Circulating anti-actin and anti-ATP synthase antibodies identify a sub-set of patients with idiopathic nephrotic syndrome (original) (raw)
Abstract
Idiopathic nephrotic syndrome (iNS) with resistance or dependence to steroids is a common disease in children but in spite of an increasing clinical impact its pathogenesis is unknown. We screened for the presence of circulating antibodies against glomerular (podocytes, mesangium) and tubular cells (tubular epithelia) a cohort of 60 children with iNS including 8 patients with a familial trait of iNS or with proven mutation of NPHS1-NPHS2 and 12 with good sensitivity to steroids. Positive sera were found in 8 cases, all belonging to the category without familial trait/molecular defects. The targets of antibodies were characterized with Western blot and MALDI-Mass utilizing β-hexyl cell extracts separated with two-dimensional electrophoresis. In all cases antibodies of the IgM class were directed against ATP synthase β chain alone (4 cases) or in combination with actin (3 cases); one child presented IgG against aldose reductase. The clinical picture was nephrotic syndrome with steroid resistance or dependence and variable cyclosporin sensitivity; 3 patients developed end stage renal failure. The basic pathology picture was focal segmental glomerulosclerosis (FSGS) in 4 cases and mesangial proliferative glomerulonephrites with deposition of IgM in 2. Overall, patients with circulating auto-antibodies could not be readely differentiated on clinical grounds with the exception of 3 children who developed positivity for antinuclear antibodies during the follow-up. Affinity-purified IgM from one patient who underwent plasmapheresis for therapeutical pourposes (but not from a normal pool) induced proteinuria in Sprague-Dawley rats and concomitant human IgM deposition within glomeruli. This is the first report of circulating anti-actin/ATP synthase β chain antibodies in a subset of patients with iNS. Both pathological significance and clinical impact given by the presence of these antibodies and the relationship with other conditions such as lupus-erythematosus, characterized by their presence, must be defined.
Keywords: nephrotic syndrome, mesangial proliferative glomerulonephrites, focal segmental glomerulosclerosis, actin, ATP synthase, auto-antibodies
Introduction
Idiopathic nephrotic syndrome (iNS) with resistance or dependence to steroids, is a common disease in children. The prevalent pathological background consists in focal areas of segmental glomerulosclerosis (FSGS) [1,2] that coexist with diffuse mesangial IgM deposition, two entities that are nowadays considered as homologs. The incidence of mesangial IgM/FSGS has greatly increased in the last decade [3] and is emerging as leading glomerular cause of end stage renal failure. In spite of increasing clinical impact, the pathogenesis of mesangial IgM deposition and FSGS is unknown. Among any other, autoimmune phenomena may play a significant role and cannot be rouled out a priori. Autoimmune mechanisms have been implicated in both primary and secondary renal diseases, the paradigms being systemic lupus erythematosus (SLE), mixed cryoglobulin with active nephritis [4] and membranous glomerulonephritis (MN). In all these conditions, renal toxicity linked to auto-immunity has been mainly justified on the basis of the presence of circulating autoantibodies and data substaining fine mechanisms for immunodeposition or of in situ formation of the immunocomplex are available [5–8]. Actin is a major protein eliciting an autoimmune response in SLE. Mostoslavsky _et al._[9] found that lupus anti-DNA antibodies cross-react with glomerular actin and renal deposition of anti-actin antibodies have been demonstrated in patients with lupus nephritis [10]. It was proposed a case of molecular mimicry since this protein shares some molecular homology with alfa-actinin 4, a protein playing a major functional role within the glomerular filtration barrier [11].
The combination of FSGS and more in general of iNS with SLE is far more common than expected and is actually considered as a noncasual link. Hertig et al. [12] recently described 11 patients with iNS substained by a pathological picture of FSGS or minimal changes and clear clinical characteristics of SLE with polyarthritis, leukopenia, thrombocitemia and positive anti-DNA antibodies, proposing the idea of a concasual pathogenesis between the two clinical entities.
Based on this background, we screened a cohort of 60 patients with iNS for the presence of circulating antibodies against renal proteins and found anti-actin and anti-ATP synthase IgM in 8. Anti-actin/ATP synthase antibodies may identify a clinical variant of iNS whose clinical impact remains to be determined.
Materials and methods
Patients
Sixty subjects (40 males, 20 females), who had presented with nephrotic syndrome at various ages and variable sensitivity to steroids, were enrolled between 1998 and 2004. In parallel with classical diagnostic approches and before any therapy was started (see below), blood samples were taken and stored frozen for personalized analysis and DNA studies. According to this, all DNAs were characterized for molecular defects of podocyte genes involved in familial nephrotic syndrome (NPHS1-NPHS2). The therapeutic approach at the first episode of proteinuria consisted in steroids, following the ISKDC consolidated scheme [13,14] that utilizes prednisolone 2 mg/kg for 30–60 days followed by 10% reduction of the same drug given every other day for another month and then slow tapering over the following 2 months. Relapses of proteinuria that occurred at least after 1 month were treated with the same protocol with the difference that the attack dose of prednisolone was continued until stable reduction of proteinuria for 1 week and was followed by the same tapering protocol as above. Patients who stably responded to steroids with normalization of proteinuria but who presented at least 3 recurrences in a year were considered as frequent relapsers and were treated with cyclophosphamide (see below). Recurrence of proteinuria during the tapering of steroids was considered as steroid dependence and was treated with cyclophosphamide as above and, in case of failure, with cyclosporin (see below). In case of unresponsiveness (partial or global), steroids were associated or substituted with cyclosporin (5 mg/kg starting dose, followed by tapering to reach the minimum dose required for maintaining cyclosporin serum levels between 50 and 100 ng/ml). In case of persistent steroid-cyclosporin resistance, methyl-prednisolone was given in pulses (10 mg/kg, 6 cycles). According to the flow-chart above, patients were subdivided categories depending on the genetic background-familial trait and/or the sensitivity to steroids (Table 1). Eight patients presented a familial trait of proteinuria or proven mutations of major podocyte genes (NPHS1-NPHS2) [15,16]; among the 52 patients without genetic background, 16 were sensitive to steroids (S), 21 were dependent (D) and 15 were resistant (R). Age at onset of proteinuria and gender distribution were comparable among groups. Patients who were succesfully treated with steroids (S) had no further treatment and/or clinical diagnostic approach and none of them had progression to end stage renal failure. Most of patients with D or R to steroids received instead a diagnostic renal biopsy and further therapeutical approaches, mainly cyclosporin and 23 were sensitive to this treatment. Based on immuno-histological findings, 28 D and R patients were classified as FSGS for the presence of at least one segmental area of glomerulosclerosis associated with diffuse mesangial IgM deposition. Nine patients presented mesangial IgM deposition at immunofluorescence with mesangial matrix proliferation and had a diagnosis of mesangial proliferative glomerulonephrites with IgM deposits (Mes IgM). Thirty normal sera were obtained from the personal of our clinic staff composed of apparently normal young people (male12, female18; mean age 21 years, range 16–35).
Table 1. Clinical features of all nephrotic patients enrolled in the study that included (overall 48): (a) 8 pts with circulating auto-antibodies; (b) 5 pts with familial trait of nephritic syndrome; (c) 3 pts with NPHS1-NPHS2 mutations; (d) 32 pts with primary nephritic syndrome.
| Drug sensitivity FSGS/Mes | |||||||
|---|---|---|---|---|---|---|---|
| Age at onset (years) | Histology | ||||||
| n | Sex | mean (range) | Steroids | Cyclosporin | IgM | ESRF | |
| Nephrotic syndrome with familial trait/NPHS1-2 mut | 8 | 3F/5M | 6·2 (2–8) | 1S/2D/2R | 2S/2R | 2/2 | 2 |
| Sporadic nephrotic syndrome | 52 | 17F/35M | 7·1 (1–36) | 16S/21D/15R | 23S/5R | 28/9 | 11 |
Human podocytes and other cells ‘in culture’
Primary cultures of glomerular epithelial cells (GECs) were established and characterized as previously described [17]. Established lines of differentiated GECs were obtained by infection of primary cultures with a hybrid Adeno5/SV40 virus as previously described and characterized as above [17,18]. An immortalized GEC line was cultured in DMEM containing 25 mM glucose, 10% inactivated FCS, 100 U/ml penicillin, and 100 mg/ml streptomycin at 37 °C and used for these experiments. GECs for immunofluorescence were fixed in 1% paraformaldehyde for 15 min at room temperature and washed in PBS. Where appropriate, GECs were incubated with 0·05 ml of serum in PBS 7·4 pH and after permeabilization at 4°C in HEPES-Triton X-100 buffer for 5 min were incubated again with FITC-conjugated anti-human IgM antibodies (DAKO, Glostrup, Denmark) for 60 min at room temperature. The slides were then mounted with Vectashield mounting medium (Vector Laboratories Inc., Burlingame, CA, USA) and examined. Control experiments included incubation of cells with nonimmune isotypic control antibodies followed by the appropriate labelled secondary antibodies.
Human mesangial cells, tubular epithelia, and human skin fibroblasts were obtained and cultured following standard recipes with slight modifications [19,20].
Detection of anti-actin/ATP synthase β chain antibodies
The research of circulating antibodies in patients with iNS was done by immuno-western utilizing a panel of human cells in culture. We included both human renal cells (podocytes, mesangial cells, tubular epithelia) and skin fibroblasts. Podocytes and other cells were first extracted in β-hexyl-glucopyranoside (25 mM in Tris-HCL ph 7·4, 10 mM KCl, 0·15%β-hexyl-glucopyranoside, 1 mM EDTA plus phosphatase and protease inhibitors) and the extracted proteins were separated with one or two dimensional electrophoresis (see below). After separation, gel strips (in case of one-dimension electrophoresis) or entire gels (in case of two-dimensional electrophoresis) were incubated overnight with serum diluted 1 : 50 in TBS-T (Tris Buffer, 0·2% Tween 20, 0·5 M NaCl), at 4C° in the dark. Nitrocellulose saturation was achieved with 4% BSA in TBS-T with different washes. Alkaline-phosphatase conjugate anti-human IgG and IgM (DAKO, Copenhagen, Denmark) diluted 1 : 1·000 in 4% BSA and TBS-T were incubated for 2 h at room temperature.
Two-dimensional electrophoresis and western-blot
Two-dimensional electrophoresis, preparation and re-hydration of immobilized gradients (IPGs) and polyacrylamide gels have been described in detail elsewhere [21]. Briefly, the IPG strips were re-hydrated overnight at 4°C in 8 M urea, 0·5 M Thiourea, 2% w/v CHAPS, 0·6% w/v carrier ampholytes (IPGs, Amersham Pharmacia Biotech), and a trace of bromophenol blue. Proteins (30 µg) were solubilized with a solution containing 9 M urea, 4% w/v CHAPS, and 40 mM Tris. Isoelectric focusing was performed at 18°C. The applied voltage for electrophoresis was increased from 300 to 3500 V during the first 5 h, followed by 5000 V for a total of 100 kV h. Before the 2-D run, IPG strips were put on the strip tray for 30 min with a solution of 0·05 M Tris-HCl buffer pH 6·8, 6 M urea, 30% v/v glycerol, 2% w/v SDS, and a trace of bromophenol blue. The second dimension was performed on 180 × 160 × 1·5 mm slabs of polyacrylamide gradient gels (%T 8–16) using piperazine diacrylamide (PDA) as cross-linking agent. The gels were run at 45 mA/gel constant current and maintained at a temperature of 12°C.
For Western blot, proteins were transblotted to Hybond nitrocellulose membranes (Amersham Pharmacia Biotech, Little Chalfont, UK) with a Novablot semidry system using a continuous buffer system with 38 mM Tris, 39 mM glycine, 0·035% SDS,and 20% methanol. The transfer was achieved at 1·55 mA/cm2 for 1·5 h. Colour was developed with 4-nitro-blue tetrazolium (NBT) and 5-bromo-4-chloro-3-indolyl-phoshate (BICP) in 0·1 M bicarbonate buffer pH 9·8 plus 1 mM MgCl2.
MALDI-mass
In-gel enzymatic digestion
Following the staining procedure, the 2D gel was washed in water and the spots of interest were excised. In-gel digestion was carried out in 100 mM ammonium bicarbonate 1 mM CaCl2 pH 8·9, 30% acetonitrile and 12·5 ng/µl (1 µg) of sequencing grade modified trypsin (Promega, Madison, WI, USA) overnight at 37°C as previously described [22]. After tryptic digestion, the sample was dried in a vacuum centrifuge (Savant Instruments, Farmingdate, NY, USA) to reduce acetonitrile concentration, diluted in 0·25% formic acid and filtered using a 0·02 µm Anodisc 13 filter (Whatman) in a MicroFilter system (ProteinSolutions, Lakewood, NJ, USA).
LC/ESI-MS/MS analysis of tryptic peptides
An automated LCQ-DECA MS/MS ion trap mass spectrometer coupled to a HPLC Surveyor (Thermo Finnigan) and equipped with a 1 × 150 mm column, Vydac C18, 5 µm, 300 Å (Dionex Company, San Francisco, CA, USA) were used. Peptides were eluted from the column using an acetonitrile gradient, 5% B for 3 min followed by 5–90% B within 52 min (eluent A: 0·25% formic acid in water; eluent B: 0·25% formic acid in acetonitrile) at a flow-rate of 50 µl/min. The capillary of the ion trap was kept at 200°C and the voltage at 30 V. Spray voltage was 5·0 kV. Spectra were acquired in automated MS/MS mode: each full MS scan (in the range 400–2000 m/z) was followed by three MS/MS of the most abundant ions, using relative collision energy of 35%. Computer analysis of peptide MS/MS spectra was performed using the version 1·2 of the TurboSEQUEST software (University of Washington, licensed to ThermoFinnigan Corp.) and searched against the National Center for Biotechnology Information (NCBI) human protein database.
IgM purification and infusion in rats
Purification of serum IgM was performed by immunoaffinity chromatography utilizing a resin produced in our laboratory, where anti-human IgM antibodies (Dako, Copenhagen, DK) were linked to CNBR activated Sepharose 4B (Amersham Pharmacia Biotech) in 0·1 M borate buffer pH 8·9 +0·5 M NaCl, by overnight incubation at 4 °C. Blocking of remaining active groups was done in 1 M ethanolamine pH 8·0. At the end of the procedure the purity of IgM was tested by two dimensional electrophoresis (see above) and their isoelectric points were characterized by immuno-western with ant-human IgM antibodies. Equal amounts of IgM purified from the children with FSGS and from normal donors (from 0·1 to 1 mg) dissolved in 1 ml PBS were slowly infused three times every other day, in the tail of 6 (3 IgM from FSGS and 3 IgM from normal controls) Sprague-Dawley rats (150–200 g) placed in metabolic cages. Every 12 h urine was collected and tested for the presence of proteinuria. Rats were then sacrificed after 4 days from the start of proteinuria and renal samples were frozen in isopenthane. Immunofluorescence was done with FITC anti-human IgM and anti-rat IgG-IgM antibodies (Dako).
Characterization of serum IgM
After purification, characterization of IgM was done by two-dimensional-electrophoresis and silver staining following the method originally described by Merril and Goldman [23].
Statistical analysis
One way analysis of variance was used to compare data on proteinuria in rats infused with human IgM.
Results
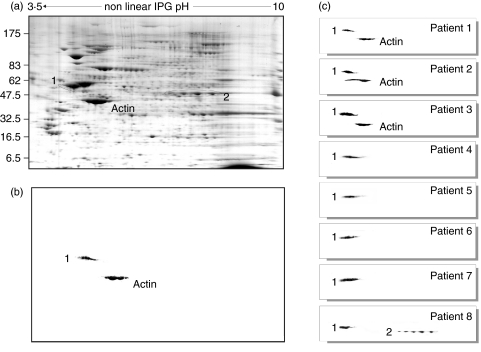
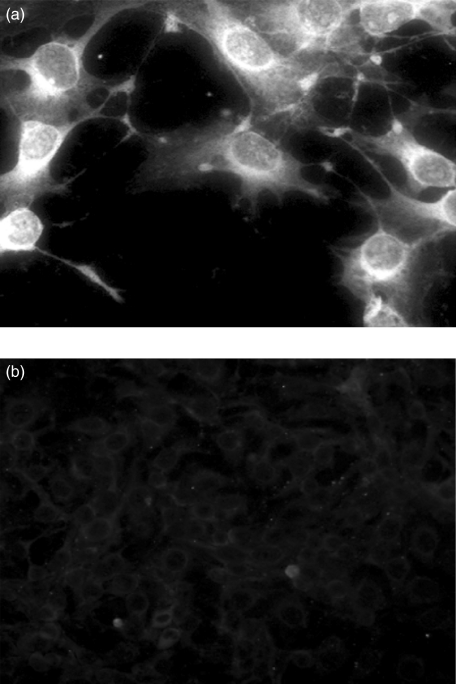
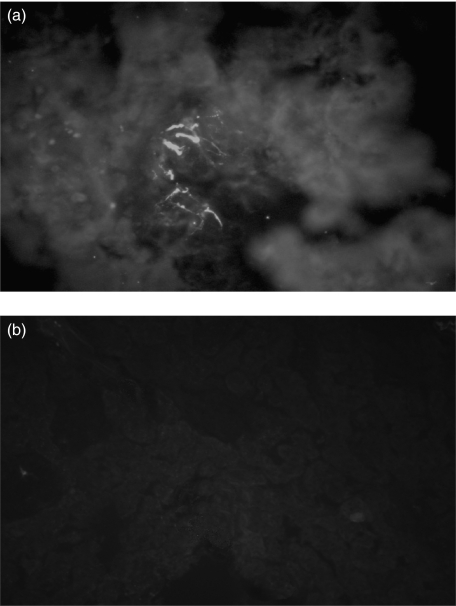
As a part of a screening study in patients with iNS that involves genetic and humoral factors, all patients enrolled between 1998 and 2004 in our department had tests for unveiling a hypothetical role of auto-immunity. Overall, 60 children with iNS were enrolled, eight showing familial trait and/or proven mutations of NPHS2. Following our clinical protocol, frozen sera were utilized for the screening on circulating antibodies against renal (podocytes, mesangial cells, tubular epithelia) and non-renal (skin fibroblasts) cells utilizing indirect Western blot. According to this procedure, serum is first incubated with extracts of different cell types previously separated by mono- or two-dimensional electrophoresis and the presence of antibodies is demonstrated by specific double-labelling with anti-serum immunoglobulins. Antibodies of the IgM class were found in eight children: in four patients IgM recognized a single protein, whereas in another three IgM recognized two spots. One child had antibodies of the IgG class. In Fig. 1b,c it is shown the western analysis of the 8 sera with podocyte extracts and, for comparison, it is reported the protein composition of a whole podocyte extract (Fig. 1a). Actin was recognized on the basis of clear electrophoretic coordinates, two other spots were characterized by MALDI-Mass as ATP synthase β chain and aldose reductase. The results are reported in Table 2, which shows the peptide composition of both ATP synthase β chain and aldose reductase and the coverage given by the sum of the molecular mass of all peptides. All cell extracts were positive to IgM auto-antibodies including skin fibroblasts (not shown) this finding excluding renal specificity of the phenomenon. Permeabilized human podocyte cells lines were also studied by immunofluorescence; as shown in Fig. 2 indirect immunofluorescence with anti-human IgM of cells preincubated with serum of patients positive to immuno-westen were positive while normal serum was negative for the presence of anti-podocyte antibodies.
Fig. 1.
(a) Two dimensional electrophoresis of a β-hexyl extract of human podocytes; (b) immuno-Western blot of a serum from one child with nephrotic syndrome; actin was identified on the basis of well-defined electrophoretic characteristics, spot 1 was characterized by MALDI-Mass; (c) immuno-Western blot of each patient with nephrotic syndrome and presence of anti-podocyte antibodies. Also in these cases, spots 1 and 2 were characterized by MALDI-Mass (see Table 2).
Table 2. Peptide composition of target antigen of serum IgM (7 cases) and IgG (1 case) immunoglobulins in children with nephrotic syndrome.
| Spot no. | Protein SWISS-PROT accession number | Peptide no. | Experimental [M + H] | Matched sequence | Position in protein |
|---|---|---|---|---|---|
| 1 | ATP synthase beta chain | 1 | 1632·88 | LVLEVAQHLGESTVR | 105–119 |
| P06576 | 2 | 1901·24 | VLDSGAPIKPPVGPETLGR | 135–153 | |
| 3 | 1020·20 | IPVGPETLGR | 144–153 | ||
| 4 | 1367·58 | IMNVIGEPIDER | 154–165 | ||
| 5 | 1070·30 | VVDLLAPYAK | 199–208 | ||
| 6 | 1388·51 | AHGGYSVFAGVGER | 236–249 | ||
| 7 | 1421·66 | VALTGLTVAEYFR | 292–304 | ||
| 8 | 1904·11 | DQEGQDVLLFIDNIFR | 305–320 | ||
| 9 | 1417·58 | FTQAGSEVSALLGR | 321–334 | ||
| 10 | 2248·55 | IPSAVGYQPTLATDMGTMQER | 335–355 | ||
| 11 | 1798·00 | IMDPNIVGSEHYDVAR | 417–432 | ||
| 12 | 2658·02 | SLQDIIAILGMDELSEEDKLTVSR | 443–466 | ||
| 2 | Aldose reductase | 1 | 823·99 | LLLNNGAK | 4–11 |
| O60218 | 2 | 1116·40 | MPILGLGTWK | 12–21 | |
| 3 | 1094·23 | SPPGQVTEAVK | 22–32 | ||
| 4 | 874·01 | VAIDVGYR | 33–40 | ||
| 5 | 1102·30 | REELFIVSK | 69–77 | ||
| 6 | 946·11 | EELFIVSK | 70–77 | ||
| 7 | 3245·64 | GIVVTAYSPLGSPDRPWAKPEDPSLLEDPR | 203–232 | ||
| 8 | 902·08 | TTAQVLIR | 243–250 | ||
| 9 | 763·98 | NLVVIPK | 256–262 |
Fig. 2.
Indirect immunofluorescence of permeabilized human podocytes ‘in culture’ incubated with (a) serum of patients with circulating anti-actin and anti-ATP synthase β chain antibodies and (b) normal serum. After several PBS washings slides were incubated with FITC conjugated anti-human IgM antibodies. In the former case, diffuse cytoplasmatic staining of human IgM was observed.
Patients with circulating auto-antibodies could not be readily differentiated from other patients with primary NS with repect to general clinical and pathological characteristics. As detailed in Table 3, all had an early onset of proteinuria (between 1 and 14 years) and had strict resistance or dependence to steroids while six out of eight patients treated with cyclosporin, two were resistant and four were sensitive. Sensitivity to cyclosporin was associated with good long-term outcome in all cases. The histological background was FSGS in four cases and MesIgM in two, while one patient had an unspecific pattern and in another one biopsy was not performed. Three patients, two with both anti-actin and anti-ATP synthase β chain antibodies and one with only the anti-ATP synthase β chain, developed after 5 years or more of follow-up positivity for antinuclear antibodies (ANA) with normal complement levels while showing a clear pathology picture of FSGS/Mes IgM excluding lupus nephrites.
Table 3. Clinical features of 8 patients with nephrotic syndrome who presented anti-podocyte antibodies.
| Drug sensitivity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type of antibody | Age (years) | Age at onset (years) | Serum IgM mg% | Parameter ANA | Steroids | Cyclosporin | Histology | IF | ESRF | TX |
| IgM-anti-Actin and ATP synthase | ||||||||||
| Patient 1 (LD) | 17·3 | 8·3 | 1·2 | Pos 1 : 360 | Resistant | Resistant | FSGS | IgM | No | |
| Patient 2 (DE) | 8·7 | 1·4 | 400 | Pos 1 : 160 | Dependent | Sensitive | Mes Prol | IgM | No | |
| Patient 3 (LS) | 7 | 3·05 | Neg | Dependent | Sensitive | Mes Prol | IgM | No | ||
| IgM-anti-ATP synthase | ||||||||||
| Patient 4 (BB) | 25 | 14 | nd | Neg | Resistant | Non utilized | Unspecific | nd | Yes | Yes |
| Patient 5 (AL) | 19 | 14 | nd | Neg | Resistant | Resistant | FSGS | nd | Yes | Yes |
| Patient 6 (ED) | 7 | 2 | 200 | Pos 1 : 80 | Dependent | Sensitive | nd | nd | No | |
| Patient 7 (VD) | 9·6 | 2·1 | 174 | Neg | Dependent | Sensitive | FSGS | IgM | No | |
| IgM anti-b-Tubulin and IgG anti-aldose reductase | ||||||||||
| Patient 8 (DV) | 16·7 | 5·2 | 269 | Neg | Resistant | Non utilized | FSGS | IgM/IgG | Yes | Yes |
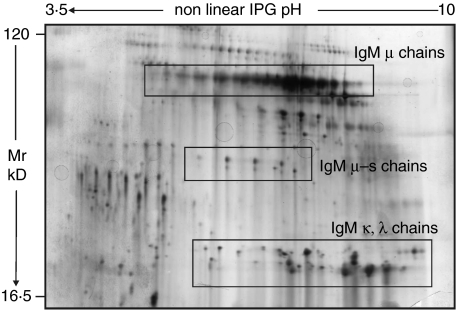
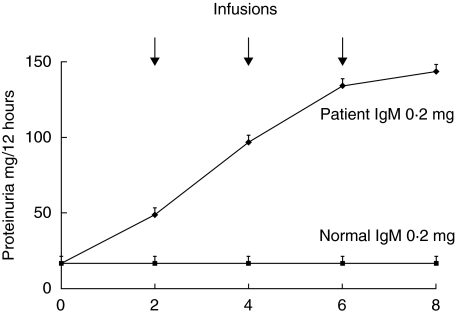
To demonstrate a cause-effect relationship between circulating antibodies and proteinuria, serum IgM were purified from plasma of the unique patients who was treated with plasmapheresis and were given to rats at doses from 0·1 to 1 mg three times every other day. Infused IgM deriving from purification steps had been preventively characterized by 2D-electrophoresis and Silver stain and the result is given in Fig. 3. Rats infused with IgM purified from the child with FSGS developed proteinuria after 6 days of treatment (Fig. 4) whereas those rats treated with normal IgM did not. Indirect immunofluorescence of rat kidneys with FITC antibodies against rat and human proteins demonstrated the presence of focal mesangial deposts of human IgM in rat glomeruli and negativity of any rat component within glomeruli. This pattern is shown in Fig. 5a and b which gives an overview at low magnification of rat glomeruli after infusion with patients’ IgM. In one case (Fig. 5a) mesangial human IgM deposition is evident, while no rat IgM, IgC and C3 complement (Fig. 5b) could be observed.
Fig. 3.
Two-dimensional electrophoresis with silver stain of plasma IgM purified from plasmapheresis eluates of a patient with both anti-actin and anti-ATP synthase antibodies. This analysis shows a high degree of purity of infused IgM, mainly consisting in heavy (µ), intermediate (µs) and light chains (kλ). Groups of proteins with anionic pIs and MW between 60 and 10 kD represent a minor fraction (10%) of this preparation.
Fig. 4.
Outcome of proteinuria in rats treated with normal IgM and with IgM purified from serum of a patient with marked hyper IgM whose electrical charge was slightly more cationic than normal. Equal amounts (0.2 mg) of purified IgM were infused in the tail of 8 rats (4 normal and 4 cationic) at time 0 and every other days. Proteinuria was determined with the Coomassie dye binding assay and results are given as Mean ± standard deviation
Fig. 5.
Indirect immunofluorescence of a 2 mm section of frozen rat kidneys stained with FITC (a) anti-human IgM (×200) and (b) anti-rat IgM (×200). Focal deposition of human IgM in mesangial areas are evident, while rat IgM were absent.
Discussion
This is the first description of circulating anti-actin/ATP synthase antibodies in a small subset of patients with iNS. On clinical grounds, they were children with poor or partial response to steroids and a pathological background of FSGS/mesangial IgM deposition and could not be readily differentiated from other FSGS patients, with the unique exception of 3 patients who developed antinuclear-antibodies during the follow-up. According to current criteria of classification the 3 children with combined positivity for antinuclear and anti-actin/ATP synthase β chain antibodies are included in the subset of patients who present serological markers of lupus still maintaining clear pathological features of FSGS/IgM or MCN. Hertig et al.[12] reported 11 patients with iNS and FSGS who developed SLE and in most cases mesangial deposition of IgM was found. They probably represent a separate cohort of all SLE patients (or, alternatively, a separate cohort of patients with iNS) and the proposed rough estimate of the incidence of patients with FSGS/mesangial IgM in SLE was about 2–4%. Thus the combination is far more common as expected and may represent a separate clinical entity. Also the characterization of actin as the target antigen of auto-antibodies seems particularly relevant since anti-actin antibodies have been characterized as a main component of lupus anti-DNA antibodies [9] and actin was recognized as the target of nephritogenic antibodies in this condition [10,24]. In spite of these supporting facts and until the pathogenesis of FSGS and more in general of iNS is better characterized [25–29] any conclusion on a pathogenesis linked to autoimmunity in a subset of patients remains speculative. The finding of IgM auto-antibodies against podocytes seems intriguing all the same since these cells have been recognized as the effective renal barrier to filtration of proteins [15,30,31] and maintainance of their integrity is critical to the overall process of ultrafiltration of proteins. Lacking proofs for a causative relationship between antipodocyte antibodies and proteinuria their presence could simply represent an epiphenomenon and further studies will define this basic aspect. We approached this problem experimentally by infusing in rats IgM purified from a single plasma eluate and were able to reproduce proteinuria and IgM mesangial deposition. Therefore, the above result supports a direct pathogenic connection between circulating anti-actin/ATP synthase β chain with mesangial IgM deposition and more in general with proteinuria. It must be stressed that this remains an isolated experience since the single donor of plasma IgM was a child treated with plasmapheresis due to the very high title of serum IgM for which the procedure was indicated. On the other hand, purification of plasma IgM for infusion in rats requires large amount of plasma to be processed and this is only possible if plasma eluates from plasmapheresis are available. Unfortunately, based on lack of evidence on potential benefits of plasmapheresis in patients with iNS no other patient was treated with this technique.
A second issue concerns specificity of antibodies, since they reacted, besides with podocytes and mesangial cells, also with skin fibroblast actin/ATP synthase β chain and this does not appear surprising based on the ubiquity of both proteins. What could mediate podocyte toxicity in glomeruli is the strong structural analogies between actin and α-actinin-4, a protein that in glomeruli plays a central role in maintaining impermeability to proteins. Actually mutations of α-actinin-4 [11] determine a familial NS with autosomal dominant inheritance and a case of molecular mimicry between actin and its glomerular homolog may determine the proteinuric response to anti-actin antibodies.
In conclusion, this paper reports the presence of anti-actin/ATP synthase β chain antibodies in serum of a subset of patients with iNS. They presented clinical and pathological hallmarks of FSGS/mesangial IgM glomerulonephritis and developed positivity to antinuclear antibodies in 3 cases. While the effective significance of these auto-antibodies remains uncertain, experiments in animals treated with IgM from a single patient suggest a pathogenic link. If this will be proved correct, removal from plasma of anti-podocyte antibodies could become an alternative therapeutic approach to those classically utilized in these patients but this approach requires more clinical and experimental evidence that we hope will be stimulated by present findings.
Acknowledgments
We acknowledege the invaluable help of Miss Capurro in preparing the text. This work was done with the financial support of the Italian Ministry of Health (Progetto Finalizzato ‘Patogenesi delle malattie della barriera di filtrazione glomerulare’, Convenzione N°178/2002). This study was partially supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC) and EU QLRT-2000–01495 Project Therapeuticantibodies. We are grateful to Dr Luciano Zardi for fruitful discussions. We finally acknowledge the financial support of KIDney FUND and in particular we thank Prof Rosanna Gusmano
References
- 1.Korbet SM, Schwartz MM, Lewis EJ. Primary focal segmental glomerulosclerosis: clinical course and response to therapy. Am J Kidney Dis. 1994;6:773–83. doi: 10.1016/s0272-6386(12)80128-4. [DOI] [PubMed] [Google Scholar]
- 2.Bonilla-Felix M, Parra C, Dajani T, Ferris M, Swinford RD, Portman RJ, Verani R. Changing patterns in the histopathology of idiopathic nephrotic syndrome in children. Kidney Int. 1999;5:1885–90. doi: 10.1046/j.1523-1755.1999.00408.x. [DOI] [PubMed] [Google Scholar]
- 3.Braden GL, Mulhern JG, O'Shea MH, Nash SV, Ucci AA, Jr, Germain MJ. Changing incidence of glomerular diseases in adults. Am J Kidney Dis. 2000;5:878–83. doi: 10.1016/s0272-6386(00)70258-7. [DOI] [PubMed] [Google Scholar]
- 4.Sabbatini A, Dolcher MP, Marchini B, et al. Alpha-enolase is a renal-specific antigen associated with kidney involvement in mixed cryoglobulinemia. Clin Exp Rheumatol. 1997;6:655–8. [PubMed] [Google Scholar]
- 5.Madaio MP, Yanase K. Cellular penetration and nuclear localization of anti-DNA antibodies. mechanisms, consequences, implications and applications. J Autoimmun. 1998;5:535–8. doi: 10.1006/jaut.1998.0217. [DOI] [PubMed] [Google Scholar]
- 6.Dolcher MP, Marchini B, Sabbatini A, et al. Autoantibodies from mixed cryoglobulinaemia patients bind glomerular antigens. Clin Exp Immunol. 1994;2:317–22. doi: 10.1111/j.1365-2249.1994.tb06560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pratesi F, Moscato S, Sabbatini A, Chimenti D, Bombardieri S, Migliorini P. Autoantibodies specific for alpha-enolase in systemic autoimmune disorders. J Rheumatol. 2000;1:109–15. [PubMed] [Google Scholar]
- 8.Wakui H, Imai H, Komatsuda A, Miura AB. Circulating antibodies against alpha-enolase in patients with primary membranous nephropathy (MN) Clin Exp Immunol. 1999;3:445–50. doi: 10.1046/j.1365-2249.1999.01080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mostoslavsky G, Fischel R, Yachimovich N, et al. Lupus anti-DNA autoantibodies cross-react with a glomerular structural protein: a case for tissue injury by molecular mimicry. Eur J Immunol. 2001;4:1221–7. doi: 10.1002/1521-4141(200104)31:4<1221::aid-immu1221>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 10.Boulila A, Hachicha J, Adyel FZ, Jlidi R, Avrameas S, Ternynck T, Ayadi H. Deposition of anti-actin antibodies in the kidney of a patient with systemic lupus erythematosus under immunosuppressive treatment. Nephrol Dial Transplant. 1996;12:2478–81. doi: 10.1093/oxfordjournals.ndt.a027218. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan JM, Kim SH, North KN, et al. Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet. 2000;3:251–6. doi: 10.1038/73456. [DOI] [PubMed] [Google Scholar]
- 12.Hertig A, Droz D, Lesavre P, Grunfeld JP, Rieu P. SLE and idiopathic nephrotic syndrome. coincidence or not? Am J Kidney Dis. 2002;6:1179–84. doi: 10.1053/ajkd.2002.36875. [DOI] [PubMed] [Google Scholar]
- 13.International Study of Kidney Disease in Children. Primary nephrotic syndrome in children: clinical significance of histopathologic variants of minimal change and of diffuse mesangial hypercellularity. A Report of the ISKDC. Kidney Int. 1981;6:765–71. doi: 10.1038/ki.1981.209. [DOI] [PubMed] [Google Scholar]
- 14.International Study of Kidney Disease in Children. Prospective, controlled trial of cyclophosphamide therapy in children with nephrotic syndrome. Report of the ISKDC. Lancet. 1974;7878:423–7. [PubMed] [Google Scholar]
- 15.Caridi G, Bertelli R, Di Duca M, et al. Broadening the spectrum of diseases related to podocin mutations. J Am Soc Nephrol. 2003;5:1278–86. doi: 10.1097/01.asn.0000060578.79050.e0. [DOI] [PubMed] [Google Scholar]
- 16.Caridi G, Bertelli R, Carrea A, et al. Prevalence, genetics, and clinical features of patients carrying podocin mutations in steroid-resistant nonfamilial focal segmental glomerulosclerosis. J Am Soc Nephrol. 2001;12:2742–6. doi: 10.1681/ASN.V12122742. [DOI] [PubMed] [Google Scholar]
- 17.Conaldi PG, Biancone L, Bottelli A, De Martino A, Camussi G, Toniolo A. Distinct pathogenic effects of group B coxsackieviruses on human glomerular and tubular kidney cells. J Virol. 1997;12:9180–7. doi: 10.1128/jvi.71.12.9180-9187.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conaldi PG, Biancone L, Bottelli A, et al. HIV-1 kills renal tubular epithelial cells in vitro by triggering an apoptotic pathway involving caspase activation and Fas upregulation. J Clin Invest. 1998;12:2041–9. doi: 10.1172/JCI3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Striker GE, Striker LJ. Glomerular cell culture. Laboratory Invest. 1985;2:122–31. [PubMed] [Google Scholar]
- 20.Candiano G, Gusmano R, Altieri P, et al. Extracellular matrix formation by epithelial cells from human polycystic kidney cysts in culture. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;1:1–9. doi: 10.1007/BF02899238. [DOI] [PubMed] [Google Scholar]
- 21.Bruschi M, Musante L, Candiano G, Ghiggeri GM, Herbert B, Antonucci F, Righetti PG. Soft immobilized pH gradient gels in proteome analysis: a follow-up. Proteomics. 2003;6:821–5. doi: 10.1002/pmic.200300361. [DOI] [PubMed] [Google Scholar]
- 22.Bottino C, Castriconi R, Pende D, et al. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med. 2003;4:557–67. doi: 10.1084/jem.20030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merril CR, Goldman D. Quantitative two-dimensional protein electrophoresis for studies of inborn errors of metabolism. Clin Chem. 1982;4:1015–20. [PubMed] [Google Scholar]
- 24.Migliorini P, Pratesi F, Bongiorni F, Moscato S, Scavuzzo M, Bombardieri S. The targets of nephritogenic antibodies in systemic autoimmune disorders. Autoimmun Rev. 2002;3:168–73. doi: 10.1016/s1568-9972(02)00028-9. [DOI] [PubMed] [Google Scholar]
- 25.Dantal J, Bigot E, Bogers W, et al. Effect of plasma protein adsorption on protein excretion in kidney-transplant recipients with recurrent nephrotic syndrome. N Engl J Med. 1994;1:7–14. doi: 10.1056/NEJM199401063300102. [DOI] [PubMed] [Google Scholar]
- 26.Savin VJ, Sharma R, Sharma M, et al. Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N Engl J Med. 1996;14:878–83. doi: 10.1056/NEJM199604043341402. [DOI] [PubMed] [Google Scholar]
- 27.Ghiggeri GM, Artero M, Carraro M, Perfumo F. Permeability plasma factors in nephrotic syndrome: more than one factor, more than one inhibitor. Nephrol Dial Transplant. 2001;5:882–5. doi: 10.1093/ndt/16.5.882. [DOI] [PubMed] [Google Scholar]
- 28.Sharma M, Sharma R, McCarthy ET, Savin VJ. ‘The FSGS factor’: enrichment and in vivo effect of activity from focal segmental glomerulosclerosis plasma. J Am Soc Nephrol. 1999;3:552–61. doi: 10.1681/ASN.V103552. [DOI] [PubMed] [Google Scholar]
- 29.Musante L, Candiano G, Bruschi M, et al. Characterization of plasma factors that alter the permeability to albumin within isolated glomeruli. Proteomics. 2002;2:197–205. doi: 10.1002/1615-9861(200202)2:2<197::aid-prot197>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 30.Boute N, Gribouval O, Roselli S, et al. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid—resistant nephrotic syndrome. Nat Genet. 2000;4:349–54. doi: 10.1038/74166. [DOI] [PubMed] [Google Scholar]
- 31.Saleem MA, Ni L, Witherden I, Tryggvason K, Ruotsalainen V, Mundel P, Mathieson PW. Co-localization of nephrin, podocin, and the actin cytoskeleton: evidence for a role in podocyte foot process formation. Am J Pathol. 2002;4:1459–66. doi: 10.1016/S0002-9440(10)64421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]