Importance of Vascular Phenotype by Basic Fibroblast Growth Factor, and Influence of the Angiogenic Factors Basic Fibroblast Growth Factor/Fibroblast Growth Factor Receptor-1 and Ephrin-A1/EphA2 on Melanoma Progression (original) (raw)
Abstract
The expression of several angiogenic factors and receptors was examined in a series of vertical growth phase cutaneous melanomas using high-throughput tissue microarray technology and immunohistochemistry. The results were correlated with microvessel density, clinicopathological features, and patient survival. Expression of basic fibroblast growth factor (bFGF) was significantly associated with increased microvessel density. Also, we found an independent prognostic importance of vascular phenotype by endothelial cell expression of bFGF; cases with positive vessels had the best prognosis and these tumors revealed a low frequency of vascular invasion (14%) when compared with bFGF-negative vessels (47%). This bFGF-negative phenotype was significantly increased in metastatic lesions. Strong tumor cell expression of FLT-4, ephrin-A1, and EphA2 was associated with increased melanoma thickness, and ephrin-A1 staining was related to decreased survival (P = 0.039). Expression of EphA2 in tumor cells was associated with increased tumor cell proliferation (Ki-67 positivity), indicating possible autocrine growth stimulation. Thus, our findings indicate the presence of phenotypic diversity among tumor-associated vessels, and subgroups defined by bFGF expression may be of clinical importance. bFGF was associated with microvessel density, whereas the ephrin-A1/EphA2 pathway might also be important for tumor cell proliferation and patient survival.
Extensive vascularization must occur if a tumor mass is to exceed 1 mm in diameter. 1 The process of tumor-associated angiogenesis, which is vital also for invasion and metastatic spread, is regulated by networks of proangiogenic and anti-angiogenic molecules. 2,3 Recent studies have focused on this complex balance, and the possibility of effective anti-angiogenic treatment is presently being considered. 4,5 Microvessel density (MVD), a commonly applied estimate of tumor angiogenesis, has proved effective as a prognostic indicator in several types of malignant tumors, such as breast cancer, 6 endometrial cancer, 7 and prostate cancer, 8 whereas its importance in malignant melanoma has been more controversial. 9-14 Furthermore, new concepts such as vasculogenic mimicry 15-18 and mosaic tumor vessels, 19 as well as the impact of tumor-associated lymphangiogenesis, 20-22 are being examined.
In general, several growth factors are important for endothelial cell proliferation and migration. Vascular endothelial growth factor (VEGF) seems to have a fundamental role in tumor vessel formation, 23 and VEGF expression has been associated with increased angiogenesis in clinical 24-27 and experimental studies. 28 The VEGF receptors FLT-1 and KDR are restricted primarily to vascular endothelium, 23,29,30 although expression has also been found on tumor cells 31-33 such as malignant melanoma, 14,34-36 indicating the possibility of autocrine growth stimulation.
Other important factors for neoplastic progression and angiogenesis are the basic fibroblastic growth factor (bFGF) and its receptors, 37-39 and interleukin (IL)-8. 40-42 VEGF-C and the receptor protein FLT-4 are thought to be important growth regulators for lymphatic endothelial cells, 21,43-46 and the relative importance of lymphangiogenesis has been focused. 21,22,45 The EPH family, which is the largest subfamily of receptor tyrosine kinases, 47,48 were originally isolated with unknown ligands 49 and found to have roles in the regulation of neurons and neural crest cells. 50 The first ligand to be identified, ephrin-A1, was up-regulated in activated endothelial cells after cytokine stimulation. 51
Regarding malignant melanoma, previous studies have indicated that several angiogenic growth factors and receptors might be important, both for tumor-associated angiogenesis, and possibly also acting as autocrine or paracrine growth factors on tumor cells. 36,52-56 Increased expression of VEGF has been associated with malignant progression in melanocytic tumors, 13,57,58 and one study found that VEGF increased the proliferation of KDR-positive melanoma cells in vitro. 52 Further, bFGF and its receptor FGFR-1 are important for melanoma angiogenesis, 38 and several studies indicate that these factors might also be of importance for autocrine growth control and melanoma progression. 59-63 Studies also indicate that IL-8 can act as an autocrine factor for melanoma cells, 64 and IL-8 mRNA expression was associated with increased tumor progression in cutaneous melanoma. 65 In experimental studies, IL-8 was found to enhance invasive growth and metastatic potency of melanoma cells by various mechanisms. 41,66,67
Recently, ephrin-A1 was found to be a melanoma growth factor, 68 and it was up-regulated during melanoma progression and possibly implicated in angiogenesis. 53 Its receptor EphA2 might be important for aggressive behavior and vasculogenic mimicry properties in melanoma cell lines. 17
In our study of angiogenesis in vertical growth phase melanomas, 14 we found that MVD, as estimated by two different endothelial cell markers (F-VIII and CD105/endoglin), was an independent prognostic factor, although of only moderate strength. Most cases were positive for VEGF, but there was no strong association with MVD or survival for VEGF and its receptors. Possible autocrine loops were suggested by co-expression of VEGF and its two receptors in tumor cells, and by a significant correlation between KDR and tumor cell proliferation (Ki-67). On this background, the present study was performed to examine the importance of other angiogenic factors and some of their receptors, such as VEGF-C, VEGFR-3 (FLT-4), bFGF, FGFR-1, IL-8, ephrin-A1, and EphA2. It was of particular interest to see whether these regulators correlated with MVD and indicators of tumor growth, as well as with survival, in advanced primary melanomas. We especially focused on vascular phenotype by endothelial cell expression of bFGF. The study was performed using high-throughput tissue microarray (TMA) technique with sensitive immunohistochemistry protocols, and expression data were related to clinicopathological variables and follow-up information.
Materials and Methods
Patients
The patient series is described in detail elsewhere. 69 Briefly, 202 vertical growth phase melanomas occurring during 1981 to 1997 were included. The presence of a vertical growth phase, and the lack of a radial growth phase, ie, adjacent in situ or microinvasive component, were used as inclusion criteria for the present study. 70 In addition, 68 separate biopsies of local (skin; n = 17), regional (lymph nodes; n = 44), or distant (n = 7) metastases from 58 patients with recurrent disease were available for analyses.
Complete information on patient survival and time and cause of death was available in all 202 cases. Last date of follow-up was December 18, 1998, and median follow-up time for all survivors was 76 months (range, 13 to 210 months). During this period, 69 patients died of malignant melanoma. Clinical follow-up (with respect to recurrences) was not performed in 14 (mostly older) patients, and 21 patients were not treated with complete local excision. Thus, recurrence-free time could be studied in 167 patients.
TMA
The technique of TMA was recently introduced 71 and validated by independent studies of several tumor markers. 72,73 TMA slides were used for most markers in this study (VEGF-C, FLT-4, FGFR-1, Il-8, ephrin-A1, and EphA2), whereas bFGF was examined on standard slides. For TMA construction, 71,73 representative tumor areas were identified on hematoxylin and eosin slides. Tissue cylinders with a diameter of 0.6 mm were then punched from selected areas of the donor block and mounted into a recipient paraffin block using a custom-made precision instrument (Beecher Instruments, Silver Spring, MD). Sections of the resulting TMA blocks (5 μm) were then made by standard technique. In our experience, TMA blocks with ∼300 samples and standard sectioning gave better results than using the tape transfer technique (to support cohesion of samples) on sections from recipient blocks with larger number of cylinders. As recommended, 72 three parallel tissue cylinders were sampled from each case, and these were taken from the suprabasal areas of the primary tumors. For internal validation, TMA sections from 50 randomly selected cases were stained for Ki-67 as previously described, 69 and the labeling index (percent positive tumor cell nuclei) was determined. We found a highly significant correlation between results from TMA sections and standard slides (P < 0.0005; r = 0.69; κ = 0.76).
Immunohistochemistry
The immunohistochemical staining was performed on formalin-fixed and paraffin-embedded archival tissue (5 μm sections). In some cases, a sufficient amount of tumor tissue was not available in the remaining paraffin blocks. For bFGF, sections from 176 cases could be included and 147 primary tumors and 56 metastases were available using the TMA technique. There was no significant difference regarding MVD or survival between the 147 cases included and those without sufficient material left for the TMA technique. Regarding staining procedures, the conditions were optimized for each antibody, and some important steps in the respective protocols are summarized in Table 1 ▶ . All negative controls were negative. The staining procedures and evaluation of VEGF, FLT-1, KDR, TSP-1, p16, p53, and Ki-67 expression, as well as MVD estimates, have been described previously. 14,69 The results on these markers have been included in the present study for comparisons (see Results).
Table 1.
Immunohistochemical Staining Methods
| Antibody | Provider | Epitope retrieval | Dilution | Incubation | Controls |
|---|---|---|---|---|---|
| pAb SC-1881, VEGF-C | Santa Cruz | 4× 5 minute MW in Target retrieval solution (TRS, DAKO) | 1:75 | Overnight, RT | Pos: Adult heart muscle Neg: Blocking peptide |
| pAb SC-321, Flt-4 | Santa Cruz | 4× 5 minute MW in citrate buffer (pH = 6) at 500 W | 1:800 | 1 hour, RT | Pos: Liver Neg: Blocking peptide |
| mAb GF22, bFGF | Oncogene | 4× 5 minute MW in citrate buffer (pH = 6) at 500 W | 1:200 | Overnight, RT | Pos: Colon carcinoma Neg: Irrelevant mouse MoAb |
| pAb SC-121, Flg (FGFR-1) | Santa Cruz | 4 × 5 minute MW in citrate buffer (pH = 6) at 500 W | 1:100 | 1 hour, RT | Pos: Colon carcinoma Neg: Blocking peptide |
| pAb AF-208-NA, IL-8 | R&D | 4× 5 minute MW in Target retrieval solution (TRS, DAKO) | 1:50 | Overnight, RT* | Pos: Granulocytes Neg: Blocking peptide |
| pAb SC-911, Ephrin-A1 | Santa Cruz | 4× 5 minute MW in citrate buffer (pH = 6) at 500 W | 1:250 | 1 hour, RT | Pos: Colon carcinoma Neg: Blocking peptide |
| pAb SC-924, EphA2 | Santa Cruz | 4× 5 minute MW in citrate buffer (pH = 6) at 500 W | 1:100 | 1 hour, RT | Pos: Colon carcinoma Neg: Blocking peptide |
Staining was performed on a DAKO TechMate 500 slide processing equipment (DAKO, Copenhagen, Denmark), using the standard avidin-biotin method. Finally, the peroxidase was localized by the 3-amino-9-ethylcarbazole-peroxidase reaction with Harris hematoxylin as counterstain.
To control for the possibility of misinterpreting bFGF-negative stromal cells as endothelial cells in cases with uncertain morphology, sections from 10 cases were double stained for bFGF and Factor-VIII. An indirect/indirect simultaneous method was used. bFGF (1:50) and Factor-VIII (pAb A-082, 1:800; DAKO) was incubated overnight at room temperature, after epitope retrieval by both proteinase K and microwave treatment. Factor-VIII was detected by horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (P0448, DAKO) at 1:50 for 30 minutes followed by 3-amino-9-ethylcarbazole for 10 minutes. bFGF was detected by a biotinylated goat anti-mouse secondary antibody (E0433, DAKO), followed by a 30-minute incubation with a streptavidin/biotinylated alcalic phosphatase (AP) complex (K0391, DAKO), and 20 minutes with Fast blue as chromogen for AP.
Evaluation of Staining Results
For all markers, both staining intensity and positive area were recorded. A staining index (values 0 to 9), obtained as a product of staining intensity (0 to 3) and proportion of immunopositive cells of interest (≤10% = 1, 10 to 50% = 2, >50% = 3), was calculated. For bFGF, both tumor cell expression (staining index) and staining in tumor-associated endothelial cells (absent or present) was determined. For other markers, only tumor cell expression was evaluated on the TMA sections. For statistical purposes, cut points for continuous variables and staining index categories were based on the distribution of the values.
Statistics
Analyses were performed using the statistical package SPSS. 74 Associations between different categorical variables were assessed by Pearson’s chi-square test. Continuous variables not following the normal distribution were compared between two or more groups using the Mann-Whitney U or Kruskal-Wallis H tests. Wilcoxon signed ranks test was used to compare related samples. Univariate analyses of time to death because of malignant melanoma or time to recurrence (recurrence-free survival) were performed using the product-limit procedure (Kaplan-Meier method), with date of histological diagnosis as the starting point. Patients who died of other causes were censored at the time of death. Differences between categories were tested by the log-rank test. The influence of covariates on patient survival was analyzed by the proportional hazards method, 75 including all variables with a P value ≤0.15 in univariate analyses, and tested by the likelihood ratio (l-ratio) test. Model assumptions were tested by log-minus-log plots, and significant variables were tested for interactions. Estimated hazard ratio, 95% CI for hazard ratio, and P values are given in the tables. Prognostic information on standard variables, which has been presented elsewhere, 14 was included for comparison in multivariate analyses (see Results).
Results
Expression results for each individual marker were recorded, with reference to staining pattern, ie, cytoplasmic or nuclear staining signals, and intensity and positive area of expression (staining index). Also, results from our previous study on VEGF, VEGFR-1 (FLT-1), VEGFR-2 (FLK-1/KDR), p16, p53, Ki-67, TSP-1, and MVD were included for comparison. 14 Table 2 ▶ summarizes the associations between the angiogenic markers studied and MVD, proliferative rate, and tumor thickness.
Table 2.
Microvessel Density (MVD), Proliferative Rate (Ki-67), and Tumor Thickness Related to Angiogenic Markers in Patients with Vertical Growth Phase Melanoma
| Marker | No | MVD* | _P_† | Ki-67 | _P_† | Tumor thickness | _P_† |
|---|---|---|---|---|---|---|---|
| VEGF-C‡ | |||||||
| Weak/absent | 42 | 118 | 0.24 | 31% | 0.72 | 4.2 mm | 0.33 |
| Moderate/strong | 105 | 125 | 28% | 3.7 mm | |||
| FLT-4‡ | |||||||
| Weak/absent | 46 | 125 | 0.77 | 30% | 0.41 | 3.4 mm§ | 0.62 |
| Moderate/strong | 99 | 125 | 29% | 4.0 mm | |||
| bFGF‡- tumor cells | |||||||
| Weak/absent | 124 | 118 | 0.022 | 28% | 0.17 | 3.8 mm | 0.91 |
| Moderate/strong | 52 | 138 | 27% | 3.3 mm | |||
| bFGF¶- endothelium | |||||||
| Absent | 38 | 115 | 0.045 | 28% | 0.82 | 4.3 mm | 0.30 |
| Present | 138 | 128 | 27% | 3.7 mm | |||
| FGFR-1‡ | |||||||
| Weak/absent | 54 | 121 | 0.44 | 32% | 0.18 | 4.2 mm | 0.060 |
| Moderate/strong | 91 | 125 | 26% | 3.6 mm | |||
| IL-8‡ | |||||||
| Weak/absent | 49 | 125 | 0.57 | 34% | 0.46 | 3.8 mm | 0.52 |
| Moderate/strong | 95 | 125 | 28% | 4.0 mm | |||
| Ephrin-A1∥ | |||||||
| Weak/moderate | 123 | 121 | 0.077 | 29% | 0.64 | 3.7 mm | 0.017 |
| Strong | 23 | 138 | 26% | 4.8 mm | |||
| EphA2∥ | |||||||
| Weak/moderate | 122 | 125 | 0.87 | 28% | 0.049 | 3.7 mm | 0.066 |
| Strong | 23 | 125 | 37% | 4.8 mm |
VEGF-C
All cases showed some tumor cell positivity for VEGF-C, and the staining pattern was predominantly cytoplasmic, with some nuclear reactivity in most cases. Low-grade staining (index < 4) was present in 28.6% of the cases, whereas 29 cases (19.7%) revealed strong staining (index = 9) (Figure 1a) ▶ .
Figure 1.
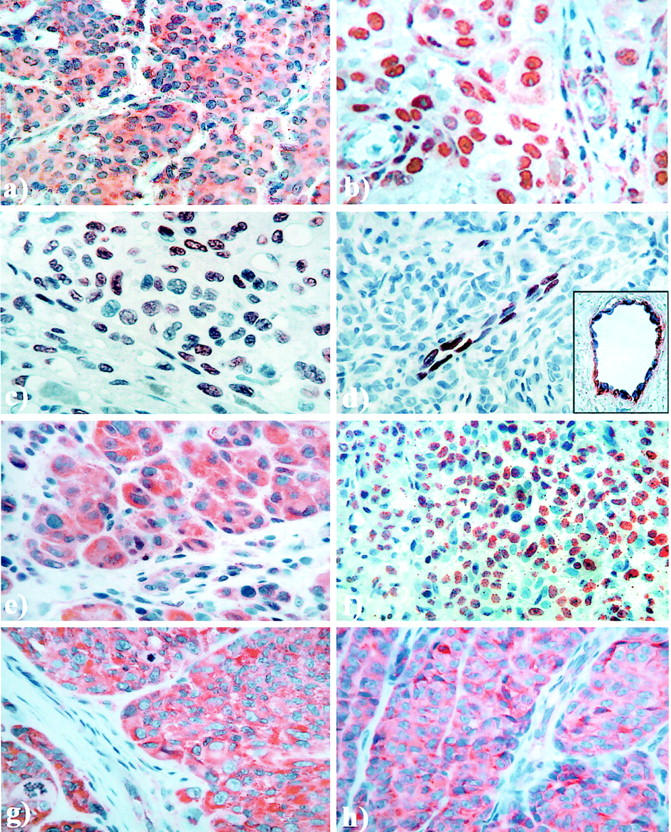
Immunohistochemical staining pattern of VEGF-C (a), VEGF-C receptor FLT-4 (b), bFGF in tumor cells (c), and bFGF in tumor-associated endothelial cells (d); inset, double staining of bFGF (Fast Blue) and Factor-VIII (3-amino-9-ethylcarbazole, red) in tumor-associated endothelial cells, FGFR-1 (e), interleukin-8 (f), ephrin-A1 (g), and ephrin receptor EphA2 (h). Original magnifications, ∼×400.
There was no significant association with MVD. Increased staining of VEGF-C (low grade versus high grade) was associated with reduced frequency of vascular invasion (P = 0.010). Also, a significant co-expression was found between VEGF-C expression and staining for FLT-1 (P = 0.009) and KDR (P = 0.04) in tumor cells.
FLT-4
Staining for FLT-4 was found in all tumors, and most cases showed mixed cytoplasmic and nuclear staining. Low-grade positivity (index < 4) was observed in 31.7%, whereas strong expression (index = 9) was observed in 20 cases (13.8%). Looking at nuclear FLT-4 staining only, 11.7% of the cases were negative, whereas low-grade positivity (index < 4) was found in 49.7% of the tumors. Strong nuclear expression (index = 9) was found in 14.5% of the cases (Figure 1b) ▶ .
Strong FLT-4 expression (index = 9) was significantly associated with increased histological tumor thickness (P = 0.043, Mann Whitney test), as well as with histological tumor ulceration (P = 0.014). FLT-4 revealed no significant association with VEGF-C; in contrast, VEGF expression was related to FLT-4 staining (P = 0.021). Regarding nuclear staining, this was significantly associated with expression of ephrin-A1 (P = <0.0005), FGFR-1 (P = 0.007), and tumor ulceration (P = 0.001).
bFGF
Tumor cell staining for bFGF was negative or showed only minimal reactivity in 70.5% of all examined cases, whereas 29.5% of the tumors were positive and showed distinct nuclear staining. Also, 6.8% showed moderate to strong expression (index 6 to 9) (Figure 1c) ▶ .
bFGF expression in tumor cells showed a significant association with increased MVD, when cases with no or minimal staining (index 0 to 1) were compared with the others (P = 0.022, Mann Whitney test). Positive cases showed increased MVD, 138 versus 118 mm−2. This relationship was especially evident in the subgroup of thick melanomas, ie, greater than median value 3.55 mm (P = 0.023, Mann Whitney test), as well as in tumors without microscopic ulceration (P = 0.022). Cases with co-expression of bFGF (index > 1) and VEGF (index > 4) showed increased MVD (P = 0.019, Mann Whitney test) and tumor cell proliferation (P = 0.030, Mann Whitney test). Lack of p16 staining showed a significant association with increased expression of bFGF (P = 0.047).
Expression of bFGF in tumor-associated endothelial cells was found in 78.4% of the cases, whereas 21.6% were negative (Figure 1d) ▶ . Double staining for bFGF and Factor-VIII confirmed the presence and absence of bFGF staining related to endothelial cells (Figure 1d ▶ , inset). Positive cases showed significantly increased MVD, 128 versus 115 mm−2 in negative tumors (P = 0.04, Mann Whitney test). There was also a significant association between expression of bFGF in endothelial cells and tumor cells (P = 0.012). In endothelial cells, bFGFand KDR were significantly co-expressed (P = 0.019), and positive endothelial cell staining of bFGF was significantly associated with FGFR-1 expression in tumor cells (P = 0.022). Also, bFGF staining in endothelial cells was inversely associated with vascular invasion; negative cases showed 47% vascular invasion, compared with 14% in positive cases (P < 0.0005).
FGF Receptor (FGFR-1)
Five cases were completely negative, whereas the rest showed various degrees of positive cytoplasmic staining in the tumor cells. Some positive nuclei were observed in a few cases, and nuclear staining was also found in some stromal cells. Low-grade expression (index < 4) was present in 37.2% of the cases, whereas 17 tumors (11.7%) revealed strong expression (index = 9) (Figure 1e) ▶ .
There was no simple association with MVD. However, co-expression of FGFR-1 and bFGF in tumor cells was associated with increased MVD (P = 0.021, Mann Whitney test).
IL-8
All cases except one tumor showed some positive staining, which was granular and both cytoplasmic and nuclear. Low-grade expression (index < 4) was found in 34.0% of the cases. Strong staining (index = 9) was present in eight cases (5.6%) (Figure 1f) ▶ .
No association with MVD was found. Increased expression was significantly associated with tumors on the head/neck area and the extremities, when compared with the trunk (P = 0.002). Also, females revealed significant stronger expression (P = 0.011). Other associations were not found.
Ephrin-A1
All cases showed some tumor cell staining of ephrin-A1, although it varied considerably. The positivity was mainly cytoplasmic. Low-grade staining (index < 4) was present in 39.0% of the cases, whereas the rest revealed stronger expression. Twenty-three cases (15.8%) showed the strongest staining (index = 9) (Figure 1g) ▶ .
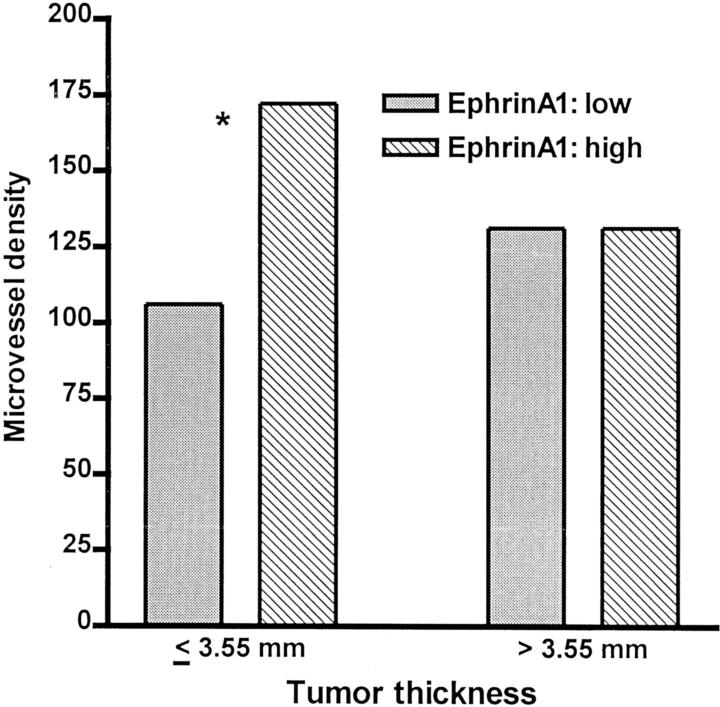
The cases with strong expression (index = 9) were significantly associated with increased histological thickness (P = 0.017, Mann Whitney test), advanced Clark’s level of invasion (P = 0.004) and histological tumor ulceration (P = 0.006). In thin melanomas (below median value, 3.55 mm), there was a significant relation between ephrin-A1 expression and MVD (P = 0.013, Mann Whitney test; Figure 2 ▶ ).
Figure 2.
The correlation between ephrin-A1 and MVD in subgroups according to median tumor thickness. *, The difference is statistically significant (Mann-Whitney, P = 0.013).
Ephrin Receptor A2 (EphA2)
All cases except one tumor showed some positive tumor cell staining for this marker, mainly in the cytoplasm. Low-grade expression (index < 4) was found in 37.9% of the cases. Twenty-three cases (15.9%) showed the strongest staining (index = 9) (Figure 1h) ▶ . There was also a significant co-expression between ephrin-A1 and its receptor EphA2 (index < 4 versus the rest, for both markers; P = 0.007).
The cases with strong expression (index = 9) tended to be associated with increased histological thickness (P = 0.066, Mann Whitney test), and histological tumor ulceration (P = 0.009). Also, there was a significant association between this strong expression and proliferation in tumor cells, as estimated by Ki-67 expression (P = 0.049, Mann Whitney test).
Expression in Metastases
Using pairwise testing (Wilcoxon signed ranks test), we found that expression of VEGF-C tended to be increased in the metastases when compared with corresponding primary tumors (P = 0.07), whereas the staining of FLT-4 was significantly increased (P < 0.0005). Expression of bFGF was not different, whereas FGFR-1 was increased (P = 0.005). However, the staining of bFGF in tumor-associated endothelial cells was found to be significantly decreased (P = 0.009). Also, there was a significant association between bFGF expression in endothelial cells and reduced tumor cell proliferation (Ki-67) in metastases (P = 0.03, Mann Whitney test). The other angiogenic factors (IL-8, ephrin-A1, EphA2) showed no significant differences in expression between primary tumors and metastatic lesions.
Survival Analyses
A nonsignificant tendency between increased bFGF expression and reduced survival was observed (P = 0.12, log rank test). In contrast, lack of bFGF expression in tumor-associated endothelial cells was associated with a significantly reduced survival (P = 0.012, log rank test; Figure 3A ▶ ); cases with bFGF+ vessels had a 59% 10-year survival, compared with 35% for patients with the bFGF− vascular phenotype. Figure 3B ▶ shows the estimated survival curves for subgroups defined by combinations of endothelial cell bFGF expression and MVD. The cases with strong ephrin-A1 expression showed significantly reduced survival (P = 0.04, log rank test; Figure 3C ▶ ), whereas no significant survival differences were present for EphA2. No survival differences were found for VEGF-C, FLT-4, FGFR-1, or IL-8.
Figure 3.
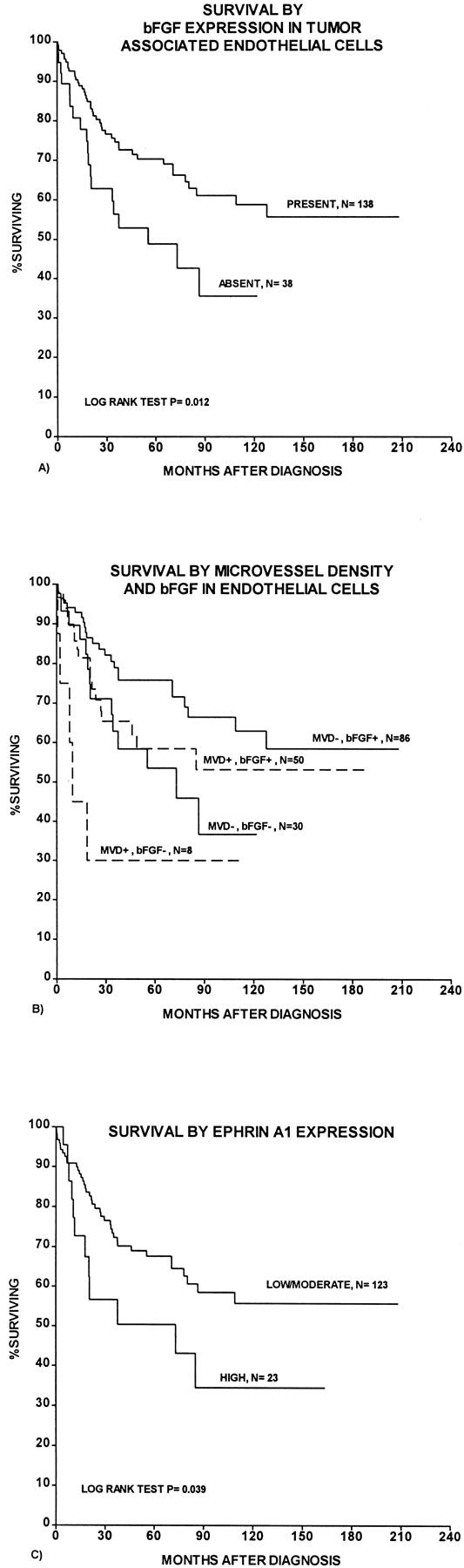
Survival curves were estimated according to the Kaplan-Meier method with death because of melanoma as end point. A: Survival by bFGF expression in tumor-associated endothelial cells. B: Survival by combinations of vascular phenotype by bFGF expression and MVD (MVD+, MVD >67th percentile; MVD−, MVD ≤67th percentile). C: Survival by ephrin-A1 expression.
In multivariate analysis of patient survival (Cox’ proportional hazards method), including tumor variables with significant impact on survival in univariate analyses (tumor thickness, Clark’s level of invasion, tumor ulceration, vascular invasion, tumor cell proliferation by Ki-67 expression), both endothelial staining of bFGF (P = 0.02, l-ratio test) and MVD (P = 0.03, l-ratio test) were found to be of independent prognostic importance, in addition to Clark’s level of invasion, tumor ulceration, and tumor cell proliferation (Table 3) ▶ . Estimates of hazard ratio showed an equally strong influence in the final model of these two angiogenesis related variables. Prognostic information on standard variables has been presented elsewhere. 14
Table 3.
Multivariate Survival Analysis of Tumor-Associated Characteristics, According to the Proportional Hazards Method for Patients with Vertical Growth Phase Melanoma, Using Death from Melanoma as End Point
| Variable | Categories | n | HR* | P value† |
|---|---|---|---|---|
| Level of invasion (Clark) | II, III, IV | 138 | 1 | |
| V | 33 | 3.3 | <0.0001 | |
| Tumor ulceration | Absent | 96 | 1 | |
| Present | 75 | 1.9 | 0.02 | |
| Ki-67 expression | Low‡ | 42 | 1 | |
| High | 129 | 2.6 | 0.03 | |
| bFGF, endothelial cells | Absent | 36 | 2.0 | |
| Present | 135 | 1 | 0.02 | |
| MVD | Low§ | 115 | 1 | |
| High | 56 | 1.9 | 0.03 |
Discussion
We previously found a more than 10-fold difference between low-grade and high-grade angiogenesis in vertical growth phase cutaneous melanoma, 76 and MVD was a significant prognostic factor of moderate strength in multivariate analysis. 14 However, no marked associations between angiogenesis and expression of VEGF and its receptors FLT-1 and KDR were found. In the present study, a panel of other angiogenic factors and some of their receptors have been examined with reference to MVD, tumor cell proliferation, and survival in vertical growth phase melanoma. As the only factor, bFGF showed a clear association with MVD in these tumors. Our findings support previous experimental data that bFGF is an important factor for melanoma angiogenesis. 38,77 However, melanoma vascularity is not a very strong prognostic factor, 14 and expression of bFGF was not significantly associated with survival in this study, in accordance with others. 78 Still, studies indicate that bFGF is important for melanoma growth and progression, and this is probably related to autocrine growth stimulation 59-63,79 in addition to angiogenesis.
Endothelial cell expression of bFGF in tumor vessels showed a significant association with improved patient survival, when compared with cases having a bFGF-negative vascular phenotype. Other studies suggest that bFGF might influence not only endothelial cell proliferation, invasion, and migration, but also vascular morphogenesis. 80 Thus, bFGF and Angiopoietin-1, which promotes vessel maturation and integrity, was found to be co-expressed in MCF-7 tumor cells. 81 The bFGF-negative vascular phenotype was associated with a strikingly increased frequency of vascular invasion, supporting a functional significance of bFGF expression in tumor vessels. Further, lack of bFGF expression in endothelial cells showed an independent prognostic impact in multivariate analysis of patient survival, when compared with MVD, which is by far the most commonly applied indicator of tumor-associated angiogenesis in clinical studies. A similar inverse relation between vessel bFGF expression and metastasis has also been reported in non-small cell lung cancers. 82 The proportion of this aggressive bFGF-negative vascular phenotype was increased in metastases, further supporting its importance in melanoma progression. Our findings indicate the presence of phenotypic diversity among tumor-associated vessels, and different subgroups or vascular differentiation grades defined by endothelial cell expression patterns may be of clinical importance.
There was no strong influence of VEGF-C, FLT-4, IL-8, ephrin-A1, and its receptor EphA2 on melanoma angiogenesis, as estimated by MVD. This lack of association with most angiogenic factors is in line with our recent findings that VEGF and its receptors FLT-1 and KDR showed no marked relationship with angiogenesis and survival, 14 and the same has been found for other tumors such as breast cancer. 83 Previously, VEGF has been associated with malignant progression in melanocytic lesions. 13,57,58 In our recent study, VEGF was up-regulated in smaller tumors, whereas weak expression was found in thicker and more angiogenic lesions, 14 suggesting that a lower baseline level of VEGF might be sufficient for an established vascular system, with VEGF acting as a survival factor for endothelial cells. 84 In these advanced primary melanomas, the impact of molecular cross-talk and synergistic effects of several factors such as VEGFs, bFGF, IL-8, and ephrins, might possibly be more important for angiogenesis and survival than single growth factors, and different regulatory subgroups may be present. This is supported by experimental data indicating that vessel formation in poorly angiogenic melanomas is promoted solely by VEGF, whereas multiple factors are involved in highly vascularized melanomas. 55 bFGF was most clearly associated with increased angiogenesis (MVD) in the thicker tumors.
VEGF-C, which is thought to be a relatively specific lymphatic endothelial growth factor, 85 showed strong tumor cell expression in ∼20% of the primary melanomas, and only few tumors were completely negative. Expression of VEGF-C has been found in other tumors like breast cancer, 43 and its up-regulation in tumor cells may act as an angiogenic factor for blood vessels. 86 Similarly, the VEGF-C receptor FLT-4, which was considered a predominantly lymphatic marker, 87 has been found on blood vessels. 86 This indicates that VEGF-C and FLT-4 expression may promote tumor-associated angiogenesis, although a direct relationship to MVD was not found in the present study. Significant associations between VEGF-C and FLT-1 or KDR, as well as between tumor cell expression of VEGF and FLT-4, further support the functional importance of angiogenic cross-talk and cross-over interactions in the VEGF family of multiple ligands and receptors.
Several of the angiogenic factors were related to indicators of tumor growth. Increasing evidence supports the importance of VEGF receptors in nonendothelial cell types 31,32,35,36,88-90 , and functional evidence showing increased proliferation of KDR-positive melanoma cells has been published. 52 Growth stimulation by possible autocrine loops was suggested by co-expression of VEGF and its two receptors in tumor cells, and by a significant correlation between KDR and tumor cell proliferation (Ki-67). 14 In our study, tumor thickness was related to expression of ephrin-A1 and EphA2. The association between EphA2 receptors on melanoma cells and increased tumor cell proliferation, as indicated by Ki-67 expression, support the existence of autocrine or paracrine growth stimulation. Whereas ephrin-A1 showed an angiogenic effect among thinner tumors, the relationship between EphA2 and tumor cell proliferation indicates a dual role for the ephrin-A1/EphA2 system. Thus, our previous 14 and present data suggest that certain factors might switch from angiogenic action to autocrine growth stimulation of tumor cells in these advanced primary melanomas. As suggested by the recent vasculogenic mimicry concept, the appearance of endothelial cell markers on tumor cells might indicate a reversion to more embryonic-like phenotypes, possibly also promoting the formation of tubular structures by tumor cells and thereby enhancing perfusion by extra-angiogenic networks. 16,17 These properties seem to indicate increasing aggressiveness in melanoma cell lines as well as in human tumors. 91
The presence of tumor ulceration was significantly associated with increased expression of angiogenic factors such as FLT-4, ephrin-A1, and EphA2, indicating that ulceration might co-activate these regulators. We previously found that the relationship between level of TSP-1 expression and MVD was also different in ulcerated and nonulcerated tumors, 14 supporting a possible interaction with angiogenic factors associated with ulceration. Alternatively, these factors might stimulate tumor growth and indirectly increase the risk for microscopic ulceration.
In conclusion, we found that vascular phenotype by endothelial cell expression of bFGF showed a significant association with patient survival in this series of human vertical growth phase melanomas of the skin. Cases with bFGF+ vessels had the best prognosis, and these tumors also revealed a strikingly low frequency of vascular invasion (14%), when compared with bFGF− vessels (47%). Expression of bFGF in tumor cells was significantly associated with MVD, whereas tumor cell bFGF was in itself not a significant prognostic factor. In multivariate survival analyses, both vascular phenotype by bFGF status and MVD had an independent prognostic importance. Also, the expression of receptors such as EphA2 and KDR on tumor cells, being associated with increased tumor cell proliferation (Ki-67), indicate a regulatory role of autocrine or paracrine growth stimulation.
Acknowledgments
We thank Mrs. Gerd Lillian Hallseth and Mr. Bendik Nordanger for excellent technical assistance.
Footnotes
Address reprint requests to Lars A. Akslen M.D., Ph.D., Department of Pathology, The Gade Institute, Haukeland University Hospital, N-5021 Bergen, Norway. E-mail: lars.akslen@gades.uib.no.
Supported by contract grant 94070 from the Norwegian Cancer Society.
References
- 1.Folkman J: Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995, 1:27-31 [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Folkman J: Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996, 86:353-364 [DOI] [PubMed] [Google Scholar]
- 3.Fidler IJ: Angiogenesis and cancer metastasis. Cancer J Sci Am 2000, 6(Suppl 2):S134-S141 [PubMed] [Google Scholar]
- 4.Ferrara N, Alitalo K: Clinical applications of angiogenic growth factors and their inhibitors. Nat Med 1999, 5:1359-1364 [DOI] [PubMed] [Google Scholar]
- 5.Burke PA, DeNardo SJ: Antiangiogenic agents and their promising potential in combined therapy. Crit Rev Oncol Hematol 2001, 39:155-171 [DOI] [PubMed] [Google Scholar]
- 6.Weidner N, Semple JP, Welch WR, Folkman J: Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med 1991, 324:1-8 [DOI] [PubMed] [Google Scholar]
- 7.Salvesen HB, Iversen OE, Akslen LA: Prognostic significance of angiogenesis and Ki-67, p53, and p21 expression: a population-based endometrial carcinoma study. J Clin Oncol 1999, 17:1382-1390 [DOI] [PubMed] [Google Scholar]
- 8.Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J: Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol 1993, 143:401-409 [PMC free article] [PubMed] [Google Scholar]
- 9.Barnhill RL, Fandrey K, Levy MA, Mihm Jr MC, Hyman B: Angiogenesis and tumor progression of melanoma. Quantification of vascularity in melanocytic nevi and cutaneous malignant melanoma. Lab Invest 1992, 67:331–337 [PubMed]
- 10.Ribatti D, Vacca A, Palma W, Lospalluti M, Dammacco F: Angiogenesis during tumor progression in human malignant melanoma. EXS 1992, 61:415-420 [DOI] [PubMed] [Google Scholar]
- 11.Graham CH, Rivers J, Kerbel RS, Stankiewicz KS, White WL: Extent of vascularization as a prognostic indicator in thin (<0.76 mm) malignant melanomas. Am J Pathol 1994, 145:510-514 [PMC free article] [PubMed] [Google Scholar]
- 12.Busam KJ, Berwick M, Blessing K, Fandrey K, Kang S, Karaoli T, Fine J, Cochran AJ, White WL, Rivers J: Tumor vascularity is not a prognostic factor for malignant melanoma of the skin. Am J Pathol 1995, 147:1049-1056 [PMC free article] [PubMed] [Google Scholar]
- 13.Marcoval J, Moreno A, Graells J, Vidal A, Escriba JM, Garcia Ramirez M, Fabra A: Angiogenesis and malignant melanoma. Angiogenesis is related to the development of vertical (tumorigenic) growth phase. J Cutan Pathol 1997, 24:212–218 [DOI] [PubMed]
- 14.Straume O, Akslen LA: Expression of vascular endothelial growth factor, its receptors (flt-1, kdr) and tsp-1 related to microvessel density and patient outcome in vertical growth phase melanomas. Am J Pathol 2001, 159:223-235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, Trent JM, Meltzer PS, Hendrix MJ: Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol 1999, 155:739-752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendrix MJ, Seftor EA, Meltzer PS, Gardner LM, Hess AR, Kirschmann DA, Schatteman GC, Seftor RE: Expression and functional significance of VE-cadherin in aggressive human melanoma cells: role in vasculogenic mimicry. Proc Natl Acad Sci USA 2001, 98:8018-8023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hess AR, Seftor EA, Gardner LM, Carles-Kinch K, Schneider GB, Seftor RE, Kinch MS, Hendrix MJ: Molecular regulation of tumor cell vasculogenic mimicry by tyrosine phosphorylation: role of epithelial cell kinase (Eck/EphA2). Cancer Res 2001, 61:3250-3255 [PubMed] [Google Scholar]
- 18.Warso MA, Maniotis AJ, Chen X, Majumdar D, Patel MK, Shilkaitis A, Gupta TK, Folberg R: Prognostic significance of periodic acid-Schiff-positive patterns in primary cutaneous melanoma. Clin Cancer Res 2001, 7:473-477 [PubMed] [Google Scholar]
- 19.Chang YS, di Tomaso E, McDonald DM, Jones R, Jain RK, Munn LL: Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci USA 2000, 97:14608-14613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Achen MG, Williams RA, Minekus MP, Thornton GE, Stenvers K, Rogers PA, Lederman F, Roufail S, Stacker SA: Localization of vascular endothelial growth factor-D in malignant melanoma suggests a role in tumour angiogenesis. J Pathol 2001, 193:147-154 [DOI] [PubMed] [Google Scholar]
- 21.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M: Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med 2001, 7:192-198 [DOI] [PubMed] [Google Scholar]
- 22.Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa Si S, Kubo H, Achen MG: VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med 2001, 7:186–191 [DOI] [PubMed]
- 23.Ferrara N: Molecular and biological properties of vascular endothelial growth factor. J Mol Med 1999, 77:527-543 [DOI] [PubMed] [Google Scholar]
- 24.Guidi AJ, Schnitt SJ, Fischer L, Tognazzi K, Harris JR, Dvorak HF, Brown LF: Vascular permeability factor (vascular endothelial growth factor) expression and angiogenesis in patients with ductal carcinoma in situ of the breast. Cancer 1997, 80:1945-1953 [DOI] [PubMed] [Google Scholar]
- 25.Kang SM, Maeda K, Onoda N, Chung YS, Nakata B, Nishiguchi Y, Sowa M: Combined analysis of p53 and vascular endothelial growth factor expression in colorectal carcinoma for determination of tumor vascularity and liver metastasis. Int J Cancer 1997, 74:502-507 [DOI] [PubMed] [Google Scholar]
- 26.Takahashi Y, Bucana CD, Cleary KR, Ellis LM: p53, vessel count, and vascular endothelial growth factor expression in human colon cancer. Int J Cancer 1998, 79:34-38 [DOI] [PubMed] [Google Scholar]
- 27.Fontanini G, Boldrini L, Calcinai A, Chine S, Lucchi M, Mussi A, Angeletti CA, Basolo F, Bevilacqua G: Thrombospondins I and II messenger RNA expression in lung carcinoma: relationship with p53 alterations, angiogenic growth factors, and vascular density. Clin Cancer Res 1999, 5:155-161 [PubMed] [Google Scholar]
- 28.Claffey KP, Brown LF, del Aguila LF, Tognazzi K, Yeo KT, Manseau EJ, Dvorak HF: Expression of vascular permeability factor/vascular endothelial growth factor by melanoma cells increases tumor growth, angiogenesis, and experimental metastasis. Cancer Res 1996, 56:172-181 [PubMed] [Google Scholar]
- 29.de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT: The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science 1992, 255:989-991 [DOI] [PubMed] [Google Scholar]
- 30.Terman BI, Dougher-Vermazen M, Carrion ME, Dimitrov D, Armellino DC, Gospodarowicz D, Bohlen P: Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun 1992, 187:1579-1586 [DOI] [PubMed] [Google Scholar]
- 31.Boocock CA, Charnock-Jones DS, Sharkey AM, McLaren J, Barker PJ, Wright KA, Twentyman PR, Smith SK: Expression of vascular endothelial growth factor and its receptors flt and KDR in ovarian carcinoma. J Natl Cancer Inst 1995, 87:506-516 [DOI] [PubMed] [Google Scholar]
- 32.de Jong JS, van Diest PJ, van der Valk P, Baak JP: Expression of growth factors, growth inhibiting factors, and their receptors in invasive breast cancer. I: An inventory in search of autocrine and paracrine loops. J Pathol 1998, 184:44–52 [DOI] [PubMed]
- 33.Bunone G, Vigneri P, Mariani L, Buto S, Collini P, Pilotti S, Pierotti MA, Bongarzone I: Expression of angiogenesis stimulators and inhibitors in human thyroid tumors and correlation with clinical pathological features. Am J Pathol 1999, 155:1967-1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gitay-Goren H, Halaban R, Neufeld G: Human melanoma cells but not normal melanocytes express vascular endothelial growth factor receptors. Biochem Biophys Res Commun 1993, 190:702-708 [DOI] [PubMed] [Google Scholar]
- 35.Stitt AW, Simpson DA, Boocock C, Gardiner TA, Murphy GM, Archer DB: Expression of vascular endothelial growth factor (VEGF) and its receptors is regulated in eyes with intra-ocular tumours. J Pathol 1998, 186:306-312 [DOI] [PubMed] [Google Scholar]
- 36.Graeven U, Fiedler W, Karpinski S, Ergun S, Kilic N, Rodeck U, Schmiegel W, Hossfeld DK: Melanoma-associated expression of vascular endothelial growth factor and its receptors FLT-1 and KDR. J Cancer Res Clin Oncol 1999, 125:621-629 [DOI] [PubMed] [Google Scholar]
- 37.Rak J, Kerbel RS: bFGF and tumor angiogenesis—back in the limelight? Nat Med 1997, 3:1083-1084 [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Becker D: Antisense targeting of basic fibroblast growth factor and fibroblast growth factor receptor-1 in human melanomas blocks intratumoral angiogenesis and tumor growth. Nat Med 1997, 3:887-893 [DOI] [PubMed] [Google Scholar]
- 39.Compagni A, Wilgenbus P, Impagnatiello MA, Cotten M, Christofori G: Fibroblast growth factors are required for efficient tumor angiogenesis. Cancer Res 2000, 60:7163-7169 [PubMed] [Google Scholar]
- 40.Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM: Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 1992, 258:1798-1801 [DOI] [PubMed] [Google Scholar]
- 41.Luca M, Huang S, Gershenwald JE, Singh RK, Reich R, Bar-Eli M: Expression of interleukin-8 by human melanoma cells up-regulates MMP-2 activity and increases tumor growth and metastasis. Am J Pathol 1997, 151:1105-1113 [PMC free article] [PubMed] [Google Scholar]
- 42.Bar-Eli M: Role of interleukin-8 in tumor growth and metastasis of human melanoma. Pathobiology 1999, 67:12-18 [DOI] [PubMed] [Google Scholar]
- 43.Salven P, Lymboussaki A, Heikkila P, Jaaskela-Saari H, Enholm B, Aase K, von Euler G, Eriksson U, Alitalo K, Joensuu H: Vascular endothelial growth factors VEGF-B and VEGF-C are expressed in human tumors. Am J Pathol 1998, 153:103-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsurusaki T, Kanda S, Sakai H, Kanetake H, Saito Y, Alitalo K, Koji T: Vascular endothelial growth factor-C expression in human prostatic carcinoma and its relationship to lymph node metastasis. Br J Cancer 1999, 80:309-313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Birner P, Schindl M, Obermair A, Breitenecker G, Kowalski H, Oberhuber G: Lymphatic microvessel density as a novel prognostic factor in early-stage invasive cervical cancer. Int J Cancer 2001, 95:29-33 [DOI] [PubMed] [Google Scholar]
- 46.Karpanen T, Egeblad M, Karkkainen MJ, Kubo H, Yla-Herttuala S, Jaattela M, Alitalo K: Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res 2001, 61:1786-1790 [PubMed] [Google Scholar]
- 47.Drescher U: The Eph family in the patterning of neural development. Curr Biol 1997, 7:R799-R807 [DOI] [PubMed] [Google Scholar]
- 48.Pasquale EB: The Eph family of receptors. Curr Opin Cell Biol 1997, 9:608-615 [DOI] [PubMed] [Google Scholar]
- 49.Pandey A, Lindberg RA, Dixit VM: Cell signalling. Receptor orphans find a family. Curr Biol 1995, 5:986-989 [DOI] [PubMed] [Google Scholar]
- 50.Zisch AH, Stallcup WB, Chong LD, Dahlin-Huppe K, Voshol J, Schachner M, Pasquale EB: Tyrosine phosphorylation of L1 family adhesion molecules: implication of the Eph kinase Cek5. J Neurosci Res 1997, 47:655-665 [DOI] [PubMed] [Google Scholar]
- 51.Holzman LB, Marks RM, Dixit VM: A novel immediate-early response gene of endothelium is induced by cytokines and encodes a secreted protein. Mol Cell Biol 1990, 10:5830-5838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu B, Earl HM, Baban D, Shoaibi M, Fabra A, Kerr DJ, Seymour LW: Melanoma cell lines express VEGF receptor KDR and respond to exogenously added VEGF. Biochem Biophys Res Commun 1995, 217:721-727 [DOI] [PubMed] [Google Scholar]
- 53.Easty DJ, Hill SP, Hsu MY, Fallowfield ME, Florenes VA, Herlyn M, Bennett DC: Up-regulation of ephrin-A1 during melanoma progression. Int J Cancer 1999, 84:494-501 [DOI] [PubMed] [Google Scholar]
- 54.Herold-Mende C, Steiner HH, Andl T, Riede D, Buttler A, Reisser C, Fusenig NE, Mueller MM: Expression and functional significance of vascular endothelial growth factor receptors in human tumor cells. Lab Invest 1999, 79:1573-1582 [PubMed] [Google Scholar]
- 55.Rofstad EK, Halsor EF: Vascular endothelial growth factor, interleukin 8, platelet-derived endothelial cell growth factor, and basic fibroblast growth factor promote angiogenesis and metastasis in human melanoma xenografts. Cancer Res 2000, 60:4932-4938 [PubMed] [Google Scholar]
- 56.Westphal JR, Hullenaar RV, Peek R, Willems RW, Crickard K, Crickard U, Askaa J, Clemmensen I, Ruiter DJ, De Waal RMW: Angiogenic balance in human melanoma: expression of VEGF, bFGF, IL-8, PDGF and angiostatin in relation to vascular density of xenografts in vivo. Int J Cancer 2000, 86:768-776 [DOI] [PubMed] [Google Scholar]
- 57.Erhard H, Rietveld FJ, van Altena MC, Brocker EB, Ruiter DJ, de Waal RM: Transition of horizontal to vertical growth phase melanoma is accompanied by induction of vascular endothelial growth factor expression and angiogenesis. Melanoma Res 1997, 7(Suppl 2):S19-S26 [PubMed] [Google Scholar]
- 58.Salven P, Heikkila P, Joensuu H: Enhanced expression of vascular endothelial growth factor in metastatic melanoma. Br J Cancer 1997, 76:930-934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Halaban R, Kwon BS, Ghosh S, Delli Bovi P, Baird A: bFGF as an autocrine growth factor for human melanomas. Oncogene Res 1988, 3:177–186 [PubMed]
- 60.Becker D, Meier CB, Herlyn M: Proliferation of human malignant melanomas is inhibited by antisense oligodeoxynucleotides targeted against basic fibroblast growth factor. EMBO J 1989, 8:3685-3691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahmed NU, Ueda M, Ito A, Ohashi A, Funasaka Y, Ichihashi M: Expression of fibroblast growth factor receptors in naevus-cell naevus and malignant melanoma. Melanoma Res 1997, 7:299-305 [DOI] [PubMed] [Google Scholar]
- 62.Nesbit M, Nesbit HK, Bennett J, Andl T, Hsu MY, Dejesus E, McBrian M, Gupta AR, Eck SL, Herlyn M: Basic fibroblast growth factor induces a transformed phenotype in normal human melanocytes. Oncogene 1999, 18:6469-6476 [DOI] [PubMed] [Google Scholar]
- 63.Meier F, Nesbit M, Hsu MY, Martin B, Van Belle P, Elder DE, Schaumburg-Lever G, Garbe C, Walz TM, Donatien P, Crombleholme TM, Herlyn M: Human melanoma progression in skin reconstructs: biological significance of bFGF. Am J Pathol 2000, 156:193-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schadendorf D, Moller A, Algermissen B, Worm M, Sticherling M, Czarnetzki BM: IL-8 produced by human malignant melanoma cells in vitro is an essential autocrine growth factor. J Immunol 1993, 151:2667-2675 [PubMed] [Google Scholar]
- 65.Nurnberg W, Tobias D, Otto F, Henz BM, Schadendorf D: Expression of interleukin-8 detected by in situ hybridization correlates with worse prognosis in primary cutaneous melanoma. J Pathol 1999, 189:546-551 [DOI] [PubMed] [Google Scholar]
- 66.Singh RK, Varney ML, Bucana CD, Johansson SL: Expression of interleukin-8 in primary and metastatic malignant melanoma of the skin. Melanoma Res 1999, 9:383-387 [DOI] [PubMed] [Google Scholar]
- 67.Singh RK, Varney ML: IL-8 expression in malignant melanoma: implications in growth and metastasis. Histol Histopathol 2000, 15:843-849 [DOI] [PubMed] [Google Scholar]
- 68.Easty DJ, Guthrie BA, Maung K, Farr CJ, Lindberg RA, Toso RJ, Herlyn M, Bennett DC: Protein B61 as a new growth factor: expression of B61 and up-regulation of its receptor epithelial cell kinase during melanoma progression. Cancer Res 1995, 55:2528-2532 [PubMed] [Google Scholar]
- 69.Straume O, Sviland L, Akslen LA: Loss of nuclear p16 protein expression correlates with increased tumor cell proliferation (Ki-67) and poor prognosis in patients with vertical growth phase melanoma. Clin Cancer Res 2000, 6:1845-1853 [PubMed] [Google Scholar]
- 70.Elder DE, Murphy GF: Melanocytic tumors of the skin. Rosai J Sobin LH eds. Atlas of Tumor Pathology. 1991, :pp 119-131 Armed Forces Institute of Pathology, Washington DC [Google Scholar]
- 71.Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP: Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 1998, 4:844-847 [DOI] [PubMed] [Google Scholar]
- 72.Hoos A, Urist MJ, Stojadinovic A, Mastorides S, Dudas ME, Leung DH, Kuo D, Brennan MF, Lewis JJ, Cordon-Cardo C: Validation of tissue microarrays for immunohistochemical profiling of cancer specimens using the example of human fibroblastic tumors. Am J Pathol 2001, 158:1245-1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nocito A, Bubendorf L, Maria Tinner E, Suess K, Wagner U, Forster T, Kononen J, Fijan A, Bruderer J, Schmid U, Ackermann D, Maurer R, Alund G, Knonagel H, Rist M, Anabitarte M, Hering F, Hardmeier T, Schoenenberger AJ, Flury R, Jager P, Luc Fehr J, Schraml P, Moch H, Mihatsch MJ, Gasser T, Sauter G: Microarrays of bladder cancer tissue are highly representative of proliferation index and histological grade. J Pathol 2001, 194:349–357 [DOI] [PubMed]
- 74.Norusis M: SPSS Advanced Statistics 6. 1, Chicago, IL, SPSS Inc. 1994, ,
- 75.Cox DR: Regression models and life-tables. J R Stat Soc 1972, 34:187-222 [Google Scholar]
- 76.Straume O, Salvesen HB, Akslen LA: Angiogenesis is prognostically important in vertical growth phase melanomas. Int J Oncol 1999, 15:595-599 [DOI] [PubMed] [Google Scholar]
- 77.Berking C, Takemoto R, Satyamoorthy K, Elenitsas R, Herlyn M: Basic fibroblast growth factor and ultraviolet B transform melanocytes in human skin. Am J Pathol 2001, 158:943-953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.al-Alousi S, Barnhill R, Blessing K, Barksdale S: The prognostic significance of basic fibroblast growth factor in cutaneous malignant melanoma. J Cutan Pathol 1996, 23:506–510 [DOI] [PubMed]
- 79.Graeven U, Rodeck U, Karpinski S, Jost M, Philippou S, Schmiegel W: Modulation of angiogenesis and tumorigenicity of human melanocytic cells by vascular endothelial growth factor and basic fibroblast growth factor. Cancer Res 2001, 61:7282-7290 [PubMed] [Google Scholar]
- 80.Kloth S, Gerdes J, Wanke C, Minuth WW: Basic fibroblast growth factor is a morphogenic modulator in kidney vessel development. Kidney Int 1998, 53:970-978 [DOI] [PubMed] [Google Scholar]
- 81.Hayes AJ, Huang WQ, Yu J, Maisonpierre PC, Liu A, Kern FG, Lippman ME, McLeskey SW, Li LY: Expression and function of angiopoietin-1 in breast cancer. Br J Cancer 2000, 83:1154-1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guddo F, Fontanini G, Reina C, Vignola AM, Angeletti A, Bonsignore G: The expression of basic fibroblast growth factor (bFGF) in tumor-associated stromal cells and vessels is inversely correlated with non-small cell lung cancer progression. Hum Pathol 1999, 30:788-794 [DOI] [PubMed] [Google Scholar]
- 83.Relf M, LeJeune S, Scott PA, Fox S, Smith K, Leek R, Moghaddam A, Whitehouse R, Bicknell R, Harris AL: Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor beta-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res 1997, 57:963-969 [PubMed] [Google Scholar]
- 84.Stone J, Itin A, Alon T, Pe’er J, Gnessin H, Chan-Ling T, Keshet E: Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci 1995, 15:4738-4747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K: Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 1997, 276:1423-1425 [DOI] [PubMed] [Google Scholar]
- 86.Valtola R, Salven P, Heikkila P, Taipale J, Joensuu H, Rehn M, Pihlajaniemi T, Weich H, deWaal R, Alitalo K: VEGFR-3 and its ligand VEGF-C are associated with angiogenesis in breast cancer. Am J Pathol 1999, 154:1381-1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K: Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci USA 1995, 92:3566-3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Clauss M, Weich H, Breier G, Knies U, Rockl W, Waltenberger J, Risau W: The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J Biol Chem 1996, 271:17629-17634 [DOI] [PubMed] [Google Scholar]
- 89.Wang H, Keiser JA: Vascular endothelial growth factor upregulates the expression of matrix metalloproteinases in vascular smooth muscle cells: role of flt-1. Circ Res 1998, 83:832-840 [DOI] [PubMed] [Google Scholar]
- 90.Xie B, Tam NN, Tsao SW, Wong YC: Co-expression of vascular endothelial growth factor (VEGF) and its receptors (flk-1 and flt-1) in hormone-induced mammary cancer in the Noble rat. Br J Cancer 1999, 81:1335-1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sood AK, Seftor EA, Fletcher MS, Gardner LM, Heidger PM, Buller RE, Seftor RE, Hendrix MJ: Molecular determinants of ovarian cancer plasticity. Am J Pathol 2001, 158:1279-1288 [DOI] [PMC free article] [PubMed] [Google Scholar]