Pigment Analysis of “Candidatus Chlorothrix halophila,” a Green Filamentous Anoxygenic Phototrophic Bacterium (original) (raw)
Abstract
The pigment composition of “Candidatus Chlorothrix halophila,” a filamentous anoxygenic phototrophic bacterium found in Baja California Sur, Mexico, was determined. Previous work showed that bacteriochlorophyll c (BChl c) was the major pigment in “Ca. Chlorothrix halophila,” but it was not clear if this bacterium also contains BChl a (J. A. Klappenbach and B. K. Pierson, Arch. Microbiol. **181:**17-25, 2004). Here we show that in addition to BChl c, a small amount of a pigment that is spectrally indistinguishable from BChl a is present in cell extracts of “Ca. Chlorothrix halophila.” Nevertheless, the BChl a_-like pigment from “_Ca. Chlorothrix halophila” has a different molecular weight and a different high-performance liquid chromatography elution time than BChl a from other photosynthetic bacteria. Based on mass spectrometry and other spectroscopic analysis, we determined that the BChl a_-like pigment in “_Ca. Chlorothrix halophila” contains a tetrahydrogeranylgeraniol tail rather than the phytol tail that is present in BChl a. The carotenoids and major BChl c homologs in “Ca. Chlorothrix halophila” were also identified. BChls c were found to be farnesol esterified and geranylgeraniol esterified.
The photosynthetic prokaryotes consist of five major groups. These groups are the cyanobacteria and four groups of anoxygenic photosynthetic bacteria which are purple, green sulfur, green filamentous, and gram-positive bacteria. All of these organisms contain photosynthetic pigments, either chlorophyll (Chl) or bacteriochlorophyll (BChl), which are involved in excitation energy transfer and the electron transport system of photosynthesis. The green sulfur bacteria (Chlorobiaceae) and the green filamentous bacteria (Chloroflexaceae) comprise two families of photosynthetic green bacteria that contain a unique light-harvesting antenna structure called a chlorosome, which is located on the inner side of the cytoplasmic membrane (4). This light-harvesting antenna complex contains a large amount of BChl c, d, or e and a small amount of BChl a (13, 32). The total amount of BChl in each chlorosome has been estimated to be ∼200,000 molecules (21). The two members of the photosynthetic green bacteria that have been studied most are Chlorobium tepidum in the green sulfur group and Chloroflexus aurantiacus in the green filamentous group.
Recently, “Candidatus Chlorothrix halophila,” a green filamentous anoxygenic phototrophic (FAP) bacterium, was obtained from an enrichment culture from a hypersaline microbial mat community in Guerrero Negro, Mexico (17). This organism is provisionally named pending a formal microbiological description. This bacterium has gliding motility and requires a solid substrate to grow. All other green FAP organisms contain BChl a; however, this pigment has not been detected previously in “Ca. Chlorothrix halophila” (17).
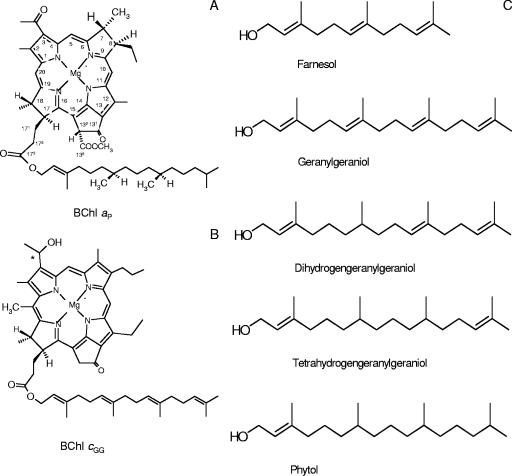
BChl c and BChl a have generally similar chemical structures and contain a chlorin or bacteriochlorin macrocycle, which is composed of four pyrrole rings (rings A to D) and an isocyclic ring (ring E), plus an esterified alcohol tail (Fig. 1). However, BChl c is different from BChl a because it has an oxidized ring B and the methoxycarbonyl group is not attached at C-132 on ring E (3). Since the spectroscopic properties of all BChls result primarily from the bacteriochlorin head group, reduction of ring B causes the absorption spectrum of BChl a to be redshifted to a longer wavelength compared to the spectrum of BChl c. In the Qy region of the spectrum, BChl a has maximum absorption at 772 nm in acetone (28), while BChl c has maximum absorption at 662 nm in organic solvents (2, 15). Both C. tepidum and C. aurantiacus contain both BChl a and BChl c (23, 31). BChl a is found in most of the photosynthetic bacterial reaction centers and antenna proteins, but the amount of BChl a is highly variable in different organisms. In C. tepidum, BChl a accounts for ∼3% of the total pigments (12). In contrast to BChl a, BChl c is only a peripheral light-harvesting antenna pigment in the chlorosome (4). BChl c is the major pigment of green photosynthetic bacteria and can account for 90 to 95% of the total pigment. It is usually present as a mixture of closely related pigments (25).
FIG. 1.
Molecular structures of BChl _a_P (A), BChl _c_GG (B), and esterifying alcohols (C).
Bacteriochlorins of BChls are esterified at C-172 with different alcohol ester groups. The most frequent esterifying alcohol in BChl is phytol (5, 8, 29). The BChl a macrocycle is commonly esterified with phytol (BChl _a_P). However, other alcohols, such as geranylgeraniol (GG), dihydrogeranylgeraniol (DHGG), and tetrahydrogeranylgeraniol (THGG), have also been observed in purple bacteria, usually at trace levels (20). In plants, these esterifying alcohols are thought to represent biosynthetic intermediates of Chl _a_GG to Chl _a_P (22, 30). Nevertheless, only the chemical structure of the geranylgeranyl-esterifying tail in Rhodospirillum rubrum BChl a has been determined by nuclear magnetic resonance and mass spectrometry (MS) (16). In C. tepidum and C. aurantiacus, BChl a is esterified with a phytol tail. BChl c is esterified with farnesol in C. tepidum (12), while it is esterified with a variety of straight and branched hydrocarbons in C. aurantiacus (9).
Besides a variety of esterifying alcohols at C-172, BChls c, d, and e are usually found as mixtures due to different alkyl substituents at C-7, C-8, C-12, and C-20. Molecules with different substituents at these positions make up a complex collection of chlorosome light-harvesting pigments. The porphyrin ring systems of these BChls allow different combinations of alkyl groups at each of these five positions; consequently, one BChl may consist of a large number of structurally related molecules (6, 7). In Chlorobium limicola, a green sulfur bacterium, up to six different BChls c and six different BChls d are present (10). Although they have different alkyl substituents at C-8 and C-12 and different esterifying alcohols at C-172, these BChl c homologs exhibit nearly indistinguishable absorption characteristics in solution, although there are spectral differences in the intact chlorosomes (7, 10, 24). BChl c has some special characteristics, including the absence of the bulky carboxymethyl group at C-132 that allows tight packing of BChls to form large oligomers and the presence of the hydroxyethyl substituent at C-3, which together play major roles in pigment oligomerization and chlorosome formation (3).
In addition to BChls, green anoxygenic phototrophs also contain a variety of carotenoids, most of which are located in the chlorosomes (13). In photosynthesis, carotenoids play important roles in light energy harvesting and photoprotection (3). Four different types of carotenes have been found in C. tepidum, including γ-carotene, chlorobactene, 1′,2′-dihydro-γ-carotene, and 1′,2′-dihydrochlorobactene (31). Compared with C. tepidum, C. aurantiacus contains other carotenoids, such as β-carotene and OH-γ-carotene glucoside ester in addition to γ-carotene (23).
In this work, we analyzed the pigment composition of “Ca. Chlorothrix halophila” to verify the presence of the pigments that play important roles in the photosynthetic apparatus. Identification of the pigments should permit a closer look at the photosynthetic apparatus and should aid in resolving the mechanisms that are used for energy and electron transfer in “Ca. Chlorothrix halophila.”
MATERIALS AND METHODS
Cell culture.
Cells of “Ca. Chlorothrix halophila” were grown in MCLO medium (17) on a sandy substrate in anaerobic conditions in culture bottles. The bottles were filled to the top and sealed to reduce the oxygen present in the medium. The temperature was maintained at 35 to 38°C by using a 60-W soft white incandescent bulb, which also provided 20 μmol photons m−2 s−1.
Electron microscopy.
Cells of “Ca. Chlorothrix halophila” were fixed semisimultaneously for 30 min by addition of glutaraldehyde and osmium tetroxide at final concentrations of 0.5 and 1%, respectively, directly to the culture medium. The cells were rinsed with cacodylate buffer that had been made in MCLO medium, followed by a 1:1 solution of cacodylate buffer and double-distilled H2O and then pure double-distilled H2O before dehydration in acetone and embedding in Spurr's resin (17). Thin (70-nm) sections were cut using an Ultracut R microtome (Leica, Vienna, Austria), poststained with 2% uranyl acetate in 50% ethanol for 5 min and then with Reynolds' lead citrate for 5 min (27), and then viewed with a CM12S transmission electron microscope (Philips Electronic Instruments Co., Mahwah, NJ) at 80 kV. Images were captured digitally with a 1,024- by 1,024-pixel charge-coupled device camera (Gatan Inc., Pleasanton, CA) using Digital Micrograph software.
Pigment analysis.
Approximately 0.5 g (wet weight) of cells was used for pigment extraction. Cells were suspended in 0.5 ml of acetone-methanol (7:3, vol/vol) and sonicated for 2 min, and the cell pellet was removed by centrifugation. The pigment extraction procedure was repeated three times to ensure that most pigments from the cells were extracted in the organic solvent. The supernatants from centrifugations were pooled and concentrated in vacuo. The pigment extract was applied to an Agilent series 1100C high-performance liquid chromatography (HPLC) system using an XDB C18 reversed-phase column (4.6 by 150 mm; Agilent Technologies). Pigments were eluted with an acetone-water elution gradient that started with 75% acetone and increased to 85% acetone in 55 min. To elute carotenoids, the solvent was changed from 85% acetone to 100% acetone for 5 min and kept constant for another 10 min. The flow rate was 1 ml/min. All pigments were detected at 370 nm; however, BChl a was also detected at 770 nm and carotenoids were also detected at 490 nm. Pigments eluted by HPLC were collected for further analysis.
The ratio of BChl a to BChl c was calculated from the absorption maxima and the extinction coefficients. The extinction coefficient of BChl a in acetone is 69.0 mM−1 cm−1 at 772 nm (28), and the extinction coefficient of BChl c in acetone is 64.0 mM−1 cm−1 at 662 nm (2, 15).
MS.
Matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) MS and tandem MS were used to determine the molecular masses of individual pigments obtained from the HPLC analysis (Applied Biosystems Mass Spec Voyager DE-STR). Individual pigments were resuspended in acetone and mixed with a terthiophene matrix at a ratio of 1:1.
To verify the identities of the esterifying alcohols in BChls c, we performed gas chromatography (GC)-MS with the esterifying alcohols which were separated from bacteriochlorins by a transesterification reaction (10). Briefly, BChls c from “Ca. Chlorothrix halophila” and other green photosynthetic bacteria separated by HPLC were hydrolyzed in 30% methanolic KOH with agitation for 60 min at room temperature. The esterifying alcohol was extracted in diethyl ether and washed with water three or four times. The ether layer was dried under a nitrogen gas stream and resuspended in dichloromethane for GC-MS analysis.
Not enough BChl a was collected by HPLC to be analyzed by GC-MS, but the BChl a was analyzed by MALDI-TOF MS.
RESULTS
Morphological characteristics of “Ca. Chlorothrix halophila.”
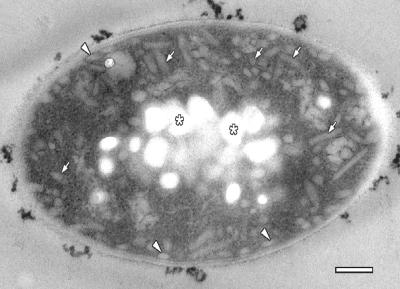
“Ca. Chlorothrix halophila” cells are approximately 5 μm long and 1 μm wide (Fig. 2). The cytoplasm of the cell has two distinct morphologies. The inner central region of each cell is filled with electron-translucent granules, which are probably composed of polyhydroxyalkanoate. The cytoplasm from the granules to the cytoplasmic membrane contains chlorosomes that appear to be dispersed throughout this region; however, the chlorosomes are most likely associated with invaginated membrane components, as observed in previous studies (26). Chlorosomes are also appressed to the cytoplasmic membrane, as they are in other green photosynthetic bacteria. The cytoplasmic membrane and cell wall form the outermost layers of the cell.
FIG. 2.
Electron micrograph of a tangential section through a cell of “Ca. Chlorothrix halophila.” Chlorosomes are present throughout the cytoplasm (arrows) and are most likely attached to invaginated membrane structures. Chlorosomes are also appressed to the cytoplasmic membrane (arrowheads). The chlorosomes are sectioned at various angles and appear as small circles or oblong structures. The center of the cell contains granules that are probably composed of polyhydroxyalkanoate (asterisks). Scale bar = 200 nm.
Absorption properties.
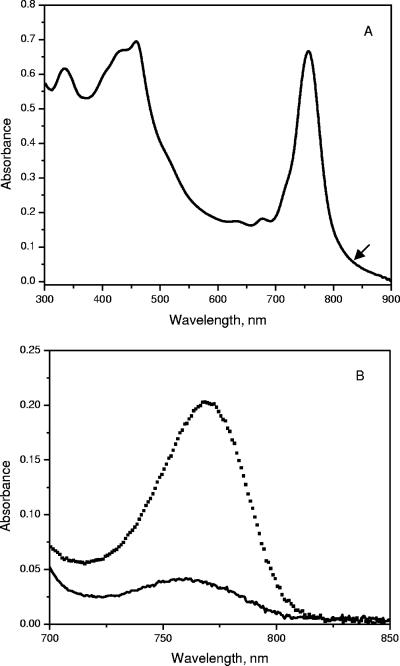
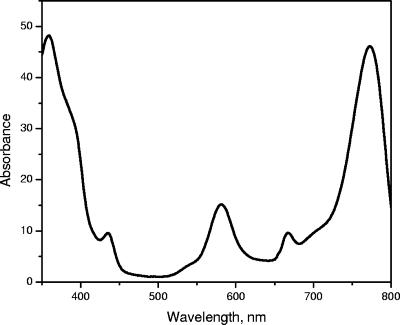
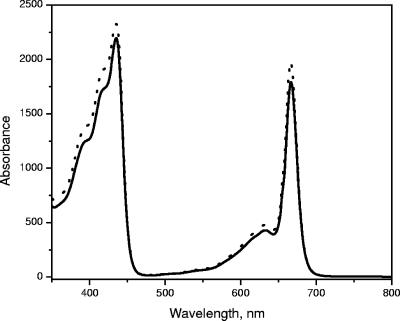
The absorption spectra of whole cells of “Ca. Chlorothrix halophila” have peaks at 461, 683, 759 nm and a shoulder around 850 nm (Fig. 3A). In organic solvent, the cell extract has an absorption band at 772 nm, which indicates that BChl a or a related pigment is present (Fig. 3B). The amount of BChl a in “Ca. Chlorothrix halophila” was compared to the amount in Chlorobium tepidum by normalization at the absorption maximum of BChl c at 662 nm. Based on the absorption maximum of BChl a at 772 nm and its extinction coefficient at this wavelength, the amount of BChl a in “Ca. Chlorothrix halophila” appeared to be less than one-fifth the amount in C. tepidum relative to the BChl c content.
FIG. 3.
Absorption spectra of whole cells of “Ca. Chlorothrix halophila” (A) and of the BChl a region of a “Ca. Chlorothrix halophila” cell extract in acetone-methanol (7:3, vol/vol) (solid line) and Chlorobium tepidum (dotted line) normalized at 662 nm (B).
HPLC and MS analysis of pigment composition. (i) BChl a.
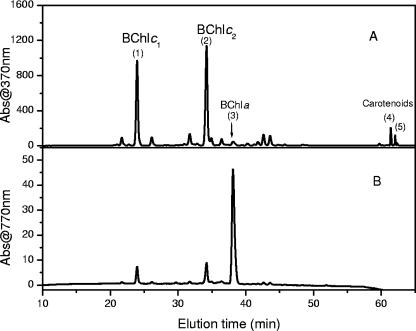
The HPLC elution profile of a “Ca. Chlorothrix halophila” pigment extract is shown in Fig. 4. The presumptive identities of individual peaks based on absorption spectra are indicated in Fig. 4A. The HPLC chromatogram obtained at 770 nm was used to detect the presence of BChl a. The absorption spectrum for this peak recorded by the HPLC detector had a maximum at 775 nm in the Qy region and a maximum at 584 nm in the Qx region (Fig. 5). A small shoulder around 670 nm was due to minor contamination by BChl c homologs in the BChl a peak. The BChl _a_-like pigment peak was collected, and the molecular mass was determined by MALDI-TOF MS.
FIG. 4.
HPLC chromatograms of “Ca. Chlorothrix halophila” extracted in acetone-methanol (7:3, vol/vol) detected at 370 and 770 nm.
FIG. 5.
Absorption spectrum of “Ca. Chlorothrix halophila” BChl a in acetone. The spectrum was recorded by using a DAD detector with an Agilent series 1100 HPLC. The small peak at 665 nm was due to a small amount of BChl c that eluted at the same time as BChl a.
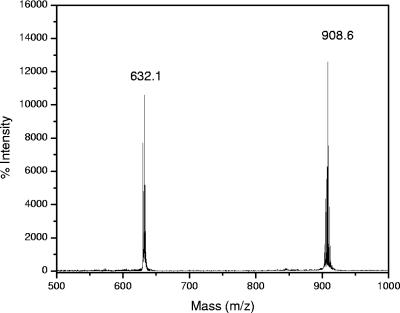
The results of MS showed that there was a major [M+H+] peak at 908.6 Da. In comparison with the molecular mass of BChl _a_P from Rhodobacter sphaeroides, which was 910.6 Da, the molecular mass of the BChl _a_-like pigment was 2 Da less. For further mass analysis, we performed postsource decay MS (MS-MS) with this pigment. The principle of this method is that only a specific selected ion (a precursor ion) and its fragment (daughter ion) are allowed to pass through a well-defined mass gate and reach the detector. For our purposes, the BChl _a_-like pigment with a molecular mass of 908.6 Da was used as the precursor ion. The results of the MS-MS analysis showed that there was a peak at 632.1 Da and that there was a peak at 908.6 Da with a higher intensity (Fig. 6). The molecular mass of the first peak is the molecular mass of BChl a bacteriochlorin macrocycle, and the molecular mass of the second peak is the molecular mass of the precursor ion. The difference between these two masses is 276.5 Da, which is the mass of the esterifying alcohol. Similar tests were performed with Rhodospirillum rubrum BChl _a_GG and R. sphaeroides BChl _a_P. Our MS-MS results showed that there was a 272.4-Da difference between the macrocycle and the precursor ion for BChl _a_GG and that there was 278.4-Da difference between the macrocycle and the precursor ion for BChl _a_P. This indicates that the esterifying alcohol of the BChl a_-like pigment from “_Ca. Chlorothrix halophila” contains four H more atoms than GG contains but two fewer H atoms than phytol contains. These results strongly suggest that the esterifying alcohol in this pigment is THGG.
FIG. 6.
Postsource decay mass spectrum of “Ca. Chlorothrix halophila” BChl _a_-like pigment. The peak at a molecular mass of 632.1 Da is the bacteriochlorin macrocycle, and the peak at a molecular mass of 908.6 Da is the intact BChl _a_-like pigment.
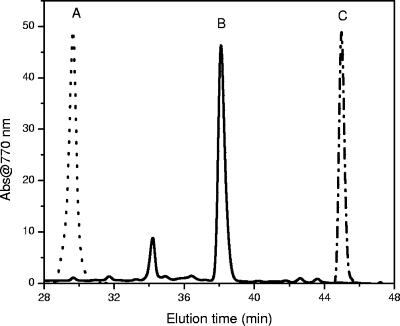
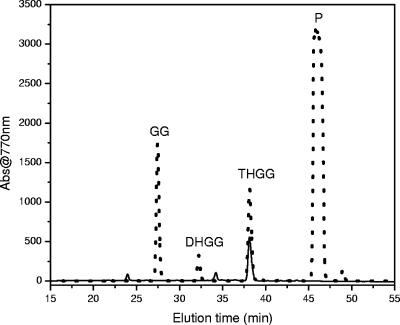
To confirm that our BChl a sample was not a product of reduction of GG or oxidation of phytol during processing, we compared the HPLC elution times of the BChl a_-like pigment from “_Ca. Chlorothrix halophila” to the HPLC elution times of BChl _a_GG from R. rubrum and BChl _a_P from C. tepidum. Consistent with the MS-MS results, the BChl _a_-like pigment had a different elution time than BChl _a_GG or BChl _a_P. The BChl _a_-like pigment eluted at 38.4 min, compared to 29.8 min for BChl aGG and 44.6 min for BChl aP (Fig. 7). Since the bacteriochlorin macrocycles of BChl _a_GG, BChl _a_P, and BChl a_THGG are identical, the different HPLC elution times result from the different alcohols esterifying these BChls a. The position of the carbon-carbon double bond was determined by comparison of the HPLC elution time of BChl a from “_Ca. Chlorothrix halophila” with the elution times of Rhodopseudomonas palustris BChls a (Fig. 8). Previous studies showed that the pigments of R. palustris included BChl _a_GG, BChl _a_DHGG, BChl _a_THGG, and BChl a_P, and the positions of the carbon-carbon double bonds in the esterifying alcohols were determined (20). Our results show that the elution time of BChl a from “_Ca. Chlorothrix halophila” is identical to the elution time of R. palustris BChl _a_THGG. Therefore, the carbon-carbon double bond is at the two ends of the esterifying alcohol, as reported previously (20). We were not able to identify the minor peak at 34 min in Fig. 7, since the amount of this peak was insufficient.
FIG. 7.
HPLC chromatograms of BChls a from different green photosynthetic bacteria detected at 770 nm. Line A, R. rubrum; line B, “Ca. Chlorothrix halophila”; line C, Chlorobium tepidum.
FIG. 8.
HPLC chromatograms of BChls a from “Ca. Chlorothrix halophila” (solid line) and R. palustris (dotted line). P, phytol.
(ii) BChl c.
Although the elution times of the two major BChl c peaks from HPLC were very distinct, the absorption spectra had almost identical maxima, which were at 665 nm in the Qy region and at 445 nm in the Soret region (Fig. 9). MS of these two BChls c revealed [M+H+] peaks at 820.3 Da (BChl _c_1) and 888.3 Da (BChl _c_2). Using an approach similar to that used to determine the identity of the BChl a esterified alcohol tail, MS-MS was performed for both BChls c. Although the precursor ions chosen for fragmentation had different masses, 820.3 and 888.3 Da, both daughter ions of these BChls c had the same mass, 616.0 Da. This is the molecular mass expected for a BChl c bacteriochlorin macrocycle substituted either with propyl and ethyl or with isobutyl and methyl at C-8 and C-12, respectively. Based on the MS-MS findings, BChl _c_1 and BChl _c_2 have the same bacteriochlorin macrocycle but different esterifying alcohols. The 68.0-Da difference corresponds to the molecular mass of one isoprene unit that makes up the esterifying alcohols in these BChls.
FIG. 9.
Absorption spectra of BChl _c_1 (solid line) and BChl _c_2 (dotted line) recorded with an Agilent HPLC UV-VIS detector.
Gas chromatograms showed that the elution times of the esterifying alcohols from BChl _c_1 and BChl _c_2 were identical to those of C. tepidum farnesol and R. rubrum GG, respectively. In addition, the mass spectra of these alcohols, BChl _c_1 with farnesol and BChl _c_2 with GG, were 90 to 95% identical (data not shown). The remaining 5 to 10% was due to random noise.
Based on the MALDI-TOF MS and GC-MS results, we propose that BChl _c_1 is esterified with farnesol and that BChl _c_2 is esterified with GG. A summary of the molecular masses of BChls a and c determined by MS and MS-MS is shown in Table 1.
TABLE 1.
Summary of determination of pigments in “Ca. Chlorothrix halophila” by MS
| Peak | Pigment | [M+H+] peak (Da) | Esterifying alcohol | Mass peaks determined by MS-MS (Da) | ||
|---|---|---|---|---|---|---|
| Intact BChl | BChl macrocycle | Alkyl fragment | ||||
| 1 | BChl _c_1 | 820.3 | Farnesol | 820.3 | 616.0 | 204.3 |
| 2 | BChl _c_2 | 888.3 | GG | 888.3 | 615.9 | 272.3 |
| 3 | BChl a | 908.6 | THGG | 908.6 | 632.2 | 276.4 |
| 4 | γ-Carotene | 536.4 | NAa | NA | NA | NA |
| 5 | β-Carotene | 536.4 | NA | NA | NA | NA |
(iii) Carotenoids.
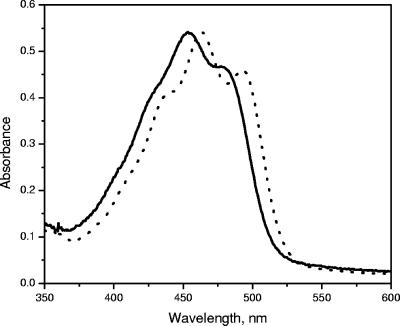
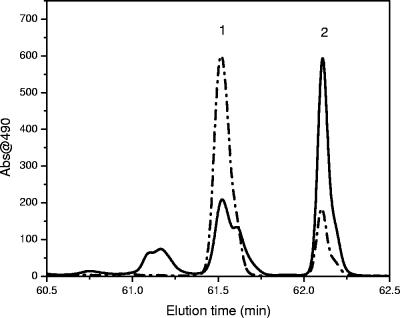
“Ca. Chlorothrix halophila” contains two major carotenoids that eluted very close to each other on HPLC. The first carotenoid eluted at 61.5 min, and the second carotenoid eluted at 62.1 min (Fig. 4). The absorption spectra of these carotenoids are shown in Fig. 10. HPLC chromatograms of “Ca. Chlorothrix halophila” and Chloroflexus aurantiacus detected at 490 nm were used to determine the identities of these carotenoids (Fig. 11). The elution time of carotenoid 1 matched that of γ-carotene, and the elution time of carotenoid 2 matched that of β-carotene from C. aurantiacus.
FIG. 10.
Absorption spectra of carotenoids 1 (solid line) and 2 (dotted line) from “Ca. Chlorothrix halophila.”
FIG. 11.
HPLC chromatograms of carotenoids from “Ca. Chlorothrix halophila” (dashed and dotted line) and Chloroflexus aurantiacus (solid line). Peak 1 is γ-carotene, and peak 2 is β-carotene.
γ-Carotene and β-carotene from C. aurantiacus and “Ca. Chlorothrix halophila” were collected from HPLC and analyzed using MS. The results of the MS analysis showed that both of the carotenoids from C. aurantiacus, as well as the carotenoid from “Ca. Chlorothrix halophila,” had the same molecular mass, 536.4 Da. Based on the similarity of the “Ca. Chlorothrix halophila” and C. aurantiacus carotenoids determined in the HPLC and MS analyses, the two major carotenoids in “Ca. Chlorothrix halophila” were identified as γ-carotene (carotenoid 1) and β-carotene (carotenoid 2).
DISCUSSION
The Chlorobiaceae and the Chloroflexaceae, the two major families in the photosynthetic green bacterial class, are distinctive because of their unique light-harvesting antennae (chlorosomes) containing an enormous amount of BChl c, BChl d, or BChl e. A comparison of the pigment compositions of two representatives of these groups, Chlorobium tepidum and Chloroflexus aurantiacus, respectively, showed that the chlorosomes of both organisms contain BChls c as the major pigments and a small amount of BChl a. In addition, a variety of carotenoids have been studied and identified. In C. tepidum, the major carotenoid is chlorobactene, while C. aurantiacus contains other carotenes, such as β-carotene and γ-carotene (12). Carotenoids play major roles in light harvesting and in quenching the triplet state of BChl a and BChl c in these organisms (11, 18).
The Chloroflexaceae family is divided into two major groups, the green FAP group, whose members contain both BChl c and BChl a, and the red FAP group, whose members contain only BChl a (17). Phylogenetic and physiological characterizations of “Ca. Chlorothrix halophila” have classified this organism in the green FAP group, which also contains C. aurantiacus.
The pigment composition analysis of “Ca. Chlorothrix halophila” in this work revealed the presence of BChl a, BChl c, and two major carotenoids and no evidence of Chl a, BChl d, or BChl e. BChl a esterified with THGG is a minor pigment, while the major pigment is BChl c esterified with GG. No BChl a esterified with phytol was found. The two major carotenoids are γ-carotene and β-carotene.
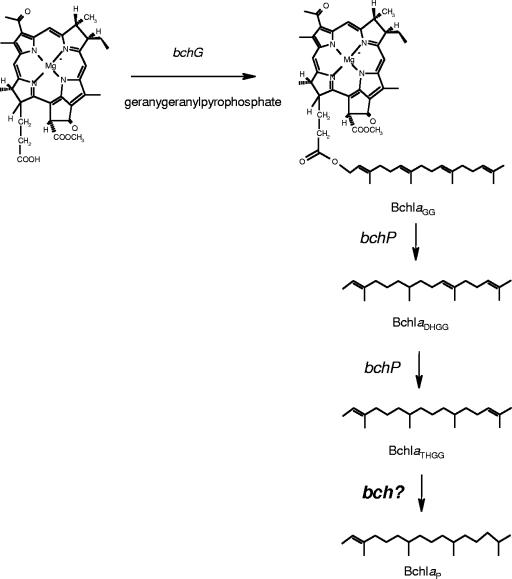
The natural occurrence of the esterifying alcohol THGG in BChl a is the most unusual feature of the pigment composition of “Ca. Chlorothrix halophila.” Previously, THGG has been found to be an esterifying alcohol in R. palustris (20) and to be a minor product resulting from two-step GG hydrogenation in R. sphaeroides (1). It is almost certainly a biosynthetic intermediate in which the precursor GG, which has four double bonds, is only partially reduced on the way to phytol, which has one double bond (Fig. 12). This reduction is carried out by the BchP gene product, which performs three successive reductions to convert GG to phytol as the final step in BChl biosynthesis (1). THGG has also been found in Chl a and green angiosperms, but it is subsequently converted to phytol as the final product (1, 22, 30). Since an insignificant amount of this alcohol was found, its photosynthetic function was questionable. Here, we report that in “Ca. Chlorothrix halophila” BChl _a_THGG is a final product of BChl a biosynthesis and is not an intermediate in the hydrogenation from GG.
FIG. 12.
Proposed pathway for biosynthesis of BChl _a_THGG to BChl _a_P and hypothetical presence of an additional bch gene.
A pathway for phytylation of bacleriochlorophyllide a in R. sphaeroides has been proposed, in which bacleriochlorophyllide a is initially esterified with GG but is quickly hydrogenated and reduced to DHGG, THGG, and finally phytol (1). In addition, Addlesee and Hunter also suggested that there is a BchP gene product that carries out all three hydrogenation reactions. We have shown that in “Ca. Chlorothrix halophila” the final product is not phytol but THGG. This suggests that a BchP-like gene product might be responsible for the first two hydrogenation reactions but not for the last hydrogenation reaction or that the BchP gene product in “Ca. Chlorothrix halophila” has a specificity different than that found in other anoxygenic phototrophs. It is conceivable that in R. sphaeroides another gene product is responsible for the conversion of BChl _a_THGG to BChl a_P and that “_Ca. Chlorothrix halophila” lacks this gene (Fig. 11).
Like many green phototrophic bacteria, “Ca. Chlorothrix halophila” contains a large number of BChl c homologs (12 different types based on the HPLC chromatogram and the absorption spectra). However, the two major esterifying alcohols in “Ca. Chlorothrix halophila” are GG and farnesol. GG was found to be a major alcohol of only BChl a in R. rubrum, and its structure was determined (16). In green sulfur bacteria, a small amount of this esterifying alcohol was found in BChl c of C. tepidum (14) and C. limicola (10). This is another distinctive feature of the pigment composition of “Ca. Chlorothrix halophila.”
As in C. aurantiacus, both γ-carotene and β-carotene were found in “Ca. Chlorothrix halophila.” Although both of these green FAP organisms contain γ-carotene and β-carotene, in “Ca. Chlorothrix halophila” γ-carotene is the dominant carotenoid instead of β-carotene, which is the dominant carotenoid in C. aurantiacus. Although γ-carotene and β-carotene are isomers, γ-carotene has absorption maxima at 439, 461, and 439 nm in acetone that are red shifted almost 10 nm compared to the absorption maxima of β-carotene (19).
In a previous publication, the authors indicated that “Ca. Chlorothrix halophila” is a member of the green FAP group, the same group that contains C. aurantiacus, which contains both BChl c and BChl a, in contrast to the red FAP group, in which only BChl a is found (17). These two organisms have similar photosynthetic antenna pigments, including BChl c, BChl a, and carotenoids. The nature of the reaction center and integral membrane antenna complexes in “Ca. Chlorothrix halophila” is not yet clear; however, our preliminary studies of this organism have suggested that the reaction center and integral membrane antenna complexes in “Ca. Chlorothrix halophila” are significantly different from the reaction center and integral membrane antenna complexes found in any other known phototroph.
Since the amount of BChl a in the cell extracts of “Ca. Chlorothrix halophila” was very small, we considered the possibility that the unusual BChl a_-like pigment was a contaminant from purple bacteria that may have been present in the enrichment culture as minor constituents. However, fluorescence excitation spectra showed that photons absorbed by BChl c were transferred to BChl a, clearly indicating that the two pigments are present in the same cell (33). Experiments to isolate an axenic culture of “_Ca. Chlorothrix halophila” are in progress in our laboratories, and this should help in the effort to verify the pigment composition of this bacterium. “Ca. Chlorothrix halophila” is the only known photosynthetic bacterium in which the bulk of the BChl a is esterified with THGG.
Conclusions.
We determined the pigment composition of cell extracts of “Ca. Chlorothrix halophila,” which contained BChl c, BChl a, γ-carotene, and β-carotene. This is a typical pigment composition for the green filamentous phototrophs, which include “Ca. Chlorothrix halophila.” The most unusual features of the pigment composition of “Ca. Chlorothrix halophila” are the presence of BChl c that is esterified with GG and the presence of BChl a that is esterified with THGG.
Acknowledgments
We thank Joanne Williams for providing R. rubrum and R. sphaeroides cultures and Michael Madigan for providing a culture of R. palustris. We are thankful for discussions with and technical assistance with MS provided by Daniel Brune in the Proteomics and Protein Chemistry Laboratory at Arizona State University.
This work was supported by grant DE-FG02-04ER15550 from the Energy Biosciences program of the Basic Energy Sciences Division of the U.S. Department of Energy to R.E.B.
Footnotes
▿
Published ahead of print on 16 March 2007.
REFERENCES
- 1.Addlesee, H. A., and N. C. Hunter. 1999. Physical mapping and functional assignment of the geranylgeranyl-bacteriochlorophyll reductase gene, bchP, of Rhodobacter sphaeroides. J. Bacteriol. 181**:**7248-7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartczak, A., A. Dudkowiak, and D. Frackowiak. 2003. Dipole strengths of the Qy (0, 0) bacteriochlorophyll c transition. Photochem. Photobiol. 78**:**525-528. [DOI] [PubMed] [Google Scholar]
- 3.Blankenship, R. E. 2002. Molecular mechanisms of photosynthesis. Blackwell Science, Oxford, United Kingdom.
- 4.Blankenship, R. E., J. M. Olson, and M. Miller. 1995. Antenna complexes from green photosynthetic bacteria, p. 399-435. In R. E. Blankenship, M. T. Madigan, and C. E. Bauer (ed.), Anoxygenic phototrophic bacteria. Kluwer, Dordrecht, The Netherlands.
- 5.Bollivar, D. W., S. Wang, J. Allen, and C. E. Bauer. 1994. Molecular genetic analysis of terminal steps in bacteriochlorophyll a biosynthesis: characterization of Rhodobacter capsulatus strain that synthesizes geranylgeraniol-esterified bacteriochlorophyll a. Biochemistry 33**:**12763-12768. [DOI] [PubMed] [Google Scholar]
- 6.Borrego, C. M., J. B. Arellano, C. A. Abella, T. Gillbro, and L. J. Garcia-Gil. 1999. The molar extinction coefficient of bacteriochlorophyll e and the pigment stoichiometry in Chlorobium phaeobacteroides. Photosynth. Res. 60**:**257-264. [Google Scholar]
- 7.Borrego, C. M., and L. J. Garcia-Gil. 1994. Separation of bacteriochlorophyll homologs from green phototrophic sulfur bacteria by reversed-phase HPLC. Photosynth. Res. 41**:**157-163. [DOI] [PubMed] [Google Scholar]
- 8.Brown, A. E., and J. Lascelles. 1972. Phytol and bacteriochlorophyll synthesis in Rhodopseudomonas spheroides. Plant Physiol. 50**:**747-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brune, D. C., T. Nozawa, and R. E. Blankenship. 1987. Antenna organization in green photosynthetic bacteria. 1. Oligomeric bacteriochlorophyll c as a model for the 740nm absorbing bacteriochlorophyll c in Chloroflexus aurantiacus chlorosomes. Biochemistry 26**:**8644-8652. [DOI] [PubMed] [Google Scholar]
- 10.Caple, M. B., H.-C. Chow, and C. E. Strouse. 1978. Photosynthetic pigments of green sulfur bacteria—the esterifying alcohols of bacteriochlorophylls c from Chlorobium limicola. J. Biol. Chem. 253**:**6730-6737. [PubMed] [Google Scholar]
- 11.Carbonera, D., E. Bordignon, G. Giacometti, G. Agostini, A. Vianelli, and C. Vannini. 2001. Fluorescence and absorption detected magnetic resonance of chlorosomes from green bacteria Chlorobium tepidum and _Chloroflexus aurantiacus_—a comparative study. J. Phys. Chem. B 105**:**246-255. [Google Scholar]
- 12.Frigaard, N.-U., and D. A. Bryant. 2004. Seeing green bacteria in a new light: genomics-enabled studies of the photosynthetic apparatus in green sulfur bacteria and filamentous anoxygenic phototrophic bacteria. Arch. Microbiol. 182**:**265-276. [DOI] [PubMed] [Google Scholar]
- 13.Frigaard, N.-U., S. Takaichi, M. Hirota, K. Shimada, and K. Matsuura. 1997. Quinones in chlorosomes of green sulfur bacteria and their role in the redox-dependent fluorescence studied in chlorosome-like bacteriochlorophyll c aggregates. Arch. Microbiol. 167**:**343-349. [Google Scholar]
- 14.Frigaard, N.-U., G. D. Voigt, and D. A. Bryant. 2002. Chlorobium tepidum mutant lacking bacteriochlorophyll c made by inactivation of the bchK gene, encoding bacteriochlorophyll c synthase. J. Bacteriol. 184**:**3368-3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoff, A. J., and J. Amesz. 1991. Visible absorption spectroscopy of chlorophylls, p. 723-738. In H. Scheer (ed.), Chlorophylls. CRC Press, Boca Raton, FL.
- 16.Katz, J. J., H. H. Strain, A. L. Harkness, M. H. Studier, W. A. Svec, T. R. Janson, and B. T. Cope. 1972. Esterifying alcohols in the chlorophylls of purple photosynthetic bacteria. A new chlorophyll bacteriochlorophyll (gg), all-trans-geranylgeranyl bacteriochlorophyllide a. J. Am. Chem. Soc. 94**:**7938-7939. [DOI] [PubMed] [Google Scholar]
- 17.Klappenbach, J. A., and B. K. Pierson. 2004. Phylogenetic and physiological characterization of a filamentous anoxygenic photoautotrophic bacterium “Candidatus Chlorothrix halophila” gen. nov., sp. nov., recovered from hypersaline microbial mats. Arch. Microbiol. 181**:**17-25. [DOI] [PubMed] [Google Scholar]
- 18.Melo, T. B., N.-U. Frigaard, K. Matsuura, and K. R. Naqvi. 2000. Electronic energy transfer involving carotenoid pigments in Chlorosomes of two green bacteria: Chlorobium tepidum and Chloroflexus aurantiacus. Spectrochim. Acta Part A 56**:**2001-2010. [DOI] [PubMed] [Google Scholar]
- 19.Mercadante, A. Z., and E. S. Egeland. 2004. Carotenoids handbook. Birkhauser Verlag, Basel, Stwitzerland.
- 20.Mizoguchi, T., J. Harada, and H. Tamiaki. 2006. Structural determination of dihydro- and tetrahydrogeranylgeraniol groups at the 17-propionate of bacteriochlorophylls a. FEBS Lett. 580**:**6644-6648. [DOI] [PubMed] [Google Scholar]
- 21.Montano, G. A., B. P. Bowen, J. T. LaBelle, N. W. Woodbury, V. B. Pizziconi, and R. E. Blankenship. 2003. Characterization of Chlorobium tepidum chlorosomes: a calculation of bacteriochlorophyll c per chlorosomes and oligomer modeling. Biophys. J. 85**:**2560-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura, A., S. Tanaka, and T. Watanabe. 2001. Normal-phase HPLC separation of possible biosynthetic intermediates of pheophytin a and chlorophyll a. Anal. Sci. 17**:**509-513. [DOI] [PubMed] [Google Scholar]
- 23.Oelze, J., and J. R. Golecki. 1995. Membranes and chlorosomes of green bacteria: structure, composition and development, p. 259-278. In R. E. Blankenship, M. T. Madigan, and C. E. Bauer (ed.), Anoxygenic photosynthetic bacteria. Kluwer, Dordrecht, The Netherlands.
- 24.Otte, S. C. M., E. J. van de Meen, P. E. van Veenlen, A. S. Pundsnes, and J. Amesz. 1993. Identification of the major chlorosomal bacteriochlorophylls of the green sulfur bacteria Chlorobium vibrioforme and Chlorobium phaeovibrioides: their function in lateral energy transfer. Photosynth. Res. 35**:**159-169. [DOI] [PubMed] [Google Scholar]
- 25.Pierson, B. K., and R. W. Castenholz. 1974. Studies of pigments and growth in Chloroflexus aurantiacus, a phototrophic filamentous bacterium. Arch. Microbiol. 100**:**283-305. [DOI] [PubMed] [Google Scholar]
- 26.Pierson, B. K., D. Valdez, M. Larsen, E. Morgan, and E. E. Mack. 1994. _Chloroflexus_-like organisms from marine and hypersaline environments: distribution and diversity. Photosynth. Res. 41**:**35-52. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds, E. S. 1963. Use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 17**:**208-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sauer, K., J. H. L. Smith, and A. J. Schulz. 1966. The dimerization of chlorophyll a, chlorophyll b, and bacteriochlorophyll in solution. J. Am. Chem. Soc. 88**:**2681-2688. [Google Scholar]
- 29.Scheer, H. 1991. Structure and occurrence of chlorophylls, p. 3-30. In H. Scheer (ed.), Chlorophylls. CRC Press, Boca Raton, FL.
- 30.Schoch, S. 1978. The esterification of chlorophyllide a in green bean leaves. Z. Naturforsch. Sect. C 33**:**712-714. [Google Scholar]
- 31.Takaichi, S., Z.-Y. Wang, M. Umetsu, T. Nozawa, K. Shimada, and M. T. Madigan. 1997. New carotenoids from the thermophilic green sulfur bacterium Chlorobium tepidum: 1′,2′-dihydro-γ-carotene, 1′,2′ dihydrochlorbactene, and OH-chlorobactene glucoside ester, and the carotenoid composition of different strains. Arch. Microbiol. 168**:**270-276. [DOI] [PubMed] [Google Scholar]
- 32.Tsuji, K., S. Takaichi, K. Matsuura, and K. Shimada. 1995. Specificity of carotenoids in chlorosomes of the green filamentous bacterium, Chloroflexus aurantiacus, p. 99-102. In P. Mathis (ed.), Photosynthesis: from light to biosphere, vol. 4. Kluwer, Dordrecht, The Netherlands. [Google Scholar]
- 33.van de Meene, A. M. L., T. L. Olson, A. M. Collins, and R. E. Blankenship. 2007. Initial characterization of the photosynthetic apparatus of “Candidatus Chlorothrix halophila,” a filamentous, anaerobic photoautotroph. J. Bacteriol. 189**:**4196-4203. [DOI] [PMC free article] [PubMed] [Google Scholar]