Latent Transforming Growth Factor-β Activation in Mammary Gland: Regulation by Ovarian Hormones Affects Ductal and Alveolar Proliferation (original) (raw)
Abstract
Transforming growth factor-β1 (TGF-β1) is a pluripotent cytokine that can inhibit epithelial proliferation and induce apoptosis, but is also widely implicated in breast cancer progression. Understanding its biological action in mammary development is critical for understanding its role in cancer. TGF-β1 is produced as a latent complex that requires extracellular activation before receptor binding. To better understand the spatial and temporal regulation of its action during mammary gland development, we examined the pattern of activation in situ using antibodies selected to distinguish between latent and active TGF-β. Activation was highly restricted. TGF-β1 activation was localized primarily to the epithelium, and within the epithelium it was restricted to luminal epithelial cells but absent from either cap or myoepithelial cells. Within the luminal epithelium, we noted a further restriction. During periods of proliferation (ie, puberty, estrus and pregnancy), which are stimulated by ovarian hormones, TGF-β1 activation decreased in some cells, consistent with preparation for proliferation. Paradoxically, other cells simultaneously increase TGF-β1 immunoreactivity, which suggests that TGF-β1 differentially restrains epithelial subpopulations from responding to hormonal signals to proliferate. These data suggest that endogenous TGF-β1 activation and thus activity are regulated by ovarian hormones. To determine the specific consequences of TGF-β1 activity, we manipulated TGF-β1 levels in vivo using Tgfβ1 knockout mice and undertook tissue recombination experiments with heterozygous tissue. In Tgfβ1 heterozygous mice, which have <10% wild-type levels of TGF-β1, ductal development during puberty and alveolar development during pregnancy were accelerated, consistent with its role as a growth inhibitor. The proliferative index of Tgfβ1+/− epithelium was increased approximately twofold in quiescent tissue and fourfold in proliferating tissue but both ducts and alveoli were grossly and histologically normal. To test whether epithelial TGF-β1 was critical to the proliferative phenotype, Tgfβ1+/+ and +/− epithelium were transplanted into +/+ mammary stroma. The outgrowth of Tgfβ1+/− epithelium was accelerated in wild-type hosts, indicating that the phenotype was intrinsic to the epithelium. Moreover, proliferation was 15-fold greater in Tgfβ1+/− than wild-type mice after ovariectomy and treatment with estrogen and progesterone, suggesting that TGF-β1 acts in an autocrine or juxtacrine manner to regulate epithelial proliferation. Together these data indicate that ovarian hormones regulate TGF-β1 activation, which in turn restricts proliferative response to hormone signaling.
Transforming growth factor-β1 (TGF-β) is the founding member of a superfamily of proteins involved in regulation of proliferation, apoptosis, and extracellular matrix and protease production. 1,2 In epithelial cells, TGF-β signaling mediates G1 arrest by indirectly inducing or activating cyclin-dependent kinase inhibitors. 3,4 Normal breast epithelial cells are profoundly growth inhibited by TGF-β1 but breast cancer cells are widely resistant to growth suppression, 5 although other targets are still responsive. 6 Overexpression of TGF-β1 suppresses mammary tumorigenesis, 7 but TGF-β1 can also convert to a tumor-promoting role in late carcinogenesis in skin, mammary gland, and colon. 8-10 TGF-β1 isoforms are often highly expressed in more advanced tumors and could promote tumorigenesis by a number of means. 11 High levels of TGF-β1 activity may promote angiogenesis 12 and expression of metalloproteases 13,14 that would be conducive to expansion and invasion. Because TGF-β1 also inhibits immune cell function, 15 its production by tumors has been suggested to reduce immune surveillance. At the autocrine level, resistance to TGF-β-mediated G1 arrest is one of the salient steps in progression of breast cell lines to less stable phenotypes. 16 Furthermore TGF-β1 is strongly implicated in epithelial-to-mesenchymal transitions in tumor cells. 8-10,17 The complexity of TGF-β1 signaling and effects, 18 underscore the need to understand its function in situ during both normal tissue development and carcinogenesis.
Defining the specific consequences of TGF-β1 activity is further complicated because TGF-β1 responses are modulated by cell type, 19 differentiation, 20 and the microenvironment 21 and because of differences in the expression of various receptors and intracellular signaling components. Elegant studies by Daniel and colleagues 22,23 were among the first to demonstrate that TGF-β1 acts as an epithelial growth inhibitor in the mouse mammary gland. Although the mRNA of all three mammalian TGF-β1 isoforms are expressed in the epithelium and stroma during all phases of mammary development and differentiation, 24 the responses to TGF-β1 seem to be finely orchestrated during mammary gland development. TGF-β1 administered from slow-release pellets causes endbuds to regress during puberty but does not inhibit lateral branching in adults or the alveolar outgrowth necessary for secretory differentiation during pregnancy. 25,26 It is of interest to define the basis of this differential sensitivity to exogenous TGF-β, because loss of TGF-β1 responsiveness is frequently associated with breast cancer cells. 5
An additional factor in understanding the biological action of TGF-β1 is its production as a latent complex, which consists of the 24-kd cytokine and an 80-kd dimer of its pre-pro region called the latency-associated peptide (LAP). 27 Because LAP contains the signal sequence for secretion, 28 cells secrete latent TGF-β1 (LTGF-β1). As a result, extracellular processes that release TGF-β1 from LAP essentially control its biological availability, and thus its action. 29 Overexpression of wild-type TGF-β, which results in elevated LTGF-β1 production, in general does not result in a phenotypic change, whereas expression of a constitutively active mutant form of TGF-β1 leads to dramatic phenotypes. 25,30 Such studies support the contention that the key to understanding TGF-β1 bioavailability is to identify the sites and circumstances of its activation.
Studies to localize TGF-β1 activation in response to physiological stimuli in vivo have not been adequately addressed, primarily because of the lack of appropriate reagents to evaluate activation in situ. We have determined that certain antibodies directed against TGF-β1 discriminate between latent and active TGF-β1 in situ by defining their immunoreactivity in tissues engineered to produce TGF-β1 in either a constitutively active form or the latent complex. 31,32 We have demonstrated in several models that increased staining intensity using antibodies selected in this manner, concomitant with loss of LAP immunoreactivity, is indicative in situ of LTGF-β1 activation. 33-35 With such information, tissue-specific actions of TGF-β1 can be more readily studied.
To better understand the role of TGF-β1 in mammary gland, we used immunofluorescence to localize LAP and sites of TGF-β1 activation during mammary development and differentiation. To define the specific consequences of TGF-β, we compared the pattern of immunoreactivity during specific developmental events with the effect of depleting TGF-β1 by targeted gene knockout in the corresponding stage of mammary gland development. 36 _Tgfβ1_−/− mice die of gross inflammation at 3 weeks of age, thus precluding analysis of mammary maturation. However, Tgfβ1+/− mice are viable even though there is a significant reduction in TGF-β1 protein levels. 37 These mice provide an experimental model of TGF-β1 depletion during mammary gland development. Our studies demonstrate that TGF-β1 activation is highly regulated in a cell-restricted manner during mammary development and secretory differentiation. The phenotype of the Tgfβ1+/− mice supports the action of TGF-β1 as an inhibitor of mammary epithelial proliferation. Together these studies demonstrate that TGF-β1 activation is controlled by hormonal milieu, and that TGF-β1 in turn restricts proliferation in response to hormones.
Materials and Methods
Animals
All experiments were conducted with institutional review and approval. Animals were euthanized by CO2 inhalation and cervical dislocation at the indicated times in accordance with AAALAC guidelines. Mammary glands were collected from FVB mice bred at Lawrence Berkeley National Laboratory, commercially obtained BALB/c mice, 129SV/C57BL/6 Tgfβ1+/+ or +/− mice bred in-house at the National Institutes of Health or the Lawrence Berkeley National Laboratory, and congenic 129S2/SV Hsdc Tgfβ1+/+ or +/− mice and F1 outcrosses of 129S2/SV Hsd and C57BL/6 Tac Tgfβ1+/+ or +/− mice bred at University of California at San Francisco. Unless otherwise specified, immunostaining data are from FVB mice and transgenic phenotype data are from 129SV/C57BL/6 Tgfβ1+/+ or +/− mice. Estrus was staged using cytological characteristics of vaginal smears and confirmed postmortem by uterine wet weight. Pregnancy was determined by vaginal plug and confirmed by embryos. Ten-week-old mice were ovariectomized for 14 to 17 days and injected intraperitoneally with estradiol (1 μg) and progesterone (1 mg) daily for 3 days. Control animals were injected with vehicle throughout the same time course.
The inguinal (fourth pair) mammary glands were dissected free of the skin and were either placed in Carnoy’s fixative for whole-mount staining, or embedded in OCT compound (Miles Inc., Elkhart, IN). Frozen tissue blocks were stored at −70°C until the time of sectioning. Whole-mount staining of glands with 1% carmine alum were done as described 38 but the glands were fixed in Carnoy’s fixative overnight; stained glands were stored in methyl salicylate after poststaining dehydration. After photography, mammary whole mounts were embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin to score for pyknotic nuclei.
Grafting of Mammary Epithelium to Cleared Fat Pads
Three-week-old F1 129S2/SV Hsd × C57BL/6 Tac Tgfβ1+/+ or +/− mice were anesthetized with 0.01 ml per 10 g weight of 40 mg/ml of xylazine and 25 mg/ml of ketamine. The skin was opened aseptically at the anterior midline and along each hock; one skin flap was pinned back to expose the fourth (inguinal) gland. 39 The nipple, the tissue above and including the lymph node, and the connection to the fifth gland were cauterized and removed. A single tissue fragment (1 mm3) from the inguinal gland of an F1 129S2/SV Hsd × C57BL/6 Tac Tgfβ1+/+ or +/− adult donor animal was transplanted to a slit in the ventral anterior edge of the cleared fat pad. This procedure was repeated with the contralateral inguinal gland. The skin of the host animal was then closed up with wound clips; these were removed after 1 week. Host animals were euthanized 6 weeks after the operation to examine ductal growth in the inguinal glands.
Antibodies
Antibodies designated chNTGF-β1 were affinity-purified immunoglobulin fractions of polyclonal affinity-purified chicken anti-TGF-β1 (AF-101-NA, Lot no. FS03 and no. FS08; R&D Systems, Minneapolis, MN), which preferentially react with the active form of TGF-β1. 34 Goat polyclonal antibodies to recombinant human TGF-β1 LAP (R&D Systems) are specific for latent TGF-β1 as previously described. 33,34 Fluorescein-labeled anti-goat IgG (Pierce, Rockford, IL, or Southern Biotechnology Associates, Inc., Birmingham, AL) was used for the detection of LAP antibody. Texas Red-labeled anti-chicken antibody (Sigma, St. Louis, MO) was used for detection of TGF-β. Fluorescein-conjugated monoclonal mouse antibody to proliferating cell nuclear antigen (PCNA) was used at 1 to 10 dilution in accordance with the manufacturer’s protocol (DAKO, Carpinteria, CA). The Intergen kit (Purchase, New York) was used for terminal dUTP nick-end labeling staining; the manufacturer’s instructions were used for staining.
Immunohistochemistry
Immunostaining to differentiate between active and latent active TGF-β1 immunostaining was conducted as previously described. 34 Briefly, frozen embedded mammary gland were sectioned onto gelatin-coated coverslips, then fixed using 2% buffered paraformaldehyde for 20 minutes at room temperature, followed by a 0.1 mol/L glycine/phosphate-buffered saline (PBS) wash. Nonspecific sites were blocked using the supernatant from a 0.5% casein/PBS solution (pH 7.4) for 60 minutes. The sections were incubated simultaneously overnight with primary antibodies to LAP and TGF-β1 antibodies diluted in blocking buffer. After washes, each primary was detected by sequential incubations with species-specific secondary antibodies. For PCNA staining, sections were fixed for 5 minutes in 2% paraformaldehyde in PBS, washed, and fixed for 10 minutes in methanol at 4°C. Sections were blocked as described above, then incubated in the fluorescein isothiocyanate-conjugated antibody for 1 hour. Nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI). The sections were mounted in Vectashield (Vector Laboratories, Burlingame, CA) and stored at −20°C until evaluated. Deletion of primary antibody controls in each experiment and assays of secondary antibody cross-reactivity were routinely negative.
Image Acquisition and Processing
Immunofluorescence images were obtained using a ×40, 0.75 numerical aperture Zeiss Neofluar objective on a Zeiss Axiovert equipped with epifluorescence. A multiband pass dichroic mirror, barrier filter, and differential wavelength filter wheel combination was used to selectively excite fluorochromes in sequence. Images were captured using a scientific-grade 12-bit charged coupled device (KAF-1400, 1317 × 1035 6.8 μm square pixels) digital camera (Xillix, Vancouver, Canada). Relative intensity of images were maintained when constructing figures by using Scilimage (TNO Institute of Applied Physics, Delft, The Netherlands) to scale the 12-bit data to a common 8-bit scale using the data set minimum and maximum. Internal standardization was achieved by comparing only images stained with the same antibodies in the same experiment, captured with identical parameters and scaled and displayed identically. Relative quantitation of fluorescence intensity was analyzed by defining a region of interest, representative of a cell, positioned between epithelial or stromal nuclei using the DAPI image without reference to the fluorochrome-labeled image to avoid selection bias. Background fluorescence was subtracted from the image before calculating the total fluorescence intensities within each region of interest and is displayed graphically for selected treatment groups or as mean immunoreactivity intensity (arbitrary units) for a population. Some images are shown in false-color composites.
Statistics
Two-tailed unpaired Student’s _t_-test or Fisher’s exact test were used to evaluate whether control and treatment groups differed significantly using Prism Version 2.01 (GraphPad Inc.). Kolmogorov-Smirnov two-sample goodness of fit test was used to compare distributions using S-Plus (Mathsoft, Inc.).
TGF-β1 Bioassay
Acid ethanol extracts of Tgfβ1+/+ and +/− mammary gland tissue were made as described. 40 Bioassays of these extracts in triplicate were performed using mink lung epithelial cells that had been transfected with a plasminogin activator inhibitor (PAI) promoter-luciferase vector construct. 41 Luciferase readings were compared with a standard curve constructed with triplicate dilutions of recombinant active TGF-β1 (R&D Systems, Minneapolis, MN).
Results
TGF-β1 Activation Is Restricted within the Mammary Epithelium of Pubertal Glands
To gain insight regarding the bioavailability of TGF-β1 during mammary gland development, we examined the distribution of immunoreactive LAP (as an indicator of latent TGF-β) and immunostaining indicative of active TGF-β1 using dual immunofluorescence in cryosections of mammary gland from juvenile FVB mice (Figure 1A) ▶ . The mammary gland develops postnatally in two stages: formation of a ductal tree at puberty and full functional differentiation during pregnancy. These stages are regulated by the ovarian hormones, estrogen and progesterone. 42 Mammary ductal morphogenesis occurs during puberty via the outgrowth of endbuds, which are multicellular epithelial structures that invade the adipose stroma to establish the mammary ductal tree. The endbud is a site of actively proliferating epithelial cells and is surrounded by a monolayer of cap cells, a specialized epithelial cell that forms a interface between the endbud and adipose stroma. 43 The subtending ducts that lead into the endbuds are relatively quiescent.
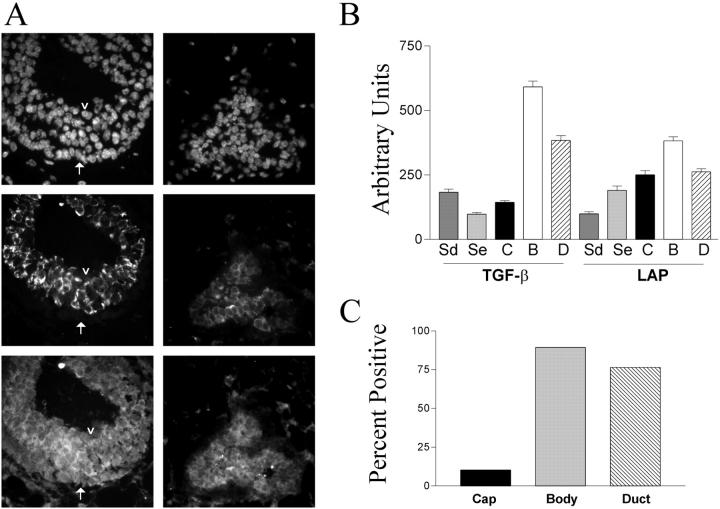
Figure 1.
Mammary epithelial LAP and TGF-β1 immunostaining are regulated during ductal morphogenesis. A: Nuclear DAPI staining (top) and dual immunolocalization of active TGF-β1 (middle) and LAP (bottom) of endbuds (left) and distal ducts (right). During ductal morphogenesis, most body cells in endbuds showed LAP and strong TGF-β1 immunostaining, but some lacked active TGF-β1 (arrowhead). Cap cells (arrows) at the interface between the endbud and adipose stroma were negative for TGF-β1 even though they stained with antibodies to LAP. Ductal epithelium proximal to the region of endbuds exhibited less TGF-β1 immunostaining. Tissue was from FVB mice. Scale bar, 20 μm. B: The mean (±SD) intensity of TGF-β1 and LAP were quantified in the peri-epithelial stroma of the ducts (Sd) and endbuds (Se) and for epithelial cells from the cap cell layer (C), endbud body cell (B), and distal ducts (D). Endbud body cells had significantly more TGF-β1 immunoreactivity than any other population. C: To evaluate the frequency of TGF-β1-positive cells in different epithelial populations, cells with immunoreactivity greater than the mean +2 SD of the stromal cell TGF-β1 intensity were defined as TGF-β1-positive. The majority of endbud body and duct epithelial cells were TGF-β1-positive, whereas cap cells were rarely positive.
LAP immunoreactivity was distributed throughout the epithelium, fibrous stroma, and adipose. Relative differences in intensity between different cell compartments were determined using digital image analysis (Figure 1B) ▶ . The mean intensity of LAP immunoreactivity was lowest in the ducts leading to endbuds; endbud stroma was approximately twice as intense and epithelial cells exhibited immunoreactivity between 2 to 4 times greater than that of distal ducts. Immunostaining indicative of TGF-β1 activation was prominent in a subset of endbud epithelial cells (Figure 1A) ▶ . In contrast, endbud cap cells exhibited fourfold less TGF-β1 immunoreactivity than endbud body cells despite relatively high LAP immunoreactivity. The epithelium of subtending ducts, which are quiescent, showed a relatively homogenous pattern of active TGF-β1 immunostaining compared to that in the endbud body epithelium. The intensity of TGF-β1 immunoreactivity in ductal epithelial cells was two thirds that of endbud body cells.
TGF-β1 immunoreactivity in the stroma was virtually undetectable and the measured intensity was sixfold lower than that in endbud body cells. This was not because of differential sensitivity of the immunostaining technique because TGF-β1 activation was readily detected in both adipose and fibrous stroma in irradiated mammary gland. 34 Using a threshold of twice the mean TGF-β1 intensity of the distal stroma, 75% or more of endbud body and ductal epithelial cells were TGF-β1-positive whereas only 10% of cap cells were positive (Figure 1C) ▶ . These data demonstrate that although LAP and TGF-β1 are widely distributed in the mammary gland, their relative abundance depends on cell type.
Because LAP and TGF-β1 are secreted together in a complex, disparate immunolocalization of LAP and TGF-β1 is consistent with cryptic TGF-β1 epitopes that are revealed after removal and/or distortion of LAP during activation or binding to receptor. 31 The restricted localization of TGF-β1 relative to the wide distribution of LAP immunostaining suggests that endogenous TGF-β1 activation in the mammary gland is regulated on a cell by cell basis during puberty. One would predict that any effects of TGF-β1 depletion would primarily affect endbud, rather than ductal, epithelial cells, because they exhibit higher levels of TGF-β1 activation.
The Pattern of TGF-β1 Activation Is Modulated by the Estrus Cycle
In nulliparous adult female mice, the mammary epithelium is relatively quiescent but undergoes modest rounds of proliferation and abbreviated lateral budding as a function of the ovarian hormones produced during the estrus cycle. LAP immunoreactivity was widespread throughout the adult mammary epithelium, peri-epithelial stroma, and adipose stroma, whereas active TGF-β1 immunostaining was restricted to epithelial cells. Analysis as a function of estrus revealed distinct patterns of TGF-β1 immunostaining associated with different stages of the estrus cycle (Figure 2) ▶ . During diestrus, the epithelium was uniformly immunoreactive for both LAP and TGF-β1 antibodies, similar to that seen in the distal ducts of the immature mammary gland. At proestrus, TGF-β1 was selectively reduced such that some cells were TGF-β1-positive but adjacent cells were almost negative. During estrus, the heterogeneity of TGF-β1 immunostaining in the epithelium increased, resulting in a checkerboard pattern that consisted of intensely positive cells adjacent to negative cells. This heterogeneity was particularly evident in transverse sections of ducts and was most frequently observed in small ducts with minimal peri-epithelial stroma (Figure 2E) ▶ . Larger ducts with distinct peri-epithelial fibroblast cuffs tended to exhibit relatively homogenous TGF-β1 immunoreactivity (not shown). This striking pattern of prominent TGF-β1-positive cells during estrus was similar to the restricted immunoreactivity observed in endbud body cells. To ensure that this phenotype was a feature of estrus cycle rather than mouse strain, three strains of mice were examined: BALB/c, 129SV/C57BL/6, and FVB, all of which showed the features described above; the data shown are from FVB mice.
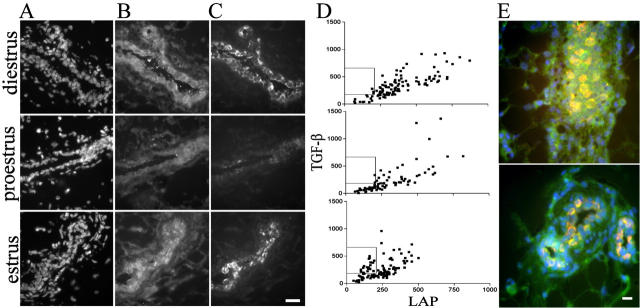
Figure 2.
LAP and TGF-β1 immunostaining is highly heterogeneous during the estrus cycle. A–C: Mammary epithelial immunoreactivity of nulliparous FVB animals as a function of the estrus cycle; DAPI-stained nuclei (A), LAP (B) and active TGF-β1 (TGF-β) (C) immunoreactivity. In diestrus, most epithelial cells stained with both antibodies. During proestrus, a transition occurs in which epithelial ducts show heterogeneous TGF-β1 staining. During estrus, the heterogeneity of TGF-β1 immunoreactivity increased, such that the epithelium contains TGF-β1-negative cells adjacent to positive cells. LAP is more homogeneous. Occasional variation was observed in that a few epithelial ducts from estrus exhibited low homogeneous staining similar to that seen in diestrus (not shown). D: Quantitative image analysis of the intensity of LAP and TGF-β1 immunoreactivity per cell. In diestrus, the relative intensity of LAP per cell was linearly correlated with TGF-β1 in most cells. In proestrus, a population of cells with very low TGF-β1 is evident. At estrus, an additional population appears consisting of cells exhibiting high TGF-β1 and low LAP immunoreactivity. These cells correspond to those with intense TGF-β1 shown above and in E. E: False-color digital micrographs of the dual immunolocalization of antigen-purified TGF-β1 antibodies (red) and LAP antibodies (green) visualized simultaneously with DAPI-stained nuclei (blue). Mammary gland tissue was obtained from animals sacrificed at estrus. LAP immunoreactivity (green) was relatively uniform in the peri-epithelial stroma and adipose stroma, whereas immunoreactive TGF-β1 was not evident in the stroma. Concordant TGF-β1 and LAP staining appeared yellow-orange. Note that all cells stain with LAP. The discordance of LAP and TGF-β1 immunoreactivity suggests that this TGF-β1 epitope is masked in the majority of stromal cells and in some epithelial cells. This highly localized TGF-β1 is indicative of restricted activation. Scale bar, 10 μm.
A cell by cell analysis of the relative immunoreactivity indicated that LAP and TGF-β1 intensities are linearly related to each other during proestrus and diestrus (Figure 2D) ▶ . During estrus two populations are evident: one similar to that found in diestrus and proestrus, and another that exhibited low LAP (<250) and relatively high TGF-β1 (>200). This pattern is consistent with activation, in which LAP is altered in a manner that reveals a previously masked TGF-β1 epitope. 33 Thus, we concluded that TGF-β1 activation is a function of the hormonal status of the adult animal, and further, that activation seems to be highly restricted, as in puberty, to certain cells within the mammary epithelium.
TGF-β1 Activation Is Restricted to a Subpopulation of Epithelial Cells During Early Pregnancy
Lobular alveolar development at pregnancy, like ductal elongation at puberty, is characterized by high levels of proliferation. Dual-immunofluorescence staining of tissue sections from nulliparous and pregnant mice demonstrated that during early pregnancy (day 6), LAP immunoreactivity was similar to estrus (Figure 3, B versus C) ▶ whereas active TGF-β1 immunostaining was less pronounced and restricted to a subset of cells in both ductal and alveolar epithelium. During late pregnancy (day 14), TGF-β1-positive cells were hardly discernable and LAP immunostaining was greatly decreased compared to the other stages (Figure 3, D versus A, B, and C) ▶ . This is consistent with the previously reported decrease in TGF-β1 message levels during later pregnancy. 24 However, secretory differentiation was accompanied by apical localization of active TGF-β1 immunoreactivity, consistent with its putative role inhibiting milk secretion. 44 Lactating tissue exhibited little LAP or TGF-β1 immunoreactivity (not shown).
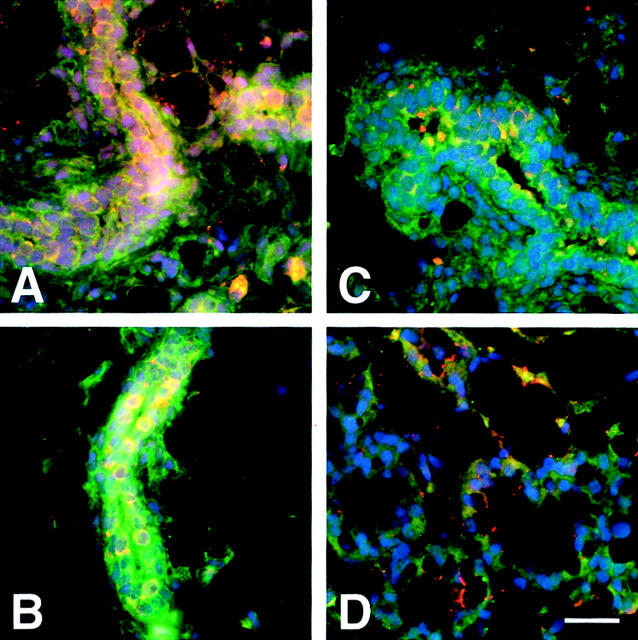
Figure 3.
Transition of adult mammary gland from estrus cycle to lobular-alveolar differentiation during pregnancy is accompanied by progressive loss of both active and latent TGF-β. A: During diestrus, chNTGF-β1 immunoreactivity is relatively homogeneous in nulliparous mammary gland. B: In contrast the epithelium was distinctly heterogeneous during estrus. C: Mammary tissue from early (6 day) pregnant mice exhibited less intense LAP immunoreactivity and less chNTGF-β, suggesting that gradual loss of both TGF-β1 production and activation is reduced. D: Epithelium undergoing functional differentiation during late (18 day) pregnancy exhibited barely detectable TGF-β1 and LAP immunostaining. The tissue sections were stained and images acquired together. False-color digital micrographs of the dual immunolocalization of antigen-purified TGF-β1 antibodies (red) and LAP antibodies (green) visualized simultaneously with DAPI-stained nuclei (blue). The images are scaled identically to allow comparison within the figure of the pattern of TGF-β1 and LAP immunoreactivity. Scale bar, 20 μm.
Tgfβ1+/− Mice Exhibit Accelerated Mammary Ductal Growth and Increased Proliferation During Puberty
TGF-β1 is widely described as an epithelial cell growth inhibitor. If so, prominent activation in the mammary endbud, which is the site of proliferation, seems somewhat paradoxical. Thus we examined the consequences of TGF-β depletion using Tgfβ1 knockout mice. Adult tissues from Tgfβ1+/− have only 10 to 30% of wild-type TGF-β1 protein levels. 37 To confirm that endogenous TGF-β1 protein levels were reduced in the pubertal mammary gland, and to determine whether either the epithelium and/or activation was specifically affected, we compared the intensity of LAP and active TGF-β1 of Tgfβ1+/− to that of wild-type littermates. The immunoreactivity of both proteins were significantly reduced in heterozygotes during puberty (Figure 4, A and B) ▶ . The mean intensities of TGF-β1 and LAP immunoreactivity in the distal duct epithelium of the heterozygote were reduced by 64% and 76% respectively, confirming that both active and latent TGF-β1 were similarly affected. However, the pattern of activation was unchanged, indicating that the phenotype of the Tgfβ1+/− mice would be informative regarding the consequences of its activity.
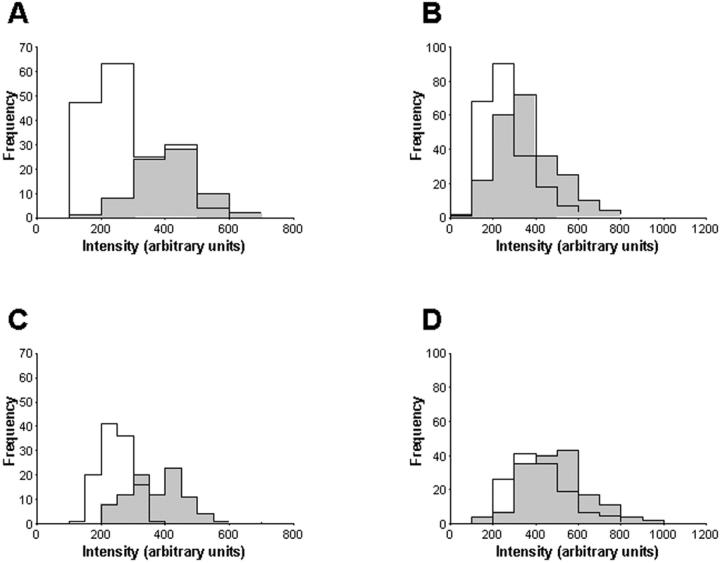
Figure 4.
LAP and TGF-β1 immunoreactivity are both decreased in Tgfβ1+/− mice compared to +/+ mice. Comparison of relative LAP (A and C) and TGF-β1 (B and D) immunoreactivity intensity in the epithelium of Tgfβ1+/+ (shaded bars) and +/− (open bars) littermates. A and B: The staining intensity of LAP and TGF-β1 in the distal ducts at puberty was significantly decreased (P < 0.01, Kolmogorov-Smirnov test) in Tgfβ1 heterozygotes. C and D: Similarly, both LAP and TGF-β1 were decreased in Tgfβ1+/− mice in the mammary epithelium of adult mice in estrus.
To determine the functional consequences of TGF-β1 in puberty, mammary glands of 129SV/C57BL/6 Tgfβ1+/+ or +/− littermates were examined at 6 weeks of age. Comparisons of the mammary whole mounts from heterozygote versus wild-type mice indicated accelerated ductal outgrowth (Figure 5) ▶ . Quantitation confirmed that duct outgrowth is twofold greater in Tgfβ1+/− compared to the same age wild-type littermates (Table 1) ▶ . Notably, both gross morphology and histology were normal despite the accelerated development. To determine whether this phenotype was strain-dependent, we examined the extent of duct outgrowth of Tgfβ1+/+ and +/− mice backcrossed to purity on the 129S2/Sv Hsd genetic background. 129S2/Sv Hsd Tgfβ1+/− mice exhibited similarly accelerated outgrowth (66% of the fat pad filled) compared to wild-type littermates (32% fat pad filled) although ductal outgrowth occurred at an earlier age (5 weeks) in this genetic background. Thus, the pubertal phenotype does not seem to be strain-dependent.
Figure 5.
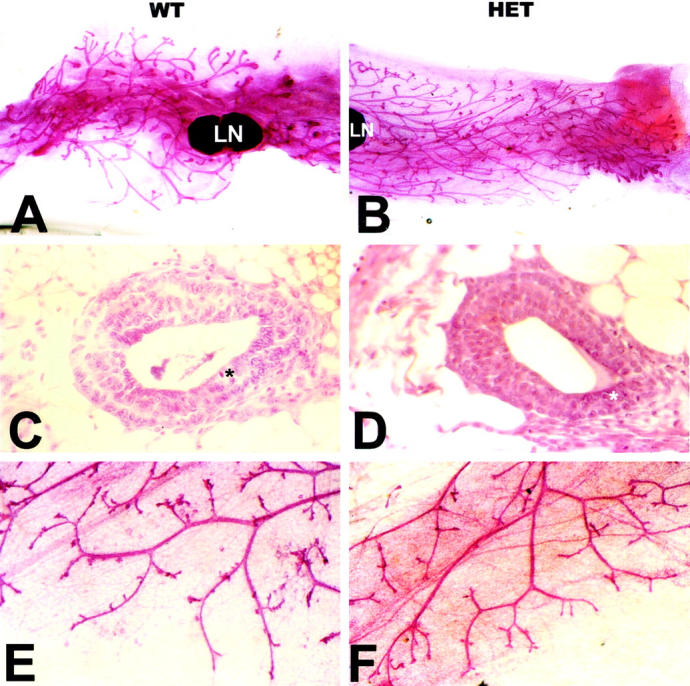
Accelerated mammary ductal growth in Tgfβ1+/− mice. Mammary gland whole mounts and histology from Tgfβ1+/+ (A, C, and E) and +/− (B, D, and F) littermates. A and B: Low-magnification views of mammary gland whole mounts from 6-week-old mice. In wild-type mice, epithelial outgrowth has reached the lymph node (LN), whereas in the heterozygotes the fat pad is two-thirds full. The nipple is to the left in both cases. C and D: The endbud epithelium of both genotypes is similar and well organized (H&E stain). Asterisks indicate pyknotic nuclei. E and F: High-magnification photomicrographs of mammary gland whole mounts show similar branching patterns in wild-type and heterozygote mice.
Table 1.
Mammary Ductal Elongation and Proliferation Are Increased in Tgfβ1 Heterozygote Mice
| Genotype | % Fatpad filled* | % PCNA labeled† | % Pyknotic endbud nuclei‡ | |
|---|---|---|---|---|
| Endbuds | Ducts | |||
| Wild type | 27 ± 3.9 | 2.7 | 1.7 | 2.2 |
| n = 11 | (34/1265) | (19/1091) | (227/9603) | |
| Heterozygote | 61 ± 7.8§ | 8.0¶ | 4.3¶ | 2.8 |
| n = 18 | (117/1460) | (44/1015) | (248/9420) |
To evaluate DNA synthesis, PCNA was localized by immunofluorescence. The frequency of PCNA-positive epithelial cells was elevated approximately threefold in Tgfβ1+/− endbud epithelial cells and more than twofold in ductal epithelium (Table 1) ▶ . This 1.5-fold differential between endbud and ductal epithelium correlated with the difference in TGF-β1 mean intensity between these epithelial compartments.
Endbuds not only represent sites of active proliferation but also of apoptosis, which is thought to play a role in lumen formation. 45 Expression of constitutively active TGF-β1 leads to mammary epithelial apoptosis during pregnancy, 38 thus one might predict that depletion of TGF-β1 might lead to decreased apoptosis. We found that the endbud morphology was not affected and there was no difference in the frequency of apoptotic nuclei in heterozygote versus wild-type mammary endbuds (Table 1) ▶ . The pyknotic indices for mammary glands of wild-type pubertal mice were in agreement with those reported by others. 46 Thus, mammary gland morphogenesis was accelerated in Tgfβ1+/− primarily because of increased mammary epithelial proliferation. This phenotype is consistent with a model in which TGF-β1 activation inhibits epithelial proliferation, but is not required for the decision to apoptose.
The Phenotype in Tgf-β1 Heterozygote Mammary Gland Is Because of Epithelial, Rather than Systemic/Stromal, TGF-β1 Depletion
Loss of TGF-β1 function studies using dominant-negative TGF-β1 receptor transgene expression in the mammary epithelium versus the stroma indicate that the roles of TGF-β1 signaling in the epithelium or the stroma are distinct during mammary gland morphogenesis. 47,48 It has also been suggested that stromal TGF-β1 mediates proliferation in the epithelium. 49 Although we did not detect appreciable active TGF-β1 immunoreactivity in the stroma of the mammary gland at any stage of normal development, latent TGF-β1 is ubiquitous. Thus it is possible that some TGF-β1 activation occurs below the limit of detection of our antibody. Moreover, activation of TGF-β1 does occur in the mammary stroma after irradiation. 33,34 To address whether depletion of stromal TGF-β1 modulates proliferation in the epithelium, we transplanted mammary gland fragments (∼ 1 mm3) from an adult wild type into cleared (ie, mammary epithelial-free) fat pads of 3-week-old F1 129S2/SV Hsd × C57BL/6 Tac Tgfβ1+/+ or +/− host mice. Six weeks after transplantation, we examined the resulting ductal outgrowths and quantified the area occupied by the outgrowth as a percentage of total fat pad area. The extent of ductal outgrowths in wild-type glands was not significantly different from ductal outgrowths in Tgfβ1 heterozygote hosts (not shown). However ductal outgrowths of Tgfβ1+/− mammary gland transplanted into cleared fat pads of +/+ or +/− hosts occupied three times (28.3 ± 2.4%, n = 5) the average area of similarly transplanted wild-type epithelium (9.1 ± 2.2% SEM; n = 8; _t_-test P < 0.001). Thus, TGF-β1 depletion in the stroma has little effect on the rate of ductal outgrowth, whereas depletion of epithelial TGF-β1 accelerates ductal outgrowth.
Tgfβ1+/− Mice Exhibit Increased Cell Turnover in Adult Mammary Epithelium
To confirm that TGF-β1 was reduced in the mammary gland of adult Tgfβ1 heterozygotes, we compared the intensity of both LAP and TGF-β1 to that of wild-type littermates (Figure 4, C and D) ▶ . The mean intensities of TGF-β1 and LAP at estrus of the heterozygote mammary epithelium were reduced by 66% and 69%, respectively, but the overall heterogeneous pattern of activation was unaffected.
Because the estrus cycle affects the pattern of TGF-β1 activation, we next asked whether the frequency of epithelial proliferation in Tgfβ1 heterozygote mammary gland was different from controls as a function of the stage of estrus. Mammary epithelial cell proliferation is dictated by the endogenous hormonal milieu such that epithelial proliferation peaks at estrus. 50 Both genotypes exhibited the maximum number of PCNA-positive cells at estrus. However, the proliferative index at estrus was four times greater in Tgfβ1+/− compared to wild-type littermates (Table 2) ▶ . A small but significant increase in PCNA index was also found during diestrus in the Tgfβ1 heterozygotes. Thus the phenotype of the Tgfβ1 heterozygote depended on the hormonal status of the adult animal.
Table 2.
Proliferation and Apoptosis Are Altered in Adult Tgfβ1 Heterozygote Mice
| Estrus cycle status | Genotype | |
|---|---|---|
| Wild type | Heterozygote | |
| % PCNA labeled* | ||
| Proestrus | 0.14 (3/2211) | 0.05 (1/2091) |
| Estrus | 0.57 (18/3142) | 2.3§ (48/2114) |
| Diestrus | 0 (0/1958) | 0.34‡ (7/2085) |
| % Pyknotic nuclei† | ||
| Proestrus | 0.23 (14/6177) | 0.93§ (59/6311) |
| Estrus | 0.47 (30/6370) | 0.23‡ (14/6177) |
| Diestrus | 0.45 (27/6064) | 0.60 (36/6034) |
Surprisingly, and in contrast to glands of pubertal mice, the morphology of the heterozygote mammary gland whole mount was indistinguishable from wild-type littermates (Figure 5, E and F) ▶ . This was confirmed by quantifying the percentage of mammary gland tissue sections occupied by ducts (not shown). The lack of hyperplastic morphology in the presence of increased proliferation suggested that the equilibrium in the heterozygote adult mammary epithelium is maintained by altered cell loss.
Therefore, we examined the frequency of nuclear pyknotic morphology, which is the final stage of apoptosis, in the mammary epithelium as a function of estrus cycle. Both the frequency and pattern of apoptosis was altered in the Tgfβ1 heterozygote. Although the frequency of pyknotic nuclei was lowest at proestrus in wild-type mice, in the Tgfβ1 heterozygotes, the apoptotic index was lowest in estrus. Apoptotic nuclei were half as frequent in Tgfβ1+/− as in wild-type mice in estrus (Table 2) ▶ . These data are consistent with the pro-apoptotic phenotype observed when constitutively active TGF-β1 is expressed during pregnancy, 38 and suggests that TGF-β1 does contribute to the regulation of apoptosis in adult tissue. However, this decrease would contribute to hyperplasia. In contrast, at proestrus null heterozygote mammary glands exhibited more than four times the number of apoptotic cells. Thus, increased cell proliferation and decreased apoptosis during estrus in the Tgfβ1+/− were offset by increased apoptosis during proestrus. This compensation most likely accounts for minimal differences in the whole-mount profiles of adult nulliparous mammary glands.
Lobular-Alveolar Development Is Accelerated in Tgfβ1 Null Heterozygotes
Alveolar development in early pregnant glands is resistant to inhibition by exogenous TGF-β1 via implanted pellets, 26 which might lead one to predict that depletion would have little effect. However, overexpression of constitutively active TGF-β1 during pregnancy leads to stunted alveolar development despite high levels of proliferation. 38 Thus we examined the impact of TGF-β1 depletion on alveolar development during pregnancy. First, we determined the amount of endogenous protein in Tgfβ1 heterozygote and wild-type mammary glands by bioassay of acid ethanol extracts of mammary gland tissue at 6 days of pregnancy. At this stage, the mammary glands of Tgfβ1 heterozygote mice have less than one-tenth the amount of TGF-β1 protein found in wild types (34.3 pg TGF-β/mg protein versus 440.1 pg TGF-β/mg protein), which is a decrease similar to those found in other tissues of the Tgfβ1 heterozygote. 37 Consistent with decreased lobular-alveolar development in whey acid protein-TGF-β223–225 mice during pregnancy, 51 and with the accelerated ductal outgrowth, depletion of TGF-β1 increased the rate of lobular-alveolar development during pregnancy. The area of mammary gland occupied by epithelium of Tgfβ1 +/− was twofold greater at day 6 and fourfold at day 14 relative to the area of mammary epithelium in wild-type tissue. Thus, the epithelium is still sensitive to TGF-β1 during pregnancy.
At day 6 of pregnancy, the PCNA index in alveolar epithelium of Tgfβ1 heterozygote mice was almost threefold that of wild-type mice and was significantly higher in ductal cells as well (Table 3) ▶ . Although PCNA indices in ductal and alveolar epithelium were lower in both Tgfβ1 heterozygote and wild-type mammary gland at day 10 and 14 of pregnancy than at day 6, proliferation in Tgfβ1 heterozygote epithelium remained twofold to threefold higher than wild type.
Table 3.
Proliferation and Apoptosis Are Altered During Pregnancy in Tgfβ1 Heterozygotes
| Ducts | Alveoli | |||
|---|---|---|---|---|
| Wild type | Heterozygote | Wild type | Heterozygote | |
| % PCNA* | ||||
| Day 6 | 3.7 (51/1363) | 6.7‡ (75/1113) | 4.8 (53/1101) | 13.8§ (131/952) |
| Day 10 | 3.2 (36/1966) | 5.3§ (100/1950) | 1.3 (17/1877) | 4.6§ (79/1830) |
| Day 14 | 2.0 (36/1903) | 6.3§ (177/2507) | 2.8 (36/1835) | 5.1§ (110/2517) |
| % TUNEL† | ||||
| Day 6 | 0.6 (18/3178) | 1.9§ (53/2750) | 0.7 (19/2899) | 2.5§ (50/1979) |
| Day 10 | 1.2 (23/1842) | 1.3 (25/1926) | 1.1 (11/1033) | 0.9 (9/976) |
| Day 14 | 0.4 (8/1802) | 0.4 (16/2223) | 1.0 (10/997) | 1.7 (35/2026) |
| Alveolar area | Wild type | Heterozygote | ||
| Day 6 | 5 | 11 | ||
| Day 14 | 11 | 42 |
Surprisingly, and in contrast to either puberty or estrus, apoptosis, as measured by terminal dUTP nick-end labeling, at day 6 of pregnancy was threefold greater in both ductal and alveolar epithelium of Tgfβ1 heterozygote compared to wild-type mammary gland. This effect was transient because the frequency of terminal dUTP nick-end labeling-positive cells was not significantly different at the later stages of pregnancy. Increased apoptosis in early pregnancy may be because of the compensatory mechanism suggested by the dual response observed in nulliparous adult tissue, or may indicate that TGF-β1 can act as a survival factor under certain conditions.
Ovarian Hormones Elicit the Tgfβ1+/− Phenotype in the Mammary Epithelium
The stage-specific phenotype of the Tgfβ1 null genotype led us to consider whether TGF-β1 activation mediates mammary epithelial responsiveness to estrogen and progesterone. If so, one would predict that administration of these hormones to ovariectomized Tgfβ1 heterozygote mice would result in increased proliferation compared to wild-type mice. 52 Ovarectomized heterozygotes were not different from wild-type controls in either the frequency of proliferation or apoptosis (not shown). Administration of estrogen and progesterone for 3 days increased proliferation more than 15-fold in TGFβ1 null heterozygote mice compared to wild-type mammary glands (7.7 ± 4.2% SD versus 0.5 ± 0.15% SD). Estradiol and progesterone treatment resulted in TGF-β1 immunoreactivity, in which activation was restricted, but prominent, in a subset of epithelial cells (not shown). Thus, both the phenotype of increased proliferation and the specific pattern of immunoreactivity could be elicited by exposing ovariectomized mice to the ovarian steroid hormones that dictate estrus.
Discussion
Mammary developmental biology involves a complex interplay between its various cell types and, therefore, the validity of any proposed mechanisms must take into account the physiological state and localization of regulatory molecules in vivo. Our immunohistochemical data are the first to demonstrate that there are significant cellular constraints on TGF-β activation. To define which cellular events are mediated by TGF-β1 in situ, we manipulated the levels of endogenous TGF-β1 by using the Tgfb1 null heterozygote and performed tissue recombination experiments thereon. Although several studies have attempted to decipher TGF-β1 effects using exogenous application, ectopic expression of constitutively active protein, or dominant-negative receptors, this is the first study to determine the mammary phenotype as a consequence of decreasing endogenous TGF-β1 levels in mammary gland, and are thus of significant physiological relevance. These data are the basis for the novel observation that hormonal regulation differentially controls activation within the mammary gland, which in turn controls the proliferative response to ovarian hormones. Further support of this hypothesis was obtained by demonstration that treatment of ovariectomized mice with estrogen and progesterone elicit both heterogeneous activation and the proliferative phenotype. Integration of immunolocalization with the phenotype of TGF-β1 depletion has thus provided cellular mechanistic insights into TGF-β1’s role in mammary biology and cancer.
A schematic summarizing the relative immunostaining of TGF-β1 in relation to the proliferative phenotype of the adult Tgfβ1+/− mammary epithelium as a function of the estrus cycle is shown in Figure 6 ▶ . Quiescent epithelium during diestrus, similar to the distal ducts of the pubescent mammary gland, showed relatively homogeneous TGF-β1 staining in wild-type mice and increased proliferation in Tgfβ1+/− mice. The decrease of TGF-β1 immunoreactivity observed during proestrus is also consistent with this model as the epithelium prepares for proliferation as estrogen levels rise. However, this model predicts neither the subset of mammary epithelial cells exhibiting intense active TGF-β1 immunoreactivity during estrus and other proliferative stages (ie, during puberty and pregnancy), nor the proliferative phenotype seen in Tgfβ1+/− mice at these stages. The apparent paradox of a growth inhibitor functioning during periods of proliferation led us to postulate that the restricted cellular activation of TGF-β1 inhibits specific cells from responding to the proliferative stimulus. It seems that TGF-β1 activation may be regulated by the same events that trigger proliferation in mammary gland. This suggests a relationship between TGF-β1 activity and ovarian hormone signaling.
Figure 6.
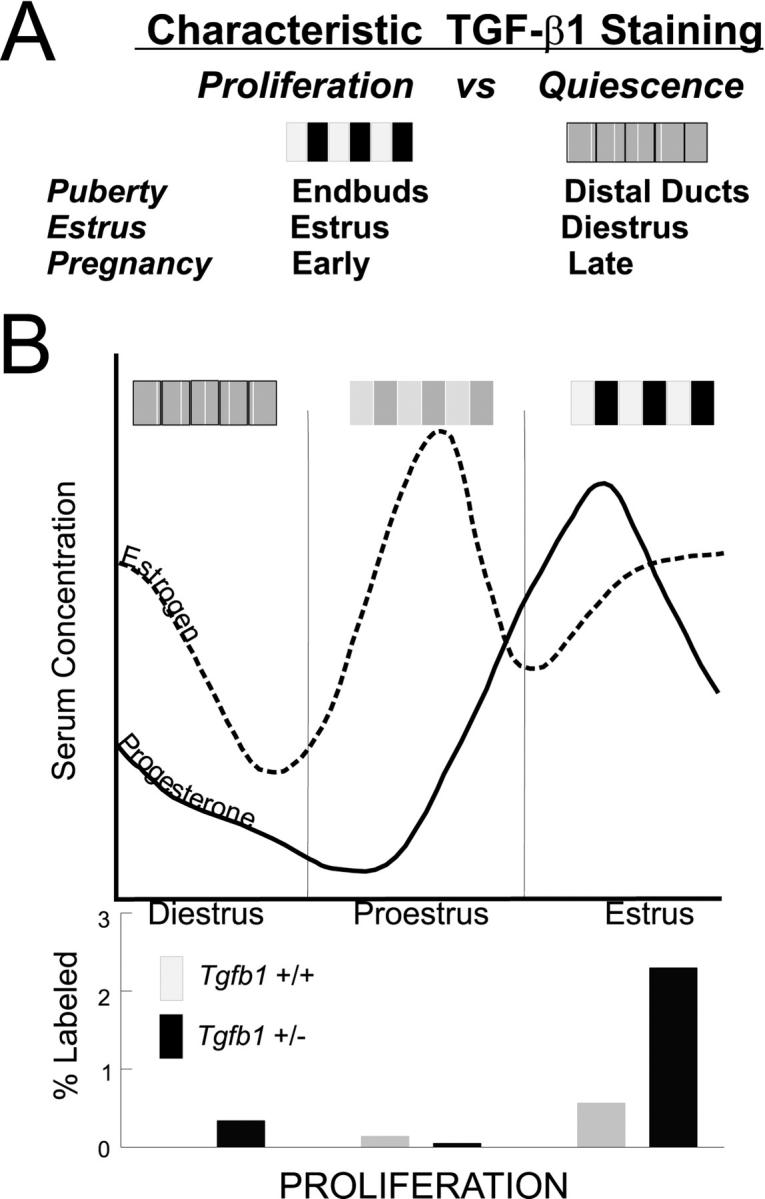
Schematic of the pattern of TGF-β1 immunoreactivity and proliferation in mammary epithelium relative to ovarian hormones during the estrus cycle. A: Characteristic patterns of active TGF-β in mammary epithelium are represented as a shaded bar representing the relative intensity of epithelial TGF-β1 as a function of mammary gland development. The pattern is heterogeneous during periods of proliferation and relatively homogeneous during quiescent stages. B: During the estrus cycle, highly heterogeneous TGF-β1 immunoreactivity at estrus correlates with the phenotype of increased proliferation and decreased apoptosis (not shown) in Tfgβ1+/− mice. The relative levels of estrogen (dotted line) and progesterone (solid line) serum concentrations are graphed and the proliferative indices of Tfgβ1+/+ and +/− mammary epithelium are summarized in the bar graph below. The functional link between the role of TGF-β as a growth inhibitor and ovarian hormones regulating proliferation in the mammary gland is supported by the dramatic proliferative response of ovarectomized Tfgβ1+/− mice to estrogen and progesterone.
These data lead to several novel conclusions. First, the highly restricted pattern of TGF-β1 activation during the estrus cycle suggests that particular cells activate TGF-β1 under specific hormonal conditions. Mammary epithelium is heterogeneous in respect to the expression of numerous markers of proliferation, differentiation, and hormone responsiveness. Ovarian hormones, estrogen and progesterone, signal through their cognate receptors localized in the nucleus of certain cells. 53 Peak proliferative periods are stimulated by increased levels of estrogen and progesterone. Recent studies have shown that in both mouse and human mammary gland, proliferating cells are usually adjacent to, rather than consonant with, those expressing estrogen or progesterone receptors. 54 Indeed, these authors have postulated that a growth inhibitor actively restrains hormone receptor-positive cells from responding to the proliferative signal resulting from hormone stimulation. 55 Our preliminary data indicate that cells exhibiting TGF-β1 activation at estrus rarely co-localize with proliferation, as one would expect, but frequently co-localize with steroid hormone nuclear receptors, which supports a functional relationship between TGF-β1 activation and hormonal stimuli (K Ewan, G Shyamala, and MH Barcellos-Hoff, manuscript in preparation).
Second, increased proliferation of Tgfβ1+/− adult mice was most prominent at estrus and in pregnancy, indicating that TGF-β1 activity restricts the response to hormonal stimuli to proliferate. This conclusion was supported by the dramatic response of ovarectomized Tgfβ1+/− to estrogen and progesterone administration.
Third, even though the amount of total TGF-β1 was severely depressed in the Tgfβ1 heterozygote, the relative distribution of latent and active TGF-β1 immunoreactivity was similar to the wild-type controls. This suggests that external events, rather than TGF-β1 itself, control TGF-β1 activation. Our contention that spatial and temporal regulation of TGF-β1 activation is a critical determinant of biological responses in mammary gland is also supported by transgenic models expressing a constitutively active TGF-β, designated TGF-β 223-225. 56 The phenotype resulting from TGF-β 223-225 expression using a mouse mammary tumor virus promoter is a transiently hypoplastic ductal morphogenesis, 25 whereas that driven by whey acidic protein exhibits greatly compromised alveolar development. 51 Differences because of timing or localization of promoter-driven TGF-β 223–225 expression relative to endogenous TGF-β1 activation in the mammary gland thus lead to different biological effects.
Fourth, despite the significant increase in the rate of mammary ductal morphogenesis and epithelial proliferation seen in Tgfβ1 heterozygote mice, morphogenesis is not abnormal, suggesting that proliferation, although increased, is not out of place. This model then provides a means of dissociating the role of TGF-β1 in production of new cells versus tissue-specific remodeling and organization. Because morphogenesis was not grossly affected when TGF-β1 is severely depleted, it suggests that TGF-β1 is more important in regulating epithelial proliferation versus pattern formation. To test what cellular source of TGF-β1 led to the proliferative phenotype of the Tgfβ1 heterozygote, we created chimeric glands by reciprocal transplantation of Tgfβ1 heterozygote epithelium into wild-type stroma and vice versa. Whether the host was wild type or transgenic, Tgfβ1+/− epithelial cells formed outgrowths that significantly more developed than transplants of wild-type epithelium to Tgfβ1 heterozygote stroma and host. These experiments demonstrate that the Tgfβ1 heterozygote mammary proliferative phenotype is independent of either stromal or systemic factors and that regulation of proliferation is primarily because of epithelial TGF-β. Joseph and colleagues 48 have shown that blunting the response to TGF-β in the stroma using a metallothionein promoter to drive a dominant-negative TGF-β type II receptor resulted in increased ductal branching whereas the same receptor expressed in the mammary epithelium via a _MMTV-LTR-_promoter resulted in alveolar hyperplasia and premature functional differentiation. 47 Because all TGF-β signaling depends on the type II receptor, the dominant-negative model inhibits all three TGF-β1 isoforms whereas our data concern the locale and timing of TGF-β1 activation. Together, our data argue for highly restricted and discrete parenchymal regulation of TGF-β1 activation and proliferative response.
Lastly, although TGF-β1 plays a prominent role in mediating apoptosis in hormone-dependent tissues such as the prostate 57 and uterus, 58 and has been postulated to have a similar function in mammary gland, 38,58 our study suggests a restricted and complex role in tissue homeostasis. A transgenic model in which constitutively active protein is driven by whey acid protein promoter results in elevated apoptosis during both estrus and pregnancy. 38 Moreover, expression of TGF-β3 in late pregnancy and lactation via a β-lactoglobulin promoter results in apoptosis in the mammary gland at these stages. 59 In studies using Tgfβ1+/− mice from puberty through late pregnancy, we found that the frequency of apoptosis cells in Tgfβ1+/− mice decreased only at estrus. Apoptosis in endbuds, was recently reported as 2 to 3% in outbred mouse strains, 46 which is thought to be necessary for lumen formation. Our data indicate that TGF-β1 is not required for lumen formation because the frequency of apoptosis in endbuds was similar in heterozygote and wild-type littermates. In contrast, apoptosis was elevated in the Tgfβ1+/− epithelium during proestrus and in early pregnancy, suggesting either a homeostatic compensatory mechanism, or direct or indirect action of TGF-β1 as a survival factor for mammary epithelial cells. 38,60 Thus, our data are in agreement with TGF-β1 acting as a pro-apoptotic agent but in a restricted manner, ie, only during estrus, and raises the possibility that it acts as an important survival factor.
TGF-β1 has been broadly implicated in breast cancer. 11,18,61-63 Recent evidence links a functional TGFβ1 polymorphism that increases serum TGF-β levels to decreased breast cancer incidence in women. 64 Furthermore various reports link TGF-β1 activation to estrogen and progesterone treatment in cultured breast cancer cells, 65 and to the action of the anti-estrogen tamoxifen. 66 We and others have argued that conversion from TGF-β1 growth sensitivity to TGF-β1 resistance during breast cancer progression is a critical juncture in establishing malignant behavior. 7,21 TGF-β1 resistant neoplastic cells could arise by positive selection in a genomically diverse population when TGF-β1 activation is elevated, as in wounding or after radiation exposure. This hypothesis is based on the premise that although latent TGF-β1 is abundant, TGF-β1 activity is normally restricted by tight regulation of its activation, thus limiting the selective pressure for resistance. The current studies are the first to show such restricted activation and to relate these events to ovarian hormone function in situ. The focus of future studies will be to define which actions predominate under particular cellular and physiological contexts. Our ongoing studies address the mechanism of latent TGF-β1 activation in normal mammary gland and further explore how misregulation of TGF-β1 activity contributes to carcinogenesis in the mammary gland.
Acknowledgments
We thank Drs. Mina J. Bissell, Carlos Ortiz De Solorzano, and Joel Chassis for helpful discussion, and Mr. Kevin Benson for skilled technical assistance.
Footnotes
Address reprint requests to M. H. Barcellos-Hoff, Life Sciences Division, Bldg. 74-174, 1 Cyclotron Rd., Lawrence Berkeley National Laboratory, Berkeley, CA 94720. E-mail: mhbarcellos-hoff@lbl.gov.
Supported by the California Breast Cancer Research Program (grant 4BP-0136), NIH CA66541 (to G.S.), and the Office of Health and Environmental Research, Health Effects Research Division, United States Department of Energy (contract no. DE-AC-03-76SF00098).
References
- 1.Moses HL, Coffey RL, Jr, Leof EB, Lyons RM, Keski-Oja J: Transforming growth factor β regulation of cell proliferation J Cell Physiol 1987, 5:S1-S7 [DOI] [PubMed] [Google Scholar]
- 2.Roberts AB: Molecular and cell biology of TGF-beta. Miner Electrolyte Metab 1998, 24:111-119 [DOI] [PubMed] [Google Scholar]
- 3.Ewen ME, Sluss HK, Whitehouse LL, Livingston DM: TGFβ inhibition of Cdk4 synthesis is linked to cell cycle arrest. Cell 1993, 74:1009-1020 [DOI] [PubMed] [Google Scholar]
- 4.Sandhu C, Garbe J, Bhattacharya N, Daksis J, Pan C-H, Yaswen P, Koh J, Slingerland JM, Stampfer MR: TGF-beta stabilizes p15INK4B protein, increases p15INK4B/cdk4 complexes and inhibits cyclin D1/cdk4 association in human mammary epithelial cells. Mol Cell Biol 1997, 17:2458-2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fynan TM, Reiss M: Resistance to inhibition of cell growth by transforming growth factor-β and its role in oncogenesis. Crit Rev Oncog 1993, 4:493-540 [PubMed] [Google Scholar]
- 6.Stampfer MR, Yaswen P, Alhadeff M, Hosoda J: TGF-β induction of extracellular matrix associated proteins in normal and transformed human mammary epithelial cells in culture is independent of growth effects. J Cell Physiol 1993, 155:210-221 [DOI] [PubMed] [Google Scholar]
- 7.Pierce DF, Gorska AE, Chythil A, Meise KS, Page DL, Coffey RJ, Jr, Moses HL: Mammary tumor suppression by transforming growth factor β1 transgene expression Proc Natl Acad Sci USA 1995, 92:4254-4258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui W, Fowlis DJ, Bryson S, Duffie E, Ireland H, Balmain A, Akhurst RJ: TGFβ1 inhibits the formation of benign skin tumors, but enhances progression to invasive spindle carcinomas in transgenic mice. Cell 1996, 86:531-542 [DOI] [PubMed] [Google Scholar]
- 9.Oft M, Heider K-H, Beug H: TGFβ signaling is necessary for carcinoma cell invasiveness and metastasis. Curr Biol 1998, 8:1243-1252 [DOI] [PubMed] [Google Scholar]
- 10.Oft M, Peli J, Rudaz C, Schwarz H, Beug H, Reichmann E: TGF-beta1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Genes Dev 1996, 10:2462-2477 [DOI] [PubMed] [Google Scholar]
- 11.Dumont N, Arteaga CL: Transforming growth factor-β and breast cancer: tumor promoting effects of transforming growth factor-β. Breast Cancer Res 2000, 2:125-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pepper MS: Transforming growth factor-beta: vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev 1997, 8:21-43 [DOI] [PubMed] [Google Scholar]
- 13.Samuel SK, Hurta RAR, Kndaiah P, Khalil N, Turley EA, Wright JA, Greenberg AH: Autocrine induction of tumor protease production and invasion by a metallothionein-regulated TGF-β1 (Ser223, 225). EMBO J 1992, 11:1599-1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sehgal I, Baley PA, Thompson TC: Transforming growth factor β1 stimulates contrasting responses in metastatic versus primary mouse prostate cancer-derived cell lines in vitro. Cancer Res 1996, 56:3359-3365 [PubMed] [Google Scholar]
- 15.Letterio JJ, Roberts AB: Regulation of immune responses by TGF-β. Annu Rev Immunol 1998, 16:137-161 [DOI] [PubMed] [Google Scholar]
- 16.Stampfer MR, Bodnar A, Garbe J, Wong M, Pan A, Villeponteau B, Yaswen P: Gradual phenotypic conversion associated with immortalization of cultured mammary epithelial cells. Mol Biol Cell 1997, 8:2391-2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Portella G, Cumming SA, Liddell J, Cui W, Ireland H, Akhurst RJ, Balmain A: Transforming growth factor beta is essential for spindle cell conversion of mouse skin carcinoma in vivo: implications for tumor invasion. Cell Growth Differ 1998, 9:393-404 [PubMed] [Google Scholar]
- 18.Derynck R, Ackhurst RJ, Balmain A: TGF-b signaling in tumor suppression and cancer progression. Nat Genet 2001, 29:117-129 [DOI] [PubMed] [Google Scholar]
- 19.Westergren-Thorsson G, Hernnas J, Sarnstrand B, Oldberg A, Heinegard D, Malmstrom A: Altered expression of small proteoglycans, collagen, and transforming growth factor-β1 in developing bleomycin-induced pulmonary fibrosis in rats. J Clin Invest 1993, 92:632-637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vollberg TM, George MD, Jetten AM: Induction of extracellular matrix gene expression in normal human keratinocytes by transforming growth factor β is altered by cellular differentiation. Exp Cell Res 1991, 193:93-100 [DOI] [PubMed] [Google Scholar]
- 21.Reiss M, Barcellos-Hoff MH: Transforming growth factor-β in breast cancer: a working hypothesis. Br Cancer Res Treat 1997, 45:81-95 [DOI] [PubMed] [Google Scholar]
- 22.Daniel CW, Robinson SD: Regulation of mammary growth and function by TGF-β. Mol Reprod Dev 1992, 32:145-151 [DOI] [PubMed] [Google Scholar]
- 23.Daniel CW, Robinson S, Silberstein GB: The role of TGF-β in patterning and growth of the mammary ductal tree. J Mammary Gland Biol Neoplasia 1996, 1:331-341 [DOI] [PubMed] [Google Scholar]
- 24.Robinson SD, Silberstein GB, Roberts AB, Flanders KC, Daniel CD: Regulated expression and growth inhibitory effects of transforming growth factor-β isoforms in mouse mammary gland development. Development 1991, 113:867-878 [DOI] [PubMed] [Google Scholar]
- 25.Pierce DFJ, Johnson MD, Matsui Y, Robinson SD, Gold LI, Purchio AF, Daniel CW, Hogan BLM, Moses HL: Inhibition of mammary duct development but not alveolar outgrowth during pregnancy in transgenic mice expressing active TGF-β 1. Genes Dev 1993, 7:2308-2317 [DOI] [PubMed] [Google Scholar]
- 26.Silberstein GB, Daniel CW: Reversible inhibition of mammary gland growth by transforming growth factor-β. Science 1987, 237:291-293 [DOI] [PubMed] [Google Scholar]
- 27.Gentry LE, Nash BW: The pro domain of pre-pro-transforming growth factor β1 when independently expressed is a functional binding protein for the mature growth factor. Biochemistry 1990, 29:6851-6857 [DOI] [PubMed] [Google Scholar]
- 28.Derynck R, Jarrett JA, Chen EY, Eaton DH, Bell JR, Assoian RK, Roberts AB, Sporn MB, Goeddel DV: Human transforming growth factor-β complementary DNA sequence and expression in normal and transformed cells. Nature 1985, 316:701-705 [DOI] [PubMed] [Google Scholar]
- 29.Flaumenhaft R, Rifkin DB: The extracellular regulation of growth factor action. Mol Biol Cell 1992, 3:1057-1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J: Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest 1997, 100:768-776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barcellos-Hoff MH, Ehrhart EJ, Kalia M, Jirtle R, Flanders K, Tsang ML-S: Immunohistochemical detection of active TGF-β in situ using engineered tissue. Am J Pathol 1995, 147:1228-1237 [PMC free article] [PubMed] [Google Scholar]
- 32.Barcellos-Hoff MH: Latency and activation in the regulation of TGF-β. J Mammary Gland Biol Neoplasia 1996, 3:353-363 [PubMed] [Google Scholar]
- 33.Barcellos-Hoff MH, Derynck R, Tsang ML-S, Weatherbee JA: Transforming growth factor-β activation in irradiated murine mammary gland. J Clin Invest 1994, 93:892-899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ehrhart EJ, Carroll A, Segarini P, Tsang ML-S, Barcellos-Hoff MH: Latent transforming growth factor-β activation in situ: quantitative and functional evidence following low dose irradiation. FASEB J 1997, 11:991-1002 [DOI] [PubMed] [Google Scholar]
- 35.Chong H, Vodovotz Y, Cox G, Barcellos-Hoff MH: Immunocytochemical detection of latent transforming growth factor-β activation in cultured macrophages. J Cell Physiol 1999, 178:275-283 [DOI] [PubMed] [Google Scholar]
- 36.Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S: Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA 1993, 90:770-774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang B, Bottinger EP, Jakowlew SB, Bangall KM, Mariano J, Anver MR, Letterio JJ, Wakefield LM: Transforming growth factor-beta1 is a new form of tumor suppressor with true haploid insufficiency. Nat Med 1998, 4:802-807 [DOI] [PubMed] [Google Scholar]
- 38.Kordon EC, McKnight RA, Jhappan C, Hennighausen L, Merlino G, Smith GH: Ectopic TGF beta 1 expression in the secretory mammary epithelium induces early senescence of the epithelial stem cell population. Dev Biol 1995, 168:47-61 [DOI] [PubMed] [Google Scholar]
- 39.DeOme KB, Faulkin LJJ, Bern HA, Blair PB: Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res 1959, 19:515-520 [PubMed] [Google Scholar]
- 40.Danielpour D, Dart LL, Flanders KC, Roberts AB, Sporn MB: Immunodetection and quantitation of the two forms of transforming growth factor-beta (TGF-β1 and TGF-β2). J Cell Physiol 1989, 138:79-86 [DOI] [PubMed] [Google Scholar]
- 41.Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB: An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Analyt Biochem 1994, 216:276-284 [DOI] [PubMed] [Google Scholar]
- 42.Shyamala G: Roles of estrogen and progesterone in normal mammary gland development: insights from progesterone null receptor mutant mice and in situ localization of receptor. Trends Endocrinol Metab 1997, 8:34-39 [DOI] [PubMed] [Google Scholar]
- 43.Daniel CW, Silberstein GB: Postnatal development of the rodent mammary gland. Neville M Daniel C C eds. The Mammary Gland: Development, Regulation and Function. 1987:pp 3-36 New York, Plenum Press Publishing Corp.
- 44.Robinson SD, Roberts AB, Daniel CW: TGFβ suppresses casein synthesis in mouse mammary explants and may play a role in controlling milk levels. J Cell Biol 1993, 120:245-251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Humphreys RC, Krajewska M, Krnacik S, Jaeger R, Weiher H, Krajewski S, Reed JC, Rosen JM: Apoptosis in the terminal endbud of the murine mammary gland: a mechanism of ductal morphogenesis. Development 1996, 122:4013-4022 [DOI] [PubMed] [Google Scholar]
- 46.Klinowska T, Soriano J, Edwards GM, Oliver J, Valentijn A, Montesano R, Streuli C: Laminin and β1 integrins are crucial for normal mammary gland development in the mouse. Dev Biol 1999, 215:13-32 [DOI] [PubMed] [Google Scholar]
- 47.Gorska AE, Joseph H, Derynck R, Moses HL, Serra R: Dominant-negative interference of the transforming growth factor-beta type II receptor in mammary gland epithelium results in alveolar hyperplasia and differentiation in virgin mice. Cell Growth Differ 1998, 9:229-238 [PubMed] [Google Scholar]
- 48.Joseph H, Gorska AE, Sohn P, Moses HL, Serra R: Overexpression of a kinase-deficient transforming growth factor-β type II receptor in mouse mammary stroma results in increased epithelial branching. Mol Biol Cell 1999, 10:1221-1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silberstein GB: Role of the stroma in mammary development. Breast Cancer Res 2001, 3:218-223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeps N, Bentel JM, Papadimitriou JM, Dawkins HJ: Murine progesterone receptor expression in proliferating mammary epithelial cells during normal pubertal development and adult estrous cycle. Association with ERα and ERβ status. J Histochem Cytochem 1999, 47:1323-1330 [DOI] [PubMed] [Google Scholar]
- 51.Jhappan C, Geiser AG, Kordon EC, Bagheri D, Hennighausen L, Roberts AB, Smith GH, Merlino G: Targeting expression of a transforming growth factor β1 transgene to the pregnant mammary gland inhibits alveolar development and lactation. EMBO J 1993, 12:1835-1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bresciani F: Topography of DNA synthesis in the mammary gland of the C3H mouse and its control by ovarian hormones: an autoradiographic study. Cell Tissue Kinet 1968, 1:51-63 [Google Scholar]
- 53.Shyamala G: Progesterone signaling and mammary gland morphogenesis. J Mammary Gland Biol Neoplasia 1999, 4:89-104 [DOI] [PubMed] [Google Scholar]
- 54.Clarke RB, Howell A, Potten CS, Anderson E: Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res 1997, 57:4987-4991 [PubMed] [Google Scholar]
- 55.Shoker BS, Jarvis C, Clarke RB, Anderson E, Hewlett J, Davies MP, Sibson DR, Sloane JP: Estrogen receptor-positive proliferating cells in the normal and precancerous breast. Am J Pathol 1999, 155:1811-1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brunner AM, Marquardt H, Malacko AR, Lioubin MN, Purchio AF: Site-directed mutagenesis of cysteine residues in the pro region of the transforming growth factor β 1 precursor. J Biol Chem 1989, 264:13660-13664 [PubMed] [Google Scholar]
- 57.Martikainen P, Kyprianou N, Isaacs JT: Effect of transforming growth factor-β1 on proliferation and death of rat prostatic cells. Endocrinology 1990, 127:2963-2968 [DOI] [PubMed] [Google Scholar]
- 58.Rotello RJ, Lieberman RC, Purchio AF, Gerschenson LE: Coordinated regulation of apoptosis and cell proliferation by transforming growth factor β 1 in cultured uterine epithelial cells. Proc Natl Acad Sci USA 1991, 88:3412-3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nguyen AV, Pollard JW: Transforming growth factor b3 induces cell death during the first stage of mammary gland involution. Development 2000, 127:3107-3118 [DOI] [PubMed] [Google Scholar]
- 60.Strange R, Li F, Saurer S, Burkhardt A, Friis RR: Apoptotic cell death and tissue remodeling during mouse mammary gland involution. Development 1992, 115:49-58 [DOI] [PubMed] [Google Scholar]
- 61.Wakefield l, Colletta AA, McCune BK, Sporn MB: Roles for transforming growth factors-β in the genesis, prevention and treatment of breast cancer. Genes, Oncogens, and Hormones: Advances in Cellular and Molecular Biology of Breast Cancer. Dickson RB Lippman ME eds. 1991:pp 97-136 Kluwer Academic Publishers, Boston
- 62.Wakefield LM, Yang Y, Dukhanina O: TGF-β and breast cancer: lessons learned from genetically altered mouse models. Breast Cancer Res 2000, 2:100-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Massague J, Blain SW, Lo RS: TGF-β signaling in growth control, cancer, and heritable disorders. Cell 2000, 103:295-309 [DOI] [PubMed] [Google Scholar]
- 64.Ziv E, Cauley J, Morin PA, Saiz R, Browner WS: Association between the T29–>C polymorphism in the transforming growth factor beta1 gene and breast cancer among elderly white women: the study of osteoporotic fractures. JAMA 2001, 285:2859-2863 [DOI] [PubMed] [Google Scholar]
- 65.Wakefield L, Kim S-J, Glick A, Winokur T, Colletta A, Sporn M: Regulation of transforming growth factor-β subtypes by members of the steroid hormone superfamily. J Cell Sci 1990, 13:S139-S148 [DOI] [PubMed] [Google Scholar]
- 66.Colletta AA, Wakefield LM, Howell FV, van Roozendaal KE, Danielpour D, Ebbs SR, Sporn MB, Baum M: Anti-oestrogens induce the secretion of active transforming growth factor beta from human fetal fibroblasts. Br J Cancer 1990, 62:405-409 [DOI] [PMC free article] [PubMed] [Google Scholar]