Identification of a T lineage committed progenitor in adult blood (original) (raw)
. Author manuscript; available in PMC: 2007 Mar 19.
Abstract
With help of a hCD25 reporter controlled by Ptcra regulatory elements T cell precursors were identified in peripheral blood. Sca-1+, IL-7Rα+, Flt3− precursors that were c-kitlo and Thy-1hi generated T lineage cells when cultured on OP9-DL1 stromal cells and upon transfer into Rag−/−γc−/− mice. No B cells were generated in vivo and only few in vitro. The circulating T cell progenitors (CTP) were found at the same frequency in Foxn1nu/nu thymus deficient mice and wt mice indicating that they were pre- rather than post-thymic, consistent with the observation that inhibition of Notch signaling in vivo did reduce the frequency of intrathymic ETP, but not CTP. Thus, extrathymic T cell commitment is Notch-independent even in adult mice.
Introduction
All blood cell lineages are derived from self-renewing hematopoietic stem cells (HSC) that reside in fetal liver or adult bone marrow (BM). HSC can generate Fms-like tyrosine kinase receptor 3 (Flt3) positive multi-potent progenitors (MPP), that are likewise lineage negative, Sca-1 positive, and c-kit high (LSK) (Adolfsson et al., 2001), as well as more committed precursors such as RAG-1 positive early lymphoid progenitors (ELP) (Igarashi et al., 2002), L-selectin positive progenitors (LSP) (Perry et al., 2004), or common lymphoid progenitors (CLP) (Kondo et al., 1997). T cell development in the adult thymus depends on continuous recruitment of progenitors from the bone marrow via the blood stream. However the nature of these thymic immigrants has remained largely elusive. Based on phenotypic similarity to the most primitive early T cell progenitors (ETP) multipotent LSK cells or a subfraction thereof in blood have been suggested to constitute physiologic thymic immigrants in adult mice (Allman et al., 2003; Benz and Bleul, 2005; Schwarz and Bhandoola, 2004). However, immigration of these cells into the thymus has not been directly demonstrated. In addition, ETP differ from circulating LSK cells with respect to their dependence on Notch signals: Abrogation or reduction of Notch signals led to a massive reduction in ETP numbers, whereas blood LSK cells were not affected, suggesting that Notch signals are required for the generation of ETP, but not LSK cells (Sambandam et al., 2005; Tan et al., 2005). In addition, we have recently demonstrated that extrathymic precursors display a marked phenotypic plasticity upon exposure to Notch signals, thus putting into question the use of surface markers such as c-kit to suggest precursor-product relationships (Krueger et al., 2006).
CLP-2 cells that were identified in our laboratory using transgenic reporter mice that express human CD25 (hCD25) under control of the Ptcra promoter and enhancer may also constitute a population of thymic progenitors (Gounari et al., 2002; Martin et al., 2003). Such cells in BM have a lin−c-kit−/loB220+ phenotype and originate from lin−c-kit+B220−IL-7Rα+ CLP cells. CLP-2 efficiently enter the thymus upon intravenous transfer and give rise to a single wave of T cells indicating limited self renewal capacity (Martin et al., 2003; Scimone et al., 2006). CLP-2 progress developmentally along the T lineage in vitro with kinetics similar to ETP (Krueger et al., 2006). Others have shown that early thymic immigrants after BM transfer are c-kit− and enriched for B220+ cells (Mori et al., 2001). However, CLP-2 have not yet been detected in the circulation under steady state conditions.
With regard to homing of extrathymic progenitors to the thymus it became recently apparent that P-selectin-PSGL-1 interactions are critical for thymus seeding. In fact, P-selectin binding capacity was detected on both BM and circulating LSK as well as CLP and ETP cells (Rossi et al., 2005). In addition, the CCL25/CCR9 chemokine receptor system appears to play a role in thymus homing as CCR9−/− BM derived progenitors displayed reduced thymic repopulation efficiency (Rossi et al., 2005; Scimone et al., 2006; Uehara et al., 2002).
The most potent T cell progenitors in the thymus are found in the heterogenous double-negative (DN) 1 thymocyte subset (CD44+CD25−) and were originally characterized as CD4loc-kit+ (Moore and Zlotnik, 1995; Wu et al., 1991). These cells were shown to have the potential to generate T, B, NK and lymphoid dendritic cells, although initially no clonogenic assay was available to directly show pluripotentiality. More detailed analysis of the DN1 subset led to the identification of lineage negative, Sca-1 high, c-kit high (LSK) and IL-7Rα−/lo early T lineage progenitors (ETP) (Allman et al., 2003). These cells were shown to have high T, but only limited B and myeloid potential. ETP could be further subdivided according to their expression levels of Flt3 or a CC chemokine receptor 9 (CCR9)-eGFP reporter gene (Benz and Bleul, 2005; Sambandam et al., 2005). ETP expressing Flt3 were shown to constitute a more immature subset and loss of Flt3 or CCR9-eGFP expression correlated with loss of B cell potential. Another study employing subfractionation of DN1 cells according to c-kit and heat stable antigen (HSA) expression showed that even though most DN1 subsets are able to generate T cells the most potent T cell progenitors were localized in the DN1a and b subsets that correspond to ETP and express high levels of c-kit and are HSA negative/low or HSA positive and presumably reprensenting a precursor-product relationship (Porritt et al., 2004).
Progressive loss of B cell potential during maturation of ETP (Benz and Bleul, 2005; Sambandam et al., 2005) and clonal analysis of the most immature ETP subfraction (Benz and Bleul, 2005) has led to the hypothesis that the decision between B and T lineage commitment of thymic progenitors in adult mice is an intrathymic event, although this has been of considerable debate (Balciunaite et al., 2005; Jenkinson et al., 2006; Lu et al., 2005). In contrast, T lineage commitment during fetal hematopoiesis appears to occur pre-thymically. Of note, no common lymphoid progenitor has been identified during fetal lymphoid development (Douagi et al., 2002; Kawamoto et al., 1997). It has been suggested that in fetal liver T cell progenitors emerge earlier than B cell progenitors during ontogeny (Kawamoto et al., 2000). In addition, T cell progenitors that apparently lack B or myeloid potential have been identified in fetal blood at different stages of development and were characterized as c-kit+IL-7R+ (Ikawa et al., 2004) and expressing paired immunoglobulin-like receptors (Masuda et al., 2005) or to be of the c-kitloThy-1+ phenotype (Rodewald et al., 1994). T cell progenitors are enriched in fetal blood during the first wave of thymus seeding suggesting a selective release of these precursors when compared to B cell progenitors (Ikawa et al., 2004).
Signaling from Notch transmembrane receptors has been implicated in lineage fate decisions of cells in multiple developmental systems in various species (Artavanis-Tsakonas et al., 1999). It has been suggested that Notch-mediated signals determine T versus B lineage choice. Conditional deletion of Notch1 in hematopoietic progenitors results in failure of T cell development and accumulation of B cells in the thymus (Radtke et al., 1999; Wilson et al., 2001). Similar results were obtained by inhibition of Notch-mediated transcription or interference with Notch-Notch ligand interactions (Koch et al., 2001; Maillard et al., 2004). Conversely, over-expression of constitutively active Notch1 leads to aberrant T cell development in the BM (Pui et al., 1999). However, it has recently been suggested that fetal thymic precursors are already T lineage committed prior to receiving intrathymic Notch signals and independent of Hes1, a downstream target of Notch (Harman et al., 2005; Masuda et al., 2005).
Here we identified prethymic T cell progenitors circulating in blood of adult mice using transgenic reporter mice that express hCD25 under control of the Ptcra promoter. These cells have a c-kitloThy-1+ phenotype. In contrast to BM CLP and CLP-2 cells, these cells possess efficient T, but only very limited B and NK potential. Of note, these progenitors are not affected by inhibition of Notch signaling suggesting that early T lineage commitment may occur independently of Notch signals while Notch signals are required at later stages of T cell development.
Results
Lineage negative hCD25+ cells in peripheral blood of adult mice
T cell development in the thymus depends on continuous recruitment of hematopoietic progenitors from the bone marrow via the blood. hCD25+ T cell precursors have so far been identified in bone marrow and thymus, and it has been shown that hCD25+ CLP-2 can efficiently seed the thymus upon intravenous transfer. Here we have analyzed whether lin−hCD25+ cells can be detected in the blood of mice under steady state conditions. We found that approximately 0.06% of lineage negative cells in peripheral blood expressed the hCD25 marker, whereas no background staining was detected in reporter negative control mice (Fig. 1A). This corresponds to a frequency of 4.1±0.3 per 105 leukocytes or 330±24 lin−hCD25+ cells per mL blood. Further surface marker characterziation revealed that most of these cells are c-kit−/lo and some express B220. In both BM derived CLP-1 and CLP-2 cells expression of the hCD25 transgene corresponds to expression of pre-TCRα mRNA, whereas hCD25+CD19+ cells in BM, which are already committed to the B lineage, lack pre-TCRα expression (Gounari et al., 2002). Thus, we analyzed pre-TCRα expression in peripheral lin−hCD25+ cells as an indicator of T cell potential. Figure 1B shows that pre-TCRα message is clearly present in circulating lin−hCD25+ cells, suggesting that these cells might contain T lineage potential. Next, we compared the surface phenotype of circulating lin−B220+hCD25+ and lin− B220−hCD25+ cells with that of lin−hCD25+ CLP-1 (lin−hCD25+B220−c-kit+) and CLP-2 (lin−hCD25+B220+c-kit−/lo) cells from bone marrow and hCD25+ DN1 (lin−hCD25+CD25−CD44+) cells from thymus, the majority of which were shown to be DN1a/b cells (Krueger et al., 2006) (Fig. 1C). CLP-1 cells are mostly c-kit+IL-7Rα+Flt3hiSca-1+CD44hiThy-1.1−, with a minor fraction of cells expressing the Thy-1.1 marker. CLP-2 cells are c-kit−/loIL-7Rα+Flt3−/loSca-1−/loCD44hiThy-1.1−. In contrast, circulating lin−hCD25+B220+ cells are c-kit−IL-7Rα−Flt3−Sca-1+CD44+ and heterogenous for Thy-1.1+ expression and thus do not correspond phenotypically to BM derived CLP-2 cells. Circulating lin−hCD25+B220− cells are c-kit+IL-7Rα+Flt3−/loSca-1+CD44hiThy-1.1+ and thymic DN1 hCD25+ cells are c-kithiIL-7Rα−/loFlt3loSca-1+CD44hiThy-1.1lo. Approximately 4% of cells with a lin−B220−c-kitloIL-7RαloFlt3−/loSca-1+Thy-1.1+ phenotype in blood express hCD25 and it is presently not known whether the hCD25 negative subset of these cells has similar functional properties as the hCD25 positive subset (Supplemental Figure 1). This is similar to the previous analysis of CLP-1 cells in bone marrow where only a fraction of these cells express hCD25 (Martin et al., 2003). Thus, blood derived lin−hCD25+B220− cells display a surface phenotype somewhat similar to CLP-1 cells, but differing with respect to expression of Flt3 and, markedly, by expression of Thy-1.1 suggesting that these cells may constitute an adult counterpart of previously described c-kitloThy-1+ T cell progenitors from fetal blood (Rodewald et al., 1994). In fact, these precursors in fetal blood were likewise found to express Ptcra (Bruno et al., 1995).
Figure 1.
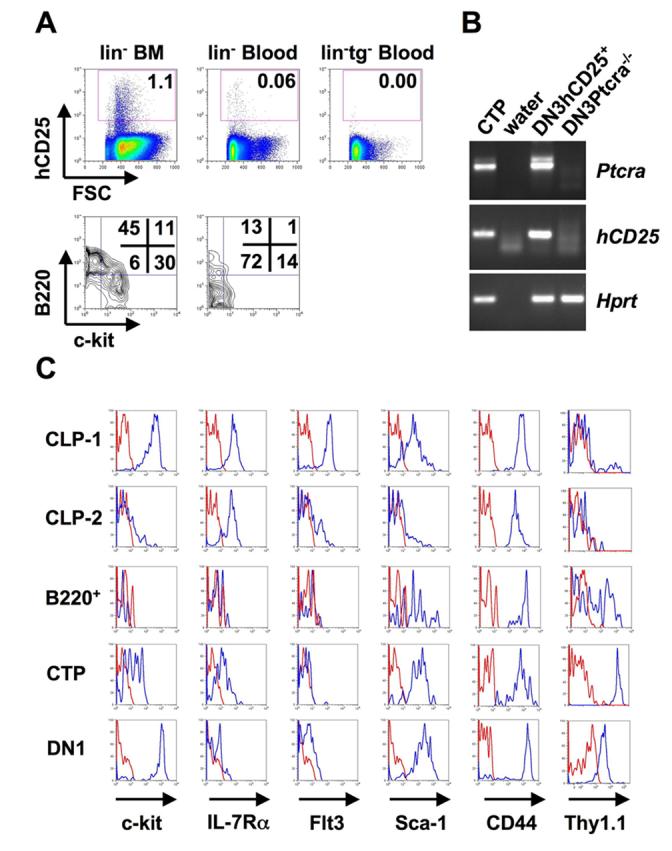
Circulating lin−hCD25+ precursors. (A) Lineage depleted BM and blood cells from hCD25 transgenic and non-transgenic mice were stained for lineage markers, hCD25, c-kit and B220. The lower panels show the expression of c-kit and B220 of electronically gated lin−hCD25+ cells. Numbers in FACS plots indicate percentages of cells within gates or quadrants. (B) Expression of pre-TCRα in circulating lin−hCD25+ cells. RT-PCR was performed on 250 CTP cells. The same amount of cDNA from hCD25+ DN3 cells and DN3 cells from Ptcra−/− mice was used as positive and negative controls, respectively. One representative out of 2 independent experiments is shown. (C) Expression of surface markers on different lin−hCD25+ populations. BM CLP-1 (lin−hCD25+ckit+B220−) and CLP-2 (lin−hCD25+c-kit−/loB220+) cells, blood lin−hCD25+B220+ cells (“B220+”) and CTP (lin−hCD25+B220−) and thymic hCD25+ DN1 (lin−CD25−CD44hihCD25+) cells were stained for c-kit, IL-7Rα, Flt3, Sca-1, CD44 and Thy-1.1. Histograms show expression levels of the respective surface markers (blue histograms) or unstained controls (red histograms) of electronically gated populations as indicated above. One representative out of 2 independent experiments is shown.
Developmental potential of lin−hCD25+ cells
In order to assess the developmental potential of hCD25+ cells from peripheral blood we employed OP9-GFP and OP9-DL1 stromal cell co-cultures (Schmitt and Zuniga-Pflucker, 2002). After 18 days of co-culture on OP9-DL1 cells CD4/CD8 double positive T cell progeny could be detected in cultures of hCD25+c-kitloB220− cells, whereas no T cell progeny was found after co-culture of hCD25+B220+ cells (Fig. 2A). Co-culture of hCD25+c-kitloB220− cells with OP9-GFP cells showed that these cells also contain some NK and B cell potential (Fig. 2A). No T cell development was observed in these cultures as evident by the absence of Thy-1.1 positive cells, suggesting that T cell development from these precursors is Notch-dependent (Fig. 2A). Co-culture of hCD25+B220+ cells on OP9-GFP cells again did not result in any detectable progeny (data not shown). In order to quantitate T, B and NK lineage potential of blood derived hCD25+c-kitloB220− cells and BM derived CLP-2 cells we sorted different numbers of precursor cells onto OP9-DL1 and OP9-GFP cell containing cultures and determined the frequency of lineage positive outgrowth. This analysis revealed that the potential of hCD25+c-kitloB220− cells to generate T cells is much higher than to generate B or NK cells (Fig 2B). Approximately 1 in 11 cells was able to give rise to T cells, whereas B or NK potential amounted to 1 in 390 or 1 in 330, respectively. In contrast, BM derived CLP-2 cells had similar T and B cell potential (1 in 14 and 1 in 12, respectively), but lower NK potential (1 in 63) (Krueger et al., 2006). The relatively low NK lineage potential in both assays might be due to suboptimal conditions, i.e. assays were performed in the absence of IL-15. At concentrations of 200 cells per well (Fig. 2A) we did not detect any wells containing cells of both lineages in the analysis of B and NK lineage potential on OP9-GFP cells. Thus, whereas lineage potential analysis of CLP-2 cells revealed a similar frequency of B and T lineage precursors (indicating that this assay is sufficiently sensitive to detect B lineage potential), the frequency of T cell precursors among hCD25+c-kitloB220− peripheral blood cells is much higher than that of B cell precursors. Therefore, we termed this population of cells “Circulating T cell Progenitors” (CTP). Expression of the hCD25 transgene regulated by Ptcra promoter and enhancer elements is indicative of lymphoid commitment (Gounari et al., 2002; Martin et al., 2003). However, in order to formally test for myeloid potential, we performed colony-forming assays using methylcellulose cultures (Fig. 2C). Whereas BM derived LSK cells showed robust myeloid potential, no colonies were formed in cultures starting with BM derived CLP-1, CLP-2 cells or CTP. In order to test the developmental potential of CTP in vivo, we injected hCD25+B220+ and hCD25+ckitloB220− cells (CD45.1) into irradiated Rag−/−γc−/− recipients (CD45.2). 5 weeks after transfer we analyzed for donor derived cells in the spleen. No donor derived cells could be detected after transfer of hCD25+B220+ cells. However, we cannot conclude from these experiments that hCD25+B220+ cells lack any precursor potential, because only rather limited numbers, which may be too limited for a meaningful characterization of this population, could be isolated. Upon transfer of CTP, we detected donor derived T and NK cells, but no B cells (Fig. 2D), in line with the results obtained in in vitro culture. A fraction of donor-derived cells were negative for all markers tested. These could constitute T cells of the γδ lineage. After five weeks, no donor-derived T cells could be detected in the thymus, suggesting a single wave of T cell development originating from CTP, similar to what has been described previously for bone marrow derived CLP-2 cells (Martin et al., 2003).
Figure 2.
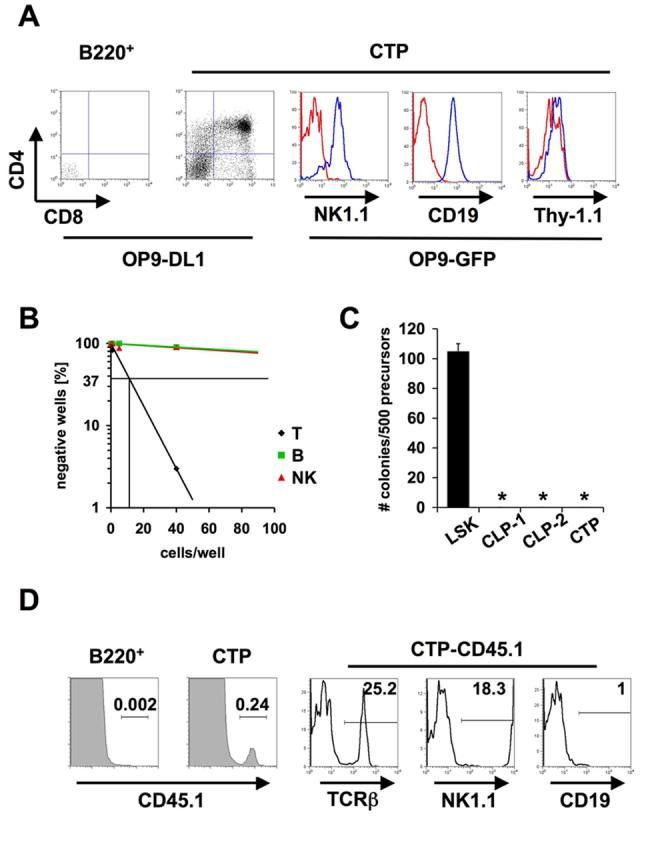
Developmental potential of circulating lin−hCD25+ cells. (A) Sorted lin−hCD25+B220− CTP and lin−hCD25+B220+ cells were co-cultured on OP9-DL1 or OP9-GFP cells for 18 days. Cells were stained for CD4, CD8, CD19, NK1.1 and Thy-1.1 and individual wells were analyzed by FACS. Blue histograms represent specific staining, red histograms represent unstained controls. 800 CD19+ (center) and 550 NK1.1+ (left) cells were recovered from starting cultures of 200 CTP. One representative out of three independent experiments is shown. (B) Limiting dilution analysis of T, B and NK potential of lin−hCD25+B220− cells (CTP) from peripheral blood. 1, 5 or 40 cells were directly sorted onto OP9-DL1 or OP9-GFP cells and analyzed by FACS after 18 days. Wells containing >50 (B and NK potential) or >100 (T potential) lineage positive cells were scored positive. (C) Analysis of myeloid potential of CTP cells. 500 CTP, BM derived LSK, CLP-1 and CLP-2 cells were cultured in methylcellulose containing SCF, IL-3, IL-6 and Erythropoietin and colonies were counted microscopically. (D) 1000 sorted CTP or 50 lin−hCD25+B220+ cells (CD45.1) were injected intravenously into irradiated Rag2−/−γc−/− recipients and spleens were analyzed 5 weeks after transfer by flow cytometry for expression of TCRβ, NK1.1 and CD19. One representative out of 2 independent experiments with 2 mice per group is shown.
Developmental progression and expansion of CTP
During the course of intrathymic differentiation T cell precursors undergo extensive proliferation. In order to test whether CTP have the potential to proliferate and thus represent potent T cell progenitors we analyzed the expansion of CTP derived cells in OP9-DL1 co-cultures in comparison to blood derived LSK cells, which have been suggested to constitute a source of T cell progenitors (Schwarz and Bhandoola, 2004). Both progenitor populations underwent proliferation during a culture period of 14 days, resulting in an approximately 1000-fold expansion (Fig. 3A). Of note, the kinetics of expansion differed between LSK cells and CTP: Whereas LSK cell derived cultures showed only moderate expansion during the first 4 days of cultures, CTP expanded almost 100-fold during this period of time, which may be due to the differences in IL-7Rα expression and, thus, due to a faster response to IL-7 present in the cultures. To analyze whether this difference was reflected by differences in developmental progression we analyzed the surface phenotype of LSK cell and CTP derived cultures at various time points (Fig. 3B). After 4 days most LSK derived cells displayed a CD44+CD25− DN1 phenotype which predominantly reached the CD44−CD25+ DN3 stage after 11 days. No DP cells were detected during this culture period. In contrast, CTP derived cultures contained a high proportion of DP cells already after 4 days of cultures with the remaining DN cells mainly being of the DN3 and CD44−CD25− DN4 phenotypes. These results indicate that the accelerated initial expansion of CTP correlates with more rapid differentiation when compared to blood derived LSK cells.
Figure 3.
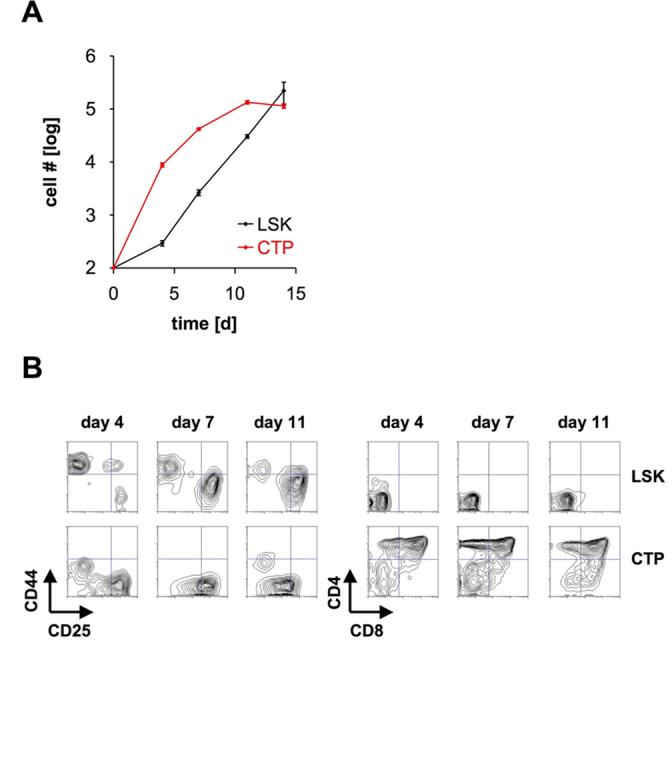
Developmental progression and expansion of CTP. (A) 100 blood derived LSK cells and 100 CTP were sorted and co-cultured on OP9-DL1 cells. Cell numbers were assessed by FACS. Data are shown ± SEM (n=4). (B) 100 blood derived LSK cells and 100 CTP were co-cultured on OP9-DL1 cells. After 4, 7, and 11 days cells were analyzed for the expression of CD4 and CD8 (right panels) and electronically gated CD4−CD8− DN cells for the expression of CD44 and CD25 (left panels). One representative out of 2 independent experiments is shown.
CTP express thymic homing molecules and home to the thymus
The signals that mediate thymus homing of circulating progenitors are still largely unknown. However, there is evidence that the CCL25/CCR9 chemokine receptor system and P-selectin-PSGL-1 interactions are involved in thymus homing (Rossi et al., 2005; Scimone et al., 2006; Uehara et al., 2002). Therefore, we addressed the question whether CTP expressed P-selectin ligands and CCR9 on their surface. BM derived CLP-1 and CLP-2, circulating CTP and thymic hCD25+CD44+c-kithiCD25− ETP were stained with P-selectin-Ig fusion proteins in the presence and absence of Calcium. Calcium-dependent binding was detected on all populations, indicating that CLP-1, CLP-2 and CTP cells express similar amounts of P-selectin ligands (Fig. 4A). ETP displayed heterogenous expression of P-selectin ligands, consistent with previously published results (Scimone et al., 2006). CTP exhibited CCR9 surface expression to a similar extent as CLP-2 cells, whereas CLP-1 cells expressed somewhat lower levels of CCR9 (Fig. 4B). Expression of CCR9 on ETP was heterogenous, which is consistent with data from previous studies and the implication that loss of CCR9 expression on ETP correlates with progressive differentiation (Benz and Bleul, 2005; Scimone et al., 2006). These data indicate that CTP have a similar pattern of homing receptors as CLP-2 cells that efficiently home to the thymus (Martin et al., 2003; Scimone et al., 2006).
Figure 4.
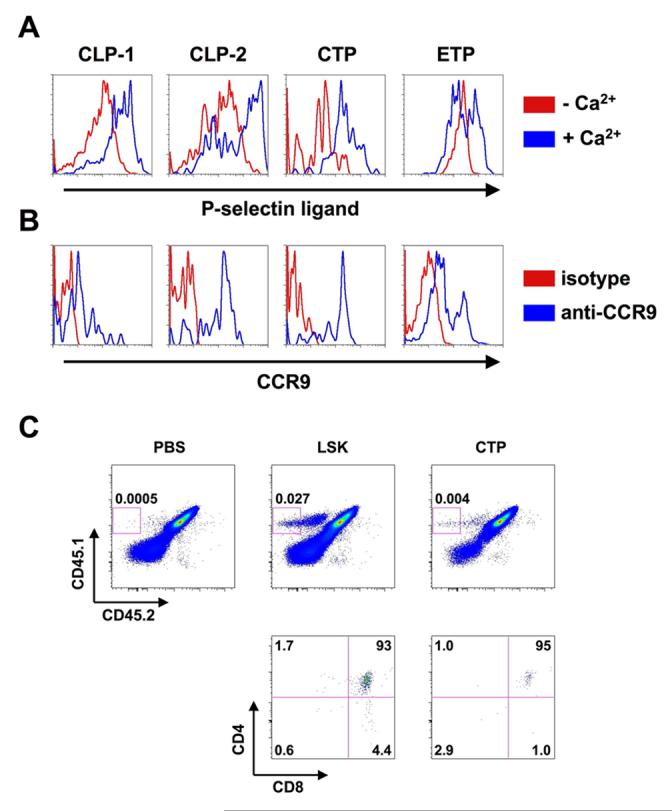
CTP express thymus homing markers and home to the thymus. (A) BM derived CLP-1 and CLP-2 cells, CTP (identified as described in Figure 1) and thymic ETP (hCD25+CD44+c-kithiCD25−) were stained with P-selectin-Ig fusion proteins in the presence (blue histograms) and absence (red histograms) of free Ca2+. (B) BM derived CLP-1 and CLP-2 cells, CTP and thymic ETP were stained with anti-CCR9 antibodies (blue histograms). Red histograms represent staining with an isototype control. One representative out of 3 independent experiments is shown. (C) 1.5 × 103 CTP or LSK cells from blood (CD45.1+CD45.2−) were injected intravenously into sub-lethally irradiated (FVB × C57BL/6)F1 mice (CD45.1+CD45.2+). Thymi were analyzed 2 weeks after transfer by flow cytometry for expression of CD45.1, CD45.2, CD4 and CD8.
Because of the limited number of CTP only a few thymic homing experiments were carried out after intravenous injection of CTP or LSK from blood. Analysis of donor-derived cells was performed at 2 weeks after transfer. As shown in Fig. 4C donor-derived thymocytes at this stage mostly exhibited the CD4+CD8+ DP phenotype with LSK cells producing seven times more DP cells when compared to CTP. This confirms previous results with CTP from fetal blood (Rodewald et al., 1994), but leaves open the question whether the difference in LSK and CTP derived DP cells reflects differences in thymic homing or differences in the kinetics of generating DP thymocytes.
CTP are present in blood of Foxn1nu/nu mice
Recently, it has been proposed that T cell precursors can emigrate from the thymus (Lambolez et al., 2006). Since the expression of thymus homing markers does not rule out the possibility that CTP might originate in the thymus rather than the bone marrow, we tested this possibility by generating athymic hCD25 transgenic Foxn1nu/nu mice and analyzing lineage negative hCD25+ cells in both BM and peripheral blood (Fig. 5). FACS analysis revealed that the frequency of lin−hCD25+ cells in BM and peripheral blood of Foxn1nu/nu mice was very similar to that of wild-type littermates (Fig. 5A). In addition, the ratio of reporter positive B220+ cells and CTP was virtually the same in Foxn1nu/nu mice when compared to wild-type littermates and CTP from Foxn1nu/nu mice did express Thy-1.1. In order to functionally analyze CTP from Foxn1nu/nu mice we assessed their developmental potential in OP9-DL1 co-cultures. As shown in Fig. 5B Foxn1nu/nu mouse derived CTP were able to generate DP cells to a similar extent as their wt derived counterparts. Foxn1nu/nu derived CTP also displayed similar kinetics of developmental progression as their wt counterparts (Fig. 5C). These results indicate that CTP are not derived from the thymus.
Figure 5.
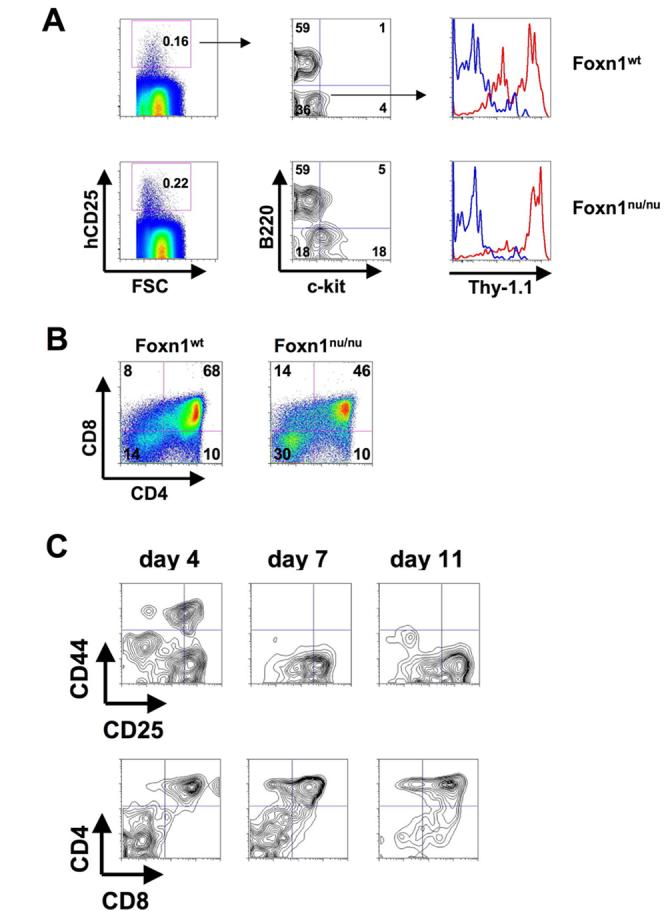
CTP are present in peripheral blood of Foxn1nu/nu mice. (A) CTP from hCD25 transgenic Foxn1nu/nu mice and hCD25 transgenic Foxn1wt littermates were analyzed by FACS. Cells were stained with antibodies against lineage markers, hCD25, c-kit, B220 and Thy-1.1 to reveal the frequency of lin−hCD25+ CTP. Numbers in FACS plots indicate percentages of cells within gates or quadrants. One representative out of 4 independent experiments is shown. (B) 200 CTP from hCD25 transgenic Foxn1nu/nu mice and hCD25 transgenic Foxn1wt littermates were sorted and co-cultured on OP9-DL1 cells. After 14 days cells were analyzed for the expression of CD4 and CD8. Numbers in FACS plots indicate percentages of cells within quadrants. One representative out of 3 independent experiments is shown (C) 100 CTP from hCD25 transgenic Foxn1nu/nu mice were sorted and co-cultured on OP9-DL1 cells. After 4, 7, and 11 days cells were analyzed for the expression of CD4 and CD8 (lower panels) and electronically gated CD4−CD8− DN cells for the expression of CD44 and CD25 (upper panels). One representative out of 2 independent experiments is shown.
CTP in the absence of Notch induced transcription
As shown in Figure 2 CTP display a clear bias towards the T lineage in terms of developmental potential. Notch signaling plays a major role in T lineage commitment and we have shown in Figure 2A that CTP are dependent on Notch signals to further differentiate into T cells. However, it was not clear whether the observed T cell bias depended on Notch-dependent transcription. This was analyzed by adoptive transfer experiments of lineage negative BM cells from hCD25 transgenic mice that were retrovirally transduced to express either a dominant negative form of the co-activator mastermind-like fused to eGFP (DN-MAML) or eGFP alone (MigR1). Six to eight weeks after transfer BM, peripheral blood and thymus from recipient mice were analyzed by FACS and the frequency of eGFP expressing cells in different populations was determined. eGFP expression was detectable at similar levels in all populations of control transduced mice (Fig. 6A, upper panels; Fig. 6C, black bars). DN-MAML transduced cells were completely absent among thymic DN3 cells and almost completely absent among DN2 cells, indicating the functionality of the construct used. Interestingly, the frequency of DN-MAML transduced ETP cells was only reduced by 80 %, suggesting that this population contains precursors that do not require Notch-dependent transcription (Fig. 6A, lower panel; Fig. 6C, red bars). Although we detected more Thy-1.1− cells among the reporter positive lin−B220− c-kitlo cells in the adoptive transfer experiment when compared to the direct ex vivo analysis (irrespective of whether cells were mock-transduced or transduced with DN-MAML) (Fig.1C), the frequency of eGFP+Thy-1.1+ CTP in DN-MAML transduced cells was identical to control transduced cells (Fig. 6B), indicating that CTP do not require Notch-dependent transcription.
Figure 6.
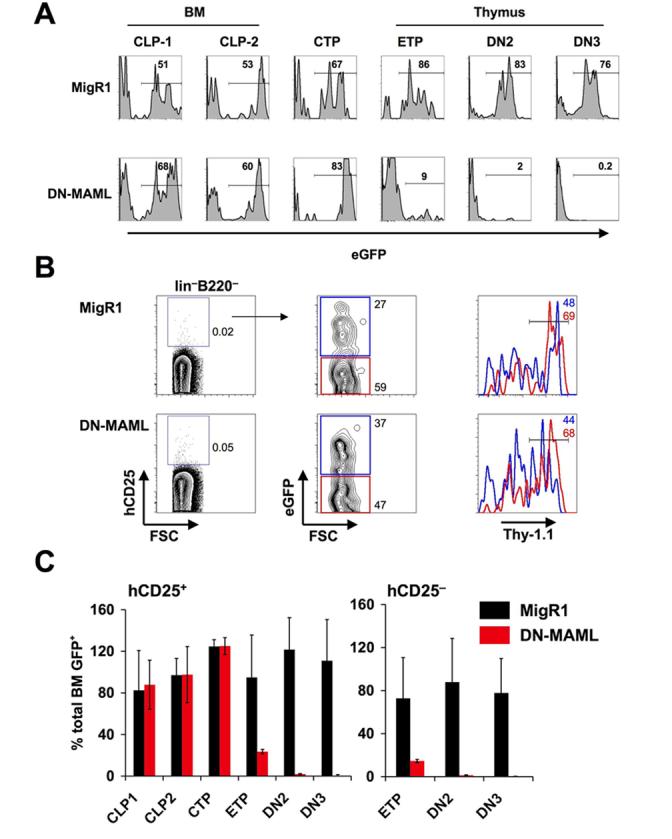
CTP do not require Notch-induced transcription. Sorted lineage negative cells from hCD25 transgenic BM were infected with DN-MAML or MigR1 control retrovirus and transferred into irradiated hosts. BM, peripheral blood and thymus were analyzed by FACS 6 - 10 weeks after transfer. Numbers in histograms indicate the frequency of eGFP+ cells. (A) eGFP expression in different populations of one representative experiment of 6 mice per group. (B) Analysis of Thy-1.1 expression in DN-MAML or MigR1 transduced blood derived lin−B220−c-kitlo cells. Numbers in FACS plots indicate percentages of cells within gates or quadrants. Blue gates and histograms indicate eGFP+ cells; red histograms indicate eGFP− cells. (C) Statistical analysis of 4 independent experiments. The percentage of eGFP positive cells was normalized to the percentage of eGFP positive cells of total BM for each independent experiment. Data are shown ± SEM.
Discussion
The identity of thymus colonizing T cell progenitors in the adult mouse remains largely elusive, although a number of different candidate populations have been described. Using a hCD25 reporter gene under control of Ptcra regulatory elements our laboratory has previously identified a lin−c-kit−/loB220+ common lymphoid progenitor “CLP-2” in bone marrow, which efficiently enters the thymus upon intravenous transfer (Martin et al., 2003; Scimone et al., 2006) and progresses developmentally along the T lineage in vitro with kinetics similar to ETP (Krueger et al., 2006). In the adult organism T cell progenitors most likely enter the blood prior to thymic colonization. We therefore searched for reporter positive cells in blood. In fact, such cells can be found in blood, but their phenotype differs from reporter positive cells in bone marrow in that the majority is B220− and expresses low levels of c-kit and IL-7Rαas well as high levels of Thy-1.1. The few reporter positive B220 positive cells from blood differ phenotypically from BM-derived B220 positive CLP-2 cells as the former, but not the latter, lack expression of IL-7Rα and are heterogeneous for expression of the Thy-1.1 marker. These cells could not be functionally analyzed, i.e. no progeny was detected when these cells were injected intravenously or cultured on OP9-DL1 feeders. However, we cannot exclude that BM derived CLP-2 circulate in blood in numbers that are below our limits of detection. The B220− cells produced T cells when injected intravenously and produced CD4+CD8+ DP thymocytes in culture. The estimated frequency of these cells in the circulation of approximately 330 per ml of blood is similar to that reported for LSK cells (Schwarz and Bhandoola, 2004). In addition, we observed a similar capacity of CTP and LSK cells to undergo a proliferative burst in vitro. Considering that a thymus may contain 100 - 300 progenitor niches of which 2 - 3 % are replenished per day (Donskoy and Goldschneider, 1992; Spangrude and Scollay, 1990) and considering that CTP have the potential of extensive expansion it is well conceivable that these cells alone could be capable of generating all T lineage cells. This would also be compatible with the finding that CTP express CCR9 and PSGL-1, which are critical for homing of progenitors to the thymus (Rossi et al., 2005; Scimone et al., 2006; Uehara et al., 2002). Subfractionation of the thymic DN1 population according to expression of c-kit and HSA led to the identification of five DN1 subsets, of which DN1a and DN1b correspond to ETP (Porritt et al., 2004). Thy-1 positive cells were found within the heterogeneous DN1c, d, and e subfractions. However, in contrast to CTP these populations did not exhibit a proliferative burst capacity, which is characteristic for ETP. When analyzing reporter positive DN1 cells, which are enriched in the DN1a and b ETP populations (Krueger et al., 2006), we found low levels of Thy-1.1 expression, which appears to be in contrast to the original characterisation of DN1a and b cells. This may be due to differences in mouse strains expressing different Thy-1 alleles as has been described for HSC (Spangrude and Brooks, 1992).
Interestingly, intravenous injection did not yield any B cells and the frequency of B cell precursors in this subset in OP9 cultures was much lower than the frequency of T cell precursors. Thus, we termed these cells circulating T cell progenitors (CTP). In fact, given the absolute number of CTP in the bloodstream the degree of B lineage potential detected in vitro can probably be considered negligible. Although we were able to detect NK potential in vivo, the in vitro assay revealed a very low NK precursor frequency, similar to that for B cells. This may be due to the fact that the in vitro assay was performed without addition of IL-15, i.e. under conditions that are suboptimal for NK cell differentiation. The absence of B lineage potential in CTP is in stark contrast to LSK cells and other extrathymic subsets with T cell progenitor potential such as MPP, ELP and LSP as well as from CLP and CLP-2, although a certain T lineage bias has also been described for LSP (Perry et al., 2004). In contrast, different circulating fetal thymic progenitors have been demonstrated to be T lineage restricted prior to thymic colonization (Ikawa et al., 2004; Masuda et al., 2005; Rodewald et al., 1994). Notably, one population, initially characterized by Rodewald et al. also phenotypically resembles the adult CTP population by being c-kitloThy-1+ and expressing pre-TCRα mRNA (Bruno et al., 1995; Rodewald et al., 1994). In fact, these cells like their equivalents in adult blood could migrate into and differentiate in the thymus. Thus, thymus-seeding CTP are apparently present in both fetal and adult blood. Our isolation of adult CTP was based on expression of a hCD25 reporter gene driven by regulatory elements of Ptcra. Isolation of CTP from wild-type mice could be attempted from a lin−Thy-1.1+Sca-1+c-kitloIL-7Rαlo population, which includes all lin−B220−Flt3− reporter positive cells in hCD25 transgenic mice. Conversely, only about 4% of these lin−Thy-1.1+Sca-1+c-kitloIL-7Rαlo cells express hCD25 raising the possibility that only a fraction of CTP express the reporter much like only a fraction of CLP-1 cells in bone marrow is reporter positive (Martin et al., 2003).
Signaling through Notch receptors has been suggested to mediate T versus B lineage decisions. Thus, constitutive Notch signaling leads to extrathymic T cell development, whereas abrogation of Notch signaling results in accumulation of B cells in the thymus (Koch et al., 2001; Maillard et al., 2004; Pui et al., 1999; Radtke et al., 1999; Wilson et al., 2001). Inhibition of Notch signaling using a dominant-negative mutant of the Notch co-activator Mastermind-like did not reduce the frequency of CTP in peripheral blood and did not abrogate Thy-1 expression on those cells, which correlates with T lineage commitment. On the other hand, further development along the T lineage was clearly Notch dependent as DP thymocytes could only be generated on Notch-ligand expressing OP9-DL1 feeder cells, but not on OP9-GFP controls feeder cells. These data suggest that terminal differentiation of CTP into T lineage cells is Notch dependent, whereas early lineage commitment is not. This is consistent with a recent study postulating that fetal thymic precursors are T lineage restricted prior to receiving intrathymic Notch signals (Harman et al., 2005). Another study using mice deficient in the Notch target gene Hes1 and showing that fetal thymic precursors, which are characterized by the expression of paired-immunoglobulin like receptors, are independent of this transcription factor, could further support the idea of Notch-independent early T lineage commitment (Masuda et al., 2005). Thus, our results and results by others are consistent with a Notch-independent early T lineage commitment step which is further enforced by intrathymic Notch signals. This indicates that, at least for certain precursors such as CTP, adult T lineage commitment parallels fetal T cell development. However, additional studies are required to address molecular mechanisms of Notch-independent T lineage commitment.
Experimental Procedures
Mice
hCD25 transgenic mice (FVB, Thy-1.1, CD45.1) have been described (Gounari et al., 2002; Martin et al., 2003). C57BL/6 Rag2−/−γc−/− and NCR-Foxn1 mice were purchased from Taconic Farms (Germantown, NY). NCR-Foxn1 __were crossed with hCD25 transgenic mice for 2 generations to generate hCD25 transgenic Foxn1 nu/nu mice. hCD25 transgenic Foxn1 nu/nu mice homozygous for the Thy-1.1 allele were used in experiments. (FVB × C57BL/6)F1 mice (CD45.1+CD45.2+) were generated by crossing hCD25 transgenic mice with C57BL/6 mice (CD45.2) for 1 generation. All mice were maintained in the specific-pathogen-free animal facilities of the Dana-Farber-Cancer Institute and all animal procedures were done in compliance with the guidelines of the DFCI Animal Resources Facility, which operates under regulatory requirements of the U.S. Department of Agriculture and Association for Assessment and Accreditation of Laboratory Animal Care.
Cell lines and cell preparations
OP9 bone marrow stromal cells expressing the Notch ligand delta-like ligand 1 (OP9-DL1) and OP9-control cells (OP9-GFP) were provided by Dr. Juan Carlos Zúñiga-Pflücker (University of Toronto, Toronto, Canada) and maintained in αMEM supplemented with 55 μM 2-mercaptoethanol, 10 mM HEPES (pH 7.5), 1mM sodium pyruvate, 100 U/ml penicillin, 0.1 mg/ml streptomycin, 50 μg/ml gentamycine and 20% heat-inactivated fetal bovine serum (FBS) and passaged as described (Schmitt and Zuniga-Pflucker, 2002). Blood was obtained from anesthetized mice through the retro-orbital venous sinus or cardiac puncture with identical results. Between 0.6 and 1 mL of blood were obtained per mouse and coagulation was prevented through addition of 20 U/mL heparin (Abbott Labs, IL, USA). Red blood cells were removed by centrifugation over Ficoll-Paque (Amersham). Typically, blood from 20 – 30 mice was used, allowing the isolation of approximately 500 lin−hCD25+ cells by cell sorting. BM cells and thymocytes were obtained as described previously (Martin et al., 2003).
Flow cytometry and cell sorting
Monoclonal antibodies specific for CD4 (RM4-5, GK1.5), CD8 (53-6.7), CD25 (PC61), CD44 (IM7), TCRβ (H57-597), TCRγδ (GL3), Gr-1 (RB6-8C5), erythroid cell marker (Ter-119), CD19 (1D3), CD11c (HL3), CD11b (M1/70), pan-NK (DX5), NK1.1 (PK136), CD45.1 (A20), B220 (RA3-6B2), c-kit (2B8), Sca-1 (E13-161.7), Thy-1.1 (CD90.1, OX-7), and human CD25 (M-A251) were purchased from BD Biosciences and were used as biotin, fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinin chlorophyll protein (PerCP), PerCP-Cy5.5, PE-Cy7, allophycocyanin (APC) or APC-Cy7 conjugates. Monoclonal antibodies specific for Flt3 (A2F10) and IL-7Rα (A7R34) were purchased from eBioscience. Anti-CCR9 (rat IgM) and P-selectin-Ig were provided by Dr. Ulrich von Andrian. FITC-conjugated anti-human Fc was from Caltag. PE-Texas Red or PerCP conjugated streptavidin was used to reveal staining with biotinylated mAb. Four-color flow cytometry was performed on a FACSCalibur (BD, San Jose, CA). Six-color and seven-color flow cytometry was performed on a FACSAria (BD). Data were analyzed with FlowJo software (Treestar). For analysis, dead cells and debris were excluded by appropriate gating of forward and sideward scatter. Lineage negative cells were isolated from total thymocytes by staining cell suspensions with a biotinylated lineage-specific antibody cocktail, followed by incubation with streptavidin-conjugated magnetic beads (Dynal) and magnetic bead depletion of mature lineages. Enriched cell suspensions were surface stained with streptavidin-PE-Texas Red or streptavidin-PerCP. Cells were sorted using a FACSAria (BD). All population were re-sorted; sorted cells were of ≥ 99% purity, as determined by post-sort analysis.
OP9 co-cultures
OP9 co-culture assays were essentially performed as described (Schmitt and Zuniga-Pflucker, 2002). Precursors were plated at an initial density of 1 - 5 × 102cells onto subconfluent OP9-GFP or OP9-DL1 monolayers at 5×104 cells/well in a 24 well plate. All co-cultures were performed in the presence of 1 ng/ml IL-7 and 5 ng/ml Flt3 ligand (Flt3L) for OP9-DL1 T cell differentiation assays and 5 ng/ml IL-7 and 5 ng/ml Flt3L for OP9-GFP co-cultures. In certain experiments Flt3L was replaced by 100 ng/ml SCF as indicated. At day 4 of differentiation the culture medium was exchanged. Contaminating OP9 cells were eliminated by filtering the harvested co-cultured cells through a 70 μm cell strainer prior to flow cytometric analysis. For cultures of less than 102 precursors cells were plated directly onto 96-well plates containing 104 γ-irradiated OP9-GFP or OP9-DL1 cells (15 Gy) using a FACSAria cell sorter.
Methylcellulose cultures
To determine erythroid and myeloid potential cells were cultured in Methocult M3434 (StemCell Technologies, Vancouver, Canada) containing rmSCF, rmIL-3, rhIL-6 and rhErythropoietin according to the manufacturer's instructions. Plates were inspected and numbers of colonies were determined 8-10 days after the start of cultures.
Retroviral infections
The retroviral construct encoding a truncated N-terminal fragment of mastermind-like 1 fused to eGFP (DN-MAML) and the control vector MigR1 were provided by Dr Jon C. Aster (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) and have been described (Maillard et al., 2004; Weng et al., 2003). Retroviral supernatants were generated by transient transfections of 293T cells with these retroviral constructs and appropriate packaging plasmids (Ory et al., 1996). Lineage negative BM cells from hCD25 transgenic mice were retrovirally transduced as described (Aifantis et al., 2002) and intravenously injected into irradiated (8 Gy) syngeneic hosts. The resultant chimeric mice were analyzed after 6 - 10 weeks.
Adoptive transfers
50 to 1,500 sorted lineage negative hCD25+ cells from peripheral blood were intravenously injected into irradiated Rag2−/−γc−/− (5 Gy) mice or (FVB × C57BL/6)F1 (6.5 Gy). Mice were analyzed 2 or 5 weeks after transfer by flow cytometry. Donor cells were distinguished from host cells by expression of CD45.1 and absence of expression of CD45.2.
RT-PCR
Cells were sorted and mRNA was extracted using the High Pure total mRNA isolation kit (Roche, Basel, Switzerland). cDNA was prepared with Superscript II RT kit (Invitrogen) and PCR was performed according to standard procedures. Oligonucleotide primer sequences were: hCD25-5′: 5′-TGAGAACTTCAGGCTCCTGGGC-3′; hCD25-3′: 5′-TGGCTTTGAATGTGGCGTGTGG-3′; pTa-5′: 5′-GGCACCCCCTTTCCGTCTCT-3′; pTa-3′: 5′-GTCCAAATTCTGTGGGTGGGA-3′; HPRT-5′: 5′-CACAGGACTAGAACACCTGC-3′; HPRT-3′: 5′-GCTGGTGAAAAGGACCTCT-3′.
Supplementary Material
suppfig
Acknowledgements
We would like to thank Drs. Ulrich von Andrian, Juan Carlos Zúñiga-Pflücker and Jon C. Aster for providing reagents and Drs. M. Lucila Scimone and Fotini Gounari for helpful discussions. The authors are grateful to Valentina Schmidt for technical assistance and to Linnea Benson for editorial help. This work was supported by grants from the Lymphoma Research Foundation and the German Research Foundation (DFG, Emmy-Noether Fellowship, KR2320/1-1) (to A.K.) and the NIH (AI45846) (to H.v.B.). The authors have no conflicting financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolfsson J, Borge OJ, Bryder D, Theilgaard-Monch K, Astrand-Grundstrom I, Sitnicka E, Sasaki Y, Jacobsen SE. Upregulation of Flt3 expression within the bone marrow Lin(−)Sca1(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15:659–669. doi: 10.1016/s1074-7613(01)00220-5. [DOI] [PubMed] [Google Scholar]
- Aifantis I, Borowski C, Gounari F, Lacorazza HD, Nikolich-Zugich J, von Boehmer H. A critical role for the cytoplasmic tail of pTalpha in T lymphocyte development. Nat Immunol. 2002;3:483–488. doi: 10.1038/ni779. [DOI] [PubMed] [Google Scholar]
- Allman D, Sambandam A, Kim S, Miller JP, Pagan A, Well D, Meraz A, Bhandoola A. Thymopoiesis independent of common lymphoid progenitors. Nat Immunol. 2003;4:168–174. doi: 10.1038/ni878. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Balciunaite G, Ceredig R, Rolink AG. The earliest subpopulation of mouse thymocytes contains potent T, significant macrophage, and natural killer cell but no B-lymphocyte potential. Blood. 2005;105:1930–1936. doi: 10.1182/blood-2004-08-3087. [DOI] [PubMed] [Google Scholar]
- Benz C, Bleul CC. A multipotent precursor in the thymus maps to the branching point of the T versus B lineage decision. J Exp Med. 2005;202:21–31. doi: 10.1084/jem.20050146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno L, Rocha B, Rolink A, von Boehmer H, Rodewald HR. Intra- and extra-thymic expression of the pre-T cell receptor alpha gene. Eur J Immunol. 1995;25:1877–1882. doi: 10.1002/eji.1830250713. [DOI] [PubMed] [Google Scholar]
- Donskoy E, Goldschneider I. Thymocytopoiesis is maintained by blood-borne precursors throughout postnatal life. A study in parabiotic mice. J Immunol. 1992;148:1604–1612. [PubMed] [Google Scholar]
- Douagi I, Colucci F, Di Santo JP, Cumano A. Identification of the earliest prethymic bipotent T/NK progenitor in murine fetal liver. Blood. 2002;99:463–471. doi: 10.1182/blood.v99.2.463. [DOI] [PubMed] [Google Scholar]
- Gounari F, Aifantis I, Martin C, Fehling HJ, Hoeflinger S, Leder P, von Boehmer H, Reizis B. Tracing lymphopoiesis with the aid of a pTalpha-controlled reporter gene. Nat Immunol. 2002;3:489–496. doi: 10.1038/ni778. [DOI] [PubMed] [Google Scholar]
- Harman BC, Jenkinson WE, Parnell SM, Rossi SW, Jenkinson EJ, Anderson G. T/B lineage choice occurs prior to intrathymic Notch signaling. Blood. 2005;106:886–892. doi: 10.1182/blood-2004-12-4881. [DOI] [PubMed] [Google Scholar]
- Igarashi H, Gregory SC, Yokota T, Sakaguchi N, Kincade PW. Transcription from the RAG1 locus marks the earliest lymphocyte progenitors in bone marrow. Immunity. 2002;17:117–130. doi: 10.1016/s1074-7613(02)00366-7. [DOI] [PubMed] [Google Scholar]
- Ikawa T, Masuda K, Lu M, Minato N, Katsura Y, Kawamoto H. Identification of the earliest prethymic T-cell progenitors in murine fetal blood. Blood. 2004;103:530–537. doi: 10.1182/blood-2003-06-1797. [DOI] [PubMed] [Google Scholar]
- Jenkinson EJ, Jenkinson WE, Rossi SW, Anderson G. The thymus and T-cell commitment: the right niche for Notch? Nat Rev Immunol. 2006;6:551–555. doi: 10.1038/nri1883. [DOI] [PubMed] [Google Scholar]
- Kawamoto H, Ikawa T, Ohmura K, Fujimoto S, Katsura Y. T cell progenitors emerge earlier than B cell progenitors in the murine fetal liver. Immunity. 2000;12:441–450. doi: 10.1016/s1074-7613(00)80196-x. [DOI] [PubMed] [Google Scholar]
- Kawamoto H, Ohmura K, Katsura Y. Direct evidence for the commitment of hematopoietic stem cells to T, B and myeloid lineages in murine fetal liver. Int Immunol. 1997;9:1011–1019. doi: 10.1093/intimm/9.7.1011. [DOI] [PubMed] [Google Scholar]
- Koch U, Lacombe TA, Holland D, Bowman JL, Cohen BL, Egan SE, Guidos CJ. Subversion of the T/B lineage decision in the thymus by lunatic fringe-mediated inhibition of Notch-1. Immunity. 2001;15:225–236. doi: 10.1016/s1074-7613(01)00189-3. [DOI] [PubMed] [Google Scholar]
- Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- Krueger A, Garbe AI, von Boehmer H. Phenotypic plasticity of T cell progenitors upon exposure to Notch ligands. J Exp Med. 2006;203:1977–1984. doi: 10.1084/jem.20060731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambolez F, Arcangeli ML, Joret AM, Pasqualetto V, Cordier C, Di Santo JP, Rocha B, Ezine S. The thymus exports long-lived fully committed T cell precursors that can colonize primary lymphoid organs. Nat Immunol. 2006;7:76–82. doi: 10.1038/ni1293. [DOI] [PubMed] [Google Scholar]
- Lu M, Tayu R, Ikawa T, Masuda K, Matsumoto I, Mugishima H, Kawamoto H, Katsura Y. The earliest thymic progenitors in adults are restricted to T, NK, and dendritic cell lineage and have a potential to form more diverse TCRbeta chains than fetal progenitors. J Immunol. 2005;175:5848–5856. doi: 10.4049/jimmunol.175.9.5848. [DOI] [PubMed] [Google Scholar]
- Maillard I, Weng AP, Carpenter AC, Rodriguez CG, Sai H, Xu L, Allman D, Aster JC, Pear WS. Mastermind critically regulates Notch-mediated lymphoid cell fate decisions. Blood. 2004;104:1696–1702. doi: 10.1182/blood-2004-02-0514. [DOI] [PubMed] [Google Scholar]
- Martin CH, Aifantis I, Scimone ML, von Andrian UH, Reizis B, von Boehmer H, Gounari F. Efficient thymic immigration of B220+ lymphoid-restricted bone marrow cells with T precursor potential. Nat Immunol. 2003;4:866–873. doi: 10.1038/ni965. [DOI] [PubMed] [Google Scholar]
- Masuda K, Kubagawa H, Ikawa T, Chen CC, Kakugawa K, Hattori M, Kageyama R, Cooper MD, Minato N, Katsura Y, Kawamoto H. Prethymic T-cell development defined by the expression of paired immunoglobulin-like receptors. Embo J. 2005;24:4052–4060. doi: 10.1038/sj.emboj.7600878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TA, Zlotnik A. T-cell lineage commitment and cytokine responses of thymic progenitors. Blood. 1995;86:1850–1860. [PubMed] [Google Scholar]
- Mori S, Shortman K, Wu L. Characterization of thymus-seeding precursor cells from mouse bone marrow. Blood. 2001;98:696–704. doi: 10.1182/blood.v98.3.696. [DOI] [PubMed] [Google Scholar]
- Ory DS, Neugeboren BA, Mulligan RC. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc Natl Acad Sci U S A. 1996;93:11400–11406. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SS, Wang H, Pierce LJ, Yang AM, Tsai S, Spangrude GJ. L-selectin defines a bone marrow analog to the thymic early T-lineage progenitor. Blood. 2004;103:2990–2996. doi: 10.1182/blood-2003-09-3030. [DOI] [PubMed] [Google Scholar]
- Porritt HE, Rumfelt LL, Tabrizifard S, Schmitt TM, Zuniga-Pflucker JC, Petrie HT. Heterogeneity among DN1 prothymocytes reveals multiple progenitors with different capacities to generate T cell and non-T cell lineages. Immunity. 2004;20:735–745. doi: 10.1016/j.immuni.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Pui JC, Allman D, Xu L, DeRocco S, Karnell FG, Bakkour S, Lee JY, Kadesch T, Hardy RR, Aster JC, Pear WS. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. 1999;11:299–308. doi: 10.1016/s1074-7613(00)80105-3. [DOI] [PubMed] [Google Scholar]
- Radtke F, Wilson A, Stark G, Bauer M, van Meerwijk J, MacDonald HR, Aguet M. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- Rodewald HR, Kretzschmar K, Takeda S, Hohl C, Dessing M. Identification of pro-thymocytes in murine fetal blood: T lineage commitment can precede thymus colonization. Embo J. 1994;13:4229–4240. doi: 10.1002/j.1460-2075.1994.tb06743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi FM, Corbel SY, Merzaban JS, Carlow DA, Gossens K, Duenas J, So L, Yi L, Ziltener HJ. Recruitment of adult thymic progenitors is regulated by P-selectin and its ligand PSGL-1. Nat Immunol. 2005;6:626–634. doi: 10.1038/ni1203. [DOI] [PubMed] [Google Scholar]
- Sambandam A, Maillard I, Zediak VP, Xu L, Gerstein RM, Aster JC, Pear WS, Bhandoola A. Notch signaling controls the generation and differentiation of early T lineage progenitors. Nat Immunol. 2005;6:663–670. doi: 10.1038/ni1216. [DOI] [PubMed] [Google Scholar]
- Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- Schwarz BA, Bhandoola A. Circulating hematopoietic progenitors with T lineage potential. Nat Immunol. 2004;5:953–960. doi: 10.1038/ni1101. [DOI] [PubMed] [Google Scholar]
- Scimone ML, Aifantis I, Apostolou I, von Boehmer H, von Andrian UH. A multistep adhesion cascade for lymphoid progenitor cell homing to the thymus. Proc Natl Acad Sci U S A. 2006;103:7006–7011. doi: 10.1073/pnas.0602024103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangrude GJ, Brooks DM. Phenotypic analysis of mouse hematopoietic stem cells shows a Thy-1-negative subset. Blood. 1992;80:1957–1964. [PubMed] [Google Scholar]
- Spangrude GJ, Scollay R. Differentiation of hematopoietic stem cells in irradiated mouse thymic lobes. Kinetics and phenotype of progeny. J Immunol. 1990;145:3661–3668. [PubMed] [Google Scholar]
- Tan JB, Visan I, Yuan JS, Guidos CJ. Requirement for Notch1 signals at sequential early stages of intrathymic T cell development. Nat Immunol. 2005;6:671–679. doi: 10.1038/ni1217. [DOI] [PubMed] [Google Scholar]
- Uehara S, Grinberg A, Farber JM, Love PE. A role for CCR9 in T lymphocyte development and migration. J Immunol. 2002;168:2811–2819. doi: 10.4049/jimmunol.168.6.2811. [DOI] [PubMed] [Google Scholar]
- Weng AP, Nam Y, Wolfe MS, Pear WS, Griffin JD, Blacklow SC, Aster JC. Growth suppression of pre-T acute lymphoblastic leukemia cells by inhibition of notch signaling. Mol Cell Biol. 2003;23:655–664. doi: 10.1128/MCB.23.2.655-664.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, MacDonald HR, Radtke F. Notch 1-deficient common lymphoid precursors adopt a B cell fate in the thymus. J Exp Med. 2001;194:1003–1012. doi: 10.1084/jem.194.7.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Scollay R, Egerton M, Pearse M, Spangrude GJ, Shortman K. CD4 expressed on earliest T-lineage precursor cells in the adult murine thymus. Nature. 1991;349:71–74. doi: 10.1038/349071a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
suppfig