Role of Mitochondrial Remodeling in Programmed Cell Death in Drosophila melanogaster (original) (raw)
Summary
The role of mitochondria in Drosophila programmed cell death remains unclear, although certain gene products that regulate cell death seem to be evolutionarily conserved. We find that developmental programmed cell death stimuli in vivo and multiple apoptotic stimuli ex vivo induce dramatic mitochondrial fragmentation upstream of effector caspase activation, phosphatidylserine exposure, and nuclear condensation in Drosophila cells. Unlike genotoxic stress, a lipid cell death mediator induced an increase in mitochondrial contiguity prior to fragmentation of the mitochondria. Using genetic mutants and RNAi-mediated knockdown of drp-1, we find that Drp-1 not only regulates mitochondrial fission in normal cells, but mediates mitochondrial fragmentation during programmed cell death. Mitochondria in drp-1 mutants fail to fragment, resulting in hyperplasia of tissues in vivo and protection of cells from multiple apoptotic stimuli ex vivo. Thus, mitochondrial remodeling is capable of modifying the propensity of cells to undergo death in Drosophila.
Keywords: CELLCYCLE
Introduction
Programmed cell death (PCD) plays an important role in sculpting tissues during animal development (Adams and Cory, 2002; Danial and Korsmeyer, 2004). The molecular regulators that are central to this process seem to be evolutionarily conserved from worms to mammals (Danial and Korsmeyer, 2004; Horvitz, 1999) and include autocatalytic initiator caspases, _trans_-activable effector caspases, cytosolic activating factors (APAF-1), and multidomain Bcl-2 proteins (Danial and Korsmeyer, 2004; Horvitz, 1999). The proapoptotic Bcl-2-family proteins oligomerize and permeabilize mitochondria, releasing intermembrane space components such as cytochrome-C and Smac/DIABLO into the cytosol, where they activate initiator caspases by an ATP-dependent mechanism (Danial and Korsmeyer, 2004). Initiator caspases _trans_-activate effector caspases that cleave multiple cellular substrates, resulting in DNA degradation, nuclear condensation, and loss of cell integrity (Danial and Korsmeyer, 2004; Wang, 2001).
Mitochondrial outer-membrane permeabilization has been proposed to depend on the mitochondrial fission and fusion machinery (Perfettini et al., 2005; Youle and Karbowski, 2005). Consistent with this, mitochondria undergo dramatic fragmentation very close in time to cytochrome-C release during mammalian cell death (Frank et al., 2001; Mancini et al., 1997). Furthermore, an increase in mitochondrial fragmentation and a block in mitochondrial fusion are essential for cell death progression (Frank et al., 2001; Karbowski et al., 2002; Yu et al., 2005). In normal cells, the balance in the rates of mitochondrial fission and fusion regulated by Dynamin-related protein-1 (Drp-1), Fis-1 and endophilin (fission), or Mitofusins and Opa-1 (fusion) maintains the dynamic, interconnected mitochondrial tubules (Meeusen and Nunnari, 2005; Okamoto and Shaw, 2005; Yaffe, 1999). An increase in recruitment of Drp-1 to the mitochondria accentuates staurosporine, lipid, and free oxygen radical stress-induced mitochondrial outer-membrane permeabilization (Breckenridge et al., 2003; Szabadkai et al., 2004). Moreover, multiple apoptotic stimuli induce mitochondrial recruitment of the proapoptotic Bcl-2-family protein, Bax, to Drp-1 and Mitofusin-2-positive putative mitochondrial fragmentation sites (Karbowski et al., 2002; Neuspiel et al., 2005) in a Fis-1-dependent manner (Lee et al., 2004), consistent with a role for mitochondrial fission and fusion machinery in cell death.
In Drosophila, RHG-family proteins (Richardson and Kumar, 2002), genotoxic stresses, and protein synthesis inhibitors (Chew et al., 2004; Zimmermann et al., 2002) antagonize Drosophila inhibitor of apoptosis protein-1 (DIAP-1)-mediated inhibition of the activation of the apical caspase Dronc in an ARK- (Drosphila APAF-1) and ATP-dependent manner (Mills et al., 2006), leading to effector caspase activation and cell death. The role of mitochondria in this process is unclear. Cytochrome-C has been shown to be differentially displayed from the mitochondria during cell death (Varkey et al., 1999). Knockdown of Drosophila cytochrome-C did not affect cell death triggered by genotoxic stress in vitro (Means et al., 2006) and ex vivo (Zimmermann et al., 2002) or developmental stimuli in vivo (Dorstyn et al., 2004), although certain nonapoptotic caspase activation pathways utilized during sperm individualization were affected (Arama et al., 2003). Furthermore, mitochondrial morphology during Drosophila PCD has not been previously reported.
In this study, we show that multiple apoptotic stimuli result in mitochondrial fragmentation upstream of caspase activation, phosphatidylserine exposure, and nuclear condensation in Drosophila cells. While etoposide induced mitochondrial fragmentation, C6-ceramide resulted in an increase in mitochondrial contiguity prior to its fragmentation. drp-1 mutant or RNAi-treated S2R+ cells are considerably protected from multiple apoptotic stimuli, consistent with reduced mitochondrial fragmentation. Thus, mitochondrial remodeling plays an important role in modifying the propensity of cells to undergo PCD in Drosophila.
Results
Mitochondria Undergo Fragmentation during PCD in Drosophila
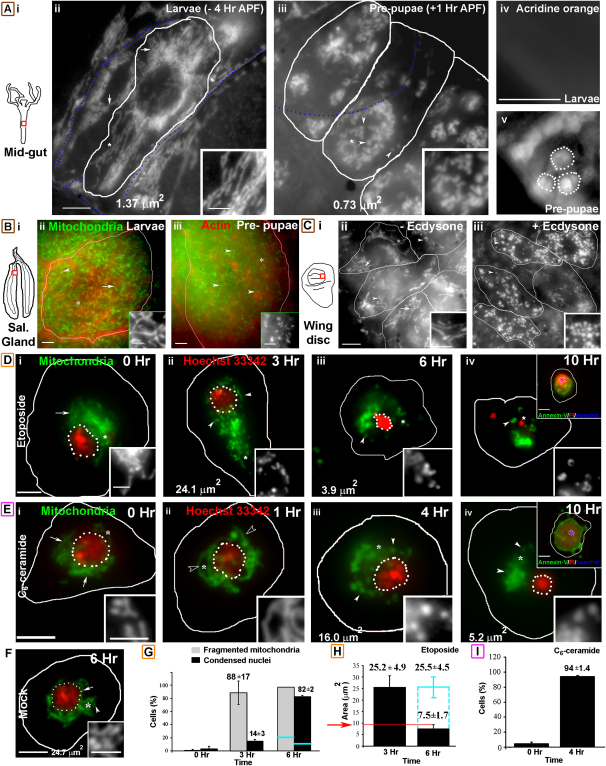
Precisely timed ecdysone pulses induce Reaper and Hid expression in the Drosophila larval midgut (0 hr after puparium formation [APF]) or the salivary gland (10 hr APF) and trigger developmental PCD (Yin and Thummel, 2005). Mitochondria, visualized by using matrix-targeted GFP (Mito-GFP) (Pilling et al., 2006) in acridine orange-positive, dying prepupal midgut cells (1 hr APF, Figure 1Aiii) and salivary glands (4 hr APF, Figure 1Biii), were remarkably fragmented, unlike third-instar larval (−4 hr APF) mitochondria. Quantification revealed a dramatic decrease in the prepupal mitochondrial cross-sectional area (CSA; midgut [∼0.7 μm2 from ∼1.4 μm2, Figure 1A] and salivary gland [Figure S1A, see the Supplemental Data available with this article online]) and a significant increase in the number of mitochondria per cell (Figure S1A). Moreover, ecdysone-induced mitochondrial fragmentation was mimicked ex vivo on third-instar larval wing discs by using 1 mM ecdysone (Kilpatrick et al., 2005) for 2 hr (Figure 1C; Figure S1B). In addition, overexpression of Hid resulted in mitochondrial fragmentation in acridine orange-positive eye disc cells (Figure S1C). Thus, mitochondria in Drosophila tissues fragment during PCD, as has been reported in C. elegans (Jagasia et al., 2005) and mammalian cells (Frank et al., 2001).
Figure 1.
Mitochondria Underwent Fragmentation during PCD
(A–C) (i) Regions (red squares) of Drosophila third-instar larval (A) midgut, (B) salivary gland, or (C) wing imaginal disc that were imaged. (A) Mitochondria in (ii) Mito-GFP larval and (iii) prepupal midgut cells (outlined) with the average CSA indicated. Acridine orange staining of (iv) larval or (v) prepupal midgut cells revealed positive nuclei (outlined in [v]) in prepupal cells. Blue, dotted lines mark trachea. (Bii–Biii) Mitochondria (green) in (ii) third-instar larval or (iii) prepupal salivary gland cells (outlined; A568 Phalloidin [red]). (Cii–Ciii) Mitochondria in wing disc cells (outlined) incubated for 2 hr with (iii) ecdysone or (ii) mock.
(D–F) Mitochondrial morphology (green) and nuclear morphology (Hoechst-33342 [D]–[F]; red, dotted outline) in hemocytes incubated with (D) 10 μM etoposide, (E) 20 μM C6-ceramide, or (F) mock for indicated times. The nuclear CSA in the hemocyte shown is indicated. Top insets show cells positive for FITC-Annexin V (AnV, green) and PI (red) with highly condensed nuclei (Hoechst-33342; blue, dotted outline) at 10 hr.
(G) Histogram showing the fraction of etoposide- or mock-treated (blue line) hemocytes (n = 100) with fragmented mitochondria (gray) or apoptotic nuclei (black) at specified times (mean ± SD; n = 2).
(H) Nuclear CSA in etoposide- (black) or mock-treated (blue) hemocytes showed condensed nuclei (CSA < 10 μm2) in AnV-positive cells (6 hr).
(I) Histogram showing the number of C6-ceramide-treated hemocytes that had fragmented mitochondria at indicated times (mean ± SD; n = 2).
Lower insets show a magnified view of the region marked with an asterisk. Arrows, arrowheads, and open arrowheads indicate tubular, fragmented, and extensively tubular mitochondria, respectively. The panel label of cells exposed to ecdysone, genotoxic stress, or ceramide has been outlined brown, orange, or pink, respectively.
The scale bars in the panels are 5 μm; those in the top insets of the panel are 5 μm, and those in the lower insets are 2.5 μm.
Multiple Apoptotic Stimuli Trigger Distinct Mitochondrial Remodeling Events during Cell Death
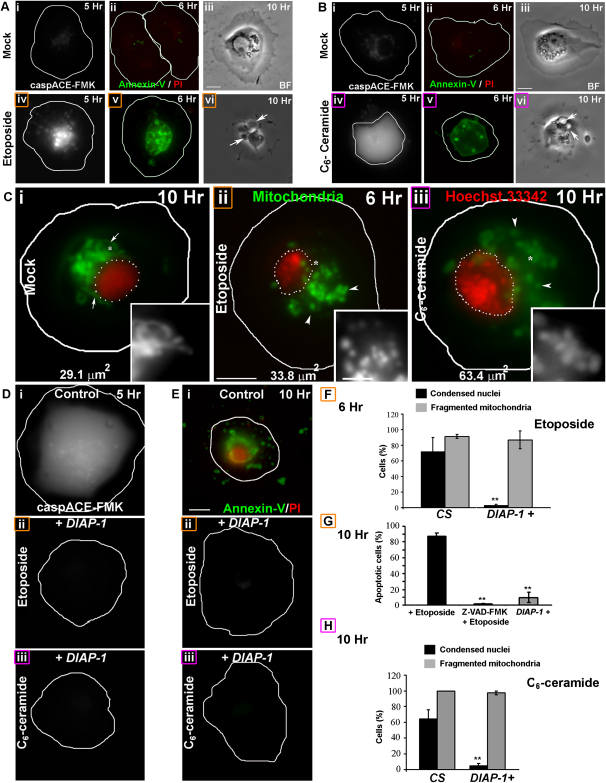
To assess the role of mitochondrial remodeling in PCD, mitochondrial morphology was temporally characterized in etoposide-, actinomycin-D-, cycloheximide-, or C6-ceramide (a lipid cell death mediator)-treated (Walter and Hajnoczky, 2005) larval hemocytes (Sriram et al., 2003) and the S2R+ cell line. A 3- to 4-fold increase in nuclear condensation (6 hr, Figure 1H) was preceded by effector caspase activation (5 hr, Figures 2A–2Biv) and phosphatidylserine (PS) exposure in propidium iodide (PI)-negative hemocytes (6 hr, Figures 2A–2Bv). These cells subsequently (10 hr) became characteristically blebbed (Figures 2A–2Bvi) and PI permeable (Figures 1D–1Eiv, top inset). The number of etoposide-treated apoptotic hemocytes increased with time (Figure 1G). Interestingly, mitochondrial fragmentation (Figures 1Dii and 1G), as confirmed by quantifying functionally isolated mitochondria (Figure S2 and Movie S1) (Collins et al., 2002) at 3 hr, preceded the onset of PS exposure (Figure 2Av) or nuclear damage (Figures 1G and 1H). Quantification showed an increase in the number of mitochondria and the contribution of fragmented mitochondria to the mitochondrial CSA (Figures S3B, S3D, and S3E). Mitochondrial fragmentation was also observed in cycloheximide- or actinomycin-D-treated, Mito-YFP-transfected S2R+ cells (6 hr, Figures S3G and S3H).
Figure 2.
Mitochondrial Fragmentation Is Upstream of Effector Caspase Activation
(A and B) (iii and vi) Phase-contrast and fluorescence images of (Aiv and Av) etoposide-, (Biv and Bv) C6-ceramide-, or (A and B) (i and ii) mock-treated hemocytes incubated with (i and iv) caspACE-FMK substrate or (ii and v) AnV (green), and PI (red) at the indicated times. Arrows indicate blebs.
(C) Mitochondria (Mitotracker Red; green) and nuclei ([ii–iii] Hoechst-33342, [I] nuclear GFP; red, dotted outline) in (i) mock-, (ii) etoposide-, or (iii) C6-ceramide-treated _DIAP-1_+ (Col-GAL4,UAS-GFP; UAS-DIAP-1/+) hemocytes at indicated times. The nuclear CSA is indicated. Insets show a magnified view of the regions marked with an asterisk.
(D and E) (i) Control and (ii and iii) DIAP-1+ hemocytes incubated with (ii) etoposide or (i and iii) C6-ceramide stained for (D) active effector caspases or (E) AnV (green) and PI (red) positivity at indicated times.
(F–H) (F and H) Histogram showing the fraction of (F) etoposide- or (H) C6-ceramide-treated _DIAP-1_+ hemocytes (n = 100) with fragmented mitochondria (gray) or apoptotic nuclei (black) at the indicated times (mean ± SD; n = 3). (G) Histogram showing the fraction of etoposide-treated mock or _DIAP-1_+ and 50 μM zVAD-fmk-treated hemocytes (n = 100) with apoptotic nuclei (mean ± SD; n = 3). ∗∗p < 0.005.
The scale bars in the panels are 5 μm; those in the insets are 2.5 μm.
Surprisingly, mitochondria in C6-ceramide-treated (30–60 min) hemocytes that had normal nuclei were highly contiguous (open arrowheads, Figure 1Eii). Quantifying functionally isolated mitochondrial CSA per cell showed a significant increase in the contribution of tubular or extensively tubular mitochondria in these cells when compared with untreated cells (Figure S3F). However, by 4 hr, these extensively tubular mitochondria underwent fragmentation in FITC-Annexin V (AnV)-negative hemocytes that had normal nuclei (Figures 1Eiii and 1I), similar to what was observed with genotoxic stress (Figure 1Dii).
Therefore, genotoxic stresses triggered mitochondrial fragmentation, while the lipid cell death mediator induced increased mitochondrial contiguity and subsequent fragmentation prior to PS exposure, nuclear condensation, and finally plasma membrane permeability during Drosophila cell death.
Mitochondrial Fragmentation Is Upstream of Effector Caspase Activation
In hemocytes incubated with an apoptotic stimulus, mitochondrial fragmentation (3–4 hr) preceded any detectable effector caspase activation (5 hr [Figures 2A and 2Biv]). Furthermore, inhibiting caspases with zVAD-fmk or by overexpressing DIAP-1 (DIAP-1+) (Hay et al., 1995) did not affect mitochondrial fragmentation (Figure 2C), although hemocyte death was inhibited (Figures 2F–2H), as revealed by a lack of apoptotic markers (Figures 2D and 2E; zVAD-fmk; data not shown). In addition, overexpression of Dcp-1, a Drosophila effector caspase, did not affect mitochondrial morphology (Figure S4). Thus, mitochondrial fragmentation is upstream of effector caspase activation.
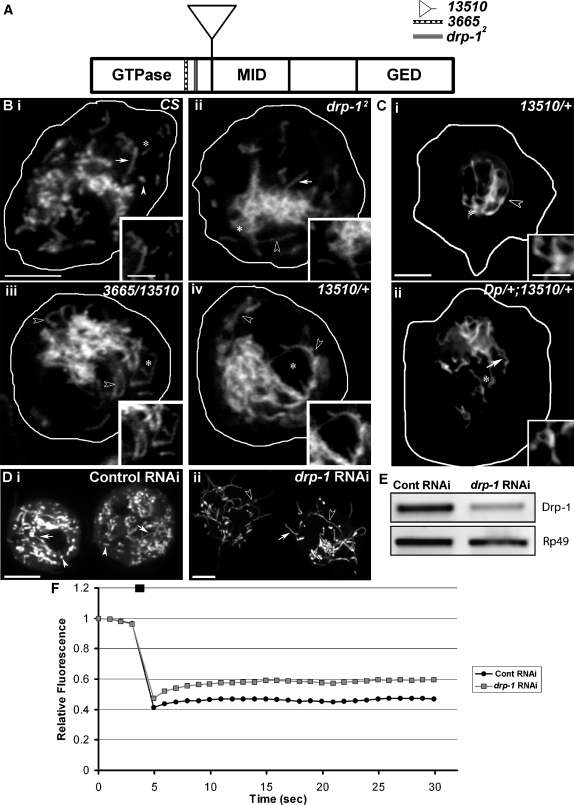
Mitochondrial Morphology Is Extensively Tubular in drp-1 Mutants
The drp-1 mutants used to study the role of mitochondrial remodeling during Drosophila PCD are functional null alleles, _drp-1_2 (Gly293Ser mutation), picked in a forward screen for genes affecting neurotransmission and _drp-1_[KG 03815], a P element insertion between the first two exons of drp-1 (13510 in this study) and a hypomorph, _nrd_D46 (Arg278Trp mutation; 3665 in this study) (Rikhy et al., 2007; Verstreken et al., 2005). _drp-1_2, 13510, and the deficiency Df Exel6008 were second-instar larval lethal (data not shown); however, _drp-1_2 yielded bang-sensitive escapers (Verstreken et al., 2005). The hypomorphic _trans_-allelic combination of 3665/13510 was third-instar larval lethal, although it yielded a few temperature-sensitive adults (Rikhy et al., 2007). A genomic duplication of drp-1 (Dp [2;1] JS13 [Rikhy et al., 2007]) completely rescued the lethality associated with _drp-1_2, 13510, and 3665/13510.
Mitochondria in _drp-1_2 and 3665/13510 hemocytes were extensively tubular when compared with wild-type mitochondria (open arrowheads, Figures 3Bi–3Biii). Quantifying mitochondrial morphology revealed a 2-fold decrease in the number of mitochondria and a significant increase in the contribution of tubular and extensively tubular mitochondria to the total mitochondrial CSA in drp-1 mutant hemocytes when compared with wild-type cells (Figure S5). Interestingly, 13510/+ hemocytes (Figures 3Biv and 3Ci) or eye disc cells (Figure S5) displayed a dominant mitochondrial fission defect that was completely rescued by a genomic duplication of drp-1 (Figure 3Cii). The mitochondrial fission defect in mutant cells could result from a reduced mitochondrial association of Drp-1 (Figure S6).
Figure 3.
Mitochondrial Morphology Is Extensively Tubular in drp-1 Mutants
(A) Drosophila Drp-1 has an N-terminal GTPase domain, the middle domain, and a C-terminal GTPase effector domain. The mutations map to the GTPase domain.
(B) Single-plane reconstruction of confocal images of mitochondria (Mitotracker Green) in (i) wild-type, (ii) _drp-1_2, (iii) 3665/13510, and (iv) 13510/+ larval hemocytes.
(C) Wide-field images of (i) 13510/+ and (ii) Dp/+; 13510/+ mitochondria.
(D) Mitochondria (Mito-YFP) in (i) mock- or (ii) drp-1 dsRNA-treated cells. Arrows, arrowheads, and open arrowheads indicate tubular, fragmented, and extensively tubular mitochondria, respectively. Insets show magnified views of areas marked with an asterisk.
(E) RT-PCR showed reduced levels of drp-1 RNA, not Rp49 RNA, in drp-1 dsRNA-treated cells.
(F) FRAP of Mito-YFP measured in a defined mitochondrial region in mock- (black) or drp-1 (gray) dsRNA-treated S2R+ cells showed increased recovery in drp-1 dsRNA-treated cells (n = 35).
The scale bars in the panels are 5 μm; those in the insets are 2.5 μm.
An increase in mitochondrial contiguity due to a loss of Drp-1 function was also confirmed by measuring fluorescence recovery after photobleaching (FRAP) of Mito-YFP (Collins et al., 2002) in drp-1 RNAi-treated S2R+ cells that had extensively tubular mitochondria (Figure 3D). Relative FRAP of Mito-YFP in a defined mitochondrial region in drp-1 RNAi-treated cells was significantly (p < 0.01) higher than that observed in mock RNAi-treated cells (Figure 3F).
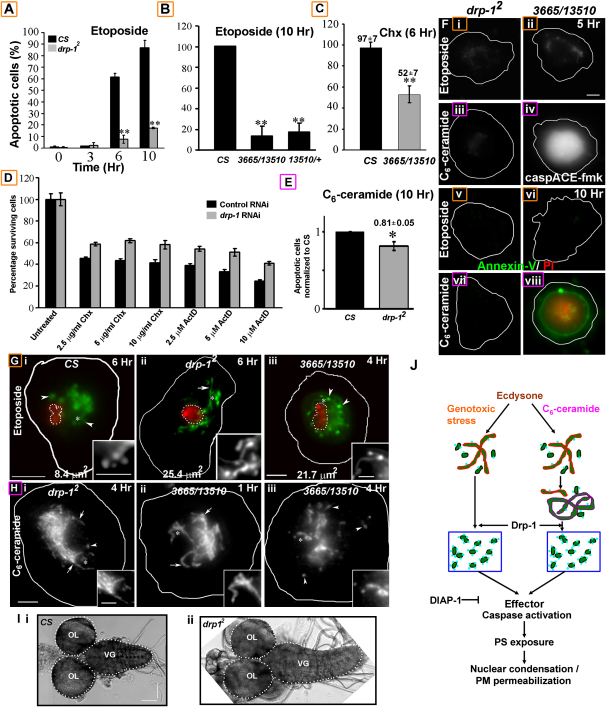
Drp-1-Mediated Fragmentation of Mitochondria Affects PCD
drp-1 mutant hemocytes were protected from etoposide-induced death up to at least 10 hr (Figures 4A and 4B), as revealed by a lack of caspase activation, PS exposure, or PI permeability in the majority (∼80%) of these cells (Figures 4Fi, 4Fii, 4Fv, and 4Fvi). Furthermore, drp-1 mutant and dsRNA-treated S2R+ cells were significantly protected (p < 0.01) from cycloheximide-, actinomycin-D- (Figures 4C and 4D), or UV-B-induced (Figure S7) death. Consistent with increased protection, mitochondria in the majority (∼98%) of etoposide-treated _drp-1_2 hemocytes failed to fragment (6 hr; compare Figure 4Gii with Figure 4Gi). Interestingly, mitochondria in etoposide-treated 3665/13510 hemocytes revealed a tubular, yet beaded and swollen intermediate in mitochondrial fragmentation by 4 hr (Figure 4Giii) that yielded some fragmented mitochondria in few (∼25%) cells later (6 hr; data not shown). Therefore, reduced (_drp-1_2) or delayed (3665/13510) mitochondrial fragmentation decreased effector caspase activation and protected cells from genotoxic stress. Moreover, an increase in expression of Drp-1 in hemocytes resulted in enhancement of etoposide-induced cell death (Figure S8).
Figure 4.
PCD Is Suppressed in drp-1 Mutants
(A) Histogram showing the fraction of etoposide-treated wild-type (black) and _drp-1_2 (gray) hemocytes (n = 100) with apoptotic nuclei (CSA < 10 μm2) at indicated times (mean ± SD; n = 3).
(B and C) Histogram showing the fraction of (B) etoposide- or (C) cycloheximide-treated (0.5 μg/ml) wild-type, 3665/13510, or 13510/+ hemocytes (n = 100) with apoptotic nuclei ([B], mean ± propagated error; [C], mean ± SD; n = 3).
(D) Histogram showing the number of surviving YFP-positive cells incubated for 5 days with either mock (black) or drp-1 (gray) dsRNA and treated with cycloheximide and actinomycin-D (18 hr), normalized to the number of untreated cells.
(E) Histogram showing the fraction of C6-ceramide-treated (10 hr) _drp-1_2 hemocytes (n = 100) with apoptotic nuclei, normalized to apoptotic wild-type cells (mean ± SD; n = 3).
(F) (i, ii, v, and vi) Etoposide- or (iii, iv, vii, and viii) C6-ceramide-treated (i, iii, v, and vii) _drp-1_2 and (ii, iv, vi, and viii) 3665/13510 hemocytes stained for (i–iv) active effector caspases or (v–viii) AnV (green) and PI (red) accessibility.
(G) Mitochondria (Mitotracker Green; green) and nuclei (Hoechst-33342; red, dotted outline) in etoposide-treated (i) wild-type, (ii) _drp-1_2, and (iii) 3665/13510 hemocytes showed a mitochondrial fragmentation defect and a block in nuclear condensation, as indicated by the nuclear CSA.
(H) Mitochondria (anti-Biotin) in C6-ceramide-treated (i) _drp-1_2 and (ii and iii) 3665/13510 hemocytes at indicated times. The insets show magnified views of the regions marked with an asterisk. Arrows and arrowheads indicate tubular and fragmented mitochondria, respectively.
(I) Optic lobes (OL) and ventral ganglion (VG) of the (i) wild-type or (ii) _drp-1_2 third-instar larval CNS. ∗p < 0.05; ∗∗p < 0.005. The scale bar is 100 μm in (I), 5 μm in all other panels, and 2.5 μm in all insets.
(J) In Drosophila, multiple apoptotic stimuli such as ecdysone, genotoxic stresses, or C6-ceramide result in fragmentation (blue box) of elongated (red outline) mitochondria (green). Unlike with genotoxic stress, there is an increase in mitochondrial contiguity (purple outline) prior to its fragmentation during lipid-mediated cell death. Mitochondrial fragmentation mediated by Drp-1 (light-blue dots) upstream of effector caspase activation, PS exposure, nuclear condensation, and plasma membrane permeability affects cell death. Thus, mitochondrial remodeling modifies the susceptibility of cells to PCD.
The majority (∼70%) of the C6-ceramide-treated _drp-1_2 hemocytes did not show effector caspase activation or PS exposure and displayed significant (p < 0.05) protection (Figures 4E, 4Fiii, and 4Fvii), similar to what was observed with etoposide, although hemocytes derived from the weaker allelic combination, 13510/3665, were apoptotic (Figures 4Fiv and 4Fviii). Unlike 13510/3665 mitochondria, _drp-1_2 mitochondria failed to fragment (Figure 4Hi), consistent with an essential role for Drp-1-mediated mitochondrial fragmentation during apoptosis in Drosophila. Moreover, developmental PCD in _drp-1_2 mutant larvae was considerably reduced, as revealed by the enlarged central nervous system and a prominently elongated ventral ganglion (Figure 4I), similar to other PCD-defective mutants reported (Mills et al., 2006).
Discussion
During metamorphosis, the first ecdysone pulse triggers mitochondrial fragmentation in prepupal tissues, although it is after the second ecdysone pulse that salivary gland histolysis occurs. It is likely that DIAP-1 inhibits caspases in these cells that have fragmented mitochondria until it is downregulated at the transcriptional level or degraded after the second ecdysone pulse (Yin and Thummel, 2005). Interestingly, this was mimicked ex vivo in etoposide-treated _DIAP-1_+ hemocytes.
The data presented here show involvement of mitochondrial fragmentation for ARK-mediated Dronc activation during cell death. The RHG-family proteins that localize to the mitochondria (Claveria et al., 2002; Haining et al., 1999; Olson et al., 2003) might activate Drp-1-mediated mitochondrial fragmentation. This could result in exposure of cytochrome-C (Varkey et al., 1999) or release of Peanut (Gottfried et al., 2004), which antagonize DIAP-1-mediated suppression of Dronc. However, as Drosophila PCD was unaffected upon knockdown of cytochrome-C (Dorstyn et al., 2004), mitochondrial fragmentation in Drosophila and mammalian cells would increase mitochondrial surface area and perhaps the concentration of bulky head group lipids on the outer mitochondrial membrane, facilitating recruitment of proapoptotic proteins. Drp-1 might organize sites for Drosophila Bcl-2-family protein Debcl function on mitochondria (Dorstyn et al., 2002) that are similar to mitochondrial sites of Bax recruitment in mammalian cells (Karbowski et al., 2002).
These results provide the first, to our knowledge, evidence that Drp-1-mediated mitochondrial fragmentation upstream of effector caspase activation modifies apoptotic sensitivity (Figure 4J). Thus, mitochondrial fragmentation, like caspase activation, plays a conserved and unifying role in diverse cell death pathways from worms to mammals (Frank et al., 2001; Jagasia et al., 2005). Although the function of the highly contiguous mitochondria during lipid-induced cell death remains poorly understood, this study brings to the forefront a modulatory role for mitochondrial remodeling in determining the susceptibility of Drosophila cells to death.
Experimental Procedures
Materials
Media and chemicals were obtained from GIBCO-BRL or Sigma-Aldrich unless otherwise specified. Fluorescent dyes for tagging and Mitotracker dyes were purchased from Molecular Probes. Secondary antibodies (Jackson ImmunoResearch Laboratories) were conjugated to fluorescent dyes as recommended by the manufacturer.
Drosophila Stocks
Drosophila stocks were grown at 25°C in cornmeal agar bottles. drp-1 mutants, Collagen-GAL4, and UAS-Mito-GFP were kindly provided by Hugo J. Bellen (Baylor College of Medicine, TX), K.S. Krishnan (DBS, TIFR, Mumbai), Charles R. Dearolf (Duke University, NC), and William Saxton (Indiana University, IN).
Treatment of Tissues and Cells Ex Vivo
Third-instar larval tissues were dissected in Schneider insect medium supplemented with 10% non-heat-inactivated fetal bovine serum and 1 μg/ml bovine pancreatic insulin (SCM). They were washed in 1× PBS (pH 7.4) and incubated with 1 mM water-soluble ecdysone (AG Scientific, Inc.) diluted in PBS for 2 hr (20°C), fixed with 4% formaldehyde (30 min), permeabilized with 0.37% Igepal (13 min), and labeled with Alexa 568 Phalloidin (1:200; Molecular Probes) for 30 min. The tissues were mounted beneath a coverslip and imaged. Mitochondria were selected based on a threshold and were quantified by using Metamorph (Molecular Devices Corporation).
Hemocytes derived from late third-instar larvae and S2R+ cells were plated in 35 mm coverslip-bottom dishes. Hemocytes at ∼1 hr postdissection were incubated with etoposide (10 μM), cycloheximide (0.5 μg/ml), or C6-ceramide (20 μM or 40 μM; Matreya, Inc.) diluted in 1× Medium 1 (M1) supplemented with 2 mg/ml glucose (imaging medium [IM]) at 20°C for the indicated times prior to staining nuclei with 5 μg/ml Hoechst-33342 (5 min). Hemocytes were preincubated (30 min) with 50 μM Caspase inhibitor-1 (zVAD-fmk; Calbiochem) in IM before incubation with apoptotic stimuli in the presence of 50 μM zVAD-fmk in order to inhibit caspases. Immunofluorescence staining was carried out as described earlier (Sriram et al., 2003), by using affinity-purified rabbit anti-Drp-1 antibody (1:200), mouse Biotin antibody (1:500), and appropriate secondary antibodies (1:500). Drp-1 punctae were selected by using a threshold on background corrected images and were quantified.
A total of 1 × 106 S2R+ cells were cotransfected with 1 μg siRNAi (Dharmacon, Inc.) and 0.25 μg pAVW vector (expressing EYFP only) (Drosophila Genomics Resource Centre, Indiana University), by using Cellfectin transfection reagent (Invitrogen). After 5 days, cells were treated with prescribed concentrations of actinomycin-D or cycloheximide for 18 hr, and surviving YFP-expressing cells in five randomly selected fields were counted (n = 5; total cells = 14,020). Counts were normalized to the untreated control; error bars represent standard error of the mean for normalized data.
Detection of Active Caspases and PI, AnV Staining
Hemocytes were incubated with 10 μM caspACE substrate (FITC-VAD-FMK, Promega) for 20 min (20°C) or stained with PI (20 μg/ml), AnV (2 μg/ml) (Santa Cruz Biotechnology), and Hoechst-33342 (5 μg/ml) diluted in M1 supplemented with 2.5 mM calcium chloride and 2 mg/ml glucose (20 min) and were imaged.
Imaging
Tissues and cell cultures in 35 mm coverslip-bottom dishes were imaged by using 60× or 100×, 1.4 NA, oil-emersion objectives on a Nikon TE 2000-U epi-fluorescent inverted microscope with optimized dichroics and filters and a Cascade 512B EM-CCD camera (Photometrics) controlled by Metamorph (Molecular Devices Corporation). Confocal images were acquired by using either a 60×, 1.4 NA or a 100×, 1.45 NA objective on a Biorad MRC 1024 or Zeiss LSM 510 (Carl Zeiss MicroImaging, Inc.) microscope equipped with optimized dichroics and filters. Images were processed by using Metamorph (Molecular Devices Corporation) and Adobe Photoshop software.
Mitochondrial Morphology
Tubulin-GAL4 or Collagen-GAL4 was used to express Mito-GFP in multiple tissues by using the GAL4-UAS system (Brand and Perrimon, 1993). Hemocytes were incubated with 100 nM Mitotracker diluted in IM supplemented with 1.5 mg/ml BSA (IM+BSA) for 20 min (20°C) and were imaged. S2R+ cells were grown in Schneider's medium supplemented with 10% fetal bovine serum (Gemini Bio-Products) at 25°C. A total of 1 × 106 cells were plated and transfected with 1 μg EYFP-Mito vector (LaJeunesse et al., 2004) by using 5 μl Cellfectin transfection reagent (Invitrogen). Mitochondria were selected by using defined thresholds post background correction and were quantified by using Metamorph (Molecular Devices Corporation).
UV-B Induced Apoptosis in Drosophila Third-Instar Larvae
Third-instar larvae were exposed (90 s) on a dual intensity transilluminator (TM20; UVP, Inc.) fitted with 6 UV-B tubes (20W × 6). A total of 6 hr later, wing discs were dissected and stained with 3 μg/ml acridine orange in PBS (5 min), washed, and imaged on the confocal microscope.
TMRM Fluorescence-Loss Assay
Hemocytes were incubated with 10 nM TMRM diluted in SCM or IM+BSA (15 min) and were exposed to 555 nm wavelength light on a wide-field microscope. Time-lapse images (0.2 s) were acquired every 1 s until TMRM was released from all the mitochondria. Individual mitochondria were outlined and quantified by using Metamorph (Molecular Devices Corporation) (n = 5–10).
FRAP Assay
S2R+ cells that were cotransfected with 1 μg siRNAi (Dharmacon, Inc.) and 1 μg EYFP-Mito by using Cellfectin transfection reagent (Invitrogen) according to the manufacturer's instructions were analyzed 5 days posttransfection. Drp siRNA was targeted to 5′-ACACTCCGGTTCACAATAA-3′ (NM_134850 bases 1994–2012), and siCONTROL nontargeting siRNA #1 (Dharmacon, Inc.) was used as a control. For FRAP, a mitochondrial region of interest (ROI, ∼1.75 μm in diameter) was selected and bleached (after 4 s, indicated by a solid bar) with high-intensity 488 nm wavelength light (∼1 s), and fluorescence in the ROI was measured for an additional 25 s.
RT-PCR
RNA was extracted from transfected S2R+ cells by using the RNeasy Mini Kit (QIAGEN), and RT-PCR was conducted by using the QIAGEN OneStep RT-PCR kit (QIAGEN). The Drp-specific 5′ primer 5′-TCATTCACGAGGAGATGCAG-3′ (designed to NM_134850 bases 1298–1317) and the 3′ primer 5′-TGCTTGGTGTTGATGTAGGC-3′ (designed to NM_134850 bases 1490–1509) were used to amplify a 208 bp product. Ribosomal protein 49 (rp49) primers (5′-ATGACCATCCGCCCAGCATAC-3′ and 5′-GAGAACGCAGGCGACCGTTGG-3′) amplified a 391 bp fragment between bases 1 and 391 of the coding region (GenBank accession number Y13939) (Ge et al., 2004).
Acknowledgments
We thank Hugo J. Bellen (Baylor College of Medicine, TX); Charles R. Dearolf (Duke University, NC); William Saxton (Indiana University, IN); K.S. Krishnan (Department of Biological Sciences [DBS], TIFR, Mumbai); the Bloomington (Indiana University), National Center for Biological Sciences (NCBS), and DBS Stock Centers for fly strains; and the NCBS Central Imaging Facility for confocal microscopy. We are grateful to Veronica Rodrigues for her keen interest, constant support, and valuable suggestions. V.S. thanks Satyajit Mayor and K.S. Krishnan for their support. V.S also thanks Appam and Chakli. G.G. thanks his family and friends for their support. This work was supported by intramural funds from the NCBS (V.S.); the National Institute of Neurological Disorders and Stroke, National Institutes of Health (R.J.Y.); and Wellcome Trust International Senior Research Fellowship, the Wellcome Trust, U.K. (A.S.).
Supplemental Data
Supplemental Document S1. Supplemental Figures
Movie S1
References
- Adams J.M., Cory S. Apoptosomes: engines for caspase activation. Curr. Opin. Cell Biol. 2002;14:715–720. doi: 10.1016/s0955-0674(02)00381-2. [DOI] [PubMed] [Google Scholar]
- Arama E., Agapite J., Steller H. Caspase activity and a specific cytochrome C are required for sperm differentiation in Drosophila. Dev. Cell. 2003;4:687–697. doi: 10.1016/s1534-5807(03)00120-5. [DOI] [PubMed] [Google Scholar]
- Brand A.H., Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Breckenridge D.G., Stojanovic M., Marcellus R.C., Shore G.C. Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol. J. Cell Biol. 2003;160:1115–1127. doi: 10.1083/jcb.200212059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew S.K., Akdemir F., Chen P., Lu W.J., Mills K., Daish T., Kumar S., Rodriguez A., Abrams J.M. The apical caspase dronc governs programmed and unprogrammed cell death in Drosophila. Dev. Cell. 2004;7:897–907. doi: 10.1016/j.devcel.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Claveria C., Caminero E., Martinez A.C., Campuzano S., Torres M. GH3, a novel proapoptotic domain in Drosophila Grim, promotes a mitochondrial death pathway. EMBO J. 2002;21:3327–3336. doi: 10.1093/emboj/cdf354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T.J., Berridge M.J., Lipp P., Bootman M.D. Mitochondria are morphologically and functionally heterogeneous within cells. EMBO J. 2002;21:1616–1627. doi: 10.1093/emboj/21.7.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial N.N., Korsmeyer S.J. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Dorstyn L., Read S., Cakouros D., Huh J.R., Hay B.A., Kumar S. The role of cytochrome c in caspase activation in Drosophila melanogaster cells. J. Cell Biol. 2002;156:1089–1098. doi: 10.1083/jcb.200111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorstyn L., Mills K., Lazebnik Y., Kumar S. The two cytochrome c species, DC3 and DC4, are not required for caspase activation and apoptosis in Drosophila cells. J. Cell Biol. 2004;167:405–410. doi: 10.1083/jcb.200408054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S., Gaume B., Bergmann-Leitner E.S., Leitner W.W., Robert E.G., Catez F., Smith C.L., Youle R.J. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev. Cell. 2001;1:515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- Ge X., Hannan F., Xie Z., Feng C., Tully T., Zhou H., Zhong Y. Notch signaling in Drosophila long-term memory formation. Proc. Natl. Acad. Sci. USA. 2004;101:10172–10176. doi: 10.1073/pnas.0403497101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried Y., Rotem A., Lotan R., Steller H., Larisch S. The mitochondrial ARTS protein promotes apoptosis through targeting XIAP. EMBO J. 2004;23:1627–1635. doi: 10.1038/sj.emboj.7600155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haining W.N., Carboy-Newcomb C., Wei C.L., Steller H. The proapoptotic function of Drosophila Hid is conserved in mammalian cells. Proc. Natl. Acad. Sci. USA. 1999;96:4936–4941. [Google Scholar]
- Hay B.A., Wassarman D.A., Rubin G.M. Drosophila homologs of baculovirus inhibitor of apoptosis proteins function to block cell death. Cell. 1995;83:1253–1262. doi: 10.1016/0092-8674(95)90150-7. [DOI] [PubMed] [Google Scholar]
- Horvitz H.R. Genetic control of programmed cell death in the nematode Caenorhabditis elegans. Cancer Res. 1999;59:1701s–1706s. [PubMed] [Google Scholar]
- Jagasia R., Grote P., Westermann B., Conradt B. DRP-1-mediated mitochondrial fragmentation during EGL-1-induced cell death in C. elegans. Nature. 2005;433:754–760. doi: 10.1038/nature03316. [DOI] [PubMed] [Google Scholar]
- Karbowski M., Lee Y.J., Gaume B., Jeong S.Y., Frank S., Nechushtan A., Santel A., Fuller M., Smith C.L., Youle R.J. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J. Cell Biol. 2002;159:931–938. doi: 10.1083/jcb.200209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick Z.E., Cakouros D., Kumar S. Ecdysone-mediated up-regulation of the effector caspase DRICE is required for hormone-dependent apoptosis in Drosophila cells. J. Biol. Chem. 2005;280:11981–11986. doi: 10.1074/jbc.M413971200. [DOI] [PubMed] [Google Scholar]
- LaJeunesse D.R., Buckner S.M., Lake J., Na C., Pirt A., Fromson K. Three new Drosophila markers of intracellular membranes. Biotechniques. 2004;36:784–788, 790. doi: 10.2144/04365ST01. [DOI] [PubMed] [Google Scholar]
- Lee Y.J., Jeong S.Y., Karbowski M., Smith C.L., Youle R.J. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol. Biol. Cell. 2004;15:5001–5011. doi: 10.1091/mbc.E04-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini M., Anderson B.O., Caldwell E., Sedghinasab M., Paty P.B., Hockenbery D.M. Mitochondrial proliferation and paradoxical membrane depolarization during terminal differentiation and apoptosis in a human colon carcinoma cell line. J. Cell Biol. 1997;138:449–469. doi: 10.1083/jcb.138.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means J.C., Muro I., Clem R.J. Lack of involvement of mitochondrial factors in caspase activation in a Drosophila cell-free system. Cell Death Differ. 2006;13:1222–1234. doi: 10.1038/sj.cdd.4401821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeusen S.L., Nunnari J. How mitochondria fuse. Curr. Opin. Cell Biol. 2005;17:389–394. doi: 10.1016/j.ceb.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Mills K., Daish T., Harvey K.F., Pfleger C.M., Hariharan I.K., Kumar S. The Drosophila melanogaster Apaf-1 homologue ARK is required for most, but not all, programmed cell death. J. Cell Biol. 2006;172:809–815. doi: 10.1083/jcb.200512126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuspiel M., Zunino R., Gangaraju S., Rippstein P., McBride H. Activated mitofusin 2 signals mitochondrial fusion, interferes with Bax activation, and reduces susceptibility to radical induced depolarization. J. Biol. Chem. 2005;280:25060–25070. doi: 10.1074/jbc.M501599200. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Shaw J.M. Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu. Rev. Genet. 2005;39:503–536. doi: 10.1146/annurev.genet.38.072902.093019. [DOI] [PubMed] [Google Scholar]
- Olson M.R., Holley C.L., Gan E.C., Colon-Ramos D.A., Kaplan B., Kornbluth S. A GH3-like domain in reaper is required for mitochondrial localization and induction of IAP degradation. J. Biol. Chem. 2003;278:44758–44768. doi: 10.1074/jbc.M308055200. [DOI] [PubMed] [Google Scholar]
- Perfettini J.L., Roumier T., Kroemer G. Mitochondrial fusion and fission in the control of apoptosis. Trends Cell Biol. 2005;15:179–183. doi: 10.1016/j.tcb.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Pilling A.D., Horiuchi D., Lively C.M., Saxton W.M. Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol. Biol. Cell. 2006;17:2057–2068. doi: 10.1091/mbc.E05-06-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson H., Kumar S. Death to flies: Drosophila as a model system to study programmed cell death. J. Immunol. Methods. 2002;265:21–38. doi: 10.1016/s0022-1759(02)00068-6. [DOI] [PubMed] [Google Scholar]
- Rikhy R., Kamat S., Ramagiri S., Sriram V., Krishnan K. Mutations in dynamin-related protein result in gross changes in mitochondrial morphology and affect synaptic vesicle recycling at the Drosophila neuromuscular junction. Genes Brain Behavior. 2007;6:42–53. doi: 10.1111/j.1601-183X.2006.00218.x. [DOI] [PubMed] [Google Scholar]
- Sriram V., Krishnan K.S., Mayor S. deep-orange and carnation define distinct stages in late endosomal biogenesis in Drosophila melanogaster. J. Cell Biol. 2003;161:593–607. doi: 10.1083/jcb.200210166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadkai G., Simoni A.M., Chami M., Wieckowski M.R., Youle R.J., Rizzuto R. Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol. Cell. 2004;16:59–68. doi: 10.1016/j.molcel.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Varkey J., Chen P., Jemmerson R., Abrams J.M. Altered cytochrome c display precedes apoptotic cell death in Drosophila. J. Cell Biol. 1999;144:701–710. doi: 10.1083/jcb.144.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreken P., Ly C.V., Venken K.J., Koh T.W., Zhou Y., Bellen H.J. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Walter L., Hajnoczky G. Mitochondria and endoplasmic reticulum: the lethal interorganelle cross-talk. J. Bioenerg. Biomembr. 2005;37:191–206. doi: 10.1007/s10863-005-6600-x. [DOI] [PubMed] [Google Scholar]
- Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. [Google Scholar]
- Yaffe M.P. Dynamic mitochondria. Nat. Cell Biol. 1999;1:E149–E150. doi: 10.1038/14101. [DOI] [PubMed] [Google Scholar]
- Yin V.P., Thummel C.S. Mechanisms of steroid-triggered programmed cell death in Drosophila. Semin. Cell Dev. Biol. 2005;16:237–243. doi: 10.1016/j.semcdb.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Youle R.J., Karbowski M. Mitochondrial fission in apoptosis. Nat. Rev. Mol. Cell Biol. 2005;6:657–663. doi: 10.1038/nrm1697. [DOI] [PubMed] [Google Scholar]
- Yu T., Fox R.J., Burwell L.S., Yoon Y. Regulation of mitochondrial fission and apoptosis by the mitochondrial outer membrane protein hFis1. J. Cell Sci. 2005;118:4141–4151. doi: 10.1242/jcs.02537. [DOI] [PubMed] [Google Scholar]
- Zimmermann K.C., Ricci J.E., Droin N.M., Green D.R. The role of ARK in stress-induced apoptosis in Drosophila cells. J. Cell Biol. 2002;156:1077–1087. doi: 10.1083/jcb.20112068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Document S1. Supplemental Figures
Movie S1