Pancreatic Expression of Keratinocyte Growth Factor Leads to Differentiation of Islet Hepatocytes and Proliferation of Duct Cells (original) (raw)
Abstract
Keratinocyte growth factor, (KGF), a member of the fibroblast growth factor (FGF) family, is involved in wound healing. It also promotes the differentiation of many epithelial tissues and proliferation of epithelial cells as well as pancreatic duct cells. Additionally, many members of the highly homologous FGF family (including KGF), influence both growth and cellular morphology in the developing embryo. We have previously observed elevated levels of KGF in our interferon-γ transgenic mouse model of pancreatic regeneration. To understand the role of KGF in pancreatic differentiation, we generated insulin promoter-regulated KGF transgenic mice. Remarkably, we have found that ectopic KGF expression resulted in the emergence of hepatocytes within the islets of Langerhans in the pancreas. Additionally, significant intra-islet duct cell proliferation in the pancreata of transgenic KGF mice was observed. The unexpected appearance of hepatocytes and proliferation of intra-islet duct cells in the pancreata of these mice evidently stemmed directly from local exposure to KGF.
Pathological consequences often follow targeted cell loss or aberrant cell growth and development. For example, in insulin-dependent diabetes mellitus (IDDM), autoimmune mechanisms contribute to the loss of insulin-producing β-cells in the pancreas during disease progression. In addition, the proliferation of duct cells appears to be associated with pancreatic disease. 1-4 As yet, however, the factors regulating pancreatic growth and development have not been fully defined. In this regard, systemic administration of keratinocyte growth factor (KGF) to rats previously induced pancreatic duct cell proliferation adjacent to or within the islets of Langerhans but without physical injury to the pancreas. 5 As KGF clearly influenced pancreatic growth, further investigation of its role in this process was warranted.
KGF belongs to the fibroblast growth factor (FGF) family, the members of which influence such processes as cell proliferation, migration, and differentiation. 6 KGF is a mesenchymally derived mitogen that acts as a paracrine effector of epithelial cell growth. 7 Indeed, the ability of KGF to induce epithelial cell proliferation has been demonstrated throughout the rat gastrointestinal tract. 8 In addition, KGF is important in the wound-healing process 9 as well as in epithelial cell differentiation. 10-13 However, studies of mice in which the KGF gene was inactivated demonstrated that KGF was not necessary for development or viability; other than deficiencies in hair development, these mice had no significant abnormalities. 14 Although other growth factors may compensate for the lack of KGF, this factor seems to play a role in mesenchymal stimulation of epithelial cell proliferation in vivo. 15
To assess the influence of KGF on growth and development of the pancreas, we developed a transgenic mouse model in which KGF was overexpressed in pancreatic β-cells. In this model (Ins-KGF transgenic mice), the human insulin promoter was used to drive expression of the murine KGF coding sequence. As a consequence, cells with the morphology and antigenicity of hepatocytes formed within the islets and intra-islet duct cells proliferated, as identified by α-fetoprotein (AFP) and carbonic anhydrase (CAII), respectively. However, no pancreatic dysfunction followed despite the novel cellular differentiation pathway induced in this model.
Materials and Methods
Animal Husbandry
All animals were kept in a specific-pathogen-free (SPF) facility at The Scripps Research Institute in accordance with the rules and regulations of the Institutional Animal Care and Use Committee. Food and water were provided ad libitum, and animals were housed under a controlled 12-hour light and dark cycle.
Transgenic Mouse Generation
To prepare Ins-KGF transgenic mice, 585-bp KGF cDNA was obtained by RT-PCR of mRNA from mouse salivary glands and modified to include the Kozak consensus sequence. The KGF cDNA was cloned into a vector containing the human insulin promoter and the hepatitis B 3′ untranslated sequence. The Ins-KGF fragment was isolated by low-melt agarose, purified using Geneclean (BIO101, La Jolla, CA) and NACS Prepac DNA purification columns (BRL, Gaithersburg, MD), and microinjected into fertilized zygotes from BALB/c × C57BL/6 F2 mice. Transgene-positive mice were then bred with BALB/c mice.
In Situ Hybridization
Hybridization was carried out as described previously. 16 Antisense and sense riboprobes were prepared by in vitro transcription of a linearized plasmid containing KGF cDNA using [35S]UTP. After in situ hybridization, sections were covered with photographic emulsion and exposed for 4 weeks before developing.
Histological Analysis and Immunocytochemistry
Both pancreata and spleens were fixed overnight in 10% neutral buffered formalin (3.6% formaldehyde) and embedded in paraffin. Spleen sections were stained in conjunction with pancreatic slices as controls for pancreas-specific antibodies. The 5-μm paraffin sections were either stained with hematoxylin and eosin (H&E) for conventional histological evaluation or stained for the presence of insulin, glucagon, somatostatin, pancreatic polypeptide, amylase, CAII, Pdx-1, albumin, AFP, or BrdU using immunocytochemical techniques as described. 17 Briefly, sections were deparaffinized and blocked with 2% normal goat serum before applying the primary antibodies for insulin, glucagon, somatostatin (all from DAKO, Carpinteria, CA), Pdx-1 (a generous gift from Dr. Helena Edlund, University of Umea, Umea, Switzerland), pancreatic peptide or AFP (both from ICN Immuno Biologicals, Costa Mesa, CA), albumin (Accurate Chemical and Scientific Corp., Westbury, NY), amylase (Sigma Chemical Co., St. Louis, MO), BrdU (Accurate/Sera-Lab, Westbury, NY), or CAII (Biodesign International, Kenne-bunk, ME). Binding of the primary antibody was detected using the appropriate secondary antibody (Vector Laboratories, Burlingame, CA) and the horseradish peroxidase (HRP)-labeled avidin-biotin complex (ABC kit, Vector Laboratories). HRP was visualized using 3,3′-diaminobenzidine as a substrate. Gill’s hematoxylin was used as a counterstain for all sections. Masson’s trichrome staining was used for the identification of fibrotic tissue. Briefly, paraffin-embedded sections were fixed in Bouin’s fixative, stained successively with Weigert’s iron hematoxylin and with Biebrich scarlet-acid fuchsin acid, and then counterstained with aniline blue. Blue staining by collagen and mucin indicated the presence of fibrosis, whereas cytoplasm stained red.
Islet Measurements
To determine the relative sizes of islets in Ins-KGF transgenic and their transgene-negative littermates, anti-insulin-stained sections were examined. Islets were measured at ×10 power (Zeiss Axioscope), using the known 100-μm length of the crosshairs at ×10 power for comparison. Islets were then classed as small (<100 μm diameter), medium (200 to 400 μm), or large (>400 μm). Sixteen Ins-KGF mice and twelve negative littermates were evaluated, and ten islets were counted for each mouse. The islet sizes of young (less than 3 months) and old (greater than 3 months) mice were compared using statistical analyses described below.
Blood Glucose Measurement
Blood was obtained from the eyes of anesthetized mice, and glucose levels were determined every 2 weeks using Glucofilm blood glucose test strips (Miles Diagnostic, Elkhart, IN). Typical, nonfasting blood glucose levels for Balb/c mice in our colony ranged from 80 to 150 mg/dl.
Bromodeoxyuridine (BrdU) Labeling and Assessment of Proliferation
For assessment of cellular proliferation, BrdU labeling was completed as previously described. 18 Briefly, 100 μg/g body weight BrdU (Serva, Heidelberg, Germany) was injected intraperitoneally into mice 16 hours before sacrifice. Adult mice in our colony are, on average, 20 to 30 g. Paraffin-embedded pancreata were sectioned and stained with an anti-BrdU antibody (Accurate Chemical, Westbury, NY) as described above after treatment with 2.8 N HCl for 15 minutes. BrdU-positive cells were counted in transgenic and nontransgenic mice (n = 6 for each genotype). The mitotic index was calculated by dividing the number of positively stained nuclei of cells comprising the duct wall with that of total nuclei/duct wall in at least five randomly chosen fields in each pancreas. Ductal cells were identified by morphology and CAII staining.
Statistical Analysis
The unpaired _t_-test was used to compare differences between the groups using the Statview program by Abacus Concepts (Berkeley, CA).
Results
Generation of Ins-KGF Transgenic Mice
To assess the influence of a growth factor on pancreatic development and function, we generated transgenic mice that expressed KGF within β-cells by using the human insulin promoter (Ins-KGF mice). In situ hybridization of pancreatic sections taken from two lines of these Ins-KGF mice demonstrated that expression of the transgene was confined to their islets (Figure 1) ▶ . The KGF signal appeared to be consistent throughout all islets, including those with unusually high levels of duct cells. In fact, no signal emanated from these duct cells, even when anti-KGF monoclonal antibodies were used (data not shown), presumably due to the lability of the KGF molecule or at least the epitope recognized by these antibodies.
Figure 1.
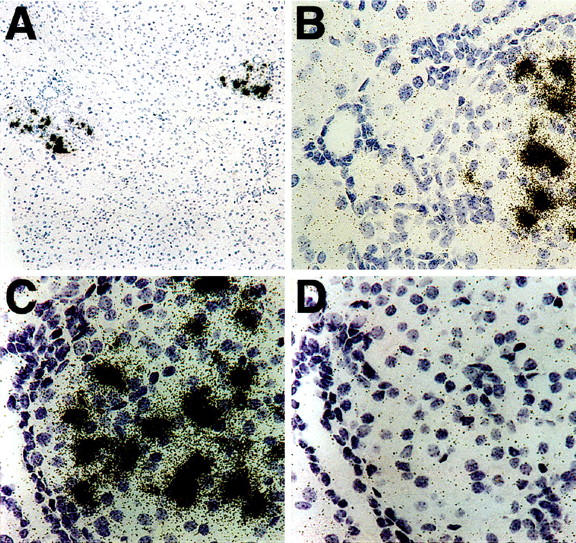
In situ hybridization indicates KGF mRNA expression is specific to pancreatic islets (A) and occurs near, but not in, ducts (B) of Ins-KGF of transgenic mice. The expression of KGF mRNA was tested using both antisense (A–C) and control sense (D) riboprobes. Original magnification, ×20 for all except A, which is ×10. Probes were prepared by in vitro transcription of a linearized plasmid containing KGF using [35S]UTP as previously described. 16 The result from a representative 10-week-old female mouse is shown.
Emergence of Pancreatic Hepatocytes in the Islets of Ins-KGF Mice
Histological analysis revealed distinct morphological changes in KGF-expressing islets of mice ranging in age from 6 weeks to 7.5 months. As Figure 2 ▶ illustrates, the cellular composition of the islets changed in that, along with some normal endocrine cells (β, δ, and PP cells; data not shown), many atypical cells were present (Figure 2, B–F) ▶ . Found in approximately one-fifth of the islets and localized in their peripheries, these novel cells encompassed more than one-half the area of the islets (Figure 2, E and F) ▶ . These cells were large, both in overall size and nuclear size (Figure 2, E and 2F ▶ , see arrows); in contrast, islets of nontransgenic control mice contained cells of a smaller and more uniform size (data not shown). Subsequent immunohistochemical analysis revealed that the larger cells did not express insulin, glucagon, somatostatin, pancreatic polypeptide, amylase, CAII, or Pdx-1 (a marker for the β and δ cell lineages in the adult), nor did they express Meca-32, a marker for endothelial cells (Figure 3E ▶ and data not shown). Thus, these atypical cells did not appear to be endocrine, exocrine, ductal, or endothelial. However, the large cells were similar in morphology to hepatocytes, appearing to take up the eosin stain more intensely than the adjacent islet cells. We tested this possibility by immunostaining pancreatic sections from transgenic mice for the expression of AFP and albumin. Hepatocytes contain AFP during liver development and regeneration 19 and express albumin during adulthood. 20 Yet, all the larger cells depicted here clearly expressed AFP (Figure 3) ▶ , although only two-thirds of them were also positive for albumin expression. Despite a significant degree of background staining for albumin (especially in the islets), the larger, putative hepatocytes stained much more intensely for albumin (Figure 3F) ▶ and were distinctly visible above background. This background most likely resulted from albumin in the serum, accentuated by the high degree of vascularization of the pancreas. No AFP-expressing cells were detected in the nontransgenic littermate controls (Figure 3A) ▶ .
Figure 2.
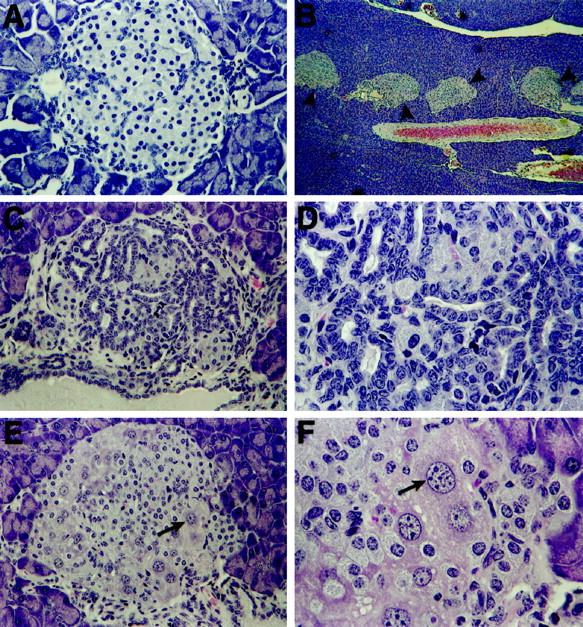
Morphological changes to islet structure in Ins-KGF mice. Shown are H&E-stained islets from Ins-KGF mice at 5 to 7 months of age; such changes are minor in younger mice (as in A, from a 6-week-old mouse). Original magnification, ×40. As the unique phenotype begins to develop (B; black arrowheads indicate islets containing a novel cell type shown at higher magnification in E and F) (7.5 months), the exocrine tissue remains typical as do many islets. Original magnification, ×4. Ins-KGF mice display distinct intra-islet ductal cell proliferation (C and D). Original magnification, ×32 and ×80, respectively. Additionally, extremely large cells with enlarged nuclei (E and F; see arrows) were observed at the peripheries of approximately one-fifth of the islets. Original magnification, ×32 and ×80, respectively.
Figure 3.
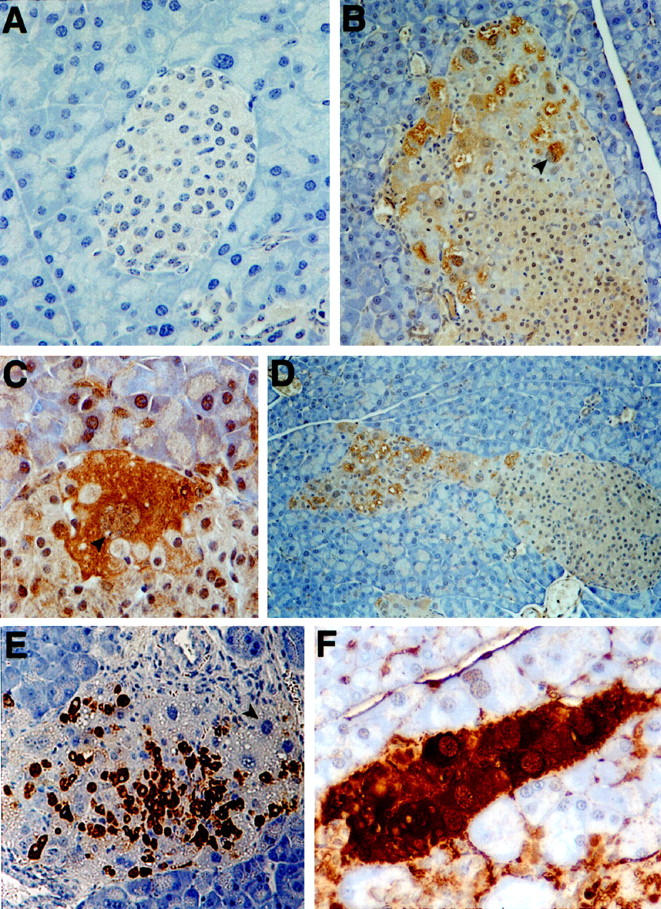
AFP and albumin staining identifies pancreatic hepatocytes within islets of Ins-KGF mice. Although AFP is not typically found within the normal adult pancreas (A, negative littermate control), it was strongly up-regulated in the large cells (B to D) first identified by H&E (see Figure 2 ▶ ). These cells were identified by their extremely large nuclei (C; see arrowhead) at the peripheries of islets (B; see arrowhead) or in trails extruding from islets (D). Although these large cells were not insulin positive (E; see arrowhead), they did express the liver protein, albumin (F). Original magnifications, ×40 (A), ×20 (B, D, and E), and ×80 (C and F).
Interestingly, comet-like clusters of these larger, AFP-positive cells often emanated from the islets or the periphery of an islet (Figure 3D) ▶ . These comet-like clusters could imply poor association with the other islet cells due to distinct membrane characteristics leading to their exclusion to the periphery of the islet structure. Some fibrosis was observed in trichrome-stained pancreata of the Ins-KGF mouse, most commonly separating hepatocyte-like cells from endocrine cells of the islets (Figure 4) ▶ . No morphological changes were evident in the livers, kidneys, or intestines of Ins-KGF mice (data not shown).
Figure 4.
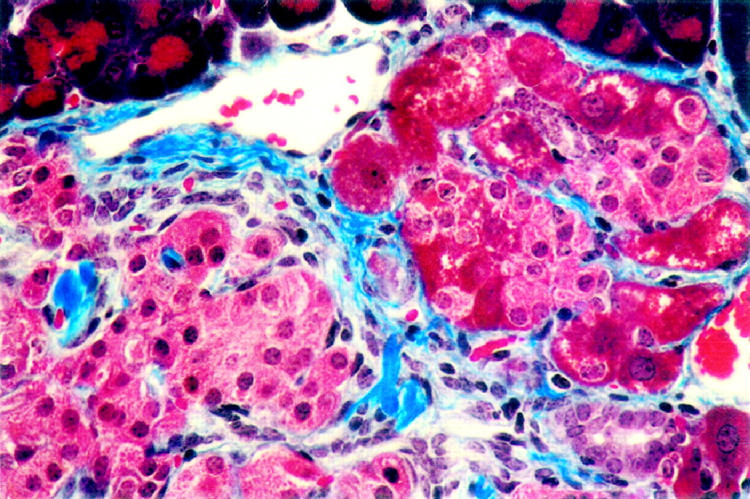
The islets of Ins-KGF transgenic mice (3-month-old female shown) have increased fibrosis in comparison with nontransgenic littermates as identified by trichrome stain. Fibrotic tissue appears blue, pancreatic hepatocytes are brilliant pink, typical islet cells are light pink, and acinar tissue is dark red. Fibrotic tissue separates areas of the islet containing hepatocytes seen on the right side of the photo from the typical cells of the islet on the left side. Original magnification, ×80.
Enhanced Duct Cell Proliferation in KGF-Expressing Mice
In general, the islets became progressively larger as the mice aged and contained distended, folded intra-islet ductal structures (Figure 2, C and D) ▶ . No such morphological changes were observed in nontransgenic littermate controls (data not shown). On average, significantly more large islets (>400 μm diameter) were found in Ins-KGF mice at 3 months of age and older than in nontransgenic littermates (P = 0.017). Although the islets of transgenic mice expressed insulin, glucagon, somatostatin, and pancreatic polypeptide just as nontransgenic mice did, these hormones in transgenic mice formed unique, small clusters surrounding the distended intra islet ducts (see Figure 3E ▶ for insulin). The cells within the distended epithelia were confirmed as ductal cells by their immunoreactivity to CAII (data not shown), a known component of pancreatic duct cells. 21 Evaluating serial sections of the same pancreas also demonstrated that, although most islet cells stained positive for Pdx-1, the intra-islet ducts did not (data not shown). These epithelial cells manifested none of the endocrine hormones, nor did they express amylase, which is normally present in acinar cells (Figure 3E ▶ and data not shown).
Often regions of the pancreas contained several highly folded ducts immediately adjacent to one another, and duct cell proliferative activity was demonstrable in experiments utilizing BrdU (Figure 5) ▶ . A mitotic index of 1.6% was measured for the duct cells of Ins-KGF mice, and that of the nontransgenic littermates was 0.16%; this latter value is comparable to that previously reported. 22 The difference in these mitotic indices is statistically significant (P < 0.001). Additionally, although the majority of BrdU-positive (proliferating) cells were ductal, both the endocrine and exocrine tissues of Ins-KGF mice contained more BrdU-positive cells than did those of the nontransgenic mice. Despite these morphological changes, transgenic mice had neither hyperglycemia nor hypoglycemia.
Figure 5.
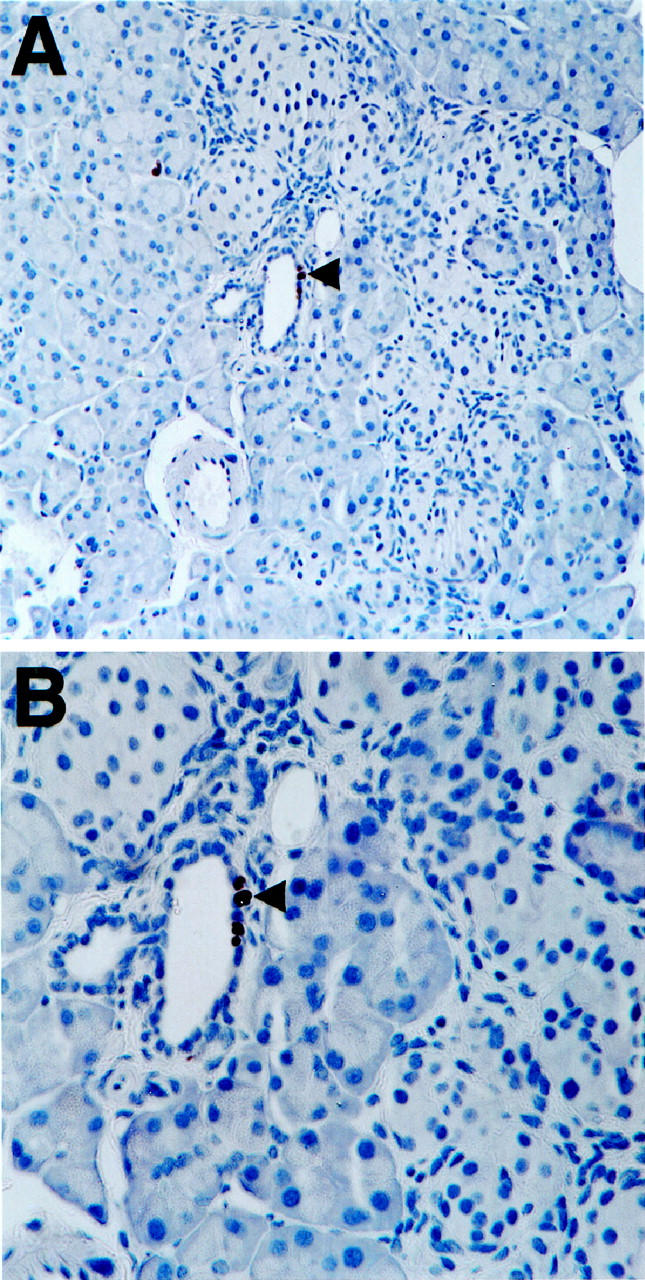
Increased proliferation within pancreata of Ins-KGF transgenic mice. A representative islet with intra-islet duct cells reveals proliferating cells staining brown, indicating that they have incorporated BrdU (male, five months). The cells in A (original magnification, ×20) can be seen in greater detail in the higher magnification of B (original magnification, ×40).
The Number of Islets Harboring Pancreatic Hepatocytes Increases with Age
To gain a more precise understanding of the timing and distribution of the pancreatic hepatocyte and duct formation, we analyzed 20 mice at 6 weeks to 12.5 months of age. Multiple pancreatic sections from individual mice were scored for the presence of islets containing either pancreatic hepatocytes or distinct duct cell proliferation. As some islets did not contain either of these two unusual phenotypes, the percentage of islets with atypical cells was compared with the total number of islets (Table 1) ▶ . Mice younger than 5 months of age had significantly fewer islets (1.3%) containing pancreatic hepatocytes (P = 0.0019) than mice between 5 and 8.99 months of age (19.3%) or 9 and 12.5 months of age (17.5%). However, all Ins-KGF mice, regardless of age, contained islets with proliferating duct cells. Although some transgenic mice younger than 5 months of age did not have pancreatic hepatocytes, these cells appeared much more frequently in older mice. Strikingly, in 19 of the 20 mice examined, any individual islet harbored either intra-islet duct cells or hepatocytes, but not both. The exception was one mouse in which two islets contained both islet hepatocytes and duct cell proliferation (male, 5.5 months). Neither the age of mice nor their gender (9 females and 11 males) had any bearing on their total number of islets or of islets containing ductal cell proliferation.
Table 1.
The Number of Pancreatic Islets Containing Hepatocytes Increases with Age
| Age (months) | Number of mice | Total number of islets counted | Islets with hepatocytes | Islets with duct cell proliferation |
|---|---|---|---|---|
| 1.5–4.99 | 4 | 182 | 1.3%* | 19.9% |
| 5.0–8.99 | 12 | 422 | 19.3% | 20.5% |
| 9.0–12.5 | 4 | 51 | 17.5%* | 23.0% |
Discussion
Dramatic morphological changes resulted from the pancreatic presence of KGF, most notably, the formation of hepatocyte-like cells in the islets of Langerhans. These hepatocytes, identifiable by their expression of AFP, edged the peripheries of most islets. Proliferating cells, identified by their expression of CAII, were also located within the islets, which exhibited normal production of all endocrine hormones. Despite these cellular abnormalities, the host Ins-KGF transgenic mice were healthy and had no obvious toxic effects nor increase in blood glucose levels compared with their age-matched, nontransgenic littermates.
KGF, which acts as a mitogen specific for epithelial cells, might participate in mesenchymal stimulation of epithelial cell proliferation in vivo. 15 Despite the described influence of KGF in vivo and in vitro, knockout mice lacking KGF do not display significant developmental abnormalities, and pancreatic and liver development appear entirely normal. 14 Thus, although KGF can induce epithelial cell proliferation and the differentiation of hepatocytes in vivo, other growth factors must also be able to perform these functions during ontogeny.
Here we observed the induction of islet hepatocytes and hyperproliferation of duct cells associated with islet production of KGF. Other growth factors have been shown to influence pancreatic growth as well. Systemic treatment with EGF in pigs induced pancreatic duct cell proliferation, 23 and transgenic mice overexpressing TGF-α displayed significant histological changes in the pancreas, including the formation of tubular complexes containing actively dividing duct cells. 24 However, no individual growth factor has previously been demonstrated to induce the formation of pancreatic hepatocytes.
The induction of hepatocytes in our transgenic model was not expected; however, KGF itself formerly induced the proliferation of hepatocytes in vitro and in vivo. 8,25 Previous studies in rodents demonstrated that hepatocytes could develop in the pancreas after a variety of exogenous treatments, including the administration of ciprofibrate, TCDD, or cadmium, as well as maintenance on a copper-deficient diet followed by transfer to a normal diet. 26-30 Furthermore, we observed hepatocyte-like cells in the pancreata of transgenic mice overexpressing interferon-γ in the islets of Langerhans. 17 In contrast to these models, Ins-KGF mice formed most of the hepatocytes we detected within the pancreatic islets, at the perimeter or in comet-like structures that extended from the islets. It is significant that the hepatocyte-like cells identified here produced AFP, an embryo-specific protein not found in fully differentiated liver cells in the pancreas. 19,20 All of the pancreatic hepatocyte-like cells observed in our Ins-KGF mice expressed AFP, whereas only two-thirds expressed albumin. As AFP is typically expressed earlier during development than albumin, 19 possibly cells expressing AFP but not albumin represent an earlier hepatocyte precursor than the cells expressing both these markers.
Not completely clear from the foregoing results is how the hepatocytes became localized to the pancreatic islets. Undoubtedly, the in situ proximity of a growth signal played a role in the resulting morphology. Others have proposed that small numbers of uncommitted stem cells in adults retain the ability to differentiate. 31,32 The potential existence of stem cells within internal organs of adults may be of great significance in understanding the effects of exogenous KGF expression within β-cells. The liver and the pancreas both arise from evagination of the primitive gut wall, or endoderm. In fact, a candidate for the liver stem cell (reviewed in Ref. 33 ) has been described. Although still debated, much evidence supports the existence of such cells and their capacity to differentiate into either bile duct cells or hepatocytes (reviewed in Refs. 31, 33, and 34). Considering the common embryonic origin of the liver and pancreas, conceivably stem cells can be induced in both these organs of adults. Such cells might also maintain the ability to act as bipotential precursors that can differentiate into hepatocytes or duct cells whether occupying the liver or pancreas. The molecules involved in activating and regulating stem cells are likely to be the same as those known to regulate cell proliferation and morphogenesis of the liver. Of such molecules thus far described, growth factors, including members of the FGF family, perform this activity (reviewed in Ref. 31 ). The pancreatic duct epithelial cell could even be the precursor for developing hepatocytes in the pancreas. For example, analysis of pancreatic hepatocyte development in rats during recovery from a copper-deficient diet suggested that these hepatocytes originate from duct cells as well as interstitial cells. 28 Other observations in the regenerating hamster pancreas indicated that pancreatic duct cells gave rise to hepatocytes. 35 Thus, the origin of pancreatic hepatocytes and proliferating ductal cells offers several alternatives. A low-frequency stem cell within the pancreas might have the capacity to differentiate toward either a pancreatic hepatocyte or a duct cell, and exposure to KGF could determine the lineage commitment. Or, two stem cells (one for each cell type) might exist at extremely low frequency, then differentiate along their precommitted pathway upon exposure to KGF. Although hepatic cells supposedly can arise from ducts, we found no lineage connection between these two cells in the Ins-KGF mouse. Finally, the islet cell types described here might also arise through trans-differentiation of existing differentiated cell types. Acinar cells have been proposed to be the cellular origin of pancreatic hepatocytes in some systems.
In former studies, 5 KGF administered daily maintained duct cell proliferation, which ceased after KGF was withdrawn. In that study, intralobular ducts adjacent to or within the islets of Langerhans were proliferating. Although such localized influence of KGF would be expected in our transgenic mice, it is not clear why systemic KGF treatment would yield such a restricted effect. Nevertheless, several authors have suggested that duct cell proliferation can contribute to the tissue damage of such pancreatic diseases as chronic pancreatitis, pancreatic cancer, and cystic fibrosis. 1-4 In addition, the proliferation and differentiation of pancreatic duct cells play critical roles in the transgenic mouse model of islet regeneration that we developed and characterized. 36 Indeed, the observation that endocrine cell differentiation frequently accompanies pancreatic duct cell carcinomas suggests that duct cell proliferation can lead to islet neogenesis, 37,38 even though no clinical symptoms were evident in our KGF transgenic mice described here.
Clearly, transgenic expression of KGF in the liver during development resulted in substantial morphological changes to the liver, the pancreas, and other sites as well as changes in epithelial growth in multiple organ systems. 39 Yet, we saw no changes in any organ other than the pancreas, a distinction that may reflect the absolute level of KGF expressed in our transgenic mice or differences in the promoters used in these two studies.
In summary, we have generated a transgenic mouse in which the differentiation of pancreatic cells to hepatocytes as well as proliferation of duct cell are induced by the expression of a discrete growth factor, KGF. Such characterization of factors influencing and controlling the proliferation and differentiation of duct epithelial cells and hepatocytes bears on the understanding of tissue breakdown in disease and the potential for recovery.
Acknowledgments
We thank Joanne Dodge and Jackie Soto for excellent administrative assistance, and the tireless work of Gail Patstone and Augusta Good, who maintained and screened the animal colony, is greatly appreciated. We greatly appreciate the work of Phyllis Minick, who corrected the manuscript for grammatical errors. Additionally, Margaret Chadwell of the TSRI Histology Department cheerfully completed all the trichrome stains for us. Finally, we also thank Dr. Marc Horwitz for his useful and timely advice on graphic layout.
Footnotes
Address reprint requests to Dr. Nora Sarvetnick, Department of Immunology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037. E-mail: noras@scripps.edu.
M. L. Krakowski is supported by a NMSS postdoctoral fellowship, E. M. Jones was supported by NIH postdoctoral fellowship DK09355-01, D. Gu was supported by a postdoctoral fellowship from the Juvenile Diabetes Foundation, and N. Sarvetnick is supported by a Diabetes Interdisciplinary Research Center from the Juvenile Diabetes Foundation and by NIH grant HD-29764 and JDFI 995010. This is publication 11056-IMM from the Department of Immunology, the Scripps Research Institute.
References
- 1.Barr HS: Fibrocystic disease of the pancreas. Lancet 1953, ii:80-83 [DOI] [PubMed] [Google Scholar]
- 2.Porta EA, Stein AA, Patterson P: Ultrastructural changes of the pancreas and liver in cystic fibrosis. Am J Clin Pathol 1964, 42:451-465 [DOI] [PubMed] [Google Scholar]
- 3.Noronha M, Bordalo O, Dreiling DA: Alcohol and the pancreas. II. Pancreatic morphology of advanced alcoholic pancreatitis. Am J Gastroenterol 1981, 76:120-124 [PubMed] [Google Scholar]
- 4.Takahashi M, Arai H, Kokubo T, Furukawa F, Kurata Y, Ito N: An ultrastructural study of precancerous and cancerous lesions of the pancreas in Syrian golden hamsters induced by N-nitrosobis(2-oxopropyl)amine. Gann 1981, 71:825-831 [PubMed] [Google Scholar]
- 5.Yi ES, Yin S, Harclerode DL, Bedoya A, Bikhazi NB, Housley RM, Aukerman SL, Morris CF, Pierce GF, Ulich TR: Keratinocyte growth factor induces pancreatic ductal epithelial proliferation. Am J Pathol 1994, 145:80-85 [PMC free article] [PubMed] [Google Scholar]
- 6.Brown KD: The epidermal growth factor/transforming growth factor-alpha family and their receptors. Eur J Gastroenterol Hepatol 1995, 7:914-922 [DOI] [PubMed] [Google Scholar]
- 7.Finch PW, Rubin JS, Miki T, Ron D, Aaronson SA: Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science 1989, 245:752-755 [DOI] [PubMed] [Google Scholar]
- 8.Housley RM, Morris CF, Boyle W, Ring B, Biltz R, Tarpley JE, Aukerman SL, Devine PL, Whitehead RH, Pierce GF: Keratinocyte growth factor induces proliferation of hepatocytes and epithelial cells throughout the rat gastrointestinal tract. J Clin Invest 1994, 94:1764-1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Werner S, Peters K, Longaker M, Fuller-Pace F, Banda M, Williams L: Large induction of keratinocyte growth factor expression in the dermis during wound healing. Proc Natl Acad Sci USA 1992, 89:6896-6900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alarid ET, Rubin JS, Young P, Chedid M, Ron D, Aaronson SA, Cunha GR: Keratinocyte growth factor functions in epithelial induction during seminal vesicle development. Proc Natl Acad Sci USA 1994, 91:1074-1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo L, Yu G-C, Fuchs E: Targeting expression of keratinocyte growth factor to keratinocytes elicits striking changes in epithelial differentiation in transgenic mice. EMBO J 1993, 12:973-986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ulich TR, Yi ES, Longmuir K, Yin S, Biltz R, Morris CF, Housley RM, Pierce GF: Keratinocyte growth factor is a growth factor for type II pneumocytes in vivo. J Clin Invest 1994, 93:1298-1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Post M, Souza P, Liu J, Tseu I, Wang J, Kuliszewski M, Tanswell AK: Keratinocyte growth factor and its receptor are involved in regulating early lung branching. Development 1996, 122:3107-3115 [DOI] [PubMed] [Google Scholar]
- 14.Guo L, Degenstein L, Fuchs E: Keratinocyte growth factor is required for hair development but not for wound healing. Genes Dev 1996, 10:165-175 [DOI] [PubMed] [Google Scholar]
- 15.Rubin JS, Hiroyuki O, Finch PW, Taylor WG, Rudikoff S, Aaronson SA: Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc Natl Acad Sci USA 1989, 86:802-806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnush M, Gu D, Baugh C, Sawyer SP, Davis D, Mroczkowski B, Krahl T, Sarvetnick N: Growth factors in the regenerating pancreas of gamma-interferon transgenic mice. Lab Invest 1995, 74:985-990 [PubMed] [Google Scholar]
- 17.Gu D, Sarvetnick N: Epithelial cell proliferation and islet neogenesis in IFN-γ transgenic mice. Development 1993, 118:33-46 [DOI] [PubMed] [Google Scholar]
- 18.Lee M-S, Gu D, Feng L, Curriden S, Arnush M, Krahl T, Gurushanthaiah D, Wilson C, Loskutoff D, Fox H, Sarvetnick N: Accumulation of extracellular matrix and developmental dysregulation in the pancreas by transgenic production of transforming growth factor-β1. Am J Pathol 1995, 147:42-52 [PMC free article] [PubMed] [Google Scholar]
- 19.Moorman AFM, De Boer PAJ, Evans D, Charles R, Lamers WH: Expression patterns of mRNAs for alpha-fetoprotein and albumin in the developing rat: the ontogenesis of hepatocyte heterogeneity. Histochem J 1990, 22:653-660 [DOI] [PubMed] [Google Scholar]
- 20.Gleiberman AS, Kudrjavtseava EI, Sharovskaya YY, Abelev GI: Synthesis of alpha-fetoprotein in hepatocytes is co-ordinately regulated with cell-cell and cell-matrix interactions. Mol Biol Med 1989, 6:95-107 [PubMed] [Google Scholar]
- 21.Hootman SR, Ondarza JD: Overview of pancreatic duct physiology and pathophysiology. Digestion 1993, 54:323-330 [DOI] [PubMed] [Google Scholar]
- 22.Githens S: The pancreatic duct cell: proliferative capabilities, specific characteristics, metaplasia, isolation, and culture. J Pediatr Gastroenterol Nutrition 1988, 7:486-506 [PubMed] [Google Scholar]
- 23.Vinter-Jensen L, Juhl CO, Teglbjaerg PS, Poulsen SS, Dajani EZ, Nexo E: Systemic treatment with epidermal growth factor in pigs induces ductal proliferations in the pancreas. Gastroenterology 1997, 113:1367-1374 [DOI] [PubMed] [Google Scholar]
- 24.Jhappan C, Stahle C, Harkins RN, Fausto N, Smith GH, Merlino GT: TGFα overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell 1990, 61:1137-1146 [DOI] [PubMed] [Google Scholar]
- 25.Itoh T, Suzuki M, Mitsui Y: Keratinocyte growth factor as a mitogen for primary culture of rat hepatocytes. Biochem Biophys Res Commun 1993, 192:1011-1015 [DOI] [PubMed] [Google Scholar]
- 26.Scarpelli DG, Rao MS: Differentiation of regenerating pancreatic cells into hepatocyte-like cells. Proc Natl Acad Sci USA 1981, 78:2577-2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao MS, Subbarao V, Scarpelli DG: Development of hepatocytes in the pancreas of hamsters treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Toxicol Environ Health 1988, 25:201-205 [DOI] [PubMed] [Google Scholar]
- 28.Rao MS, Dwivedi RS, Yeidandi AV, Subbarao V, Tan X, Usman MI, Thangada S, Neimali MR, Kumar S, Scarpelli DG, Reddy JK: Role of periductal and ductal epithelial cells of the adult rat pancreas in pancreatic hepatocyte lineage: a change in the differentiation commitment. Am J Pathol 1989, 134:1069-1086 [PMC free article] [PubMed] [Google Scholar]
- 29.Reddy JK, Rao MS, Qureshi SA, Reddy MK, Scarpelli DG, Lalwani ND: Induction and origin of hepatocytes in rat pancreas. J Cell Biol 1984, 98:2082-2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waalkes MP, Cherian MG, Ward JM, Goyer RA: Immunohistochemical evidence of high concentrations of metallothionein in pancreatic hepatocytes induced by cadmium in rats. Toxicol Pathol 1992, 20:323-326 [DOI] [PubMed] [Google Scholar]
- 31.Sirica A: Ductular hepatocytes. Histol Histopathol 1995, 10:433-456 [PubMed] [Google Scholar]
- 32.Reddy J, Rao S, Yeldandi A, Tan X, Dwivdedi R: Pancreatic hepatocytes: an in vivo model for cell lineage in pancreas of adult rat. Dig Dis Sci 1991, 36:502-509 [DOI] [PubMed] [Google Scholar]
- 33.Fausto N: Hepatocyte differentiation and liver progenitor cells. Curr Opin Cell Biol 1990, 2:1036-1042 [DOI] [PubMed] [Google Scholar]
- 34.Sell S: Is there a liver stem cell? Cancer Res 1990, 50:3811-3815 [PubMed] [Google Scholar]
- 35.Makino T, Usuda N, Rao S, Reddy JK, Scarpelli DG: Transdifferentiation of ductular cells into hepatocytes in regenerating hamster pancreas. Lab Invest 1990, 62:552-561 [PubMed] [Google Scholar]
- 36.Sarvetnick N, Shizuru J, Liggitt D, Martin L, McIntyre B, Gregory A, Parslow T, Stewart T: Loss of pancreatic islet tolerance induced by beta-cell expression of interferon-gamma. Nature 1990, 346:844-847 [DOI] [PubMed] [Google Scholar]
- 37.Dawiskiba S, Pour PM, Stenram U, Sundler F, Andren-Sandberg A: Immunohistochemical characterization of endocrine cells in experimental exocrine pancreatic cancer in the Syrian golden hamster. Int J Pancreatol 1992, 11:87-96 [DOI] [PubMed] [Google Scholar]
- 38.Terada T, Ohta T, Kitamura Y, Ashida K, Matsunaga Y, Kato M: Endocrine cells in intraductal papillary-mucinous neoplasms of the pancreas: a histochemical and immunohistochemical study. Virchows Arch 1997, 431:31-36 [DOI] [PubMed] [Google Scholar]
- 39.Nguyen HQ, Danilenko DM, Bucay N, DeRose ML, Van GY, Thomason A, Simonet WS: Expression of keratinocyte growth factor in embryonic liver of transgenic mice causes changes in epithelial growth and differentiation resulting in polycystic kidneys and other organ malformations. Oncogene 1996, 12:2109-2119 [PubMed] [Google Scholar]