pro-B cells
To gain insight into the early transcriptional effects of the C/EBPα and GATA factors, we infected wild-type and _Pax5_–/– pro-B cells with the different retroviruses, isolated the hCD2+ cells by FACS sorting after 3 days of infection and investigated the expression of selected erythroid, myeloid and lymphoid genes by semi-quantitative RT–PCR (Figure 6). This analysis indicated that the endogenous GATA1, GATA2, GATA3 and _C/EBP_α genes were either not transcribed or expressed below the detection limit in both wild-type (+/+) and Pax5-deficient (–/–) pro-B cells infected with the control MiCD2 virus (Figure 6). Ectopic expression of GATA2 or GATA3 was able to activate the endogenous GATA1 gene in _Pax5_–/– pro-B cells, thus indicating that the GATA1 locus is in an accessible chromatin configuration in these cells (Figure 6). The GATA1 gene is known to be under auto-regulatory control by its own protein in erythroblasts (Tsai et al., 1991), which may also explain its cross-regulation by GATA2 and GATA3 in _Pax5_–/– pro-B cells. This cross-regulation was, however, not observed in committed wild-type pro-B cells (Figure 6). Moreover, forced expression of C/EBPα and GATA factors also failed to cross-activate the endogenous GATA2, GATA3 and _C/EBP_α genes in both pro-B cell types (Figure 6). Interestingly, the myeloid-inducing activity of the erythroid GATA1 protein does not result from the absence of FOG1, an essential cofactor of GATA1 (Tsang et al., 1997), as FOG1 expression was detected in both wild-type and _Pax5_–/– pro-B cells (Figure 6).
Fig. 6. Gene expression changes in wild-type and _Pax5_–/– pro-B cells upon expression of C/EBPα and GATA factors. Wild-type (+/+) and Pax5 mutant (–/–) pro-B cells were infected in IL-7 medium for 3 days on GP+E-86 cells producing the indicated retroviruses. Immediately after infection, the hCD2+ cells were sorted, and total RNA was prepared. Transcripts of the indicated genes were quantitated by RT–PCR, and the PCR products were visualized on agarose gels by ethidium bromide staining. Retroviral transcripts are indicated by asterisks.
All three GATA factors strongly activated transcription of the βmaj-globin gene in _Pax5_–/– pro-B cells (Figure 6) in agreement with the fact that GATA1 binds to multiple regulatory elements in the β-globin locus (reviewed by Andrews and Orkin, 1994). The three GATA factors also induced expression of the _Bcl-x_L and myeloperoxidase (MPO) genes in _Pax5_–/– pro-B cells and in turn repressed the B-lymphoid genes Blk, RAG1, EBF and Pax5 (monitored as lacZ transcript of the targeted allele; Urbánek et al., 1994). The myeloid M-CSFR (c-fms), Fc_γ_RI (CD64) and lysozyme M (lysM) genes were, however, not yet activated within the first 3 days of infection (Figure 6), consistent with the observation that the three GATA factors induced macrophage differentiation with delayed kinetics in _Pax5_–/– pro-B cells (Figure 4A and B). In wild-type pro-B cells, only the βmaj-globin gene was weakly activated and the RAG1 gene was repressed, thus confirming that the B-lymphoid phenotype of committed pro-B cells is maintained upon ectopic expression of GATA factors (Figure 6).
C/EBPα, like the GATA factors, had no effect on the expression of B-lymphoid genes in wild-type pro-B cells, but repressed their transcription in _Pax5_–/– pro-B cells (Figure 6). Contrary to the GATA factors, C/EBPα failed, however, to activate the erythroid GATA1 and βmaj-globin genes. Instead, it strongly stimulated transcription of the myeloid MPO, M-CSFR, lysM and Fc_γ_RI genes in _Pax5_–/– pro-B cells (Figure 6), thus providing molecular evidence for the rapid C/EBPα-induced transdifferentiation of these cells into macrophages (Figure 4). C/EBPα is known to directly regulate the MPO (Ford et al., 1996), M-CSFR (Zhang et al., 1996) and lysM genes (Wang and Friedman, 2002) in myeloid cells. All three target genes were also strongly activated by C/EBPα in wild-type pro-B cells (Figure 6). Consequently, these wild-type cells expressed myeloid genes in addition to the B-lymphoid gene program and yet remained committed to the B-lymphoid lineage due to their normal expression of Pax5 (Figure 6). These molecular data further demonstrate the dominance of the B-lineage commitment factor Pax5 over the myeloid regulator C/EBPα in wild-type B-lymphocytes.
Discussion
Pax5 was identified as the B-lineage commitment factor based on the discovery that the _Pax5_–/– pro-B cells possess a broad lymphomyeloid developmental potential (Nutt et al., 1999; Rolink et al., 1999). _Pax5_–/– pro-B cells realize, however, their myeloid potential with low efficiency in vivo, as they give rise only to a partial rescue of osteoclast development in c-_fos_–/– mice (Nutt et al., 1999) and to a delayed appearance of granulocytes, macrophages and erythroid cells in transplanted _RAG2_–/– mice (Schaniel et al., 2002a). Here we have shown that the ectopic expression of C/EBPα and the three hematopoietic GATA factors induces a myeloid lineage switch in _Pax5_–/– pro-B cells. All four transcription factors activated the myeloid colony-forming potential of _Pax5_–/– pro-B cells and promoted efficient macrophage differentiation even under lymphoid growth conditions. This myeloid cell fate conversion was accompanied by the rapid activation of myeloid genes and concomitant repression of B-lymphoid genes by all four proteins. To the best of our knowledge, this is the first example of a transcription factor-mediated lineage switch from lymphoid to myeloid cells, while several transcriptional regulators have previously been shown to induce cell fate changes within the erythroid-myeloid compartment (reviewed by Graf, 2002; Heyworth et al., 2002).
GATA2 and GATA3 expression activated the myeloid potential of _Pax5_–/– pro-B cells also in vivo, which resulted in strong engraftment and efficient differentiation to myeloid progenitors, granulocytes and monocytes within 12 days after transplantation. Three independently derived _Pax5_–/– pro-B cell lines gave rise to similar in vivo engraftment and myeloid differentiation upon GATA2 and GATA3 expression (data not shown). This response was, however, not observed after infection of the same pro-B cells with the parental MiCD2 virus. Hence, the rapid engraftment and myeloid development of _Pax5_–/– pro-B cells depends on the expression of GATA2 or GATA3 and is unlikely to be caused by the inadvertent generation of a mutation in these cells. Moreover, histological examinations failed to reveal any infiltration of lymphoid or myeloid cells in non-hematopoietic organs such as the lung, liver and kidney at 5 weeks after pro-B cell transfer (data not shown). Hence, _Pax5_–/– pro-B cells expressing GATA2 or GATA3 give rise to a myeloid hyperplasia in vivo in the absence of leukemic transformation.
C/EBPα is an essential regulator of granulopoiesis, as granulocytes are specifically lost, while macrophages develop normally in _C/EBP_α–/– mice (Zhang et al., 1997). In gain-of-function experiments, C/EBPα can act as a myeloid differentiation switch, as bipotential myeloid precursors develop into granulocytes upon conditional expression of C/EBPα (Radomska et al., 1998; Wang et al., 1999). It was therefore surprising to see that ectopic expression of the same transcription factor induces macrophage differentiation in _Pax5_–/– pro-B cells. The related C/EBPβ protein is the most prominent isoform in monocytic cells, is required for normal macrophage function (Tanaka et al., 1995), can compensate for the loss of C/EBPα in knock-in mice (Jones et al., 2002) and elicits similar responses as C/EBPα upon ectopic expression in hematopoietic cells (Hu et al., 1998; Nerlov et al., 1998). Hence, C/EBPα is likely to mimic C/EBPβ in inducing macrophage differentiation in _Pax5_–/– pro-B cells. The lymphoid culture conditions and regulatory milieu of _Pax5_–/– pro-B cells may additionally modify the differentiation response of C/EBPα particularly in view of the fact that the stromal ST2 cells used express the cytokine M-CSF, but neither G-CSF nor GM-CSF (Yamane et al., 1997; data not shown). In addition to its differentiation function, C/EBPα is also known to inhibit the proliferation of hematopoietic cells (Wang et al., 1999). This growth-inhibitory property is likely to contribute to the rapid transdifferentiation of C/EBPα-expressing _Pax5_–/– pro-B cells into macrophages in vitro, whereas it appears to prevent the engraftment of these cells in vivo.
In contrast, the GATA factors are known to be key regulators of proliferation and survival of early hematopoietic cells (Tsai et al., 1994; Weiss and Orkin, 1995; Tsai and Orkin, 1997; Gregory et al., 1999). Consistent with this notion, the expression of GATA factors strongly promoted the survival and myeloid colony formation of _Pax5_–/– pro-B cells in the absence of the lymphoid proliferation and survival signal IL-7. The GATA1/2/3 proteins also transiently stimulated expression of the early marker c-Kit, suggesting that these regulators initially induce retrodifferentiation of _Pax5_–/– pro-B cells to earlier hematopoietic progenitors followed by subsequent differentiation to myeloid cells. Indeed, the induction of myeloid differentiation and gene expression by the three GATA factors was delayed compared with that of C/EBPα. In addition, most GATA2- and GATA3-expressing cells in the bone marrow of transplanted mice appeared to be early progenitors as judged by their cell surface phenotype. GATA1 differed, however, from GATA2 and GATA3 in two aspects, as only GATA1 efficiently induced erythroid differentiation in vitro and yet failed to give rise to long-lasting engrafting of _Pax5_–/– cells in vivo. These differences must be caused by the activation of distinct target genes, which could result from subtle variations of the DNA-binding specificity of GATA1 compared with GATA2 and GATA3 (Ko and Engel, 1993).
The myeloid-inducing activity of GATA3 in _Pax5_–/– pro-B cells is surprising in view of the fact that GATA3 is essential for early thymocyte and Th2 cell development (Ting et al., 1996; Zheng and Flavell, 1997). The T-lymphoid function of GATA3 may therefore depend on intrathymic signaling possibly involving the Notch1 receptor. GATA3 is, however, able to partially rescue the loss of GATA1 function in erythroid cells of a knock-in mouse (Tsai et al., 1998). Moreover, forced expression of all three GATA factors similarly induces megakaryocyte differentiation in a myeloid cell line (Visvader and Adams, 1993). The GATA1/2/3 proteins are highly conserved only in their zinc-finger DNA-binding domains, which display similar DNA sequence specificities (Ko and Engel, 1993; Merika and Orkin, 1993) and additionally bind the cofactor FOG1 (Tsang et al., 1997). The DNA-binding domain of GATA1 or GATA2 is primarily responsible for erythroid and eosinophilic differentiation of early progenitor cells (Weiss et al., 1997; Hirasawa et al., 2002) and is even sufficient for inducing megakaryocytic differentiation of a primitive myeloid cell line (Visvader et al., 1995). In analogy, the conserved zinc-finger domain of the three GATA factors may also be the main determinant of their myeloid-inducing activity in _Pax5_–/– pro-B cells.
B cell commitment in wild-type pro-B cells was not affected by C/EBPα or the GATA factors, as these regulators were unable to induce myeloid differentiation in committed pro-B cells despite activation of some myeloid target genes. Importantly, all four factors failed to down-regulate the expression of Pax5, which we have shown to be a prerequisite for the decommitment and subsequent differentiation of pro-B cells along other hematopoietic lineages (Mikkola et al., 2002). Indeed, conditional Pax5 inactivation was required for inducing myeloid differentiation of _Pax5_F/F pro-B cells in response to retroviral C/EBPα expression. Hence, the function of Pax5 is dominant over the activity of other lineage-specific transcription factors in committed B-lymphocytes. We have recently described an inverse hierarchical relationship between Pax5 and myeloid transcription factors in myeloid cells, where ectopic expression of Pax5 from the Ikaros locus was unable to interfere with granulocyte and macrophage differentiation in vivo (Souabni et al., 2002).
The CLP, giving rise to B, T, NK and DC cells (Kondo et al., 1997; Traver et al., 2000), can be induced in vitro and in vivo to undergo a lymphoid-to-myeloid lineage conversion by signaling through exogenously expressed IL-2 or GM-CSF receptors (Kondo et al., 2000; Iwasaki-Arai et al., 2003). This cytokine-induced transdifferentiation to granulocytes and macrophages uncovered a latent myeloid potential of the CLP and thus resembles the transcription factor-induced myeloid lineage switch of _Pax5_–/– pro-B cells described in this study. In further analogy, committed wild-type pro-B cells could not be diverted to myeloid lineages by IL-2 signaling (Kondo et al., 2000) similar to the resistance of these cells to myeloid transcription factors (Figure 5). The CLP loses its developmental plasticity within 2 days after its isolation, as it can thereafter no longer be redirected to myeloid lineages by stimulation of the transgenic IL-2 receptor (Kondo et al., 2000). Instead, the CLP undergoes commitment to the B-lymphoid lineage (Kondo et al., 2000) and adopts its primary fate of B cell development (Schebesta et al., 2002). In contrast to the CLP, the _Pax5_–/– pro-B cell maintains its developmental plasticity due to the absence of B cell commitment and can therefore realize its latent myeloid potential with low efficiency over an extended time period (Nutt et al., 1999; Schaniel et al., 2002a). In conclusion, we have shown that the Pax5-deficient pro-B cells are lymphoid progenitors with a latent myeloid developmental potential similar to the CLP.
Materials and methods
Mice
Pax5 and RAG2 mutant mice were maintained on the C57BL/6 background (Urbánek et al., 1994; Rolink et al., 1999).
Pro-B cell culture
B220+ pro-B cells were enriched from the bone marrow of 2 week old mice by magnetic cell sorting (MACS) and propagated on a semi-confluent layer of γ-irradiated stromal ST2 cells in IL-7 medium as described (Nutt et al., 1997).
Retroviral vectors
The parental virus MiCD2 was derived from the MSCV vector MigR1 (Pear et al., 1998) by replacing the IRES-GFP gene with an IRES-hCD2t gene coding for a C-terminally truncated (t) human (h) CD2 protein (Deftos et al., 1998). Retroviral vectors were generated by cloning the mouse GATA1 (2 kb), human GATA2 (2 kb), mouse GATA3 (2 kb) and rat _C/EBP_α (1.4 kb) cDNAs into the MiCD2 vector.
Retroviral infection of pro-B cells
Stable transfectants of the GP+E-86 packaging cell line were generated by a two-step infection procedure. Phoenix (φNX) cells were transfected with bicistronic retroviral vectors by using lipofectamine (Gibco-BRL). The viral supernatants were harvested 48 h later and added together with 4 µg/ml polybrene to GP+E-86 cells, which were pre-treated overnight with 1 µg/ml tunicamycin. Two days after infection, trypsinized GP+E-86 cells were sorted with anti-hCD2 immunomagnetic beads (Miltenyi Biotec). The hCD2+ GP+E-86 cells were resorted 1 week later to obtain a stable retrovirus-producing cell line.
Pro-B cells were infected in IL-7 medium for 3 days by co-culture with retrovirus-producing GP+E-86 cells that were γ-irradiated with 12 Gy. Infected hCD2+ pro-B cells were isolated by MACS or FACS sorting. The expression of GATA and C/EBPα proteins was verified by western blot analysis (Supplementary figure 3). Several pro-B cell lines were established from the bone marrow of _Pax5_–/– mice, as the infection frequency of different cell lines varied considerably for unknown reasons. Only pro-B cell lines with an infection efficiency of >10% were used for in vitro and in vivo differentiation experiments.
Antibodies and flow cytometry
The following monoclonal antibodies were obtained from PharMingen as fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)- or allophycocyanin (APC)-labeled antibodies: anti-B220 (RA3-6B2), anti-CD19 (1D3), anti-Gr-1 (RB6-8C5), anti-c-Kit (2B8), anti-Mac-1/CD11b (M1/70), anti-Ter119 (TER-119) and anti-human CD2 (RPA-2.10) antibodies. PE-conjugated anti-F4/80 (C1:A3-1) antibody was purchased from Serotec and PE-labeled streptavidin from Southern Biotechnology Associates. Single-cell suspensions were stained with the respective antibodies and analyzed on a FACSCalibur flow cytometer (Becton-Dickinson). Unspecific antibody binding was suppressed by the pre-incubation of cells with CD16/CD32 Fc-block solution (PharMingen). A wide FSC/SSC gate, which included all hematopoietic cells except for the small erythrocytes, was used for the analysis of bone marrow, spleen and in vitro cultured cells.
Pro-B cell transfer into RAG2–/– mice
_Pax5_–/– pro-B cells were first propagated on ST2 cells in IL-7 medium for 3 months and then infected by co-culture with virus-producing GP+E-86 cells. The infected hCD2+ pro-B cells were isolated by FACS sorting on day 3 of co-culture, and 3 × 105 sorted pro-B cells were intravenously injected into 8 to 12-week-old _RAG2_–/– mice that were γ-irradiated with 5.5 Gy 15 h prior to injection.
In vitro macrophage differentiation
Following retroviral infection, the _Pax5_–/– pro-B cells were continuously cultured on ST2 cells in IL-7 medium (containing 0.5% supernatant of rIL-7 producing J558L cells) prior to flow cytometry and cytospin preparation.
Colony assays
In vitro colony-forming assays were carried out in duplicate by plating 500 infected hCD2+ _Pax5_–/– pro-B cells (3 days after infection) with 1 ml of MethoCult™ M3434 medium (StemCell Technologies) in 35 mm Petri dishes (for details see Souabni et al., 2002). Colonies were counted after 4–5 days. The methylcellulose was washed away from the cells of one dish prior to flow cytometric analysis and May–Grünwald Giemsa staining. Alternatively, 5000 infected _Pax5_–/– pro-B cells were plated in 1 ml of Epo-containing MethoCult™ M3334 supplemented with SCF (50 ng/ml), and the colonies were scored after 8 days.
RT–PCR analysis
hCD2+ _Pax5_–/– pro-B cells were isolated by FACS sorting 3 days after the initiation of retroviral infection, and total RNA was prepared using the Trizol Reagent (Gibco-BRL). Reverse transcription (with random hexamer primers) and semi-quantitative PCR were performed as described (Horcher et al., 2001). The input cDNA template was normalized to generate an equivalent amount of HPRT cDNA. The primer sequences and details of the RT–PCR analysis are shown in Supplementary table I.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank S.Orkin and S.McKnight for providing GATA1/2/3 and _C/EBP_α cDNA clones, Q.Sun for technical assistance and G.Stengl and K.Paiha for FACS sorting. This research was supported by Boehringer Ingelheim, the Austrian Industrial Research Promotion Fund, a short-term EMBO (B.H.) and Marie Curie (C.C.) fellowship.
References
- Akashi K., Traver,D., Miyamoto,T. and Weissman,I.L. (2000) A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature, 404, 193–197. [DOI] [PubMed] [Google Scholar]
- Allman D., Sambandam,A., Kim,S., Miller,J.P., Pagan,A., Well,D., Meraz,A. and Bhandoola,A. (2003) Thymopoiesis independent of common lymphoid progenitors. Nat. Immunol., 4, 168–174. [DOI] [PubMed] [Google Scholar]
- Andrews N.C. and Orkin,S.H. (1994) Transcriptional control of erythropoiesis. Curr. Opin. Hematol., 1, 119–124. [PubMed] [Google Scholar]
- Deftos M.L., He,Y.-W., Ojala,E.W. and Bevan,M.J. (1998) Correlating Notch signaling with thymocyte maturation. Immunity, 9, 777–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford A.M., Bennett,C.A., Healy,L.E., Towatari,M., Greaves,M.F. and Enver,T. (1996) Regulation of the myeloperoxidase enhancer binding proteins Pu1, C-EBPα, -β and -δ during granulocyte-lineage specification. Proc. Natl Acad. Sci. USA, 93, 10838–10843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara Y., Browne,C.P., Cunniff,K., Goff,S.C. and Orkin,S.H. (1996) Arrested development of embryonic red cell precursors in mouse embryos lacking transcription factor GATA-1. Proc. Natl Acad. Sci. USA, 93, 12355–12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T. (2002) Differentiation plasticity of hematopoietic cells. Blood, 99, 3089–3101. [DOI] [PubMed] [Google Scholar]
- Gregory T., Yu,C., Ma,A., Orkin,S.H., Blobel,G.A. and Weiss,M.J. (1999) GATA-1 and erythropoietin cooperate to promote erythroid cell survival by regulating bcl-xL expression. Blood, 94, 87–96. [PubMed] [Google Scholar]
- Heyworth C., Pearson,S., May,G. and Enver,T. (2002) Transcription factor-mediated lineage switching reveals plasticity in primary committed progenitor cells. EMBO J., 21, 3770–3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa R. et al. (2002) Essential and instructive roles of GATA factors in eosinophil development. J. Exp. Med., 195, 1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horcher M., Souabni,A. and Busslinger,M. (2001) Pax5/BSAP maintains the identity of B cells in late B lymphopoiesis. Immunity, 14, 779–790. [DOI] [PubMed] [Google Scholar]
- Hu H.M., Baer,M., Williams,S.C., Johnson,P.F. and Schwartz,R.C. (1998) Redundancy of C/EBPα, -β and -δ in supporting the lipopolysaccharide-induced transcription of IL-6 and monocyte chemoattractant protein-1. J. Immunol., 160, 2334–2342. [PubMed] [Google Scholar]
- Igarashi H., Gregory,S.C., Yokota,T., Sakaguchi,N. and Kincade,P.W. (2002) Transcription from the RAG1 locus marks the earliest lymphocyte progenitors in bone marrow. Immunity, 17, 117–130. [DOI] [PubMed] [Google Scholar]
- Iwasaki-Arai J., Iwasaki,H., Miyamoto,T., Watanabe,S. and Akashi,K. (2003) Enforced granulocyte/macrophage colony-stimulating factor signals do not support lymphopoiesis, but instruct lymphoid to myelomonocytic lineage conversion. J. Exp. Med., 197, 1311–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L.C., Lin,M.-L., Chen,S.-S., Krug,U., Hofmann,W.-K., Lee,S., Lee,Y.-H. and Koeffler,H.P. (2002) Expression of C/EBPβ from the C/ebpa gene locus is sufficient for normal hematopoiesis in vivo. Blood, 99, 2032–2036. [DOI] [PubMed] [Google Scholar]
- Ko L.J. and Engel,J.D. (1993) DNA-binding specificities of the GATA transcription factor family. Mol. Cell. Biol., 13, 4011–4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M., Weissman,I.L. and Akashi,K. (1997) Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell, 91, 661–672. [DOI] [PubMed] [Google Scholar]
- Kondo M., Scherer,D.C., Miyamoto,T., King,A.G., Akashi,K., Sugamura,K. and Weissman,I.L. (2000) Cell-fate conversion of lymphoid-committed progenitors by instructive actions of cytokines. Nature, 407, 383–386. [DOI] [PubMed] [Google Scholar]
- Landschulz W.H., Johnson,P.F., Adashi,E.Y., Graves,B.J. and McKnight,S.L. (1988) Isolation of a recombinant copy of the gene encoding C/EBP. Genes Dev., 2, 786–800. [DOI] [PubMed] [Google Scholar]
- Leonard M., Brice,M., Engel,J.D. and Papayannopoulou,T. (1993) Dynamics of GATA transcription factor expression during erythroid differentiation. Blood, 82, 1071–1079. [PubMed] [Google Scholar]
- Manaia A., Lemarchandel,V., Klaine,M., Max-Audit,I., Romeo,P.-H., Dieterlen-Lièvre,F. and Godin,I. (2000) Lmo2 and GATA-3 associated expression in intraembryonic hemogenic sites. Development, 127, 643–653. [DOI] [PubMed] [Google Scholar]
- Martin D.I., Zon,L.I., Mutter,G. and Orkin,S.H. (1990) Expression of an erythroid transcription factor in megakaryocytic and mast cell lineages. Nature, 344, 444–447. [DOI] [PubMed] [Google Scholar]
- Merika M. and Orkin,S.H. (1993) DNA-binding specificity of GATA family transcription factors. Mol. Cell. Biol., 13, 3999–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkola I., Heavey,B., Horcher,M. and Busslinger,M. (2002) Reversion of B cell commitment upon loss of Pax5 expression. Science, 297, 110–113. [DOI] [PubMed] [Google Scholar]
- Mouthon M.A., Bernard,O., Mitjavila,M.T., Romeo,P.H., Vainchenker,W. and Mathieu-Mahul,D. (1993) Expression of tal-1 and GATA-binding proteins during human hematopoiesis. Blood, 81, 647–655. [PubMed] [Google Scholar]
- Nerlov C., McNagny,K.M., Doderlein,G., Kowenz-Leutz,E. and Graf,T. (1998) Distinct C/EBP functions are required for eosinophil lineage commitment and maturation. Genes Dev., 12, 2413–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt S.L., Urbánek,P., Rolink,A. and Busslinger,M. (1997) Essential functions of Pax5 (BSAP) in pro-B cell development: difference between fetal and adult B lymphopoiesis and reduced _V_-to-DJ recombination at the IgH locus. Genes Dev., 11, 476–491. [DOI] [PubMed] [Google Scholar]
- Nutt S.L., Morrison,A.M., Dörfler,P., Rolink,A. and Busslinger,M. (1998) Identification of BSAP (Pax-5) target genes in early B-cell development by loss- and gain-of-function experiments. EMBO J., 17, 2319–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt S.L., Heavey,B., Rolink,A.G. and Busslinger,M. (1999) Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature, 401, 556–562. [DOI] [PubMed] [Google Scholar]
- Pear W.S. et al. (1998) Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood, 92, 3780–3792. [PubMed] [Google Scholar]
- Pevny L., Simon,M.C., Robertson,E., Klein,W.H., Tsai,S.-F., D’Agati,V., Orkin,S.H. and Costantini,F. (1991) Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature, 349, 257–260. [DOI] [PubMed] [Google Scholar]
- Radomska H.S., Huettner,C.S., Zhang,P., Cheng,T., Scadden,D.T. and Tenen,D.G. (1998) CCAAT/enhancer binding protein alpha is a regulatory switch sufficient for induction of granulocytic development from bipotential myeloid progenitors. Mol. Cell. Biol., 18, 4301–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolink A., Kudo,A., Karasuyama,H., Kikuchi,Y. and Melchers,F. (1991) Long-term proliferating early pre B cell lines and clones with the potential to develop to surface Ig-positive, mitogen reactive B cells in vitro and in vivo. EMBO J., 10, 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolink A.G., Nutt,S.L., Melchers,F. and Busslinger,M. (1999) Long-term in vivo reconstitution of T-cell development by Pax5-deficient B-cell progenitors. Nature, 401, 603–606. [DOI] [PubMed] [Google Scholar]
- Schaniel C., Bruno,L., Melchers,F. and Rolink,A.G. (2002a) Multiple hematopoietic cell lineages develop in vivo from transplanted _Pax5_-deficient pre-B I-cell clones. Blood, 99, 472–478. [DOI] [PubMed] [Google Scholar]
- Schaniel C., Gottar,M., Roosnek,E., Melchers,F. and Rolink,A.G. (2002b) Extensive in vivo self-renewal, long-term reconstitution capacity and hematopoietic multipotency of Pax5-deficient precursor B-cell clones. Blood, 99, 2760–2766. [DOI] [PubMed] [Google Scholar]
- Schebesta M., Heavey,B. and Busslinger,M. (2002) Transcriptional control of B cell development. Curr. Opin. Immunol., 14, 216–223. [DOI] [PubMed] [Google Scholar]
- Scott L.M., Civin,C.I., Rorth,P. and Friedman,A.D. (1992) A novel temporal expression pattern of three C/EBP family members in differentiating myelomonocytic cells. Blood, 80, 1725–1735. [PubMed] [Google Scholar]
- Shivdasani R.A., Fujiwara,Y., McDevitt,M.A. and Orkin,S.H. (1997) A lineage-selective knockout establishes the critical role of transcription factor GATA-1 in megakaryocyte growth and platelet development. EMBO J., 16, 3965–3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souabni A., Cobaleda,C., Schebesta,M. and Busslinger,M. (2002) Pax5 promotes B lymphopoiesis and blocks T cell development by repressing Notch1. Immunity, 17, 781–793. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Akira,S., Yoshida,K., Umemoto,M., Yoneda,Y., Shirafuji,N., Fujiwara,H., Suematsu,S., Yoshida,N. and Kishimoto,T. (1995) Targeted disruption of the NF-IL6 gene discloses its essential role in bacteria killing and tumor cytotoxicity by macrophages. Cell, 80, 353–361. [DOI] [PubMed] [Google Scholar]
- Ting C.N., Olson,M.C., Barton,K.P. and Leiden,J.M. (1996) Transcription factor GATA-3 is required for development of the T-cell lineage. Nature, 384, 474–478. [DOI] [PubMed] [Google Scholar]
- Traver D., Akashi,K., Manz,M., Merad,M., Miyamoto,T., Engleman,E.G. and Weissman,I.L. (2000) Development of CD8α-positive dendritic cells from a common myeloid progenitor. Science, 290, 2152–2154. [DOI] [PubMed] [Google Scholar]
- Tsai F.-Y. and Orkin,S.H. (1997) Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood, 89, 3636–3643. [PubMed] [Google Scholar]
- Tsai S.F., Martin,D.I., Zon,L.I., D’Andrea,A.D., Wong,G.G. and Orkin,S.H. (1989) Cloning of cDNA for the major DNA-binding protein of the erythroid lineage through expression in mammalian cells. Nature, 339, 446–451. [DOI] [PubMed] [Google Scholar]
- Tsai S.-F., Strauss,E. and Orkin,S.H. (1991) Functional analysis and in vivo footprinting implicate the erythroid transcription factor GATA-1 as a positive regulator of its own promoter. Genes Dev., 5, 919–931. [DOI] [PubMed] [Google Scholar]
- Tsai F.-Y., Keller,G., Kuo,F.C., Weiss,M., Chen,J., Rosenblatt,M., Alt,F.W. and Orkin,S.H. (1994) An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature, 371, 221–226. [DOI] [PubMed] [Google Scholar]
- Tsai F.-Y., Browne,C.P. and Orkin,S.H. (1998) Knock-in mutation of transcription factor GATA-3 into the GATA-1 locus: partial rescue of GATA-1 loss of function in erythroid cells. Dev. Biol., 196, 218–227. [DOI] [PubMed] [Google Scholar]
- Tsang A.P., Visvader,J.E., Turner,C.A., Fujiwara,Y., Yu,C., Weiss,M.J., Crossley,M. and Orkin,S.H. (1997) FOG, a multitype zinc finger protein, acts as a cofactor for transcription factor GATA-1 in erythroid and megakaryocytic differentiation. Cell, 90, 109–119. [DOI] [PubMed] [Google Scholar]
- Urbánek P., Wang,Z.-Q., Fetka,I., Wagner,E.F. and Busslinger,M. (1994) Complete block of early B cell differentiation and altered patterning of the posterior midbrain in mice lacking Pax5/BSAP. Cell, 79, 901–912. [DOI] [PubMed] [Google Scholar]
- Visvader J. and Adams,J.M. (1993) Megakaryocytic differentiation induced in 416B myeloid cells by GATA-2 and GATA-3 transgenes or 5-azacytidine is tightly coupled to GATA-1 expression. Blood, 82, 1493–1501. [PubMed] [Google Scholar]
- Visvader J.E., Crossley,M., Hill,J., Orkin,S.H. and Adams,J.M. (1995) The C-terminal zinc finger of GATA-1 or GATA-2 is sufficient to induce megakaryocytic differentiation of an early myeloid cell line. Mol. Cell. Biol., 15, 634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.-F. and Friedman,A.D. (2002) CCAAT/enhancer-binding proteins are required for granulopoiesis independent of their induction of the granulocyte colony-stimulating factor receptor. Blood, 99, 2776–2785. [DOI] [PubMed] [Google Scholar]
- Wang X., Scott,E., Sawyers,C.L. and Friedman,A.D. (1999) C/EBPα bypasses granulocyte colony-stimulating factor signals to rapidly induce PU.1 gene expression, stimulate granulocytic differentiation and limit proliferation in 32D cl3 myeloblasts. Blood, 94, 560–571. [PubMed] [Google Scholar]
- Weiss M.J. and Orkin,S.H. (1995) Transcription factor GATA-1 permits survival and maturation of erythroid precursors by preventing apoptosis. Proc. Natl Acad. Sci. USA, 92, 9623–9627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M.J., Yu,C. and Orkin,S.H. (1997) Erythroid-cell-specific properties of transcription factor GATA-1 revealed by phenotypic rescue of a gene-targeted cell line. Mol. Cell. Biol., 17, 1642–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane T., Kunisada,T., Yamazaki,H., Era,T., Nakano,T. and Hayashi,S.-I. (1997) Development of osteoclasts from embryonic stem cells through a pathway that is c-fms but not c-kit dependent. Blood, 90, 3516–3523. [PubMed] [Google Scholar]
- Zhang D.E., Hetherington,C.J., Meyers,S., Rhoades,K.L., Larson,C.J., Chen,H.M., Hiebert,S.W. and Tenen,D.G. (1996) CCAAT enhancer-binding protein (C/EBP) and AML1 (CBFα2) synergistically activate the macrophage colony-stimulating factor receptor promoter. Mol. Cell. Biol., 16, 1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D.E., Zhang,P., Wang,N.D., Hetherington,C.J., Darlington,G.J. and Tenen,D.G. (1997) Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein α-deficient mice. Proc. Natl Acad. Sci. USA, 94, 569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W. and Flavell,R.A. (1997) The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell, 89, 587–596. [DOI] [PubMed] [Google Scholar]