Toll-like receptor (TLR) expression and TLR-mediated cytokine/chemokine production by human uterine epithelial cells (original) (raw)
Abstract
The objective of this study was to examine the expression of toll-like receptors (TLRs) by the uterine epithelial cell line ECC-1 and to determine if stimulation of the expressed TLRs induces changes in cytokine and/or chemokine secretion. The expression of TLR1 to TLR9 by ECC-1 cells was demonstrated by reverse transcription polymerase chain reaction, with only TLR10 not being expressed. Stimulation of ECC-1 cells using agonists to TLR2, TLR4 and TLR5 induced the expression of the chemokines interleukin-8 (IL-8) and monocyte chemotactic protein-1 (MCP-1), as well as the pro-inflammatory cytokine IL-6, and occurred in a dose-dependent manner. In response to zymosan and flagellin, pathogen-associated molecular patterns (PAMP) that are recognized by TLR2 and TLR5 respectively, ECC-1 cells secreted significantly more IL-8, MCP-1 and IL-6 than in response to other TLR agonists. In contrast, agonists to TLR3, TLR7, and TLR9 had no effect on the secretion of the 13 cytokines or chemokines analysed. These results indicate that uterine epithelial cells are important sentinels of the innate immune system. Further it indicates that all but one of the known TLRs are expressed by ECC-1 cells and that stimulation through specific TLRs mediates changes in the expression of key chemokines and pro-inflammatory cytokines that aid in the defence of the uterus against potential pathogens.
Keywords: chemokine, cytokine, epithelial, TLR, uterus
Introduction
The human uterine endometrium has evolved to orchestrate many essential functions for the host, ranging from fertilization, implantation and pregnancy to defence from sexually transmitted diseases and other invading pathogens. In the endometrium, the cells responsible for the first line of defence are probably the epithelial cells that line the mucosal surface. Known to be an efficient physical barrier to infection, epithelial cells have evolved innate immune antimicrobial functions as well as the ability to modulate the recruitment and activity of immune cells of both the innate and adaptive immune systems.1 For the purpose of innate immune defence and activation of the adaptive immune response, endometrial epithelial cells secrete antimicrobial factors, such as secretory leucocyte protease inhibitor and β-defensins, that eliminate potential pathogens.2–4 By secreting chemokines such as interleukin-8 (IL-8), monocyte chemoattractant protein (MCP-1), and RANTES (regulated upon activation, normal, T-cell- expressed and secreted), epithelial cells recruit immune cells to the sites of infection.5–7 In addition, epithelial cells secrete cytokines such as IL-6 and IL-1β to activate and regulate the inflammatory and immune responses of both the innate and adaptive immune systems.8,9
The innate immune system, which has evolved to protect the host at the onset of infectious microbial challenge, relies on germ-line-encoded pattern recognition receptors that recognize conserved pathogen-associated molecular patterns (PAMP) synthesized by micro-organisms but not by the host. One group of receptors that recognize PAMP are the toll-like receptors (TLRs), which are expressed on effector cells including T and B lymphocytes, dendritic cells, macrophages and epithelial cells. At least 10 TLRs have been identified, each having specificity to various bacterial, fungal and viral products. These pathogen-related products are often highly conserved structures that are critical for microbial survival. Recognition of PAMP by TLRs ensures that pathogens with these conserved structures, despite their mutational potential, rarely escape surveillance by the innate immune system.10 TLR2 recognizes Gram-positive bacteria, mycobacteria, the haemagglutinin protein of wild-type measles virus, and a broad range of microbial components, including zymosan, peptidoglycan, lipopolysaccharide (LPS), and Pam3Cys-Ser-(Lys)4 (pam3cys).11–15 Recognition of these microbial components by TLR2 is achieved at least in part by heterodimerization with TLR6 or TLR1.16 TLR3 recognizes double-stranded RNA, and more recently has been shown to be stimulated when exposed to messenger RNA (mRNA), potentially from cells killed by an invading pathogen.17,18 TLR4, in association with a co-receptor CD14 and the extracellular accessory molecule MD-2, recognizes the major component of the outer membrane of Gram-negative bacteria.19–21 TLR5 recognizes a highly conserved structure specific to bacterial flagellin. Required for flagellar protofilament assembly and bacterial motility, flagellin is unlikely to mutate under selective pressures.22,23 TLR7 and TLR8 have not been shown to recognize a naturally occurring microbial product, although small antiviral molecules, such as imiquimod and resiquimod, stimulate cells through TLR7 and TLR8.24,25 Similarly, a guanosine analogue, loxoribine, is known to stimulate through TLR7.26 TLR9 binds unmethylated DNA found in bacteria.27 More recently, TLR10 mRNA was identified as being expressed in lymphoid tissues such as spleen, lymph node, thymus, and tonsil, but a ligand has yet to be demonstrated.28
Recognition of microbial products by TLRs activates not only innate immunity but also adaptive immunity. Ligation of TLRs (TLR2, TLR3 and TLR4) by PAMP agonists results in the activation of NF-κb and JNK and p38 MAP-kinase, leading to the expression of pro-inflammatory cytokines such as tumour necrosis factor-α (TNF-α), IL-1 and IL-6.10 Type I interferon (IFN-α and IFN-β) is induced through certain TLRs (TLR3 and TLR4) in response to bacterial and viral products.29,30 Furthermore, TLR recognition of PAMP induces the expression of cytokines such as IL-10 and IL-12, that are essential for the adaptive immune response, as well as those required for antigen presentation, such as major histocompatibility complex class II, CD80 and CD86.31 Furthermore, TLR signalling in dendritic cells is known to be critical in determining the T helper type 1 (Th1)/Th2 balance.32 TLR stimulation also plays an important role in macrophage responsiveness by up-regulating inducible NO synthase and inducing macrophage apoptosis, of which the latter suggests the involvement of TLRs in infection-induced cell death.33,34
While little has been done to identify TLR expression on epithelial cells of the endometrium, epithelial cells at other anatomical sites are known to express TLRs. For example, airway epithelial cells express TLR1 to TLR10, intestinal epithelial cells express TLR1 to TLR4, TLR6 and TLR9 and gastric epithelial cells express TLR2, TLR4 and TLR5.35–39 Within the female reproductive tract, vaginal and cervical epithelial cell lines have been reported to express TLR1, TLR2, TLR3, TLR5 and TLR6, while primary endocervical epithelial cells expressed TLR1, TLR2, TLR3 and TLR6.40 Others have shown that stimulation of epithelial TLRs induces expression of various immunologically relevant molecules. For example, exposure of bacterial lipoprotein to airway and lung epithelial cells induces the expression of β-defensin-2 via TLR2.41,42 Gastric epithelial cells express macrophage inflammatory protein (MIP-3α), IL-8, and growth regulated oncogene alpha (GROα) when stimulated via TLR2 and to a lesser extent TLR5.39 Human intestinal epithelial cells increase expression of IL-6 and IL-8 upon exposure to CpG and LPS, which act via TLR9 and TLR4, respectively.36,43 Finally, endocervical epithelial cells express IL-6 and IL-8 via TLR2 when exposed to Neisseria gonorrheoa lipoprotein.44
Despite the crucial role played by epithelial cells in protecting the human uterine endometrium, little is known about the innate mechanisms of epithelial cell activation by pathogens and the receptors that mediate and regulate these responses. To identify the TLRs expressed and to study epithelial–microbial interactions that occur in the endometrium, human endometrium epithelial cell line ECC-1 was used; this cell line was generated from an adenocarcinoma of human endometrium, EnCa101.45 Based on morphology, the presence of epithelial antigens, and responsiveness to female sex steroid hormones, the ECC-1 cell line is considered a unique well-differentiated human uterine epithelial cell line. Given that epithelial cells are the first cells to encounter potential pathogens, our goal in these studies was to test the hypothesis that ECC-1 cells express TLR and that stimulation of TLR by specific PAMP may induce the secretion of cytokines and/or chemokines that would initiate an inflammatory response and the recruitment of immune cells to the site of infection. The goals of this study were to identify the TLRs expressed on ECC-1 cells and determine if stimulation through any of the expressed TLRs results in the secretion of cytokines and/or chemokines.
Materials and methods
Reverse transcription polymerase chain reaction (RT-PCR) analysis
Total RNA was isolated from cells using TRIzol Reagent according to the manufacturer's recommendations (Invitrogen, Carlsbad, CA) and treated with DNA-free (Ambion, Austin, TX). Two micrograms of total RNA was reverse transcribed using the Superscript First-Strand Synthesis System for RT-PCR according to the manufacturer's recommendations (Invitrogen, Austin, TX). PCR amplification was performed using Platinum PCR Supermix (Invitrogen, Austin, TX). PCR amplification was performed on the PTC-100 Thermal Cycler (MJ Research, Waltham MA) for 35 cycles using the following cycling conditions: 94° for 1 min, followed by 35 cycles of 94° for 30 seconds, 55° for 30 seconds, 72° for 1 min, and then a final extension of 72° for 2 min Forward and reverse primer pairs are listed in Table 1. Lack of DNA contamination in the RNA preparations was verified by PCR amplification in the absence of reverse transcription. Using tissue biopsies from various anatomical sites within the female reproductive tract it was verified that all TLR primer pairs used in this study would amplify their respective TLR if expressed.
Table 1.
PCR primer pairs for the amplification of human TLR mRNA
| Product | Forward primer | Reverse primer | Amplicon size (bp) |
|---|---|---|---|
| TLR1 | GGTCTTGCTGGTCTTAGGAGAGAC | CTGAAGTCCAGCTGACCCTGTAGCTTCACG | 372 |
| TLR2 | GGCCAGCAAATTACCTGTGTG | CTGAGCCTCGTCCATGGGCCACTCC | 637 |
| TLR3 | CGGGCCAGCTTTCAGGAACCTG | GGCATGAATTATATATGCTGC | 400 |
| TLR4 | TGCAATGGATCAAGGACCAGAGGC | GTGCTGGGACACCACAACAATCACC | 449 |
| TLR5 | CCTCATGACCATCCTCACAGTCAC | GGCTTCAAGGCACCAGCCATCTC | 355 |
| TLR6 | CCAAGTGAACATATCAGTTAATACTTTAGGGTGC | CTCAGAAAACACGGTGTACAAAGCTG | 358 |
| TLR7 | CTCCCTGGATCTGTACACCTGTGAG | CTCCCACAGAGCCTTTTCCGGAGCT | 551 |
| TLR8 | GTCCTGGGGATCAAAGAGGGAAGAG | CTCTTACAGATCCGCTGCCGTAGCC | 581 |
| TLR9 | GCGAGATGAGGATGCCCTGCCCTACG | TTCGGCCGTGGGTCCCTGGCAGAAG | 510 |
| TLR10 | CAGAGGTCATGATGGTTGGATGG | GACCTAGCATCCTGAGATACCAGGGCAG | 256 |
Cell culture and reagents
To establish a cell culture system of polarized human uterine epithelial cells with both apical and basolateral compartments, the human uterine epithelial cell line ECC-1 (originally established by Dr Pondichery Satyaswaroop and kindly provided by Dr George Olt, Penn State College of Medicine, Milton S. Hershey Medical Center, PA) was cultured in Falcon cell culture inserts in 24-well culture dishes designed for these cell inserts (Fisher Scientific, Pittsburgh, PA). For these experiments, apical and basolateral compartments had 300 and 850 μl complete medium, respectively. The medium was changed every 2 days. Complete medium was supplemented with 20 mm HEPES, 50 U/ml penicillin, 50 mg/ml streptomycin, 2 mm l-glutamine (all from Life Technologies) and 10% heat-inactivated defined fetal bovine serum (Hyclone, Logan, UT) and did not contain phenol red. Following 24-hr incubation with various PAMPs, ECC-1 apical and basolateral conditioned media were centrifuged for 5 min at 10 000 g. Ultra-pure lipopolysaccharide from Salmonella minnesota (List Biological Laboratories, Inc, Campbell, CA) was used at a final concentration of 1 μg/ml; Pam3cys (EMC Microcollections, Tübingen, Germany), 1 μg/ml; poly(I:C) (Invivogen, San Diego, CA), 25 μg/ml; flagellin from Escherichia coli (Inotek Pharmaceuticals, Beverly, MA), 100 ng/ml; Zymosan from Saccharomyces cerevisiae (Invivogen), 10 μg/ml; peptidoglycan from Staphylococcus aureus (Invivogen), 10 μg/ml: CpG oligonucleotide (Invivogen), 1 μm; loxoribine (Invivogen), 100 μm.
Measurement of transepithelial resistance
As an indicator of tight junction formation of epithelial cell monolayers, transepithelial resistance (TER) was periodically assessed using an EVOM electrode and Voltohmmeter (World Precision Instruments, Inc., Sarasota, FL), as described previously.46
Multiplex cytokine assays
Cytokines were measured using Bio-Plex human cytokine multiplex kits (Bio-Rad, Hercules, CA). Calibration curves from recombinant cytokine standards were prepared with serial dilutions in the same media as the culture supernatants (RPMI-1640 medium containing 10% fetal bovine serum). High and low reference points were included to determine cytokine recovery. Standards and reference points were measured in triplicate, each sample was measured once, and blank values were subtracted from all readings. All assays were carried out directly in a 96-well filtration plate (Millipore, Billerica, MA) at room temperature and protected from light. Briefly, wells were pre-wetted with 100 μl phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA), then beads (5000 beads per cytokine) together with a either a standard, sample, reference point, or blank were added in a final volume of 100 μl, and incubated together at room temperature for 30 min with continuous shaking. Beads were washed three times with 100 μl PBS containing 1% BSA and 0·05% Tween-20. A cocktail of biotinylated antibodies (50 μl/well) was added to the beads for a further 30-min incubation with continuous shaking. Beads were washed three times, then streptavidin-phycoerythrin was added for 10 min Beads were again washed three times and resuspended in 125 μl of PBS containing 1% BSA and 0·05% Tween-20. The fluorescence intensity of the beads was measured using the Bio-Plex array reader. bio-plex manager software with five-parametric-curve fitting (Bio-Rad technical note 2861 at http://www.bio-rad.com) was used for data analysis. The levels of detection for IL-8, granulocyte–macrophage colony-stimulating factor (GM-CSF), MCP-1, and IL-6 were 0·8 pg/well, 2·0 pg/well, 1·6 pg/well and 0·6 pg/well, respectively.
Measurement of IL-8, MCP-1 and IL-6
Concentrations of IL-8, MCP-1 and IL-6 in the apical and basolateral supernatants from polarized ECC-1 uterine epithelial cells were each determined with an enzyme-linked immunosorbent assay (ELISA) test kit (R & D Systems, Minneapolis, MN) according to the manufacturer's protocol. The IL-8, MCP-1 and IL-6 ELISAs had minimum detection levels of 0·8 pg/well, 0·8 pg/well and 0·1 pg/well, respectively. Calculations of IL-8, MCP-1 and IL-6 were determined from a standard curve after optical density measurements at 450 nm on an ELISA reader (Dynex).
Statistics
The data are presented as the mean ± standard error of the mean. One-way analysis of variance with Dunnett's post test was performed using graphpad instat version 3·0a (GraphPad Software, San Diego, CA). When an analysis of variance indicated that significant differences existed among means, preplanned paired comparisons were made using the Dunnett's post test method to adjust _P_-values. A _P_-value of less than 0·05 was taken as indicative of statistical significance.
Results
Toll-like receptor mRNA expression in the human uterine epithelial cell line ECC-1
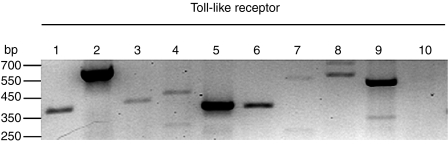
The human uterine epithelial cell line ECC-1 was evaluated for expression of mRNA for the various TLRs by RT-PCR. ECC-1 cells were grown to confluence on cell inserts and shown to have formed tight junctions as indicated by TER > 900 ohms/well or greater, control wells had TER of 130–150 ohms/well. PCR primers are listed in Table 1. As seen in Fig. 1, ECC-1 cells express mRNA for TLR1–TLR9, while expression of TLR10 mRNA was not observed.
Figure 1.
Toll-like receptor expression in uterine ECC-1 epithelial cells by RT-PCR. Lanes 1–10 correspond to TLR1–TLR10, respectively. Total RNA was isolated from ECC-1 cells and examined by RT-PCR for TLR mRNA expression.
Analysis of cytokines in apical secretions of ECC-1 cells treated with ligands of TLRs using the Luminex multiplex system
Having demonstrated that ECC-1 cells express mRNA for TLR1–TLR9, we sought to determine which, if any, TLRs were functional and able to play a role in initiating innate immune responses by uterine epithelial cells. PAMP used in these experiments for binding to TLR2, in possible co-operation with TLR1 and/or TLR6, were peptidoglycan (Staphylococcus aureus), pam3cys, and zymosan (Saccharomyces cerevisiae); TLR3, poly(I:C); TLR4, LPS (Salmonella minnesota); TLR5, flagellin (Escherichia coli); TLR7, loxoribine; and TLR9, CpG. Following a 24-hr PAMP-exposure, conditioned apical medium was collected from ECC-1 cells (five inserts per treatment), pooled, and screened for the expression of several cytokines and chemokines using the Luminex multiplex system. Of the eight PAMPs examined, only flagellin and zymosan displayed an effect on the secretion of cytokines by ECC-1 cells. Both flagellin and zymosan induced the secretion of IL-8, IL-6 and MCP-1 (data not shown). The six other PAMPs screened had no effect on the secretion of IL-8, IL-6, or MCP-1, or on the secretion of the other cytokines examined: IFN-γ, TNF-α, IL-4, IL-10, GM-CSF, IL-1β, IL-12, IL-17, G-CSF, and MIP-1β (data not shown). Using the Luminex multiplex system, three cytokines, IL-8, IL-6 and MCP-1, were identified whose secretion by ECC-1 cells was induced by exposure to both zymosan and flagellin (via TLR2 and TLR5, respectively).
Effect of flagellin and zymosan on apical and basolateral secretion of IL-8 by ECC-1 cells
Having shown through Luminex multiplex screening that ECC-1 cells in response to specific PAMP secrete IL-8 into the apical compartment, we sought to confirm and extend this result by ELISA as well as examine the basolateral release of IL-8 by ECC-1 cells exposed to the panel of TLR agonists. ECC-1 cells were grown on cell inserts until TER measurements indicated that tight junctions had occurred (>900 ohms/well). Cell inserts that were absent from ECC-1 cells or that had failed to form a confluent monolayer displayed mean TER measurements of 130–150 ohms/well.
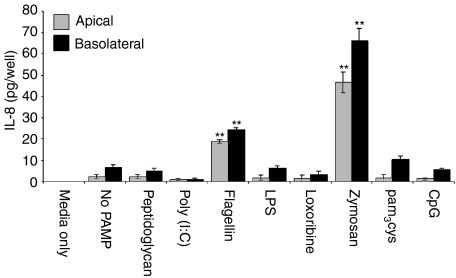
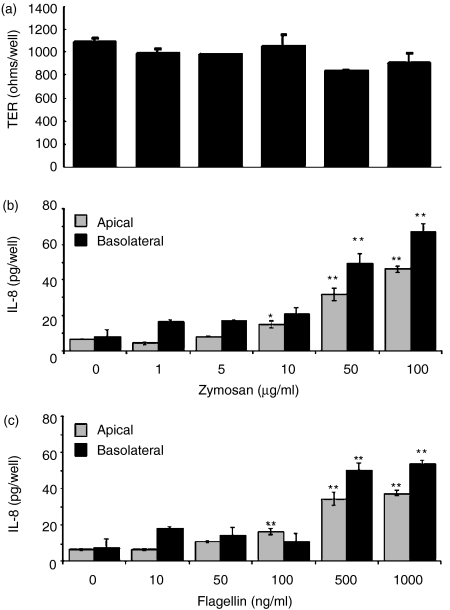
ECC-1 cells were treated with the panel of TLR agonists at concentrations similar to those used for the Luminex multiplex analyses. At 24 hr post-exposure, apical and basolateral supernatants were collected and analysed for IL-8 secretion by ELISA. As seen in Fig. 2, in the absence of PAMP, ECC-1 cells constitutively secreted IL-8 into apical and basolateral compartments with no significant difference in either direction. In response to flagellin (TLR5) and zymosan (TLR2) IL-8 secretion into both the apical and basolateral compartments was significantly greater than that seen with control cells which constitutively secreted low levels of IL-8 (Fig. 2). Similar to the constitutive expression of IL-8 by ECC-1 cells, basolateral secretion was not significantly different to that of the apical compartment. We next sought to determine if the induction of IL-8 secretion was dose-dependent and if increased doses of flagellin and zymosan had any effect on ECC-1 cell integrity. As shown in Fig. 3, both flagellin and zymosan displayed dose-dependent effects on apical and basolateral IL-8 secretion, with flagellin and zymosan inducing significant increases in IL-8 secretion at 500 and 1000 ng/ml and 50 and 100 μg/ml, respectively. Interestingly, the addition of zymosan had no effect on TER, indicating that the ECC-1 cells maintained tight junctions and that cell integrity was not affected by high doses of the TLR2 agonist (Fig. 3a). Similarly, high doses of flagellin failed to influence TER (data not shown). In separate experiments, soluble CD14, which has been shown to be involved in TLR4-dependent recognition of LPS, was added with LPS but failed to induce any changes in IL-8 expression (data not shown). These results indicate that ECC-1 cells, when stimulated via TLR5 or TLR2, secrete increased amounts of IL-8 both apically and basolaterally.
Figure 2.
IL-8 production by uterine ECC-1 epithelial cells treated with ligands to several TLR. Cultured media were collected following 24-hr PAMP stimulation and analysed for IL-8 protein expression by ELISA. Cells treated with ultra pure lipopolysaccharide from Salmonella minnesota were used at a final concentration of 1 μg/ml; pam3cys-ser-(lys)4, 1 μg/ml; Poly(I:C), 25 μg/ml; flagellin from Escherichia coli, 100 ng/ml; zymosan from Saccharomyces cerevisiae, 10 μg/ml; peptidoglycan from Staphylococcus aureus, 10 μg/ml; CpG oligonucleotide, 1 μm; loxoribine, 100 μm. Apical and basolateral IL-8 protein expression are shown. **Significantly different (P < 0·01) from control. Number of experiments n = 4).
Figure 3.
Dose-dependent apical production of IL-8 by uterine ECC-1 epithelial cells grown to maximal transepithelial resistance (TER). (a) TER following 24 hr zymosan stimulation. Cultured media were collected following 24 hr (b) zymosan or (c) flagellin stimulation and the apical media were analysed for IL-8 protein expression by ELISA. Apical and basolateral IL-8 protein expression are shown. Significantly different from control: *P < 0·05; **P < 0·01; (n = 3).
Effect of TLR agonists on MCP-1 secretion into the apical compartment
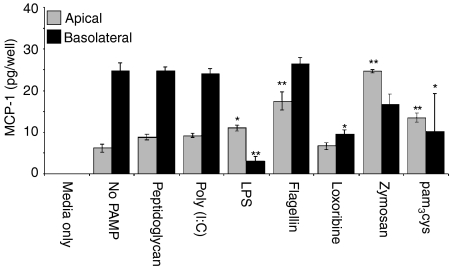
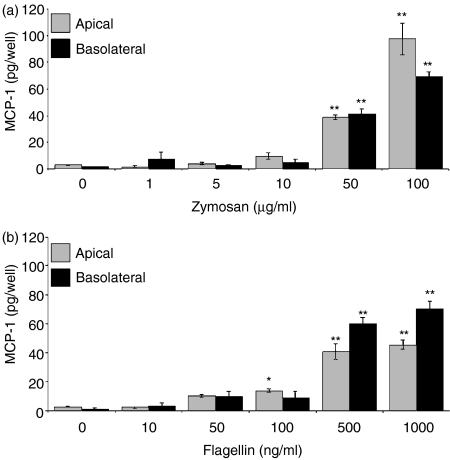
To characterize more fully the release of MCP-1, ECC-1 cells were incubated with a range of TLR agonists. As seen in Fig. 4, in the absence of TLR agonists, MCP-1 was constitutively produced and preferentially released into the basolateral compartment. When ECC-1 cells were incubated for 24 hr, LPS, flagellin, zymosan, and/or pam3cys induced MCP-1 secretion into the apical compartment (Fig. 4). In contrast, peptidoglycan, poly(I:C), and loxoribine had no effect on MCP-1 secretion by ECC-1 cells. Overall, apical secretion of MCP-1 either remained unchanged or was induced in the presence of PAMP. In contrast to apical secretion, basolateral release of MCP-1 by ECC-1 cells remained either unchanged or was significantly decreased upon exposure to the various PAMP. Of the TLR agonists analysed, LPS, loxoribine, and pam3cys had a significant inhibitory effect on MCP-1 basolateral secretion (Fig. 4). To define more fully the MCP-1 response to PAMP, ECC-1 cells were incubated with various amounts of flagellin and zymosan. Interestingly, while a significant increase in basolateral secretion of MCP-1 was not observed at a dose of 100 ng/ml (flagellin) and 10 μg/ml (zymosan) (Fig. 4), flagellin and zymosan at 500 and 1000 ng/ml and 50 and 100 μg/ml, respectively, increased MCP-1 secretion into both the apical and basolateral chambers (Fig. 5a,b). These results indicate that ECC-1 cells stimulated via TLR5, TLR2 and to a lesser extent TLR4 induce MCP-1 secretion apically, while only stimulation through TLR4 appears to result in a reduction of MCP-1 secretion basolaterally.
Figure 4.
MCP-1 production by uterine ECC-1 epithelial cells treated with ligands to several TLR. Cultured media were collected following 24 hr PAMP stimulation and analysed for the presence of MCP-1 protein expression by ELISA. Apical and basolateral protein expression are shown. Significantly different from control: *P < 0·05; **P < 0·01; (n = 4).
Figure 5.
Dose-dependent production of MCP-1 by uterine ECC-1 epithelial cells. Cultured media were collected following 24 hr (a) zymosan or (b) flagellin stimulation and analysed for MCP-1 protein expression by ELISA. Apical and basolateral MCP-1 protein expression are shown. Significantly different from control: *P < 0·05; **P < 0·01; (n = 3).
Exposure of ECC-1 cells to flagellin and zymosan induces apical secretion of IL-6 in a dose-dependent manner
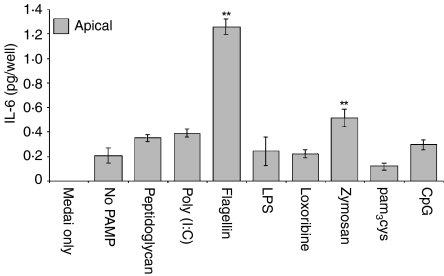
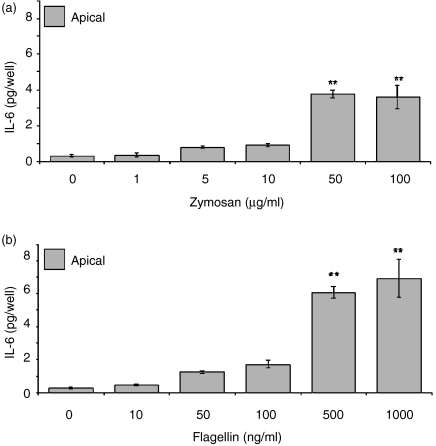
Analysis of basolateral and apical secretions of ECC-1 cells indicated that, whereas IL-6 is produced constitutively, secretion is confined to the apical compartment, albeit to a low level. In agreement with the Luminex data, IL-6 secretion into the apical compartment was induced by flagellin and zymosan (Fig. 6). Basolateral secretion of IL-6 was not observed, either constitutively or upon exposure of ECC-1 cells to various PAMPs. Similar to the results obtained with IL-8 and MCP-1, IL-6 secretion was induced in a dose-dependent manner in response to flagellin and zymosan (Fig. 7). Moreover, even at high doses of flagellin and zymosan, IL-6 secretion was not observed in the basolateral compartment (Fig. 7). These data demonstrate that signalling through TLR2 and TLR5 induces the apical release of IL-6 in a dose-dependent manner.
Figure 6.
Apical IL-6 production by uterine ECC-1 epithelial cells treated with ligands to several TLR. Cultured media were collected following 24 hr PAMP stimulation and analysed for IL-6 protein expression by ELISA. Apical IL-6 protein expression is shown. Significantly different from control: *P < 0·05; **P < 0·01; (n = 4).
Figure 7.
Dose-dependent apical production of IL-6 by uterine ECC-1 epithelial cells. Cultured media were collected following 24 hr (a) zymosan or (b) flagellin stimulation and analysed for IL-6 protein expression by ELISA. Apical IL-6 protein expression is shown. Significantly different from control: **P < 0·01; (n = 3).
Discussion
These studies demonstrate that the uterine epithelial cell line ECC-1 expresses mRNA for TLR1–TLR9, with only the expression of TLR10 mRNA not observed. Exposure of these cells to specific TLR ligands induced changes in the secretion of the chemokines IL-8 and MCP-1 and of the pro-inflammatory cytokine IL-6. Apical and basolateral secretion of IL-8 was induced upon exposure of uterine epithelial cells to flagellin and zymosan. MCP-1 apical secretion was induced in response to flagellin, zymosan, pam3cys, and LPS, while basolateral secretion was reduced following LPS exposure. Finally, IL-6 secretion, which was detected only in the apical compartment, was induced in response to flagellin and zymosan. The other PAMP examined, peptidoglycan, poly(I:C), loxoribine, and CpG, had no effect on cytokines released by uterine epithelial cells. These studies indicate that while uterine epithelial cells express TLR1–TLR9, only selected TLR agonists affect the expression of chemokines and pro-inflammatory cytokines. Overall, these findings demonstrate that uterine ECC-1 epithelial cells recognize TLR2, TLR5, and to a lesser extent TLR4 agonists. Furthermore, these studies suggest that PAMP initiate innate immune responses by inducing the production of pro-inflammatory chemokines and cytokines that are essential for host defence and survival.
The lumen of the lower reproductive tract (vagina and ectocervix) is lined with squamous epithelium, whereas the upper reproductive tract (endocervix, uterus and Fallopian tubes) is lined with columnar epithelium. While both types of cells form physical barriers to invading microbes, the means by which they mediate innate immune protection remains unclear. Little work has been performed to identify the TLR expressed on the epithelial cells of the lower and upper reproductive tract. Vaginal and cervical epithelial cells have been shown to express TLR1–TLR3, TLR5, and TLR6, constitutively but fail to express TLR4.40 More recently, it was demonstrated that tissues from the vagina, cervix, uterus and Fallopian tubes all express TLR1–TLR6, suggesting that the entire female reproductive tract is part of the innate immune system (P. Pioli, Dartmouth Medical School, personal communication). In this study, we report that the uterine epithelial cell line ECC-1 expresses TLR1–TLR9, but failed to detect TLR10. Whereas others have identified TLR expression on epithelial cells of the female reproductive tract, our study with ECC-1 cells demonstrates for the first time that TLRs are functional.
Our studies indicate that stimulation of endometrial epithelial ECC-1 cells with ligands to TLR2 and TLR5 uniformly induces the expression of chemokines IL-8, MCP-1 and IL-6. TLR4 stimulation also resulted in the induction of MCP-1. To our knowledge, this is the first time that stimulation through TLR5 has been shown to induce a response by epithelial cells of uterine origin. The consequences of chemokine and pro-inflammatory cytokine induction can have profound effects on the initiation of innate and adaptive immune responses. IL-8, MCP-1 and IL-6 are important mediators of the immune response with regard to Neisseria gonorrheoae and Chlamydia trachomatis infection of the female reproductive tract. These organisms are responsible for the majority of cases of pelvic inflammatory disease.1 Endocervical epithelial cells respond to a conserved surface antigen of N. gonorrheoae called the Lip lipoprotein by secreting IL-8, an α-chemokine, and the pro-inflammatory cytokine IL-6 in a TLR2-dependent manner.44 Our studies demonstrate that stimulation through TLR2 induces both IL-8 and IL-6. As a potent chemoattractant and activator of neutrophils and T lymphocytes, IL-8 is produced by a variety of cells types, including monocytes, fibroblasts, lymphocytes and epithelial and endothelial cells.47–49 IL-6 is a pro-inflammatory cytokine that induces terminal differentiation of proliferating B cells to plasma cells, stimulates antibody secretion by plasma cells, promotes leucocyte recruitment, and enhances T-lymphocyte responses in secondary lymphoid organs.50,51 Induction of IL-8 and IL-6 secretion, usually in concert with IL-1α and TNF-α expression, elicits the recruitment and activation of macrophages, dendritic cells, neutrophils and T lymphocytes to the site of infection, as well as inducing B-cell responses and enhancing T-cell help, the latter having been shown to be important in control of Neisserial infections.52,53 However, although it is likely that zymosan and flagellin are stimulating ECC-1 cells specifically through TLR2 and TLR5, respectively, the possibility that these PAMP may be binding other, as yet uncharacterized, receptors and/or multiple TLR working in concert with each other cannot be excluded.
Unexpectedly, whereas ECC-1 cells express TLR1–TLR9, only stimulation of TLR2, TLR5 and to a lesser extent TLR4 resulted in the induction of chemokine and/or cytokine secretion. While the secretion of 13 different cytokines and chemokines was examined following stimulation of various TLRs, it is possible that stimulation of one or more TLRs resulted in the differential secretion of chemokines/cytokines that were not measured in this study. Another possibility is that a secondary molecule and/or signal may be needed for TLR stimulation. For example, others have shown that inflammatory mediators such as IFN-γ or TNF-α enhance the stimulation signal of selected PAMP. The endometrium, once thought to be a sterile environment, is now known to be periodically exposed to commensal organisms and potential pathogens that are present in the vagina.54,55 Since particles and/or dyes deposited in the vagina and/or cervix move through the cervix and uterus into the Fallopian tubes within hours of deposition, it is likely that microbes colonizing the vagina and cervix routinely reach the uterus.56 Under these conditions, secondary signals would be essential in distinguishing between a commensal or pathogenic organism that is present in the endometrium.
In addition to the regulation of leucocyte migration and responsiveness during infection and inflammation, chemokines such as IL-8 and MCP-1 are crucial to normal reproductive physiology. These chemokines are synthesized in a timed and well-regulated manner and play important roles in endometrial angiogenesis, apoptosis, proliferation and differentiation.57 IL-8 and MCP-1 expression levels are high during all phases of the menstrual cycle, except during early pregnancy. IL-8 is especially important in cervical relaxation and parturition.58,59 On the other hand, not all effects of IL-8 and MCP-1 are beneficial. Elevated levels of IL-8 and MCP-1 are reported in women with endometriosis and correlate with the size and number of active lesions.60,61 Therefore, although these chemokines play important roles in both uterine defence from infection and normal progression through the menstrual cycle, aberrant production of these chemokines can cause damage to the endometrium and can lead in some cases to infertility.
In conclusion, our findings with ECC-1 cells suggest that uterine epithelial cells are important sentinels of the innate immune system. They express all but one of the known TLR, and upon recognition of specific pathogen-associated molecular patterns, they induce changes in the expression of key chemokines and pro-inflammatory cytokines that aid in the defence of the uterus from infection.
Acknowledgments
This work was supported by NIH grants AI51877 (C.R.W.) and immunology training grant T32 AI07363-12. We thank Dr Jacqueline Smith for advice and assistance on the Luminex multiplex system and Jacqueline A. Wright for assistance with ELISA analyses.
References
- 1.Quayle AJ. The innate and early immune response to pathogen challenge in the female genital tract and the pivotal role of epithelial cells. J Reprod Immunol. 2002;57:61–79. doi: 10.1016/s0165-0378(02)00019-0. [DOI] [PubMed] [Google Scholar]
- 2.King AE, Critchley HO, Kelly RW. Presence of secretory leukocyte protease inhibitor in human endometrium and first trimester decidua suggests an antibacterial protective role. Mol Hum Reprod. 2000;6:191–6. doi: 10.1093/molehr/6.2.191. [DOI] [PubMed] [Google Scholar]
- 3.Fleming DC, King AE, Williams AR, Critchley HO, Kelly RW. Hormonal contraception can suppress natural antimicrobial gene transcription in human endometrium. Fertil Steril. 2003;79:856–63. doi: 10.1016/s0015-0282(02)04930-0. [DOI] [PubMed] [Google Scholar]
- 4.King AE, Fleming DC, Critchley HO, Kelly RW. Differential expression of the natural antimicrobials, beta-defensins 3 and 4, in human endometrium. J Reprod Immunol. 2003;59:1–16. doi: 10.1016/s0165-0378(02)00083-9. [DOI] [PubMed] [Google Scholar]
- 5.Arici A, Head JR, MacDonald PC, Casey ML. Regulation of interleukin-8 gene expression in human endometrial cells in culture. Mol Cell Endocrinol. 1993;94:195–204. doi: 10.1016/0303-7207(93)90168-j. [DOI] [PubMed] [Google Scholar]
- 6.Arici A, MacDonald PC, Casey ML. Regulation of monocyte chemotactic protein-1 gene expression in human endometrial cells in cultures. Mol Cell Endocrinol. 1995;107:189–97. doi: 10.1016/0303-7207(94)03442-v. [DOI] [PubMed] [Google Scholar]
- 7.Hornung D, Ryan IP, Chao VA, Vigne JL, Schriock ED, Taylor RN. Immunolocalization and regulation of the chemokine RANTES in human endometrial and endometriosis tissues and cells. J Clin Endocrinol Metab. 1997;82:1621–8. doi: 10.1210/jcem.82.5.3919. [DOI] [PubMed] [Google Scholar]
- 8.Laird SM, Li TC, Bolton AE. The production of placental protein 14 and interleukin 6 by human endometrial cells in culture. Hum Reprod. 1993;8:793–8. doi: 10.1093/oxfordjournals.humrep.a138144. [DOI] [PubMed] [Google Scholar]
- 9.Simon C, Piquette GN, Frances A, Westphal LM, Heinrichs WL, Polan ML. Interleukin-1 type I receptor messenger ribonucleic acid expression in human endometrium throughout the menstrual cycle. Fertil Steril. 1993;59:791–6. doi: 10.1016/s0015-0282(16)55861-0. [DOI] [PubMed] [Google Scholar]
- 10.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–45. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 11.Lien E, Sellati TJ, Yoshimura A, et al. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J Biol Chem. 1999;274:33419–25. doi: 10.1074/jbc.274.47.33419. [DOI] [PubMed] [Google Scholar]
- 12.Bieback K, Lien E, Klagge IM, et al. Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. J Virol. 2002;76:8729–36. doi: 10.1128/JVI.76.17.8729-8736.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirschning CJ, Schumann RR. TLR2: cellular sensor for microbial and endogenous molecular patterns. Curr Top Microbiol Immunol. 2002;270:121–44. doi: 10.1007/978-3-642-59430-4_8. [DOI] [PubMed] [Google Scholar]
- 14.Takeuchi O, Hoshino K, Kawai T, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–51. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 15.Muroi M, Ohnishi T, Azumi-Mayuzumi S, Tanamoto K. Lipopolysaccharide-mimetic activities of a Toll-like receptor 2-stimulatory substance(s) in enterobacterial lipopolysaccharide preparations. Infect Immun. 2003;71:3221–6. doi: 10.1128/IAI.71.6.3221-3226.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozinsky A, Underhill DM, Fontenot JD, et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA. 2000;97:13766–71. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–8. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 18.Kariko K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for toll-like receptor 3. J Biol Chem. 2004;279:12542–50. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- 19.da Silva CJ, Soldau K, Christen U, Tobias PS, Ulevitch RJ. Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex. transfer from CD14 to TLR4 and MD-2. J Biol Chem. 2001;276:21129–35. doi: 10.1074/jbc.M009164200. [DOI] [PubMed] [Google Scholar]
- 20.Nagai Y, Akashi S, Nagafuku M, et al. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3:667–72. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- 21.Akashi S, Nagai Y, Ogata H, et al. Human MD-2 confers on mouse Toll-like receptor 4 species-specific lipopolysaccharide recognition. Int Immunol. 2001;13:1595–9. doi: 10.1093/intimm/13.12.1595. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi F, Smith KD, Ozinsky A, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 23.Smith KD, Andersen-Nissen E, Hayashi F, et al. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat Immunol. 2003;4:1247–53. doi: 10.1038/ni1011. [DOI] [PubMed] [Google Scholar]
- 24.Jurk M, Heil F, Vollmer J, et al. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat Immunol. 2002;3:499. doi: 10.1038/ni0602-499. [DOI] [PubMed] [Google Scholar]
- 25.Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 26.Lee J, Chuang TH, Redecke V, She L, Pitha PM, Carson DA, Raz E, Cottam HB. Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: activation of Toll-like receptor 7. Proc Natl Acad Sci U S A. 2003;100:6646–51. doi: 10.1073/pnas.0631696100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 28.Chuang T, Ulevitch RJ. Identification of hTLR10: a novel human Toll-like receptor preferentially expressed in immune cells. Biochim Biophys Acta. 2001;1518:157–61. doi: 10.1016/s0167-4781(00)00289-x. [DOI] [PubMed] [Google Scholar]
- 29.Servant MJ, Grandvaux N, Hiscott J. Multiple signaling pathways leading to the activation of interferon regulatory factor 3. Biochem Pharmacol. 2002;64:985–92. doi: 10.1016/s0006-2952(02)01165-6. [DOI] [PubMed] [Google Scholar]
- 30.Kawai T, Takeuchi O, Fujita T, Inoue J, Muhlradt PF, Sato S, Hoshino K, Akira S. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J Immunol. 2001;167:5887–94. doi: 10.4049/jimmunol.167.10.5887. [DOI] [PubMed] [Google Scholar]
- 31.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–50. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 32.Pulendran B, Kumar P, Cutler CW, Mohamadzadeh M, Van Dyke T, Banchereau J. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J Immunol. 2001;167:5067–76. doi: 10.4049/jimmunol.167.9.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thoma-Uszynski S, Stenger S, Takeuchi O, et al. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science. 2001;291:1544–7. doi: 10.1126/science.291.5508.1544. [DOI] [PubMed] [Google Scholar]
- 34.Aliprantis AO, Yang RB, Mark MR, et al. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–9. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 35.Muir A, Soong G, Sokol S, Reddy B, Gomez M, Van Heeckeren A, Prince A. Toll like receptors in normal and cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol. 2003;30:777–830. doi: 10.1165/rcmb.2003-0329OC. [DOI] [PubMed] [Google Scholar]
- 36.Akhtar M, Watson JL, Nazli A, McKay DM. Bacterial DNA evokes epithelial IL-8 production by a MAPK-dependent, NF-kappaB-independent pathway. FASEB J. 2003;17:1319–21. doi: 10.1096/fj.03-0950fje. [DOI] [PubMed] [Google Scholar]
- 37.Melmed G, Thomas LS, Lee N, et al. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implications for host–microbial interactions in the gut. J Immunol. 2003;170:1406–15. doi: 10.4049/jimmunol.170.3.1406. [DOI] [PubMed] [Google Scholar]
- 38.Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010–17. doi: 10.1128/iai.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith MF, Mitchell A, Li G, Ding S, Fitzmaurice AM, Ryan K, Crowe S, Goldberg JB., Jr Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-kappa B activation and chemokine expression by epithelial cells. J Biol Chem. 2003;278:32552–60. doi: 10.1074/jbc.M305536200. [DOI] [PubMed] [Google Scholar]
- 40.Fichorova RN, Cronin AO, Lien E, Anderson DJ, Ingalls RR. Response to Neisseria gonorrhoeae by cervicovaginal epithelial cells occurs in the absence of toll-like receptor 4-mediated signaling. J Immunol. 2002;168:2424–32. doi: 10.4049/jimmunol.168.5.2424. [DOI] [PubMed] [Google Scholar]
- 41.Hertz CJ, Wu Q, Porter EM, et al. Activation of toll-like receptor 2 on human tracheobronchial epithelial cells induces the antimicrobial peptide human beta defensin-2. J Immunol. 2003;171:6820–6. doi: 10.4049/jimmunol.171.12.6820. [DOI] [PubMed] [Google Scholar]
- 42.Birchler T, Seibl R, Buchner K, Loeliger S, Seger R, Hossle JP, Aguzzi A, Lauener RP. Human Toll-like receptor 2 mediates induction of the antimicrobial peptide human beta-defensin 2 in response to bacterial lipoprotein. Eur J Immunol. 2001;31:3131–7. doi: 10.1002/1521-4141(200111)31:11<3131::aid-immu3131>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 43.Bocker U, Yezerskyy O, Feick P, et al. Responsiveness of intestinal epithelial cell lines to lipopolysaccharide is correlated with Toll-like receptor 4 but not Toll-like receptor 2 or CD14 expression. Int J Colorectal Dis. 2003;18:25–32. doi: 10.1007/s00384-002-0415-6. [DOI] [PubMed] [Google Scholar]
- 44.Fisette PL, Ram S, Andersen JM, Guo W, Ingalls RR. The Lip lipoprotein from Neisseria gonorrhoeae stimulates cytokine release and NF-kappaB activation in epithelial cells in a Toll-like receptor 2-dependent manner. J Biol Chem. 2003;278:46252–60. doi: 10.1074/jbc.M306587200. [DOI] [PubMed] [Google Scholar]
- 45.Satyaswaroop PG, Tabibzadeh SS. Extracellular matrix and the patterns of differentiation of human endometrial carcinomas in vitro and in vivo. Cancer Res. 1991;51:5661–6. [PubMed] [Google Scholar]
- 46.Richardson JM, Kaushic C, Wira CR. Polymeric immunoglobin (Ig) receptor production and IgA transcytosis in polarized primary cultures of mature rat uterine epithelial cells. Biol Reprod. 1995;53:488–98. doi: 10.1095/biolreprod53.3.488. [DOI] [PubMed] [Google Scholar]
- 47.Mukaida N, Shiroo M, Matsushima K. Genomic structure of the human monocyte-derived neutrophil chemotactic factor IL-8. J Immunol. 1989;143:1366–71. [PubMed] [Google Scholar]
- 48.Larsen CG, Anderson AO, Appella E, Oppenheim JJ, Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989;243:1464–6. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- 49.Baggiolini M, Dahinden CA. CC chemokines in allergic inflammation. Immunol Today. 1994;15:127–33. doi: 10.1016/0167-5699(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 50.Kishimoto T, Taga T, Yamasaki K, et al. Normal and abnormal regulation of human B cell differentiation by a new cytokine, BSF2/IL-6. Adv Exp Med. 1989;254:135–43. doi: 10.1007/978-1-4757-5803-0_16. [DOI] [PubMed] [Google Scholar]
- 51.Romano M, Sironi M, Toniatti C, et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6:315–25. doi: 10.1016/s1074-7613(00)80334-9. [DOI] [PubMed] [Google Scholar]
- 52.Brooks GF, Lammel CJ. Humoral immune response to gonococcal infections. Clin Microbiol Rev. 1989;2(Suppl.):S5–10. doi: 10.1128/cmr.2.suppl.s5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsirpouchtsidis A, Hurwitz R, Brinkmann V, Meyer TF, Haas G. Neisserial immunoglobulin A1 protease induces specific T-cell responses in humans. Infect Immun. 2002;70:335–44. doi: 10.1128/IAI.70.1.335-344.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heinonen PK, Teisala K, Punnonen R, Miettinen A, Lehtinen M, Paavonen J. Anatomic sites of upper genital tract infection. Obstet Gynecol. 1985;66:384–90. [PubMed] [Google Scholar]
- 55.Spence MR, Blanco LJ, Patel J, Brockman MT. A comparative evaluation of vaginal, cervical and peritoneal flora in normal, healthy women: a preliminary report. Sex Transm Dis. 1982;9:37–40. doi: 10.1097/00007435-198201000-00007. [DOI] [PubMed] [Google Scholar]
- 56.Kunz G, Beil D, Deininger H, Wildt L, Leyendecker G. The dynamics of rapid sperm transport through the female genital tract: evidence from vaginal sonography of uterine peristalsis and hysterosalpingoscintigraphy. Hum Reprod. 1996;11:627–32. doi: 10.1093/humrep/11.3.627. [DOI] [PubMed] [Google Scholar]
- 57.Kayisli UA, Mahutte NG, Arici A. Uterine chemokines in reproductive physiology and pathology. Am J Reprod Immunol. 2002;47:213–21. doi: 10.1034/j.1600-0897.2002.01075.x. [DOI] [PubMed] [Google Scholar]
- 58.Ito A, Hiro D, Sakyo K, Mori Y. The role of leukocyte factors on uterine cervical ripening and dilation. Biol Reprod. 1987;37:511–17. doi: 10.1095/biolreprod37.3.511. [DOI] [PubMed] [Google Scholar]
- 59.Osmers RG, Blaser J, Kuhn W, Tschesche H. Interleukin-8 synthesis and the onset of labor. Obstet Gynecol. 1995;86:223–9. doi: 10.1016/0029-7844(95)93704-4. [DOI] [PubMed] [Google Scholar]
- 60.Jolicoeur C, Boutouil M, Drouin R, Paradis I, Lemay A, Akoum A. Increased expression of monocyte chemotactic protein-1 in the endometrium of women with endometriosis. Am J Pathol. 1998;152:125–33. [PMC free article] [PubMed] [Google Scholar]
- 61.Iwabe T, Harada T, Tsudo T, Tanikawa M, Onohara Y, Terakawa N. Pathogenetic significance of increased levels of interleukin-8 in the peritoneal fluid of patients with endometriosis. Fertil Steril. 1998;69:924–30. doi: 10.1016/s0015-0282(98)00049-1. [DOI] [PubMed] [Google Scholar]