NPFXD-mediated Endocytosis Is Required for Polarity and Function of a Yeast Cell Wall Stress Sensor (original) (raw)
Abstract
The actin-associated protein Sla1p, through its SHD1 domain, acts as an adaptor for the NPFX(1,2)D endocytic targeting signal in yeast. Here we report that Wsc1p, a cell wall stress sensor, depends on this signal-adaptor pair for endocytosis. Mutation of NPFDD in Wsc1p or expression of Sla1p lacking SHD1 blocked Wsc1p internalization. By live cell imaging, endocytically defective Wsc1p was not concentrated at sites of endocytosis. Polarized distribution of Wsc1p to regions of cell growth was lost in the absence of endocytosis. Mutations in genes necessary for endosome to Golgi traffic caused redistribution of Wsc1p from the cell surface to internal compartments, indicative of recycling. Inhibition of Wsc1p endocytosis caused defects in polarized deposition of the cell wall and increased sensitivity to perturbation of cell wall synthesis. Our results reveal that the NPFX(1,2)D-Sla1p system is responsible for directing Wsc1p into an endocytosis and recycling pathway necessary to maintain yeast cell wall polarity. The dynamic localization of Wsc1p, a sensor of the extracellular wall in yeast, resembles polarized distribution of certain extracellular matrix-sensing integrins through endocytic recycling.
INTRODUCTION
Endocytosis plays a fundamental role in regulating the dynamic organization of the plasma membrane in eukaryotic cells (Conner and Schmid, 2003). A well-characterized endocytic entry route involves formation of clathrin-coated vesicles (CCV). Proteins destined for uptake via CCV generally harbor endocytic targeting signals that direct incorporation into emergent vesicles (Traub, 2005). Such signals are recognized by adaptors that link the cargo to core components of the clathrin coat. Identifying endocytic targeting signals and their partner adaptors, and defining the roles of signal/adaptor pairs in cell physiology are key issues in understanding the endocytic process.
Several types of endocytic targeting signals for CCV have been identified in mammalian cells including YXXΦ, FXNPXY (where X is any amino acid and Φ is a bulky hydrophobic amino acid), and ubiquitin (Hicke and Dunn, 2003; Traub, 2005). The YXXΦ signal, present in many endocytic cargo proteins, is recognized by the AP-2 adaptor complex, a core structural component of CCV that plays important roles in clathrin coat assembly. In contrast, the less common FXNPXY and ubiquitin signals are recognized by alternative adaptors known as CLASPs (clathrin-associated sorting proteins), that appear to act more specifically in cargo collection (Traub, 2005).
In the yeast Saccharomyces cerevisiae, two distinct classes of endocytic targeting signals have been defined: ubiquitin, which is added post-translationally to lysine residues in endocytic cargo and the peptide signal NPFX(1,2)D (Hicke and Riezman, 1996; Tan et al., 1996). Monoubiquitylation is sufficient to direct internalization and this signal is likely recognized by components of the endocytic machinery such as the epsins Ent1p and Ent2p and the Eps15 homologue Ede1p, all of which contain ubiquitin-binding domains (Hicke and Dunn, 2003; Dupre et al., 2004). However, definitive genetic demonstration that these proteins act as CLASPs for the ubiquitin signal has been complicated by possible roles for ubiquitin binding in regulating assembly of the endocytic machinery (Shih et al., 2002).
The NPFX(1,2)D signal was first discovered during characterization of a chimeric protein containing the cytoplasmic domain of the furin-like protease Kex2p (Tan et al., 1996). Although Kex2p normally cycles between the _trans_-Golgi network (TGN) and endosomes without transit through the plasma membrane, the sequence NPFSD in the cytoplasmic domain was found to be both necessary and sufficient for rapid internalization when appended to an otherwise endocytically inactive plasma membrane protein. Within this sequence, each residue except serine was important for endocytosis. NPFSD-related motifs are present in the cytoplasmic domains of the mating pheromone receptors Ste2p (GPFSD) and Ste3p (NPFSTD), which also are ubiquitylated (Hicke and Riezman, 1996; Roth and Davis, 1996; Tan et al., 1996; Howard et al., 2002). The NPFX(1,2)D motifs were dispensible for internalization of otherwise normal receptors but became important for uptake in receptors lacking ubiquitylation sites, suggesting redundancy between the two types of endocytic signals in these proteins (Tan et al., 1996; Howard et al., 2002).
A screen for NPFSD-interacting proteins yielded Sla1p, a component of the actin- and clathrin-based endocytic machinery of yeast (Howard et al., 2002; Warren et al., 2002; Kaksonen et al., 2005). NPFX(1,2)D binding mapped to a discrete domain in Sla1p, SHD1 (Sla1-homology domain; Ayscough et al., 1999), and deletion of SHD1 completely abolished endocytosis of an NPFX(1,2)D-dependent reporter but had no effect on uptake of a ubiquitin-dependent reporter (Howard et al., 2002). Thus, through SHD1, Sla1p can serve as an NPFX(1,2)D-specific endocytic adaptor. Although Sla1p does not display clear sequence similarity with mammalian proteins, there are structural parallels and common interactions to conserved actin cytoskeletal and clathrin coat components between Sla1p and the mammalian endocytic proteins intersectin and the CIN85/CD2AP family (Howard et al., 2002; Stamenova et al., 2004). In the case of CIN85 and CD2AP, the analogy to Sla1p is strengthened by findings that the mammalian proteins are part of a complex that can provide cargo-selective CLASP-like activity linking receptor tyrosine kinases to the endocytic machinery (Szymkiewicz et al., 2004).
Because the only two native proteins analyzed to date, Ste2p and Ste3p, do not require NPFX(1,2)D signals for endocytosis, specific roles for NPFX(1,2)D/Sla1p-mediated endocytosis in yeast have not been defined. A database search for yeast plasma membrane proteins carrying cytoplasmic NPFX(1,2)D sequences yielded only a few candidates, suggesting that the NPFX(1,2)D/Sla1p signal/adaptor system may be dedicated to a select subset of endocytic cargo (Howard et al., 2002). One NPFX(1,2)D-containing protein, Wsc1p, is a major cell wall stress sensor. Wsc1p is one of four Wsc proteins in yeast, three of which (Wsc1–3p) function in response to cell wall stress (Levin, 2005). Of these, only Wsc1p contains an NPFX(1,2)D sequence and this sequence is present in the cytoplasmic domain where it could function as an endocytic targeting signal. The Wsc4p cytoplasmic domain contains an SPFRD sequence, but this protein is localized to the endoplasmic reticulum so the significance of this possible signal is uncertain (Mamoun et al., 1999).
Of the Wsc proteins, Wsc1p has been most extensively characterized (Levin, 2005). Wsc1p is normally localized to sites of new cell surface growth. There it helps coordinate response to cell wall perturbations through a Rho1p GTPase pathway that activates cell wall β (1–3) glucan synthase as well as a protein kinase C signal transduction pathway that elicits transcriptional up-regulation of cell wall biosynthesis genes. Here we report that Wsc1p requires NPFX(1,2)D and the Sla1p SHD1 domain for internalization. In the absence of NPFX(1,2)D-mediated endocytosis, Wsc1p becomes depolarized on the cell surface, and there is an accompanying depolarization of cell wall synthesis. Our results indicate that NPFX(1,2)D/Sla1p-mediated endocytosis of Wsc1p plays an important role in maintaining yeast cell polarity.
MATERIALS AND METHODS
Yeast Media, Plasmids, and Strains
Yeast strains were grown in YPD (1% Bacto-yeast extract [DifCo, Detroit, MI], 2% Bacto-peptone [Difco], 2% dextrose) or SD (0.067% yeast nitrogen base [Difco], 2% dextrose) supplemented with 20 μg/ml l-histidine, uracil, and l-tryptophan, and 30 μg/ml l-leucine, adenine, and l-lysine. Solid media contained 2% agar. Yeast transformation was performed by the lithium acetate method as described (Ito et al., 1983). Cell densities were measured by spectrophotometry. One OD600 corresponds to 107 cells per milliliter. Sequences of oligonucleotides used in this study are available on request.
Plasmids pRS314-SLA1 and pRS314-sla1-Δshd1 were derived from 5.8-kb PvuI fragment of pKA51 (pRS313-SLA1) and pKA51Δshd1, respectively (Ayscough et al., 1999; Howard et al., 2002), and cloned into the PvuI site in pRS314. To generate pGAD-SHD1, PCR-amplified SHD1 (aa 471–555) was inserted into the BamHI and PstI sites of pGAD-C1 (James et al., 1996). To generate pGBDU-Wsc1 and pGBDU-Wsc1AAA sequences encoding the C-terminal tails (aa 291–378) of Wsc1p or Wsc1pAAA were amplified by PCR from GPY3527 or GPY3544 genomic DNA. The PCR products were then cloned into the BamHI and PstI sites of pGBDU-C1 (James et al., 1996). All constructs were confirmed by DNA sequencing.
Table 1 lists the strains used in this study. GFP and mRFP were introduced by standard PCR-based methods (Sikorski and Hieter, 1989; Longtine et al., 1998) to generate GPY3527, GPY4185, and GPY4187. The GPY3544 (Wsc1AAA-GFP) mutant strain was generated by PCR using a forward primer encoding the NPF to AAA mutations in Wsc1 and the same 3′ end primer used to generate the GPY3527 strain. Functionality of Wsc1p-GFP was assessed by growth at 37°C, a temperature at which Wsc1p-deficient cells do not grow (Gray et al., 1997; Verna et al., 1997). All other strains were obtained by mating 3527 or 3544 to congenic strains carrying the relevant mutant alleles, followed by sporulation, and isolation of haploid strains, except for GPY3546, which was obtained from a cross of GPY3527 to DDY958. All insertions were confirmed by PCR amplification of the genomic loci, immunoblotting, and DNA sequencing in the case of Wsc1AAA-GFP.
Table 1.
Yeast strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| GPY3130 | MATα ura3-52 leu2-3,112 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 sla1Δ::URA3 | This study |
| GPY3527 | MATα ura3-52 leu2-3,112 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 WSC1-GFP::HIS3 | This study |
| GPY3532 | MATa ura3-54 leu2-3,112 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 WSC1-GFP::HIS3 end3Δ::LEU2 | This study |
| GPY3536 | MATa ura3-52 leu2-3,112 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 WSC1-GFP::HIS3 pep4Δ::LEU2 | This study |
| GPY3544 | MATα ura3-52 leu2-3,112 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 WSC1AAA-GFP::HIS3 | This study |
| GPY3546 | MATa ura3-52 leu2-3,112 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 WSC1-GFP::HIS3 sla1Δ::URA3 | This study |
| GPY3567 | MATa ura3-52 leu2-3,112 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 WSC1-GFP::HIS3 sla2Δ::HIS3 | This study |
| GPY3602 | MATa ura3-52 leu2-3,112 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 WSC1-GFP::HIS3 vps27Δ::HIS3 | This study |
| GPY3625 | MATa ura3-52 leu2-3,112 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 WSC1AAA-GFP::HIS3 pep4Δ::LEU2 | This study |
| GPY4112 | MATα ura3-52 leu2-3,112 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 WSC1-GFP::HIS3 ric1Δ::TRP1 | This study |
| GPY4120 | MATα ura3-52 leu2-3,112 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 WSC1AAA-GFP::HIS3 ric1Δ::TRP1 | This study |
| GPY4185 | MATa/MATα ura3-52/ura3-52 leu2-3,112/leu2-3,112 his3-Δ200/his3-Δ200 trp1-Δ901/trp1-Δ901 lys2-801/lys2-801 suc2-Δ9/suc2-Δ9 WSC1-GFP::HIS3/WSC1-GFP::HIS3 Sla1-RFP::KanMX6/Sla1-RFP::KanMX6 | This study |
| GPY4187 | MATa/MATα ura3-52/ura3-52 leu2-3,112/leu2-3,112 his3-Δ200/his3-Δ200 trp1-Δ901/trp1-Δ901 lys2-801/lys2-801 suc2-Δ9/suc2-Δ9 WSC1AAA-GFP::HIS3/WSC1AAA-GFP::HIS3 Sla1-RFP::KanMX6/Sla1-RFP::KanMX6 | This study |
| SEY6210 | MATα ura3-52 leu2-3,112 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 | Robinson et al. (1988) |
| DDY 958 | MATa ura3-52 leu2-3 his3-Δ200 trp1-1(am) sla1Δ::URA3 | D. Drubin |
| pJ69-4A | _MATa trp1-Δ901 leu2-3,112 ura3-52 his3-Δ200 gal4_Δ _gal80_Δ GAL2-ADE2 LYS2::GAL1-HIS3 met2::GAL7-lacZ | James et al. (1996) |
Yeast Two-Hybrid Assay
Yeast strain pJ69-4A (James et al., 1996) was transformed consecutively with pGAD-SHD1 and pGBDU-Wsc1 or pGBDU-Wsc1AAA. Colonies from the second transformations were grown in supplemented SD without leucine or uracil, and fivefold serial dilutions were spotted onto supplemented SD without leucine or uracil (control) or supplemented SD without leucine, uracil, or histidine (for interaction). Colony growth was recorded after 3 d incubation at 30°C. Two independent colonies were tested in two separate experiments.
Fluorescence Microscopy
All strains were grown at 24°C in supplemented SD without histidine or, for plasmid-containing strains, supplemented SD without histidine or tryptophan. Cells grown overnight at 24°C were diluted to OD600 = 0.02–0.03 and allowed to grow for four generations. The cultures were concentrated to ∼5 × 108 cells/ml by centrifugation at 750 × g for 1 min at room temperature and resuspended in fresh media. Cells were visualized by pipetting 2 μl onto a microscope slide and applying a coverslip. To apply heat shock, cells grown and diluted at 24°C as described above were shifted to 37°C for 35 min and then immediately concentrated and visualized. All images were captured using a 100× objective on a Zeiss Axiovert 200M microscope (Thornwood, NY) with a Hamamatsu Orca II camera (Bridgewater, NJ).
Pulse-Chase Immunoprecipitation and Protease Protection Assay
Cells (1.2 × 108 final) were grown overnight to early logarithmic growth phase (OD600 = 0.3) at 24°C in supplemented SD, labeled with [35S]methionine/cysteine for 15 min and subjected to a 15-min chase regimen as described in Seeger and Payne (1992). Cells were then divided into two samples, and one sample was shifted to 37°C while the other remained at 24°C. At designated incubation times cells were collected, washed, and treated or mock-treated with pronase, and lysed by agitation with glass beads as described by Howard et al. (2002), except the carrier cells were SEY6210. Lysates were diluted and subjected to immunoprecipitation as in Seeger and Payne (1992) with polyclonal anti-GFP (AbCam, Cambridge, MA). Immunoprecipitates were resolved by 8% SDS-PAGE gels and analyzed by autoradiography.
FM4-64 Staining
Yeast grown to midlogarithmic phase (OD600 = 0.5) in supplemented SD without histidine were stained with 10 μM FM4-64 (Molecular Probes, Eugene, OR) for 15 min at 24°C and then grown for 60 min in dye-free medium as described (Vida and Emr, 1995). Cells were then concentrated and analyzed by fluorescent microscopy as described above.
Caspofungin Sensitivity Assay
Caspofungin stock solution (200 mM, prepared in sterile water) was a gift from Ainslie Parsons (University of Toronto, Canada). Cells grown to midlogarithmic phase in YPD or supplemented SD without tryptophan were spotted as serial dilutions onto solid YPD or SD containing 300 ng/ml caspofungin. Growth of cells was monitored after 3 d at 30°C.
Electron Microscopy Analysis
Cells were grown to midlogarithmic phase at 24°C in supplemented SD without tryptophan or histidine. Samples were prepared by a modification of the method of Fassel et al. (1997). Cells were prefixed with prefixation solution (2% paraformaldehyde, 2% glutaraldehyde, 1 mM MgCl2, 1 mM CaCl2, 0.1% DMSO, 0.1% ruthenium red, in 0.1 M sodium phosphate buffer, pH 7.4, and 100 mM NaCl) for 30 min at room temperature, and washed three times with sodium phosphate buffer, each time for 10 min. Then cells were fixed with postfixation solution (1% OsO4, 1% K2Cr2O7, 0.85% NaCl, 0.1% DMSO in 0.1 M sodium phosphate buffer, pH 7.4) for 30 min at room temperature and washed as with the prefixation. Cells were dehydrated and embedded as described in Fassel et al. (1997). Sections were cut on a Reichert Ultracut Ultramicrotome, poststained in aqueous 2% uranyl acetate and lead citrate, and observed in a JEOL 100CX transmission electron microscope (Peabody, MA) at 10,000×.
RESULTS
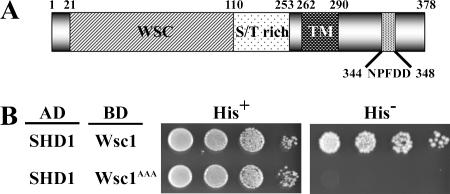
Wsc1p contains the potential SHD1-interacting sequence NPFDD starting at position 344 in the cytoplasmic domain (Figure 1A). To determine whether this sequence can bind to SHD1, we applied yeast two-hybrid interaction assays that have proved reliable measures of SHD1 binding to other NPFX(1,2)D signals (Howard et al., 2002; Costa et al., 2005). By such assays interaction was detected between SHD1 and the entire cytoplasmic domain of Wsc1p (aa 291-378) but not with a mutant form of the Wsc1 domain in which the critical NPF residues were converted to alanines (Figure 1B). These results indicate that SHD1 can recognize the Wsc1p cytoplasmic domain and this interaction depends on the NPFDD sequence.
Figure 1.
Wsc1 interacts with Sla1 homology domain 1 (SHD1) through the NPFDD motif. (A) Diagram of Wsc1. WSC, cysteine-rich WSC domain; TM, transmembrane domain; NPFDD, Sla1-SHD1 interaction motif. (B) SHD1 fragment (aa 471-555) from Sla1 fused to Gal4AD was tested for interaction with C-terminal cytoplasmic fragments of wild-type Wsc1 or Wsc1AAA fused to Gal4BD. Fivefold serial dilutions of exponentially growing cells were spotted onto supplemented SD with histidine (His+; no selection for interaction) or supplemented SD without histidine (His−; selection for interaction).
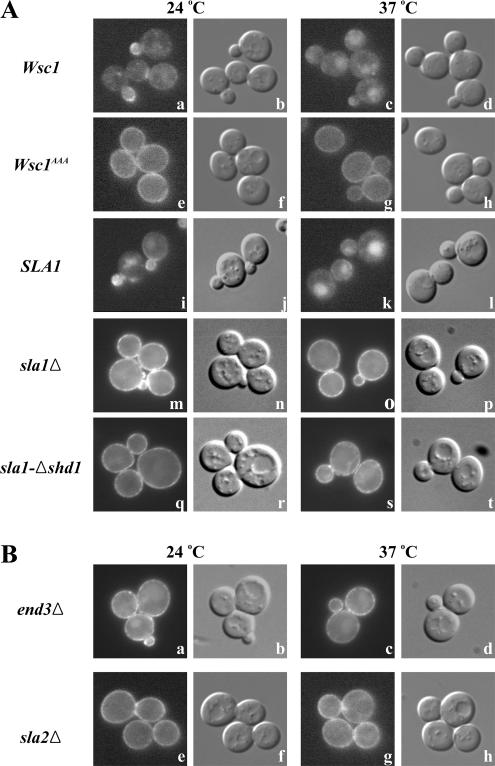
To test whether SHD1-NPFDD interaction is required for Wsc1p endocytosis, we generated strains encoding, at the endogenous WSC1 locus, a functional Wsc1p fusion protein appended at the C-terminus with GFP or a mutant Wsc1-GFP fusion carrying the NPF to AAA substitutions (Wsc1AAA-GFP). Consistent with prior studies (Delley and Hall, 1999), at 24°C Wsc1p-GFP displayed mostly polarized cell surface distribution along with some intracellular localization in living cells (Figure 2A, a and b). Surface Wsc1p-GFP localized at sites of surface growth, with strong accumulation in small- and medium-sized buds and localization to the neck in large-budded cells. After cell wall stress imposed by heat shock (shift to 37°C; Kamada et al., 1995), surface Wsc1p-GFP polarization was less evident and much of the GFP signal was present in the vacuole (Figure 2A, c and d), suggesting that cell wall stress leads to Wsc1p down-regulation. This pattern differs from that described for an HA-tagged Wsc1p, which was predominantly depolarized at the cell surface after heat shock (Delley and Hall, 1999), probably because degradation of the HA tag in the vacuole prevented detection by immunofluorescence. Strikingly, at both temperatures, the Wsc1p NPF mutant (Wsc1AAA-GFP) accumulated uniformly along the cell surface, suggesting defects in internalization (Figure 2A, e–h).
Figure 2.
Wsc1 polarized localization at the plasma membrane depends on the NPFDD signal, Sla1p SHD1, and general endocytosis factors. Early log phase cells were treated with or without heat shock at 37°C for 35 min and observed by epifluorescence (left panel in each pair) and differential interference contrast (DIC) microscopy (right panel in each pair). (A) Wsc1-GFP (GPY3527; a–d) and Wsc1AAA-GFP (GPY3544; e–h) in wild-type cells and Wsc1-GFP sla1Δ cells (GPY3546) carrying pRS314-SLA1 (SLA1; i–l), pRS314 (sla1Δ; m–p), or pRS314-sla1-Δshd1 (sla1-Δshd1; q–t). (B) Wsc1-GFP end3Δ (GPY3532; a–d) and Wsc1-GFP sla2Δ (GPY3567; e–h) cells were grown and visualized as in A.
In a reciprocal set of experiments, localization of Wsc1p-GFP was visualized in wild-type cells, cells harboring a complete deletion of SLA1, or cells expressing a mutant form of Sla1p lacking SHD1. Unlike complete deletion of SLA1, Sla1p-Δshd1 maintains function in ubiquitin-mediated endocytosis and actin organization (Howard et al., 2002), thereby allowing a more specific test of NPFX(1,2)D-mediated endocytosis. Mirroring the results with Wsc1AAA-GFP, wild-type Wsc1p-GFP was localized almost exclusively along the cell surface in the absence of Sla1p (Figure 2A, m–p) or in cells expressing Sla1p without SHD1, regardless of the temperature (Figure 2A, q–t). Taken together these results provide strong evidence that internalization of Wsc1p requires interaction between the NPFDD signal and Sla1p SHD1 and suggest that endocytosis is necessary for polarized localization of Wsc1p at the cell surface.
As an independent approach to assess the role of endocytosis in Wsc1p localization, we monitored Wsc1-GFP distribution in mutants carrying deletions of general endocytosis factors End3p and Sla2p. End3p is part of a complex, also containing Sla1p and Pan1p, that associates relatively early with forming endocytic vesicles (Tang et al., 2000; Kaksonen et al., 2003). Deletion of END3 is known to block both ubiquitin- and NPFX(1,2)D-mediated endocytosis (Benedetti et al., 1994; Tan et al., 1996). Sla2p, homologous to mammalian Hip1 and Hip1R, is thought to couple vesicle formation with actin polymerization (Kaksonen et al., 2003). Deletion of SLA2 freezes endocytic structures at the plasma membrane. In both mutants, Wsc1p was primarily localized in a depolarized manner at the plasma membrane (Figure 2B), further supporting a role for endocytosis in maintaining Wsc1p polarity at the cell surface.
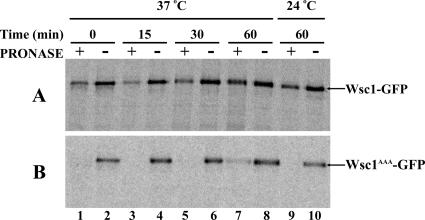
The fluorescence experiments with Wsc1p-GFP provided a steady state measure of Wsc1p localization. We also applied a kinetic assay of endocytosis to assess NPFX(1,2)D-mediated uptake of Wsc1p (Davis et al., 1993; Tan et al., 1996). For these experiments, cells were metabolically pulse-labeled with [35S]methionine/cysteine and then subjected to a chase regimen. At designated intervals, cells were harvested and treated intact with protease. Under such conditions cell surface proteins are sensitive to the exogenous protease, but internal proteins remain protected (Davis et al., 1993; Tan et al., 1996; Deloche and Schekman, 2002). These experiments were carried out in pep4Δ cells that lack active vacuolar proteases to prevent degradation of Wsc1p in the vacuole. Thus, newly synthesized Wsc1p that has reached the cell surface is sensitive to exogenous protease but then becomes resistant once internalized by endocytosis. After a 15-min labeling and a 15-min chase at 24°C, the majority of labeled Wsc1p was sensitive to protease (Figure 3A, lanes 1 and 2), indicating that most Wsc1p was present at the cell surface. After a shift to 37°C, the percentage of Wsc1p protected from external protease slightly declined at the 15-min point (Figure 3A, lanes 3 and 4) and then increased over the next 45 min (Figure 3A, lanes 5–8). These results indicate that the population of pulse-labeled Wsc1p almost completely reached the cell surface by the 15-min time point at 37°C and then was internalized over the ensuing 45 min. At 24°C the level of internal Wsc1p increased only marginally after 60 min at 24°C (Figure 3A, lanes 9 and 10), consistent with the steady state plasma membrane localization of Wsc1p at this temperature. In comparison to wild-type Wsc1p, Wsc1AAA was dramatically more sensitive to protease at all times tested, indicating continued presence of Wsc1AAA at the cell surface (Figure 3B). These results provide more direct evidence that the rate of internalization of Wsc1p is dependent on the NPFDD signal.
Figure 3.
Internalization of Wsc1 depends on the NPFDD signal. (A) Wsc1-GFP pep4Δ (GPY3536) or (B) Wsc1AAA-GFP pep4Δ (GPY3624) cells were metabolically labeled for 15 min, subjected to a chase for 15 min at 24°C, and then shifted to 37°C or incubated at 24°C for 60 min. Samples were collected at the designated times and divided into two aliquots that were treated with (+) or without (−) pronase. Cell lysates were prepared, Wsc1p was immunoprecipitated with GFP antibodies, and immunoprecipitates were analyzed by SDS-PAGE and autoradiography.
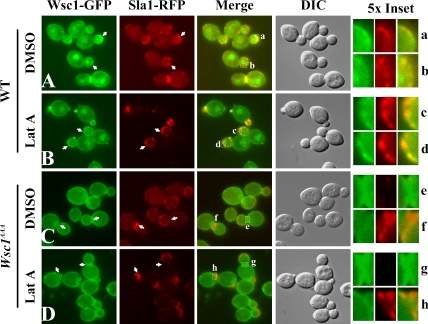
If Sla1p acts as an endocytic adaptor for NPFX(1,2)D-dependent cargo, then cargo collection into nascent endocytic vesicles should depend on recognition of the NPFX(1,2)D signal. Recent studies indicate that cortical patches of actin and actin-associated proteins represent sites of endocytosis (Kaksonen et al., 2003, 2005; Newpher et al., 2005). Furthermore, Sla1p is recruited relatively early to these structures (Kaksonen et al., 2003). Thus, to test more directly whether an NPFX(1,2)D signal specifies incorporation into forming endocytic vesicles, we assessed colocalization of either Wsc1-GFP or Wsc1AAA-GFP with Sla1p-mRFP in living cells. To enhance the chances of detecting transient colocalization, we also treated cells with latrunculin A (LatA), an actin monomer-sequestering drug that allows Sla1p recruitment but prevents actin assembly and inward progression of endocytic structures (Kaksonen et al., 2003). In untreated cells at 24°C, patches of Wsc1p-GFP were occasionally colocalized with cortical Sla1p patches (Figure 4A). LatA treatment substantially increased the degree of colocalization (Figure 4B), with intense puncta of Wsc1p colocalizing with Sla1p in very small buds as well as weaker Wsc1p patches overlapping with Sla1p in other regions of the cells. In contrast, Wsc1AAA-GFP was relatively uniformly distributed along the cell surface and rarely displayed concentration at Sla1p-mRFP patches under either condition (Figure 4, C and D). Quantitation of colocalization in LatA–treated cells revealed that 46.6% of Sla1p puncta colocalized with patches of wild-type Wsc1p-GFP (n = 249 Sla1p patches), whereas only 10.8% of Sla1p puncta overlapped with patches of Wsc1AAA-GFP (n = 415 Sla1p patches). In fact, Wsc1AAAp and Sla1p often exhibited reciprocal localization patterns, with areas of Sla1p concentration mostly devoid of Wsc1AAA and vice versa (arrows in Figure 4, C and D). Similar results were obtained at 37°C (unpublished results). These results suggest that recognition of the NPFDD targeting signal in Wsc1p by Sla1p is an essential step in packaging Wsc1p into forming endocytic vesicles.
Figure 4.
Colocalization of Wsc1p but not Wsc1AAA with Sla1p. Sla1-RFP Wsc1-GFP cells (GPY4185; A and B) or Wsc1AAA-GFP (GPY4187; C and D) cells were grown to early log phase at 24°C and treated with DMSO (A and C) or 200 μM LatA (B and D) for 20 min at 24°C. Cells were analyzed by epifluorescence or DIC (4th column). Arrows indicate patches of Wsc1-GFP that colocalize with Sla1-RFP in Wsc1-GFP strains (A and B); arrowheads indicate reciprocal localization of Wsc1AAA-GFP and Sla1p-RFP in Wsc1AAA cells (C and D). Boxed areas in the merged panels are 5× magnified on the right. Insets b and g were rotated 90° counterclockwise.
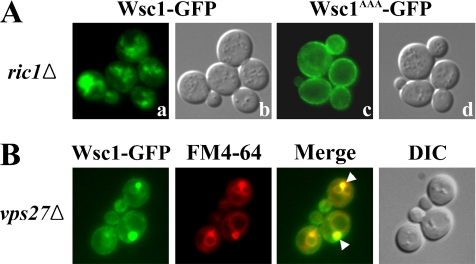
The results of kinetic analysis of Wsc1p internalization at 24°C in Figure 3 indicated that levels of cell surface Wsc1p did not substantially decline over 60 min. This observation, combined with the presence of some internal Wsc1p-GFP at 24°C, suggested that Wsc1p might be constitutively internalized and recycled at this temperature. To address this possibility, Wsc1p-GFP was visualized in strains carrying ric1Δ, a mutation that prevents traffic from endosomes to the Golgi (Siniossoglou et al., 2000; Bensen et al., 2001). As shown in Figure 5A, a and b, ric1Δ caused internal accumulation of Wsc1p and depletion from the cell surface. In contrast, endocytosis-defective Wsc1AAA-GFP still localized to the surface of ric1Δ cells (Figure 5A, c and d), indicating that the effect of ric1Δ occurs after Wsc1p internalization. Wsc1p-GFP also accumulated intracellularly in vps27Δ cells where exit from a late endosomal compartment is delayed (Figure 5B; Piper et al., 1995). As assessed by labeling with the endocytic tracer FM4-64, Wsc1p-GFP primarily accumulated in the exaggerated late endosomal compartment present in vps27Δ cells. These results indicate that, at 24°C, Wsc1p is constitutively internalized in an NPFX(1,2)D-dependent process and then recycled from endosomes through the Golgi to the cell surface. Because Wsc1p is constitutively recycled, and its polarity depends on endocytosis, our findings collectively provide evidence that the polarized distribution of this stress sensor is maintained by endocytic recycling and localized exocytosis. By this model, the increased vacuolar localization of Wsc1p after shift to 37°C would reflect a heat-shock–triggered rerouting of Wsc1p to the vacuole, most likely from endosomes.
Figure 5.
Wsc1p constitutively recycles at 24°C. (A) Wsc1-GFP ric1Δ (GPY4112) and Wsc1AAA-GFP ric1Δ (GPY4120) cells were grown at 24°C and analyzed as in Figure 2B. (B) Wsc1-GFP vps27Δ (GPY3602) cells were grown to midlog phase, incubated with FM4-64 to label vacuoles and exaggerated prevacuolar endosomes, and analyzed by epifluorescence and DIC microscopy. Arrowheads indicate Wsc1-GFP accumulation in prevacuolar endosomes.
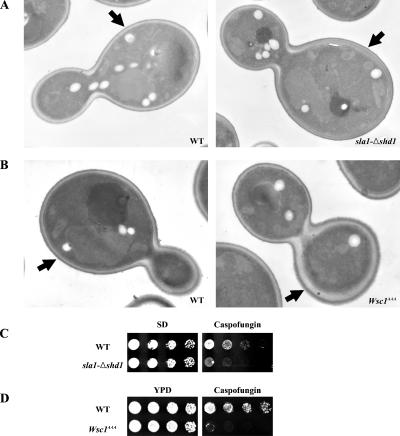
Wsc1p activates cell wall biosynthetic pathways to maintain cell integrity in response to perturbations of the cell wall (Levin, 2005). To probe the physiological role of endocytosis-dependent Wsc1p polarization, cell wall structure was examined in wild-type, sla1-Δshd1, and Wsc1AAA cells by thin-section electron microscopy. In wild-type cells, the thickness of the walls in mother cells and the daughter buds was commonly the same (Figure 6A; 41/50 cells with mother and daughter cell walls the same thickness). Strikingly, in the sla1-Δshd1 cells, most mother cell walls were significantly thicker than the daughter cells (Figure 6A; 57/66 cells with thicker mother cell wall). This effect is likely caused by continued low level signaling from Wsc1p abnormally localized in the mother cell after bud emergence due to the defect in endocytosis. Similar results were obtained with Wsc1-GFP (Figure 6B; 52/69 cells with mother and daughter cell walls the same thickness) and Wsc1AAA-GFP cells (70/93 cells with thicker mother cell wall). Thus, endocytic polarization of Wsc1p is necessary to ensure polarized cell wall expansion.
Figure 6.
Wsc1p endocytosis is required for cell wall polarity and response to cell wall stress. (A) Thin section electron micrographs of sla1Δ cells (GPY3130) containing pRS314-SLA1 (WT) or pRS314-sla1-Δshd1 (sla1-Δshd1). (B) Wsc1-GFP (GPY3527) and Wsc1AAA-GFP (GPY3544) cells. Arrows point to mother cell walls. (C) sla1Δ cells carrying pRS314-SLA1 (WT), or pRS314-sla1-Δshd1 (sla1-Δshd1) were grown in synthetic media to select for the plasmid and serial dilutions were spotted synthetic media (SD) without or with 300 ng/ml caspofungin. (D) Wild-type (WT; GPY3527) and Wsc1AAA (GPY3544) cells were grown in YPD media, and fivefold serial dilutions were spotted onto YPD or YPD containing 300 ng/ml caspofungin.
As an alternative approach to monitor the effects of Wsc1p depolarization, we induced cell wall stress by treating wild-type and mutant cells with the β(1–3) glucan synthase inhibitor caspofungin (Reinoso-Martin et al., 2003). Both Wsc1AAA and sla1-Δshd1 cells exhibited hypersensitivity to caspofungin compared with their wild-type counterparts (Figure 6, C and D), indicating that the capacity of Wsc1p to respond to this cell wall stress is compromised when Wsc1p polarization is not properly maintained by endocytosis.
DISCUSSION
We have demonstrated that the yeast cell wall stress sensor Wsc1p requires a cytoplasmic NPFX(1,2)D sequence and Sla1p SHD1 for endocytosis and undergoes constitutive recycling from endosomes. In the absence of endocytosis, Wsc1p becomes depolarized at the cell surface resulting in defects in cell wall polarity and hypersensitivity to inhibition of cell wall synthesis. These results provide a physiological role in cell polarity for the NPFX(1,2)D/SHD1 endocytosis signal/adaptor pair and indicate that Wsc1p is localized through a constitutive cycle of endocytosis and exocytosis.
Only seven known or putative plasma membrane proteins were identified in a search for NPFX(1,2)D-containing proteins, suggesting that NPFX(1,2)D sequences represent a specialized class of endocytosis signals. Two of the proteins uncovered in the search are the Ste3p and Ste2p pheromone receptors. However, in Ste2p, the signal (GPFSD) was weakly active, and effects of mutating the signal were not apparent unless ubiquitylation sites were removed (Howard et al., 2002). In the case of Ste3p, the receptor normally undergoes constitutive ligand-independent endocytosis, and this process was not affected by mutations of the NPFSTD signal; dependence on NPFSTD was manifested only when a ubiquitylated PEST-like sequence was removed, resulting in a receptor that requires ligand binding for endocytosis (Davis et al., 1993; Tan et al., 1996). Thus, the importance of NPFX(1,2)D signals for uptake of native pheromone receptors was not clear. The results reported here identify the NPFDD sequence in Wsc1p as necessary for uptake, thereby establishing that this class of internalization signal functions in a native context.
We have proposed that Sla1p through SHD1 acts as an endocytic adaptor for internalization of NPFX(1,2)D-containing plasma membrane proteins (Howard et al., 2002). With identification of Wsc1p as an NPFX(1,2)D-dependent cargo and recent development of live cell imaging approaches to visualize endocytosis, we have been able to test this model more directly. We observed limited concentration of Wsc1p but not Wsc1pAAA at cortical patches of Sla1p that are sites of endocytosis in living cells. Furthermore, wild-type but not mutant Wsc1p colocalization with Sla1p patches was enhanced when progression of endocytic vesicles was blocked with LatA. These results provide evidence that the NPFX(1,2)D signal is required for association with Sla1p in vivo and that this interaction mediates Wsc1p incorporation into nascent endocytic vesicles. These are precisely the features predicted for an endocytosis signal-adaptor pair, defining the NPFX(1,2)D-SHD1 interaction as a key first step in endocytic cargo packaging.
What advantage might a specialized endocytosis signal such as NPFX(1,2)D provide? In yeast the more common endocytosis signal seems to be ubiquitin (Hicke and Dunn, 2003; Dupre et al., 2004). However, ubiquitin also acts as a sorting signal in endosomes (Katzmann et al., 2002), guiding proteins into the internal vesicles of multivesicular endosomes (also known as multivesicular bodies or MVBs). From endosomes, the internal vesicles are delivered to vacuoles and degraded. Because of this dual targeting function, when ubiquitin is added to a protein at the plasma membrane, the protein is committed to destruction unless the ubiquitin is removed, making this system well suited for down-regulation. In contrast, NPFX(1,2)D-dependent internalization of Wsc1p at 24°C leads not to vacuolar degradation but rather to recycling from endosomes to the plasma membrane. Thus, ubiquitin-independent internalization provided by NPFX(1,2)D offers a simple mechanism for Wsc1p to avoid the degradative vacuolar pathway. In a similar manner, the truncated form of Ste3p that requires ligand for endocytosis relies on an NPFX(1,2)D signal for uptake and, once internalized, is rapidly recycled rather than delivered to the vacuole (Chen and Davis, 2000). Full-length Ste3p also appears to recycle when bound to ligand, raising the possibility that ligand-binding induces the receptor to switch from use of ubiquitin to NPFX(1,2)D as an endocytosis signal, thereby altering the downstream itinerary of the receptor. On the basis of the examples of Wsc1p and Ste3p, we suggest that NPFX(1,2)D signals are specialized for endocytosis of proteins that recycle between the plasma membrane and endosomes in yeast.
When cells were shifted from 24°C to 37°C, the principal site of Wsc1p localization changed from the plasma membrane to vacuoles. Because Wsc1p is internalized and recycled at 24°C, the effect of temperature shift is most likely due to a novel type of intracellular sorting process: temperature-regulated sorting from endosomes to the vacuole. The nature of this sorting process remains to be explored, but our results provide some insights. The GFP signal from vacuolar Wsc1p was predominantly luminal in wild-type cells, suggesting that the cytoplasmic GFP was internalized into the vacuole. This localization is a signature of proteins that are sorted to the vacuole by incorporation into intralumenal vesicles of MVBs (Pelham, 2002). Consistent with temperature-dependent sorting of Wsc1p into the MVB pathway, deletion of a key pathway component, Vps27p, caused Wsc1p accumulation in perivacuolar endosomes and weak localization to the vacuolar membrane (Figure 5), phenotypes diagnostic for MVB pathway cargo (Pelham, 2002). Because heat shock triggers the cell wall integrity signal transduction pathway (Kamada et al., 1995), heat shock-induced sorting of Wsc1p into the MVB pathway probably leads to down-regulation and attenuation of signaling, similar to MVB pathway-mediated down-regulation of signaling receptors in mammalian cells (Katzmann et al., 2002; Gruenberg and Stenmark, 2004). Ubiquitin serves as the sorting signal for most, but not all, proteins transported into the MVB pathway (Reggiori and Pelham, 2001). Additional experiments will be required to determine whether heat shock–induced ubiquitylation of Wsc1p dictates MVB sorting.
Our data indicate that endocytic recycling of Wsc1p is necessary to maintain its polarized distribution on the plasma membrane. Because exocytosis is naturally polarized by the actin cytoskeleton in yeast to sites of new surface growth (Pruyne and Bretscher, 2000), endocytic recycling provides a simple mechanism to polarize Wsc1p to these sites. Dynamic localization of Wsc1p through recycling has several potential benefits to the cell. First, localization of Wsc1p to regions of cell growth allows the cell to coordinate cell wall remodeling with polarized expansion of the plasma membrane. Indeed, the cell wall integrity signal transduction pathway is activated during periods of polarized cell growth (Zarzov et al., 1996). Although the role of Wsc1p in this activation in response to polarized cell growth has not been probed, the localization pattern of Wsc1p makes it a much better candidate for cell cycle cell wall stress sensing than the other major sensor, Mid2p, which lacks an NPFX(1,2)D signal and is uniformly distributed along the cell surface (Ketela et al., 1999; Rajavel et al., 1999). Such a role would account for the caspofungin sensitivity of cells in which Wsc1p is no longer concentrated at sites of cell growth because of endocytic defects. Second, continuous Wsc1p recycling allows rapid redistribution in response to external cell wall perturbation such as the heat shock–induced sorting to the vacuole. Third, Wsc1p in transit through the recycling pathway can serve as a stress probe in internal compartments. Yeast cells repress ribosomal protein and RNA expression upon inhibition of secretion or endosome to Golgi traffic, but not in response to blocks of endocytosis or Golgi-to-endosome transport (Mizuta and Warner, 1994; Nanduri et al., 1999; Nanduri and Tartakoff, 2001). At least three of the four Wsc proteins contribute to this repression pathway (Li et al., 2000). Nanduri and Tartakoff reported that the response required Wsc2p along the secretory pathway (Nanduri and Tartakoff, 2001). They also observed that Wsc1p and Wsc2p are not interchangeable. We submit that the recycling itinerary of Wsc1p is well-suited to monitor the status of endosome to Golgi traffic and this represents the specific role of Wsc1p in the ribosome biosynthesis repression pathway. Analysis of Wsc2p trafficking and the roles of Wsc1p and Wsc2p in repressing ribosome biosynthesis in endosome to Golgi mutants will be needed to test this model.
A role for Wsc1p as a general cell wall stress sensor has been inferred from the susceptibility of wsc1Δ cells to various forms of stress including caspofungin treatment and heat shock (Gray et al., 1997; Verna et al., 1997; Jacoby et al., 1998; Reinoso-Martin et al., 2003). Our finding that Wsc1AAA and sla1-Δshd1 cells are hypersensitive to caspofungin indicates that polarized localization of Wsc1p is important to counter cell wall stress triggered by inhibition of glucan synthase. However, unlike our wsc1Δ strain, these mutants were not sensitive to growth at elevated temperatures (Howard et al., 2002), another condition thought to trigger cell wall stress (Kamada et al., 1995). The basis for this difference is currently unclear but could reflect the nature of the stress. Because caspofungin targets glucan synthase, which is localized to regions of new cell surface growth (Drgonova et al., 1996; Qadota et al., 1996), the principal cell wall defects are likely to be concentrated at those sites. Consequently, stimulation of cell wall stress response pathways from Wsc1p localized at sites of new cell wall synthesis may be especially important. In contrast, heat shock may cause a more even cell wall stress so that global activation of stress response pathways from delocalized Wsc1p may suffice to maintain cell wall integrity. In support of this view, Wsc1p and glucan synthase become transiently depolarized in response to heat shock, suggesting that a more uniform distribution of these proteins is normally involved in the heat-shock response (Delley and Hall, 1999).
It has been suggested that Wsc1p has similarities to mammalian integrins, acting to probe an extracellular matrix (the cell wall) through bidirectional signaling with the actin cytoskeleton (Delley and Hall, 1999). The view that actin can signal to Wsc1p was based on the observation that Wsc1p became depolarized in response to actin depolymerization by LatA. Given the essential role for actin dynamics in endocytosis in yeast, our findings indicate that the basis for this effect is most likely a block in Wsc1p endocytosis, which prevents polarized localization through endocytic recycling.
The discovery that Wsc1p achieves polarized localization through endocytosis and recycling extends analogies with integrins in mammalian cells. Like Wsc1p, certain integrins undergo cycles of internalization and recycling to the plasma membrane, a process thought to be crucial for the polarized distribution of integrins at the leading edge of migrating cells (Bretscher, 1996; Caswell and Norman, 2006). Our results reveal that endocytic recycling represents a common evolutionary strategy to polarize the cell surface localization of proteins that monitor the status of extracellular matrices.
ACKNOWLEDGMENTS
We thank Marianne Cilluffo and Sergey Ryazantsev for help with electron microscopy and Esteban Fernandez, Giancarlo Costaguta, and Mara Duncan for assistance with fluorescence microscopy. We are grateful to members of the Payne laboratory for helpful discussions and comments on the manuscript. This work was supported by National Institutes of Health Grant GM39040 to G.P.
Abbreviations used:
CCV
clathrin-coated vesicles
GFP
green fluorescence protein
LatA
latrunculin A
SHD1
Sla1 homology domain 1
TGN
_trans_-Golgi network.
Footnotes
REFERENCES
- Ayscough K. R., Eby J. J., Lila T., Dewar H., Kozminski K. G., Drubin D. G. Sla1p is a functionally modular component of the yeast cortical actin cytoskeleton required for correct localization of both Rho1p-GTPase and Sla2p, a protein with talin homology. Mol. Biol. Cell. 1999;10:1061–1075. doi: 10.1091/mbc.10.4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti H., Raths S., Crausaz F., Riezman H. The END3 gene encodes a protein that is required for the internalization step of endocytosis and for actin cytoskeleton organization in yeast. Mol. Biol. Cell. 1994;5:1023–1037. doi: 10.1091/mbc.5.9.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensen E. S., Yeung B. G., Payne G. S. Ric1p and the ypt6p gtpase function in a common pathway required for localization of trans-golgi network membrane proteins. Mol. Biol. Cell. 2001;12:13–26. doi: 10.1091/mbc.12.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S. Moving membrane up to the front of migrating cells. Cell. 1996;85:465–467. doi: 10.1016/s0092-8674(00)81246-5. [DOI] [PubMed] [Google Scholar]
- Caswell P. T., Norman J. C. Integrin trafficking and the control of cell migration. Traffic. 2006;7:14–21. doi: 10.1111/j.1600-0854.2005.00362.x. [DOI] [PubMed] [Google Scholar]
- Chen L., Davis N. G. Recycling of the yeast a-factor receptor. J. Cell Biol. 2000;151:731–738. doi: 10.1083/jcb.151.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner S. D., Schmid S. L. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- Costa R., Warren D. T., Ayscough K. R. Lsb5p interacts with actin regulators Sla1p and Las17p, ubiquitin and Arf3p to couple actin dynamics to membrane trafficking processes. Biochem. J. 2005;387:649–658. doi: 10.1042/BJ20041729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. G., Horecka J. L., Sprague G. F., Jr Cis- and trans-acting functions required for endocytosis of the yeast pheromone receptors. J. Cell Biol. 1993;122:53–65. doi: 10.1083/jcb.122.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delley P. A., Hall M. N. Cell wall stress depolarizes cell growth via hyperactivation of RHO1. J. Cell Biol. 1999;147:163–174. doi: 10.1083/jcb.147.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deloche O., Schekman R. W. Vps10p cycles between the TGN and the late endosome via the plasma membrane in clathrin mutants. Mol. Biol. Cell. 2002;13:4296–4307. doi: 10.1091/mbc.02-07-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drgonova J., Drgon T., Tanaka K., Kollar R., Chen G. C., Ford R. A., Chan C. S., Takai Y., Cabib E. Rho1p, a yeast protein at the interface between cell polarization and morphogenesis. Science. 1996;272:277–279. doi: 10.1126/science.272.5259.277. [DOI] [PubMed] [Google Scholar]
- Dupre S., Urban-Grimal D., Haguenauer-Tsapis R. Ubiquitin and endocytic internalization in yeast and animal cells. Biochim. Biophys. Acta. 2004;1695:89–111. doi: 10.1016/j.bbamcr.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Fassel T. A., Sohnle P. G., Kushnaryov V. M. The use of dimethylsulfoxide for fixation of yeasts for electron microscopy. Biotech. Histochem. 1997;72:268–272. doi: 10.3109/10520299709082251. [DOI] [PubMed] [Google Scholar]
- Gray J. V., Ogas J. P., Kamada Y., Stone M., Levin D. E., Herskowitz I. A role for the Pkc1 MAP kinase pathway of Saccharomyces cerevisiae in bud emergence and identification of a putative upstream regulator. EMBO J. 1997;16:4924–4937. doi: 10.1093/emboj/16.16.4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J., Stenmark H. The biogenesis of multivesicular endosomes. Nat. Rev. Mol. Cell Biol. 2004;5:317–323. doi: 10.1038/nrm1360. [DOI] [PubMed] [Google Scholar]
- Hicke L., Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu. Rev. Cell Dev. Biol. 2003;19:141–172. doi: 10.1146/annurev.cellbio.19.110701.154617. [DOI] [PubMed] [Google Scholar]
- Hicke L., Riezman H. Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endosytosis. Cell. 1996;84:277–287. doi: 10.1016/s0092-8674(00)80982-4. [DOI] [PubMed] [Google Scholar]
- Howard J. P., Hutton J. L., Olson J. M., Payne G. S. Sla1p serves as the targeting signal recognition factor for NPFX(1,2)D-mediated endocytosis. J. Cell Biol. 2002;157:315–326. doi: 10.1083/jcb.200110027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby J. J., Nilius S. M., Heinisch J. J. A screen for upstream components of the yeast protein kinase C signal transduction pathway identifies the product of the SLG1 gene. Mol. Gen. Genet. 1998;258:148–155. doi: 10.1007/s004380050717. [DOI] [PubMed] [Google Scholar]
- James P., Halladay J., Craig E. A. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics. 1996;144:1425–1436. doi: 10.1093/genetics/144.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaksonen M., Sun Y., Drubin D. G. A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell. 2003;115:475–487. doi: 10.1016/s0092-8674(03)00883-3. [DOI] [PubMed] [Google Scholar]
- Kaksonen M., Toret C. P., Drubin D. G. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell. 2005;123:305–320. doi: 10.1016/j.cell.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Kamada Y., Jung U. S., Piotrowski J., Levin D. E. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 1995;9:1559–1571. doi: 10.1101/gad.9.13.1559. [DOI] [PubMed] [Google Scholar]
- Katzmann D. J., Odorizzi G., Emr S. D. Receptor downregulation and multivesicular-body sorting. Nat. Rev. Mol. Cell Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- Ketela T., Green R., Bussey H. Saccharomyces cerevisiae mid2p is a potential cell wall stress sensor and upstream activator of the PKC1-MPK1 cell integrity pathway. J. Bacteriol. 1999;181:3330–3340. doi: 10.1128/jb.181.11.3330-3340.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. E. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2005;69:262–291. doi: 10.1128/MMBR.69.2.262-291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Moir R. D., Sethy-Coraci I. K., Warner J. R., Willis I. M. Repression of ribosome and tRNA synthesis in secretion-defective cells is signaled by a novel branch of the cell integrity pathway. Mol. Cell Biol. 2000;20:3843–3851. doi: 10.1128/mcb.20.11.3843-3851.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine M. S., McKenzie A., Demarini D. J., Shah N. G., Wach A., Brachat A., Philippsen P., Pringle J. R. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Mamoun C. B., Beckerich J. M., Gaillardin C., Kepes F. Disruption of YHC8, a member of the TSR1 gene family, reveals its direct involvement in yeast protein translocation. J. Biol. Chem. 1999;274:11296–11302. doi: 10.1074/jbc.274.16.11296. [DOI] [PubMed] [Google Scholar]
- Mizuta K., Warner J. R. Continued functioning of the secretory pathway is essential for ribosome synthesis. Mol. Cell Biol. 1994;14:2493–2502. doi: 10.1128/mcb.14.4.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanduri J., Mitra S., Andrei C., Liu Y., Yu Y., Hitomi M., Tartakoff A. M. An unexpected link between the secretory path and the organization of the nucleus. J. Biol. Chem. 1999;274:33785–33789. doi: 10.1074/jbc.274.47.33785. [DOI] [PubMed] [Google Scholar]
- Nanduri J., Tartakoff A. M. The arrest of secretion response in yeast: signaling from the secretory path to the nucleus via Wsc proteins and Pkc1p. Mol. Cell. 2001;8:281–289. doi: 10.1016/s1097-2765(01)00312-4. [DOI] [PubMed] [Google Scholar]
- Newpher T. M., Smith R. P., Lemmon V., Lemmon S. K. In vivo dynamics of clathrin and its adaptor-dependent recruitment to the actin-based endocytic machinery in yeast. Dev. Cell. 2005;9:87–98. doi: 10.1016/j.devcel.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Insights from yeast endosomes. Curr. Opin. Cell Biol. 2002;14:454–462. doi: 10.1016/s0955-0674(02)00352-6. [DOI] [PubMed] [Google Scholar]
- Piper R. C., Cooper A. A., Yang H., Stevens T. H. VPS27 controls vacuolar and endocytic traffic through a prevacuolar compartment in Saccharomyces cerevisiae. J. Cell Biol. 1995;131:603–617. doi: 10.1083/jcb.131.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne D., Bretscher A. Polarization of cell growth in yeast II. The role of the cortical actin cytoskeleton. J. Cell Sci. 2000;113(Pt 4):571–585. doi: 10.1242/jcs.113.4.571. [DOI] [PubMed] [Google Scholar]
- Qadota H., Python C. P., Inoue S. B., Arisawa M., Anraku Y., Zheng Y., Watanabe T., Levin D. E., Ohya Y. Identification of yeast Rho1p GTPase as a regulatory subunit of 1,3-beta-glucan synthase. Science. 1996;272:279–281. doi: 10.1126/science.272.5259.279. [DOI] [PubMed] [Google Scholar]
- Rajavel M., Philip B., Buehrer B. M., Errede B., Levin D. E. Mid2 is a putative sensor for cell integrity signaling in Saccharomyces cerevisiae. Mol. Cell Biol. 1999;19:3969–3976. doi: 10.1128/mcb.19.6.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiori F., Pelham H. R. Sorting of proteins into multivesicular bodies: ubiquitin-dependent and -independent targeting. EMBO J. 2001;20:5176–5186. doi: 10.1093/emboj/20.18.5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinoso-Martin C., Schuller C., Schuetzer-Muehlbauer M., Kuchler K. The yeast protein kinase C cell integrity pathway mediates tolerance to the antifungal drug caspofungin through activation of Slt2p mitogen-activated protein kinase signaling. Eukaryot. Cell. 2003;2:1200–1210. doi: 10.1128/EC.2.6.1200-1210.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth A. F., Davis N. G. Ubiquitination of the yeast a-factor receptor. J. Cell Biol. 1996;134:661–674. doi: 10.1083/jcb.134.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger M., Payne G. S. Selective and immediate effects of clathrin heavy chain mutations on Golgi membrane protein retention in Saccharomyces cerevisiae. J. Cell Biol. 1992;118:531–540. doi: 10.1083/jcb.118.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih S. C., Katzmann D. J., Schnell J. D., Sutanto M., Emr S. D., Hicke L. Epsins and Vps27p/Hrs contain ubiquitin-binding domains that function in receptor endocytosis. Nat. Cell Biol. 2002;4:389–393. doi: 10.1038/ncb790. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siniossoglou S., Peak-Chew S. Y., Pelham H. R. Ric1p and Rgp1p form a complex that catalyses nucleotide exchange on Ypt6p. EMBO J. 2000;19:4885–4894. doi: 10.1093/emboj/19.18.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamenova S. D., Dunn R., Adler A. S., Hicke L. The Rsp5 ubiquitin ligase binds to and ubiquitinates members of the yeast CIN85-endophilin complex, Sla1-Rvs167. J. Biol. Chem. 2004;279:16017–16025. doi: 10.1074/jbc.M313479200. [DOI] [PubMed] [Google Scholar]
- Szymkiewicz I., Shupliakov O., Dikic I. Cargo- and compartment-selective endocytic scaffold proteins. Biochem. J. 2004;383:1–11. doi: 10.1042/BJ20040913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan P. K., Howard J. H., Payne G. S. The sequence NPFXD defines a new class of endocytosis signal in Saccharomyces cerevisiae. J. Cell Biol. 1996;135:1789–1800. doi: 10.1083/jcb.135.6.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H. Y., Xu J., Cai M. Pan1p, End3p, and Sla1p, three yeast proteins required for normal cortical actin cytoskeleton organization, associate with each other and play essential roles in cell wall morphogenesis. Mol. Cell Biol. 2000;20:12–25. doi: 10.1128/mcb.20.1.12-25.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub L. M. Common principles in clathrin-mediated sorting at the Golgi and the plasma membrane. Biochim. Biophys. Acta. 2005;1744:415–437. doi: 10.1016/j.bbamcr.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Verna J., Lodder A., Lee K., Vagts A., Ballester R. A family of genes required for maintenance of cell wall integrity and for the stress response in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 1997;94:13804–13809. doi: 10.1073/pnas.94.25.13804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida T. A., Emr S. D. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol. 1995;128:779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren D. T., Andrews P. D., Gourlay C. W., Ayscough K. R. Sla1p couples the yeast endocytic machinery to proteins regulating actin dynamics. J. Cell Sci. 2002;115:1703–1715. doi: 10.1242/jcs.115.8.1703. [DOI] [PubMed] [Google Scholar]
- Zarzov P., Mazzoni C., Mann C. The SLT2(MPK1) MAP kinase is activated during periods of polarized cell growth in yeast. EMBO J. 1996;15:83–91. [PMC free article] [PubMed] [Google Scholar]