Cytokine-dependent imatinib resistance in mouse BCR-ABL+, Arf-null lymphoblastic leukemia (original) (raw)
Abstract
Retroviral transduction of the BCR-ABL kinase into primary mouse bone marrow cells lacking the Arf tumor suppressor rapidly generates polyclonal populations of continuously self-renewing pre-B cells, virtually all of which have leukemic potential. Intravenous infusion of 20 such cells into healthy syngeneic mice induces rapidly fatal, transplantable lymphoblastic leukemias that resist imatinib therapy. Introduction of BCR-ABL into _Arf_-null severe combined immunodeficient (SCID) bone marrow progenitors lacking the cytokine receptor common γ-chain yields leukemogenic pre-B cells that exhibit greater sensitivity to imatinib in vivo. Hence, salutary cytokines in the hematopoietic microenvironment can facilitate leukemic proliferation and confer resistance to targeted therapy.
Keywords: BCR-ABL kinase, Arf tumor suppressor, imatinib (Gleevec), leukemia-initiating cells, cytokines, drug resistance
The Arf tumor suppressor protects against the emergence of oncogene-induced cancers (for reviews, see Kim and Sharpless 2006; Sherr 2006). With few exceptions, Arf is not expressed during fetal development or in young adult mice. However, elevated levels of sustained proliferative signals conveyed by oncogenes induce Arf expression. In turn, by antagonizing the p53-negative regulator Mdm2, the p19Arf protein induces a p53 transcriptional response that triggers either cell cycle arrest or apoptosis, thereby eliminating incipient tumor cells. Deletion or epigenetic silencing of Arf abrogates this form of tumor suppression and, not surprisingly, Arf inactivation is frequently observed in many forms of cancer.
Whereas cultured mouse pre-B cells ultimately senesce, their _Arf_-null counterparts continuously self-renew in culture as long as their proliferation and survival are supported by interleukin-7 (IL-7) (Randle et al. 2001). Not only are immortalized _Arf_-null pre-B cells resistant to BCR-ABL-induced apoptosis, but enforced expression of this kinase bypasses their IL-7 requirement (McLaughlin et al. 1987). Hence, introduction of BCR-ABL into _Arf_-deficient bone marrow progenitors allows rapid ex vivo outgrowth of pre-B-cell populations that induce a highly aggressive form of acute lymphocytic leukemia (ALL) when inoculated intravenously into healthy, nonconditioned syngeneic mice (Williams et al. 2006). These leukemias resist therapy with the BCR-ABL kinase inhibitor imatinib (Gleevec), and surprisingly, drug resistance is non-tumor-cell-autonomous. We have now used this system to quantify the effects of Arf inactivation on the generation of BCR-ABL-induced leukemia-initiating cells (LICs), to characterize the clonality of the LIC population, and to explore the basis of their resistance to targeted therapy.
Results and Discussion
BCR-ABL expression and Arf loss are sufficient to generate ALL
A mouse stem cell retroviral vector (MSCV) expressing the human p185 BCR-ABL isoform and encoding GFP from an internal ribosome entry site was packaged into replication-incompetent ecotropic virions. Marrow extracted from the long bones of C57BL/6 mice was infected and cultured on autologous stroma under conditions that selectively yield pre-B cells (Whitlock and Witte 1987). After 7–8 d of culture, the emerging population uniformly exhibited immunoglobulin (Ig) heavy-chain gene rearrangements (see below); expressed the B-cell markers B220, CD19, and CD24; and lacked expression of stem cell (Sca-1, Kit) or myeloid (Gr-1, Mac1) surface antigens. Thus, by these criteria, these cultures contained uniform populations of pre-B cells.
Intravenous inoculation of 200,000 p185+, Arf+/+ donor pre-B cells (or p185-negative _Arf_−/− cells) into healthy 12-wk-old syngeneic mice failed to induce disease over an 8-mo observation period. However, animals that received only 2000 p185+, _Arf_−/− donor cells were moribund by ∼17 d post-injection, and each 10-fold reduction of donor cells down to a limiting dilution of 20 extended the mean latency of disease by ∼3 d (Table 1). This implied that GFP-marked leukemia cells expanded at a rate that guaranteed a log increase in their number every 3 d (mean generation time of ∼22 h), ultimately leading to replacement of the vast majority of host cells within the bone marrow (Table 1). When retransplanted into secondary recipients, leukemic cells recovered from such mice induced ALL with similar efficiency (Supplementary Table S1). Remarkably, then, Arf inactivation increased the number of p185+ LICs by at least four orders of magnitude (i.e., 20 p185+, _Arf_−/− cells induced disease, but 200,000 p185+, Arf+/+ cells did not).
Table 1.
Leukemia induction and imatinib response of mice receiving donor p185+, _Arf_-null pre-B cells
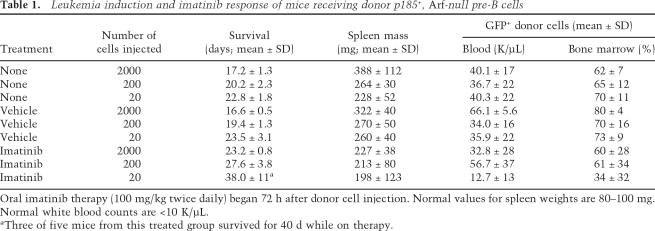
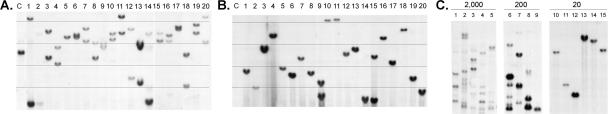
Single donor cells from primary p185+, Arf−/− pre-B-cell cultures were sorted into 96-well dishes by flow cytometry and expanded in the same medium. Each clone exhibited distinct biallelic Ig heavy-chain gene rearrangements (Fig. 1A) and contained one to two proviruses integrated at different sites (Fig. 1B). (Figure 1 shows representative data for 20 clones of >100 so analyzed.) Their polyclonality implies that individual p185+, Arf−/− pre-B cells generated in short-term cultures have not undergone further selection for emergence of more rapidly proliferating “dominant” clones. Moreover, leukemic cells isolated from moribund animals receiving as few as 200 donor cells were also oligoclonal, whereas mono- or biclonal leukemias arose only in animals receiving 20 cells (Fig. 1C). Analyses of individual donor cell clones derived by two different methods revealed that the progeny of at least one of every two cells in the polyclonal p185+, Arf −/− donor population could initiate leukemia (Fig. 2). Recloning of proviruses from leukemias generated at limiting donor cell dilution and nucleotide sequencing of flanking host cellular sequences confirmed that integrations occurred at distinct chromosomal sites (Supplementary Table S2). Although these results do not preclude that insertional mutagenesis or other genetic events might contribute to the relative rate of disease progression, the combination of p185 expression and Arf inactivation is sufficient to generate LICs. Because virtually every donor cell has leukemic capacity, we conclude that these LICs are not rare “cancer stem cells.”
Figure 1.
Assessment of pre-B-cell clonality. (A) DNA extracted from mouse tail (control, lane C) or from 20 clones derived at random from single cells (lanes 1_–_20) was digested with XbaI, electrophoretically separated on agarose gels, transferred to nylon, and hybridized with a JH probe that detects Ig heavy-chain gene rearrangements. The unrearranged germline band is visualized in lane C, whereas two bands observed in the other lanes result from rearrangements affecting the two Ig heavy-chain alleles. The panel represents a composite of data from two parallel gels. (B) The same DNAs were digested with EcoRI and probed for GFP sequences. Because EcoRI recognizes a single restriction site 5′ to the GFP gene, proviruses integrated at different sites yield hybridizing fragments of variable lengths that contain different host cell sequences adjoining their 3′ long-terminal repeat. Each hybridizing band corresponds to a single site of proviral insertion. Clones 9 and 15 sustained two integrations, whereas the remaining clones had one. (C) DNAs extracted from bone marrow of individual leukemic animals that had received 2000, 200, or 20 p185+, _Arf_-null cells (as noted at the top) were studied for viral integrations as in B. Proviruses molecularly cloned from the monoclonal leukemias illustrated in C were integrated at distinct loci on six different chromosomes (Supplementary Table S2).
Figure 2.
Survival of mice injected with clonally-derived pre-B cells. A and C schematically summarize the protocols for generating clones derived from single cells, whereas B and D illustrate the data. (A) _Arf_-null bone marrow cells transduced with p185 were cultured for 8 d to generate pre-B cells. These were subcloned from single cells and expanded for 3 wk, and 20 cells from six randomly chosen clones were infused into cohorts of five syngeneic mice. All such clones contained single proviral insertions and exhibited rearranged Ig heavy-chain alleles. Animals were monitored daily for disease and sacrificed when moribund. (B) Survival curves generated from the experiment summarized in A. (C) To further limit the period of in vitro manipulation, six primary cultures of _Arf_-null bone marrow cells were transduced with p185 and grown under B-cell selective conditions. Eight days later, single cells derived from each primary culture were seeded into individual microwells using an automated cell sorter. Five colonies appearing after only six additional days of culture, each containing ∼50 cells and chosen at random from each 96-well dish, were injected into individual mice (total, 30). (D) The time course and percentage of clones inducing fatal ALL from the experiment outlined in C are indicated.
Imatinib resistance can be conferred by the hematopoietic microenvironment
Mice inoculated with p185+, _Arf_−/− donor cells were treated by oral gavage with a maximum tolerated dose of 100 mg/kg imatinib twice daily. This regimen effectively inhibits the BCR-ABL kinase and induces durable remissions of chronic myelogenous leukemia (CML) in mouse models (Wolff and Ilaria 2001; Hu et al. 2004, 2006) and, at lower doses, in human patients (Druker et al. 2001, 2006). Pharmacokinetic analysis has indicated that this dosing schedule maintains plasma drug levels >1 μM for >8 h (Wolff et al. 2004); this is sufficient to inhibit substrate phosphorylation by BCR-ABL, blocks cyclin D2 synthesis, and prevents the proliferation of leukemic cells in culture (Supplementary Fig. S1A; Williams et al. 2006). Although recipient animals were highly resistant to treatment by imatinib (Table 1), recovered leukemia cells were as sensitive as the donor cells to imatinib-induced proliferative arrest (IC50 ∼100 nM) (Supplementary Fig. S1A). Thus, p185 overexpression or kinase mutations affecting drug binding to p185 did not contribute to imatinib resistance, as directly confirmed by nucleotide sequencing of human BCR-ABL genes amplified by PCR and recloned from leukemic cells (Supplementary Table S3).
Although BCR-ABL alleviates the cytokine dependence of primary _Arf_-null pre-B cells, the IL-7 signaling pathway remains functional; hence, IL-7 addition can attenuate the imatinib response, enabling cells to remain in cycle even when exposed to micromolar drug concentrations (Supplementary Fig. S1A; Williams et al. 2006). To determine whether the failure of ALL cells to respond to imatinib in vivo might be due to cytokine-mediated rescue within the host hematopoietic microenvironment, severe combined immunodeficient (SCID) mice that lack the gene encoding the common γ-chain (γc) required for signaling by multiple cytokine receptors (IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21) were backcrossed onto the _Arf_-null C57BL/6 background (Shou et al. 2006). Bone marrow cells from these mice were briefly transduced in the absence of cytokine support with the p185 vector, and infected cells were infused without intervening culture into lethally irradiated syngeneic recipients. Under these conditions, irradiated mice infused with p185+ wild-type donor cells developed ALL, which was accelerated to a similar degree by Arf or p53 inactivation (Supplementary Fig. S2). Disruption of γc attenuated disease on the _Arf_-null background, although six of nine injected mice still succumbed to ALL during a 2-mo observation period. The pre-B immunophenotype of these leukemic cells was indistinguishable from that of ALLs arising in their γc+ counterparts. Therefore, not only were uncultured p185+, _Arf_-null, γc-null donor cells leukemogenic in irradiated hosts, but BCR-ABL kinase activity can completely bypass the requirement for cytokines normally needed for the commitment, differentiation, proliferation, and survival of pre-B cells.
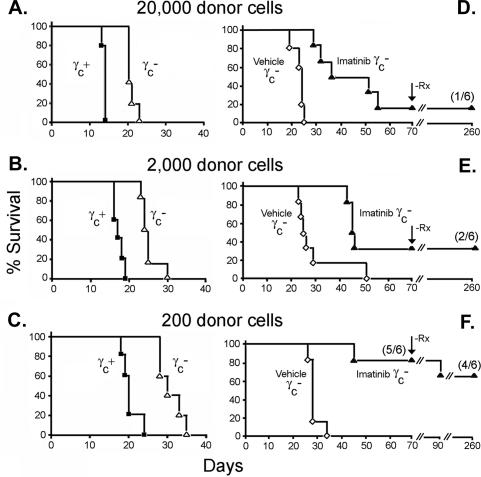
Similarly, when bone marrow cells from _Arf_-null, γc-null SCID mice were transduced with the vector encoding p185+ and GFP, pre-B cells could be expanded in culture. These γc-null pre-B variants were again indistinguishable from their γc+ counterparts with regard to lymphoid morphology, Ig gene rearrangements, and expression of B220, CD19, and CD24. However, as expected, IL-7 addition to p185+, Arf −/−, γc-null pre-B cells ex vivo no longer attenuated their response to imatinib (Supplementary Fig. S1B). These p185+, _Arf_-null, γc-null donor cells induced ALL in healthy mice (Fig. 3A–C). Animals receiving 20,000 p185+ donor cells were moribund by ∼21 d post-injection, whereas each 10-fold reduction of donor cells extended the mean latency by ∼5 d (mean generation time, ∼36 h) as compared with 3 d for their γc+ counterparts (Fig. 3A–C). Thus, although BCR-ABL supplants the cytokine requirement for pre-B-cell development in vitro or in vivo, cytokine signaling contributes significantly to the aggressiveness of leukemia.
Figure 3.
Disease incidence and therapeutic response of leukemias induced with p185+, Arf−/−, γc-null donor cells. Six cohorts of six mice receiving different numbers of donor cells as indicated above the panels were left untreated (A–C) or were treated twice daily with vehicle or imatinib (D–F). Animals were sacrificed when moribund and studied histopathologically to confirm the presence of ALL. Treatment with vehicle alone was without effect. (D–F) Imatinib administration was discontinued in mice that entered clinical remission after 70 d of treatment (vertical arrow, −Rx), and mice were followed for disease reappearance for 180 more days. (F) Only one mouse that received 200 donor cells underwent relapse during the latter period.
This begged the question of whether imatinib treatment might prove effective in animals receiving p185+, _Arf_−/−, γc-null donor cells. Because γc-null LICs were less potent than their γc+ counterparts, we adjusted the donor cell inocula so that vehicle-treated control mice would all become moribund with a mean latency of <30 d. A proportion of mice still failed therapy (Fig. 3D–F). Leukemia cells isolated from diseased animals again lacked BCR-ABL mutations. When necropsied, two mice with extensive dissemination of leukemia cells to spleen and blood exhibited brain involvement, consistent with the inability of imatinib to cross the blood–brain barrier (Wolff et al. 2003). However, gross central nervous system disease was infrequent. In contrast, durable remissions were obtained in a significant number of imatinib-treated mice, yielding long-term survivors (Fig. 3D–F). As would be expected, the frequency of imatinib-induced remissions was highest in the cohort that received the fewest donor cells (five of six animals). After 70 d of treatment, imatinib therapy was discontinued in animals that had survived. Only one of nine such mice that received only 200 donor cells relapsed over an additional 20-d observation period, whereas eight others have remained healthy 180 d post-therapy. Although multiple signaling pathways might well provide barriers to the eradication of these tumors, we conclude that abrogation of cytokine signaling can significantly improve the therapeutic response to BCR-ABL inhibition.
A role for INK4A/ARF in human Philadelphia chromosome-positive (Ph+) ALL?
Targeted inhibition of the BCR-ABL kinase by imatinib has revolutionized the therapy of CML (Wong and Witte 2004; Druker et al. 2006) in which the Ph+ chromosome is detected in long-term repopulating hematopoietic stem cells (HSCs) (Fialkow et al. 1978; Clarkson et al. 2003; Clarke et al. 2006). The disease presents as an indolent myelodysplastic syndrome (the chronic phase) that, when untreated, leads to aggressive myeloid or lymphoid blast crisis. CML conforms to the emerging “cancer stem cell” model (Clarke et al. 2006) in that it arises from a small reservoir of tumorigenic, HSC-like Ph+ progenitor cells that self-renew but also differentiate into heterogeneous blood cell types that, although comprising the bulk of the leukemia, are themselves nontumorigenic and relatively short-lived. In the chronic phase of CML, deletions of CDKN2A (INK4A/ARF) have not been observed, presumably because the locus is normally silenced in stem cells (Jacobs et al. 1999; Park et al. 2003), thereby abrogating its oncogene-induced activation and subsequent selection for loss of function. In this setting, mutations of BCR-ABL account for most drug resistance (Shah et al. 2002), and second-generation inhibitors like dasatinib (Sprycel) and nilotinib that inhibit many mutant forms of the kinase can effectively induce second remissions (Kantarjian et al. 2006; Talpaz et al. 2006; Hochhaus et al. 2007).
In contrast, imatinib has proven less successful in treating Ph+ ALL (Druker et al. 2001; Talpaz et al. 2006; Alvarado et al. 2007; de Labarthe et al. 2007). Although clinical trials of both imatinib and dasatinib in Ph+ ALL have clearly demonstrated the efficacy of both drugs, remissions are of relatively shorter duration due to more rapid outgrowth of resistant subclones bearing BCR-ABL kinase mutations (Talpaz et al. 2006; Hochhaus et al. 2007; O’Hare et al. 2007). In Ph+ ALL arising de novo (and distinguished from lymphoid blast crisis in CML), the LICs appear to be committed lymphoid progenitors (Castor et al. 2005) in which the INK4A/ARF checkpoint can be activated. In turn, deletion of the INK4A/ARF locus occurs in Ph+ ALL cases (Heerema et al. 2004; Primo et al. 2005; Mullighan et al. 2007); indeed, a recent survey of 21 pediatric and 22 adult cases has documented INK4A–ARF deletion in Ph+ ALL blasts taken from ∼50% of patients at diagnosis (Mullighan et al. 2007) (J. Downing, C. Mullighan, R.T. Williams, and C.J. Sherr, unpubl.). In our mouse model, the use of _Arf_-null donor cells negates the requirement for p185-induced deletion and instead leads rapidly to fatal drug-resistant disease, thereby leaving little time to accrue leukemic subpopulations that acquire mutations in BCR-ABL itself. We speculate that in human Ph+ ALL, INK4A–ARF deletion might help maintain LIC survival in the face of targeted drug therapy and facilitate the emergence of drug resistant BCR-ABL variants. As such, INK4A–ARF deletion might prove to be a poor prognostic indicator.
Materials and methods
Gene transduction, cell culture, and transplantation
_Arf_-null mice backcrossed onto a C57Bl/6 background from our own colony (Kamijo et al. 1997) were intercrossed with syngeneic γc-null mice to generate _Arf_-null SCID animals (Shou et al. 2006). MSCV vectors coexpressing human p185 and GFP were provided by Owen Witte (University of California at Los Angeles, Los Angeles, CA) and were packaged into replication-incompetent ecotropic virions as described (Zindy et al. 1998). Marrow extracted from the long bones of mice was suspended in vector-containing supernatant and plated to derive pre-B cells on autologous stroma (Whitlock and Witte 1987; Williams et al. 2006). After 7–8 d of culture, defined numbers of pre-B cells were infused intravenously into cohorts of healthy 12-wk-old C57Bl/6 mice. For some experiments, single cells were sorted into 96-well culture dishes by flow cytometry, and cultures were expanded in the same medium. For transplantation of irradiated recipients, donor bone marrow cells were transduced for 3 h in the absence of cytokine support with a vector encoding p185, and infected cells (1 × 106 per recipient mouse) were infused without intervening culture into lethally irradiated (11 Gy in two fractions) animals. To quantify cytostatic effects of imatinib, cells plated at 2 × 104 per well in 96-well plates were cultured in the presence or absence of 10 ng/mL recombinant IL-7 (R&D Systems), and imatinib (Novartis) was added to achieve the indicated concentrations. Cell growth was quantified 72 h later by a methane–thiosulfonate–base assay (CellTiter 96 Aqueous One Solution reagent, Promega).
Southern blotting and molecular cloning of proviral integrations
Genomic DNA extracted from single-cell-derived pre-B clones or from leukemic bone marrow obtained from moribund mice was digested with XbaI or EcoRI to detect Ig heavy-chain gene rearrangements or proviral integrations, respectively. DNA digests (20 μg per lane) were separated on 0.7% agarose gels and transferred to Nytran SuPerCharge (SPC) nylon membranes (Whatman, Inc.). Membranes were prehybridized at 68°C for at least 1 h with PerfectHyb Plus (Sigma-Aldrich, Inc.) containing 100 μg/mL salmon sperm DNA. Radiolabeled probes prepared with [α-32P] dATP by random priming (Roche Diagnostics) were then added, and hybridization was continued for 16 h. Ig heavy-chain gene rearrangements were visualized using a radiolableled 1.2-kb BamHI–XbaI JH fragment cloned from Balb/c mouse liver (Borzillo and Sherr 1989); a 1.4-kb EcoRI–ClaI IRES-GFP fragment isolated from the MSCV-IRES-GFP retroviral vector was used to detect proviral integrations. Membranes were briefly rinsed in 2× SSC (1× SSC is 0.15 M NaCl/0.015 M Na citrate) containing 0.1% SDS at room temperature followed by a stringent wash in 0.1× SSC/0.1% SDS for 30 min at 60°C. Dried membranes were exposed to Kodak BioMax MR film (Kodak) at −80°C. Proviral integrations were cloned using an inverse PCR technique (Dominici et al. 2004). A detailed description of PCR conditions and primers appears in the legend for Supplementary Table S2.
Monitoring of disease and imatinib treatment
Cohorts of male mice injected intravenously with donor cells were observed daily and sacrificed when moribund (dehydration, ruffled fur, poor mobility, respiratory distress). Treatment was initiated 3 d after cell injection by twice-daily oral gavage with vehicle alone or with imatinib (100 mg/kg) and continued until animals became sick or until the indicated times if mice remained healthy.
Acknowledgments
We gratefully thank S. Wilkerson, H. Briley, and R. Wilson for expert assistance; R.A. Ashmun for flow cytometry; and D. Yons for help maintaining our animal colony. We also acknowledge other members of the Sherr-Roussel laboratories for helpful suggestions and criticisms throughout the course of this work. These studies were supported in part by Cancer Center Core Grant CA-21765 and ALSAC of St. Jude Children’s Research Hospital. C.J.S. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
References
- Alvarado Y., Apostolidou E., Swords R., Giles F.J., Apostolidou E., Swords R., Giles F.J., Swords R., Giles F.J., Giles F.J. Emerging therapeutic options for Philadelphia-positive acute lymphocytic leukemia. Expert Opin. Emerg. Drugs. 2007;12:165–179. doi: 10.1517/14728214.12.1.165. [DOI] [PubMed] [Google Scholar]
- Borzillo G.V., Sherr C.J., Sherr C.J. Early pre-B-cell transformation induced by the v-fms oncogene in long-term mouse bone marrow cultures. Mol. Cell. Biol. 1989;9:3973–3981. doi: 10.1128/mcb.9.9.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castor A., Nilsson L., Astrand-Grundstrom I., Buitenhuis M., Ramirez C., Anderson K., Strombeck B., Garwicz S., Bekassy A.N., Schmiegelow K., Nilsson L., Astrand-Grundstrom I., Buitenhuis M., Ramirez C., Anderson K., Strombeck B., Garwicz S., Bekassy A.N., Schmiegelow K., Astrand-Grundstrom I., Buitenhuis M., Ramirez C., Anderson K., Strombeck B., Garwicz S., Bekassy A.N., Schmiegelow K., Buitenhuis M., Ramirez C., Anderson K., Strombeck B., Garwicz S., Bekassy A.N., Schmiegelow K., Ramirez C., Anderson K., Strombeck B., Garwicz S., Bekassy A.N., Schmiegelow K., Anderson K., Strombeck B., Garwicz S., Bekassy A.N., Schmiegelow K., Strombeck B., Garwicz S., Bekassy A.N., Schmiegelow K., Garwicz S., Bekassy A.N., Schmiegelow K., Bekassy A.N., Schmiegelow K., Schmiegelow K., et al. Distinct patterns of hematopoietic stem cell involvement in acute lymphoblastic leukemia. Nat. Med. 2005;11:630–637. doi: 10.1038/nm1253. [DOI] [PubMed] [Google Scholar]
- Clarke M.F., Dick J.E., Dirks P.B., Eaves C.J., Jamieson C.H.M., Jones D.L., Visvader J., Weissman I.L., Wahl G.M., Dick J.E., Dirks P.B., Eaves C.J., Jamieson C.H.M., Jones D.L., Visvader J., Weissman I.L., Wahl G.M., Dirks P.B., Eaves C.J., Jamieson C.H.M., Jones D.L., Visvader J., Weissman I.L., Wahl G.M., Eaves C.J., Jamieson C.H.M., Jones D.L., Visvader J., Weissman I.L., Wahl G.M., Jamieson C.H.M., Jones D.L., Visvader J., Weissman I.L., Wahl G.M., Jones D.L., Visvader J., Weissman I.L., Wahl G.M., Visvader J., Weissman I.L., Wahl G.M., Weissman I.L., Wahl G.M., Wahl G.M. Cancer stem cells—Perspectives on current status and future directions: AACR workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- Clarkson B., Strife A., Wisniewski D., Lambek C.J., Liu C., Strife A., Wisniewski D., Lambek C.J., Liu C., Wisniewski D., Lambek C.J., Liu C., Lambek C.J., Liu C., Liu C. Chronic myelogenous leukemia as a paradigm of early cancer and possible curative strategies. Leukemia. 2003;17:1211–1262. doi: 10.1038/sj.leu.2402912. [DOI] [PubMed] [Google Scholar]
- de Labarthe A., Rousselot P., Huguet-Rigal F., Delabesse E., Witz F., Maury S., Rea D., Cayuela J.-M., Vekemans M.-C., Reman O., Rousselot P., Huguet-Rigal F., Delabesse E., Witz F., Maury S., Rea D., Cayuela J.-M., Vekemans M.-C., Reman O., Huguet-Rigal F., Delabesse E., Witz F., Maury S., Rea D., Cayuela J.-M., Vekemans M.-C., Reman O., Delabesse E., Witz F., Maury S., Rea D., Cayuela J.-M., Vekemans M.-C., Reman O., Witz F., Maury S., Rea D., Cayuela J.-M., Vekemans M.-C., Reman O., Maury S., Rea D., Cayuela J.-M., Vekemans M.-C., Reman O., Rea D., Cayuela J.-M., Vekemans M.-C., Reman O., Cayuela J.-M., Vekemans M.-C., Reman O., Vekemans M.-C., Reman O., Reman O., et al. Imatinib combined with induction or consolidation chemotherapy in patients with de novo Philadelphia chromosome-positive acute lymphoblastic leukemia: Results of the GRAAPH-2003 study. Blood. 2007;109:1408–1413. doi: 10.1182/blood-2006-03-011908. [DOI] [PubMed] [Google Scholar]
- Dominici M., Pritchard C., Garlits J.E., Hofmann T.J., Persons D.A., Horwitz E.M., Pritchard C., Garlits J.E., Hofmann T.J., Persons D.A., Horwitz E.M., Garlits J.E., Hofmann T.J., Persons D.A., Horwitz E.M., Hofmann T.J., Persons D.A., Horwitz E.M., Persons D.A., Horwitz E.M., Horwitz E.M. Hematopoietic cells and osteoblasts are derived from a common marrow progenitor after bone marrow transplantation. Proc. Natl. Acad. Sci. 2004;101:11761–11766. doi: 10.1073/pnas.0404626101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druker B.J., Talpaz M., Resta D.J., Peng B., Buchdunger E., Ford J.M., Lydon N.B., Kantarjian H., Capdeville R., Ohno-Jones S., Talpaz M., Resta D.J., Peng B., Buchdunger E., Ford J.M., Lydon N.B., Kantarjian H., Capdeville R., Ohno-Jones S., Resta D.J., Peng B., Buchdunger E., Ford J.M., Lydon N.B., Kantarjian H., Capdeville R., Ohno-Jones S., Peng B., Buchdunger E., Ford J.M., Lydon N.B., Kantarjian H., Capdeville R., Ohno-Jones S., Buchdunger E., Ford J.M., Lydon N.B., Kantarjian H., Capdeville R., Ohno-Jones S., Ford J.M., Lydon N.B., Kantarjian H., Capdeville R., Ohno-Jones S., Lydon N.B., Kantarjian H., Capdeville R., Ohno-Jones S., Kantarjian H., Capdeville R., Ohno-Jones S., Capdeville R., Ohno-Jones S., Ohno-Jones S., et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- Druker B.J., Guilhot F., O’Brien S.G., Gathmann I., Kantarjian H., Gattermann N., Deininger M.W., Silver R.T., Goldman J.M., Stone R.M., Guilhot F., O’Brien S.G., Gathmann I., Kantarjian H., Gattermann N., Deininger M.W., Silver R.T., Goldman J.M., Stone R.M., O’Brien S.G., Gathmann I., Kantarjian H., Gattermann N., Deininger M.W., Silver R.T., Goldman J.M., Stone R.M., Gathmann I., Kantarjian H., Gattermann N., Deininger M.W., Silver R.T., Goldman J.M., Stone R.M., Kantarjian H., Gattermann N., Deininger M.W., Silver R.T., Goldman J.M., Stone R.M., Gattermann N., Deininger M.W., Silver R.T., Goldman J.M., Stone R.M., Deininger M.W., Silver R.T., Goldman J.M., Stone R.M., Silver R.T., Goldman J.M., Stone R.M., Goldman J.M., Stone R.M., Stone R.M., et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N. Engl. J. Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- Fialkow P.J., Denman A.M., Jacobsen R.J., Lowenthal M.N., Denman A.M., Jacobsen R.J., Lowenthal M.N., Jacobsen R.J., Lowenthal M.N., Lowenthal M.N. Chronic myelocytic leukemia. Origin of some lymphocytes from leukemic stem cells. J. Clin. Invest. 1978;62:815–823. doi: 10.1172/JCI109193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerema N.A., Harbott J., Galimberti S., Camitta B.M., Gaynon P.S., Janka-Schaub G., Kamps W., Basso G., Pui C.-H., Schrappe M., Harbott J., Galimberti S., Camitta B.M., Gaynon P.S., Janka-Schaub G., Kamps W., Basso G., Pui C.-H., Schrappe M., Galimberti S., Camitta B.M., Gaynon P.S., Janka-Schaub G., Kamps W., Basso G., Pui C.-H., Schrappe M., Camitta B.M., Gaynon P.S., Janka-Schaub G., Kamps W., Basso G., Pui C.-H., Schrappe M., Gaynon P.S., Janka-Schaub G., Kamps W., Basso G., Pui C.-H., Schrappe M., Janka-Schaub G., Kamps W., Basso G., Pui C.-H., Schrappe M., Kamps W., Basso G., Pui C.-H., Schrappe M., Basso G., Pui C.-H., Schrappe M., Pui C.-H., Schrappe M., Schrappe M. Secondary cytogenetic aberrations in childhood Philadelphia chromosome positive acute lymphoblastic leukemia are nonrandom and may be associated with outcome. Leukemia. 2004;18:693–702. doi: 10.1038/sj.leu.2403324. [DOI] [PubMed] [Google Scholar]
- Hochhaus A., Kantarjian H.M., Baccarani M., Lipton J.H., Apperley J.F., Druker B.J., Facon T., Goldberg S.L., Cervantes F., Niederwieser D., Kantarjian H.M., Baccarani M., Lipton J.H., Apperley J.F., Druker B.J., Facon T., Goldberg S.L., Cervantes F., Niederwieser D., Baccarani M., Lipton J.H., Apperley J.F., Druker B.J., Facon T., Goldberg S.L., Cervantes F., Niederwieser D., Lipton J.H., Apperley J.F., Druker B.J., Facon T., Goldberg S.L., Cervantes F., Niederwieser D., Apperley J.F., Druker B.J., Facon T., Goldberg S.L., Cervantes F., Niederwieser D., Druker B.J., Facon T., Goldberg S.L., Cervantes F., Niederwieser D., Facon T., Goldberg S.L., Cervantes F., Niederwieser D., Goldberg S.L., Cervantes F., Niederwieser D., Cervantes F., Niederwieser D., Niederwieser D., et al. Dasatinib induces notable hematologic and cytogenetic responses in chronic-phase chronic myeloid leukemia after failure of imatinib therapy. Blood. 2007;109:2303–2309. doi: 10.1182/blood-2006-09-047266. [DOI] [PubMed] [Google Scholar]
- Hu Y., Liu Y., Pelletier S., Buchdunger E., Warmuth M., Fabbro D., Hallek M., Van Etten R.A., Li S., Liu Y., Pelletier S., Buchdunger E., Warmuth M., Fabbro D., Hallek M., Van Etten R.A., Li S., Pelletier S., Buchdunger E., Warmuth M., Fabbro D., Hallek M., Van Etten R.A., Li S., Buchdunger E., Warmuth M., Fabbro D., Hallek M., Van Etten R.A., Li S., Warmuth M., Fabbro D., Hallek M., Van Etten R.A., Li S., Fabbro D., Hallek M., Van Etten R.A., Li S., Hallek M., Van Etten R.A., Li S., Van Etten R.A., Li S., Li S. Requirement of Src kinases Lyn, Hck, and Fgr for BCR-ABL1-induced B-lymphoblastic leukemia but not chronic myeloid leukemia. Nat. Genet. 2004;36:453–461. doi: 10.1038/ng1343. [DOI] [PubMed] [Google Scholar]
- Hu Y., Swerdlow S., Duffy T.M., Weinmann R., Lee F.Y., Li S., Swerdlow S., Duffy T.M., Weinmann R., Lee F.Y., Li S., Duffy T.M., Weinmann R., Lee F.Y., Li S., Weinmann R., Lee F.Y., Li S., Lee F.Y., Li S., Li S. Targeting multiple kinase pathways in leukemic progenitors and stem cells is essential for improved treatment of Ph+ leukemia in mice. Proc. Natl. Acad. Sci. 2006;103:16870–16875. doi: 10.1073/pnas.0606509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J.J.L., Kieboom K., Marino S., DePinho R.A., van Lohuizen M., Kieboom K., Marino S., DePinho R.A., van Lohuizen M., Marino S., DePinho R.A., van Lohuizen M., DePinho R.A., van Lohuizen M., van Lohuizen M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999;397:164–168. doi: 10.1038/16476. [DOI] [PubMed] [Google Scholar]
- Kamijo T., Zindy F., Roussel M.F., Quelle D.E., Downing J.R., Ashmun R.A., Grosveld G., Sherr C.J., Zindy F., Roussel M.F., Quelle D.E., Downing J.R., Ashmun R.A., Grosveld G., Sherr C.J., Roussel M.F., Quelle D.E., Downing J.R., Ashmun R.A., Grosveld G., Sherr C.J., Quelle D.E., Downing J.R., Ashmun R.A., Grosveld G., Sherr C.J., Downing J.R., Ashmun R.A., Grosveld G., Sherr C.J., Ashmun R.A., Grosveld G., Sherr C.J., Grosveld G., Sherr C.J., Sherr C.J. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell. 1997;91:649–659. doi: 10.1016/s0092-8674(00)80452-3. [DOI] [PubMed] [Google Scholar]
- Kantarjian H., Giles F., Wunderle L., Bhalla K., O’Brien S., Wassmann B., Tanaka C., Manley P., Rae P., Mietlowski W., Giles F., Wunderle L., Bhalla K., O’Brien S., Wassmann B., Tanaka C., Manley P., Rae P., Mietlowski W., Wunderle L., Bhalla K., O’Brien S., Wassmann B., Tanaka C., Manley P., Rae P., Mietlowski W., Bhalla K., O’Brien S., Wassmann B., Tanaka C., Manley P., Rae P., Mietlowski W., O’Brien S., Wassmann B., Tanaka C., Manley P., Rae P., Mietlowski W., Wassmann B., Tanaka C., Manley P., Rae P., Mietlowski W., Tanaka C., Manley P., Rae P., Mietlowski W., Manley P., Rae P., Mietlowski W., Rae P., Mietlowski W., Mietlowski W., et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N. Engl. J. Med. 2006;354:2542–2551. doi: 10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- Kim W.Y., Sharpless N.E., Sharpless N.E. The regulation of INK4/ARF in cancer and aging. Cell. 2006;127:265–275. doi: 10.1016/j.cell.2006.10.003. [DOI] [PubMed] [Google Scholar]
- McLaughlin J., Chianese E., Witte O.N., Chianese E., Witte O.N., Witte O.N. In vitro transformation of immature hematopoietic cells by the P210 BCR/ABL oncogene product of the Philadelphia chromosome. Proc. Natl. Acad. Sci. 1987;84:6558–6562. doi: 10.1073/pnas.84.18.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullighan C.G., Goorha S., Radtke I., Miller C.B., Coustan-Smith E., Dalton J.D., Girtman K., Mathew S., Ma J., Pounds S.B., Goorha S., Radtke I., Miller C.B., Coustan-Smith E., Dalton J.D., Girtman K., Mathew S., Ma J., Pounds S.B., Radtke I., Miller C.B., Coustan-Smith E., Dalton J.D., Girtman K., Mathew S., Ma J., Pounds S.B., Miller C.B., Coustan-Smith E., Dalton J.D., Girtman K., Mathew S., Ma J., Pounds S.B., Coustan-Smith E., Dalton J.D., Girtman K., Mathew S., Ma J., Pounds S.B., Dalton J.D., Girtman K., Mathew S., Ma J., Pounds S.B., Girtman K., Mathew S., Ma J., Pounds S.B., Mathew S., Ma J., Pounds S.B., Ma J., Pounds S.B., Pounds S.B., et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukemia. Nature. 2007;446:758–764. doi: 10.1038/nature05690. [DOI] [PubMed] [Google Scholar]
- O’Hare T., Eide C.A., Deininger M.W., Eide C.A., Deininger M.W., Deininger M.W. Bcr-Abl kinase domain mutations, drug resistance and the road to a cure of chronic myeloid leukemia. Blood. 2007 doi: 10.1182/blood-2007-03-066936. [DOI] [PubMed] [Google Scholar]
- Park I.-K., Qian D., Kiel M., Becker M.W., Pihalja M., Weissman I.L., Morrison S.J., Clarke M.F., Qian D., Kiel M., Becker M.W., Pihalja M., Weissman I.L., Morrison S.J., Clarke M.F., Kiel M., Becker M.W., Pihalja M., Weissman I.L., Morrison S.J., Clarke M.F., Becker M.W., Pihalja M., Weissman I.L., Morrison S.J., Clarke M.F., Pihalja M., Weissman I.L., Morrison S.J., Clarke M.F., Weissman I.L., Morrison S.J., Clarke M.F., Morrison S.J., Clarke M.F., Clarke M.F. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- Primo D., Tabernero M.D., Perez J.J., Rasillo A., Sayagues J.M., Espinosa A.B., Lopez-Berges M.C., Garcia-Sanz R., Gutierrez N.C., Hernandez J.M., Tabernero M.D., Perez J.J., Rasillo A., Sayagues J.M., Espinosa A.B., Lopez-Berges M.C., Garcia-Sanz R., Gutierrez N.C., Hernandez J.M., Perez J.J., Rasillo A., Sayagues J.M., Espinosa A.B., Lopez-Berges M.C., Garcia-Sanz R., Gutierrez N.C., Hernandez J.M., Rasillo A., Sayagues J.M., Espinosa A.B., Lopez-Berges M.C., Garcia-Sanz R., Gutierrez N.C., Hernandez J.M., Sayagues J.M., Espinosa A.B., Lopez-Berges M.C., Garcia-Sanz R., Gutierrez N.C., Hernandez J.M., Espinosa A.B., Lopez-Berges M.C., Garcia-Sanz R., Gutierrez N.C., Hernandez J.M., Lopez-Berges M.C., Garcia-Sanz R., Gutierrez N.C., Hernandez J.M., Garcia-Sanz R., Gutierrez N.C., Hernandez J.M., Gutierrez N.C., Hernandez J.M., Hernandez J.M. Genetic heterogeneity of BCR/ABL+ adult B-cell precursor acute lymphoblastic leukemia: Impact on the clinical, biological, and immunophenotypical disease characteristics. Leukemia. 2005;19:713–720. doi: 10.1038/sj.leu.2403714. [DOI] [PubMed] [Google Scholar]
- Randle D.H., Zindy F., Sherr C.J., Roussel M.F., Zindy F., Sherr C.J., Roussel M.F., Sherr C.J., Roussel M.F., Roussel M.F. Differential effects of p19Arf and p16Ink4a loss on senescence of murine bone marrow-derived pre-B cells and macrophages. Proc. Natl. Acad. Sci. 2001;98:9654–9659. doi: 10.1073/pnas.171217498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah N.P., Nicoll J.M., Nagar B., Gorre M.E., Paquette R.L., Kuriyan J., Sawyers C.L., Nicoll J.M., Nagar B., Gorre M.E., Paquette R.L., Kuriyan J., Sawyers C.L., Nagar B., Gorre M.E., Paquette R.L., Kuriyan J., Sawyers C.L., Gorre M.E., Paquette R.L., Kuriyan J., Sawyers C.L., Paquette R.L., Kuriyan J., Sawyers C.L., Kuriyan J., Sawyers C.L., Sawyers C.L. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2:117–125. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- Sherr C.J. Divorcing ARF and p53: An unsettled case. Nat. Rev. Cancer. 2006;6:663–673. doi: 10.1038/nrc1954. [DOI] [PubMed] [Google Scholar]
- Shou Y., Ma Z., Lu T., Sorrentino B.P., Ma Z., Lu T., Sorrentino B.P., Lu T., Sorrentino B.P., Sorrentino B.P. Unique risk factors for insertional mutagenesis in a mouse model of XSCID gene therapy. Proc. Natl. Acad. Sci. 2006;103:11730–11735. doi: 10.1073/pnas.0603635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talpaz M., Shah N.P., Kantarjian H., Donato N., Nicoll J., Paquette R., Cortes J., O’Brien S., Nicaise C., Bleickardt E., Shah N.P., Kantarjian H., Donato N., Nicoll J., Paquette R., Cortes J., O’Brien S., Nicaise C., Bleickardt E., Kantarjian H., Donato N., Nicoll J., Paquette R., Cortes J., O’Brien S., Nicaise C., Bleickardt E., Donato N., Nicoll J., Paquette R., Cortes J., O’Brien S., Nicaise C., Bleickardt E., Nicoll J., Paquette R., Cortes J., O’Brien S., Nicaise C., Bleickardt E., Paquette R., Cortes J., O’Brien S., Nicaise C., Bleickardt E., Cortes J., O’Brien S., Nicaise C., Bleickardt E., O’Brien S., Nicaise C., Bleickardt E., Nicaise C., Bleickardt E., Bleickardt E., et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N. Engl. J. Med. 2006;354:2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- Whitlock C.A., Witte O.N., Witte O.N. Long-term culture of murine bone marrow precursors of B lymphocytes. Methods Enzymol. 1987;150:275–286. doi: 10.1016/0076-6879(87)50085-4. [DOI] [PubMed] [Google Scholar]
- Williams R.T., Roussel M.F., Sherr C.J., Roussel M.F., Sherr C.J., Sherr C.J. Arf gene loss enhances oncogenicity and limits imatinib response in mouse models of Bcr-Abl-induced acute lymphoblastic leukemia. Proc. Natl. Acad. Sci. 2006;103:6688–6693. doi: 10.1073/pnas.0602030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff N.C., Ilaria R.L., Ilaria R.L. Establishment of a murine model for therapy-related chronic myelogenous leukemia using the tyrosine kinase inhibitor STI571. Blood. 2001;98:2808–2816. doi: 10.1182/blood.v98.9.2808. [DOI] [PubMed] [Google Scholar]
- Wolff N.C., Richardson J.A., Egorin M., Ilaria R.L., Richardson J.A., Egorin M., Ilaria R.L., Egorin M., Ilaria R.L., Ilaria R.L. The CNS is a sanctuary for leukemic cells in mice receiving imatinib mesylate for Bcr/Abl-induced leukemia. Blood. 2003;101:5010–5013. doi: 10.1182/blood-2002-10-3059. [DOI] [PubMed] [Google Scholar]
- Wolff N.C., Randle D.E., Egorin M.J., Minna J.D., Ilaria R.L., Randle D.E., Egorin M.J., Minna J.D., Ilaria R.L., Egorin M.J., Minna J.D., Ilaria R.L., Minna J.D., Ilaria R.L., Ilaria R.L. Imatinib mesylate efficiently achieves therapeutic intratumor concentrations in vivo but has limited activity in a xenograft model of small cell lung cancer. Clin. Cancer Res. 2004;10:3528–3534. doi: 10.1158/1078-0432.CCR-0957-03. [DOI] [PubMed] [Google Scholar]
- Wong S., Witte O.N., Witte O.N. The BCR-ABL story: Bench to bedside and back. Annu. Rev. Immunol. 2004;22:247–306. doi: 10.1146/annurev.immunol.22.012703.104753. [DOI] [PubMed] [Google Scholar]
- Zindy F., Eischen C.M., Randle D.H., Kamijo T., Cleveland J.L., Sherr C.J., Roussel M.F., Eischen C.M., Randle D.H., Kamijo T., Cleveland J.L., Sherr C.J., Roussel M.F., Randle D.H., Kamijo T., Cleveland J.L., Sherr C.J., Roussel M.F., Kamijo T., Cleveland J.L., Sherr C.J., Roussel M.F., Cleveland J.L., Sherr C.J., Roussel M.F., Sherr C.J., Roussel M.F., Roussel M.F. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes & Dev. 1998;12:2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]