Hyaluronan in Peritumoral Stroma and Malignant Cells Associates with Breast Cancer Spreading and Predicts Survival (original) (raw)
Abstract
Hyaluronan (HA) is an extracellular matrix polysaccharide that promotes cell migration through its cell surface receptors and by effecting changes in the physical environment. HA expression is frequently increased in malignant tumors, whereas its association with the invasive potential and patient outcome in breast cancer has not been reported. The localization and signal intensity of HA was analyzed in 143 paraffin-embedded tumor samples of human breast carcinoma using a biotinylated HA-specific probe. In the immediate peritumoral stroma, HA signal was moderately or strongly increased in 39% and 56% of the cases, respectively. Normal ductal epithelium showed no HA, whereas in 57% of the tumors at least some of the carcinoma cells were HA positive. The intensity of the stromal HA signal and the presence of cell-associated HA were both significantly related to poor differentiation of the tumors, axillary lymph node positivity, and short overall survival of the patients. In Cox’s multivariate analysis, both the intensity of stromal HA signal alone and that combined with the HA positivity in tumor cells were independent prognostic factors for overall survival. These results suggest that HA is directly involved in the spreading of breast cancer and may offer a potential target for new therapies.
Hyaluronan (HA) is an ubiquitous extracellular matrix component of connective, epithelial, and neural tissues. 1-3 Its expression is increased during active tissue remodeling, eg, during morphogenesis and wound healing. 1 Elevated amounts of HA are also frequently found in malignant tumors, especially in the stroma of malignant areas, 4,5 but also in the most aggressive cancer cells. 6,7,8
HA may support tumor growth and spread by regulating cell proliferation 1,9 or by enhancing tumor neovascularization through fragmentation into angiogenic oligosaccharides. 10 HA likely promotes cell migration by effecting changes in the physical environment so that the accumulation of HA creates expanded gel-filled spaces where cells can migrate. 1,11 In addition, HA can, through its cell surface receptors for hyaluronan (HA)-mediated motility (RHAMM) and CD44, give motogenic signals that are mediated, at least in part, by the activation of focal adhesion kinase and mitogen-activated protein kinases. 12,13 HA on the cell surface may also increase the probability of metastasis by facilitating the attachment of the disseminating carcinoma cells to lymph nodes or the endothelial cells in distant organs. 14
Several lines of evidence indicate that HA metabolism is altered in breast cancer and that this may play an important role in tumor progression. Biochemical 15,16 and histochemical 17-20 studies have shown that malignant breast tissue contains more HA than normal breast tissue or benign lesions. The stroma at the invading edge of the breast carcinomas is especially enriched in HA. 16,17 The stromal fibroblasts stimulated by the tumor cells are probably responsible for most of the HA accumulation in breast tumors. 4,5,21,22 However, the most tumorigenic and phenotypically aggressive breast carcinoma cell lines also synthesize large quantities of HA, unlike the less malignant cell lines. 6 The invasive potential created by the accumulation of HA may be further aggravated by changes in the expression of HA receptors, CD44 and RHAMM, which both are frequently observed in breast cancer cells. 23,24
Despite the extensive evidence for increased HA expression in breast cancer and the general ability of HA to promote experimental tumor progression, no direct relationship between the level of HA accumulation in human breast carcinomas and patient survival rate has been reported. Here we establish a strong correlation between the progression of the disease and the degree of HA accumulation within the peritumoral stroma and cancer cells. These findings are consistent with a causal role of HA in the advancement of human breast carcinoma.
Materials and Methods
Patients
The patient material consisted of 143 women operated on for primary, invasive breast cancer at Kuopio University hospital between 1980 and 1984, and monitored until June 1990. These patients were selected from the original cohort of 237 patients. In 26 of these cases either the clinical data or histopathologic samples were not available, and in 68 cases the histopathologic reevaluation of available samples did not reveal tumor tissue. The formalin-fixed, paraffin-embedded, 5-μm-thick sections were stained with hematoxylin and eosin for histological typing and grading. Tumor size was recorded as the largest diameter in fresh mastectomy specimens. Axillary lymph node status was studied histopathologically in 93% of patients. The estrogen receptor (ER) status and progesterone receptor (PR) status were assayed as previously described. 25 ER status was available in 130 (91%) cases and PR status in 132 (92%) cases. The primary treatment was mastectomy for 139 patients (97%). Postoperative radiotherapy was given to 67 patients (47%). Adjuvant hormonal therapy and chemotherapy were given to 24 (17%) and 30 (21%) patients, respectively. The clinical data of the patients are summarized in Table 1 ▶ .
Table 1.
Clinical Data of 143 Breast Cancer Patients
| Parameter | Results |
|---|---|
| Median age (range) | 60 (27–91) years |
| Mean follow-up, years (±SD) | 5.7 (±2.6) years |
| Tumor size | |
| ≤2 cm | 52 (36%) |
| 2–5 cm | 71 (50%) |
| >5 cm | 20 (14%) |
| Axillary lymph node status (pathological) | |
| Negative | 62 (43%) |
| Positive | 71 (50%) |
| Unknown | 10 (7%) |
| Metastases at diagnosis | |
| No | 137 (96%) |
| Yes | 6 (4%) |
| Histological type | |
| Ductal carcinoma | 128 (90%) |
| Lobular carcinoma | 13 (9%) |
| Others | 2 (1%) |
| Histological grade | |
| I | 9 (6%) |
| II | 71 (50%) |
| III | 63 (44%) |
| Death during follow-up | |
| Alive | 75 (52%) |
| Breast cancer death | 55 (38%) |
| Other causes | 13 (10%) |
Preparation of the Biotinylated Complex of the Hyaluronan-Binding Region and Link Protein
The biotinylated complex of the hyaluronan-binding region and link protein (bHABC) was prepared from bovine articular cartilage as described previously. 26,27 Briefly, the proteoglycans were extracted from the cartilage with 4 mol/L guanidine chloride. The extract was dialyzed against distilled water in the presence of high-molecular-weight hyaluronan. The C terminus of the proteoglycan molecule was cleaved with trypsin, and the resultant complex of hyaluronan-binding region and link protein (HABC) with HA was purified using hydroxylapatite chromatography and gel filtration. The complex was biotinylated, and the bHABC was separated from HA using gel filtration under dissociative conditions. The purity of the preparation was tested by polyacrylamide gel electrophoresis and Western blotting.
Staining of Tissue Sections
The sections were deparaffinized in xylene, rehydrated with graded alcohols, and washed with sodium phosphate buffer (PB; 0.1 mol/L, pH 7.4). Endogenous peroxidase was blocked with 3% H2O2 for 3 minutes, and nonspecific binding was blocked with 1% bovine serum albumin in PB for 30 minutes. The sections were incubated in bHABC (2.5 μg/ml, diluted in 1% bovine serum albumin) overnight at 4°C. The slides were washed with PB and treated with avidin-biotin-peroxidase (Vector Laboratories, Irvine, CA; 1:200 dilution) for 1 hour at room temperature. After the wash with PB, the color was developed with 0.05% 3,3′-diaminobenzidine (Sigma Chemical Co., St. Louis, MO) and 0.03% H2O2 in PB at room temperature for 5 minutes. The slides were counterstained with Mayer’s hematoxylin for 2 minutes, washed, dehydrated, and mounted in DePex.
The specificity of the staining was controlled by digesting some sections with Streptomyces hyaluronidase in the presence of protease inhibitors before staining or preincubating the bHABC probe with hyaluronan oligosaccharides. 27
Evaluation of Staining
The sections were examined by a dual-head microscope simultaneously by two observers (P. A. and V-M. K.). The level of the stromal HA signal in the invasive areas of breast carcinoma was graded as weak (no intense HA signal in peritumoral stroma), moderate (<50% with intense signal), or strong (≥50% with intense signal) and recorded as 1–3, respectively. The expression of HA in carcinoma cells was graded as negative or positive and recorded as 0 or 1, respectively. The cell-associated signal was also scored according to its apparent location in cell surface, cytoplasm, and nucleus.
Statistical Analysis
The statistics were computed by using the SPSS Base 7.5 for Windows program package. The relationships between variables were tested using χ 2 analysis. Univariate survival analyses were based on the Kaplan-Meier method, and the comparisons between curves were done using the log-rank test. Overall survival analysis included as an event all deaths, whatever the cause. Recurrence-free survival was defined as the time elapsed between the primary treatment and the first recurrence of breast cancer. For disease-free survival analysis, patients with metastatic disease at the time of diagnosis were excluded. Multivariate survival analysis was done by Cox’s proportional hazards model, using the backward method (removal limit P < 0.10). The variables in multivariate analysis were tumor size, axillary lymph node status, primary metastases, histological grade, ER status, PR status, age at the time of diagnosis, HA expression in tumor stroma, and HA expression in carcinoma cells.
Results
The normal mammary gland stroma showed a weak or moderate HA signal (Figure 1A) ▶ . In malignant areas. the stromal HA staining was generally stronger than in normal stroma (Figure 1B) ▶ . The intensity of the stromal HA staining varied within each tumor, but there was no difference in HA expression between the peripheral or central parts of the tumors. However, the strongest stromal HA signal always occurred in close proximity to the carcinoma cells. In the 143 breast carcinomas examined, the intensity of stromal HA staining was weak in 7 (5%), moderate in 56 (39%) and strong in 80 (56%) cases (Figure 1, C–E) ▶ .
Figure 1.
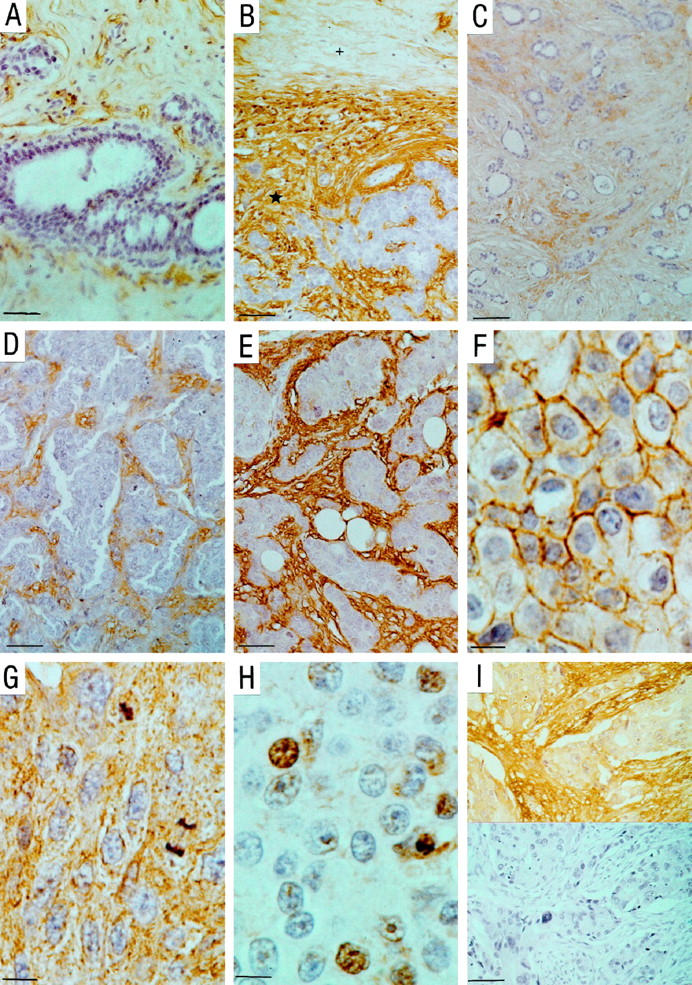
The expression patterns of hyaluronan (HA) in breast carcinoma lesions. A: Normal breast tissue. The HA signal in stroma is weak. Epithelial and myoepithelial cells in normal ducts are negative for HA (scale bar, 50 μm). B: Typical HA signal intensity difference between normal(+) and peritumoral([star]) stroma (scale bar, 90 μm). Views of breast cancer cases in which the intensity of HA signal in stroma is weak (C; scale bar, 90 μm), moderate (D; scale bar, 50 μm), or strong (E; scale bar, 30 μm). Examples of tumor cell-associated HA from areas with HA signal on plasma membranes (F; scale bar, 10 μm), cytoplasm (G; scale bar, 10 μm), and some of the nuclei (H; scale bar, 10 μm). I: A breast cancer case with cytoplasmic HA signal and its negative control treated with Streptomyces hyaluronidase before the staining (scale bar, 50 μm).
The normal ductal epithelial cells and myoepithelial cells displayed no HA (Figure 1A) ▶ . In contrast, the breast carcinomas contained HA-positive epithelial (carcinoma) cells in 81/143 patients (57%). In most of those cases, the HA-positive cells formed a minority of all carcinoma cells (5–10%), but in a few cases even 30 to 50% of them contained HA. The HA in the malignant cells was located on the plasma membrane in 77/143 (54%) cases (Figure 1F) ▶ , in cytoplasm in 65/143 (45%) cases (Figure 1G) ▶ , and in the nucleus in 21/143 (15%) cases (Figure 1H) ▶ .
The intensity of the stromal HA signal was associated with the axillary lymph node positivity (P = 0.015) and poor differentiation of breast carcinoma (P = 0.003) (Table 2) ▶ , but not with the other factors tested, eg, tumor size (P = 0.08), histological type (P = 0.7), distant metastases (P = 0.4), ER status (P = 0.7), or PR status (P = 0.2).
Table 2.
Association between HA Expression in Stroma and Axillary Lymph Node Status and Differentiation of Tumor
| Tumor characteristic | HA expression in extracellular matrix of breast carcinoma cases | |||
|---|---|---|---|---|
| Weak | Moderate | Strong | Significance* | |
| Lymph node status | ||||
| No metastases | 5 | 30 | 27 | P = 0.015 |
| Metastases | 1 | 23 | 47 | |
| Histological grade | ||||
| I | 2 | 5 | 2 | P < 0.003 |
| II | 5 | 32 | 34 | |
| III | 0 | 19 | 44 |
The presence of HA-positive carcinoma cells correlated significantly with axillary lymph node positivity (P = 0.04) and poor differentiation (P = 0.001) (Table 3) ▶ . In addition, the cases with HA-positive tumor cells were more often ER- and PR-negative (P = 0.007 and P = 0.001, respectively) than carcinomas lacking the cell-associated HA (Table 3) ▶ . The expression of cell-associated HA did not correlate with tumor size (P = 0.7), histological type (P = 0.07), or primary metastases (P = 0.2).
Table 3.
Association between HA Expression in Carcinoma Cells and Axillary Lymph Node Status, Differentiation of Tumor, ER Status, and PR Status
| Tumor characteristic | HA expression in breast carcinoma cells | ||
|---|---|---|---|
| Negative | Positive | Significance* | |
| Lymph node status | |||
| No metastases | 31 | 31 | P = 0.039 |
| Metastases | 23 | 48 | |
| Histological grade | |||
| I | 9 | 0 | P < 0.001 |
| II | 38 | 33 | |
| III | 15 | 48 | |
| ER status | |||
| Negative | 17 | 40 | P = 0.007 |
| Positive | 39 | 34 | |
| PR status | |||
| Negative | 17 | 41 | P = 0.001 |
| Positive | 44 | 30 |
The correlations were studied also by the localization of HA in carcinoma cells. Over 60% of cases with HA on the plasma membrane had metastasized to axillary lymph nodes (P = 0.037). HA signals on the plasma membrane and in the cytoplasm of carcinoma cells were both correlated with ER negativity (P < 0.02), PR negativity (P < 0.003), and with poor differentiation of tumors (P < 0.002). HA signal in the nucleus was not significantly related to any of those factors.
Both the intensity of the stromal HA signal and the presence of cell-associated HA related to the overall survival of the 143 breast cancer patients (Table 4) ▶ . The 5-year overall survival was 100%, 80%, and 50% with weak, moderate, and strong stromal HA signal, respectively (P = 0.0001) (Figure 2a) ▶ . The 5-year overall survival of the patients exhibiting HA-positive carcinoma cells was 54% compared with 81% for the patients without HA-positive carcinoma cells (P = 0.01; Figure 2b ▶ ). In analyses based on the localization of cell-associated HA, HA on the plasma membrane correlated with poor 5-year survival (36% for the patients with HA on the plasma membrane versus 57% for the patients without; P = 0.02). HA signal in the cytoplasm or in the nucleus had no prognostic value. The overall survival correlated also with general indicators like tumor size (P = 0.0001), axillary lymph node status (P = 0.0001), primary metastases (P = 0.0001), histological grade (P = 0.03), and age at diagnosis (P = 0.0001).
Table 4.
The 5-Year Overall Survival of All of Patients (n = 143) and 5-Year Disease-Free Survival of Patients without Distant Metastasis at Time of Diagnosis (n = 137)
| Metastasis | Overall survival | Disease-free survival | ||||
|---|---|---|---|---|---|---|
| % | (95% CI*) | Significance | % | (95% CI*) | Significance | |
| HA in stroma | ||||||
| Weak | 100 | (100–100) | P = 0.001 | 100 | (100–100) | P = 0.0035 |
| Moderate | 80 | (67–89) | 73 | (59–83) | ||
| Strong | 53 | (41–63) | 49 | (37–60) | ||
| HA in carcinoma cells | ||||||
| Negative | 81 | (68–86) | P = 0.01 | 63 | (49–74) | P = 0.2 |
| Positive | 54 | (43–64) | 60 | (48–70) |
Figure 2.
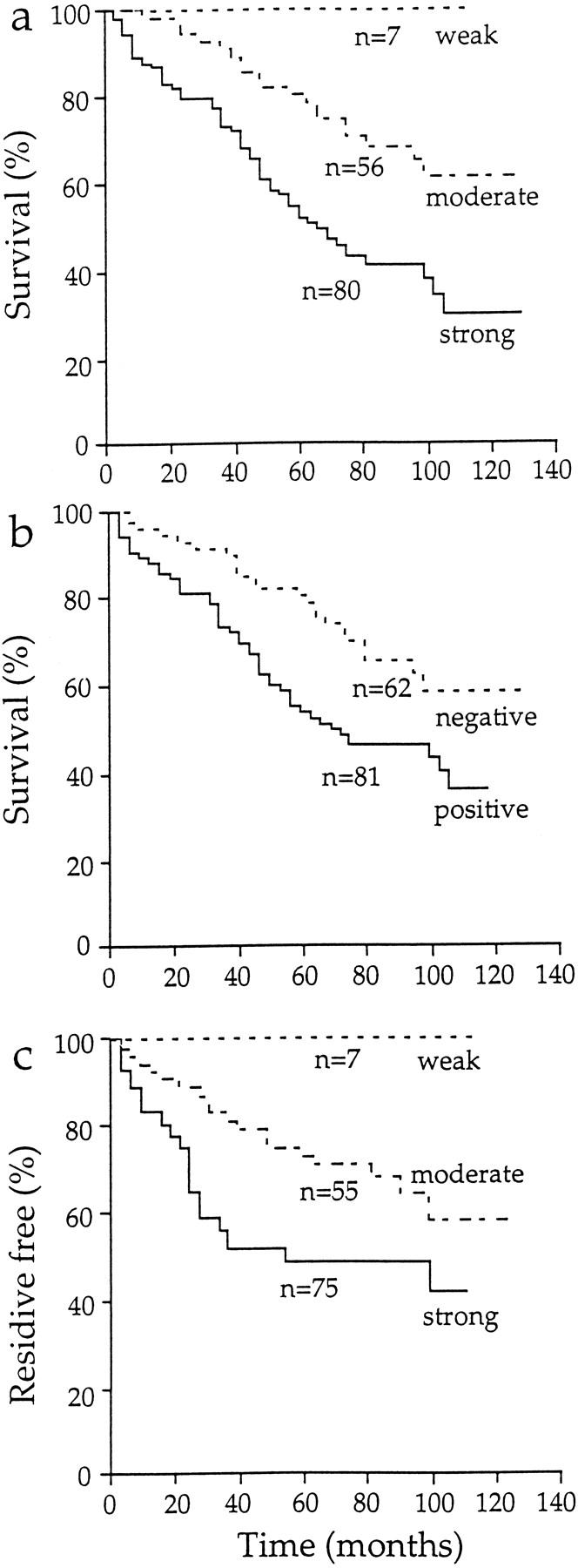
Survival plots of breast cancer patients scored for HA signal intensity in peritumoral stroma and the presence of HA in malignant cells. a: Overall survival of the 143 patients based on the level of HA signal in peritumoral stroma. b: Overall survival of the 143 patients based on the presence of HA signal on carcinoma cells. c: Disease-free survival of the 137 patients without distant metastases based on the level of HA signal in peritumoral stroma.
The disease-free survival of the patients also correlated significantly with HA expression in the tumor stroma (Table 4) ▶ . The 5-year disease-free survival of the breast carcinoma patients was 100% with weak, 73% with moderate, and 49% with strong stromal HA staining (P = 0.0035; Figure 2c ▶ ). The occurrence of HA-positive carcinoma cells did not associate with disease-free survival (P = 0.2).
In multivariate analysis the level of the stromal HA signal was a significant, independent prognostic factor (P = 0.006; Table 5 ▶ ). The other important prognostic factors were tumor size (P = 0.0001), age at diagnosis (P = 0.0006), axillary lymph node status (P = 0.0007), primary metastases (P = 0.005), and ER status (P = 0.01; Table 5 ▶ ). The multivariate analysis was done also for a group with both strong stromal HA signal and carcinoma cell-associated HA (n = 60), versus others (n = 83). In that analysis HA expression showed an even stronger predictive power (P = 0.0009).
Table 5.
Cox’s Multivariate Survival Analysis
| Variable | Odds ratio | 95% CI* of odds ratio | Significance |
|---|---|---|---|
| Tumor size | 1.03 | 1.02–1.06 | P = 0.0001 |
| Age at diagnosis | 1.04 | 1.02–1.06 | P = 0.0006 |
| pN† status | 3.1 | 1.6–5.9 | P = 0.0007 |
| Primary metastases | 5.7 | 1.7–19.0 | P = 0.003 |
| HA in stroma | 2.6 | 1.3–5.0 | P = 0.006 |
| ER status | 0.42 | 0.21–0.81 | P = 0.01 |
Discussion
Several previous investigations have demonstrated elevated HA levels in malignant breast tumors 15-19 and the ability of HA to enhance the motility and metastatic behavior of breast cancer cells. 6 The present study established a strong association between the level of HA around the malignant cells, spreading of the cancer, and patient outcome. An independent role of HA in breast cancer progression was confirmed by Cox multivariate analysis in which both the intense HA expression in stroma alone and that combined with the expression of cell-associated HA were important prognosticators. Taken together, these results predict that HA has a critical role in the spreading of breast cancer, and it can offer a good target for therapeutic purposes.
Consistent with our results, the cell surface receptors of HA, CD44, and RHAMM have been shown to play a key role in cancer cell adhesion, 28,29 cell migration, 13 tumor neovascularization, 30 and cell signaling in general. 24 Also, elevated expressions of CD44 and RHAMM have been linked to breast cancer progression. 23,24 Surprisingly, the local content of HA within tumors has received much less attention, even though the abundance of the ligand must be key to processes regulated by the receptors. 31 In fact, the present findings on breast cancer and a previous study on colon cancer 7 indicate that the level of HA in the malignant epithelium and its immediate vicinity is strongly associated with the spreading status of the tumors and with clinical outcome.
The presence of HA in a fraction of the carcinoma cells and its association with reduced overall survival in univariate analysis indicate that not only the level of surrounding stromal HA but the altered metabolism of HA in the malignant cells themselves promote tumor advancement. The fact that the combination of cell-associated HA with the elevated stromal HA enhanced the predictive power for survival supports the idea that the two parameters act individually but synergistically in promoting tumor spreading.
Although details of the process that leads to accumulation of HA in cancer cells are currently unknown, CD44 variants have been reported to regulate receptor-mediated uptake of HA in cultured breast carcinoma cells. 32 In the current study, the cell-associated HA was mostly found on plasma membrane and often in the cytoplasm, but less frequently in the nucleus. The unfavorable prognosis was also most strongly correlated with the cell surface location, suggesting that the intracellular HA may result from uptake mediated by plasma membrane receptors. The abilities of different breast cancer cell lines to bind and internalize HA through CD44 vary; the cell lines with a high binding capacity show the highest invasive potential. 32 Our in vivo data strongly support this notion. Cell-associated HA alone was also a very strong indicator of unfavorable prognosis in colon cancer. 7
Cell-associated HA, but not matrix-associated HA, was correlated with hormone receptor status. This result complements a previous in vitro work showing that ER- and PR-negative cell lines synthesized and bound more HA than otherwise comparable ER- and PR-positive lines. 33 Work in vitro suggests that enhanced synthesis by the cancer cells was also involved in the cellular accumulation of HA. 7
The degradation of stromal matrix by metalloproteinases is obviously crucial for the growth and spreading of many malignant tumors. 34 Interestingly, HA may stimulate the activity of matrix metalloproteinase 9 in a mouse mammary epithelial carcinoma cell line by a mechanism that involves ligand-induced aggregation of cell surface CD44. 35 This suggests a new, direct link between HA accumulation and matrix turnover. HA is not the only new matrix component in the breast cancer stroma, also enriched in versican, an HA-binding proteoglycan, 36 and lumican, a small proteoglycan that binds to and modulates the structure of collagen fibers. 37 This set of changes in the matrix is apparently effected by the cells on the mesenchymal side, but, perhaps triggered by active oncogenes, such as Ras, in the malignant epithelial cells. 38
Tumor size and axillary lymph node involvement are currently the most powerful prognostic factors in breast cancer. Because of the pressure to use less radical operations and neoadjuvant cytostatic therapy, additional prognostic factors like molecular markers are needed to identify the patients that benefit from the most aggressive therapy. The current, relatively simple technique could aid therapeutic decisions based on biopsies taken before the actual operation, because the predictive power of the stromal and cell-associated HA combined was in the same range as that of tumor size and nodal status, the conventional indicators.
The accumulation of HA in malignant cells and adjacent stroma may also offer possibilities for therapeutic interference. Intravenous hyaluronidase, which degrades HA, has been proved relatively nontoxic and has been successfully used to enhance the penetration of cytostatic drugs in bladder carcinomas, squamous carcinomas of neck, and also in breast cancer models. 39-41 Agents that specifically inhibit HA synthesis are not currently available, but the recent cloning of the human HA synthase genes 42 will facilitate studies on HA synthesis regulation in breast cancer, and provide potential targets of therapeutic interference. For instance, growth factors such as epidermal growth factor and transforming growth factor β stimulate HA synthesis in certain cells 43,44 ; blocking of those growth factors or their receptors could reduce HA synthesis. Down-regulation of RHAMM, the transforming and migration-stimulating receptor of HA, could reduce the effect of excess HA in the vicinity of the malignant cells. 45 Antibodies that inhibit the RHAMM function in vitro are available, 45 and blocking of HA with soluble peptides that bind HA has been tested in preliminary experiments on animals. 46 Furthermore, hyaluronan oligomers inhibit tumor growth in the mouse, presumably by displacing intact hyaluronan from its cell and matrix receptors. 47 Therefore, we conclude that further clinical studies on the synthesis and regulation of hyaluronan in malignant tissues are clearly warranted.
Acknowledgments
We thank Dr. Eva Turley for her comments on the manuscript. Technical help from Ms. Seija Eskelinen and Ms. Arja Venäläinen is gratefully acknowledged.
Footnotes
Address reprint requests to Päivi Auvinen, Department of Oncology, Kuopio University Hospital, P.O. Box 1777, FIN-70211, Kuopio, Finland. E-mail: paivi.auvinen@kuh.fi.
Supported by grants of the Finnish Cancer Union, the Finnish Breast Cancer Group, and Orion Corporation and by EVO funding of Kuopio University Hospital.
References
- 1.Toole B: Glycosaminoglycans in morphogenesis. Hay ED eds. Cell Biology of the Extracellular Matrix. 1981, :pp 259-294 Plenum Press, New York [Google Scholar]
- 2.Laurent T, Fraser J: Hyaluronan. FASEB J 1992, 6:2397-2404 [PubMed] [Google Scholar]
- 3.Tammi R, Ripellino JA, Margolis RU, Tammi M: Localization of epidermal hyaluronic acid using the hyaluronate binding region of cartilage proteoglycan as a specific probe. J Invest Dermatol 1988, 90:412-414 [DOI] [PubMed] [Google Scholar]
- 4.Knudson W, Biswas C, Li X-Q, Nemec R, Toole B: The role and regulation of tumour-associated hyaluronan. The Biology of Hyaluronan. Edited by D Evered, J Whelan. Ciba Found Symp 1989, 143:150–169 [DOI] [PubMed]
- 5.Toole B, Biswas C, Gross J: Hyaluronate and invasiveness of the rabbit V2 carcinoma. Proc Natl Acad Sci USA 1979, 76:6299-6303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimata K, Honma Y, Okayama M, Oguri K, Hozumi M, Suzuki S: Increased synthesis of hyaluronic acid by mouse mammary carcinoma cell variants with high metastatic potential. Cancer Res 1983, 43:1347-1354 [PubMed] [Google Scholar]
- 7.Ropponen K, Tammi M, Parkkinen J, Eskelinen M, Tammi R, Lipponen P, Ågren U, Alhava E, Kosma V-M: Tumor cell-associated hyaluronan as an unfavorable prognostic factor in colorectal cancer. Cancer Res 1998, 58:342-347 [PubMed] [Google Scholar]
- 8.Setälä L, Tammi M, Tammi R, Eskelinen M, Lipponen P, Ågren U, Parkkinen J, Alhava E, Kosma V-M: Hyaluronan expression in gastric cancer cells is associated with local and nodal spread and reduced survival rate. Br J Cancer 1999, 79:1133-1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brecht M, Mayer U, Schlosser E, Prehm P: Increased hyaluronate synthesis is required for fibroblast detachment and mitosis. Biochem J 1986, 239:445-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rooney P, Kumar S, Ponting J, Wang M: The role of hyaluronan in tumour neovascularization (review). Int J Cancer 1995, 60:632-636 [DOI] [PubMed] [Google Scholar]
- 11.Pauli B, Knudson W: Tumor invasion: A consequence of destructive and compositional matrix alterations. Hum Pathol 1988, 19:628-639 [DOI] [PubMed] [Google Scholar]
- 12.Ohta S, Yoshida J, Iwata H, Hamaguchi M: Hyaluronate activates tyrosine phosphorylation of cellular proteins including focal adhesion kinase via CD44 in human glioma cells. Int J Oncol 1997, 10:561-564 [DOI] [PubMed] [Google Scholar]
- 13.Turley E: Hyaluronan and cell locomotion. Cancer Met Rev 1992, 11:21-30 [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Underhill C, Chen L: Hyaluronan on the surface of tumor cells is correlated with metastatic behavior. Cancer Res 1995, 55:428-433 [PubMed] [Google Scholar]
- 15.Takeuchi J, Sobue M, Sato E, Shamoto M, Miura K, Nakagaki S: Variation in glycosaminoglycan components of breast tumors. Cancer Res 1976, 36:2133-2139 [PubMed] [Google Scholar]
- 16.Bertrand P, Girard N, Delpech B, Duval C, d‘Anjou J, Dauce JP: Hyaluronan and hyaluronectin in the extracellular matrix of human breast carcinomas: comparison between invasive and non-invasive areas. Int J Cancer 1992, 52:1–6 [DOI] [PubMed]
- 17.Ponting J, Kumar S, Pye D: Co-localisation of hyaluronan and hyaluronectin in normal and neoplastic breast tissues. Int J Oncol 1993, 2:889-893 [DOI] [PubMed] [Google Scholar]
- 18.Shuster S, Smith HS, Thor AD, Stern R: Enhanced deposition of hyaluronan in the stroma of human breast cancers. Proc Am Assoc Cancer Res 1993, 34:2 [Google Scholar]
- 19.de la Torre M, Wells A, Bergh J, Lindgren A: Localization of hyaluronan in normal breast tissue, radial scar, and tubular breast carcinoma. Hum Pathol 1993, 24:1294-1297 [DOI] [PubMed] [Google Scholar]
- 20.Auvinen P, Parkkinen J, Johansson R, Ågren U, Tammi R, Eskelinen M, Kosma V-M: Expression of hyaluronan in benign and malignant breast lesions. Int J Cancer (Pred Oncol) 1997, 74:477-481 [DOI] [PubMed] [Google Scholar]
- 21.Schor SL, Schor AM, Grey AM, Chen J, Rushton G, Grant ME, Ellis I: Mechanism of action of the migration stimulating factor produced by fetal and cancer patient fibroblasts: effect on hyaluronic acid synthesis. In Vitro Cell Dev Biol 1989, 25:737-746 [DOI] [PubMed] [Google Scholar]
- 22.Cedeno D, Stern R: Stimulation of hyaluronic acid synthesis in fibroblasts by cocultivation with human breast tumor cell lines. J Cell Biol 1986, 103:101a [Google Scholar]
- 23.Joensuu H, Klemi P, Toikkanen S, Jalkanen S: Glycoprotein CD44 expression and its association with survival in breast cancer. Am J Pathol 1993, 143:867-874 [PMC free article] [PubMed] [Google Scholar]
- 24.Wang C, Thor A, Moore D, II, Zhao Y, Kerschmann R, Stern R, Watson P, Turley E: The overexpression of RHAMM, a hyaluronan-binding protein that regulates ras signaling, correlates with overexpression of mitogen-activated protein kinase and is a significant parameter in breast cancer progression. Clin Cancer Res 1998, 4:567-576 [PubMed] [Google Scholar]
- 25.Vihko R, Jänne O, Kontula K, Syrjälä P: Female sex steroid receptor status in primary and metastatic breast carcinoma and its relationship to serum steroid and peptide hormone levels. Int J Cancer 1980, 26:13-21 [DOI] [PubMed] [Google Scholar]
- 26.Wang C, Tammi M, Hongtao G, Tammi R: Hyaluronan distribution in the normal epithelium of esophagus, stomach and colon and their cancers. Am J Pathol 1996, 148:1861-1869 [PMC free article] [PubMed] [Google Scholar]
- 27.Tammi R, Ågren U, Tuhkanen A, Tammi M: Hyaluronan metabolism in skin. Prog Histochem Cytochem 1994, 29:1-77 [DOI] [PubMed] [Google Scholar]
- 28.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B: CD44 is the principal cell surface receptor for hyaluronate. Cell 1990, 61:1303-1313 [DOI] [PubMed] [Google Scholar]
- 29.Yeo TK, Nagy JA, Yeo KT, Dvorak HF, Toole BP: Increased hyaluronan at sites of attachment to mesentery by CD44-positive mouse ovarian and breast tumor cells. Am J Pathol 1996, 148:1733-1740 [PMC free article] [PubMed] [Google Scholar]
- 30.Trochon V, Mabilat C, Bertrand P, Legrand Y, Smadja-Joffe F, Soria C, Delpech B, Lu H: Evidence of involvement of CD44 in endothelial cell proliferation, migration and angiogenesis in vitro. Int J Cancer 1996, 66:664-668 [DOI] [PubMed] [Google Scholar]
- 31.Bartolazzi A, Peach R, Aruffo A, Stamenkovic I: Interaction between CD44 and hyaluronate is directly implicated in the regulation of tumor development. J Exp Med 1994, 180:53-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Culty M, Shizari M, Thompson EW, Underhill CB: Binding and degradation of hyaluronan by human breast cancer cell lines expressing different forms of CD44: correlation with invasive potential. J Cell Physiol 1994, 160:275-286 [DOI] [PubMed] [Google Scholar]
- 33.Heldin P, de la Torre M, Ytterberg D, Bergh J: Differential synthesis and binding of hyaluronan by human breast cancer cell lines: relationship to hormone receptor status. Oncol Rep 1996, 3:1011-1016 [DOI] [PubMed] [Google Scholar]
- 34.Kleiner DE, Stetler-Stevenson WG: Matrix metalloproteinases and metastasis (review). Cancer Chemother Pharmacol 1999, 43(Suppl):S42-S51 [DOI] [PubMed] [Google Scholar]
- 35.Yu Q, Stamenkovic I: Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Dev 1999, 13:35-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nara Y, Kato Y, Torii Y, Tsuji Y, Nakagaki S, Goto S, Isobe H, Nakashima N, Takeuchi J: Immunohistochemical localization of extracellular matrix components in human breast tumors with special reference to PG-M/versican. Histochem J 1997, 29:21-30 [DOI] [PubMed] [Google Scholar]
- 37.Leygue E, Snell L, Dotzlaw H, Hole K, Hiller-Hitchcock T, Roughley PJ, Watson PH, Murphy LC: Expression of lumican in human breast carcinoma. Cancer Res 1998, 58:1348-1352 [PubMed] [Google Scholar]
- 38.Chin L, Tam A, Pomerantz J, Wong M, Holash J, Bardeesy N, Shen Q, O’Hagant R, Pantginis J, Zhoul H, Horner JW, Cordon-Cardo C, Yancopoulos GD, DePinho RA: Essential role for oncogenic Ras in tumor maintenance. Nature 1999, 400:468-472 [DOI] [PubMed] [Google Scholar]
- 39.Höbarth K, Maier U, Marberger M: Topical chemoprophylaxis of superficial bladder cancer with mitomycin C and adjuvant hyaluronidase. Eur Urol 1992, 21:206-210 [DOI] [PubMed] [Google Scholar]
- 40.Kohno N, Ohnuma T, Truog P: Effects of hyaluronidase on doxorubicin penetration into squamous carcinoma multicellular tumor spheroids and its cell lethality. J Cancer Res Clin Oncol 1994, 120:293-297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beckenlehner K, Bannke S, Spruss T, Bernhardt G, Schonenberg H, Schiess W: Hyaluronidase enhances the activity of adriamycin in breast cancer models in vitro and in vivo. J Cancer Res Clin Oncol 1992, 118:591-596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weigel PH, Hascall VC, Tammi M: Hyaluronan synthases. J Biol Chem 1997, 272:13997-14000 [DOI] [PubMed] [Google Scholar]
- 43.Tirone E, D’Alessandris C, Hascall VC, Siracusa G, Salustri A: Hyaluronan synthesis by mouse cumulus cells is regulated by interactions between follicle-stimulating hormone (or epidemal growth factor) and a soluble oocyte factor (or transforming growth factor β1). J Biol Chem 1997, 272:4748-4794 [DOI] [PubMed] [Google Scholar]
- 44.Samuel SK, Hurta RA, Spearman MA, Wright JA, Turley EA, Greenberg AH: TGF-β 1 stimulation of cell locomotion utilizes the hyaluronan receptor RHAMM, and hyaluronan. J Cell Biol 1993, 123:749-758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohapatra S, Yang X, Wright JA, Turley EA, Greenber AH: Soluble hyaluronan receptor RHAMM induces mitotic arrest by suppress-ing Cdc2 and cyclin B1 expression. J Exp Med 1996, 183:1663-1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Savani RC, Khalil N, Turley EA: Hyaluronan receptor antagonists alter skin inflammation and fibrosis following injury. Proc West Pharmacol Soc 1995, 38:131-136 [PubMed] [Google Scholar]
- 47.Zeng C, Toole BP, Kinney SD, Kuo J-W, Stamenkovic I: Inhibition of tumor growth in vivo by hyaluronan oligomers. Int J Cancer 1998, 77:396-401 [DOI] [PubMed] [Google Scholar]