Positive feedback between Dia1, LARG, and RhoA regulates cell morphology and invasion (original) (raw)
Abstract
The RhoA-effector Dia1 controls actin-dependent processes such as cytokinesis, SRF transcriptional activity, and cell motility. Dia1 polymerizes actin through its formin homology (FH) 2 domain. Here we show that Dia1 acts upstream of RhoA independently of its effects on actin assembly. Dia1 binds to the leukemia-associated Rho-GEF (LARG) through RhoA-dependent release of Dia1 autoinhibition. The FH2 domain stimulates the guanine nucleotide exchange activity of LARG in vitro. Our results reveal that Dia1 is necessary for LPA-stimulated Rho/ROCK signaling and bleb-associated cancer cell invasion. Thus, Dia1-dependent RhoA activation constitutes a positive feedback mechanism to modulate cell behavior.
Keywords: Diaphanous-related formins, RhoA, LARG, actin polymerization, LPA, Rho-kinase
Diaphanous-related formins (DRFs) are Rho–GTPase-binding proteins that possess conserved functions in actin cytoskeleton dynamics exerted through their formin homology (FH) 2 domains (Goode and Eck 2007). DRFs are involved in essential cellular processes such as cytokinesis, cell movement, and polarity (Faix and Grosse 2006; Gomez et al. 2007), which are frequently deregulated during pathological situations like tumor cell transformation and metastasis (Sahai 2005). The dormant conformation of the DRF Dia1 is maintained by intramolecular association of its regulatory N terminus to the diaphanous autoregulatory domain (DAD), which is relieved through binding of active RhoA (Lammers et al. 2005; Otomo et al. 2005a). The catalytic FH2 domain is believed to become “exposed” by conformational changes in the DRFs to promote barbed end actin polymerization by forming a tethered dimer (Xu et al. 2004; Otomo et al. 2005a). The FH2 domain of Dia1 promotes stress fiber formation and transcriptional activation of the MAL/SRF pathway through its actin-polymerizing activity (Copeland and Treisman 2002; Grosse et al. 2003; Miralles et al. 2003). A Dia1 mutant defective in FH2 dimerization interferes with lysophosphatidic acid (LPA)-induced stress fiber formation and SRF activity (Copeland and Treisman 2002), suggesting that Dia1 is part of LPA signal transduction known to play an important role in cell proliferation and metastasis of a variety of human cancers (Mills and Moolenaar 2003). LPA receptors belong to the group of G-protein-coupled receptors that activate the heterotrimeric G-proteins G12 and G13, which can bind to RGS-containing Rho-GEFs such as leukemia-associated Rho-GEF (LARG), initially isolated from a patient with acute myeloid leukemia (AML) (Kourlas et al. 2000; Vazquez-Prado et al. 2004). Rho-dependent mechanisms have emerged as critical processes in tumor progression (Sahai and Marshall 2002; Lozano et al. 2003), and evidence exists that Rho/ROCK function is essential to promote a specific type of rounded bleb-associated mode of cell invasion (Sahai and Marshall 2003; Wyckoff et al. 2006). However, the role of DRFs in tumor cell behavior has not been investigated.
In this study, we provide evidence for an essential role of the Rho-effector Dia1 in LPA-mediated Rho/ROCK activity for tumor cell morphology and invasion that involves LARG, thereby constituting a positive feedback loop toward RhoA.
Results and Discussion
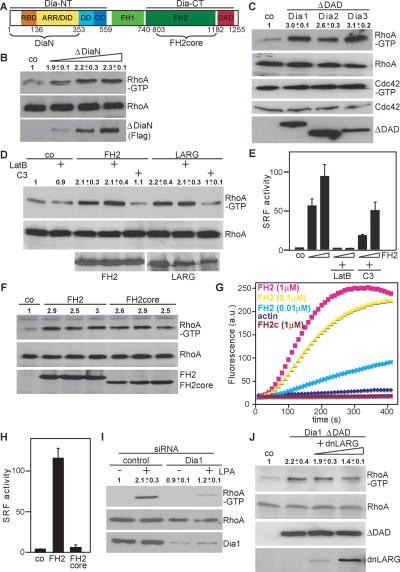
To explore the role of Dia1 in the regulation of RhoA, we performed GTPase activity assays (Goulimari et al. 2005). HEK293 cells were used to achieve transfection efficiencies >70% to determine whether versions of Dia1 lacking the regulatory N terminus (ΔDiaN) (Fig. 1A) required for autoinhibition could induce endogenous RhoA activity. Interestingly, ΔDiaN caused substantial activation of RhoA (Fig. 1B). Active DAD-deletion mutants (ΔDAD) of mouse Dia1, Dia2, and Dia3 also stimulated RhoA but not Cdc42 activity, indicating that this is a shared characteristic among DRFs of this family (Fig. 1C). Dia1 also activated Rac as previously suggested (Tsuji et al. 2002; data not shown). Further analysis revealed that the FH2 domain was sufficient to activate RhoA (Fig. 1D). Since the FH2 is also sufficient for actin assembly and activation of SRF, we tested their possible involvement, indicative of an indirect mechanism. For this we treated cells with Latrunculin B, which sequesters actin monomers and prevents SRF activation (Sotiropoulos et al. 1999). Although Latrunculin B effectively blocked FH2-induced SRF activation as expected (Fig. 1E), it failed to inhibit RhoA activation by FH2 and LARG, while transfection of C3 transferase as a control blocked RhoA activity (Fig. 1D). Interestingly and in agreement with previous findings (Copeland and Treisman 2002), we observed that FH2-induced SRF activity was significantly inhibited by C3 (Fig. 1E), suggesting that upstream RhoA contributes to FH2-exerted actin dynamics. The FH2 domain dimerizes involving its N-terminal lasso loop linker in order to nucleate actin filaments, whereas the core FH2 (FH2core) lacking the lasso behaves as a barbed end capper (Shimada et al. 2004; Otomo et al. 2005b). Therefore, we compared FH2core and FH2 for RhoA activity and actin polymerization. We found that both domains activate RhoA (Fig. 1F), but only the FH2 was able to stimulate SRF and actin polymerization, whereas the FH2core inhibited SRF and basal actin polymerization due to its capping activity (Fig. 1G,H). These data demonstrate that Dia1 stimulates RhoA activity independently of its actin nucleation activity, thus representing a novel function of this conserved protein.
Figure 1.
Role of Dia1 in activation of RhoA. (A) Schematic representation of mDia1. (B) Cells were transfected with control plasmid or increasing amounts of plasmids (0.2, 0.5, 1.0 μg) encoding ΔDiaN and subjected to RhoA-GTP pull-down assays. RhoA and Flag-ΔDiaN were analyzed by Western blotting. (C) Activation of RhoA but not Cdc42 by ΔDAD expression plasmids for Dia1–3 is shown. Active RhoA and Cdc42 were precipitated simultaneously from cell extracts. (D) FH2-induced RhoA activity in the absence or presence of 0.5 μM Latrunculin B (LatB) or in the absence or presence of C3 plasmid (0.5 μg). (E) Cells expressing FH2 (0.1 and 0.3 μg) together with the SRF reporter were cotransfected with C3 (0.1 μg) or pretreated with LatB as indicated. Reporter activity is shown as the mean ± SEM of three independent experiments. (F) FH2 or FH2core was transfected. The amount of Rho-GTP bound, of total RhoA, and of expressed FH2 and FH2core proteins was analyzed by Western blotting. (G) Shown is FH2-induced actin polymerizing versus FH2core capping activity using pyrene-actin assembly assays with indicated components. (H) Expression plasmids for FH2 or FH2core were transfected with an SRF reporter. Data represent the mean ± SEM of three independent experiments. (I) Dia1 is required for RhoA activation by LPA as shown by siRNA. Cells were untreated or stimulated with LPA (10 μM, 5 min) as indicated, and activated RhoA was precipitated. Cell extracts were immunoblotted for the indicated proteins. (J) HEK293 cells were transfected with Flag-Dia1ΔDAD and increasing amounts of expression plasmids encoding dnLARG (1.0, 5.0 μg). Activated RhoA and cell extracts were immunoblotted for the indicated proteins. All Western blot quantifications shown are the mean ± SEM of at least two independent experiments..
Next we tested the possibility that Dia1 may be part of a signal transduction pathway toward RhoA. For this we stimulated cells with LPA under conditions in which Dia1 expression was suppressed by RNA interference. We observed that Dia1 is required for full RhoA activation by LPA (Fig. 1I), indicating that Dia1 is an essential component of LPA signal transduction toward RhoA. Since this function is separable from the actin nucleation activity, it is an independent and additional function of Dia1. Activation of RhoA requires GEF activity. We speculated about an involvement of LARG, based on the facts that (1) LARG, like Dia1, is implicated in LPA signaling by interaction with Gα12/13 as well as by using a dominant-negative mutant of LARG (dnLARG) (Vogt et al. 2003; Tanabe et al. 2004; Vazquez-Prado et al. 2004); and (2) the Drosophila LARG homolog dRhoGEF2 colocalizes with Diaphanous at the furrow canal during cellularization (Grosshans et al. 2005). To investigate whether LARG is involved in Dia1-induced stimulation of RhoA, we used the previously characterized dnLARG lacking the DH/PH domains (Fig. 2A; Vogt et al. 2003). Despite relatively low expression levels, dnLARG partially but significantly inhibited Dia1–ΔDAD-induced RhoA activity (Fig. 1J), indicating that LARG is involved in RhoA-GTP formation through Dia1.
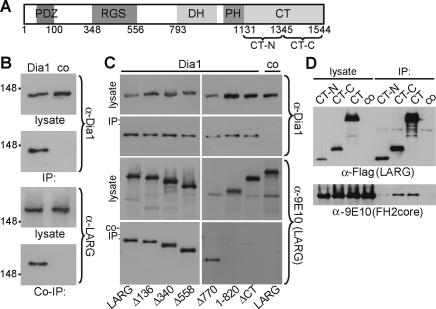
Figure 2.
Dia1 interacts with LARG. (A) Schematic representation of LARG. (RGS) Regulator of G protein signaling; (DH) Dbl homology; (PH) pleckstrin homology. (B) Cells maintained in 10% serum were lysed before immunoprecipitation with α-Dia1 or control antiserum (co). Cell lysates and precipitates (IP) were analyzed by immunoblot as indicated. (C) Lysates were prepared from cells expressing myc-LARG derivatives or full-length LARG. Immunoprecipitates (IP) prepared using α-Dia1 were analyzed for associating LARG derivatives (Co-IP) by immunoblotting as indicated. (D) Lysates from cells expressing FH2core and LARG C-terminal mutants were immunoprecipitated and analyzed by immunoblotting as indicated.
How can Dia1 regulate RhoA activity? Since dnLARG interfered with Dia1-induced RhoA activity and both proteins function in LPA signaling toward RhoA, we tested whether LARG and Dia1 associate. Using Dia1 antibodies, we were able to coimmunoprecipitate Dia1 together with endogenous or transfected LARG (Fig. 2B; Supplementary Fig. 1). We also tested if dishevelled is present, as it was shown to bind the DRF Daam1 for Wnt/Frizzled activation of RhoA during Xenopus gastrulation (Habas et al. 2001), but we did not detect dishevelled in the Dia1/LARG complex (data not shown).
LARG belongs to the RGS domain-containing RhoGEFs, consisting of two additional members, p115RhoGEF and PDZ-RhoGEF (PRG) (Vazquez-Prado et al. 2004). Hence, we assessed the specificity of the Dia1/LARG association. We found that Dia1 interaction with LARG is specific among this RhoGEF family since PRG or p115RhoGEF did not coimmunoprecipitate with Dia1 (Supplementary Fig. 1). However, we cannot exclude the possibility that Dia1 influences additional RhoGEFs or RhoGTPase-regulating proteins.
To determine the regions required for association with Dia1, a series of LARG truncation mutants was tested. Only LARG mutants containing the C terminus coimmunoprecipitated with Dia1 (Fig. 2C). Since the FH2core domain of Dia1 is sufficient to induce RhoA activity, we tested whether the LARG C terminus and the FH2core domain would associate. Indeed, we could coimmunoprecipitate the FH2core domain with the C terminus of LARG (Fig. 2D). To further define the region within the LARG C terminus, we coexpressed its N- or C-terminal regions (CT-N and CT-C, respectively) with FH2core. As shown in Figure 2D, only the C-terminal half of the LARG C terminus efficiently coimmunoprecipitated the FH2core domain, indicating that these regions are responsible for the association. These data were confirmed using protein overlay assays (Supplementary Fig. 2).
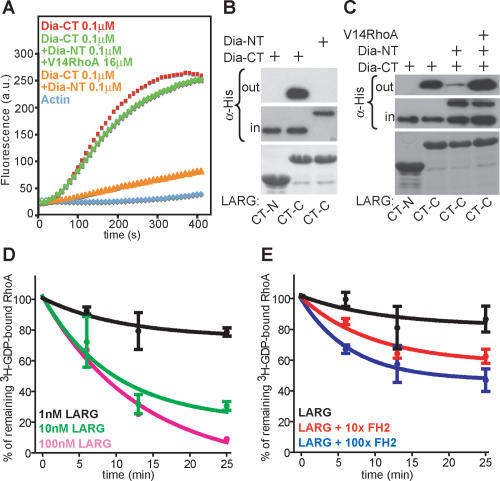
The association of FH2core with the C terminus of LARG suggested that activated Dia1 regulates the LARG C terminus. The release of Dia1 autoinhibition through binding of active RhoA to Dia1-NT (Fig. 1A) can be reconstituted in vitro by measuring FH2-DAD (CT) domain-induced actin polymerization (Fig. 3A; Li and Higgs 2005; Brandt et al. 2007). To address whether autoinhibition of Dia1 regulates its interaction with LARG, we performed GST pull-down assays with LARG CT-C and the Dia1-NT and Dia1-CT fragments in the absence or presence of active RhoAV14. Interestingly, association between Dia1-CT and LARG-CT-C was strongly inhibited by the addition of Dia1-NT (Fig. 3B). Moreover, this inhibition was overcome by the addition of active RhoA to the complex (Fig. 3C). These data demonstrate that the interaction between Dia1 and LARG is controlled by RhoA-induced release of Dia1 autoinhibition. This suggests the existence of a positive feedback loop involving RhoA, Dia1, and LARG. Interestingly, conformational changes of the C terminus of LARG (CT) appear to be mediated by the interaction of N- and C-terminal regions, while the FH2core domain can interfere with CT-N/CT-C binding in a dose-dependent manner (Supplementary Fig. 2), indicating that Dia1 may modulate LARG C-terminal conformation, known to control LARG activity in vivo (Chikumi et al. 2004).
Figure 3.
Dia1 stimulates LARG GEF activity. (A) Actin assembly assays of Dia1-CT show release of NT-induced inhibition by RhoAV14. (B) His-Dia-NT and His-Dia-CT proteins were incubated with GST-LARG C-terminal regions, and complexes were analyzed by immunoblot using α-His antibody. Coomassie staining for GST-LARG CT-N and CT-C is shown in the bottom panel. (C) GST pull-downs show association of LARG CT-C with Dia1-CT and the release of NT-induced binding inhibition by RhoAV14. Complexes were analyzed by immunoblot as indicated. Coomassie staining for GST-LARG CT-N and CT-C is shown in the bottom panel. One representative out of four independent experiments is shown. (D) Indicated concentrations of GST-LARG incubated with [8-3H]GDP-loaded RhoA. [8-3H]GDP-bound RhoA (%) is plotted against incubation time. Shown is the mean ± SEM of three independent experiments. (E) One nanomolar GST-LARG was incubated with [8-3H]GDP-loaded RhoA with 100-fold molar excess of GST or with 10- and 100-fold molar excess of GST-FH2. [8-3H]GDP-bound RhoA (%) is plotted against incubation time. Shown is the mean ± SEM of three independent experiments.
To directly assess if Dia1 affects LARG activity, we performed in vitro GEF assays using a recombinant LARG consisting of the DH/PH domain and the C terminus. LARG efficiently accelerated the release of GDP from RhoA in a dose-dependent manner (Fig. 3D). Under these conditions, the lowest measurable concentration of LARG was used as a baseline activity to which recombinant FH2 was added. This resulted in a concentration-dependent acceleration of LARG activity (Fig. 3E). These results show that Dia1 can stimulate the GEF activity of LARG. We propose that the Dia1/LARG interaction results in conformational changes of regulatory regions within the LARG C terminus.
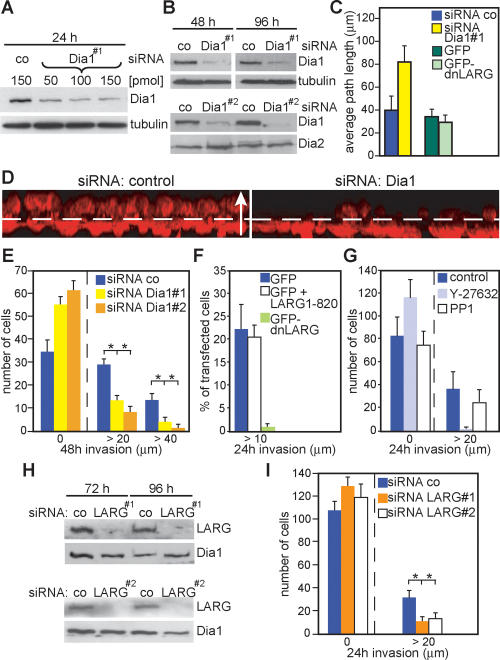
LARG was identified as a gene fusion rearranged in AML (Kourlas et al. 2000). Up-regulated activities of RhoA have been reported for a variety of human cancers (Sahai and Marshall 2002; Lozano et al. 2003). Here we show that Dia1 promotes RhoA activity via interaction with LARG and that Dia1 is required for LPA-mediated RhoA activity. Since LPA is known to play an important role in tumor development and progression promoting cancer cell metastasis and invasion (Mills and Moolenaar 2003), we tested the role of Dia1 in tumor cell invasion. One mode of tumor cell invasion in three-dimensional (3D) matrices is represented by the rounded mode of motility, for which Rho/ROCK signaling is necessary and sufficient (Sahai and Marshall 2003; Wyckoff et al. 2006). After optimizing the conditions for small interfering RNA (siRNA_ toward Dia1 (Fig. 4A,B), we used highly invasive MDA-MB-435 human cancer cells (Sellappan et al. 2004; Rae et al. 2006), which express Dia1, Dia2 (Fig. 4B), and Dia3 (data not shown), and tested their ability to migrate into LPA-containing 3D matrices when Dia1 or Dia2 expression was down-regulated. Interestingly, knockdown of Dia1 efficiently and significantly inhibited the total number of tumor cells that have invaded as well as the overall invasion distance (Fig. 4D,E; Supplementary Videos 1, 2), whereas random MDA-MB-435 cell migration under two-dimensional (2D) tissue culture conditions was slightly increased (Fig. 4C). Knockdown of Dia2 did not inhibit cell invasion (data not shown). Similar results were obtained in A375m2 melanoma cells (data not shown), which also use the rounded mode of motility (Sahai and Marshall 2003). We next determined the involvement of LARG in MDA-MB-435 cell invasion. For this, we transfected GFP-dnLARG, GFP alone, or cotransfected with LARG1–820 as a control (transfection efficiencies were ∼1%) and assessed the GFP-positive cells invaded into the Matrigel. This revealed that dnLARG inhibited cell invasion (Fig. 4F) but not random 2D migration (Fig. 4C). Inhibition of ROCK with 10 μM Y27632 blocked invasion of MDA-MB-435 cells as expected, while treatment with the Src-kinase inhibitor PP1 had no effect (Fig. 4G). Consistently, usage of siRNAs against LARG significantly reduced cell invasion (Fig. 4H,I). Together, these data suggest that LARG is involved in cancer cell invasion.
Figure 4.
Dia1 is required for MDA-MB-435 cancer cell invasion. (A) Cells treated with different amounts of siRNA as indicated and analyzed by immunoblotting. (B) Cells treated with control siRNA or siRNA against Dia1 were immunoblotted for the indicated proteins. (C) Cells transfected with indicated siRNAs or plasmids were analyzed for random motility. Data are expressed as mean ± SD of three independent experiments. (D) Representative 3D reconstructions of invaded cells transfected with siRNA as indicated. Cells were stained for F-actin (red); dashed line indicates the approximate position of the Transwell membrane; the arrow (50 μm) indicates the direction of movement. (E) Cells were allowed to invade for 48 h into Matrigel before counting at indicated invasion distances. The average of cell number from three independent experiments ±SD is shown. (F) MDA-MB-435 cells transfected with indicated plasmids were analyzed in invasion assays. The percentage of GFP-positive cells invaded is shown from two independent experiments counting >300 cells. (G) The number of cells invaded into Matrigel containing LPA and indicated inhibitors is shown. (H,I) Cells treated with indicated siRNAs were analyzed for invasion.
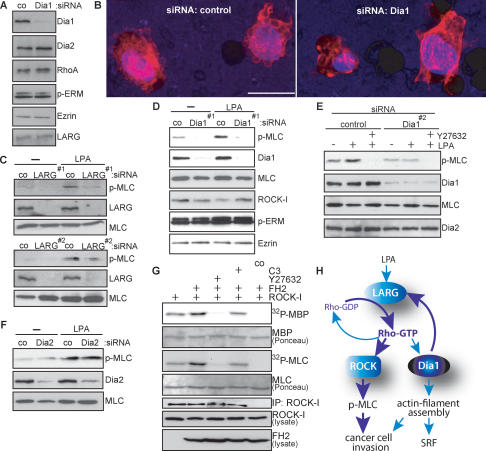
In order to understand better the mechanisms underlying the dependence on Dia1 for tumor cell invasion, we analyzed the MDA-MB-435 cell morphologies in Matrigel. We observed that these cells use the rounded bleb-associated mode of motility (Supplementary Video 3), while in 2D culture these cells displayed a mesenchymal-like cell morphology (data not shown). Analysis of F-actin using phalloidin staining revealed that untreated or control siRNA-treated MDA-MB-435 cells showed extensive formation of membrane blebs when contacting and invading Matrigel (Fig. 5B; Supplementary Video 4). However, cells that were silenced for Dia1 (Fig. 5A) displayed strikingly different morphologies and lost their ability to promote membrane blebbing (Fig. 5B; Supplementary Video 5).
Figure 5.
Dia1 is required for ROCK signaling and cancer cell morphology. (A) MDA-MB-435 cells were treated with control siRNA or siRNA against Dia1, and cell extracts were immunoblotted for the indicated proteins. (B) Representative 3D reconstructions of MDA-MB-435 cells treated in A invading through the porous membrane into the Matrigel. Cells were stained using DAPI (blue) to visualize nuclei and rhodamine-phalloidin (red) to visualize F-actin. Bar, 20 μm. (C_–_F) MDA-MB-435 cells treated with indicated siRNAs were stimulated with 10 μM LPA for 10 min. Cell extracts were immunoblotted for the indicated proteins. (G) myc-ROCK-I was immunoprecipitated from HEK293 cells cotransfected with Flag-FH2 or C3 or treated with 10 μM Y27632 as indicated, and subjected to kinase assays using MBP or MLC as substrates. Kinase reactions were separated by SDS-PAGE, and phosphorylated substrates were visualized by autoradiography (32P-MBP, 32P-MLC). Immobilized rabbit IgG was used as a control (co). One representative out of three independent experiments with similar results is shown. (H) Proposed hypothetical model for Dia1-mediated feedback activation of RhoA through LARG.
Bleb-associated cell invasion requires ROCK (Sahai and Marshall 2003) to control contractile force through myosin activity (Riento and Ridley 2003). When basal as well as LPA-stimulated phosphorylation of myosin light chain (MLC), a ROCK substrate (Riento and Ridley 2003), was determined in MDA-MB-435 cells in the presence or absence of siRNA against LARG or Dia1, we found that MLC phosphorylation was strongly reduced (Fig. 5C–E) in contrast to Dia2 siRNA (Fig. 5F). To investigate whether Dia1 promotes ROCK activity through RhoA, we used HEK293 cells to efficiently express the FH2 domain with ROCK-I before determining in vitro kinase activities using either MBP or MLC as substrates. Interestingly, we found that active Dia1 increased ROCK activity in a Rho-dependent manner (Fig. 5G). These results clearly show that activation of RhoA by Dia1 leads to an increase in ROCK activity to promote the MLC phosphorylation required for bleb-associated cell invasion.
Dia1 is a well-established downstream effector of active RhoA. Our results presented here reveal an unexpected and essential role for Dia1 in the activation of the Rho/ROCK signal transduction pathway and subsequent bleb-associated cancer cell motility. We provide evidence that Dia1 is required and sufficient for full LPA-induced activation of RhoA and downstream ROCK signaling. This effect can be mediated through interaction of Dia1 with LARG. The data shown here imply a novel signaling module by which Dia1, in addition to its role as a downstream RhoA effector, can function upstream of RhoA. This constitutes a positive feedback mechanism (Fig. 5H) amplifying signal-regulated cellular effects such as tumor cell invasion by activating RhoA and its effector ROCK.
Materials and methods
Plasmids, siRNA, transfections, and cell-based assays
Plasmids were generated using standard procedures. All siRNA oligos were from IBA. GTPase pull-down assays, immunoprecipitations, and Rho-kinase and SRF activity assays were performed in HEK293 or NIH3T3 cells. See the Supplemental Material for details.
Protein purification, actin assembly, and guanyl-nucleotide exchange assays
Proteins were produced and purified in Escherichia coli strain DE3 as GST or His fusions. Actin polymerization was monitored as described (Brandt et al. 2007). Guanyl-nucleotide exchange assays were performed using GST-RhoA loaded with [8-3H]GDP. See the Supplemental Material for full details.
3D matrigel invasion and confocal analysis
Human MDA-MB-435 cancer cells were used for invasion assays. Assays were analyzed using confocal microscopy (Leica TCS SP2). See the Supplemental Material for full details.
Acknowledgments
We thank R. Treisman and B. Di Ventura for critical reading of the manuscript. We are indebted to G. Stier for plasmids, reagents, and expertise in protein purification. We thank S. Offermanns for support. We thank S. Narumiya and S. Gutkind for plasmids and A. Rippberger and J. Stastná for technical assistance. O.T.F. is supported by the CHS Stiftung. This work was funded by the Emmy Noether Program of the DFG (GR2111/1-2) to R.G.
Footnotes
References
- Brandt D.T., Marion S., Griffiths G., Watanabe T., Kaibuchi K., Grosse R., Marion S., Griffiths G., Watanabe T., Kaibuchi K., Grosse R., Griffiths G., Watanabe T., Kaibuchi K., Grosse R., Watanabe T., Kaibuchi K., Grosse R., Kaibuchi K., Grosse R., Grosse R. Dia1 and IQGAP1 interact in cell migration and phagocytic cup formation. J. Cell Biol. 2007 doi: 10.1083/jcb.200612071. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikumi H., Barac A., Behbahani B., Gao Y., Teramoto H., Zheng Y., Gutkind J.S., Barac A., Behbahani B., Gao Y., Teramoto H., Zheng Y., Gutkind J.S., Behbahani B., Gao Y., Teramoto H., Zheng Y., Gutkind J.S., Gao Y., Teramoto H., Zheng Y., Gutkind J.S., Teramoto H., Zheng Y., Gutkind J.S., Zheng Y., Gutkind J.S., Gutkind J.S. Homo- and hetero-oligomerization of PDZ-RhoGEF, LARG and p115RhoGEF by their C-terminal region regulates their in vivo Rho GEF activity and transforming potential. Oncogene. 2004;23:233–240. doi: 10.1038/sj.onc.1207012. [DOI] [PubMed] [Google Scholar]
- Copeland J.W., Treisman R., Treisman R. The diaphanous-related formin mDia1 controls serum response factor activity through its effects on actin polymerization. Mol. Biol. Cell. 2002;13:4088–4099. doi: 10.1091/mbc.02-06-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faix J., Grosse R., Grosse R. Staying in shape with formins. Dev. Cell. 2006;10:693–706. doi: 10.1016/j.devcel.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Gomez T.S., Kumar K., Medeiros R.B., Shimizu Y., Leibson P.J., Billadeau D.D., Kumar K., Medeiros R.B., Shimizu Y., Leibson P.J., Billadeau D.D., Medeiros R.B., Shimizu Y., Leibson P.J., Billadeau D.D., Shimizu Y., Leibson P.J., Billadeau D.D., Leibson P.J., Billadeau D.D., Billadeau D.D. Formins regulate the actin-related protein 2/3 complex-independent polarization of the centrosome to the immunological synapse. Immunity. 2007;26:177–190. doi: 10.1016/j.immuni.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode B.L., Eck M.J., Eck M.J. Mechanism and function of formins in control of actin assembly. Annu. Rev. Biochem. 2007;76:32.1–32.35. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- Goulimari P., Kitzing T.M., Knieling H., Brandt D.T., Offermanns S., Grosse R., Kitzing T.M., Knieling H., Brandt D.T., Offermanns S., Grosse R., Knieling H., Brandt D.T., Offermanns S., Grosse R., Brandt D.T., Offermanns S., Grosse R., Offermanns S., Grosse R., Grosse R. Gα12/13 is essential for directed cell migration and localized Rho-Dia1 function. J. Biol. Chem. 2005;280:42242–42251. doi: 10.1074/jbc.M508690200. [DOI] [PubMed] [Google Scholar]
- Grosse R., Copeland J.W., Newsome T.P., Way M., Treisman R., Copeland J.W., Newsome T.P., Way M., Treisman R., Newsome T.P., Way M., Treisman R., Way M., Treisman R., Treisman R. A role for VASP in RhoA-diaphanous signalling to actin dynamics and SRF activity. EMBO J. 2003;22:3050–3061. doi: 10.1093/emboj/cdg287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans J., Wenzl C., Herz H.M., Bartoszewski S., Schnorrer F., Vogt N., Schwarz H., Muller H.A., Wenzl C., Herz H.M., Bartoszewski S., Schnorrer F., Vogt N., Schwarz H., Muller H.A., Herz H.M., Bartoszewski S., Schnorrer F., Vogt N., Schwarz H., Muller H.A., Bartoszewski S., Schnorrer F., Vogt N., Schwarz H., Muller H.A., Schnorrer F., Vogt N., Schwarz H., Muller H.A., Vogt N., Schwarz H., Muller H.A., Schwarz H., Muller H.A., Muller H.A. RhoGEF2 and the formin Dia control the formation of the furrow canal by directed actin assembly during Drosophila cellularisation. Development. 2005;132:1009–1020. doi: 10.1242/dev.01669. [DOI] [PubMed] [Google Scholar]
- Habas R., Kato Y., He X., Kato Y., He X., He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- Kourlas P.J., Strout M.P., Becknell B., Veronese M.L., Croce C.M., Theil K.S., Krahe R., Ruutu T., Knuutila S., Bloomfield C.D., Strout M.P., Becknell B., Veronese M.L., Croce C.M., Theil K.S., Krahe R., Ruutu T., Knuutila S., Bloomfield C.D., Becknell B., Veronese M.L., Croce C.M., Theil K.S., Krahe R., Ruutu T., Knuutila S., Bloomfield C.D., Veronese M.L., Croce C.M., Theil K.S., Krahe R., Ruutu T., Knuutila S., Bloomfield C.D., Croce C.M., Theil K.S., Krahe R., Ruutu T., Knuutila S., Bloomfield C.D., Theil K.S., Krahe R., Ruutu T., Knuutila S., Bloomfield C.D., Krahe R., Ruutu T., Knuutila S., Bloomfield C.D., Ruutu T., Knuutila S., Bloomfield C.D., Knuutila S., Bloomfield C.D., Bloomfield C.D., et al. Identification of a gene at 11q23 encoding a guanine nucleotide exchange factor: Evidence for its fusion with MLL in acute myeloid leukemia. Proc. Natl. Acad. Sci. 2000;97:2145–2150. doi: 10.1073/pnas.040569197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers M., Rose R., Scrima A., Wittinghofer A., Rose R., Scrima A., Wittinghofer A., Scrima A., Wittinghofer A., Wittinghofer A. The regulation of mDia1 by autoinhibition and its release by Rho*GTP. EMBO J. 2005;24:4176–4187. doi: 10.1038/sj.emboj.7600879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Higgs H.N., Higgs H.N. Dissecting requirements for auto-inhibition of actin nucleation by the formin, mDia1. J. Biol. Chem. 2005;280:6986–6992. doi: 10.1074/jbc.M411605200. [DOI] [PubMed] [Google Scholar]
- Lozano E., Betson M., Braga V.M., Betson M., Braga V.M., Braga V.M. Tumor progression: Small GTPases and loss of cell–cell adhesion. Bioessays. 2003;25:452–463. doi: 10.1002/bies.10262. [DOI] [PubMed] [Google Scholar]
- Mills G.B., Moolenaar W.H., Moolenaar W.H. The emerging role of lysophosphatidic acid in cancer. Nat. Rev. Cancer. 2003;3:582–591. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- Miralles F., Posern G., Zaromytidou A.I., Treisman R., Posern G., Zaromytidou A.I., Treisman R., Zaromytidou A.I., Treisman R., Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329–342. doi: 10.1016/s0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- Otomo T., Otomo C., Tomchick D.R., Machius M., Rosen M.K., Otomo C., Tomchick D.R., Machius M., Rosen M.K., Tomchick D.R., Machius M., Rosen M.K., Machius M., Rosen M.K., Rosen M.K. Structural basis of Rho GTPase-mediated activation of the formin mDia1. Mol. Cell. 2005a;18:273–281. doi: 10.1016/j.molcel.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Otomo T., Tomchick D.R., Otomo C., Panchal S.C., Machius M., Rosen M.K., Tomchick D.R., Otomo C., Panchal S.C., Machius M., Rosen M.K., Otomo C., Panchal S.C., Machius M., Rosen M.K., Panchal S.C., Machius M., Rosen M.K., Machius M., Rosen M.K., Rosen M.K. Structural basis of actin filament nucleation and processive capping by a formin homology 2 domain. Nature. 2005b;433:488–494. doi: 10.1038/nature03251. [DOI] [PubMed] [Google Scholar]
- Rae J.M., Creighton C.J., Meck J.M., Haddad B.R., Johnson M.D., Creighton C.J., Meck J.M., Haddad B.R., Johnson M.D., Meck J.M., Haddad B.R., Johnson M.D., Haddad B.R., Johnson M.D., Johnson M.D. MDA-MB-435 cells are derived from M14 melanoma cells—A loss for breast cancer, but a boon for melanoma research. Breast Cancer Res. Treat. 2006 doi: 10.1007/s10549-006-9392-8. [DOI] [PubMed] [Google Scholar]
- Riento K., Ridley A.J., Ridley A.J. Rocks: Multifunctional kinases in cell behaviour. Nat. Rev. Mol. Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- Sahai E. Mechanisms of cancer cell invasion. Curr. Opin. Genet. Dev. 2005;15:87–96. doi: 10.1016/j.gde.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Sahai E., Marshall C.J., Marshall C.J. RHO–GTPases and cancer. Nat. Rev. Cancer. 2002;2:133–142. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- Sahai E., Marshall C.J., Marshall C.J. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat. Cell Biol. 2003;5:711–719. doi: 10.1038/ncb1019. [DOI] [PubMed] [Google Scholar]
- Sellappan S., Grijalva R., Zhou X., Yang W., Eli M.B., Mills G.B., Yu D., Grijalva R., Zhou X., Yang W., Eli M.B., Mills G.B., Yu D., Zhou X., Yang W., Eli M.B., Mills G.B., Yu D., Yang W., Eli M.B., Mills G.B., Yu D., Eli M.B., Mills G.B., Yu D., Mills G.B., Yu D., Yu D. Lineage infidelity of MDA-MB-435 cells: Expression of melanocyte proteins in a breast cancer cell line. Cancer Res. 2004;64:3479–3485. doi: 10.1158/0008-5472.CAN-3299-2. [DOI] [PubMed] [Google Scholar]
- Shimada A., Nyitrai M., Vetter I.R., Kuhlmann D., Bugyi B., Narumiya S., Geeves M.A., Wittinghofer A., Nyitrai M., Vetter I.R., Kuhlmann D., Bugyi B., Narumiya S., Geeves M.A., Wittinghofer A., Vetter I.R., Kuhlmann D., Bugyi B., Narumiya S., Geeves M.A., Wittinghofer A., Kuhlmann D., Bugyi B., Narumiya S., Geeves M.A., Wittinghofer A., Bugyi B., Narumiya S., Geeves M.A., Wittinghofer A., Narumiya S., Geeves M.A., Wittinghofer A., Geeves M.A., Wittinghofer A., Wittinghofer A. The core FH2 domain of diaphanous-related formins is an elongated actin binding protein that inhibits polymerization. Mol. Cell. 2004;13:511–522. doi: 10.1016/s1097-2765(04)00059-0. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos A., Gineitis D., Copeland J., Treisman R., Gineitis D., Copeland J., Treisman R., Copeland J., Treisman R., Treisman R. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell. 1999;98:159–169. doi: 10.1016/s0092-8674(00)81011-9. [DOI] [PubMed] [Google Scholar]
- Tanabe S., Kreutz B., Suzuki N., Kozasa T., Kreutz B., Suzuki N., Kozasa T., Suzuki N., Kozasa T., Kozasa T. Regulation of RGS-RhoGEFs by Gα12 and Gα13 proteins. Methods Enzymol. 2004;390:285–294. doi: 10.1016/S0076-6879(04)90018-3. [DOI] [PubMed] [Google Scholar]
- Tsuji T., Ishizaki T., Okamoto M., Higashida C., Kimura K., Furuyashiki T., Arakawa Y., Birge R.B., Nakamoto T., Hirai H., Ishizaki T., Okamoto M., Higashida C., Kimura K., Furuyashiki T., Arakawa Y., Birge R.B., Nakamoto T., Hirai H., Okamoto M., Higashida C., Kimura K., Furuyashiki T., Arakawa Y., Birge R.B., Nakamoto T., Hirai H., Higashida C., Kimura K., Furuyashiki T., Arakawa Y., Birge R.B., Nakamoto T., Hirai H., Kimura K., Furuyashiki T., Arakawa Y., Birge R.B., Nakamoto T., Hirai H., Furuyashiki T., Arakawa Y., Birge R.B., Nakamoto T., Hirai H., Arakawa Y., Birge R.B., Nakamoto T., Hirai H., Birge R.B., Nakamoto T., Hirai H., Nakamoto T., Hirai H., Hirai H., et al. ROCK and mDia1 antagonize in Rho-dependent Rac activation in Swiss 3T3 fibroblasts. J. Cell Biol. 2002;157:819–830. doi: 10.1083/jcb.200112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Prado J., Basile J., Gutkind J.S., Basile J., Gutkind J.S., Gutkind J.S. Modular architecture and novel protein–protein interactions regulating the RGS-containing Rho guanine nucleotide exchange factors. Methods Enzymol. 2004;390:259–285. doi: 10.1016/S0076-6879(04)90017-1. [DOI] [PubMed] [Google Scholar]
- Vogt S., Grosse R., Schultz G., Offermanns S., Grosse R., Schultz G., Offermanns S., Schultz G., Offermanns S., Offermanns S. Receptor-dependent RhoA activation in G12/G13-deficient cells: Genetic evidence for an involvement of Gq/G11. J. Biol. Chem. 2003;278:28743–28749. doi: 10.1074/jbc.M304570200. [DOI] [PubMed] [Google Scholar]
- Wyckoff J.B., Pinner S.E., Gschmeissner S., Condeelis J.S., Sahai E., Pinner S.E., Gschmeissner S., Condeelis J.S., Sahai E., Gschmeissner S., Condeelis J.S., Sahai E., Condeelis J.S., Sahai E., Sahai E. ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr. Biol. 2006;16:1515–1523. doi: 10.1016/j.cub.2006.05.065. [DOI] [PubMed] [Google Scholar]
- Xu Y., Moseley J.B., Sagot I., Poy F., Pellman D., Goode B.L., Eck M.J., Moseley J.B., Sagot I., Poy F., Pellman D., Goode B.L., Eck M.J., Sagot I., Poy F., Pellman D., Goode B.L., Eck M.J., Poy F., Pellman D., Goode B.L., Eck M.J., Pellman D., Goode B.L., Eck M.J., Goode B.L., Eck M.J., Eck M.J. Crystal structures of a Formin Homology-2 domain reveal a tethered dimer architecture. Cell. 2004;116:711–723. doi: 10.1016/s0092-8674(04)00210-7. [DOI] [PubMed] [Google Scholar]