emb-4 Is a Conserved Gene Required for Efficient Germline-Specific Chromatin Remodeling During Caenorhabditis elegans Embryogenesis (original) (raw)
Abstract
In C. elegans, germline blastomeres are initially kept transcriptionally quiescent by the maternally loaded CCCH zinc-finger protein PIE-1. PIE-1 disappears upon the birth of the primordial germ cells Z2 and Z3, yet these cells appear to remain quiescent. We have previously demonstrated that there is a chromatin-based repression that succeeds PIE-1 degradation. The chromatin in Z2/Z3 loses certain histone modifications, including histone H3 lysine 4 dimethylation (H3K4me2), a conserved marker for transcriptionally competent chromatin. We find that mutations in the maternal-effect gene emb-4 cause defects in both PIE-1 degradation and germline-specific chromatin remodeling. emb-4 encodes a highly conserved protein with orthologs in fly, mouse, and human and has a subtle role in Notch signaling. The embryonic phenotype of emb-4 is consistent with a defect in the efficient and timely activation of developmental programs, including germline chromatin remodeling. We also find that, as in early somatic blastomeres, the degradation of PIE-1 in Z2/Z3 is facilitated by zinc-finger-interacting protein ZIF-1, and in the absence of either zif-1 or emb-4, PIE-1 is abnormally retained in Z2/Z3.
UNDERSTANDING the processes that establish and maintain the germ lineage has long been a fundamental goal of developmental biology research. Throughout embryogenesis, regulatory mechanisms are thought to safeguard the germline to maintain its pluripotent capacity and protect it from factors that promote somatic differentiation (Seydoux and Strome 1999). One such regulatory mechanism conserved in many organisms is the transcriptional inactivation of the embryonic germline (Seydoux and Schedl 2001). In both Caenorhabditis elegans and Drosophila melanogaster, for example, transcriptional quiescence has been shown to be an essential requirement for formation and survival of a functional germline (Seydoux and Strome 1999; Leatherman and Jongens 2003).
In the early C. elegans embryo, RNA polymerase II (RNAPII) transcription is repressed in the “P”-lineage germline blastomeres by the maternally synthesized protein PIE-1 (Batchelder et al. 1999). PIE-1 is a CCCH zinc-finger protein that segregates asymmetrically to remain concentrated within the posterior daughter cell at each P-blastomere division. In pie-1 mutant embryos, transcription is aberrantly activated in the P2 blastomere, causing the P2-lineage to undergo somatic transformation and to behave identically to the descendants of the EMS blastomere (the somatic sister of P2) (Mello et al. 1992, 1996; Seydoux et al. 1996). This strongly indicates that in C. elegans transcriptional repression is necessary for maintaining germ cell identity, and in the absence of transcriptional repression, the default cell fate of the germ lineage is somatic.
PIE-1 has been proposed to repress transcription by sequestering factors necessary for transcriptional elongation (Zhang et al. 2003). Whereas the early somatic blastomeres are transcriptionally engaged at the four-cell stage and exhibit phospho-epitopes of RNAPII that correspond to both transcriptional initiation and elongation (phosSer5 and phosSer2, respectively), the early P-lineage expresses only phosSer5 (Seydoux and Dunn 1997). The phosSer2 epitope is observed in the P-lineage only after PIE-1 is degraded, which occurs after the division of P4 and yields the “germline restricted” primordial germ cells Z2 and Z3 (Z2/Z3). The term “germline restricted” is used to describe P4 and Z2/Z3 since they, unlike the other P blastomeres, do not contribute to any somatic lineages.
Although the phosSer2 epitope appears in Z2/Z3, there is little evidence for robust transcription in these cells, which remain mitotically inactive throughout embryogenesis (Seydoux and Dunn 1997). Only two zygotic transcripts have been identified as being produced in Z2/Z3, and both accumulate late in embryogenesis (Subramaniam and Seydoux 1999; Kawasaki et al. 2004). Recent evidence suggests that the apparent paucity of transcription in Z2/Z3 may be due to the initiation of a specific chromatin-based repressive mechanism in these cells (Schaner et al. 2003). Higher-order chromatin assembly can have direct effects on the global regulation of gene expression, and a variety of post-translational modifications on the N-terminal tails of nucleosomal core histones are thought to influence chromatin organization (Kornberg and Lorch 1999; Strahl and Allis 2000; Fischle et al. 2003). A number of histone modifications strongly correlate with transcriptionally competent chromatin. Two of these modifications, dimethylation of lysine 4 on histone 3 and aceylation of lysine 8 on histone 4 (H3K4me2 and H4K8ac, respectively), selectively disappear from Z2/Z3, while remaining present in most other cells in the embryo. In addition, the DNA in Z2/Z3 appears more condensed than the DNA in neighboring somatic nuclei, suggesting that loss of H3K4me2 and H4K8ac staining correlates with a physical change in the chromatin architecture of Z2/Z3 (Schaner et al. 2003). PIE-1 protein is degraded in Z2/Z3 coincident with the disappearance of these modifications, suggesting a possible linkage between the two events. However, the mechanism(s) controlling the temporal regulation of PIE-1 degradation in the nascent germ cells, as well as loss of H3K4me2 and H4K8ac from Z2/Z3, are still poorly understood.
The absence of H3K4me2 from primordial germ cell (PGC) chromatin is a conserved characteristic, as it is also observed in Drosophila (Schaner et al. 2003). Furthermore, the conserved maternal factor Nanos is required for the continued absence of H3K4me2 from PGC chromatin in both worms and flies (Schaner et al. 2003). We also observed that a mutation in another locus, emb-4, caused H3K4me2 retention and defective PIE-1 protein degradation in Z2/Z3 (Schaner et al. 2003). While it was not determined whether the persistence of PIE-1 in emb-4 embryos is causal to the retention of H3K4me2 in Z2/Z3, their correlation suggested a potential link between the two processes.
Here we report the further characterization of the emb-4 phenotype and the molecular identification of emb-4. We (and others) found emb-4 to be allelic to mutations in mal-2 and sel-6 (Katic and Greenwald 2006, accompanying article in this issue). The mal-2 and sel-6 mutations were isolated in genetic screens for maternal-effect morphologically abnormal mutants and as genetic suppressors of a dominant lin-12 [_lin-12(d)_] (Notch) gain-of-function mutation, respectively (Hekimi et al. 1995; Tax et al. 1997; Katic and Greenwald 2006, this issue). We found that EMB-4 is a highly conserved protein with homologs in fly, mouse, and human, for which a function has not been previously described. We show that severe emb-4 alleles are defective in germline-specific chromatin remodeling during embryogenesis and that this phenotype correlates with high lethality of emb-4 null (emb-4[0]) alleles. Less severe emb-4 alleles have defects restricted to lin-12(d) suppression [the emb-4(sel) alleles] (Katic and Greenwald 2006, this issue). We have also further investigated the degradation of PIE-1 in Z2/Z3 and show that this occurs by a mechanism similar to its degradation in somatic lineages, but is delayed in the germline until the birth of Z2/Z3. The accompanying article by Katic and Greenwald (2006, this issue) provides evidence that EMB-4 has a genetically defined role in LIN-12/Notch signaling in postembryonic lineages and indicates that EMB-4 is a nuclear protein. This is consistent with our results, which suggest that EMB-4 plays an important role in processes that perform the unique chromatin remodeling that occurs in the embryonic germline.
MATERIALS AND METHODS
Alleles and strain maintenance:
Maintenance and genetic analysis of worms were performed as described in Brenner (1974). C. elegans var. Bristol (N2) was used as the wild-type strain. The transgenic elt-7∷gfp worms used were an integrated line provided as a gift from J. Rothmann (University of California at Santa Barbara). The following mutations were used in this study: LG IV, lag-1(om13); LG V, emb-4(hc60), mal-2(qm16, qm31, qm35), and sel-6(n1256, sa44). The presumed null alleles of emb-4 (hc60, qm16, qm31, and qm35) are referred to collectively as emb-4(0), and the alleles isolated as suppressors of lin-12(d), (n1256 and sa44) are referred to as the emb-4(sel) alleles. Strains were maintained at either 16° or 20°, unless otherwise noted. The temperature-sensitive emb-4(0) embryos used in immunocytochemistry were the progeny of L4 larvae raised to 25° overnight.
Immunofluorescence analysis:
Whole-mount embryos were fixed using a methanol/acetone procedure followed by antibody staining (Strome and Wood 1983). In some cases (e.g., staining for anti-PIE-1), whole-mount embryos were fixed in 2.5% formaldehyde, followed by a 2-min postfix in −20° (95%) ethanol prior to washing and antibody addition.
The stage of each embryo was determined by counting DAPI-stained nuclei in optical Z-sections using a Leica DMRA microscope outfitted with a Cooke Sensicam. Collection and analysis of the images were performed using Volume Scan (Vaytek, Fairfield, IA) and Image-Pro Plus (Media Cybernetics) software.
Immunostaining of embryos was performed as in Strome and Wood (1983). The following primary antibodies were used at the indicated dilutions: rabbit antiacetylated histone H4 acetyl K8 diluted 1:500 (Serotec, Oxford, UK), rabbit antihistone H3 dimethyl K4 1:500 (a gift from. C. David Allis, Rockefeller University), and goat anti-PIE-1 diluted 1:100 (Santa Cruz Biotechnology). P granules were visualized using either affinity-purified monoclonal anti-P granule OIC1D4 (undiluted) or rabbit affinity-purified anti-PGL-1 diluted 1:10,000 (gifts from S. Strome, Indiana University). The following secondary antibodies from Molecular Probes (Eugene, OR) were all used at 1:500 dilutions: Alexa Fluor 596 goat anti-rabbit IgG diluted, Alexa Fluor 568 donkey anti-goat IgG diluted, Alexa Fluor 488 goat anti-mouse IgG diluted, and Alexa Fluor 488 donkey anti-rabbit IgG diluted. DAPI (1 μg/ml; Sigma, St. Louis) was used to counterstain DNA.
RNA-mediated interference analysis:
All RNA-mediated interference experiments in this study were performed at 20°, using the feeding method, as described in Timmons et al. (2001). For emb-4 (RNAi) experiments, L4 larvae were fed a Y80D3A.h RNAi-inducing HT115(DE3) bacteria strain from an available RNAi feeding library (Kamath et al. 2003). As a control, animals were fed the same bacteria, except that it was transformed with the empty RNAi feeding vector, L4440. Progeny from the fed animals were transferred to fresh RNAi feeding plates and the gravid F1 adults were subsequently dissected. Additional RNAi constructs generated from an emb-4 full-length cDNA (yk1132d03) cloned into the L4440 vector yielded similar phenotypes, but for this study all images and scoring were done using the Y80D3A.h RNAi construct.
RESULTS
emb-4 encodes a highly conserved protein with putative helicase and RNA-binding motifs:
Mutations in emb-4 cause high levels of maternal-effect embryonic lethality with 100% embryonic arrest if the mothers are shifted to 25° (Miwa et al. 1980; Schierenberg et al. 1980). We previously reported that defects in both primordial germ-cell chromatin remodeling and PIE-1 degradation are observed in emb-4 embryos (Schaner et al. 2003). We wanted to more closely examine these defects and to try to understand more clearly the role of emb-4 in the chromatin-remodeling process. To identify the mutation(s) causing these defects, we positionally mapped the emb-4(hc60) locus to a narrow region near the right end of linkage group V. During the course of this mapping, we found that another maternal-effect mutation that mapped within the emb-4 interval, mal-2(qm31), failed to complement emb-4(hc60). mal-2 was identified in a screen for maternal-effect morphologically abnormal mutants (Hekimi et al. 1995). As was observed with emb-4 mutants, shifting mal-2 mothers to 25° results in 100% embryonic lethality in the F1 generation. emb-4(hc60) fails to complement all three mal-2 alleles (qm16, qm31, and qm35) for embryonic lethality, and emb-4/mal-2 embryos are phenotypically identical to either homozygous mutation alone (data not shown).
Recent data have also linked emb-4/mal-2 to a third mutant, sel-6, which was isolated in a screen for suppressors of lin-12(n302), a weak gain-of-function allele of the Notch-type receptor LIN-12 (Tax et al. 1997; Katic and Greenwald 2006, this issue). sel-6 mutants suppress uncontrolled Notch signaling from a constitutively activated LIN-12/Notch receptor, suggesting that the normal function of the gene is to help activate the pathway downstream of the receptor signal. The identity of sel-6 has been determined to be the Y80D3A.2 locus by RNA interference (RNAi). RNAi of the Y80D3A.2 locus phenocopies the sel-6 phenotype by suppressing a lin-12(n302) gain -of-function mutation, suggesting that EMB-4 acts as a positive effector in Notch signaling (Katic and Greenwald 2006, this issue).
We sequenced the Y80D3A.2 locus in DNA isolated from the emb-4(hc60), mal-2(qm35), and mal-2(qm31) alleles and found nonsense mutations in all three alleles (Katic and Greenwald 2006, this issue; data not shown). Thus, emb-4, mal-2, and sel-6 mutations are all alleles of the same gene. The emb-4(hc60) allele contains a nonsense mutation within the fifth exon, and the mal-2(qm35) and mal-2(qm31) alleles contain nonsense mutations within the 1st and 13th exon, respectively, and therefore all are predicted to be null or strong loss-of-function alleles. Both emb-4(sel) alleles isolated in the lin-12(d) suppression screen contain missense mutations, suggesting that they are hypomorphic (Katic and Greenwald 2006, this issue). Therefore, only the embryonic lethal alleles contain premature stop codons and presumably encode truncated and/or unstable proteins. Indeed, the embryonic lethal phenotype is not notably more severe in animals that carry emb-4(hc60) over a deficiency for Y80D3A.2 (data not shown). The strongest and putative null phenotype for the gene defined by this collection of allelic mutations is embryonic lethality, and because emb-4(hc60) was the first to be isolated of the allelic series, we will hereafter refer to the gene as emb-4. Because the sel alleles define a unique, hypomorphic class of emb-4 alleles, they are referred to as emb-4(sel).
The predicted structure of the unspliced emb-4 gene is >34 kb, and it encodes a previously uncharacterized protein of ∼1467 amino acids that contains an AAA ATPase motif and a putative DNA/RNA helicase motif (Katic and Greenwald 2006, issue). The gene is highly conserved in all eukaryotes examined with the exception of Saccharomyces cerevisiae. Relative to fly, mouse, and human orthologs, the predicted EMB-4 protein shares >50% overall identity, and it appears to be present as a single-copy gene in the genomes of these organisms. The mouse homolog, named AQUARIUS, was identified in a promoter trap screen; the trap did not affect the expression of the locus, so no phenotypic information is available (Sam et al. 1998).
Chromatin-remodeling mechanisms are disrupted in emb-4 embryos:
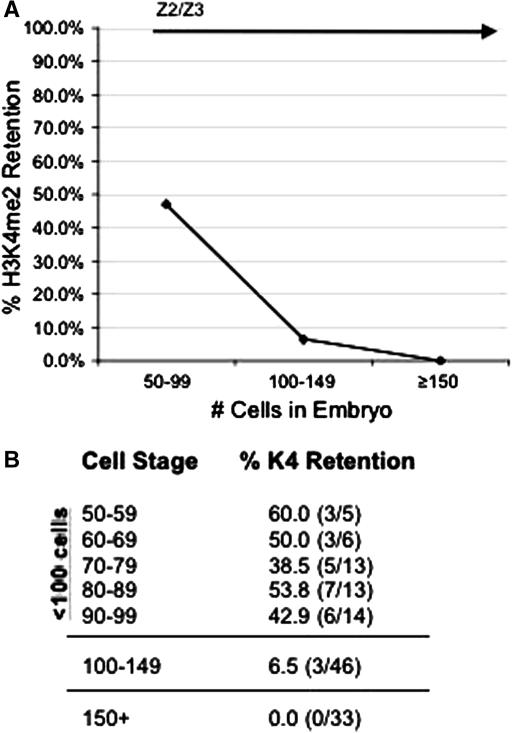
In wild-type embryos, the germline blastomere P4 moves to the inside of the embryo during gastrulation and subsequently divides to form the PGCs, Z2/Z3. We have previously demonstrated that following their birth, the PGCs undergo a chromatin-remodeling mechanism that is unique to these cells (Schaner et al. 2003). The histone modification H3 lysine 4 dimethylation (H3K4me2) is specifically lost from Z2/Z3, while all of the somatic cells in the embryo retain this mark (Figure 1, C and D). We wanted to more accurately elucidate the time course during which chromatin remodeling is initiated in Z2/Z3, so we scored for the presence or absence of H3K4me2 staining in closely staged embryos just prior to and after the birth of Z2/Z3. We stained whole-mount embryos with an antibody against H3K4me2, and the germline precursor cells Z2/Z3 were identified by costaining embryos with OIC1D4, a monoclonal antibody that binds to germline-specific components named P granules (Strome and Wood 1983). Interestingly, H3K4me2 is still observed in Z2/Z3 in nearly 50% of all wild-type embryos just after the division of P4 that marks their birth, suggesting that there can be a slight delay between PGC birth and the onset or completion of chromatin remodeling (Figure 2; Table 1). However, it is clear that the signals required for initiation of chromatin remodeling are not always precise nor invariantly timed, since a significant decrease in the amount of H3K4me2 staining was often observed in P4, just prior to its division (Figure 1B; data not shown). Furthermore, a small fraction of embryos between 100 and 150 cells were observed to still exhibit H3K4me2 staining in Z2/Z3 (Figure 2). However, in wild-type embryos with >150 cells, we did not observe any significant levels of H3K4me2 staining in Z2/Z3 relative to neighboring somatic cells in the assays presented (Figure 2B). It should be noted that when including a number of further studies, we have on rare occasion observed H3K4me2 in Z2/Z3 in later-stage wild-type embryos, but the frequency was very low and was not deemed to be significant (P. Checchi, H. Furuhashi and W. Kelly, unpublished results). In contrast to wild-type, H3K4me2 was retained in Z2/Z3 in emb-4 mutant embryos at all stages assayed, and retention of this mark was frequently observed at the point of arrest in late stages of embryogenesis (Table 1; Figure 3).
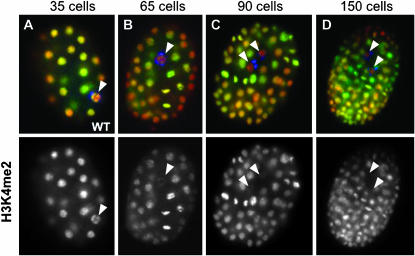
Figure 1.—
H3K4me2 disappearance in the embryonic germline. Mixed-stage populations of fixed, whole-mount wild-type embryos were costained with an antibody against the histone modification H3K4me2 (green, bottom row) and the monoclonal antibody OIC1D4 to mark P granules/germ lineage (blue) and were counterstained with DAPI (red). H3K4me2 is present in all cells, including the P4 germline blastomeres, of the early embryo (arrowhead in A), but declines or is absent in later-stage germ cells (B–D, arrowheads). H3K4me2 staining begins to disappear either in the germline blastomere P4 prior to its division (B, arrowhead) or shortly after the birth of Z2/Z3 (C, arrowheads). By the 150-cell stage (D), Z2/Z3 are nearly devoid of H3K4me2 staining.
Figure 2.—
Stage-specific disappearance of H3K4me2 in wild-type embryos. Fixed, whole-mount embryos from parents grown overnight at 25° were stained as in Figure 1. Since early embryos exhibit asynchronous cell divisions, only embryos with two OIC1D4-staining cells (Z2/Z3) were scored for the presence or absence of H3K4me2 within the PGCs. (A) In embryos with <100 cells, H3K4me2 can be still detected in Z2/Z3 in ∼50% of embryos, but shortly after the 100-cell stage, the percentage of embryos with H3K4me2 staining in Z2/Z3 drops off dramatically and was rarely observed. (B) The numbers of embryos scored for the data in A. (Note that the three embryos that retained H3K4me2 staining in the 100- to 149-cell group each had <115 cells.)
TABLE 1.
Quantitation of the emb-4 H3K4me2 retention phenotype
| Strain | <100 cells | ≥100 cellsa |
|---|---|---|
| % embryos with H3K4me staining in Z2/Z3b | ||
| Wild type (N2)c | 47 (24/51) | 3.8 (3/79) |
| emb-4(qm35)c | 79.2 (19/24) | 63.2 (36/57) |
| emb-4(qm31)c | 70.8 (34/48) | 72.7 (72/99) |
| emb-4(qm16)c | 75.0 (24/32) | 65.8 (73/111) |
| emb-4(hc60)c | 86.0 (49/57) | 59.6 (28/47) |
| emb-4(sa44)c | 51.2 (21/41) | 13.9 (5/36) |
| emb-4(n1256)c | 48.4 (15/31) | 4.6 (3/65) |
| emb-4(RNAi)d | 72.9 (35/48) | 23.9 (11/46) |
| emb-4(RNAi); eri-1(mg366)d | 70.0 (14/20) | 40.4 (19/47) |
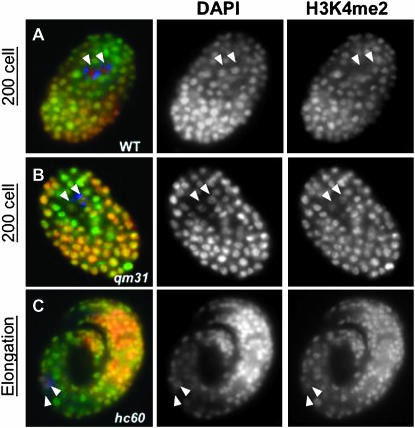
Figure 3.—
Later-stage emb-4 embryos retain H3K4me2 in Z2/Z3. Wild-type (A) and emb-4(0) (B and C) hermaphrodites were shifted overnight to 25° and dissected. Embryos from parents grown overnight at 25° were fixed as before. By the 200-cell stage, all wild-type embryos lack H3K4me2 staining in Z2/Z3 (A, arrowheads) while emb-4 embryos stain with H3K4me2 in Z2/Z3 at a level comparable to staining within the somatic nuclei (B, arrowheads). In some emb-4 embryos, Z2/Z3 never lose H3K4me2 (C, arrowheads), and this mark remains detectable until the embryos arrest prior to hatching.
emb-4(0) embryonic lethality is less penetrant at 16° and 20° than at 25°, with most escapers grown at either at 16° or 20° becoming fertile adults. We therefore scored for retention of H3K4me2 in Z2/Z3 in emb-4(0) embryos at both at 16° and 20° to try to correlate the penetrance of the embryonic lethal phenotype with the penetrance of H3K4me2 retention. Similar to what is observed for embryonic lethality, a reduced number of embryos retain H3K4me2 in Z2/Z3 at 20° as compared to experiments conducted at 25° (i.e., 35% at 20° vs. 60% at 25°; Table 1; data not shown). There is therefore a correlation between penetrance of both phenotypes at the different temperatures. Wild-type embryos showed no temperature-dependent differences in H3K4me2 retention in Z2/Z3, suggesting that elevated temperature alone does not significantly affect chromatin remodeling in Z2/Z3.
emb-4 is not essential for histone deacetylation in Z2/Z3:
We have previously demonstrated that acetylation of histone H4 at lysine 8 (H4K8ac) also selectively disappears from Z2/Z3 in wild-type embryos (Schaner et al. 2003). Similar to the chromatin remodeling event involving loss of H3K4me2, the H4K8ac epitope disappears from PGC chromatin following the birth of Z2/Z3. Like H3K4me2, H4K8ac is also a highly conserved modification correlating with transcriptionally competent chromatin (Strahl and Allis 2000; Margueron et al. 2005). To determine whether emb-4 mutants have any defects in loss of H4K8ac, we stained both wild-type and emb-4(0) embryos of all stages with an antibody against H4K8ac and costained with an antibody against a P-granule component (OIC1D4), scoring for H4K8ac retention in Z2/Z3 (Table 2). While H4K8ac retention was observed in Z2/Z3 in a small percentage of emb-4 embryos scored at all stages, the defect was not as penetrant as what was observed for H3K4me2 removal (see Table 1). The disappearance of the H4K8ac epitope in wild-type embryos also appeared to be more rapid than that of H3K4me2. Wild-type embryos with H4K8ac retained in Z2/Z3 were never observed, even right after their birth, whereas H3K4me2 was often observed at these earlier stages (Tables 1 and 2). These data suggest that EMB-4 more specifically regulates the loss of H3K4me2 from Z2/Z3, and the removal of H4K8ac may not be mechanistically linked to the EMB-4-dependent process.
TABLE 2.
Quantitation of H4K8ac retention in Z2/Z3
| Strain | <100 cells | ≥100 cellsa |
|---|---|---|
| % embryos with H4K8ac staining in Z2/Z3b | ||
| Wild type (N2) | 0.0 (0/10) | 0.0 (0/23) |
| emb-4(qm31) | 9.0 (2/22) | 11.4 (5/44) |
emb-4 allelic series reveals differential effects on chromatin remodeling in Z2/Z3:
We scored embryos from each of the six emb-4 alleles as well as embryos obtained from emb-4 (RNAi) animals for defects in PGC chromatin remodeling. The emb-4(0) alleles are 100% embryonic lethal at 25° and exhibit high (∼50–70%) lethality at the “permissive” temperatures (data not shown). In contrast, the emb-4(sel) alleles are viable and do not have any obvious phenotype on their own (Tax et al. 1997; Katic and Greenwald 2006, accompanying article in this issue). We therefore tested whether the reduction of function of emb-4 that occurs in the sel alleles would affect chromatin regulation in Z2/Z3. The emb-4(0) alleles all exhibited aberrant H3K4me2 retention in Z2/Z3 throughout embryonic development, whereas both emb-4(sel) alleles (n1256 and sa44) were normal (Table 1; Figure 4, F and G).
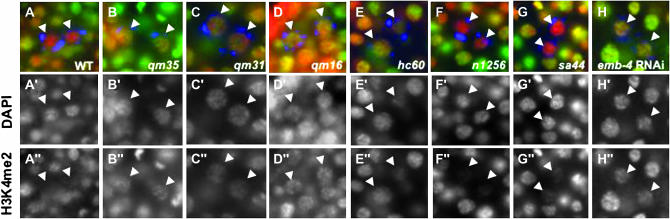
Figure 4.—
Defects in H3K4me2 loss occur only in severe emb-4 alleles. Wild-type and emb-4 hermaphrodites were shifted to 25° overnight and dissected. emb-4(RNAi) F1 hermaphrodites were maintained at 20° and dissected. Fixed, whole-mount embryos of each genotype were as before. Z2/Z3 are marked by arrowheads in all panels. All embryos are ∼100 cells. In wild-type embryos (A), and in the sel alleles, n1256 and sa44 (F and G), H3K4me2 staining is significantly reduced in Z2/Z3. In contrast, H3K4me2 is retained in Z2/Z3 in all four of the emb-4 embryonic lethal alleles (B–E) and in F1 progeny of emb-4(RNAi) parents (H).
We repeated these experiments in emb-4(RNAi) animals, feeding Y80D3A.2 double-stranded RNA (dsRNA) to both wild-type animals and eri-1(366) mutants, a strain with an enhanced RNAi response (Table 1; Figure 4H; Kennedy et al. 2004). Although both wild-type and eri-1(mg366) animals fed Y80D3A.2 dsRNA produced viable offspring, the surviving F1 progeny of eri-1(mg366);emb-4(RNAi) produced mostly arrested embryos, consistent with the maternal-effect nature of the mutations and enhanced RNAi response of the eri-1 strain. The arrested F2 embryos from both emb-4(RNAi) F1 adults as well as eri-1(mg366);emb-4(RNAi) F1 adults exhibited H3K4me2 retention in Z2/Z3, similar to the chromatin-remodeling defect in emb-4 mutants (Table 1; Figure 4H; data not shown). As expected, we observed a more penetrant phenotype when experiments were performed in an eri-1 background, and a higher number of later-stage (100+ cells) eri-1(mg366);emb-4(RNAi) embryos retained H3K4me2 staining in Z2/Z3 as compared to emb-4(RNAi) carried out in the N2 (Bristol) background (Table 1).
PIE-1 degradation in Z2/Z3 is delayed in emb-4 embryos:
The PIE-1 protein acts in the P-lineage to globally repress transcription (Mello et al. 1996; Seydoux et al. 1996). However, when P4 divides (∼60–90 cells in most embryos), the PIE-1 protein is rapidly degraded from Z2/Z3. The nature of its sudden disappearance is not well understood. In emb-4(hc60) embryos, we had previously observed PIE-1 staining in Z2/Z3 in stages where it is normally absent, suggesting a requirement for EMB-4 function in normal PIE-1 degradation (Schaner et al. 2003). We analyzed this defect more closely by comparing PIE-1 staining in wild-type vs. emb-4(0) embryos at different developmental stages. Although we were able to observe, as in our previous studies, a perdurance of PIE-1 in emb-4(0) embryos, we further observed that this was not permanent. We found that while PIE-1 is rapidly lost during the division of Z2/Z3 in wild-type embryos, emb-4 (0) embryos exhibit a significant delay in the disappearance of PIE-1 from Z2/Z3 (Figure 5; Table 3). The stabilization of PIE-1 protein in emb-4(0) embryos is therefore a transient defect, as it is not observed in late-stage emb-4(0) embryos (Table 3).
Figure 5.—
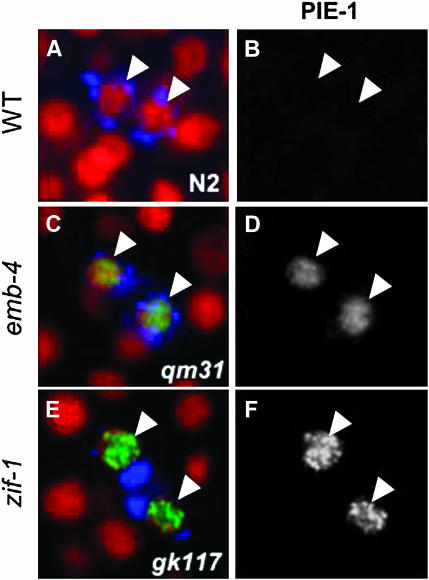
PIE-1 degradation in Z2/Z3 is delayed in emb-4(0) and zif-1 mutants. Wild-type and emb-4(qm31) hermaphrodites were shifted overnight to 25° and dissected. zif-1(gk117) embryos were dissected from parents grown at 20°. Fixed, whole-mount embryos of each genotype were costained with an antibody against PIE-1 (green in A, C, and E; channel separated in B, D, and F) and anti-PGL-1 (blue in A, C, and E), and were counterstained with DAPI (red in A, C, and E). Arrowheads mark Z2/Z3 in each panel. In wild-type embryos (A and B), PIE-1 rapidly disappears from Z2/Z3 following their birth and is undetectable (B). In contrast, PIE-1 is abnormally detected in Z2/Z3 in emb-4 embryos at similar stages (C and D). Abnormal retention of PIE-1 in Z2/Z3 is also observed in the absence of zif-1 (E and F). Somatic perdurance of PIE-1 caused by the absence of ZIF-1 is not readily observed in embryos at this stage.
TABLE 3.
Quantitation of PIE-1 retention in Z2/Z3
| Strain | <100 cells | 100–150 cells | ≥150 cellsa |
|---|---|---|---|
| % embryos with PIE-1 staining in Z2/Z3b | |||
| Wild-type (N2)c | 10.0 (2/20) | 0 (0/32) | 0 (0/27) |
| emb-4(qm31)c | 69.6 (16/23) | 12.2 (5/41) | 0 (0/49) |
| zif-1(gk117) | 85.7 (18/21) | 28.1 (9/32) | 0 (0/29) |
Similar mechanisms degrade PIE-1 in the soma and in Z2/Z3:
During the asymmetric divisions of the P-lineage, PIE-1 is actively moved to the posterior half of the cell prior to cytokinesis for enriched deposition into the posterior (germline) daughter (Tenenhaus et al. 1998; Reese et al. 2000). PIE-1 that is retained or left behind in the anterior (somatic) daughter is quickly degraded by a mechanism involving an E3 ubiquitin ligase complex that includes CUL-2 and ZIF-1 (DeRenzo et al. 2003). Degradation of PIE-1 is guided by ZIF-1 binding to one of the two CCCH zinc fingers of PIE-1. Mutations in the first CCCH domain of PIE-1 or loss of ZIF-1 activity each disrupt active destruction of PIE-1 remaining in the somatic daughters (DeRenzo et al. 2003).
To determine whether a similar mechanism may be responsible for targeting PIE-1 for degradation upon the birth of Z2/Z3, we tested whether PIE-1 was still present in Z2/Z3 in the absence of ZIF-1. We performed these experiments both in a zif-1(RNAi) background and in the zif-1(gk117) allele, which is a deletion that disrupts both the promoter region and the first exon of the zif-1 gene. In both zif-1(gk117) and zif-1(RNAi) embryos, PIE-1 was inappropriately stabilized in Z2/Z3 following the division of P4 (Figure 5, E and F; Table 3). This suggests that the same mechanism targets PIE-1 for immediate degradation in both somatic and germ lineages, but that this mechanism is repressed in the germline until after PGC specification. Similar to emb-4(0) embryos, the effect is transient; while a high percentage of <100 cell embryos and some 100–150 cell embryos retain PIE-1 in Z2/Z3 in the absence of ZIF-1, later-stage embryos do not. These results suggest that ZIF-1 mediates a “fast mode” of PIE-1 degradation, but that in its absence PIE-1 degradation can still occur, albeit with slower kinetics. Interestingly, PIE-1 retention was limited to the nucleus in Z2/Z3 of zif-1 embryos (Figure 5, E and F), which indicates a longer basal half-life for this fraction in the absence of ZIF-1.
We next tested whether the perdurance of PIE-1 in zif-1 embryos was consistent with retention of H3K4me2in Z2/Z3. Previously, we showed that there is a temporal correlation between loss of PIE-1 and the onset of chromatin remodeling in Z2/Z3 (Schaner et al. 2003). If PGC specification is repressed by PIE-1 and requires its destruction to occur, H3K4me2 might also persist in Z2/Z3 in the absence of ZIF-1. To test this, we stained zif-1(gk117) and zif-1(RNAi) embryos with antibodies against PIE-1 and H3K4me2 and looked for whether a retention of PIE-1 in Z2/Z3 correlates with failure to chromatin remodel. While we did occasionally observe this, we also observed embryos that retained PIE-1 but lacked H3K4me2 in Z2/Z3 (data not shown). These data suggest that either the two events are not dependent on one another or there is a threshold level of PIE-1 below which chromatin-remodeling events can still occur.
emb-4 is required for somatic cell organization, but not necessarily for specification, during embryogenesis:
The highly disorganized state of late-stage arrested emb-4 embryos suggests a potential role for the emb-4 gene in regulating cell fate specification. We tested whether emb-4 embryos have defects in specifying somatic cell lineages. The overall division rate in emb-4 embryos is slow compared to wild type, and strikingly, the E-lineage rate of division is reported to be ∼33% slower in emb-4 embryos vs. wild type (Schierenberg et al. 1980). We tested whether disruption of cell division rates in the E-lineage also caused misregulation of E-lineage-specific genes.
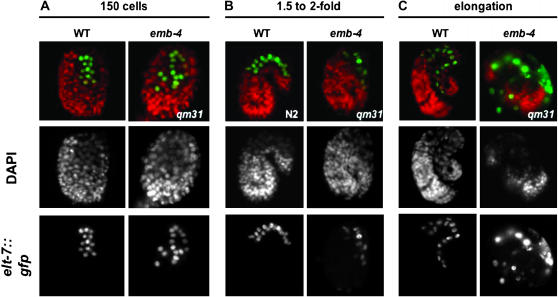
The E-lineage is clonally derived from the E blastomere and is solely responsible for giving rise to the entire C. elegans intestine (Maduro and Rothman 2002). We crossed emb-4 males into a strain carrying an elt-7:gfp transgene. Expression of elt-7, a transcription factor required for gut differentiation, correlates with gastrulation and cell movements in the embryo, and it is expressed specifically in the E-lineage throughout later stages of embryogenesis. To determine if elt-7 expression was defective in emb-4 embryos, we counted the number of GFP-positive cells at different stages in live embryos, comparing GFP expression in both wild-type and emb-4 mutant backgrounds. While there was no obvious difference in the number of GFP-expressing cells in wild-type vs. emb-4 backgrounds, elt-7∷gfp cells become abnormally localized in later-stage emb-4 embryos (Figure 6). Defects in the spatial organization of elt-7∷gfp became notable at approximately the 1.5- to 2-fold stage, which is consistent with a generalized defect in development previously seen in emb-4 embryos at this stage (Figure 6B). Many embryos exhibit severe defects, indicating that a number of tissues in addition to the gut are poorly organized (not shown). These data suggest that emb-4 is required for normal somatic cell lineage organization and development, but not necessarily tissue specification. It should be noted, however, that even in the putative null alleles a significant percentage of animals raised at lower temperatures develop normally. EMB-4 activity therefore seems to be required only during embryogenesis for a higher probability of normal development at all temperatures.
Figure 6.—
Intestinal lineage organization, but not specification, is disrupted in emb-4 embryos. emb-4(qm31) males were crossed into transgenic animals expressing the E-lineage (intestinal)-specific GFP marker elt-7 (green, bottom row). Both elt-7∷gfp and elt-7∷gfp;mal-2(qm31) transgenic animals were dissected and stained with DAPI (red/middle row). While late-stage elt-7∷gfp;emb-4(qm31) embryos arrest as masses of poorly differentiated embryos (C, right column), the number of GFP-expressing cells at all stages (A–C, right column) is identical to wild-type animals expressing elt-7∷gfp (A–C, left column and data not shown).
Notch signaling is not a direct requirement for Z2/Z3 chromatin remodeling in the embryo:
The loss of H3K4me2 after germline restriction does not occur normally in _emb-4(0)_mutants, and both emb-4(0) and emb-4(sel) alleles are required for efficient Notch signaling. While the biochemical function of EMB-4 is not known, emb-4 behaves genetically as an activator of Notch-signaling-induced transcription in postembryonic lineage specification (Tax et al. 1997; Katic and Greenwald 2006, accompanying article in this issue). A GFP-tagged version of EMB-4 localizes to the nucleus, which suggests that its biochemical role is nuclear (Katic and Greenwald 2006, this issue). EMB-4 therefore either could also act within the Notch pathway in PGC specification or could be a shared component of separate pathways within the nucleus.
There are two Notch receptors in C. elegans: LIN-12 and GLP-1. Of these, GLP-1 is the predominant Notch receptor that is used in embryonic development (Priess 2005). GLP-1 plays essential roles in embryonic somatic fate decisions and in postembryonic germ cell proliferation (Seydoux and Schedl 2001; Priess 2005). Both GLP-1 and LIN-12 signaling converge on LAG-1, a transcription factor orthologous to CBF1 in humans and Suppressor of Hairless [Su(H)] in flies. LAG-1 orthologs mediate the downstream transcriptional program of Notch receptors in all species where Notch signaling has been studied (Christensen et al. 1996).
We tested whether Notch signaling was required for chromatin remodeling by assaying for retention of H3K4me2 in Z2/Z3 in lag-1(RNAi) embryos. No significant alterations in histone modification patterns were observed in these experiments (data not shown). To ensure that we were analyzing animals that had lost both maternal and zygotic LAG-1 function, we repeated these experiments in lag-1(om13) mutant embryos, a weak temperature-sensitive mutation that causes embryonic arrest in the offspring of animals raised at 25°. Again, we did not observe any defects in the loss of H3K4me2 in Z2/Z3 (data not shown).
To determine whether emb-4 could be affecting downstream Notch target gene expression, we generated emb-4(qm31)V;ref-1∷gfp animals and tested for defects in ref-1∷gfp expression. ref-1 is a transcription factor that is downstream of lag-1, and in the absence of Notch signaling its expression is either abrogated or mislocalized (Neves and Priess 2005). While emb-4(qm31)V;ref-1∷gfp animals are extremely sick and produce very few embryos, GFP expression appears normal, suggesting that emb-4 does not directly affect expression of ref-1 (data not shown). Although most late emb-4 embryos are disorganized, earlier embryos that were normal appearing did not show obvious defects in ref-1 expression or localization, indicating that neither the _lag-1_-dependent or _lag-1_-independent pathways of ref-1 expression require emb-4 activity. Together, these experiments suggest that, unlike EMB-4's role in postembryonic vulva specification, its role in embryonic primordial germ-cell remodeling is not carried out through LAG-1/Su(H)-dependent Notch signaling.
emb-4 does not directly affect the RNAi pathway:
In recent years, the RNAi pathway has been shown to have a direct relationship to chromatin-remodeling mechanisms, and in particular, studies in both Drosophila and C. elegans have shown Polycomb group-related genes to be implicated in germline RNAi pathways (Bernstein and Allis 2005; Grishok et al. 2005). Furthermore, AQUARIUS, the mouse ortholog of EMB-4, was suggested to be an RNA-dependent RNA polymerase (RdRP) (Sam et al. 1998), orthologs of which have conserved roles in RNAi-related mechanisms. We therefore tested for RNAi defects in emb-4 animals.
To first determine whether emb-4 is defective in RNAi responses to exogenous dsRNA, we fed bacteria expressing ama-1 dsRNA to emb-4 animals at 20°. ama-1 encodes the large subunit of RNAPII in C. elegans. When fed ama-1 dsRNA, the level of embryonic lethality in emb-4(qm35) embryos was elevated to 100% (data not shown). emb-4 animals fed bacteria carrying the empty L4440 vector exhibited the normal background level (∼50–70%) of lethality at 20°. Similar results were obtained for RNAi targeting par-1 and par-3 (both maternally supplied factors). The susceptibility of emb-4 mutants to RNAi was thus neither specific to ama-1 dsRNA nor limited to zygotically expressed mRNAs (data not shown). emb-4 is therefore not required for dsRNA-induced mRNA destruction mechanisms.
We also tested whether mutations in emb-4 can result in an enhanced R_NA_i (Eri) phenotype. A number of genes whose defects enhance the effectiveness of RNAi via dsRNA feeding have been described. A standard procedure is to feed dsRNA targeting a gene in a behavioral or morphological pathway that normally shows a weak response in a wild-type background, but a more robust response in an eri genetic background (e.g., Simmer et al. 2002). Additional assays target dsRNA against GFP in strains carrying a GFP transgene that is expressed in tissue that is normally refractive to efficient RNAi by feeding, such as neurons (Kennedy et al. 2004). To avoid complications due to the morphological and behavioral defects in emb-4 animals, as well as to limit our analysis to RNAi-resistant tissues, we opted for another assay for an enhanced RNAi phenotype.
Transgenes in C. elegans are generally high-copy arrays that are targeted for repression by both RNAi and chromatin-based mechanisms (Kelly and Fire 1998; Grishok et al. 2005). In somatic lineages, these transgenes are not fully repressed and the GFP reporter is easily detected, whereas in Eri strains the reporter is more extensively repressed. Previous studies by Grishok et al. (2005) have demonstrated that the somatic expression of an elt-2∷gfp repetitive transgene is markedly decreased in rrf-3 mutants (Grishok et al. 2005). RNAi repression of the elt-2∷gfp transgene is therefore enhanced by mutations in rrf-3, which encodes a putative RdRP (Grishok et al. 2005). Mutations in another gene, eri-1, also cause an Eri phenotype and behave similarly in this assay, indicating that this is not a special property limited to the rrf-3 mutations (T. Ratliff and W. Kelly, unpublished results). Therefore, to determine whether mutations in emb-4 similarly enhance this RNAi pathway, we crossed emb-4(hc60) males into an elt-2∷gfp transgenic strain (Grishok et al. 2005). Unlike rrf-3 mutants, emb-4 had no obvious effect on somatic transgene expression in this assay (not shown), and we conclude that emb-4 is not likely to be a direct modulator of the RNAi pathways tested by these assays.
DISCUSSION
Roles for emb-4 during embryonic development:
Loss of EMB-4 results in a number of diverse developmental defects that severely perturb embryogenesis. Consequently, even at “permissive” temperatures a high percentage of emb-4 mutants either arrest in late stages of embryonic development or, in the case of escapers, may develop as adults with pronounced morphological defects. The results presented here suggest that emb-4 is required for a number of developmental processes, as loss of emb-4 has profound effects on both PGC specification and somatic cell organization and organogenesis. It is important to note that the presumed null alleles of emb-4 are temperature sensitive, in so far as there is a substantial decrease in embryonic lethality when the animals are grown at a lower temperature. This suggests that EMB-4 is not absolutely required for normal development, but that its activity ensures that normal development is the most likely outcome at all temperatures. Normal development may involve a number of inherently temperature-sensitive processes for which EMB-4 is required for efficient and timely activation. As a putative RNA or DNA helicase, there are a number of potential developmental processes that EMB-4 may be mechanistically involved in during embryogenesis. It is interesting that the initial characterization of the emb-4(hc60) allele reported that the rate of cell division of the E-lineage was slowed (Schierenberg et al. 1980). This may indicate a role in efficient DNA replication, but is also consistent with a role in efficient transcription, as the sel-6 alleles seem to suggest. The defect in PGC chromatin remodeling, as will be discussed below, may also support a role in transcription. Importantly, while the specific function of EMB-4 in the worm embryo is not yet known, its activity is unlikely to be unique to C. elegans, as it is highly conserved and, curiously, also appears to be a single-copy gene in vertebrates and plants.
PGC chromatin remodeling:
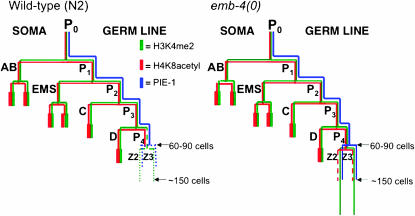
While much is already known about how the how the P-lineage germline blastomeres are specified and regulated by PIE-1 and other factors, less is known about the mechanisms that maintain germline identity in the PGCs. EMB-4 is required for an important aspect of PGC-specific chromatin establishment: the efficient genomewide disappearance of H3K4me2. In the absence of EMB-4 activity, removal of H3K4me2 from the PGCs either is significantly delayed or does not occur. In contrast, the normal disappearance of another modification, H4K8acetyl, is not as strongly affected in emb-4 mutants. The removal of H4K8acetyl is thus apparently uncoupled to the removal of H3K4me2. In addition, defects in H4K8acetyl removal correlate with few obvious germline defects, indicating a nonessential role for the deacetylation mechanism (P. Checchi and W. Kelly, unpublished results). The dynamics of these germline events in wild-type and emb-4 embryos are summarized in Figure 7.
Figure 7.—
Summary of PIE-1 and histone modification dynamics in the germline: wild type vs. emb-4. Throughout embryogenesis, a number of conserved mechanisms ensure that germ cells are kept transcriptionally quiescent. In wild-type embryos (left), PIE-1 (blue) segregates with the P-lineage and inhibits transcription until the division of P4, during which time PIE-1 protein is diluted twofold as it is segregated into Z2/Z3 (indicated by dotted blue lines) and is then subsequently degraded. The histone modifications H3K4me2 (green) and H4K8ac (red) are initially present in both the soma and the P-lineage, but in P4 the level of H3K4me2 begin to decrease (indicated by dashed dark-green lines). Shortly after the birth of Z2/Z3, H3K4me2 disappears from Z2/Z3 (indicated by dotted green lines). Like H3K4me2, H4K8ac is initially present in all cells of the embryo and disappears from the germline coincident with the birth of Z2/Z3. In emb-4(0) embryos (right), PIE-1 initially persists in Z2/Z3, but is eventually degraded by the 100- to 150-cell stage. H3K4me2 fails to disappear from Z2/Z3 in the majority of emb-4(0) embryos and can still be detected in late-stage PGCs. H4K8ac persists in Z2/Z3 in a small percentage of emb-4(0) embryos.
The activities of the worm Nanos homologs, NOS-1 and NOS-2, are redundantly required for proper PGC chromatin remodeling (Schaner et al. 2003). The nos-2;nos-1 double-knockout phenotype is complete adult sterility, but there are few other obvious defects (Subramanian and Seydoux 1999). This may indicate that the removal of H3K4me2, in contrast to H4K8ac, is more critical for germline maintenance. All other mutants thatwe have identified as essential for Z2/Z3 chromatin remodeling exhibit highly penetrant embryonic lethality, indicating that the mechanism of remodeling employs generally essential factors that may be specifically regulated by the NOS proteins in the embryonic germline (P. Checchi, H. Furuhashi and W. Kelly, unpublished results).
EMB-4 and PIE-1 degradation:
The coincidence of H3K4me2 disappearance with PIE-1 degradation is interesting, as PIE-1 is proposed to act in the germline blastomeres to repress RNAPII transcriptional elongation (Seydoux and Dunn 1997; Zhang et al. 2003). PIE-1 degradation in the PGCs is abnormally delayed in emb-4 embryos: in the mutants, it eventually disappears by the 100- to 150-cell stage in contrast to the sudden disappearance near or at PGC birth in wild-type embryos. The zinc-finger-binding protein ZIF-1 has been shown to be essential for clearance of maternal germline factors, including PIE-1, from early somatic blastomeres (DeRenzo et al. 2003). This process has been proposed to comprise a switch from oocyte/germline characteristics to zygotic developmental control (DeRenzo et al. 2003). Interestingly, we have shown that ZIF-1 is also required for timely PIE-1 degradation in the PGCs, which suggests that there is also a switch to the activation of zygotic processes in the germline that establish and/or maintain this lineage during embryonic development.
The similarity of the delayed PIE-1 degradation observed in both emb-4 and zif-1 embryos may suggest either co- or interdependent regulation. We have occasionally observed low levels of PIE-1 retained within somatic nuclei of early stage emb-4 embryos, which is also observed in the absence of ZIF-1, suggesting that EMB-4 may be functioning upstream of zif-1 (P. Checchi and W. Kelly, unpublished results). However, whereas the majority of late-stage emb-4 embryos aberrantly retain H3K4me2 staining in Z2/Z3, the effect of a lack of ZIF-1 on the disappearance of H3K4me2 is unclear. Furthermore, loss of EMB-4 function causes embryonic arrest, while deletion of zif-1 has few observable consequences other than delayed PIE-1 degradation. This suggests that if, for example, EMB-4 activates zif-1, that zif-1 is not the sole target of EMB-4 function. It also suggests that fast degradation of PIE-1 at the birth of Z2/Z3 may be a redundant process and supports previous findings that a highly concentrated level of PIE-1 is required for its full effect. Indeed, dilution of PIE-1 at the symmetric P4 cell division may lead to insufficient PIE-1 for complete repression, allowing some transcription elongation to occur (Tenenhaus et al. 1998). One early product could be ZIF-1, which could then quickly direct degradation of the remaining PIE-1. The similarity between emb-4 and zif-1 delays in PIE-1 degradation could be due to a requirement for EMB-4 in early rounds of PGC transcription (below).
Transcriptional roles for EMB-4:
What is the biochemical role of EMB-4? Katic and Greenwald (2006, accompanying article in this issue) have shown that weak alleles of emb-4 can suppress a lin-12(d) phenotype, indicating that EMB-4 acts to promote Notch signaling. Further, GFP-tagged EMB-4 is nuclear, indicating that this is the site of its function (Katic and Greenwald 2006, this issue). Little information is available about the function of emb-4 homologs in other organisms. The Schizosaccharoomyces pombe emb-4 homolog, Cwf11p, copurifies with TAP-tagged Cdc5p in an essential pre-mRNA splicing complex (Ohi et al. 2002). However, unlike other splicing factors, Cwf11 is nonessential and has been proposed to play “an ancillary role” in pre-mRNA splicing. Interestingly, there is no obvious homolog of emb-4/cwf11 in budding yeast. Furthermore, unlike all of the organisms in which an emb-4 homolog exists, S. cerevesiae lacks both RNAi machinery and conserved components of repressive chromatin architecture, such as histone H3 methylated on lysine 9 (H3K9me) as well as the protein that binds H3K9me, heterochromatin-binding protein 1 (Aravind et al. 2000). Our experiments appear to rule out a role for EMB-4 in the post-transcriptional RNAi pathways tested, but its genetic role as a nonessential positive activator of Notch signaling is intriguing. Perhaps its odd absence in S. cerevesiae indicates a requirement for EMB-4 to augment efficient gene activation in the face of an unfavorable chromatin structure that no longer exists in this organism.
A role for EMB-4 in efficient transcription activation is consistent both with its subtle role in Notch signaling activity and with recent results from our lab that indicate a requirement for transcription in PGC chromatin remodeling (H. Furuhashi, P. Checchi and W. Kelly, unpublished results). The removal of H3K4me2 in Z2/Z3 is abrogated by RNAi of ama-1 (the large subunit of RNA polymerase II), cdk-9, histone chaperones RbAp48 and ASF1, as well as conserved components of ATP-dependent chromatin-remodeling complexes. In contrast, RNAi of a number of other essential factors, including essential splicing factors, has no impact on PGC remodeling (H. Furuhashi, P. Checchi and W. Kelly, unpublished results). This could indicate a direct role for transcriptional activation, accompanied by transcription-coupled histone replacement, in the mechanism driving PGC chromatin remodeling. We have not ruled out the possibility that transcription-independent chromatin-remodeling pathways (that may require EMB-4 for their initiation or function) are also involved.
Vulva induction is one of the few postembryonic developmental specifications that occur in C. elegans, and the sel-6 alleles reveal a subtle role for EMB-4 in this pathway. It is not clear, however, why emb-4 survivors grown at lower temperatures do not exhibit vulva defects, unless the slower growth at lower temperatures allows for more leeway in the timing of developmental events, including those that occur in the larvae. Its embryonic role is essential, yet only fractionally so at lower temperatures. A role for EMB-4 in chromatin remodeling, thereby directly or indirectly affecting only the efficiency of timely gene activity, could account for this apparent conundrum. In the future it will be important to understand the biochemical activity of EMB-4 and how it relates to these developmental pathways.
Acknowledgments
The authors thank Iva Greenwald and Iskra Katic for sharing unpublished data, intellectual guidance during the course of this work, and for critical reading of the manuscript. We similarly thank the anonymous reviewers for their helpful critiques and comments. We also thank Geraldine Seydoux, Susan Strome, Steve L'Hernault, Joel Rothman, and James Priess for helpful discussions and provision of reagents and strains. We thank David C. Allis for sharing antibody reagents and Yuji Kohara for providing cDNA clones used in this study. Some of the C. elegans strains used in this study were provided by the Caenorhabditis Genetics Center, and we thank Theresa Stiernagle for continuing assistance with strain requests and Robert Herman for his enormous contributions to the Center and the worm community. This work was supported by an Emory University Research Council Grant (W.G.K.) and by grants from the National Institutes of Health to W.G.K. (GM63102 and GM077600).
References
- Aravind, L., H. Watanabe, D. J. Lipman and E. V. Koonin, 2000. Lineage-specific loss and divergence of functionally linked genes in eukaryotes. Proc. Natl. Acad. Sci. USA 97**:** 11319–11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelder, C., M. A. Dunn, B. Choy, Y. Suh, C. Cassie et al., 1999. Transcriptional repression by the Caenorhabditis elegans germ-line protein PIE-1. Genes Dev. 13**:** 202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein, E., and C. D. Allis, 2005. RNA meets chromatin. Genes Dev. 19**:** 1635–1655. [DOI] [PubMed] [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77**:** 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, S., V. Kodoyianni, M. Bosenberg, L. Friedman and J. Kimble, 1996. lag-1, a gene required for lin-12 and glp-1 signaling in Caenorhabditis elegans, is homologous to human CBF1 and Drosophila Su(H). Development 122**:** 1373–1383. [DOI] [PubMed] [Google Scholar]
- DeRenzo, C., K. J. Reese and G. Seydoux, 2003. Exclusion of germ plasm proteins from somatic lineages by cullin-dependent degradation. Nature 424**:** 685–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischle, W., Y. Wang and C. D. Allis, 2003. Histone and chromatin cross-talk. Curr. Opin. Cell Biol. 15**:** 172–183. [DOI] [PubMed] [Google Scholar]
- Grishok, A., J. L. Sinskey and P. A. Sharp, 2005. Transcriptional silencing of a transgene by RNAi in the soma of C. elegans. Genes Dev. 19**:** 683–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hekimi, S., P. Boutis and B. Lakowski, 1995. Viable maternal-effect mutations that affect the development of the nematode Caenorhabditis elegans. Genetics 141**:** 1351–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath, R. S., A. G. Fraser, Y. Dong, G. Poulin, R. Durbin et al., 2003. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421**:** 231–237. [DOI] [PubMed] [Google Scholar]
- Katic, I., and I. Greenwald, 2006. EMB-4: a predicted ATPase that facilitates lin-12 activity in Caenorhabditis elegans. Genetics 174**:** 1907–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki, I., A. Amiri, Y. Fan, N. Meyer, S. Dunkelbarger et al., 2004. The PGL family proteins associate with germ granules and function redundantly in Caenorhabditis elegans germline development. Genetics 167**:** 645–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, W. G., and A. Fire, 1998. Chromatin silencing and the maintenance of a functional germline in Caenorhabditis elegans. Development 125**:** 2451–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, S., D. Wang and G. Ruvkin, 2004. A conserved siRNA-degrading RNase negatively regulates RNA interference in C. elegans. Nature 427**:** 645–649. [DOI] [PubMed] [Google Scholar]
- Kornberg, R. D., and Y. Lorch, 1999. Twenty-five years of the nucleosome, fundamental particle of the eukaryotic chromosome. Cell 98**:** 285–294. [DOI] [PubMed] [Google Scholar]
- Leatherman, J. L., and T. A. Jongens, 2003. Transcriptional silencing and translational control: key features of early germline development. BioEssays 25**:** 326–335. [DOI] [PubMed] [Google Scholar]
- Maduro, M. F., and J. H. Rothman, 2002. Making worm guts: the gene regulatory network of the Caenorhabditis elegans endoderm. Dev. Biol. 246**:** 68–85. [DOI] [PubMed] [Google Scholar]
- Margueron, R., P. Trojer and D. Reinberg, 2005. The key to development: Interpreting the histone code? Curr. Opin. Genet. Dev. 15**:** 163–176. [DOI] [PubMed] [Google Scholar]
- Mello, C. C., B. W. Draper, M. Krause, H. Weintraub and J. R. Priess, 1992. The pie-1 and mex-1 genes and maternal control of blastomere identity in C. elegans embryos. Cell 70**:** 163–176. [DOI] [PubMed] [Google Scholar]
- Mello, C. C., C. Schubert, B. Draper, W. Zhang, R. Lobel et al., 1996. The PIE-1 protein and germline specification in C. elegans embryos. Nature 382**:** 710–712. [DOI] [PubMed] [Google Scholar]
- Miwa, J., E. Schierenberg, S. Miwa and G. von Ehrenstein, 1980. Genetics and mode of expression of temperature-sensitive mutations arresting embryonic development in Caenorhabditis elegans. Dev. Biol. 76**:** 160–174. [DOI] [PubMed] [Google Scholar]
- Neves, A., and J. R. Priess, 2005. The REF-1 family of bHLH transcription factors pattern C. elegans embryos through Notch-dependent and Notch-independent pathways. Dev. Cell 8**:** 867–879. [DOI] [PubMed] [Google Scholar]
- Ohi, M. D., A. J. Link, L. Ren, J. L. Jennings, W. H. McDonald et al., 2002. Proteomics analysis reveals stable multiprotein complexes in both fission and budding yeasts containing Myb-related Cdc5p/Cef1p, novel pre-mRNA splicing factors, and snRNAs Mol. Cell. Biol. 22**:** 2011–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priess, J. R., 2005. Notch signaling in the C. elegans embryo, in WormBook, edited by The C. elegans Research Community (doi/10.1895/wormbook.1.7.1; http://www.wormbook.org). [DOI] [PMC free article] [PubMed]
- Reese, K. J., M. A. Dunn, J. A. Waddle and G. Seydoux, 2000. Asymmetric segregation of PIE-1 in C. elegans is mediated by two complementary mechanisms that act through separate PIE-1 protein domains. Mol. Cell 6**:** 445–455. [DOI] [PubMed] [Google Scholar]
- Sam, M., W. Wurst, M. Kluppel, O. Jin, H. Heng et al., 1998. Aquarius, a novel gene isolated by gene trapping with an RNA-dependent RNA polymerase motif. Dev. Dyn. 212**:** 304–317. [DOI] [PubMed] [Google Scholar]
- Schaner, C. E., G. Deshpande, P. Schedl and W. G. Kelly, 2003. A conserved chromatin architecture marks and maintains the restricted germ cell lineage in worms and flies. Dev. Cell 5**:** 747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schierenberg, E., J. Miwa and G. von Ehrenstein, 1980. Cell lineages and developmental defects of temperature-sensitive embryonic arrest mutants in Caenorhabditis elegans. Dev. Biol. 76**:** 141–159. [DOI] [PubMed] [Google Scholar]
- Seydoux, G., and M. A. Dunn, 1997. Transcriptionally repressed germ cells lack a subpopulation of phosphorylated RNA polymerase II in early embryos of Caenorhabditis elegans and Drosophila melanogaster. Development 124**:** 2191–2201. [DOI] [PubMed] [Google Scholar]
- Seydoux, G., and T. Schedl, 2001. The germline in C. elegans: origins, proliferation, and silencing. Int. Rev. Cytol. 203**:** 139–185. [DOI] [PubMed] [Google Scholar]
- Seydoux, G., and S. Strome, 1999. Launching the germline in Caenorhabidits elegans: regulation of gene expression in early germ cells. Development 126**:** 3275–3283. [DOI] [PubMed] [Google Scholar]
- Seydoux, G., C. C. Mello, J. Pettitt, W. B. Wood, J. R. Priess et al., 1996. Repression of gene expression in the embryonic germ lineage of C. elegans. Nature 382**:** 713–716. [DOI] [PubMed] [Google Scholar]
- Simmer, F, M. Tijsterman, S. Parrish, S.P. Koushika, M. L. Nonet et al., 2002. Loss of the putative RNA-directed RNA polymerase RRF-3 makes C. elegans hypersensitive to RNAi. Curr. Biol. 12**:** 1317–1319. [DOI] [PubMed] [Google Scholar]
- Strahl, B. D., and C. D. Allis, 2000. The language of covalent histone modifications. Nature 403**:** 41–45. [DOI] [PubMed] [Google Scholar]
- Strome, S., and W. B. Wood, 1983. Generation of asymmetry and segregation of germline granules in early C. elegans embryos. Cell 35**:** 15–25. [DOI] [PubMed] [Google Scholar]
- Subramaniam, K., and G. Seydoux, 1999. nos-1 and nos-2, two genes related to Drosophila nanos, regulate primordial germ cell development and survival in Caenorhabditis elegans. Development 126**:** 4861–4871. [DOI] [PubMed] [Google Scholar]
- Tax, F. E., J. H. Thomas, E. L. Fergusonand and H. R. Horvitz, 1997. Identification and characterization of genes that interact with lin-12 in Caenorhabditis elegans. Genetics 147**:** 1675–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenenhaus, C., C. Schubert and G. Seydoux, 1998. Genetic requirements for PIE-1 localization and inhibition of gene expression in the embryonic germ lineage of Caenorhabditis elegans. Dev. Biol. 200**:** 212–224. [DOI] [PubMed] [Google Scholar]
- Timmons, L., D. L. Court and A. Fire, 2001. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263**:** 103–112. [DOI] [PubMed] [Google Scholar]
- Zhang, F., M. Barboric, T. K. Blackwell and B. M. Peterlin, 2003. A model of repression: CTD analogs and PIE-1 inhibit transcriptional elongation by P-TEFb. Genes Dev. 17**:** 748–758. [DOI] [PMC free article] [PubMed] [Google Scholar]