Suppressive effect of leflunomide metabolite (A77 1726) on metalloproteinase production in IL-1β stimulated rheumatoid synovial fibroblasts (original) (raw)
Abstract
Leflunomide, an isoxazol derivative structurally unrelated to other immunomodulatory drugs, has proven to be efficacious in the treatment of rheumatoid arthritis (RA). This study was conducted to elucidate the mechanism by which leflunomide mediated antirheumatic effects. We investigated the effects of A77 1726, leflunomide's active metabolite, on mitogen-activated protein kinase (MAPK) activation in IL-1_β_-stimulated rheumatoid synovial fibroblasts. The effects of A77 1726 on the secretion of matrix metalloproteinases (MMPs) from rheumatoid synovial fibroblasts were also examined. A77 1726 partially suppressed IL-1_β_-induced ERK1/2 and p38 kinase activation. In contrast, A77 1726 efficiently suppressed IL-1_β_-stimulated JNK1/2 kinase activation. Although no suppressive effect was demonstrated on MMP-2, A77 1726 markedly inhibited MMP-1, 3, and 13 secretions from IL-1_β_-stimulated rheumatoid synovial fibroblasts. Tissue inhibitor of metalloproteinases-1 (TIMP-1) was constitutively produced from rheumatoid synovial fibroblasts and the suppressive effects of A77 1726 on TIMP-1 production were minimal. Our results suggest that the suppression of the MAPK signalling pathway and MMP synthesis in rheumatoid synovial fibroblasts is a possible mechanism for the inhibitory activity of leflunomide against rheumatoid arthritis.
Keywords: leflunomide, matrix metalloproteinases, mitogen-activated protein kinases, rheumatoid arthritis
INTRODUCTION
Leflunomide is a novel immunomodulatory agent that has recently been approved as a disease-modifying antirheumatic drug (DMARD) for the treatment of rheumatoid arthritis [1]. This isoxazole derivative is metabolized to its active form, A77 1726 [2]. The exact mechanism by which A 77 1726 exerts its effect in vivo is as yet unknown. Previous data suggest two possible modes of action: inhibition of dihydroorate dehydrogenase (DHODH), by which A77 1726 influences de novo pyrimidine biosynthesis [3] and interaction with signalling events, thereby interfering the phosphorylation of tyrosine kinases [4]. Because the immunoregulatory effects of A77 1726 occur at doses that inhibit DHODH but not tyrosine kinases [5], the interruption of de novo pyrimidine synthesis is thought to be the primary mode of action [6]. However, additional mechanisms of action may remain to be discovered. It has been suggested that leflunomide is a potent inhibitor of the transcription factor, activating protein-1 (AP-1) [7]. AP-1 is composed of two proteins, Fos and c-Jun [8]. c-Jun is phosphorylated by mitogen-activated protein kinases [9]. Therefore, it is possible that leflunomide has an inhibitory effect on AP-1 by affecting mitogen-activated protein kinases (MAPK). MAPK activation is thought to be involved in rheumatoid synovitis, based on the fact that MAPK is activated in rheumatoid synovium [10]. Because of the importance of MAPK in rheumatoid synovitis, we examined the effects of leflunomide on MAPK activation in rheumatoid synovial fibroblasts.
MATERIALS AND METHODS
Reagents
A77 1726 was provided by Aventis Pharma Japan (Tokyo, Japan). Human recombinant IL-1_β_ (1·5 Χ 108 unit/mg) was kindly provided by Dainihon Chemical Co. (Osaka, Japan). Mouse antihuman 13 antibodies were obtained from Fuji Chemical Co. (Takaoka, Japan). Anti-phospho-specific ERK1/2, p38, JNK1/2 and antipan ERK1/2, p38, JNK1/2 antibodies were purchased from Biosource (Camarillo, CA, USA).
Isolation of synovial cells
The experimental protocol was approved by the local ethics committee, and a signed informed consent was obtained from each participant prior to commencement of the study. Synovial tissue samples were obtained from five patients with RA during synovectomy. Synovial fibroblasts were isolated from the synovial tissues by enzymatic digestion. The isolated synovial fibroblasts were used at the third or fourth passages for subsequent experiments. Synovial fibroblasts were cultured with RPMI 1640 media containing 10% FCS. For the experiment, the medium was discarded and the cells were stimulated with IL-1_β_ (20 U/ml) in serum-free RPMI 1640, with or without A77 1726.
Assesment of cell viability
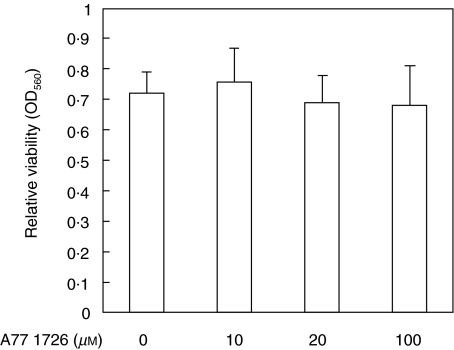
Cell viability was assessed using metyl thiazolyl tetrazolium (MTT) staining. 100 _µ_l of cell culture containing 1 × 104 cells was plated in the wells of 96-well culture plates. After incubation with A77 1726 for 24 h, 100 _µ_l of MTT solution (2·5 mg/ml) was added. After incubation at 37°C for 4 h, 100 _µ_l of acid-isopropanol (0·04 N HCl in isoporopanol) was added and mixed gently with the cell suspension, and OD560 was determined with ELISA reader.
Immunoblot analysis for MAPK activation
Synovial fibroblasts were grown to subconfluence on 10 cm culture dishes and starved in a serum-free medium with various concentrations of A77 1726 for 12 h. The synovial fibroblasts were stimulated with IL-1_β_ (20 U/ml) for 15 min. The cells were washed with cold PBS and lysed with a lysis buffer (1% Nonidet P 40, 50 mm Tris, pH 7·5, 100 mm NaCl, 50 mm NaF, 5 mm EDTA, 20 mm_β_-glycerophosphate, 1·0 mm sodium orthovanadate, 10 _µ_g/ml aprotinin and 10 _µ_g/ml leupeptin) for 20 min at 4°C. Insoluble material was removed by centrifugation at 15 000× g for 15 min at 4°C. The supernatant was saved and the protein concentration was determined using the Bio-Rad protein assay kit. An identical amount of protein (50 _µ_g) for each lysate was subjected to 10% SDS-polyacrylamide gel electrophoresis. The proteins were transferred to a nitrocellulose membrane. The filters were blocked for 1·5 h using 5% nonfat dried milk in TBS (50 mm Tris, 0·15 m NaCl, pH 7·5) containing 0·1% Tween 20, washed in TBS and incubated at room temperature, for 2 h in a 1 : 1000 dilution of phosphospecific ERK1/2, p38 or JNK1/2 antibodies (Biosource). The filters were later washed in TBS and incubated with 1 : 1000 dilution of donkey antirabbit IgG antibodies, coupled with horseradish peroxidase. An enhanced chemiluminescence (ECL) system (Amersham. Bucks. UK) was used for detection.
ELISA
MMP-1, 2, 3, and TIMP-1 in the culture supernatants were measured by sandwich enzyme linked immunoassay (ELISA, R & D Systems Inc, USA).
Immunoblot analysis for MMPs
Culture supernatants were subjected to 8% SDS-polyacrylamide gel electrophoresis (PAGE). The fractionated proteins were transferred to nitrocellulose membranes and the filters were immunoblotted by anti-MMP-13 monoclonal antibodies (Fuji Chemical, Takaoka, Japan) at a 1 : 150 dilution.
Statistical analysis
Statistical significance was performed using Student's _t_-test.
RESULTS
A77 1726 modifies the phosphorylation state of the MAPK activated by IL-1_β_
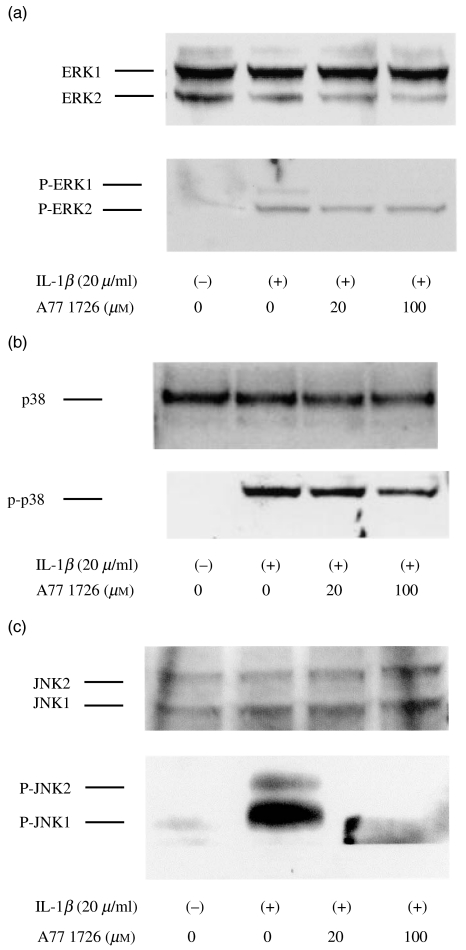
Quiescent synovial fibroblasts were incubated with A77 1726, an active metabolite of leflunomide (0–100 µ_m) for 12 h. We checked the cell viability using MTT-staining method. These concentrations of A77 1726 had no effect on the viability of these cells (Fig. 1). Synovial fibroblasts pretreated with A77 1726 were then stimulated with IL-1_β for 15 min. Cellular lysates were analysed by immunoblots using antiphospho-specific MAPK antibodies. IL-1_β_ stimulation induces ERK1/2 phosphorylation, demonstrating activation of the kinase. A77 1726 pretreatment efficiently blocked ERK1 phosphorylation, whereas A77 1726 pretreatment showed only a moderate effect on the phosphorylation status of ERK2 (Fig. 2a). Also, IL-1_β_-induced p38 phosphorylation was partially inhibited by A77 1726 pretreatment (Fig. 2b). Interestingly A77 1726 pretreatment almost completely inhibited the JNK1/2 phosphorylation induced by IL-1_β_ stimulation (Fig. 2c).
Fig. 1.
Assesment of cell viability of A77 1726-treated synovial fibroblasts Synovial fibroblasts were incubated with various concentrations of A77 1726. Twelve hours later the cell viability was determined by MTT assay. Bars show the mean (SEM) of OD560 in three separate experiments.
Fig. 2.
Effects of A77 1726 on MAPK activation of IL-1_β_-stimulated rheumatoid synovial fibroblasts Rheumatoid synovial fibroblasts were pretreated with A77 1726 for 12 h in serum-free media. These quiescent synovial fibroblasts were stimulated by IL-1_β_ for 15 min. Protein lysates from whole cell extracts were prepared as described in Materials and Methods. Protein lysates (50 µ_g) were subjected to 10% SDS-polyacrylamide gels, and immunoblotted using antiphospho-specific and pan-ERK1/2 (a), p38 (b), and JNK1/2 (c) antibodies. IL-1_β stimulation induced the phosphorylation of MAPK (P-ERK1/2, P-p38, P-JNK1/2) in rheumatoid synovial fibroblasts. Similar results were obtained in three independent experiments using different rheumatoid synovial fibroblasts.
Effects of A77 1726 on MMP production
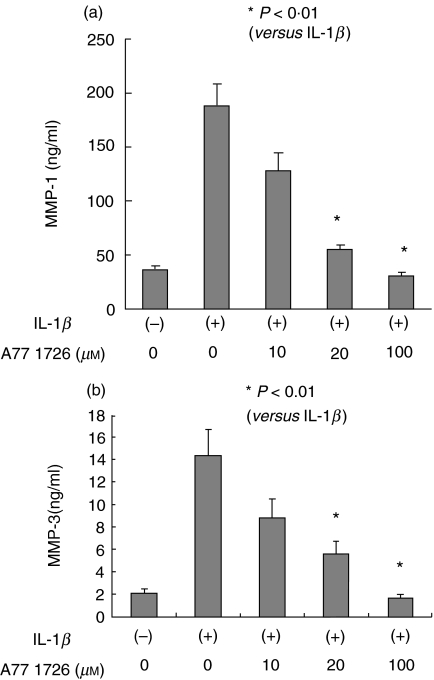
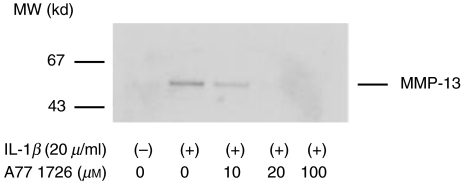
Next, we investigated the possibility that leflunomide affects MMP secretion from IL-1_β_-stimulated rheumatoid synovial fibroblasts. MMP-1, 2, 3, and TIMP-1 secretions were measured by ELISA method and MMP-13 secretions were analysed by immunoblot. Synovial fibroblast exhibited a significant increase in the production of MMP-1 (Fig. 3a) and MMP-3 (Fig. 3b) in response to IL-1_β_. A77 1726 treatment completely prevented the stimulation of synthesis of these MMPs from IL-1_β_-stimulated rheumatoid synovial fibroblasts. Although IL-1_β_ slightly increased the MMP-2 secretions from rheumatoid synovial fibroblasts, A77 1726 treatment did not influence the IL-1_β_-induced MMP-2 production of rheumatoid synovial fibroblasts significantly (Fig. 3c). A77 1726 treatment also inhibited the MMP-13 secretion from IL-1_β_-stimulated rheumatoid synovial fibroblasts (Fig. 4).
Fig. 3.
Effects of A77 1726 on the MMP-1, MMP-2, and MMP-3 secretions from IL-1_β_ stimulated rheumatoid synovial fibroblasts Rheumatoid synovial fibroblasts were stimulated by IL-1_β_ in the presence or absence of A77 1726 for 24 h. MMP-1 (a), MMP-3 (b), and MMP-2 (c) in the culture supernatants were measured by ELISA. Bars show the mean (SEM) levels of MMPs in three separate experiments.
Fig. 4.
Effects of A77 1726 on MMP-13 secretions from IL-1_β_-stimulated rheumatoid synovial fibroblasts Rheumatoid synovial fibroblasts were stimulated by IL-1_β_ in the presence or absence of A77 1726 for 24 h. The culture supernatants were collected and subjected to electrophoresis on 10% SDS-polyacrylamide gels, and immunoblotted using anti-MMP-13 antibodies. Similar results were obtained in three independent experiments using different rheumatoid synovial fibroblasts.
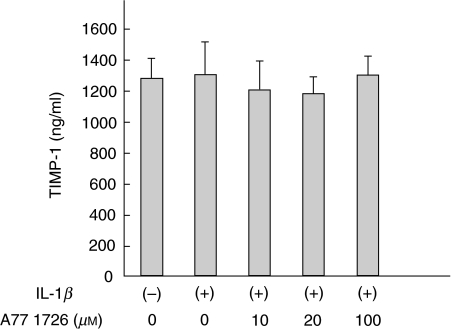
Finally we investigated the effects of A77 1726 on TIMP-1 secretions. Levels of TIMP-1 secretion were high in unstimulated synovial fibroblasts and the suppressive effect of A77 1726 on TIMP-1 was not marked (Fig. 5).
Fig. 5.
Effects of A77 1726 on the TIMP-1 secretions from IL-1_β_ stimulated rheumatoid synovial fibroblasts Rheumatoid synovial fibroblasts were stimulated by IL-1_β_ in the presence or absence of A77 1726 for 24 h. TIMP-1 in the culture supernatants was measured by ELISA. Bars show the mean (SEM) levels of TIMP-1 in three separate experiments.
DISCUSSION
It is known that leflunomide is metabolized to its active form, A77 1726, which exerts the immunosuppressive activity [2]. Previous data have suggested that inhibition of DHODH and interference with tyrosine kinases are two possible immunosuppressive properties of A77 1726 [2–4]. Additionally it has been suggested that leflunomide is a potent inhibitor of MAPK [7]. To determine whether the effects of leflunomide are attributable to the inhibition of MAPK activation, we examined the effects of A77 1726 on MAPK in IL-1_β_-stimulated synovial fibroblasts. Our results showed that leflunomide partially suppressed IL-1_β_-induced ERK1/2 kinase and p38 activation. In contrast, A77 1726 almost completely inhibited JNK1/2 kinase activation in the same cells. Our results clearly indicate that leflunomide is a potent inhibitor of MAPK in rheumatoid synovial fibroblasts.
Overproduction of MMP, a family of enzymes degrading the extracellular matrix components, mediates the irreversible cartilage degradation and joint destruction of RA [11]. It has been suggested that MAPK serve as the key regulator of matrix remodeling in RA, based on the fact that MAPK are activated in the rheumatoid synovium [12]. Despite the differential expression, a high degree similarity in the promoter regions of MMP exists [13]. The AP-1 site upstream of the transcriptional start site has been thought to play a critical role in the transcriptional activation of MMP gene [14]. Activation of p38 and JNK is commonly responsible for AP-1 activation through the phosphorylation of c-Jun [9]. It is conceivable that MAPK pathways are involved in coordinate AP-1 activation and MAPK inhibition is a potential therapeutic approach for the down-regulation of MMP by regulating the AP-1 transcriptional activity. Our data clearly indicate that A77 1726 prevented MMPs secretion from IL-1_β_-stimulated rheumatoid synovial fibroblasts. A77 1726-mediated inhibition of MAPK may decrease the MMP secretion from cytokine-stimulated rheumatoid synovial fibroblasts by affecting AP-1. Our data also indicated that A77 1726 did not influence the MMP-2 production from IL-1_β_-stimulated rheumatoid synovial fibroblasts. An AP-1 binding site is present within the promoter region of all of the MMP except MMP-2 [15]. It is possible that suppression of MAPK or AP-1 pathways may not be involved in the MMP-2 production from rheumatoid synovial fibroblasts.
Recent evidence suggests that A77 1726 is a potent inhibitor of nuclear factor-_κ_B (NF-_κ_B) activation. Manna and Aggarwal showed that 5–10 _µ_m of A77 1726 blocks TNF-mediated NF-_κ_B activation [7]. It is possible that A77 1726 inhibits MMPs secretion by affecting NF-_κ_B, since this transcription factor is also involved in MMPs transcription [16]. In our experiments, the inhibitory effects on MMPs secretions were observed in the presence of >20 _µ_m of A77 1726. This concentration of A77 1726 almost equals to the maximal plasma concentrations in RA patients with repeated oral administrations of leflunomide [1]. Previous reports also demonstrated that MMP-1 and MMP-3 secretions from rheumatoid synovial fibroblasts were inhibited at high concentrations of A 771726 concentrations (>10 _µ_m) [17,18]. Therefore, in addition to the inhibition of NF-_κ_B, other mechanisms, including MAPK suppression, could be involved in A77 1726-mediated MMPs suppression.
In conclusion, our data suggest that an alternative explanation for the antirheumatic efficacy of leflunomide is the potent inhibition of MAPK and MMP secretion from rheumatoid synovial fibroblasts.
REFERENCES
- 1.Weinblatt ME, Kremer JM, Coblyn JS, et al. Pharmacokinetics, safety, and efficacy of combination treatment with methotrexate and leflunomide in patients with active rheumatoid arthritis. Arthritis Rheum. 1999;42:1322–8. doi: 10.1002/1529-0131(199907)42:7<1322::AID-ANR4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 2.Davis JP, Cain GA, Pitts WJ, Magolda RL, Copeland RA. The immunosuppressive metabolite of leflunomide is a potent inhibitor of human dihydroorotate dehydrogenase. Biochemistry. 1996;35:1270–3. doi: 10.1021/bi952168g. [DOI] [PubMed] [Google Scholar]
- 3.Bruneau JM, Yea CM, Spinella-Jaegle S, et al. of human dihydro-orotate dehydrogenase and its inhibition by A77 1998, 1726, the active metabolite of leflunomide. Biochem J, 336: 299-303. [DOI] [PMC free article] [PubMed]
- 4.Mattar T, Kochhar K, Bartlett R, Bremer EG, Finnegan A. Inhibition of the epidermal growth factor receptor tyrosine kinase activity by leflunomide. FEBS Lett. 1993;334:161–4. doi: 10.1016/0014-5793(93)81704-4. [DOI] [PubMed] [Google Scholar]
- 5.Williamson RE, Yea CM, Robson PA, et al. Dihydroorotate dehydrogenase is a high affinity binding protein for A77 1726 and mediator of a range of biological effects of the immunomodulatory compound. J Biol Chem. 1995;270:22467–72. doi: 10.1074/jbc.270.38.22467. [DOI] [PubMed] [Google Scholar]
- 6.Fox RI. Mechanism of action of leflunomide in rheumatoid arthritis. J Rheumatol. 1998;53:20–6. [PubMed] [Google Scholar]
- 7.Manna SK, Aggarwal BB. Immunosuppressive leflunomide metabolite (A77 1999, 1726 blocks TNF–dependent nuclear factor–kappa B activation and gene expression. J Immunol. 162: 2095-102. [PubMed]
- 8.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–6. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 9.Whitmarsh AJ, Davis RJ. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- 10.Schett G, Tohidast-Akrad M, Smolen JS, et al. Activation, differential localization, and regulation of the stress-activated protein kinases, extracellular signal-regulated kinase, c-JUN N-terminal kinase, and p38 mitogen-activated protein kinase, in synovial tissue and cells in rheumatoid arthritis. Arthritis Rheum. 2000;43:2501–12. doi: 10.1002/1529-0131(200011)43:11<2501::AID-ANR18>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 11.Firestein GS. Mechanisms of tissue destruction and cellular activation in rheumatoid arthritis. Curr Opin Rheumatol. 1992;4:348–54. doi: 10.1097/00002281-199206000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Piecyk M, Anderson P. Signal transduction in rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2001;15:789–803. doi: 10.1053/berh.2001.0194. [DOI] [PubMed] [Google Scholar]
- 13.Matrisian LM. Matrix metalloproteinase gene expression. Ann NY Acad Sci. 1994;732:42–50. doi: 10.1111/j.1749-6632.1994.tb24723.x. [DOI] [PubMed] [Google Scholar]
- 14.Westermarck J, Kahari VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13:781–92. [PubMed] [Google Scholar]
- 15.Corcoran ML, Hewitt RE, Kleiner DE, Stetler-Stevenson WG. MMP-2: expression, activation and inhibition. Enzyme Protein. 1996;49:7–19. doi: 10.1159/000468613. [DOI] [PubMed] [Google Scholar]
- 16.Bondeson J, Brennan F, Foxwell B, Feldmann M. Effective adenoviral transfer of IkappaBalpha into human fibroblasts and chondrosarcoma cells reveals that the induction of matrix metalloproteinases and proinflammatory cytokines is nuclear factor-kappaB dependent. J Rheumatol. 2000;27:2078–89. [PubMed] [Google Scholar]
- 17.Burger D, Begue-Pastor N, Benavent S, Gruaz L, Kaufmann MT, Chicheportiche R, Dayer JM. inhibits the production of prostaglandin E (2), matrix metalloproteinase 1 and interleukin 6 in human fibroblast–like synoviocytes. Rheumatology. 2003;42:89–96. doi: 10.1093/rheumatology/keg038. The active metabolite of leflunomide, A77, 1726. [DOI] [PubMed] [Google Scholar]
- 18.Elkayam O, Yaron I, Shirazi I, Judovitch R, Caspi D, Yaron M. Active leflunomide metabolite inhibits interleukin 1beta, tumour necrosis factor alpha, nitric oxide, and metalloproteinase-3 production in activated human synovial tissue cultures. Ann Rheum Dis. 2003;62:440–3. doi: 10.1136/ard.62.5.440. [DOI] [PMC free article] [PubMed] [Google Scholar]