Expression of the Chemokine Receptors CCR4, CCR5, and CXCR3 by Human Tissue-Infiltrating Lymphocytes (original) (raw)
Abstract
Differential expression of adhesion molecules and chemokine receptors has been useful for identification of peripheral blood memory lymphocyte subsets with distinct tissue and microenvironmental tropisms. Expression of CCR4 by circulating memory CD4+ lymphocytes is associated with cutaneous and other systemic populations while expression of CCR9 is associated with a small intestine-homing subset. CCR5 and CXCR3 are also expressed by discrete memory CD4+ populations in blood, as well as by tissue-infiltrating lymphocytes from a number of sites. To characterize the similarities and differences among tissue-infiltrating lymphocytes, and to shed light on the specialization of lymphocyte subsets that mediate inflammation and immune surveillance in particular tissues, we have examined the expression of CCR4, CXCR3, and CCR5 on CD4+ lymphocytes directly isolated from a wide variety of normal and inflamed tissues. Extra-lymphoid tissues contained only memory lymphocytes, many of which were activated (CD69+). As predicted by classical studies, skin lymphocytes were enriched in CLA expression whereas intestinal lymphocytes were enriched in α4β7 expression. CCR4 was expressed at high levels by skin-infiltrating lymphocytes, at lower levels by lung and synovial fluid lymphocytes, but never by intestinal lymphocytes. Only the high CCR4 levels characteristic of skin lymphocytes were associated with robust chemotactic and adhesive responses to TARC, consistent with a selective role for CCR4 in skin lymphocyte homing. In contrast, CXCR3 and CCR5 were present on the majority of lymphocytes from each non-lymphoid tissue examined, suggesting that these receptors are unlikely to determine tissue specificity, but rather, may play a wider role in tissue inflammation.
Tissue-specific recruitment and retention of lymphocyte subsets compartmentalizes the immune system. Such partitioning increases the likelihood that lymphocytes bearing rare antigen specificities will encounter their cognate antigen. At the same time, compartmentalization decreases the likelihood that an expanded lymphocyte population (specific for an antigen found only within a given tissue) will be exposed to a potentially cross-reactive host-derived antigen within a different tissue. 1 Tissue-targeted recruitment and localization are thought to be achieved by regulated expression of particular homing receptors on lymphocytes (including adhesion and chemokine receptors), as well as by regulation of their counter-receptors expressed by endothelial, epithelial, inflammatory, and parenchymal cells in the target tissues. 2,3
The clearest evidence to support the concept of tissue-dedicated trafficking of peripheral blood lymphocyte subsets exists at the level of their expression of adhesion molecules. 1,2 Circulating lymphocytes associated with skin homing express the E-selectin ligand CLA (cutaneous lymphocyte antigen), whereas those associated with homing to the gastrointestinal tract express the MAdCAM-1 ligand α4β7 integrin. Although skin- and intestinal-specific lymphocyte populations are the best studied, there is reason to believe that additional populations dedicated to other tissues may exist. 1,2
In addition to differential expression of adhesion molecules, differential expression of chemokine receptors may also contribute to tissue-specific homing. This hypothesis would predict the disproportionate expression of particular chemokine receptors by lymphocytes that have infiltrated tissues that express the corresponding chemokine ligand. We have recently found support for this prediction. 4 By carefully isolating T cells from various human tissues, we demonstrated that the chemokine receptor CCR9 is highly expressed by nearly all T lymphocytes infiltrating the small intestine (the one organ outside of the thymus where the CCR9 ligand TECK is highly expressed). In contrast, CCR9 was not expressed by T lymphocytes isolated from other tissues tested, including skin, liver, synovium, and lung. In contrast to small intestine-derived lymphocytes, CCR9 was expressed by only a small percentage of colon lymphocytes, suggesting that circulating intestinal-specific lymphocytes may be further subdivided into small intestine-dedicated versus large intestine-dedicated populations. 4
To further examine similarities and differences among lymphocytes that have infiltrated diverse human tissues, we have investigated the differential expression of three more chemokine receptors (CCR4, CCR5, and CXCR3) by tissue-infiltrating lymphocytes. We previously reported that circulating skin-associated CLA+ lymphocytes uniformly express high levels of CCR4 5 and that the CCR4 ligand TARC 6 is expressed on normal and inflamed cutaneous (but not intestinal) endothelium. Taken together, these two findings suggest that CCR4 and TARC may participate in skin-specific lymphocyte recruitment. 3,5 Interestingly, CCR4 monoclonal antibodies (mAbs) also recognized a subset of CLA−/α4β7− peripheral blood memory CD4+ lymphocytes. 5 The role of CCR4 here is unknown, but these cells may represent another skin-associated subset or a subset dedicated to a systemic tissue.
CCR5 and CXCR3 have been reported to be tissue-specific receptors in multiple organs. CCR5 is expressed by lymphocytes in intestinal tissues and was suggested to be a mucosa-specific receptor. 7,8 However, other groups reported CCR5 expression on lymphocytes in the brains of multiple sclerosis patients, 9 liver, 10 and on synovial lymphocytes from arthritis patients. 11,12 CXCR3 expression was similarly reported on intestinal, 7 inflamed brain, 9 liver, 10 and synovial 11,12 lymphocytes. Thus, the roles of CCR4, CCR5, and CXCR3 in tissue-specific lymphocyte homing require further clarification.
By identifying the similarities and differences in expression of chemokine receptors by lymphocytes that have infiltrated a variety of extralymphoid tissues, we hope to gain an understanding of the specialized CD4+ lymphocyte subsets involved in inflammation and immune surveillance in particular tissue sites. Such knowledge may identify pharmacological targets that could be used to manipulate tissue-specific homing or other chemokine-mediated inflammatory functions, making possible treatments for regional autoimmune diseases (eg, psoriasis or Crohn’s disease) as well as tailored treatments to prevent rejection of transplanted tissues.
Materials and Methods
Antibodies and Reagents
Anti-human CCR4 mAbs 1G1 and 2B10 (both mouse IgG1), 5 CLA-FITC mAb HECA-452 (rat IgM), 13 and α4β7-PE mAb Act-1 (mouse IgG1) 14 have been previously described. Unconjugated mouse anti-human CD45RO (IgG2a, clone UCHL1), CCR5 (IgG2a, clone 2D7; known to correlate best with genetic expression of CCR5 15 ), CXCR3 (IgG1, clone 1C6), and directly conjugated mouse anti-human CD45RA-FITC (IgG2b, clone HI100), CD69-PE (IgG1, clone FN50), CD3-FITC (IgG1, clone UCHT1), TCRαβ-FITC (IgM, clone T10B9.1A-31), and CD4-APC (IgG1, clone RPAT4) were obtained from PharMingen, Inc. (San Diego, CA). Recombinant human SDF-1α and TARC were purchased from Peprotech (Rocky Hill, NJ). ICAM-1 was purified from human tonsils as described. 5
Tissue Sources and Lymphocyte Isolation
Human jejunum, ileum, colon, lung, tonsil, and inflamed liver were obtained from patients undergoing various surgical procedures. Synovial fluid (SF) was obtained from patients undergoing diagnostic arthrocentesis. Peripheral blood was collected in heparinized tubes from healthy donors. Bronchoalveolar lavage (BAL) fluid was obtained by optic bronchoscopy from healthy volunteers. Skin lymphocytes were obtained from suction blisters in sensitized volunteers. All human subject protocols were approved by the Institutional Review Boards at Stanford University or Leicester University.
Peripheral blood lymphocytes were isolated as described. 5 Briefly, after dextran sedimentation and separation of the mononuclear fraction over Ficoll (Amersham Pharmacia Biotech, Piscataway, NJ), monocytes were removed by two 30 minute rounds of adherence to plastic culture flasks in an incubator. Tonsil lymphocytes were obtained by dispersal of fresh tonsils through a stainless steel mesh followed by incubation in a plastic flask to deplete adherent cells. Bronchoalveolar lavage subjects were premedicated with nebulized salbutamol, lightly sedated with midazolam and the upper airway was anesthetized with 2% lignocaine. 180 ml of normal saline were inserted through the bronchoscope into the middle lobe of the right lung and the lymphocyte-containing fluid was aspirated using gentle suction (fluid recovery was 20 to 46%). Lymphocytes from the epithelium and lamina propria of human intestine were isolated as described previously. 16 Briefly, the epithelium and any resident lymphocytes were removed from the lamina propria by gentle stirring in the presence of 1 mmol/L EDTA. The epithelium-free lamina propria was then dispersed through wire mesh. Lymphocytes were isolated from inflamed human skin as described previously. 17 Briefly, skin lymphocytes were isolated from the fluid drained from suction blisters raised over the site of an epidermal delayed-type hypersensitivity reaction (to poison oak leaves or Candida albicans extract) elicited in allergic volunteers. Lymphocytes were isolated from normal lung, inflamed liver, and inflamed synovial fluid as described. 4 Briefly, lung lymphocytes were obtained by mincing the tissue with fine scissors and passing the supernatant through gauze; liver lymphocytes were obtained after mechanical dispersal of liver pieces followed by Ficoll separation, and synovial fluid mononuclear cells were isolated by Ficoll separation.
We performed confirmatory studies to investigate whether chemokine receptor expression on extracted lymphocytes might have been altered by the tissue-chelation procedure. In these parallel experiments, purified lymphocytes (from peripheral blood) were treated by chelation in exactly the same manner as the tissue samples. Donor-matched, untreated and treated lymphocytes were then stained with the same mAbs to chemokine receptors and adhesion molecules presented in the text. We found no staining differences between the treated and untreated cells (Kunkel and Campbell, unpublished findings).
Adhesion and Chemotaxis Assays
Chemotaxis assays were performed using 24-well Transwell plates (Corning Costar, Cambridge, MA; 5 μm pores) in RPMI 1640 supplemented with 0.5% bovine serum albumin for 2 hours in an incubator as described. 5 Migrated cells were stained with CD4-APC, CD45RA-CyChrome, CLA-FITC, and α4β7-PE to analyze T cell subsets (Pharmingen). Chemokine-triggered adhesion assays were performed as described. 5 Briefly, sorted lymphocytes were allowed to settle on glass slides coated with purified human ICAM-1 and stimulated to adhere with chemokines, after which the slides were gently washed and bound lymphocytes were fixed with gluteraldehyde and counted using NIH Image software (version 1.62b).
FACS Analysis
Tissue or blood lymphocytes were stained and gated for the populations of interest using markers labeled with fluorescein isothyocyanate (FITC), phycoerythrim (PE), or allophycocyanin (APC). Unconjugated (or isotype-matched control mAbs) were detected using a biotinylated horse anti-mouse IgG secondary antibody (Vector Laboratories, Burlingame, CA) and streptavidin-PerCP (Pharmingen). In some experiments, blood lymphocytes were stained for CCR4 and CD45RO and sorted on a FACSVantage SE (Becton-Dickinson). Four-color flow cytometry was carried out on a FACSCalibur (Becton-Dickinson) using CellQuest software, version 3.1 (Becton-Dickinson).
Results
Memory and Activation Status of Tissue-Infiltrating Lymphocytes
In an attempt to more thoroughly understand the phenotype of tissue-infiltrating CD4+ lymphocytes, we have undertaken to gently isolate lymphocytes from surgical specimens for direct examination. Our lymphocyte isolation techniques involved chelation at 4°C as previously described. 4 This technique allowed us to avoid prolonged (potentially destructive) exposure of lymphocytes to digestive enzymes at 37°C as entailed by conventional protocols. We first examined the activation and naive/memory status of tissue infiltrating cells. CD4+ lymphocytes from non-lymphoid tissues (eg, inflamed skin, normal jejunum, lung BAL fluid, and psoriatic arthritis SF in Figure 1, C–F ▶ ) were overwhelmingly of the memory phenotype (CD45RA−). This is a dramatic enrichment of memory cells over their representation in blood, where they usually comprise less than 50% of CD4+ T cells (Figure 1A) ▶ . In contrast, CD4+ T cells isolated from tonsil (a representative secondary lymphoid organ) maintained a naïve to memory ratio similar to that of blood (Figure 1B) ▶ . Both lymphoid and non-lymphoid tissues contained large numbers of cells of activated phenotype (as assessed by the activation marker CD69, Figure 1 ▶ ). Activated cells were exceedingly rare in the blood (Figure 1A) ▶ .
Figure 1.
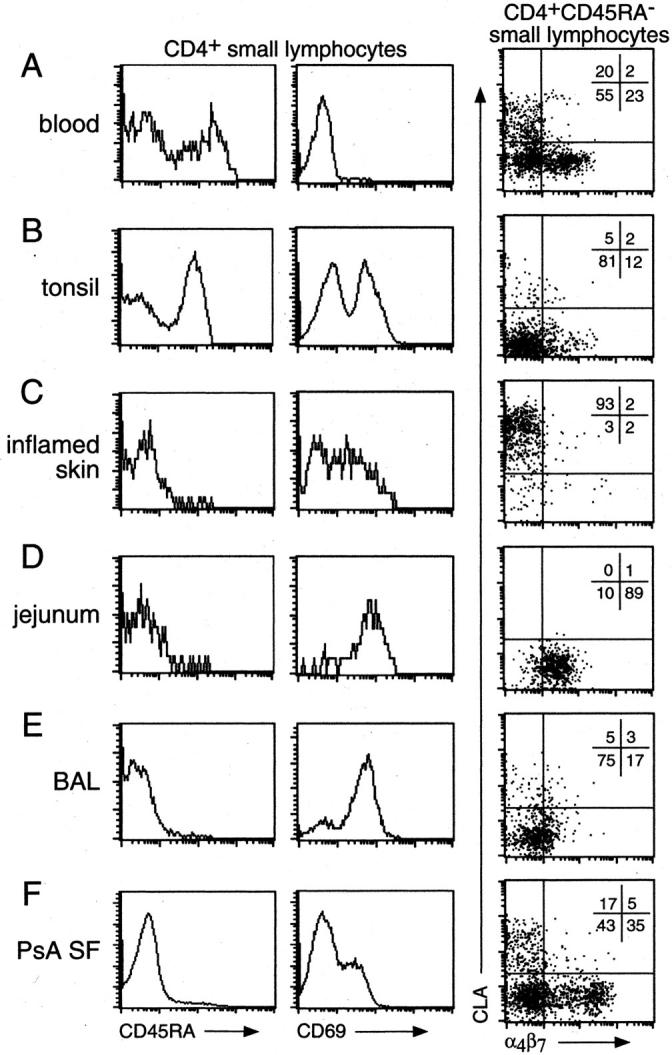
Phenotype of blood and tissue CD4+ lymphocytes. CD4+ lymphocytes in various tissues were isolated and stained for expression of CD45RA, CD69, CLA, and α4β7. Circulating blood CD4+ lymphocytes (A) are evenly divided into memory (CD45RA−) and naive (CD45RA+) populations, contain virtually no activated (CD69+) cells, and contain CLA+, α4β7hi, and CLA−α4β7lo/− memory populations. Tonsils (B) contain memory and naïve lymphocytes of which about half are activated, and very few lymphocytes with skin (CLA+) or gut (α4β7+) homing specificity. Tissue sites such as inflamed skin (C), jejunum (D), BAL (E), and arthritic synovial fluid (F) contain only memory lymphocytes, including many activated cells. In addition, all skin lymphocytes are CLA+ (C), all gut lymphocytes are α4β7+ (D), BAL lymphocytes are mostly CLA−α4β7− (E), and SF contains many subsets of lymphocytes (F). Data are representative of three or more different samples of each tissue.
Adhesion Molecule Expression on Tissue-Infiltrating Lymphocytes
To further characterize the tissue-derived lymphocytes, we examined their expression of the two best-studied adhesion molecules associated with tissue-specific homing: CLA and α4β7 integrin. CLA is a ligand for E-selectin, which allows CLA+ cells to interact with E-selectin in cutaneous venules. CLA+ cells home preferentially to cutaneous sites in vivo, and T cells specific for cutaneous antigens reside within this population. 18 The α4β7 integrin is a ligand for MAdCAM-1, which allows α4β7+ cells to interact with MAdCAM-1+ intestinal venules. α4β7- memory cells home preferentially to intestinal sites in vivo, 19 and cells specific for intestinal antigens reside within this population. 20
Memory CD4+ cells from blood contained a mixture of CLA-expressing, α4β7-expressing, and other less-well studied subsets (Figure 1, A ▶ ; far right panel). However, when we examined cells isolated directly from skin or from jejunal lamina propria we found essentially pure CLA+ or α4β7+ populations, respectively (Figure 1, C and D ▶ ; far right panels). In contrast, CD4+ populations from the lung or tonsil, where these molecules are proposed to play little or no role in homing, contained very few cells with high levels of these molecules (Figure 1, B and E ▶ ; far right panels). Interestingly, lymphocytes from the synovial fluid of autoimmune arthritis patients appeared to be an exception to the rule of tissue-specific lymphocyte homing (eg, psoriatic arthritis in Figure 1E ▶ ; far right panel). Although naïve cells were excluded from this extra-lymphoid site, the representation of memory cells expressing CLA or α4β7 was similar to that of blood.
Chemokine Receptor Expression of Tissue-Infiltrating Lymphocytes
The above data suggested that the gentle method we used for isolating lymphocytes from tissues (see Materials and Methods) 4 preserved the predicted homing phenotypes of the isolated cells. We then examined the expression of chemokine receptors by these cells, focusing on CCR4, CCR5, and CXCR3.
The vast majority of CD4+ cells isolated from the 10 different non-lymphoid tissues examined (Figure 2) ▶ expressed CCR5 and CXCR3 (Figure 2, C ▶ -L; middle and right panels). This is in dramatic contrast to tonsil, where only very small numbers of CD4+ lymphocytes expressed CCR5 or CXCR3 (Figure 2B ▶ ; far right panel). Nearly all of the CD4+ populations infiltrating non-lymphoid tissues contained far more CCR5+ and CXCR3+ cells than did memory CD4+ cells from the blood (with the exception of CXCR3 in the skin, where the pattern was similar to that on CLA+ cells in the blood 11 ) (Figure 2A ▶ ; middle and right panels). The ubiquity of CCR5+ and CXCR3+ T cells within so many diverse tissues suggests that CCR5 and CXCR3 are not involved in determining the tissue-specificity of lymphocyte homing, and that CCR5 and CXCR3 expression may be a general phenotype of tissue-infiltrating lymphocytes. CCR5 and CXCR3 may therefore be more important with respect to positioning or retention of lymphocytes after tissue entry. 21 Indeed, it is possible that these receptors may actually be induced after tissue entry.
Figure 2.
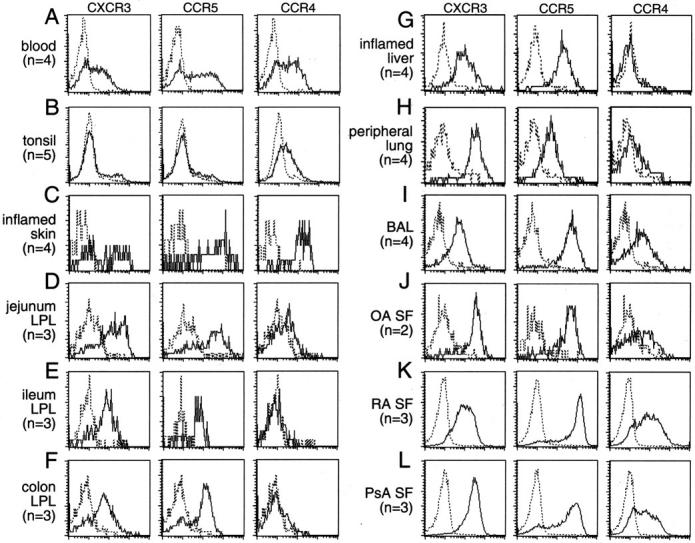
CCR4 expression is restricted to lymphocytes in skin (but not intestines) and certain other systemic sites while virtually all tissue lymphocytes express CXCR3 and CCR5. Memory lymphocytes from a variety of tissues were stained for CCR4, CXCR3, and CCR5 expression. CCR4 is expressed on a large fraction of blood (A) and a small fraction of tonsil (B) lymphocytes, but at a high level on virtually all inflamed skin (C) lymphocytes. Intestinal segments including the jejunum (D), ileum (E) and colon (F) contain virtually no lymphocytes expressing CCR4. Similarly, systemic tissues such as inflamed liver (G), peripheral lung tissue (H), and osteoarthritis synovial fluid (J) also contain few CCR4-expressing lymphocytes. Other systemic tissues such as BAL (I) and the synovial fluid from autoimmune arthropathies including rheumatoid arthritis (K), and psoriatic arthritis (L), do contain many lymphocytes that express lower levels of CCR4. Virtually all lymphocytes in normal or inflamed tissues examined express both CXCR3 and CCR5 (C–L) compared to practically none in tonsil (B) and only 30 to 50% on blood lymphocytes (A). Number of samples for each tissue (n) shown.
Unlike the ubiquitous expression pattern of CCR5 and CXCR3, lymphocyte CCR4 expression was variable among the tissues studied (Figure 2, C–L) ▶ . CCR4 was not seen on lamina propria CD4+ cells from any of the intestinal segments (including jejunum, ileum and colon, Figure 2, D–F ▶ ), or on liver-infiltrating CD4+ cells (Figure 2G) ▶ . CCR4 was expressed at very high levels only in the skin (Figure 2C) ▶ . CCR4 was expressed at lower levels by synovial fluid CD4+ cells from autoimmune arthritis patients (rheumatoid, Figure 2K ▶ and psoriatic, Figure 2L ▶ ) but not from osteoarthritis patients (Figure 2J) ▶ . CCR4 was also expressed at low levels on bronchial CD4+ cells (Figure 2I) ▶ and even more rarely by lung interstitial CD4+ cells (Figure 2H) ▶ . Like CCR5 and CXCR3, there was little CCR4 expression in the tonsil (Figure 2B) ▶ .
Relative CCR4 Expression Levels on CD4 Cells from Systemic Tissues
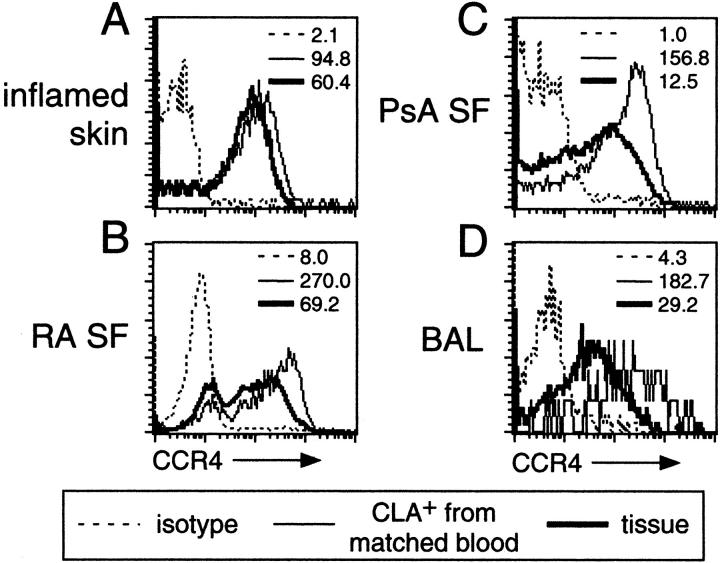
The expression of CCR4 by skin-derived lymphocytes appeared significantly higher than its expression by bronchial or synovial cells. This higher expression was confirmed by additional experiments using blood CLA+ lymphocytes from tissue donors as an internal standard (Figure 3) ▶ . Flow cytometry of CCR4 on tissue-infiltrating lymphocytes was overlaid directly on that of CD4+CLA+ blood lymphocytes from the same patient (Figure 3, A–D) ▶ . Only skin-derived cells expressed CCR4 at a level matching that of CD4+CLA+ cells from donor-matched blood (Figure 3A) ▶ . The median CCR4 expression by bronchial or synovial cells was at least an order of magnitude less that of donor-matched CD4+CLA+ blood cells (Figure 3, B–D) ▶ .
Figure 3.
Skin-infiltrating and blood CLA+ lymphocytes express higher levels of CCR4 than lymphocytes in non-cutaneous tissues. CCR4 intensity on lymphocytes from tissues that contained CCR4+ cells was compared to the level of CCR4 expression on matched donor blood CLA+ lymphocytes. Lymphocytes in inflamed skin (A) expressed levels of CCR4 comparable to circulating CLA+ skin-homing lymphocytes, whereas lymphocytes in systemic sites such as rheumatoid (B) and psoriatic (C) synovial fluid or BAL (D) expressed lower levels of CCR4 comparable to that found on the systemic and mucosae-homing populations in the blood. Data are representative of three or more independent matched samples for each tissue. The median fluorescence channel is shown for each staining panel.
Functional Significance of CCR4 Expression Levels
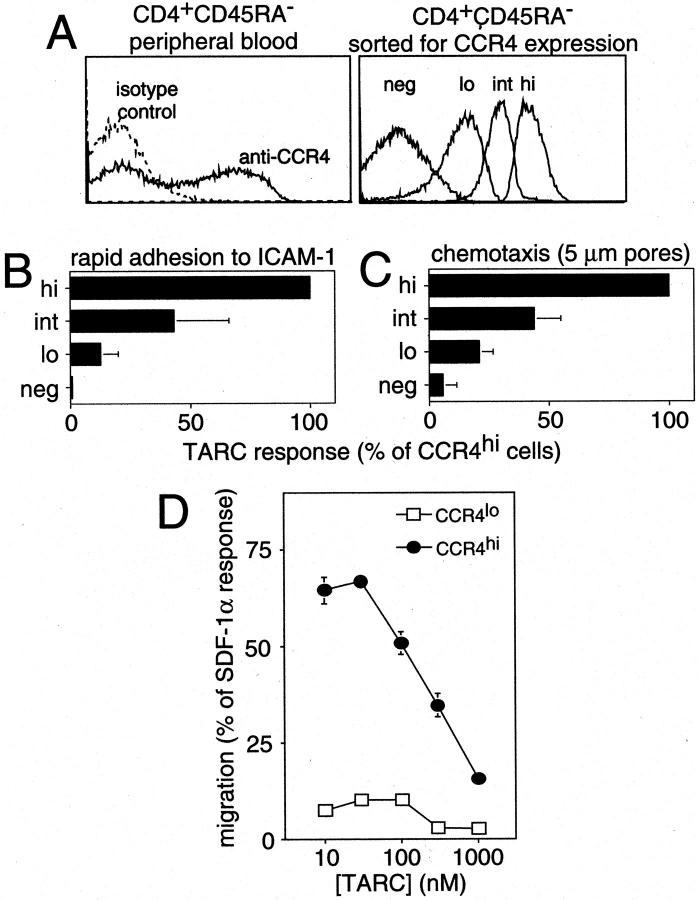
We hypothesized that differences in CCR4 expression levels may be functionally relevant in lymphocyte trafficking. We therefore sorted peripheral blood memory CD4+ lymphocytes into four categories of CCR4 expression (Figure 4A) ▶ : CCR4neg cells were sorted to match the level of staining of the isotype control antibody, CCR4lo cells were sorted to have a level of expression similar to that of synovial fluid or BAL cells (Figure 2) ▶ , CCR4hi cells were sorted to have a level of expression similar to that of skin-homing cells (Figure 2) ▶ , and CCR4int cells were sorted from the region between the CCR4lo and CCR4hi cells and contained some overlap with both populations.
Figure 4.
High CCR4 expression is required for effective triggered adhesion and chemotaxis. Peripheral blood lymphocytes were sorted into four CCR4 expression levels: CCR4hi, CCR4int, CCR4lo, and CCR4neg (A) and compared for their ability to chemotax and adhere in response to chemokine stimulation. Compared to CCR4hi lymphocytes, lower levels of CCR4 concomitantly reduced the ability of lymphocytes to undergo rapid adhesion to ICAM-1 (B) and chemotax toward TARC (C). The low chemotactic ability of CCR4lo lymphocytes was not due to reduced TARC sensitivity as shown by a dose response (D). Data are representative of three separate donors done in triplicate.
We then tested the ability of the CCR4hi, CCR4int, CCR4lo and CCR4neg lymphocytes to rapidly adhere to ICAM-1 or migrate in response to CCR4 ligands. In TARC-induced rapid adhesion to ICAM-1, 5 the CCR4hi cells adhered most efficiently while the populations expressing lower levels of CCR4 showed a concomitant reduction in firm adhesion consistent with the level of CCR4 expression (Figure 4B) ▶ . Interestingly, the small number of cells becoming adherent in the CCR4lo sorted population could be accounted for largely by overlap with the CCR4int sorted population. All populations adhered similarly in response to SDF-1α (∼90 to 95% of input, not shown), and TARC responses were normalized to the SDF-1α response for each population. As seen for adhesion, all four populations responded similarly to SDF-1α in chemotaxis assays a (∼68 to 74% of input, not shown), and the TARC responses were also normalized to the SDF-1a response for each population. CCR4hi cells showed the best TARC response (Figure 4C) ▶ . Surprisingly, lower levels of CCR4 expression corresponded with reduced chemotaxis to TARC (Figure 4C) ▶ , similar to the pattern seen in the adhesion assay. To rule out the possibility that the optimal chemotactic concentration of TARC was different for cells bearing different levels of CCR4, we titered TARC on sorted CCR4lo and CCR4hi cells in the chemotaxis assay (Figure 4D) ▶ . We found that the difference in migratory performance between the two sorted populations could not be accounted for by a difference in relative sensitivity to TARC. Similar chemotaxis results were obtained with MDC, another ligand for CCR4 (data not shown).
Discussion
In an attempt to more fully characterize tissue-infiltrating lymphocytes in a search for tissue-specific lymphocyte specialization, we have examined the cell surface phenotype of lymphocytes directly isolated from a wide variety of tissues for comparison with their circulating counterparts. One striking finding from this work was the enormous enrichment of memory cells in non-lymphoid versus lymphoid tissues. These data strongly support classical in vivo animal work 22 indicating that naive cells have a very restricted pattern of recirculation (only secondary lymphoid organs), whereas memory cell subsets can traffic to any soft tissue in the body. A corollary to this theory is the notion (recently demonstrated in mice 23,24 ), that lymphocytes gain the ability to home to a broader array of sites after encountering their cognate ligands and differentiating into memory lymphocytes. Another important finding was that most tissue infiltrating lymphocytes display an “activated” phenotype (as assessed by expression of CD69), whereas circulating populations contained essentially no activated cells.
Tissue-Specific Adhesion Molecules
Our findings strongly support the importance of adhesion molecules classically associated with tissue-specific lymphocyte homing. 1 As reported previously, CLA+ lymphocytes, ∼20% of circulating memory T cells, were enriched to almost 100% in skin-infiltrating populations, but virtually absent from the jejunal lamina propria. 17 Conversely, α4β7+ lymphocytes, also ∼20% of circulating memory CD4+ cells, were enriched to nearly 100% in jejunal lamina propria-infiltrating populations, but absent in the skin. CLA and α4β7 expressing lymphocytes were rare in memory populations isolated from lung and tonsil, where these molecules are not thought to play a role in homing. In contrast to the skin and jejunum, SF contained CLA+ and α4β7+ memory CD4+ subpopulations in proportions similar to the blood. The SF data may reflect a reduction in homing specificity at sites of intense chronic inflammation. It is noteworthy that immunophenotypically-defined skin-homing, gut-homing, and other memory lymphocyte types are found together in synovial fluid from autoimmune arthritis cases. In contrast, naive lymphocytes are apparently excluded from this compartment. This finding may imply the existence of a previously unrecognized homing pathway that distinguishes between memory and naive cells, but does not distinguish among the various tissue-specialized memory lymphocyte subsets. This may shed light on a previous finding that intestine-derived lymphocytes are able to interact with synovial endothelium. 25 Instead of implying that autoimmune arthritis is mediated by intestine-derived lymphocytes, the finding may simply demonstrate another manifestation of the notion that any type of memory cell can potentially enter this compartment.
Expression of CCR5 and CXCR3 by Tissue-Infiltrating Lymphocytes
Our survey of lymphocytes from various human tissues has shown that the vast majority of resident tissue CD4+ cells express both CXCR3 and CCR5. Expression of these inflammatory receptors has been previously reported for tissue lymphocytes in the intestines, 7 liver, 10 brain, 9 and synovial fluid, 11 and explained as particularly important for specific homing/localization to each location. The strong expression of CCR5 and CXCR3 by lymphocytes from the wide variety of tissues shown here however suggests that these receptors are unlikely to contribute to the specificity of lymphocyte homing per se. However, their near ubiquity implies important, albeit not yet understood, roles in tissue lymphocyte homing and/or function. We and others have found that only about 25 to 30% of circulating memory CD4+ lymphocytes co-express CCR5 and CXCR3 (E.J. Kunkel and J.J. Campbell, unpublished data). 11 It is possible that tissue-infiltrating lymphocytes are recruited only from this double-positive population. However, the correlation between CD69 expression (an activation marker) and CXCR3/CCR5 expression raises the possibility that both receptors may be induced after tissue entry. In either scenario, CCR5 and CXCR3 likely facilitate the movement of lymphocytes within target tissues. 26,27 The CCR5 ligands, RANTES and MIP-1α, are known to be produced by activated dendritic cells, 28 suggesting a potential role for CCR5 in mediating co-localization of lymphocytes and dendritic cells within tissues. Alternatively, one or both of these receptors may contribute to retention of activated cells within tissues. Finally, CCR5 and/or CXCR3 up-regulation on lymphocytes in tissues may enhance the ability of these lymphocytes, once they return to the circulation, to subsequently be recruited to active sites of subacute inflammation where CCR5 and CXCR3 ligands may play a more prominent role.
Expression of CCR4 by Tissue-Infiltrating Lymphocytes
In contrast to CCR5 and CXCR3, lymphocyte expression of CCR4 was only observed on lymphocytes from a subset of non-intestinal tissues, and only at high levels within the skin (as will be discussed further below). The enriched expression of CCR4 by skin-infiltrating lymphocytes supports the model that the combined expression of CLA and CCR4 is necessary for fruitful interaction between cutaneous venules and circulating skin-homing lymphocytes. 5 Cutaneous venules are known to express E-selectin, the ligand for CLA, 29 and TARC, one of the chemokine ligands for CCR4. 5
Our finding that CCR4 is strongly expressed by lymphocytes isolated directly from DTH- or contact hypersensitivity-inflamed skin in vivo would tend to argue against the notion that CCR4 is a marker for Th2 cells, as suggested by in vitro models of polarized T cell development, 30-32 because DTH and contact hypersensitivity responses are classical**,** universally accepted models for Th1-mediated inflammation.
CCR4 Expression Levels and Function
We have previously proposed that high numbers of cell-surface chemokine receptors may be necessary to mediate adhesion triggering under shear, whereas smaller numbers of receptors per cell may be sufficient to mediate chemotaxis. 33 By this argument, only skin-infiltrating lymphocytes (those with the highest CCR4 expression) might express sufficiently high levels of CCR4 to mediate rapid adhesion-triggering under shear. 5 Lymphocytes expressing lower levels of CCR4 (as in BAL fluid) could, by the same argument, use this receptor for chemotaxis. We were therefore surprised to find that peripheral blood lymphocytes sorted for low levels of CCR4 expression (as determined by two mAbs to CCR4) responded very poorly to CCR4 ligands in both adhesion and chemotaxis assays.
There is, however, recent evidence proposing a role for CCR4 in inflammation at these sites (ie, bronchi and synovium). Bronchial epithelial cells stimulated with both tumor necrosis factor-α and interferon-γ produce large amounts of TARC, 34 and activated macrophages (found in synovial tissue) are extremely potent sources of another CCR4 ligand, MDC. 35 If the low levels of CCR4 observed on BAL and synovial fluid lymphocytes are genuine, this receptor may potentially mediate chemotaxis at these sites. CCR4 on these lymphocytes may serve to chemotactically guide lung lymphocytes to the epithelial layer once they have entered peri-bronchial tissues. Similarly, synovial lymphocytes may be guided through the tissue by using gradients of MDC secreted by resident activated macrophages. Expression of CCR4 ligands may also play roles in other aspects of immunity not related to lymphocyte localization.
Synovial lymphocytes from autoimmune arthritis patients have previously been shown to have some characteristics of skin-homing cells. 36 This has led to the proposal (especially in the case of psoriatic arthritis) that attraction of cutaneous lymphocytes to the synovium may be a component of the disease mechanism. 36 Our findings demonstrate that lymphocytes from synovial fluid do indeed contain cells of the cutaneous homing phenotype, but cells of intestinal and other homing phenotypes are present as well. Thus, cutaneous lymphocytes are not enriched in the synovial fluid above their relative numbers in normal peripheral blood. These findings could be explained by the notion that control of memory T cell homing specificity has broken down in such chronic autoimmune disorders.
Conclusions
We have examined tissue-infiltrating lymphocytes from a variety of human tissues to explore the similarities and differences among tissue-infiltrating lymphocytes in various extralymphoid sites in the body, and thus shed light on the specialization of lymphocyte subsets that mediate inflammation and immune surveillance in particular tissues. We have found that 1) Naïve CD4+ T cells are restricted to blood and lymphoid organs, and are not found in non-lymphoid organs. 2) Activated CD4+ lymphocytes (as assessed by CD69 expression) are rare to non-existent in the blood, but constitute the majority of tissue-infiltrating lymphocytes. 3) The dramatic enrichment of lymphocytes expressing the adhesion molecules CLA and α4β7 within skin and intestine, respectively, is consistent with a role for these molecules in tissue specific homing. 4) CCR5 and CXCR3 are nearly ubiquitously expressed by lymphocytes that have infiltrated non-lymphoid tissues, and are thus unlikely to contribute to the determination of tissue specificity. 5) CCR4 is expressed by CD4+ T cells from only a subset of non-intestinal tissues, and expressed at the highest, most functional levels only by skin-infiltrating CD4+ cells.
Acknowledgments
We thank Maris Handley of the Dana-Farber Flow-Cytometry facility for expert cell sorting.
Footnotes
Address reprint requests to James J. Campbell, Ph.D., Joint Program in Transfusion Medicine, Children’s Hospital, 300 Longwood Avenue, Room BD-401, Boston, MA 02115. E-mail: james.campbell@tch.harvard.edu
Supported by NIH grant AI-46784 to J.J.C. and NIH grants GM-37734, AI-47822, GM-56527 and AI-37832 and a Merit Award from the Veterans Administration to E.C.B. E.J.K. is a recipient of an Arthritis Foundation Postdoctoral Fellowship.
References
- 1.Butcher EC, Picker LJ: Lymphocyte homing and homeostasis. Science 1996, 272:60-66 [DOI] [PubMed] [Google Scholar]
- 2.Butcher EC, Williams M, Youngman K, Rott L, Briskin M: Lymphocyte trafficking and regional immunity. Adv Immunol 1999, 72:209-253 [DOI] [PubMed] [Google Scholar]
- 3.Campbell JJ, Butcher EC: Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr Opin Immunol 2000, 12:336-341 [DOI] [PubMed] [Google Scholar]
- 4.Kunkel EJ, Campbell JJ, Haraldsen G, Pan J, Boisvert J, Roberts AI, Ebert EC, Vierra MA, Goodman SC, Genovese MC, Wardlaw AJ, Greenberg HB, Parker CM, Butcher EC, Andrew DP, Agace WW: Lymphocyte CCR9 and epithelial TECK expression distinguish the small intestinal immune compartment: epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med 2000, 192:761-768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell JJ, Haraldsen G, Pan J, Rottman J, Qin S, Ponath P, Andrew DP, Warnke R, Ruffing N, Kassam N, Wu L, Butcher EC: The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature 1999, 400:776-780 [DOI] [PubMed] [Google Scholar]
- 6.Imai T, Baba M, Nishimura M, Kakizaki M, Takagi S, Yoshie O: The T cell-directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4. J Biol Chem 1997, 272:15036-15042 [DOI] [PubMed] [Google Scholar]
- 7.Agace WW, Roberts AI, Wu L, Greineder C, Ebert EC, Parker CM: Human intestinal lamina propria and intraepithelial lymphocytes express receptors specific for chemokines induced by inflammation. Eur J Immunol. 2000, 30:819-826 [DOI] [PubMed] [Google Scholar]
- 8.Mackay CR: Dual personality of memory T cells. Nature 1999, 401:659-660 [DOI] [PubMed] [Google Scholar]
- 9.Sorensen TL, Tani M, Jensen J, Pierce V, Lucchinetti C, Folcik VA, Qin S, Rottman J, Sellebjerg F, Strieter RM, Frederiksen JL, Ransohoff RM: Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J Clin Invest 1999, 103:807-815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shields PL, Morland CM, Salmon M, Qin S, Hubscher SG, Adams DH: Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J. Immunol 1999, 163:6236–6243 [PubMed]
- 11.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch AE, Moser B, Mackay CR: The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest 1998, 101:746-754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki N, Nakajima A, Yoshino S, Matsushima K, Yagita H, Okumura K: Selective accumulation of CCR5+ T lymphocytes into inflamed joints of rheumatoid arthritis. Int. Immunol 1999, 11:553–559 [DOI] [PubMed]
- 13.Berg EL, Yoshino T, Rott LS, Robinson MK, Warnock RA, Kishimoto TK, Picker LJ, Butcher EC: The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med 1991, 174:1461-1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schweighoffer T, Tanaka Y, Tidswell M, Erle DJ, Horgan KJ, Luce GE, Lazarovits AI, Buck D, Shaw S: Selective expression of integrin alpha 4 beta 7 on a subset of human CD4+ memory T cells with hallmarks of gut-trophism. J Immunol 1993, 151:717-729 [PubMed] [Google Scholar]
- 15.Hill CM, Kwon D, Jones M, Davis CB, Marmon S, Daugherty BL, DeMartino JA, Springer MS, Unutmaz D, Littman DR: The amino terminus of human CCR5 is required for its function as a receptor for diverse human and simian immunodeficiency virus envelope glycoproteins. Virology 1998, 248:357-371 [DOI] [PubMed] [Google Scholar]
- 16.Fiocchi C, Youngman K: 1997. Isolation of human intestinal mucosal mononuclear cells. Current Protocols in Immunology. Edited by JE Collogan, AM Kruisbeek, DH Margulies, EM Shevach, W Strober. New York, John Wiley & Sons, Inc., pp 7.30.1–7.30.8 [DOI] [PubMed]
- 17.Picker LJ, Treer JR, Ferguson-Darnell B, Collins PA, Bergstresser PR, Terstappen LW: Control of lymphocyte recirculation in man. II. Differential regulation of the cutaneous lymphocyte-associated antigen, a tissue-selective homing receptor for skin-homing T cells. J Immunol 1993, 150:1122-1136 [PubMed] [Google Scholar]
- 18.Santamaria Babi LF, Picker LJ, Perez Soler MT, Drzimalla K, Flohr P, Blaser K, Hauser C: Circulating allergen-reactive T cells from patients with atopic dermatitis and allergic contact dermatitis express the skin-selective homing receptor, the cutaneous lymphocyte-associated antigen. J Exp Med 1995, 181:1935–1940 [DOI] [PMC free article] [PubMed]
- 19.Williams MB, Butcher EC: Homing of naive and memory T lymphocyte subsets to Peyer’s patches, lymph nodes, and spleen. J Immunol 1997, 159:1746-1752 [PubMed] [Google Scholar]
- 20.Williams MB, Rose JR, Rott LS, Franco MA, Greenberg HB, Butcher EC: The memory B cell subset responsible for the secretory IgA response and protective humoral immunity to rotavirus expresses the intestinal homing receptor, α4β7. J Immunol 1998, 161:4227-4235 [PubMed] [Google Scholar]
- 21.Luster AD: Chemokines: chemotactic cytokines that mediate inflammation. N Engl J Med 1998, 338:436-445 [DOI] [PubMed] [Google Scholar]
- 22.Mackay CR: Homing of naive, memory, and effector lymphocytes. Curr Opin Immunol 1993, 5:423-427 [DOI] [PubMed] [Google Scholar]
- 23.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK: Visualizing the generation of memory CD4 T cells in the whole body. Nature 2001, 410:101-105 [DOI] [PubMed] [Google Scholar]
- 24.Masopust D, Vezys V, Marzo AL, Lefrancois L: Preferential localization of effector memory cells in nonlymphoid tissue. Science 2001, 291:2413-2417 [DOI] [PubMed] [Google Scholar]
- 25.Salmi M, Andrew DP, Butcher EC, Jalkanen S: Dual binding capacity of mucosal immunoblasts to mucosal and synovial endothelium in humans: dissection of the molecular mechanisms. J Exp Med 1995, 181:137-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lloyd CM, Delaney T, Nguyen T, Tian J, Martinez A, Coyle AJ, Gutierrez-Ramos JC: CC chemokine receptor (CCR)3/eotaxin is followed by CCR4/monocyte-derived chemokine in mediating pulmonary T helper lymphocyte type 2 recruitment after serial antigen challenge in vivo. J Exp Med 2000, 191:265-274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sallusto F, Kremmer E, Palermo B, Hoy A, Ponath P, Qin S, Forster R, Lipp M, Lanzavecchia A: Switch in chemokine receptor expression upon TCR stimulation reveals novel homing potential for recently activated T cells. Eur J Immunol 1999, 29:2037-2045 [DOI] [PubMed] [Google Scholar]
- 28.Sallusto F, Palermo B, Lenig D, Miettinen M, Matikainen S, Julkunen I, Forster R, Burgstahler R, Lipp M, Lanzavecchia A: Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur J Immunol 1999, 29:1617-1625 [DOI] [PubMed] [Google Scholar]
- 29.Picker LJ, Kishimoto TK, Smith CW, Warnock RA, Butcher EC: ELAM-1 is an adhesion molecule for skin-homing T cells. Nature 1991, 349:796-799 [DOI] [PubMed] [Google Scholar]
- 30.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A: Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med 1998, 187:875-883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imai T, Nagira M, Takagi S, Kakizaki M, Nishimura M, Wang J, Gray PW, Matsushima K, Yoshie O: Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Immunol 1999, 11:81-88 [DOI] [PubMed] [Google Scholar]
- 32.D’Ambrosio D, Iellem A, Bonecchi R, Mazzeo D, Sozzani S, Mantovani A, Sinigaglia F: Selective up-regulation of chemokine receptors CCR4 and CCR8 upon activation of polarized human type 2 Th cells. J Immunol 1998, 161:5111-5115 [PubMed] [Google Scholar]
- 33.Campbell JJ, Qin S, Bacon KB, Mackay CR, Butcher EC: Biology of chemokine and classical chemoattractant receptors: differential requirements for adhesion-triggering versus chemotactic responses in lymphoid cells. J Cell Biol 1996, 134:255-266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sekiya T, Miyamasu M, Imanishi M, Yamada H, Nakajima T, Yamaguchi M, Fujisawa T, Pawankar R, Sano Y, Ohta K, Ishii A, Morita Y, Yamamoto K, Matsushima K, Yoshie O, Hirai K: Inducible expression of a Th2-type CC chemokine thymus- and activation-regulated chemokine by human bronchial epithelial cells. J Immunol 2000, 165:2205-2213 [DOI] [PubMed] [Google Scholar]
- 35.Godiska R, Chantry D, Raport CJ, Sozzani S, Allavena P, Leviten D, Mantovani, A, Gray PW: Human macrophage-derived chemokine (MDC), a novel chemoattractant for monocytes, monocyte-derived dendritic cells, and natural killer cells. J Exp Med 1997, 185:1595–1604 [DOI] [PMC free article] [PubMed]
- 36.Jones SM, Dixey J, Hall ND, McHugh NJ: Expression of the cutaneous lymphocyte antigen and its counter-receptor E-selectin in the skin and joints of patients with psoriatic arthritis. Br J Rheumatol 1997, 36:748-757 [DOI] [PubMed] [Google Scholar]