Rb is critical in a mammalian tissue stem cell population (original) (raw)
Abstract
The inactivation of the retinoblastoma (Rb) tumor suppressor gene in mice results in ectopic proliferation, apoptosis, and impaired differentiation in extraembryonic, neural, and erythroid lineages, culminating in fetal death by embryonic day 15.5 (E15.5). Here we show that the specific loss of Rb in trophoblast stem (TS) cells, but not in trophoblast derivatives, leads to an overexpansion of trophoblasts, a disruption of placental architecture, and fetal death by E15.5. Despite profound placental abnormalities, fetal tissues appeared remarkably normal, suggesting that the full manifestation of fetal phenotypes requires the loss of Rb in both extraembryonic and fetal tissues. Loss of Rb resulted in an increase of E2f3 expression, and the combined ablation of Rb and E2f3 significantly suppressed Rb mutant phenotypes. This rescue appears to be cell autonomous since the inactivation of Rb and E2f3 in TS cells restored placental development and extended the life of embryos to E17.5. Taken together, these results demonstrate that loss of Rb in TS cells is the defining event causing lethality of Rb−/− embryos and reveal the convergence of extraembryonic and fetal functions of Rb in neural and erythroid development. We conclude that the Rb pathway plays a critical role in the maintenance of a mammalian stem cell population.
Keywords: Rb, development, placenta, stem cells
The retinoblastoma (Rb) tumor suppressor gene was identified more than two decades ago as the gene responsible for retinoblastoma, but has since been implicated in most human cancers. In contrast to retinoblastoma patients, inheritance of one deleted copy of Rb in mice did not induce retinoblastoma but did increase risk of pituitary and thyroid cancers (Jacks et al. 1992; Hu et al. 1994; Maandag et al. 1994; Williams et al. 1994; Robanus-Maandag et al. 1998; Yamasaki et al. 1998). Deletion of both copies of Rb in mice resulted in a broad range of severe abnormalities that lead to lethality by embryonic day 15.5 (E15.5) (Clarke et al. 1992; Jacks et al. 1992; Lee et al. 1992; Wu et al. 2003). Because Rb is normally expressed in all tissues of the mouse embryo, it was assumed that these developmental abnormalities were due to the absence of Rb protein in the tissues affected. Subsequent analysis of chimeric embryos suggested that Rb function is likely to be much more complex than initially suspected (Maandag et al. 1994; Lipinski et al. 2001). Indeed, recent findings showed that _Rb_-deficient embryos supplied with a wild-type placenta could develop to term and suggested a critical function of Rb in the placenta that might underlie many of the fetal developmental abnormalities observed in Rb−/− embryos (de Bruin et al. 2003; Wu et al. 2003).
Because Rb is involved in so many important processes that are critical for embryonic development and cancer prevention there has been an intense effort to understand its biochemical functions. As a result, >110 factors have been found to bind the Rb protein, either directly or through association in a complex. Approximately two-thirds of these interacting proteins play a role in the regulation of transcription (Morris and Dyson 2001). Id2 and E2F transcription factors are among those that have emerged as relevant effectors of Rb function in vivo. For example, loss of Id2 can rescue the viability of Rb−/− embryos (Lasorella et al. 2000), at least in part, by restoring normal fetal liver macrophage differentiation and erythrocyte maturation (Iavarone et al. 2004). The loss of E2f1 or E2f3 suppresses ectopic proliferation and apoptosis in a number of _Rb_-null tissues, and extends the life of Rb−/− embryos to late gestation (Tsai et al. 1998; Ziebold et al. 2001; Saavedra et al. 2002). The mechanism by which Id2, E2Fs, and other effectors of Rb act remains hotly contested, as there is evidence to support that some functions of Rb are cell autonomous and others are non-cell autonomous (Symonds et al. 1994; Lipinski et al. 2001; MacPherson et al. 2003; Iavarone et al. 2004). A prerequisite to understanding the biochemical properties of Rb that are critical for its role in development and in cancer is to identify the cell(s) where Rb is required. Understanding the dynamic interactions of Rb with its effectors might then elucidate how Rb function in this cell(s) impacts the rest of the organism.
Mutations in Rb but not in the related p107 and p130 genes are found in many types of human cancers (Weinberg 1995). While Rb and the related p107 and p130 pocket proteins can functionally compensate for one another in a number of biological settings, they also appear to regulate some processes uniquely (Lee et al. 1996; Mulligan and Jacks 1998; Lipinski and Jacks 1999; Vanderluit et al. 2004). Rb−/− mice die during gestation with anemia, placental defects, and widespread hyperproliferation and apoptosis, whereas p130−/− and p107−/− mice can develop normally (Cobrinik et al. 1996; Lee et al. 1996; LeCouter et al. 1998a, b; Mulligan and Jacks 1998). These distinct phenotypes, however, may reflect spatial differences in their expression pattern. The only clear biochemical distinctions between the related pocket proteins are their E2F-binding preferences in vitro. Whereas E2F4 and E2F5 can associate with all pocket proteins, E2F1, E2F2, and E2F3 normally bind exclusively to Rb (Ewen et al. 1992; Faha et al. 1992; Lees et al. 1992; Li et al. 1993; Lipinski and Jacks 1999). Despite all we have learned about this family of proteins, the functions that endow Rb with tumor suppressor activity have yet to be defined. The tumor suppressor function that distinguishes Rb from the other pocket proteins might be related to the unique function Rb plays during development. This uniqueness may originate from the biochemical properties of Rb or the nature of the cell in which Rb functions.
As in cancer and other complex-trait diseases, the developmental abnormalities caused by the inactivation of Rb are likely based on multiple interactions within and between different cell types and organs. Here, we designed digital algorithm-based three-dimensional (3D) image analysis systems and lineage-specific cre mice to dissect the spatial and temporal requirement of Rb gene networks within the developing embryo. We show that Rb is required in trophoblast stem (TS) cells but not in trophoblast derivatives of the placenta. The unique requirement for Rb in the TS cell population, and not in its derivatives, points to a stem cell-specific function of Rb not shared by the related p107 and p130 family members. Further, we find that a critical role for Rb in this TS cell compartment is mediated by one of its binding partners, E2F3.
Results
3D image analysis reveals global disruption of Rb mutant placentas
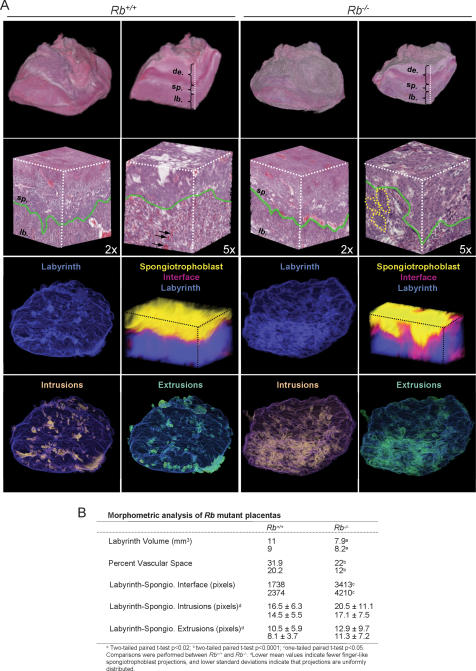
The global impact of Rb loss on the placenta was visualized by a digital 3D imaging system. The 3D renderings of the entire placenta presented in Figure 1 illustrate the cellular complexity of the maternal-derived decidual layer and two of the major extraembryonic cell derivatives: labyrinth trophoblasts and spongiotrophoblasts. The placenta vasculature embedded within the extraembryonic layer provides the main site of nutrient–waste exchange between the mother and fetus via a network of maternal sinusoids juxtaposed to fetal blood vessels. A comparison between littermates revealed a significant decrease of vascular space in Rb mutant placentas, consistent with their impaired transport function (Fig. 1B; Wu et al. 2003). This vascular defect was compounded by a concomitant decrease in the total volume of the labyrinth. In addition, complementary morphometric analyses indicated a dramatic perturbation of the mutant labyrinth–spongiotrophoblast interface. These measurements revealed the presence of shallow finger-like spongiotrophoblast projections that fail to properly invade the mutant labyrinth layer, highlighting the chaotic organization within this region of Rb mutant placentas (Fig. 1A [two bottom panels], B). Indeed, necrosis, clustering, and multinucleation of _Rb_-deficient trophoblasts were especially acute near the labyrinth–spongiotrophoblast interface. These observations illustrate the global disruption of labyrinth and vascular architecture that results from the hyperproliferation of Rb−/− trophoblasts (see Fig. 4A, below) and suggest that placental dysfunction could stem from a defect in one or more of the extraembryonic cell lineages.
Figure 1.
Three-dimensional image analysis reveals global disruption of Rb mutant placentas. (A, panels in first row) Representative 3D images derived from H&E-stained sections of whole placentas that were scanned, registered, and stitched together. A representative internal slice reveals the major cell layers of the placenta. (de) Decidua; (sp) spongiotrophoblast; (lb) labyrinth trophoblast. (Panels in second row) Higher-magnification (2× and 5×) cubic images of the labyrinth–spongiotrophoblast interface. Green lines represent the boundary between the labyrinth–spongiotrophoblast interface; dotted yellow lines encircle clusters of Rb−/− trophoblasts that are embedded within the labyrinth; arrows point to typical blood spaces visible in the Rb+/+ placenta. (Panels in third row) Volumetric rendering of the labyrinth layer (blue). Morphometric analysis of the labyrinth (blue) and spongiotrophoblast (yellow) layers are depicted by cubic images at high resolution. The magenta area represents the region of contact between the labyrinth and spongiotrophoblast layers; note the expanded interface zone between these two layers in the Rb−/− placenta. (Panels in fourth row) Morphometric analyses of spongiotrophoblast intrusions and extrusions into the labyrinth layer (blue) are visually rendered in orange and green, respectively. The genotypes of each placenta are indicated at the top (Rb+/+, n = 3; Rb−/−, n = 3). For a rotational view of whole placentas and magnified cubic images, see also http://tinyurl.com/bc6ka. (B) Morphometric analysis of Rb mutant placentas.
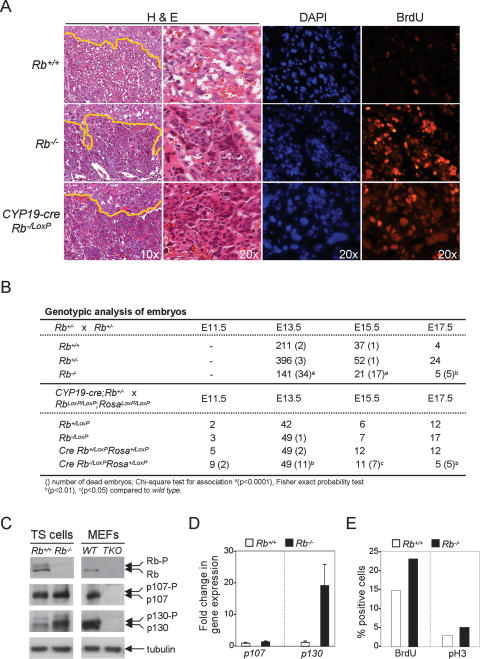
Figure 4.
Rb ablation in the placenta causes lethality and disruption of labyrinth architecture. (A) Sections of placentas were stained with H&E (two left panels) or processed for immunofluorescence using BrdU-specific antibodies and counterstained with DAPI (two right panels). Yellow lines indicate the boundary between the labyrinth and spongiotrophoblast cell layers. The original magnification is indicated within the bottom panels for each column. (B) Genotypic analysis of embryos. (C) Pocket proteins were detected by Western blot in TS cells and mouse embryonic fibroblasts (MEFs). TKO is a cell line triply deleted for Rb, p107, and p130. (D) Expression of p107 and p130 in Rb+/+ and Rb−/− TS cells was measured by real-time RT–PCR using primers listed in Supplementary Figure S3. (E) Immunofluorescence of BrdU incorporation and phosphorylation of histone H3 was used as an indicator of DNA synthesis and mitosis, respectively, in cultured TS cells.
Fetuses supplied with an Rb mutant placenta are embryonic lethal
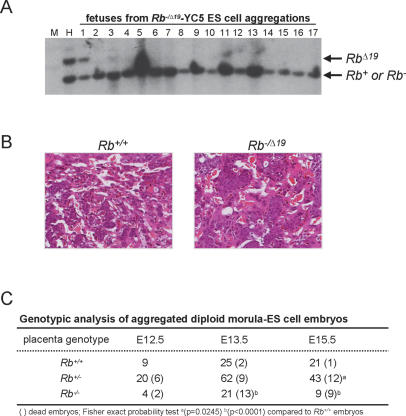
Previous embryo aggregation studies have shown that Rb+/+–_Rb_−/− chimeric fetuses, as well as _Rb_−/− fetuses, can survive to birth when supplied with a wild-type placenta (Maandag et al. 1994; Williams et al. 1994; Wu et al. 2003). To examine the requirement of Rb in extraembryonic lineages we used morula–embryonic stem (ES) cell aggregation techniques to generate chimeric fetuses that had _Rb_-deficient placentas (Wang et al. 1997). Rb+/+ and _Rb_−/Δ19 E2.5 diploid morula were aggregated with wild-type YC5 ES cells expressing the yellow fluorescent protein (YFP) (Hadjantonakis et al. 2002); the use of two different _Rb_-null alleles (Rb− and _Rb_Δ19) facilitated the genotyping of the aggregated embryos (Jacks et al. 1992; Marino et al. 2000). In this setting, diploid morula contribute to both extraembryonic and fetal lineages but ES cells contribute only to fetal lineages. Hence, the resulting placentas would be either genetically Rb+/+ or _Rb_−/Δ19 and fetuses would be chimeric (James et al. 1995). As evaluated by both fluorescent microscopy and Southern blot analysis, chimeric fetuses were uniformly enriched for wild-type YFP-positive cells (>95% Rb+/+) (Fig. 2A; data not shown). As expected, embryos reconstituted with Rb+/+ morula had normal placentas, with spongiotrophoblast finger-like projections extending into the labyrinth and trophoblasts uniformly organized around the placental vasculature (Fig. 2B). In contrast, fetuses derived from _Rb_−/Δ19 morula had placentas with a severely disrupted labyrinth architecture typical of _Rb_−/− embryos. While most fetuses associated with wild-type placentas were alive at each of the time points examined (54/57), more than half of the fetuses associated with _Rb_−/Δ19 placentas were dead by E13.5 (15/25), and all were dead by E15.5 (9/9) (Fig. 2C). Thus, in contrast to previous work showing that Rb+/+-_Rb_−/− chimeric fetuses supplied with wild-type placentas can be carried to term (Maandag et al. 1994; Williams et al. 1994), our work here shows that chimeric fetuses supplied with _Rb_−/Δ19 placentas die by E15.5. These morula–ES cell aggregation studies demonstrate that _Rb_-deficient extraembryonic lineages are functionally responsible for the global disruption of the placenta and lethality in _Rb_−/− embryos.
Figure 2.
Embryos reconstituted with Rb+/+ and Rb−/Δ19 placentas. (A) Southern blot analysis of aggregated chimeric fetuses hybridized with an Rb-specific probe that differentiates between the RbΔ19 and Rb+/Rb− alleles (indicated on the right). (M) Lane loaded with DNA ladder marker; (H) RbΔ19/+ control DNA sample. Note the low contribution of YC5 ES cells (Rb+/+) to chimeric fetus number 1; all others fetuses contained >95% contribution from YC5 ES cells. (B) Placental sections from representative aggregated embryos with the indicated genotypes were stained with H&E. Note the clusters of small cuboidal trophoblast cells in Rb−/Δ19 placentas. Original magnification, 20×. (C) Genotypic analysis of aggregated diploid morula–ES cell embryos.
Generation of extraembryonic lineage-specific cre mice
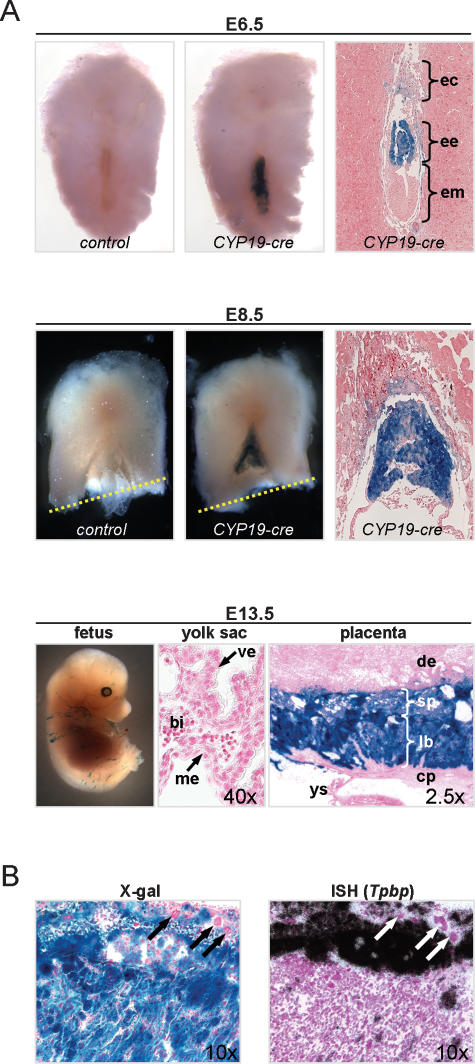
To define the contributions made by Rb in the various extraembryonic lineages we initially targeted the ablation of Rb in TS cells, which are thought to give rise to labyrinth trophoblasts, spongiotrophoblasts and giant trophoblasts. To this end, expression of cre in TS cells was driven by a transgene (CYP19-cre) containing a 504-base-pair (bp) fragment of the human CYP19 promoter previously shown to promote expression of a reporter gene in the mouse placenta (Kamat 1999; Kamat et al. 2005). As determined by X-gal histochemical staining, cre expression could be detected in CYP19-cre;Rosa+/LoxP TS cells cultured in vitro from E3.5 blastocyst explants (P. Wenzel, unpubl.). Moreover, analysis of CYP19-cre;Rosa+/LoxP reporter embryos confirmed expression of cre in the ectoplacental cone and extraembryonic ectoderm where TS cells reside at E6.5 and E8.5 (Fig. 3A; Tanaka et al. 1998; Uy et al. 2002). By the time TS cells give rise to the mature trilaminate cell layers of the placenta, which occurs at around E10.5–E13.5, cre expression could be visualized throughout the labyrinth and spongiotrophoblast of the placenta but not in the primitive endoderm and ectoderm derivatives that populate the yolk sac (Fig. 3A). In situ hybridization with a spongiotrophoblast-specific probe (Tpbp) confirmed the expression of cre in these cells and its absence in many of the adjacent giant cells (Fig. 3B). This latter unexpected observation suggests that cre expression in TS cells is either incomplete, turned on after giant cell fate determination, or restricted to a TS cell subpopulation lacking the ability to differentiate into all giant cell types. Some fetal tissues also expressed cre, but these were primarily restricted to localized areas in the skin and occasional lens cells of the eye (Fig. 3A; Supplementary Fig. S1A; P. Wenzel, unpubl.).
Figure 3.
The CYP19-cre transgene drives expression of cre in TS cell lineages. (A) CYP19-cre transgenic mice were crossed to RosaLoxP/LoxP reporter mice and analyzed for β-galactosidase activity to establish the pattern and timing of cre expression in the E6.5 and E8.5 embryo (counterstained with eosin) and E13.5 (counterstained with nuclear fast red) fetus, yolk sac, and placenta. The dotted yellow line shows the incision site made for removal of the fetus for genotyping; note that the fetus is not shown for the E8.5 panels. (ec) Ectoplacental cone; (ee) extraembryonic ectoderm; (em) embryonic endoderm; (me) extraembryonic mesoderm; (ve) visceral endoderm; (bi) blood island; (de) decidua; (sp) spongiotrophoblast; (lb) labyrinth trophoblast; (cp) chorionic plate; (ys) yolk sac. (B, right) Placenta sections from E13.5 embryos were hybridized with a spongiotrophoblast-specific (Tpbp) RNA probe and were counterstained with hematoxylin. Micrographs of sections hybridized with a sense RNA Tpbp probe are not shown. (Left) An adjacent section was stained by X-gal and counterstained with nuclear fast red. Giant trophoblasts that did not express cre are indicated by arrows.
Specific loss of Rb in extraembryonic TS cells causes death by E15.5
To evaluate the developmental impact of ablating Rb specifically in TS cells we analyzed the offspring from CYP19-cre;Rb−/+ and RbLoxP/LoxP;RosaLoxP/LoxP intercrosses. In contrast to Rb_−/LoxP;Rosa+/LoxP embryos, placentas associated with E13.5 CYP19-cre;Rb_−/LoxP; Rosa+/LoxP embryos had a severe disruption of labyrinth architecture that was characterized by clustering of trophoblasts, a marked reduction in maternal–fetal blood spaces, and occasional necrosis of trophoblasts near the labyrinth–spongiotrophoblast interface (Fig. 4A, left panels). BrdU-specific immunofluorescence assays confirmed an increase in the proliferation of trophoblasts that had the morphological features of TS cells and that we previously showed to express Eomes (Figs. 4A, 7B [below]; Wu et al. 2003). Whereas Rb_−/LoxP;Rosa+/LoxP_ offspring, which were alive at all stages of development, some CYP19-cre;Rb_−/LoxP;Rosa+/LoxP_ embryos were dead as early as E11.5 (2/9), approximately one-quarter were dead by E13.5 (11/49), two-thirds were dead by E15.5 (7/11), and all embryos were dead by E17.5 (5/5) (Fig. 4B). This range in the timing of lethality is similar to that previously found for _Rb_−/− embryos (Fig. 4B; Clarke et al. 1992; Jacks et al. 1992; Lee et al. 1992).
Figure 7.
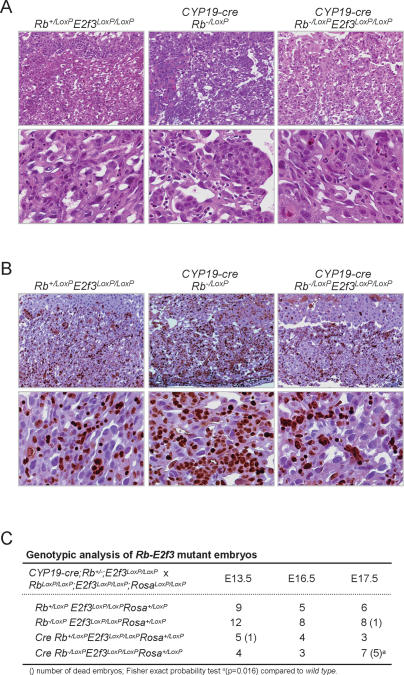
Conditional deletion of Rb and E2f3 in TS cells. (A) Placental tissue sections with the indicated genotypes were stained with H&E and micrographed at low (top panels) and high (bottom panels) magnification. (B) Similar sections as in A were processed for immunohistochemistry using Ki67-specific antibodies and counterstained with hematoxylin; top and bottom panels show micrographs at low and high magnification, respectively. (C) Genotypic analysis of embryos derived from crosses between CYP19-cre;Rb−/LoxP;E2f3LoxP/LoxP;Rosa+/LoxP and Rb−/LoxP;E2f3LoxP/LoxP;Rosa+/LoxP mice.
The early lethality of Rb mutant embryos could stem from the restricted pattern of pocket protein expression in TS cells or from a unique function of Rb in these cells that is not shared by p107 or p130. The Western blot and real-time RT–PCR analysis in Figure 4, C and D show that p107 and p130 are indeed expressed in cultured Rb+/+ TS cells. As has been shown in other cell types (Classon et al. 2000), the loss of Rb in TS cells led to the compensatory up-regulation of p130 and, to a lesser extent, p107 (Fig. 4C,D). Despite the increase in p107 and p130 expression, Rb−/− TS cells had a higher index of proliferation as determined by BrdU incorporation and histone H3 phosphorylation (Fig. 4E). Consistent with these findings, pocket protein function in trophoblasts appears to be conferred by Rb alone, since loss of Rb and not p107 or p130 causes the severe placental defects described in this study (P. Wenzel, unpubl.). Together, these data suggest that Rb is endowed with a singular role in the control of TS cell proliferation and placental development.
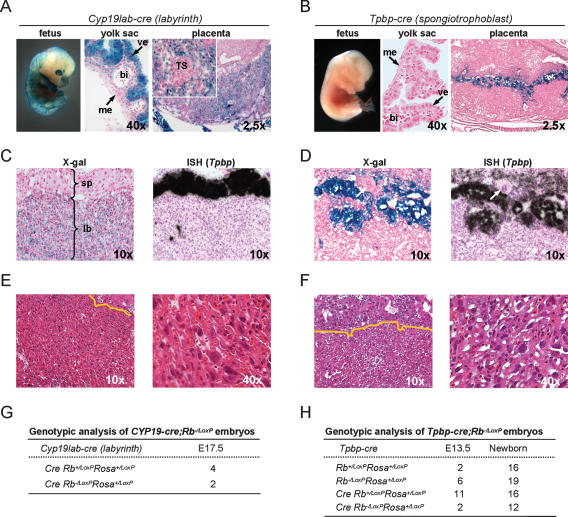
Analysis of some embryos derived from a second CYP19-cre founder line revealed that cre failed to be expressed in TS cells, but was instead expressed in labyrinth trophoblast and visceral endoderm lineages (CYP19lab-cre) (Fig. 5A,C). We also generated transgenic mice that expressed cre specifically in spongiotrophoblasts by linking the cre ORF to a 1.1-kb fragment of the mouse Tpbp promoter known to drive expression in this cell lineage (Calzonetti et al. 1995) (Tpbp-cre) (Fig. 5B,D; A.L. Fortier, H. Yamamoto, and J.C. Cross, in prep.). By similar breeding strategies as described above, we obtained offspring from crosses between CYP19lab-cre;Rb−/+ and RbLoxP/LoxP;RosaLoxP/LoxP mice as well as from Tpbp-cre;Rb−/+ and RbLoxP/LoxP;RosaLoxP/LoxP mice. Interestingly, we found that the targeted inactivation of Rb from either of these two TS cell derivatives had no adverse effect on placental development and the associated fetuses could be carried to term normally (Fig. 5E–H; data not shown). These results suggest that the critical function of Rb resides in TS cells prior to their commitment to labyrinth trophoblast and spongiotrophoblast cell fates. Together, the embryo aggregation and trophoblast-specific conditional deletion approaches define loss of Rb in TS cells as the critical event causing lethality of Rb−/− embryos.
Figure 5.
Deletion of Rb in labyrinth trophoblasts or spongiotrophoblasts does not affect embryonic development. (A,B) Whole mount of the fetus and sections of the yolk sac and placenta from CYP19lab-cre;Rosa+/LoxP (A) and Tpbp-cre;Rb−/LoxP;Rosa+/LoxP (B) embryos were stained with X-gal and counterstained with nuclear fast red. Inset represents a higher-magnification micrograph of a representative TS cell cluster, which does not stain positively with X-gal. (me) Extraembryonic mesoderm; (ve) visceral endoderm; (bi) blood island; (TS) cluster of cells with TS cell morphology. (C,D) In situ hybridization of placenta sections from E13.5 CYP19lab-cre; Rb−/LoxP;Rosa+/LoxP (C) or E13.5 Tpbp-cre;Rb−/LoxP;Rosa+/LoxP (D) embryos using an antisense RNA Tpbp probe. Micrographs of sections hybridized with a sense RNA Tpbp probe are not shown. Adjacent sections were stained with X-gal as indicated. Arrows point to a giant trophoblast. (sp) Spongiotrophoblast; (lb) labyrinth trophoblast. (E,F) Sections of E17.5 CYP19lab-cre Rb−/LoxP;Rosa+/LoxP placentas (E) and E13.5 Tpbp-cre;Rb−/LoxP;Rosa+/LoxP placentas (F) stained with H&E. (G,H) Genotypic analyses of embryos derived from CYP19lab-cre;Rb+/− and RbLoxP/LoxP;RosaLoxP/LoxP intercrosses (G), as well as from Tpbp-cre;Rb+/− and RbLoxP/LoxP;RosaLoxP/LoxP intercrosses (H).
E2F3 is a key mediator of Rb function in TS cells
Having identified the critical cell where many Rb−/− placental and fetal defects originate, we wondered if key effectors of Rb might also function in TS cells to dictate placental and fetal developmental programs. Among the many proteins that physically interact with Rb, the E2F transcription factors have emerged as critical Rb-binding partners. E2Fs consist of several family members thought to be functionally redundant that regulate similar sets of target genes; however, some studies suggest that there are at least two distinct classes of E2Fs: activators and repressors (Trimarchi and Lees 2001).
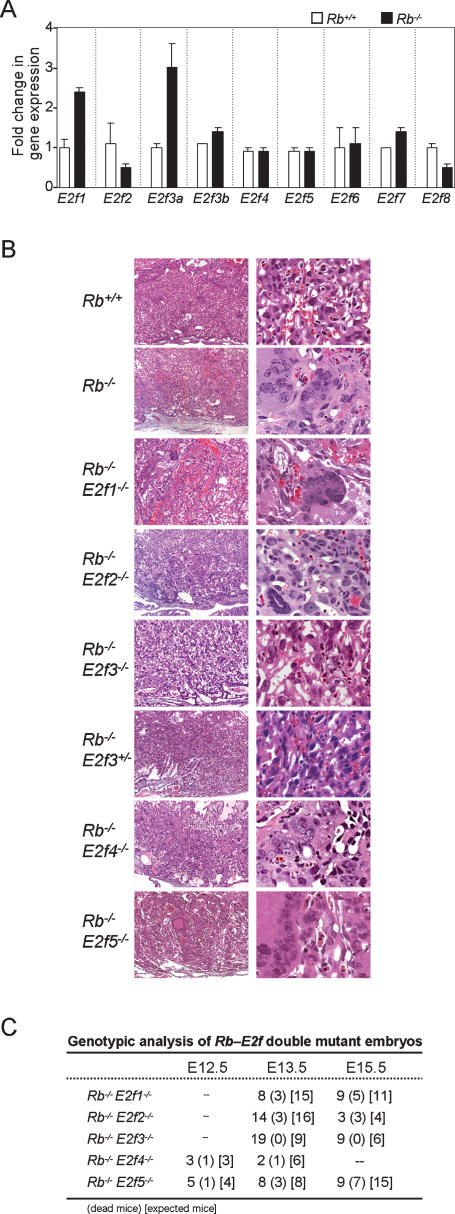
To address the potential role of E2F family members in mediating Rb mutant phenotypes we initially measured their expression in Rb mutant placentas. As determined by real-time RT–PCR assays, E2f1 and E2f3a expression was up-regulated two- to threefold in _Rb_-deficient placentas, whereas E2f2, E2f3b, E2f4, E2f5, E2f6, E2f7, and E2f8 expression remained relatively unchanged (Fig. 6A). We then analyzed the consequence resulting from the deletion of each family member in an Rb mutant context. Histological evaluation of Rb–E2F doubly deficient embryos revealed that loss of E2f3, but not E2f1, E2f2, E2f4, and E2f5, significantly suppressed the widespread overproliferation and clumping of abnormal multinucleated trophoblasts typically observed in _Rb_-deficient placentas. While a few Rb_–_E2f1 doubly deficient placentas had a milder Rb mutant placenta phenotype (data not shown), the majority of embryos collected at ages later than E13.5 were not alive (Fig. 6C). Deletion of E2f3 was able to abrogate the Rb−/− phenotype to the extent that all double-knockout embryos at E15.5 were alive (Fig. 6B,C), an age when ∼80% of _Rb_-deficient embryos are found dead (Fig. 4B). Loss of even one allele of E2f3 resulted in a partial rescue of the Rb−/− placental defect, with some variability observed between the embryos analyzed (Fig. 6B; data not shown).
Figure 6.
Loss of E2f3 in _Rb_-null TS cells suppresses mutant phenotypes. (A) Expression of E2f1–8 in Rb+/+ and Rb−/− placentas was measured by real-time RT–PCR assays using primers described in Supplementary Figure S3. (B) Placental sections from embryos with the indicated genotypes were stained with H&E. Note that loss of E2f3 suppressed the formation of large clumps of dysplastic trophoblasts. (C) Genotypic analysis of double-knockout embryos derived from crosses between Rb+/− and either E2f+/− or E2f−/− mice.
To directly test whether Rb−/− placental defects are a result of deregulated E2F3 activity in TS cells, we interbred CYP19-cre;Rb_−/+;E2f3LoxP/LoxP_ and RbLoxP/LoxP; E2f3LoxP/LoxP;RosaLoxP/LoxP mice and analyzed the resulting embryos. Placentas with _Rb–E2f3-_deficient TS cells had a relatively normal spongy architecture with little evidence of hyperproliferative trophoblasts (Fig. 7A,B). While some regional clumping could still be observed in these doubly deficient placentas, the presence of large dysplastic trophoblasts and the high degree of necrosis typically observed in Rb mutant placentas was significantly diminished. In contrast to embryos associated with placentas having _Rb-_deficient TS cells, which died by E15.5 (Fig. 4B), embryos with doubly deficient TS cells were still alive as late as E17.5 (Fig. 7C). These results phenocopy those obtained in Rb−/−E2f3−/− embryos and suggest a cell-autonomous role for E2f3 in TS cells that is responsible for the lethality of _Rb_-deficient embryos by E15.5. The fact that placentas derived from double-mutant TS cells cannot carry fetuses all the way to term, however, suggests that some functions of Rb in the placenta are independent of its interaction with E2F3 and the control of proliferation, but presumably are related to the control of trophoblast differentiation.
Loss of Rb in TS cells leads to ectopic apoptosis and erythroid defects in the fetus
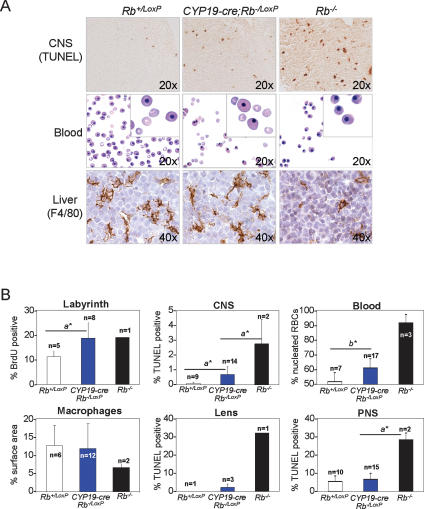
Previous work suggested that Rb function in the placenta dictates critical developmental events in the fetus, including the control of apoptosis in the CNS and of erythropoiesis in fetal livers (de Bruin et al. 2003; Wu et al. 2003). We therefore examined the developmental consequences stemming from the ablation of Rb in TS cells. As expected, CYP19-cre;Rb_−/LoxP;Rosa+/LoxP_ E13.5 fetuses had normal numbers of proliferating cells in the CNS, peripheral nervous system (PNS), lens, liver, and skeletal muscle (Supplementary Fig. S1A,B; data not shown). However, there was clear evidence of ectopic apoptosis in areas of the brainstem surrounding the fourth ventricle. The severity of this defect, though, was considerably less pronounced than in Rb−/− embryos (Fig. 8A,B). Other compartments of the brain, such as the cerebellum and midbrain, and tissues of the PNS, lens, liver, and skeletal muscle did not exhibit any signs of increased cell death (Fig. 8B; Supplementary Fig. S1A; data not shown).
Figure 8.
Loss of Rb in TS cells partially disrupts fetal development. (A) Analysis of E13.5 fetal tissues from CYP19-cre; Rb−/LoxP;Rosa+/LoxP and control embryos. Apoptosis was measured in the CNS by TUNEL assays, erythrocyte maturation in peripheral blood smears was monitored by staining with Giemsa, and the presence of macrophages in the liver was assessed by immunostaining with F4/80-specific antibodies and counterstaining with hematoxylin. Original magnifications are indicated for each panel and insets were 40×. (B) Quantification of proliferation and apoptosis of the indicated tissues as determined by BrdU- and TUNEL-assays, respectively. Note the presence of a small percentage of TUNEL-positive lens fiber cells present in CYP19-cre;Rb−/LoxP; Rosa+/LoxP embryos that arises as a consequence of occasional _cre_-expressing cells in the lens driven by this transgene. Hematopoietic differentiation was measured by the percentage of nucleated RBCs in peripheral blood (Blood) and by the amount of macrophages (% surface area) present in fetal livers (Macrophages). (a*) Mann-Whitney U-test, p < 0.05; (b*) Wilcoxon Signed Rank test, p = 0.002.
Impaired red blood cell (RBC) differentiation leading to anemia is another hallmark phenotype of Rb knockout mice. While CYP19-cre;Rb_−/LoxP;Rosa+/LoxP_ fetuses had normal coloration and appearance, they had a small but significant increase in immature nucleated RBCs (Fig. 8A,B). Erythropoietic defects in Rb mutant embryos can be mediated, at least in part, through non-cell-autonomous mechanisms involving liver-specific macrophages (Wu et al. 2003; Iavarone et al. 2004). Thus, we analyzed CYP19-cre;Rb_−/LoxP;Rosa+/LoxP_ fetal livers but found no significant change in F4/80-positive macrophages (Fig. 8A,B). These results suggest a macrophage-independent role of the placenta in RBC differentiation. Parallel analysis of chimeric embryos with Rb mutant placentas generated by tetraploid and diploid aggregation techniques yielded results identical to those obtained by the genetic ablation of Rb in TS cells (Supplementary Fig. S2; data not shown). These results demonstrate that Rb deficiency in TS cells causes some ectopic apoptosis and impaired RBC differentiation but that this non-cell-autonomous role of Rb is insufficient to induce the severe and widespread apoptosis and differentiation defects typically observed in Rb−/− embryos. Thus, we conclude that while some of the phenotypes observed in Rb−/− embryos are mainly due to loss of Rb in extraembryonic lineages and others are due to loss of Rb in fetal tissues, the massive neural apoptosis and erythroid defects observed in Rb−/− embryos are due to a deficiency of Rb protein in both. These findings address the cell-autonomous versus non-cell-autonomous controversy of how Rb regulates neuronal apoptosis and erythroid differentiation by showing that extraembryonic and fetal signaling converge to control these processes (de Bruin et al. 2003; Wu et al. 2003; Clark et al. 2004; Spike et al. 2004).
Discussion
Since the inception of gene ablation techniques in mice two decades ago, a significant but likely underrepresented number of knockout mouse models have been found to suffer from extraembryonic defects (Han and Carter 2001; Sapin et al. 2001). Some of these genes encode chromatin remodeling activities that function in the regulation of embryonic patterning, cell migration, proliferation, and apoptosis. Other genes encode activities that have not been linked to specific processes. Unfortunately, genetic systems to study the mechanism of gene networks and function in extraembryonic lineages are lacking. Here we developed novel genetic and imaging tools that provide a uniquely powerful system to identify and dissect how Rb and other gene pathways interact in the placenta and in turn impact fetal development. Three-dimensional imaging of Rb mutant placentas revealed a global disruption in placenta architecture, characterized by an increase in trophoblast proliferation, a decrease in labyrinth and vascular spaces, and a disorganization of the labyrinth–spongiotrophoblast interface. Importantly, complementary morula–ES cell aggregation and _cre_-mediated gene ablation techniques demonstrate that the global disruption of the labyrinth architecture can be manifested by the specific ablation of Rb in TS cells. We conclude that the loss of Rb in TS cells is the defining event that causes lethality of Rb−/− embryos.
Previous work suggested that lethality of Rb−/− embryos is due to massive apoptosis and severe anemia in the fetus (Clarke et al. 1992; Jacks et al. 1992; Lee et al. 1992). More recent work raised the possibility that these fetal defects could be due to non-cell-autonomous roles of Rb originating in the placenta (Wu et al. 2003). The results presented here suggest that apoptosis and anemia are unlikely to be responsible for the lethality of Rb−/− embryos since placental deletion of Rb is sufficient to cause lethality without the associated massive apoptotic and erythroid phenotypes. Rather, we suggest that fetal lethality might simply be a result of general placental dysfunction. Thus, we conclude that some phenotypes observed in Rb−/− embryos, such as mid-gestation lethality, are primarily due to loss of Rb in extraembryonic lineages. Other phenotypes such as incomplete skeletal muscle differentiation, ectopic proliferation, and apoptosis in the PNS and lens are due to loss of Rb in fetal tissues. Yet other phenotypes such as neural apoptosis and erythroid defects are due to the convergence of cell-autonomous and nonautonomous roles of Rb in the fetus and placenta (de Bruin et al. 2003; Wu et al. 2003; Clark et al. 2004; Spike et al. 2004).
Rb is expressed in all extraembryonic lineages (Wu et al. 2003) yet its loss appears to impact only TS cells. The fact that ablation of Rb in TS cells, but not in differentiated trophoblast derivatives, disrupts placental development suggests that the critical function of Rb resides in a TS cell population prior to the commitment of labyrinth trophoblast and spongiotrophoblast cell fates. These results also begin to define the window of time during which Rb function becomes pivotal. Our genetic analyses suggest that Rb is critical for TS cell homeostasis by regulating two related cellular processes: proliferation and differentiation. Here we identified E2F3 among E2F family members as one of the key downstream effectors of Rb that aids in the maintenance of the TS cell population. The specific loss of E2f3 in Rb−/− TS cells reduces the ectopic proliferation of trophoblasts and thus provides the necessary space for the branched trophoblast network to evolve. As a result, Rb–E2f3 doubly deficient placentas can support fetal life beyond the time when Rb−/− fetuses typically die. The fact that these doubly deficient placentas cannot support life to term, however, suggests that loss of E2f3 is unable to correct all defects in Rb−/− TS cells, including those that could arise from incomplete differentiation. Having identified the TS cell as a critical site of Rb action, we can now begin to unravel the biochemical interactions that are relevant to how Rb in this cell impacts the rest of the developing embryo.
Recent findings show a critical function of Rb in the maintenance of Arabidopsis root stem cells (Wildwater et al. 2005). Whereas ablation of Rb by RNAi or overexpression of E2F preserved and expanded stem cell populations in the columella and lateral root caps, overexpression of Rb or a cyclin-dependent kinase inhibitor led to depletion of this population. The role of Rb in this process appeared not to be related to the control of proliferation but rather in the maintenance of stem cell identity. Our results describing an effect of Rb in a murine extraembryonic stem cell niche extend these observations to higher eukaryotes. Moreover, we identified E2F3 as the critical Rb-binding partner involved in the proliferative maintenance of this stem cell population. We propose that TS cells, unlike their lineage derivatives (labyrinth trophoblasts and spongiotrophoblasts), represent a compartment that is particularly susceptible to loss of Rb, possibly because p107 and p130 may not have the capacity to functionally compensate, even though these related proteins are clearly present in TS cells. The basis for the specificity in pocket protein function is not yet clear, but could involve the selective binding of Rb to E2F3. The properties that make Rb unique in TS cells may also be relevant in stem cells of other tissues. These observations raise the possibility that stem cell-specific functions of Rb described here may be related to its unique ability among pocket proteins to function as a tumor suppressor.
Materials and methods
Mouse strains and genotyping
Rb+/− (Jacks et al. 1992), RbLoxP/LoxP (Marino et al. 2000), RosaLoxP reporter (Soriano 1999), CYP19-cre, and Tpbp-cre (A.L. Fortier, H. Yamamoto, and J.C. Cross, in prep.) transgenic mice were maintained on a mixed background (C57BL/6 × 129/Sv × FVB/N). The _Rb_Δ19 allele used in aggregation experiments was generated by in vivo knockout of the RbLoxP allele. PCR genotyping was used for mice, embryos, and placentas (primer sequences can be provided on request). DNA samples from transgenic cre lines were digested with BglII and hybridized with a DNA fragment encoding the cre ORF to probe Southern blots. Southern blot analysis of chimeric embryos generated by aggregation methods was carried out on EcoRV/NdeI-digested genomic DNA using a standard method.
Computational 3D imaging
Rb+/+ and Rb−/− E13.5 embryos were collected from timed pregnancies, fixed in 10% buffered formalin, embedded in paraffin, serially sectioned at 5-μm intervals, and stained with hematoxylin and eosin (H&E). Stained sections from the entire placenta (∼1200 sections; n = 6 placentas) were digitally scanned with an Aperio ScanScope using a 20× objective. Images were registered with rigid registration algorithms that employ a preprocessing _k_-means clustering step for foreground extraction and a principal component analysis (PCA) step for prealignment (Mosaliganti et al. 2006). The latter step is essential for expedient convergence of the underlying optimization procedure, an essential component of all registration algorithms (Maes et al. 1997). The registration algorithms of the NLM/NIHs’ Insight Toolkit (ITK) (Ibanez 2003) were modified to align the two-dimensional (2D) sections. Simultaneously, each of the sections was segmented into tissue regions. Initial classification of cell types in the placenta was performed at the pixel level either by a Bayesian probability-based algorithm or the _k_-means clustering algorithm. Later, through the use of features such as cytoplasm/nuclear color, size, and density of nuclei and presence of vacuoles or RBCs, regions were classified into various tissue types by a Bayesian probability-based segmentation algorithm. Training of the segmentation algorithm was performed by a pathologist. The volumetric stack containing the segmented and mutually registered images was visualized using appropriate 3D volume-rendering algorithms (Sharp et al. 2007). The Volview application from Kitware, Inc., was used to create the final images. Ten individual sections from regular intervals throughout the placenta were used for human-validated segmentation of placental cell types and for morphometric measurement of tissue volumes (Sharp et al. 2007). Two different types of morphometirc analyses were used to evaluate the labyrinth–spongiotrophoblast interface. Contact surface areas were determined by Hausdorff dimension calculations. Spongiotrophoblast intrusions/extrusions were determined by evaluating each point in the labyrinth through the use of contour-tracking transfer functions (Mosaliganti et al. 2006) and numerical values were compiled from the entire placenta. Lower mean values indicate fewer finger-like projections, and lower standard deviations indicate a more uniform distribution of finger-like projections.
Tetraploid and diploid aggregations
Tetraploid aggregations were performed as described previously, with the exception that tetraploid and diploid embryos were aggregated at a 1:1 ratio (Wang et al. 1997; Wu et al. 2003). Aggregation of diploid embryos with YC5 ES cells was performed by methods similar to the tetraploid aggregations, except without the electrofusion step at E1.5. Genotyping of the resulting placentas was facilitated by the use of two different _Rb_-null alleles (Rb− and _Rb_Δ19) (Jacks et al. 1992; Marino et al. 2000). Fluorescent microscopy and Southern blot analysis demonstrated that the reconstituted chimeric fetuses had a high degree of contribution derived from Rb+/+ YFP-positive YC5 ES cells (Hadjantonakis et al. 2002).
Histology and special stains
Embryos and placentas were collected from timed pregnancies, and whole-mount samples or 10-μm frozen sections from each embryo were stained with X-gal to monitor cre expression. Pregnant dams were injected with BrdU 1.5 h prior to harvest. Tissue from embryo aggregations was fixed in 10% buffered formalin, and tissue from transgenic crosses was either immediately fixed in 2% formaldehyde or frozen in OCT freezing medium and stored at −80°C. Proliferation was measured by immunostaining sections with BrdU-specific antibodies from DAKO (clone Bu20a), and apoptosis was detected by TUNEL assays using the Apoptag Plus Peroxidase In Situ Apoptosis Detection Kit (Chemicon International). Macrophages in fetal livers were detected using F4/80-specific antibodies (Caltag Laboratories, catalog no. MF48000) and quantified morphometrically by Matlab software. Frozen sections were used for in situ hybridization with antisense and sense RNA probes specific for the Tpbp gene. Blood smears were fixed in methanol and stained with Giemsa for quantification of nucleated RBCs.
Quantitative real-time RT–PCR
Placentas were collected from E13.5 embryos and homogenized to obtain single-cell suspensions that were used for total RNA isolation with TRIzol reagent (Life Technologies). Two micrograms to 10 μg of total RNA was used to generate cDNA using SuperScript III RT (Invitrogen). Real-time RT–PCR was performed using the Bio-Rad iCycler PCR machine. Each PCR reaction contained 2.0 μL of cDNA template, primers at a concentration of 100 nM, and 1× of SYBR Green Reaction Mix (Bio-Rad). Reactions were done in a final volume of 25 μL in triplicate, and data were analyzed using the ΔCt method, where GAPDH served as the internal control. Each PCR reaction generated only the expected amplicon as shown by the melting-temperature profiles of the final products, gel electrophoresis, and sequencing.
Acknowledgments
We thank A. Nagy for providing the YC5 ES cell line and K. Vintersten for assistance with embryo aggregations. We are grateful for technical assistance provided by J. Moffitt, J. Opavska, A. Gulacy, S. Kharzai, and Z. Barsman. We also thank M. Weinstein, A. Simcox, M. Ostrowski, and members of the Leone laboratory for critically reading the manuscript and helpful suggestions. This work was funded by NIH grants to G.L. (R01CA85619, R01CA82259, R01HD047470, P01CA097189), NIH grant to J.S. (BISTI P20 EB000591), NSF grants to J.S. (ANI-0330612, EIA-0203846, CNS-0615155, CNS-0509326, CNS-0426241), NSF grants to R.M. (0234273 and 0326386), NIH grant to L.W. (K01CA102328), and DoD award to A.d.B. (BC030892). G.L. is the recipient of The Pew Charitable Trust Scholar Award and the Leukemia and Lymphoma Society Scholar Award.
Footnotes
References
- Calzonetti T., Stevenson L., Rossant J., Stevenson L., Rossant J., Rossant J. A novel regulatory region is required for trophoblast-specific transcription in transgenic mice. Dev. Biol. 1995;171:615–626. doi: 10.1006/dbio.1995.1309. [DOI] [PubMed] [Google Scholar]
- Clark A.J., Doyle K.M., Humbert P.O., Doyle K.M., Humbert P.O., Humbert P.O. Cell-intrinsic requirement for pRb in erythropoiesis. Blood. 2004;104:1324–1326. doi: 10.1182/blood-2004-02-0618. [DOI] [PubMed] [Google Scholar]
- Clarke A.R., Maandag E.R., van Roon M., van der Lugt N.M., van der Valk M., Hooper M.L., Berns A., te Riele H., Maandag E.R., van Roon M., van der Lugt N.M., van der Valk M., Hooper M.L., Berns A., te Riele H., van Roon M., van der Lugt N.M., van der Valk M., Hooper M.L., Berns A., te Riele H., van der Lugt N.M., van der Valk M., Hooper M.L., Berns A., te Riele H., van der Valk M., Hooper M.L., Berns A., te Riele H., Hooper M.L., Berns A., te Riele H., Berns A., te Riele H., te Riele H. Requirement for a functional Rb-1 gene in murine development. Nature. 1992;359:328–330. doi: 10.1038/359328a0. [DOI] [PubMed] [Google Scholar]
- Classon M., Salama S., Gorka C., Mulloy R., Braun P., Harlow E., Salama S., Gorka C., Mulloy R., Braun P., Harlow E., Gorka C., Mulloy R., Braun P., Harlow E., Mulloy R., Braun P., Harlow E., Braun P., Harlow E., Harlow E. Combinatorial roles for pRB, p107, and p130 in E2F-mediated cell cycle control. Proc. Natl. Acad. Sci. 2000;97:10820–10825. doi: 10.1073/pnas.190343497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobrinik D., Lee M.H., Hannon G., Mulligan G., Bronson R.T., Dyson N., Harlow E., Beach D., Weinberg R.A., Jacks T., Lee M.H., Hannon G., Mulligan G., Bronson R.T., Dyson N., Harlow E., Beach D., Weinberg R.A., Jacks T., Hannon G., Mulligan G., Bronson R.T., Dyson N., Harlow E., Beach D., Weinberg R.A., Jacks T., Mulligan G., Bronson R.T., Dyson N., Harlow E., Beach D., Weinberg R.A., Jacks T., Bronson R.T., Dyson N., Harlow E., Beach D., Weinberg R.A., Jacks T., Dyson N., Harlow E., Beach D., Weinberg R.A., Jacks T., Harlow E., Beach D., Weinberg R.A., Jacks T., Beach D., Weinberg R.A., Jacks T., Weinberg R.A., Jacks T., Jacks T. Shared role of the pRB-related p130 and p107 proteins in limb development. Genes & Dev. 1996;10:1633–1644. doi: 10.1101/gad.10.13.1633. [DOI] [PubMed] [Google Scholar]
- de Bruin A., Wu L., Saavedra H.I., Wilson P., Yang Y., Rosol T.J., Weinstein M., Robinson M.L., Leone G., Wu L., Saavedra H.I., Wilson P., Yang Y., Rosol T.J., Weinstein M., Robinson M.L., Leone G., Saavedra H.I., Wilson P., Yang Y., Rosol T.J., Weinstein M., Robinson M.L., Leone G., Wilson P., Yang Y., Rosol T.J., Weinstein M., Robinson M.L., Leone G., Yang Y., Rosol T.J., Weinstein M., Robinson M.L., Leone G., Rosol T.J., Weinstein M., Robinson M.L., Leone G., Weinstein M., Robinson M.L., Leone G., Robinson M.L., Leone G., Leone G. Rb function in extraembryonic lineages suppresses apoptosis in the CNS of Rb-deficient mice. Proc. Natl. Acad. Sci. 2003;100:6546–6551. doi: 10.1073/pnas.1031853100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewen M.E., Faha B., Harlow E., Livingston D.M., Faha B., Harlow E., Livingston D.M., Harlow E., Livingston D.M., Livingston D.M. Interaction of p107 with cyclin A independent of complex formation with viral oncoproteins. Science. 1992;255:85–87. doi: 10.1126/science.1532457. [DOI] [PubMed] [Google Scholar]
- Faha B., Ewen M.E., Tsai L.H., Livingston D.M., Ewen M.E., Tsai L.H., Livingston D.M., Tsai L.H., Livingston D.M., Livingston D.M. Interaction between human cyclin A and adenovirus E1A-associated p107 protein. Science. 1992;255:87–90. doi: 10.1126/science.1532458. [DOI] [PubMed] [Google Scholar]
- Hadjantonakis A.K., Macmaster S., Nagy A., Macmaster S., Nagy A., Nagy A. Embryonic stem cells and mice expressing different GFP variants for multiple non-invasive reporter usage within a single animal. BMC Biotechnol. 2002;2:11. doi: 10.1186/1472-6750-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han V.K., Carter A.M., Carter A.M. Control of growth and development of the feto-placental unit. Curr. Opin. Pharmacol. 2001;6:632–640. doi: 10.1016/s1471-4892(01)00108-4. [DOI] [PubMed] [Google Scholar]
- Hu N., Gutsmann A., Herbert D.C., Bradley A., Lee W.H., Lee E.Y., Gutsmann A., Herbert D.C., Bradley A., Lee W.H., Lee E.Y., Herbert D.C., Bradley A., Lee W.H., Lee E.Y., Bradley A., Lee W.H., Lee E.Y., Lee W.H., Lee E.Y., Lee E.Y. Heterozygous Rb-1 Δ 20/+ mice are predisposed to tumors of the pituitary gland with a nearly complete penetrance. Oncogene. 1994;9:1021–1027. [PubMed] [Google Scholar]
- Iavarone A., King E.R., Dai X.M., Leone G., Stanley E.R., Lasorella A., King E.R., Dai X.M., Leone G., Stanley E.R., Lasorella A., Dai X.M., Leone G., Stanley E.R., Lasorella A., Leone G., Stanley E.R., Lasorella A., Stanley E.R., Lasorella A., Lasorella A. Retinoblastoma promotes definitive erythropoiesis by repressing Id2 in fetal liver macrophages. Nature. 2004;432:1040–1045. doi: 10.1038/nature03068. [DOI] [PubMed] [Google Scholar]
- Ibanez L. The ITK software guide. Kitware; Inc., Clifton Park, NY: 2003. [Google Scholar]
- Jacks T., Fazeli A., Schmitt E.M., Bronson R.T., Goodell M.A., Weinberg R.A., Fazeli A., Schmitt E.M., Bronson R.T., Goodell M.A., Weinberg R.A., Schmitt E.M., Bronson R.T., Goodell M.A., Weinberg R.A., Bronson R.T., Goodell M.A., Weinberg R.A., Goodell M.A., Weinberg R.A., Weinberg R.A. Effects of an Rb mutation in the mouse. Nature. 1992;359:295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- James R.M., Klerkx A.H., Keighren M., Flockhart J.H., West J.D., Klerkx A.H., Keighren M., Flockhart J.H., West J.D., Keighren M., Flockhart J.H., West J.D., Flockhart J.H., West J.D., West J.D. Restricted distribution of tetraploid cells in mouse tetraploid <=> diploid chimaeras. Dev. Biol. 1995;167:213–226. doi: 10.1006/dbio.1995.1018. [DOI] [PubMed] [Google Scholar]
- Kamat A. A 500-bp region, approximately ≈40 kb upstream of the human CYP19 (aromatase) gene, mediates placenta-specific expression in transgenic mice. Proc. Natl. Acad. Sci. 1999;96:4575–4580. doi: 10.1073/pnas.96.8.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat A., Smith M.E., Shelton J.M., Richardson J.A., Mendelson C.R., Smith M.E., Shelton J.M., Richardson J.A., Mendelson C.R., Shelton J.M., Richardson J.A., Mendelson C.R., Richardson J.A., Mendelson C.R., Mendelson C.R. Genomic regions that mediate placental cell-specific and developmental regulation of human Cyp19 (aromatase) gene expression in transgenic mice. Endocrinology. 2005;146:2481–2488. doi: 10.1210/en.2004-1606. [DOI] [PubMed] [Google Scholar]
- Lasorella A., Noseda M., Beyna M., Yokota Y., Iavarone A., Noseda M., Beyna M., Yokota Y., Iavarone A., Beyna M., Yokota Y., Iavarone A., Yokota Y., Iavarone A., Iavarone A. Id2 is a retinoblastoma protein target and mediates signalling by Myc oncoproteins. Nature. 2000;407:592–598. doi: 10.1038/35036504. [DOI] [PubMed] [Google Scholar]
- LeCouter J.E., Kablar B., Hardy W.R., Ying C., Megeney L.A., May L.L., Rudnicki M.A., Kablar B., Hardy W.R., Ying C., Megeney L.A., May L.L., Rudnicki M.A., Hardy W.R., Ying C., Megeney L.A., May L.L., Rudnicki M.A., Ying C., Megeney L.A., May L.L., Rudnicki M.A., Megeney L.A., May L.L., Rudnicki M.A., May L.L., Rudnicki M.A., Rudnicki M.A. Strain-dependent myeloid hyperplasia, growth deficiency, and accelerated cell cycle in mice lacking the Rb-related p107 gene. Mol. Cell. Biol. 1998a;18:7455–7465. doi: 10.1128/mcb.18.12.7455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeCouter J.E., Kablar B., Whyte P.F., Ying C., Rudnicki M., Kablar B., Whyte P.F., Ying C., Rudnicki M., Whyte P.F., Ying C., Rudnicki M., Ying C., Rudnicki M., Rudnicki M. Strain-dependent embryonic lethality in mice lacking the retinoblastoma-related p130 gene. Development. 1998b;125:4669–4679. doi: 10.1242/dev.125.23.4669. [DOI] [PubMed] [Google Scholar]
- Lee E.Y., Chang C.Y., Hu N., Wang Y.C., Lai C.C., Herrup K., Lee W.H., Bradley A., Chang C.Y., Hu N., Wang Y.C., Lai C.C., Herrup K., Lee W.H., Bradley A., Hu N., Wang Y.C., Lai C.C., Herrup K., Lee W.H., Bradley A., Wang Y.C., Lai C.C., Herrup K., Lee W.H., Bradley A., Lai C.C., Herrup K., Lee W.H., Bradley A., Herrup K., Lee W.H., Bradley A., Lee W.H., Bradley A., Bradley A. Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature. 1992;359:288–294. doi: 10.1038/359288a0. [DOI] [PubMed] [Google Scholar]
- Lee M.H., Williams B.O., Mulligan G., Mukai S., Bronson R.T., Dyson N., Harlow E., Jacks T., Williams B.O., Mulligan G., Mukai S., Bronson R.T., Dyson N., Harlow E., Jacks T., Mulligan G., Mukai S., Bronson R.T., Dyson N., Harlow E., Jacks T., Mukai S., Bronson R.T., Dyson N., Harlow E., Jacks T., Bronson R.T., Dyson N., Harlow E., Jacks T., Dyson N., Harlow E., Jacks T., Harlow E., Jacks T., Jacks T. Targeted disruption of p107: Functional overlap between p107 and Rb. Genes & Dev. 1996;10:1621–1632. doi: 10.1101/gad.10.13.1621. [DOI] [PubMed] [Google Scholar]
- Lees E., Faha B., Dulic V., Reed S.I., Harlow E., Faha B., Dulic V., Reed S.I., Harlow E., Dulic V., Reed S.I., Harlow E., Reed S.I., Harlow E., Harlow E. Cyclin E/cdk2 and cyclin A/cdk2 kinases associate with p107 and E2F in a temporally distinct manner. Genes & Dev. 1992;6:1874–1885. doi: 10.1101/gad.6.10.1874. [DOI] [PubMed] [Google Scholar]
- Li Y., Graham C., Lacy S., Duncan A.M., Whyte P., Graham C., Lacy S., Duncan A.M., Whyte P., Lacy S., Duncan A.M., Whyte P., Duncan A.M., Whyte P., Whyte P. The adenovirus E1A-associated 130-kD protein is encoded by a member of the retinoblastoma gene family and physically interacts with cyclins A and E. Genes & Dev. 1993;7:2366–2377. doi: 10.1101/gad.7.12a.2366. [DOI] [PubMed] [Google Scholar]
- Lipinski M.M., Jacks T., Jacks T. The retinoblastoma gene family in differentiation and development. Oncogene. 1999;18:7873–7882. doi: 10.1038/sj.onc.1203244. [DOI] [PubMed] [Google Scholar]
- Lipinski M.M., Macleod K.F., Williams B.O., Mullaney T.L., Crowley D., Jacks T., Macleod K.F., Williams B.O., Mullaney T.L., Crowley D., Jacks T., Williams B.O., Mullaney T.L., Crowley D., Jacks T., Mullaney T.L., Crowley D., Jacks T., Crowley D., Jacks T., Jacks T. Cell-autonomous and non-cell-autonomous functions of the Rb tumor suppressor in developing central nervous system. EMBO J. 2001;20:3402–3413. doi: 10.1093/emboj/20.13.3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maandag E.C., van der Valk M., Vlaar M., Feltkamp C., O’Brien J., van Roon M., van der Lugt N., Berns A., te Riele H., van der Valk M., Vlaar M., Feltkamp C., O’Brien J., van Roon M., van der Lugt N., Berns A., te Riele H., Vlaar M., Feltkamp C., O’Brien J., van Roon M., van der Lugt N., Berns A., te Riele H., Feltkamp C., O’Brien J., van Roon M., van der Lugt N., Berns A., te Riele H., O’Brien J., van Roon M., van der Lugt N., Berns A., te Riele H., van Roon M., van der Lugt N., Berns A., te Riele H., van der Lugt N., Berns A., te Riele H., Berns A., te Riele H., te Riele H. Developmental rescue of an embryonic-lethal mutation in the retinoblastoma gene in chimeric mice. EMBO J. 1994;13:4260–4268. doi: 10.1002/j.1460-2075.1994.tb06746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson D., Sage J., Crowley D., Trumpp A., Bronson R.T., Jacks T., Sage J., Crowley D., Trumpp A., Bronson R.T., Jacks T., Crowley D., Trumpp A., Bronson R.T., Jacks T., Trumpp A., Bronson R.T., Jacks T., Bronson R.T., Jacks T., Jacks T. Conditional mutation of Rb causes cell cycle defects without apoptosis in the central nervous system. Mol. Cell. Biol. 2003;23:1044–1053. doi: 10.1128/MCB.23.3.1044-1053.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes F., Collignon A., Vandermeulen D., Marchal G., Suetens P., Collignon A., Vandermeulen D., Marchal G., Suetens P., Vandermeulen D., Marchal G., Suetens P., Marchal G., Suetens P., Suetens P. Multimodality image registration by maximization of mutual information. IEEE Trans. Med. Imaging. 1997;16:187–198. doi: 10.1109/42.563664. [DOI] [PubMed] [Google Scholar]
- Marino S., Vooijs M., van Der Gulden H., Jonkers J., Berns A., Vooijs M., van Der Gulden H., Jonkers J., Berns A., van Der Gulden H., Jonkers J., Berns A., Jonkers J., Berns A., Berns A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes & Dev. 2000;14:994–1004. [PMC free article] [PubMed] [Google Scholar]
- Morris E.J., Dyson N.J., Dyson N.J. Retinoblastoma protein partners. Adv. Cancer Res. 2001;82:1–54. doi: 10.1016/s0065-230x(01)82001-7. [DOI] [PubMed] [Google Scholar]
- Mosaliganti K., Pan T., Sharp R., Ridgway R., Iyengar S., Gulacy A., Wenzel P., de Bruin A., Machiraju R., Huang K., Pan T., Sharp R., Ridgway R., Iyengar S., Gulacy A., Wenzel P., de Bruin A., Machiraju R., Huang K., Sharp R., Ridgway R., Iyengar S., Gulacy A., Wenzel P., de Bruin A., Machiraju R., Huang K., Ridgway R., Iyengar S., Gulacy A., Wenzel P., de Bruin A., Machiraju R., Huang K., Iyengar S., Gulacy A., Wenzel P., de Bruin A., Machiraju R., Huang K., Gulacy A., Wenzel P., de Bruin A., Machiraju R., Huang K., Wenzel P., de Bruin A., Machiraju R., Huang K., de Bruin A., Machiraju R., Huang K., Machiraju R., Huang K., Huang K., et al. Registration and 3D visualization of large microscopy images. In: Reinhardt J.M., et al., editors. Medical imaging 2006: Image processing. Proceedings of the SPIE. Vol. 6144. 2006. pp. 923–934. [Google Scholar]
- Mulligan G., Jacks T., Jacks T. The retinoblastoma gene family: Cousins with overlapping interests. Trends Genet. 1998;14:223–229. doi: 10.1016/s0168-9525(98)01470-x. [DOI] [PubMed] [Google Scholar]
- Robanus-Maandag E., Dekker M., van der Valk M., Carrozza M.L., Jeanny J.C., Dannenberg J.H., Berns A., te Riele H., Dekker M., van der Valk M., Carrozza M.L., Jeanny J.C., Dannenberg J.H., Berns A., te Riele H., van der Valk M., Carrozza M.L., Jeanny J.C., Dannenberg J.H., Berns A., te Riele H., Carrozza M.L., Jeanny J.C., Dannenberg J.H., Berns A., te Riele H., Jeanny J.C., Dannenberg J.H., Berns A., te Riele H., Dannenberg J.H., Berns A., te Riele H., Berns A., te Riele H., te Riele H. p107 is a suppressor of retinoblastoma development in pRb-deficient mice. Genes & Dev. 1998;12:1599–1609. doi: 10.1101/gad.12.11.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra H.I., Wu L., de Bruin A., Timmers C., Rosol T.J., Weinstein M., Robinson M.L., Leone G., Wu L., de Bruin A., Timmers C., Rosol T.J., Weinstein M., Robinson M.L., Leone G., de Bruin A., Timmers C., Rosol T.J., Weinstein M., Robinson M.L., Leone G., Timmers C., Rosol T.J., Weinstein M., Robinson M.L., Leone G., Rosol T.J., Weinstein M., Robinson M.L., Leone G., Weinstein M., Robinson M.L., Leone G., Robinson M.L., Leone G., Leone G. Specificity of E2F1, E2F2 and E2F3 in mediating Rb function. Cell Growth Differ. 2002;13:215–225. [PubMed] [Google Scholar]
- Sapin V., Blanchon L., Serre A.F., Lemery D., Dastugue B., Ward S.J., Blanchon L., Serre A.F., Lemery D., Dastugue B., Ward S.J., Serre A.F., Lemery D., Dastugue B., Ward S.J., Lemery D., Dastugue B., Ward S.J., Dastugue B., Ward S.J., Ward S.J. Use of transgenic mice model for understanding the placentation: Towards clinical applications in human obstetrical pathologies? Transgenic Res. 2001;10:377–398. doi: 10.1023/a:1012085713898. [DOI] [PubMed] [Google Scholar]
- Sharp R., Ridgway R., Mosaliganti K., Wenzel P., Pan T., de Bruin A., Machiraju R., Huang K., Leone G., Saltz J., Ridgway R., Mosaliganti K., Wenzel P., Pan T., de Bruin A., Machiraju R., Huang K., Leone G., Saltz J., Mosaliganti K., Wenzel P., Pan T., de Bruin A., Machiraju R., Huang K., Leone G., Saltz J., Wenzel P., Pan T., de Bruin A., Machiraju R., Huang K., Leone G., Saltz J., Pan T., de Bruin A., Machiraju R., Huang K., Leone G., Saltz J., de Bruin A., Machiraju R., Huang K., Leone G., Saltz J., Machiraju R., Huang K., Leone G., Saltz J., Huang K., Leone G., Saltz J., Leone G., Saltz J., Saltz J. Volume rendering phenotype differences in microscopy data. Journal of Computing in Science and Engineering. 2007 (in press). [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Spike B.T., Dirlam A., Dibling B.C., Marvin J., Williams B.O., Jacks T., Macleod K.F., Dirlam A., Dibling B.C., Marvin J., Williams B.O., Jacks T., Macleod K.F., Dibling B.C., Marvin J., Williams B.O., Jacks T., Macleod K.F., Marvin J., Williams B.O., Jacks T., Macleod K.F., Williams B.O., Jacks T., Macleod K.F., Jacks T., Macleod K.F., Macleod K.F. The Rb tumor suppressor is required for stress erythropoiesis. EMBO J. 2004;23:4319–4329. doi: 10.1038/sj.emboj.7600432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds H., Krall L., Remington L., Saenz-Robles M., Lowe S., Jacks T., Van Dyke T., Krall L., Remington L., Saenz-Robles M., Lowe S., Jacks T., Van Dyke T., Remington L., Saenz-Robles M., Lowe S., Jacks T., Van Dyke T., Saenz-Robles M., Lowe S., Jacks T., Van Dyke T., Lowe S., Jacks T., Van Dyke T., Jacks T., Van Dyke T., Van Dyke T. p53-dependent apoptosis suppresses tumor growth and progression in vivo. Cell. 1994;78:703–711. doi: 10.1016/0092-8674(94)90534-7. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Kunath T., Hadjantonakis A.K., Nagy A., Rossant J., Kunath T., Hadjantonakis A.K., Nagy A., Rossant J., Hadjantonakis A.K., Nagy A., Rossant J., Nagy A., Rossant J., Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282:2072–2075. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
- Trimarchi J.M., Lees J.A., Lees J.A. Sibling rivalry in the E2F family. Mol. Cell. Biol. 2001;3:11–20. doi: 10.1038/nrm714. [DOI] [PubMed] [Google Scholar]
- Tsai K.Y., Hu Y., Macleod K.F., Crowley D., Yamasaki L., Jacks T., Hu Y., Macleod K.F., Crowley D., Yamasaki L., Jacks T., Macleod K.F., Crowley D., Yamasaki L., Jacks T., Crowley D., Yamasaki L., Jacks T., Yamasaki L., Jacks T., Jacks T. Mutation of E2f-1 suppresses apoptosis and inappropriate S phase entry and extends survival of Rb-deficient mouse embryos. Mol. Cell. 1998;2:293–304. doi: 10.1016/s1097-2765(00)80274-9. [DOI] [PubMed] [Google Scholar]
- Uy G.D., Downs K.M., Gardner R.L., Downs K.M., Gardner R.L., Gardner R.L. Inhibition of trophoblast stem cell potential in chorionic ectoderm coincides with occlusion of the ectoplacental cavity in the mouse. Development. 2002;129:3913–3924. doi: 10.1242/dev.129.16.3913. [DOI] [PubMed] [Google Scholar]
- Vanderluit J.L., Ferguson K.L., Nikoletopoulou V., Parker M., Ruzhynsky V., Alexson T., McNamara S.M., Park D.S., Rudnicki M., Slack R.S., Ferguson K.L., Nikoletopoulou V., Parker M., Ruzhynsky V., Alexson T., McNamara S.M., Park D.S., Rudnicki M., Slack R.S., Nikoletopoulou V., Parker M., Ruzhynsky V., Alexson T., McNamara S.M., Park D.S., Rudnicki M., Slack R.S., Parker M., Ruzhynsky V., Alexson T., McNamara S.M., Park D.S., Rudnicki M., Slack R.S., Ruzhynsky V., Alexson T., McNamara S.M., Park D.S., Rudnicki M., Slack R.S., Alexson T., McNamara S.M., Park D.S., Rudnicki M., Slack R.S., McNamara S.M., Park D.S., Rudnicki M., Slack R.S., Park D.S., Rudnicki M., Slack R.S., Rudnicki M., Slack R.S., Slack R.S. p107 regulates neural precursor cells in the mammalian brain. J. Cell Biol. 2004;166:853–863. doi: 10.1083/jcb.200403156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.Q., Kiefer F., Urbanek P., Wagner E.F., Kiefer F., Urbanek P., Wagner E.F., Urbanek P., Wagner E.F., Wagner E.F. Generation of completely embryonic stem cell-derived mutant mice using tetraploid blastocyst injection. Mech. Dev. 1997;62:137–145. doi: 10.1016/s0925-4773(97)00655-2. [DOI] [PubMed] [Google Scholar]
- Weinberg R.A. The molecular basis of oncogenes and tumor suppressor genes. Ann. N. Y. Acad. Sci. 1995;758:331–338. doi: 10.1111/j.1749-6632.1995.tb24838.x. [DOI] [PubMed] [Google Scholar]
- Wildwater M., Campilho A., Perez-Perez J.M., Heidstra R., Blilou I., Korthout H., Chatterjee J., Mariconti L., Gruissem W., Scheres B., Campilho A., Perez-Perez J.M., Heidstra R., Blilou I., Korthout H., Chatterjee J., Mariconti L., Gruissem W., Scheres B., Perez-Perez J.M., Heidstra R., Blilou I., Korthout H., Chatterjee J., Mariconti L., Gruissem W., Scheres B., Heidstra R., Blilou I., Korthout H., Chatterjee J., Mariconti L., Gruissem W., Scheres B., Blilou I., Korthout H., Chatterjee J., Mariconti L., Gruissem W., Scheres B., Korthout H., Chatterjee J., Mariconti L., Gruissem W., Scheres B., Chatterjee J., Mariconti L., Gruissem W., Scheres B., Mariconti L., Gruissem W., Scheres B., Gruissem W., Scheres B., Scheres B. The RETINOBLASTOMA-RELATED gene regulates stem cell maintenance in Arabidopsis roots. Cell. 2005;123:1337–1349. doi: 10.1016/j.cell.2005.09.042. [DOI] [PubMed] [Google Scholar]
- Williams B.O., Schmitt E.M., Remington L., Bronson R.T., Albert D.M., Weinberg R.A., Jacks T., Schmitt E.M., Remington L., Bronson R.T., Albert D.M., Weinberg R.A., Jacks T., Remington L., Bronson R.T., Albert D.M., Weinberg R.A., Jacks T., Bronson R.T., Albert D.M., Weinberg R.A., Jacks T., Albert D.M., Weinberg R.A., Jacks T., Weinberg R.A., Jacks T., Jacks T. Extensive contribution of Rb-deficient cells to adult chimeric mice with limited histopathological consequences. EMBO J. 1994;13:4251–4259. doi: 10.1002/j.1460-2075.1994.tb06745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., de Bruin A., Saavedra H.I., Starovic M., Trimboli A., Yang Y., Opavska J., Wilson P., Thompson J.C., Ostrowski M.C., de Bruin A., Saavedra H.I., Starovic M., Trimboli A., Yang Y., Opavska J., Wilson P., Thompson J.C., Ostrowski M.C., Saavedra H.I., Starovic M., Trimboli A., Yang Y., Opavska J., Wilson P., Thompson J.C., Ostrowski M.C., Starovic M., Trimboli A., Yang Y., Opavska J., Wilson P., Thompson J.C., Ostrowski M.C., Trimboli A., Yang Y., Opavska J., Wilson P., Thompson J.C., Ostrowski M.C., Yang Y., Opavska J., Wilson P., Thompson J.C., Ostrowski M.C., Opavska J., Wilson P., Thompson J.C., Ostrowski M.C., Wilson P., Thompson J.C., Ostrowski M.C., Thompson J.C., Ostrowski M.C., Ostrowski M.C., et al. Extra-embryonic function of Rb is essential for embryonic development and viability. Nature. 2003;421:942–947. doi: 10.1038/nature01417. [DOI] [PubMed] [Google Scholar]
- Yamasaki L., Bronson R., Williams B.O., Dyson N.J., Harlow E., Jacks T., Bronson R., Williams B.O., Dyson N.J., Harlow E., Jacks T., Williams B.O., Dyson N.J., Harlow E., Jacks T., Dyson N.J., Harlow E., Jacks T., Harlow E., Jacks T., Jacks T. Loss of E2F-1 reduces tumorigenesis and extends the lifespan of Rb1+/− mice. Nat. Genet. 1998;18:360–364. doi: 10.1038/ng0498-360. [DOI] [PubMed] [Google Scholar]
- Ziebold U., Reza T., Caron A., Lees J.A., Reza T., Caron A., Lees J.A., Caron A., Lees J.A., Lees J.A. E2F3 contributes both to the inappropriate proliferation and to the apoptosis arising in Rb mutant embryos. Genes & Dev. 2001;15:386–391. doi: 10.1101/gad.858801. [DOI] [PMC free article] [PubMed] [Google Scholar]