Alterations in an inositol phosphate code through synergistic activation of a G protein and inositol phosphate kinases (original) (raw)
Abstract
In mammals, many cellular stimuli evoke a response through G protein activation of phospholipase C, which results in the lipid-derived production of inositol 1,4,5-trisphosphate (IP3). Although it is well established that IP3 is converted to numerous inositol phosphates (IPs) and pyrophosphates (PP-IPs) through the action of up to six classes of inositol phosphate kinases (IPKs), it is not clear that these metabolites are influenced by G protein signaling. Here we report that activation of Gαq leads to robust stimulation of IP3 to IP8 metabolism. To expose flux through these pathways, genetic perturbation was used to alter IP homeostasis. Coupled expression of a constitutively active GαqQL and one or more IPK gene products synergistically generated dramatic changes in the patterns of intracellular IP messengers. Many distinct IP profiles were observed through the expression of different combinations of IPKs, including changes in previously unappreciated pools of IP5 and IP6, two molecules widely viewed as stable metabolites. Our data link the activation of a trimeric G protein to a plethora of metabolites downstream of IP3 and provide a framework for suggesting that cells possess the machinery to produce an IPK-dependent IP code. We imply, but do not prove, that agonist-induced alterations in such a code would theoretically be capable of enhancing signaling complexity and specificity. The essential roles for IPKs in organism development and cellular adaptation are consistent with our hypothesis that such an IP code may be relevant to signaling pathways.
Keywords: Gq, metabolomics, phospholipase C, signal transduction, G protein-coupled receptor
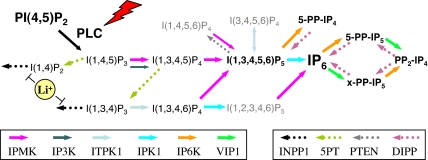
In mammals, hundreds of G protein-coupled receptors (GPCR) and receptor tyrosine kinases (RTKs) are present and link to numerous intracellular signaling modules that, in combination, enable selective cellular responses. One of the most commonly activated signaling modules is the generation of the second messengers inositol 1,4,5-trisphosphate (IP3) and 1,2-diacylglycerol by the action of phospholipase C isozymes (PLCs) on phosphatidylinositol 4,5-bisphosphate (PIP2) (1–5). The ubiquity of PLC activation in response to a wide range of stimuli supports its central role in signaling, but also emphasizes that the production of two messengers alone, IP3 and diacylglycerol, is insufficient to encode the breadth of specific cellular responses necessary for survival and adaptation. Therefore, it has been postulated that further signaling diversity could theoretically arise from the conversion of IP3 to numerous inositol tetrakisphosphate (IP4), inositol pentakisphosphate (IP5), inositol hexakisphosphate (IP6), and inositol pyrophosphate (PP-IP) molecules (2, 6–9). The production of these regulators occurs through the action of up to six classes of evolutionarily conserved IP kinase (IPK) gene products that include: IPMK/IPK2 (10–14), IPK1 (15–17), IP3K (18–20), ITPK/IP56K (21, 22), IP6K/IHPK (23–25), and VIP1 (25–27) (Fig. 1). Genetic and biochemical studies of IPKs have provided valuable insights into the essential regulatory roles and biology of their IP and PP-IP products (7–9, 28–30).
Fig. 1.
Six classes of evolutionarily conserved IPKs generate an IP code in mammalian cells: (i) IPMK (also known as IPK2 or ARG82), a multikinase capable of producing I(1,3,4,5,6)P5 through the dual phosphorylation I(1,4,5)P3 at the 3- and 6-kinase positions, as well as through a 5-kinase activity toward I(1,3,4,6)P4; (ii) IPK1, an IP5 2-kinase responsible for the generation of IP6; (iii) IP3K, a selective IP3 3-kinase that produces I(1,3,4,5)P4 from I(1,4,5)P3; (iv) ITPK1 (also known as IP56K), an I(1,3,4)P3 5- and 6-kinase capable of producing I(1,3,4,6)P4 and I(1,3,4,5)P4, and a reversible 1-kinase/phosphatase that regulates I(3,4,5,6)P4 levels; (v) IP6K/IHPK, an IP5 /IP6 /IP7 5-kinase that generates diphosphoinositol phosphates (also known as PP-IPs), including 5-PP-IP4 and 5-PP-IP5; and (vi) VIP1, an IP6 /IP7 kinase that generates a unique species of PP-IP5 (_x_-PP-IP5) and (PP)2-IP4 (IP8). In addition, the action of four IPs are relevant to this pathway: (i) INPP1, a lithium-inhibited I(1,4)P2/I(1,3,4)P3 1-phosphatase; (ii) 5PT, an I(1,4,5)P3/I(1,3,4,5)P4 5-phosphatase; (iii) PTEN, a phosphoinositide 3-phosphatase that also has been shown to dephosphorylate IP5; and (iv) DIPP, which degrades PP-IPs. The lightning bolt represents GPCR and RTK pathways that activate PLC isoforms; the yellow sphere represents lithium, a pharmacological agent used to inhibit INPP1. Legend for colored arrows indicates the six kinase (solid) and four phosphatase (dotted) enzymes.
In contrast to the wealth of evidence demonstrating that activation of GPCR and RTK pathways leads to the stimulation of PKC and calcium release through DAG and IP3 second messengers, links of receptor activation to alterations in the levels of IP4, IP5, IP6, and PP-IPs have been limited. This result has impeded acceptance that these putative messengers lie downstream of GPCR or RTK signaling. However, recent studies have shown that, in a variety of organisms, IPK gene products are required for proper development and cellular adaptation, examples of highly orchestrated receptor-mediated signaling processes, providing support that these pathways are in fact used by the cell for encoding selective responses.
In light of this apparent paradox, we considered an alternative explanation that receptor activation may indeed result in flux through these pathways, but such changes have not been observed because of rapid reequilibration, masking, or an inability to trap transient bursts of messenger synthesis and breakdown. Precedent for the latter explanation comes from monitoring agonist-induced IP3 changes in which cellular studies frequently use lithium treatment to inhibit IP phosphatases, thereby enabling accumulation of signaling intermediates that are stable and easily quantified (31, 32). Because pharmacological traps are not readily available for the IPK-mediated pathways, we examined whether genetic perturbation in combination with metabolic labeling would enable visualization of flux through these pathways. We report that coupling the overexpression of a constitutively active G protein Gαq and the IPKs exposed dramatic changes in IP3, IP4, IP5, IP6, IP7, and IP8 metabolism. Our data reveal that constitutively elevated molecules, such as IP5 and IP6, appear to have multiple pools, including those that are low at resting state and undergo dramatic elevation in response to the activation of G protein. Collectively, these data indicate that G protein activation works in concert with the six classes of IPKs to regulate flux through a complex number of IP molecules, thereby enhancing signaling complexity downstream of PLC. The ability to observe alterations in the patterns and flux of these messengers provides a framework for suggesting that individual IP species represent the building blocks of a potentially dynamic IP code.
Results
Enhancing Detection of G Protein-Activated IP3 Release by Trapping Rapidly Fluxing Metabolites.
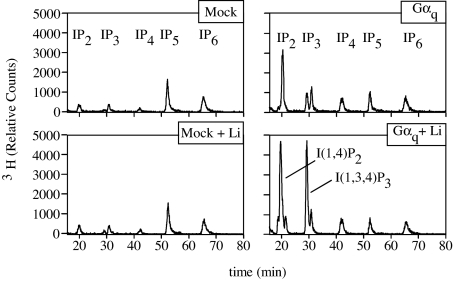
The premise of our study was that detecting signaling-dependent changes in IP metabolites would require a robust activation of IP3 production or a pharmacological or genetic mechanism to trap rapidly equilibrating, masked, or fluxing IPs or both. As a means to increase IP3 production through PLC stimulation, we overexpressed an activated Gαq mutant. The Gαq family of heterotrimeric G proteins, comprised of Gαq, Gα11, Gα14, and Gα15/16, specifically activate PLCβ isoforms (33). A mutation of a glutamine residue at position 209 to leucine in Gαq (GαqQL) inactivates the GTPase of the G protein, locking GαqQL in a constitutively active state (34). Adenoviral introduction of GαqQL into Rat-1 cells radiolabeled with [3H]inositol indeed led to substantial increases in the levels of IP2, IP3, and IP4 (Fig. 2Right Upper). To examine the second component of our hypothesis, we combined the treatment of cells expressing GαqQL with the addition of 10 mM lithium chloride in the growth medium for 1 h before harvest. Although lithium treatment alone did not result in alterations in any of the IP metabolites (Fig. 2 Left Upper), the combination of lithium plus GαqQL expression resulted in an additional increase in the levels of IP2 and I(1,3,4)P3, consistent with an inhibition of INPP1 (see Fig. 1), but did not further alter levels of I(1,4,5)P3, IP4, IP5, or IP6 (Fig. 2 Right Lower). Most notably, the levels of I(1,3,4)P3 increased >20-fold by using a combined GαqQL plus lithium as compared with control cells. These data confirm that (i) Gαq activates IP metabolism, and (ii) the stimulus-dependent increases in I(1,4)P2 and I(1,3,4)P3 are examples of lithium-trapped metabolites that serve as reporters for signaling activated flux through I(1,4,5)P3.
Fig. 2.
Enhanced detection of GαqQL-induced IP3 production by the trapping of metabolites. Rat1 cells were plated at densities of 25,000 cells per milliliter and labeled metabolically for 72 h with _myo_-[3H]inositol as described in Methods. Twenty-four hours before harvest, cells were infected with control adenovirus (labeled Mock) or GαqQL adenovirus (labeled Gαq). Lithium treatment was performed by adding LiCl to the cells at a final concentration of 10 mM 1 h before harvest. Soluble IPs were extracted by HCl and resolved by HPLC. Peaks were normalized to the total inositol counts present in the samples.
It is noteworthy that changes in I(1,4,5)P3 levels in G protein-activated cells, even in the presence of lithium, were relatively minimal compared with the 20-fold elevation observed for I(1,3,4)P3. Because the only known route of I(1,3,4)P3 synthesis occurs through the metabolism of I(1,4,5)P3 and I(1,3,4,5)P4 (2), we infer that the synthesis of I(1,4,5)P3 and I(1,3,4,5)P4 also was up-regulated 20-fold, but such changes were not observed because of their rapid equilibration. Our data highlight that indeed GαqQL overexpression induces a robust stimulation of I(1,4,5)P3 and I(1,3,4,5)P4 production, and for these untrapped molecules analysis of steady-state metabolic labeling may not accurately reflect their flux. Furthermore, the data provided evidence consistent with the notion that the homeostatic mechanisms aimed at maintaining IP levels in cells were tuned to rapidly reequilibrate to prevent inappropriate fluctuations. Thus, we next sought to perturb IP4, IP5, and IP6 homeostasis alongside PLC activation to further test our initial hypothesis.
Coupled Activation of Gαq and Distinct IP3 Kinases Generate Unique IP Signatures.
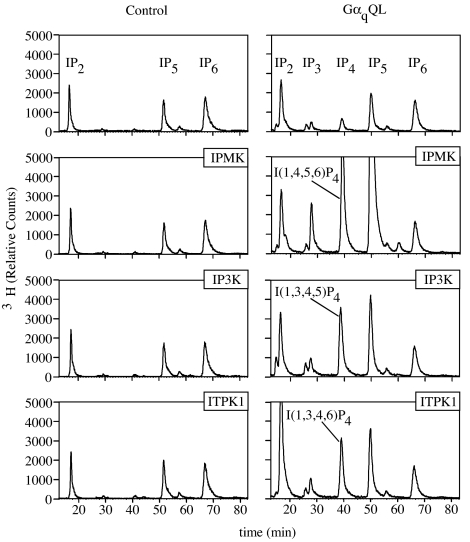
Because pharmacological regulators of IP4 or IP5 metabolism are not readily available, we examined the effects of genetic perturbation of the three classes of IP3 kinase, IPMK, IP3KA, or ITPK1, in the context of activated Gαq. To perform these studies, we switched to a human embryonic kidney cell, HEK293T, and used transient transfection methods to facilitate the introduction of multiple gene products and the rapid assessment of metabolic changes. In the absence of GαqQL, expression of any one of the three individual IP3 kinases had minimal effect on soluble IP levels (Fig. 3Left). Similar to Rat-1 cells, the expression of GαqQL alone in HEK293T cells resulted in small changes in IP3 and IP4 levels, but did not significantly alter IP5 or IP6 (Fig. 3 Right Upper). In contrast, when the different IP3 kinases were coexpressed with GαqQL, tremendous changes in the profiles of individual IP metabolites were observed (Fig. 3 Right Lower). Coexpression of GαqQL + IPMK led to massive up-regulation of IP3, IP4, and IP5, whereas coexpression of GαqQL + IP3KA and GαqQL + ITPK1 led to large increases in IP4 and smaller but significant increases in IP5. Significantly, the IP4 species produced as a result of the expression of each IPK were unique (Table 1). IPMK expression resulted in an increased production of I(1,4,5,6)P4, IP3KA expression yielded I(1,3,4,5)P4, and ITPK1 produced a mixture of IP4 composed of 60% I(1,3,4,6)P4 and 30% I(1,3,4,5)P4. The IP4 species that accumulate in response to IP3KA and ITPK1 expression are the expected products of these enzymes, whereas the I(1,4,5,6)P4 that accumulates in response to IPMK may be a mass action increase through metabolism of IP5 (see Fig. 1) because mammalian IPMK has been shown to function as a dual-specificity I(1,4,5)P3 3-kinase and I(1,3,4,5)P4 6-kinase. These results demonstrate three important findings: (i) expression of GαqQL generates a release of IP3 that is accessible to IP3 kinases, (ii) there is a synergistic activation of IP metabolism between G protein and IPKs, and (iii) the different IP3 kinases enable the generation of distinct IP profiles or patterns in response to G protein activation, thereby enhancing the complexity of signals downstream of PLC.
Fig. 3.
Coincident activation of IP3 kinases along with GαqQL synergistically enhances inositol phosphate metabolism and distinct species of IP4. HEK293T cells were plated at a density of 100,000 cells per milliliter and labeled metabolically for 72 h with _myo_-[3H]inositol. Twenty-four hours before harvest, cells were transfected by the addition of FuGENE6/DNA complexes directly into the labeling media. DNA complexes were prepared by mixing pcDNA3.1 plasmids expressing IPMK, IP3K, or ITPK1 with either pcDNA3.1 (Left) or pcDNA3.1-GαqQL (Right); 100 ng of each cDNA was used for the transfection, and empty pcDNA3.1 was used to bring the total DNA in each transfection to 500 ng per well. Soluble IPs were extracted from the cells by using HCl and resolved by HPLC.
Table 1.
Identification of IP4 species in cell extracts by enzymatic analysis
| Cell extract | Enzymatic treatment, percent conversion | Primary IP4 species | |||
|---|---|---|---|---|---|
| IPMK | IPK1 | 5-Ptase | ITPK1 | ||
| Gaq + IPMK | 82 | 22 | 11 | 18 | I(1,4,5,6)P4 |
| Gaq + IP3K | 92 | 14 | 86 | 0 | I(1,3,4,5)P4 |
| Gaq + ITPK1 | 94 | 60 | 32 | 0 | I(1,3,4,6)P4 |
Gαq Induces Robust IP5 Synthesis.
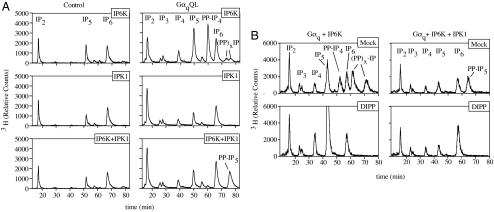
To ascertain whether similar genetic perturbation strategies could be used to expose changes in IP5 and IP6 metabolism, two molecules widely viewed as stable and whose levels are relatively high at resting state in mammalian cells (Fig. 3 Right Upper), we overexpressed IP6K1 and IPK1 in activated cells. We surmised that these experiments also would enable monitoring the effects of G protein activation at endogenous levels of the IP3 and IP4 kinases. Similar to what was observed for the IP3 kinases, overexpression of IPK1 or IP6K, or both, in the absence of GαqQL had minimal effects on IP profiles, with the exception of a decrease in IP5 levels observed in IPK1-expressing cells (Fig. 4A Left). When GαqQL was coexpressed along with IP6K1, significant increases in the levels of PP-IP4 and, to a lesser extent, two PP-IP species more polar than IP6 were observed (Fig. 4A Right Top). As a means to determine whether these pyrophosphate species emerged from flux through IP5 or IP6, or both, we treated the extracts with recombinant diphosphoinositol phosphate phosphatase (DIPP) and found that all PP-IPs were quantitatively hydrolyzed to IP5 (Fig. 4B Left), indicating that they emerged from IP5 metabolism and not from IP6. Our data provide evidence that IP5 synthesis increases significantly in response to Gαq and that IP6K1 is a useful sensor for exposing this metabolism. Importantly, we find that this flux does not require overexpression of IP3 and IP4 kinases and occurs at endogenous levels of these enzymes.
Fig. 4.
Detection of a flux of IP5 emanating from activated Gαq through the coexpression of IP5/IP6 kinases. (A) Capture of a flux of IP5 synthesis by IP5 kinases. _myo_-[3H]inositol-labeled cells were transfected with plasmids expressing IP6K1, IPK1, or IP6K1 and IPK1 with either pcDNA3.1 (Left) or pcDNA3.1-GαqQL (Right). Soluble IPs were extracted from the cells by using HCl and resolved by HPLC. (B) Enzymatic analysis of IP6K generated IP pyrophosphates. HEK293T cells were metabolically labeled and transfected with GαqQL and IP6K1 (Left) or GαqQL, IP6K1, and IPK1 (Right). Cells were harvested in boiling hot 50 mM Tris, and tubes containing collected cell debris were further heated for 5 min in boiling water to minimize phosphatase activity against soluble IPs. Extracts were incubated in reaction buffer for 30 min at 37°C in the absence or presence of 1 μg of human DIPP. Reactions were halted by the addition of 0.5 M HCl, and samples were resolved by HPLC. Slight changes in the elution times of IPs in A and B are due to the use of different HPLC columns between the experiments.
We next examined the coexpression of IPK1 with GαqQL and observed only a slight increase in IP6 levels compared with cells transfected with IPK1 alone (Fig. 4A Middle). Although these data did not corroborate a G protein-induced flux through IP5, we reasoned that if increases in IP6 were rapidly metabolized, such effects could be masked. Therefore, we coexpressed both IPK1 and IP6K1 along with GαqQL and observed a large increase in the levels of PP-IP5 (Fig. 4A Bottom). The identity of this peak was confirmed to be PP-IP5 by treatment with DIPP, which hydrolyzed the molecule to form IP6 (Fig. 4B Right). These data provide further support of a model in which activated GαqQL induces a flux of IP5 synthesis and indicate that coincidently coupling IPK1 and IP6K1 activation shifts production from PP-IP4 to PP-IP5, providing yet another layer of signaling complexity.
Another intriguing aspect of these results was that overexpression of IP6K alone resulted in minimal increases in PP-IP4 and PP-IP5 despite the presence of significant amounts of IP5 and IP6. In contrast, when IP6K1 expression was coupled to GαqQL or GαqQL + IPK1, there were relatively large elevations in the PP-IPs. These data are consistent with a model in which IP5 and IP6 are present in multiple pools, including those that are accessible to IP6K only in response to the activation of G protein. Furthermore, these data highlight that the endogenous cellular machinery has a tremendous capacity to maintain the levels of both IP5 and IP6 even in the presence of what appeared to be significant up-regulation of their synthesis in response to G protein activation.
G Protein Activation, Along with Expression of the Four Evolutionarily Conserved IPKs, Increases IP3 to IP8 Metabolism.
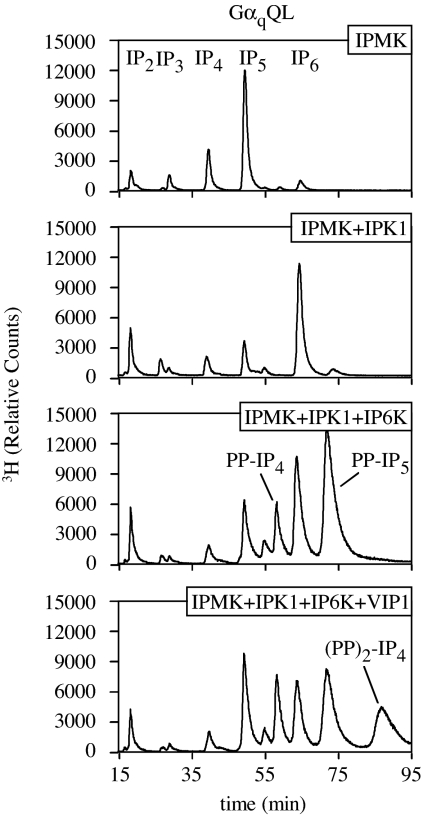
Four of the six classes of IPK gene products, IPMK/IPK2, IPK1, IP6K/KCS1, and VIP1, are present in genomes from fungi to man. To test whether all four kinases were linked to G protein-activated IP3 metabolism, we coexpressed them in combinations in activated HEK293T cells. Expression of any one individual or combinations of two, three, or all four IPKs in the absence of coexpression with GαqQL resulted in minimal observable changes in the IP profiles (data not shown). In contrast, when GαqQL was concomitantly expressed, dramatic changes were observed (Fig. 5). As reported in Fig. 3, G protein-mediated induction of IP5 synthesis was massively up-regulated by the coexpression with IPMK, which can be fully appreciated by extending the y axis of the HPLC analysis (Fig. 5 Top). The up-regulation of IP5 synthesis was quantitatively shifted to IP6 by the coexpression of IPK1, along with GαqQL and IPMK (Fig. 5 Top Middle). This result differed from GαqQL + IPK1 (Fig. 4A Right Middle), suggesting that concomitant up-regulation of both IPMK and IPK1 cooperate to boost IP6 synthesis in response to G protein activation. An increase in PP-IP5 also was observed. However, its level was 5% of the IP6 peak, similar to the relative ratio of IP6 and PP-IP5 in untreated cells, indicating that this increase is likely a mass-action effect. Remarkably, the coexpression of GαqQL, IPMK, IPK1, and IP6K1 resulted in yet another pattern of IP/PP-IP synthesis, in which the IP6 levels were comparable to those seen in GαqQL + IPMK + IPK1 cells, but the PP-IP4 and PP-IP5 levels were now dramatically elevated (Fig. 5 Bottom Middle). Last, overexpression of all four evolutionarily conserved IPKs and GαqQL resulted in the sequential conversion of IP3 to IP8 as measured by the accumulation of PP2-IP4 (Fig. 5 Bottom). Collectively, our data suggest that these four gene products link to G protein activation and indicate that an evolutionarily conserved minimum kinase requirement for conversion of IP3 to IP8 is encoded by IPMK, IPK1, IP6K, and VIP1.
Fig. 5.
Coexpression of activated Gαq with four of the six classes of IP kinases stimulates production of IP8. GαqQL and IPMK were coexpressed with IPK1, IPK1 + IP6K1, or IPK1 + IP6K + VIP1. Soluble IPs were extracted from the cells by using HCl and resolved by HPLC. Note the changes in the scale of the x and y axes from previous figures.
Discussion
Our results identify that coupled activation of a G protein and one or more of six classes of evolutionarily conserved IP kinases synergistically produce many distinct patterns of IP and PP-IP messengers. The ability to manipulate the concentrations and profiles of each IP species through differential activation of IPKs indicates that collectively these molecules could comprise an IP code capable of enhancing intracellular messenger complexity in mammalian cells. In this case, each species represents a building block of the code. By transiently assembling different combinations of IP and PP-IPs, the cell could, in theory, rapidly remodel the signaling landscape downstream of PLC activation. At this point, we have shown that the IPK machinery exists to alter IP profiles under engineered conditions. However, proof that agonist stimulation induces changes in a putative IP code remains to be established. Nonetheless, our data linking changes in this putative IP code to G protein activation in mammals provide an impetus to explore whether agonist stimulation of any of the hundreds of GPCR or possibly even the RTK pathways alters the code and whether one or more of the IPK gene products is required to mediate signaling processes. Of particular relevance, during the revision of this manuscript, Gao et al. (35) found that Wnt3a activation of Gαq (through the Frizzled-1 receptor) causes a transient increase in IP5, and indicated that siRNA knockdown of IPMK and IP3KB inhibited the ability of Wnt3a to stimulate the canonical β-catenin/lymphoid enhancer factor/T cell factor pathway.
Is there evidence that other signaling processes depend on a putative IPK-dependent IP code? Yes. Recent studies of several IPKs have demonstrated their essential involvement in organism development and function (17, 36–41). Given the highly orchestrated nature of these processes, it is reasonable to conclude that the production of an IP code that emerges from IP3 is involved in the execution of these instructions. Studies of the IPKs in yeast and Dictyostelium demonstrate their roles in cellular adaptation and survival under conditions of nutritional change and stress and have provided important insights into a wide range of cellular processes that are regulated by the IP and PP-IP messengers (10, 15, 24, 25, 42–51). On this basis, it has been proposed that a primordial role for activation of PLC is to up-regulate IPK-dependent signaling (9, 52). This notion is further supported by our current studies in mammalian cells. Additionally, data from both uni- and multicellular organisms have provided clues into the intracellular receptors that participate in decoding the IPK-dependent IP4, IP5, IP6, and PP-IP messengers, in particular with respect to mRNA export (49, 50), chromatin remodeling (46, 47), RNA editing (53), protein phosphorylation (54), phosphate signaling (25, 51), auxin biology (41), and immune function (40). Collectively, these data provide exciting evidence supporting a role for an IPK-dependent IP code in signal-transduction pathways.
Do IPK-dependent products IP4, IP5, IP6, and PP-IPs function as classical second messengers in a manner analogous to IP3 or do they regulate signaling through a novel mechanism? Evidence exists that supports each model. For molecules such as IP4 and some of the PP-IPs, there is convincing evidence that, upon stimulation, their concentrations are transiently induced and quickly returned to low levels, consistent with classic second-messenger behavior. In contrast, for molecules whose levels appear elevated even under resting conditions, such as IP6 and, in some cell types, IP5, other modes of regulation have been proposed (55). Our data support a model in which IP5 and IP6 are present in cells in multiple pools, such that there may be both stable reservoirs as well as those that are signal-induced (low at resting state and that, upon stimulation of G protein, are rapidly elevated, as highlighted by our IP6K1 overexpression studies). In this way, IP6 may serve as both a second messenger and a precursor to other signaling reactions, analogous to the lipid phosphatidylinositol 4,5-bisphosphate. Evidence for a mechanism of action for IP6 comes from elegant structural studies of an RNA editing enzyme, ADAR2, and from the auxin receptor in plants, TIR1 (41, 53). The crystal structure of ADAR2 revealed that IP6 was a structural cofactor buried within the protein whose presence was critical for deaminase activity (53). In the case of TIR1, the hormone auxin and IP6 were bound within the structure at distinct, but adjacent, sites consistent with TIR1 functioning as a coincidence detection sensor (41). IP6 appeared necessary for the stabilization of the superhelical structure of the leucine-rich repeats and is likely critical for the function of TIR1. Given the nonexchangeable nature of IP6 binding in both structures, it is unlikely that acute changes in a messenger would be rapidly transduced. In this scenario, IP6 would function as a regulatory cofactor that could alter the stability or folding of the receptor over longer periods of time.
As our studies demonstrate, cells have a tremendous capacity to maintain IP homeostasis, none more obvious than the control of IP5 and IP6. We suspect that this finding may provide a plausible explanation for why IPK-dependent changes in IP metabolism in response to agonist stimulation have been difficult to detect. Our use of genetic perturbation to trap or alter this homeostasis provides new tools to approach these types of studies. Because metabolic labeling has limitations with respect to detecting large fluxes in IP metabolism, measurements of IP metabolism in response to agonists would be greatly aided by cell-based sensors of the individual IP or PP-IP molecules or the processes they regulate. Regardless of the exact mechanisms of regulation, our work provides a compelling link between the activation of G proteins and the production of IP4, IP5, IP6, and several PP-IPs. We now have important reagents and information that enable further examination of the involvement of the IPKs as transducers of the myriad of potential signaling pathways downstream of GPCRs.
Methods
Materials and Cell Culture.
Tissue culture reagents were purchased from Sigma–Aldrich (St. Louis, MO) and Invitrogen (Carlsbad, CA). Inositol free-labeling DMEM was from Specialty Media (Phillipsburg, NJ). Molecular biology reagents used for subcloning and PCR were purchased from New England Biolabs (Ipswich, MA) and Stratagene (La Jolla, CA). Oligonucleotides were from Integrated DNA Technologies (Coralville, IA). HEK293T and Rat1 cells were obtained from the Duke University Medical Center Cell Culture Facility. All cell lines were maintained in DMEM containing 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2.
Adenovirus and Plasmid Constructs.
Construction of recombinant control adenovirus and adenovirus expressing human GαqQL were described previously (56). The cDNA for human GαqQL in pcDNA was from UMR cDNA Resource Center (Rolla, MO). The construction of the rat IPMK cDNA construct pBabePuro-GFP-rIPMK was described previously (57). The human Vip1 kinase domain (residues 1–387) CFP fusion construct pmCFP-_hs_VIP1-KD has been described (27). The cDNA for human IP6K1 (pCMV-SPORT6-hIP6K1), human IPK1 (pBluescriptR-hIPK1), human IP56K (pCMV-SPORT6-hIP56K), and human ITPKA (pBluescriptR-hITPKA) were from Open Biosystems (Huntsville, AL). The cDNA for hIPK1 was subcloned from pBluescriptR-hIPK1 into pcDNA3.1(−) by using 5′-XhoI and 3′-BamHI restriction sites that flank the ORF to generate pcDNA3.1-hIPK1. The cDNA of hITPKA from residues 185–461 was amplified by PCR by using pBluescriptR-hITPKA as a template and inserted into a pcDNA3.1 construct containing the cDNA for CFP, generating pcDNA3.1-CFP-hITPKA.
Metabolic Labeling in Cells and HPLC Analysis of Inositol Phosphates.
Cells were seeded on tissue culture plates at densities of 25,000–100,000 cells per ml. Twelve-well plates (1-ml media) were used for direct HPLC analysis of soluble IPs, and 6-well plates (2-ml media) were used to generate extracts for enzymatic analysis. After incubation overnight, cells were washed one time with inositol-free DMEM and then labeled for 72 h with 37.5 μCi/ml _myo_-[3H]inositol in inositol-free DMEM supplemented with 10% dialyzed FBS. Cells were infected with adenovirus or transfected with FuGENE 6 Transfection Reagent (Roche Diagnostics, Indianapolis, IN) after 48 h of labeling directly into the labeling media. Cell extracts destined for direct HPLC analysis were prepared by aspirating the labeling media, washing the cells with 1 ml of PBS, and adding 0.4 ml of 0.5 M HCl. HCl was left on the cells for 5 min to extract the soluble IPs and was then removed from the cells and filtered through a 0.45-μm nylon filter to remove cell debris. Samples were stored at −80°C.
Cell extracts for enzymatic analysis were prepared by aspirating the labeling media from cells, washing the cells with 2 ml of PBS, and adding 0.8 ml of boiling hot 50 mM Tris·HCl (pH 8.0). Cells were scraped from the plate, transferred to microfuge tubes, and boiled for an additional 5 min. Extracts were passed through a 22-gauge needle several times, cell debris was pelleted by centrifugation, and samples were filtered through 0.45-μm nylon filters. Samples were stored at −80°C before enzymatic analysis.
Before HPLC analysis, samples were diluted with four volumes of 10 mM NH4H2PO4 (pH 3.5). Soluble IPs were separated by HPLC on a Partisphere SAX column (4.6 × 125 mm; Whatman, Clifton, NJ) by using the following buffer profile: 10 mM NH4H2PO4 (pH 3.5) for 5 min, a linear gradient of 10 mM NH4H2PO4 to 1.7 M NH4H2PO4 for 65 min, and 1.7 M NH4H2PO4 for 30 min. Radiolabeled IPs eluting from the column were quantified by using an inline radiation detector.
Determination of IP Molecule Identity by Using Enzymatic Analysis.
The expression and purification of Arabidopsis thaliana IPK1, A. thaliana IPK2/IPMK, human I(1,4,5)P3 type I 5-phosphatase (5-Ptase), and human DIPP have been described (17, 58). Recombinant human ITPK1 was generously provided by S. Shears (National Institute of Environmental Health Sciences, Research Triangle Park, NC) and used under published conditions. Metabolically labeled cell extracts that were harvested in boiling 50 mM Tris·HCl (pH 8.0) were incubated with 1 μg of enzyme in 50 mM Hepes (pH 7.5), 50 mM KCl, 10 mM MgCl2, and 1 mM ATP at 37°C for 20 min. Reactions were halted by the addition of 0.5 M HCl, and the samples were processed for HPLC analysis as described above.
Acknowledgments
We thank the members of the J.D.Y. laboratory for helpful discussions, especially P. Friday; Dr. S. Shears (National Institute of Environmental Health Sciences, Research Triangle Park, NC) for recombinant human ITPK1; and a thoughtful colleague who provided several helpful comments and insights during the review process. This work was supported by National Institutes of Health Grants R01 HL-55672 and R33 DK-070272 (to J.D.Y.) and by the Howard Hughes Medical Institute (J.D.Y.).
Abbreviations
DIPP
diphosphoinositol phosphate phosphatase
GPCR
G protein-coupled receptor
IP
inositol phosphate
IP3
inositol 1,4,5-triphosphate
IP4
inositol tetrakisphosphate
IP5
inositol pentakisphosphate
IP6
inositol hexakisphosphate
IPK
inositol phosphate kinase
PIP2
phosphatidylinositol 4,5-bisphosphate
PLC
phospholipase C isozymes
PP-IP
inositol pyrophosphate
RTK
receptor tyrosine kinase.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Berridge MJ, Irvine RF. Nature. 1989;341:197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- 2.Majerus PW. Annu Rev Biochem. 1992;61:225–250. doi: 10.1146/annurev.bi.61.070192.001301. [DOI] [PubMed] [Google Scholar]
- 3.Exton JH. Annu Rev Pharmacol Toxicol. 1996;36:481–509. doi: 10.1146/annurev.pa.36.040196.002405. [DOI] [PubMed] [Google Scholar]
- 4.Rhee SG. Annu Rev Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harden TK, Sondek J. Annu Rev Pharmacol Toxicol. 2006;46:355–379. doi: 10.1146/annurev.pharmtox.46.120604.141223. [DOI] [PubMed] [Google Scholar]
- 6.Shears SB. Biochim Biophys Acta. 1998;1436:49–67. doi: 10.1016/s0005-2760(98)00131-3. [DOI] [PubMed] [Google Scholar]
- 7.Irvine RF, Schell MJ. Nat Rev Mol Cell Biol. 2001;2:327–338. doi: 10.1038/35073015. [DOI] [PubMed] [Google Scholar]
- 8.Bennett M, Onnebo SM, Azevedo C, Saiardi A. Cell Mol Life Sci. 2006;63:552–564. doi: 10.1007/s00018-005-5446-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.York JD. Biochim Biophys Acta. 2006;1761:552–559. doi: 10.1016/j.bbalip.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Odom AR, Stahlberg A, Wente SR, York JD. Science. 2000;287:2026–2029. doi: 10.1126/science.287.5460.2026. [DOI] [PubMed] [Google Scholar]
- 11.Saiardi A, Caffrey JJ, Snyder SH, Shears SB. FEBS Lett. 2000;468:28–32. doi: 10.1016/s0014-5793(00)01194-7. [DOI] [PubMed] [Google Scholar]
- 12.Chang SC, Miller AL, Feng Y, Wente SR, Majerus PW. J Biol Chem. 2002;277:43836–43843. doi: 10.1074/jbc.M206134200. [DOI] [PubMed] [Google Scholar]
- 13.Nalaskowski MM, Deschermeier C, Fanick W, Mayr GW. Biochem J. 2002;366:549–556. doi: 10.1042/BJ20020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevenson-Paulik J, Odom AR, York JD. J Biol Chem. 2002;277:42711–42718. doi: 10.1074/jbc.M209112200. [DOI] [PubMed] [Google Scholar]
- 15.York JD, Odom AR, Murphy R, Ives EB, Wente SR. Science. 1999;285:96–100. doi: 10.1126/science.285.5424.96. [DOI] [PubMed] [Google Scholar]
- 16.Verbsky JW, Wilson MP, Kisseleva MV, Majerus PW, Wente SR. J Biol Chem. 2002;277:31857–31862. doi: 10.1074/jbc.M205682200. [DOI] [PubMed] [Google Scholar]
- 17.Stevenson-Paulik J, Bastidas RJ, Chiou ST, Frye RA, York JD. Proc Natl Acad Sci USA. 2005;102:12612–12617. doi: 10.1073/pnas.0504172102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irvine RF, Letcher AJ, Heslop JP, Berridge MJ. Nature. 1986;320:631–634. doi: 10.1038/320631a0. [DOI] [PubMed] [Google Scholar]
- 19.Choi KY, Kim HK, Lee SY, Moon KH, Sim SS, Kim JW, Chung HK, Rhee SG. Science. 1990;248:64–66. doi: 10.1126/science.2157285. [DOI] [PubMed] [Google Scholar]
- 20.Takazawa K, Lemos M, Delvaux A, Lejeune C, Dumont JE, Erneux C. Biochem J. 1990;268:213–217. doi: 10.1042/bj2680213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson MP, Majerus PW. J Biol Chem. 1996;271:11904–11910. doi: 10.1074/jbc.271.20.11904. [DOI] [PubMed] [Google Scholar]
- 22.Yang X, Shears SB. Biochem J. 2000;351:551–555. [PMC free article] [PubMed] [Google Scholar]
- 23.Saiardi A, Erdjument-Bromage H, Snowman AM, Tempst P, Snyder SH. Curr Biol. 1999;9:1323–1326. doi: 10.1016/s0960-9822(00)80055-x. [DOI] [PubMed] [Google Scholar]
- 24.York SJ, Armbruster BN, Greenwell P, Petes TD, York JD. J Biol Chem. 2005;280:4264–4269. doi: 10.1074/jbc.M412070200. [DOI] [PubMed] [Google Scholar]
- 25.Mulugu S, Bai W, Fridy PC, Bastidas RJ, Otto JC, Dollins DE, Haystead TA, Ribeiro AA, York JD. Science. 2007;316:106–109. doi: 10.1126/science.1139099. [DOI] [PubMed] [Google Scholar]
- 26.Choi JH, Cho J, Shears SB. J Biol Chem. 2007 doi: 10.1074/jbc.M704655200. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fridy PC, Otto JC, Dollins DE, York JD. J Biol Chem. 2007 doi: 10.1074/jbc.M704656200. in press. [DOI] [PubMed] [Google Scholar]
- 28.Shears SB. Biochem J. 2004;377:265–280. doi: 10.1042/BJ20031428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhandari R, Chakraborty A, Snyder SH. Cell Metab. 2007;5:321–323. doi: 10.1016/j.cmet.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Irvine R. Science. 2007;316:845–846. doi: 10.1126/science.1143339. [DOI] [PubMed] [Google Scholar]
- 31.Burgess GM, McKinney JS, Irvine RF, Putney JW., Jr Biochem J. 1985;232:237–243. doi: 10.1042/bj2320237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inhorn RC, Majerus PW. J Biol Chem. 1987;262:15946–15952. [PubMed] [Google Scholar]
- 33.Hubbard KB, Hepler JR. Cell Signal. 2006;18:135–150. doi: 10.1016/j.cellsig.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 34.De Vivo M, Chen J, Codina J, Iyengar R. J Biol Chem. 1992;267:18263–18266. [PubMed] [Google Scholar]
- 35.Gao Y, Wang HY. J Biol Chem. 2007;282:26490–26502. doi: 10.1074/jbc.M702106200. [DOI] [PubMed] [Google Scholar]
- 36.Pouillon V, Hascakova-Bartova R, Pajak B, Adam E, Bex F, Dewaste V, Van Lint C, Leo O, Erneux C, Schurmans S. Nat Immunol. 2003;4:1136–1143. doi: 10.1038/ni980. [DOI] [PubMed] [Google Scholar]
- 37.Frederick JP, Mattiske D, Wofford JA, Megosh LC, Drake LY, Chiou S-T, Hogan BLM, York JD. Proc Natl Acad Sci USA. 2005;102:8454–8459. doi: 10.1073/pnas.0503706102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarmah B, Latimer AJ, Appel B, Wente SR. Dev Cell. 2005;9:133–145. doi: 10.1016/j.devcel.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Verbsky J, Lavine K, Majerus PW. Proc Natl Acad Sci USA. 2005;102:8448–8453. doi: 10.1073/pnas.0503656102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang YH, Grasis JA, Miller AT, Xu R, Soonthornvacharin S, Andreotti AH, Tsoukas CD, Cooke MP, Sauer K. Science. 2007;316:886–889. doi: 10.1126/science.1138684. [DOI] [PubMed] [Google Scholar]
- 41.Tan X, Calderon-Villalobos LI, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N. Nature. 2007;446:640–645. doi: 10.1038/nature05731. [DOI] [PubMed] [Google Scholar]
- 42.Dubois E, Scherens B, Vierendeels F, Ho MM, Messenguy F, Shears SB. J Biol Chem. 2002;277:23755–23763. doi: 10.1074/jbc.M202206200. [DOI] [PubMed] [Google Scholar]
- 43.Luo HR, Saiardi A, Yu H, Nagata E, Ye K, Snyder SH. Biochemistry. 2002;41:2509–2515. doi: 10.1021/bi0118153. [DOI] [PubMed] [Google Scholar]
- 44.Saiardi A, Sciambi C, McCaffery JM, Wendland B, Snyder SH. Proc Natl Acad Sci USA. 2002;99:14206–14211. doi: 10.1073/pnas.212527899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El Alami M, Messenguy F, Scherens B, Dubois E. Mol Microbiol. 2003;49:457–468. doi: 10.1046/j.1365-2958.2003.03562.x. [DOI] [PubMed] [Google Scholar]
- 46.Shen X, Xiao H, Ranallo R, Wu W-H, Wu C. Science. 2003;299:112–114. doi: 10.1126/science.1078068. [DOI] [PubMed] [Google Scholar]
- 47.Steger DJ, Haswell ES, Miller AL, Wente SR, O'Shea EK. Science. 2003;299:114–116. doi: 10.1126/science.1078062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saiardi A, Resnick AC, Snowman AM, Wendland B, Snyder SH. Proc Natl Acad Sci USA. 2005;102:1911–1914. doi: 10.1073/pnas.0409322102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alcazar-Roman AR, Tran EJ, Guo S, Wente SR. Nat Cell Biol. 2006;8:711–716. doi: 10.1038/ncb1427. [DOI] [PubMed] [Google Scholar]
- 50.Weirich CS, Erzberger JP, Flick JS, Berger JM, Thorner J, Weis K. Nat Cell Biol. 2006;8:668–676. doi: 10.1038/ncb1424. [DOI] [PubMed] [Google Scholar]
- 51.Lee YS, Mulugu S, York JD, O'Shea EK. Science. 2007;316:109–112. doi: 10.1126/science.1139080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Irvine RF. J Physiol (London) 2005;566:295–300. doi: 10.1113/jphysiol.2005.087387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Macbeth MR, Schubert HL, VanDemark AP, Lingam AT, Hill CP, Bass BL. Science. 2005;309:1534–1539. doi: 10.1126/science.1113150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saiardi A, Bhandari R, Resnick AC, Snowman AM, Snyder SH. Science. 2004;306:2101–2105. doi: 10.1126/science.1103344. [DOI] [PubMed] [Google Scholar]
- 55.Shears SB. Cell Signal. 2001;13:151–158. doi: 10.1016/s0898-6568(01)00129-2. [DOI] [PubMed] [Google Scholar]
- 56.Kelly P, Stemmle LN, Madden JF, Fields TA, Daaka Y, Casey PJ. J Biol Chem. 2006;281:26483–26490. doi: 10.1074/jbc.M604376200. [DOI] [PubMed] [Google Scholar]
- 57.Fujii M, York JD. J Biol Chem. 2005;280:1156–1164. doi: 10.1074/jbc.M412006200. [DOI] [PubMed] [Google Scholar]
- 58.Seeds AM, Bastidas RJ, York JD. J Biol Chem. 2005;280:27654–27661. doi: 10.1074/jbc.M505089200. [DOI] [PubMed] [Google Scholar]