Structure and function of the yeast U-box-containing ubiquitin ligase Ufd2p (original) (raw)
Abstract
Proteins conjugated by Lys-48-linked polyubiquitin chains are preferred substrates of the eukaryotic proteasome. Polyubiquitination requires an activating enzyme (E1), a conjugating enzyme (E2), and a ligase (E3). Occasionally, these enzymes only assemble short ubiquitin oligomers, and their extension to full length involves a ubiquitin elongating factor termed E4. Ufd2p, as the first E4 identified to date, is involved in the degradation of misfolded proteins of the endoplasmic reticulum and of a ubiquitin–β-GAL fusion substrate in Saccharomyces cerevisiae. The mechanism of action of Ufd2p is unknown. Here we describe the crystal structure of the full-length yeast Ufd2p protein. Ufd2p has an elongated shape consisting of several irregular Armadillo-like repeats with two helical hairpins protruding from it and a U-box domain flexibly attached to its C terminus. The U-box of Ufd2p has a fold similar to that of the RING (Really Interesting New Gene) domain that is present in certain ubiquitin ligases. Accordingly, Ufd2p has all of the hallmarks of a RING finger-containing ubiquitin ligase: it associates with its cognate E2 Ubc4p via its U-box domain and catalyzes the transfer of ubiquitin from the E2 active site to Ufd2p itself or to an acceptor ubiquitin molecule to form unanchored diubiquitin oligomers. Thus, Ufd2p can function as a bona fide E3 ubiquitin ligase to promote ubiquitin chain elongation on a substrate.
Keywords: E4 elongation factor, endoplasmic reticulum-associated degradation, Cdc48p/p97, polyubiquitination, Armadillo repeats
In eukaryotes, short-lived proteins are degraded by the ubiquitin (Ub) proteasome system (reviewed in ref. 1). Substrates of the proteasome include transcription factors, cell cycle regulators, signal transducers, and misfolded proteins generated under stress conditions. Protein ubiquitination is achieved by a multistep mechanism involving a cascade of enzymes. Ub-activating enzyme (E1) hydrolyzes ATP to form a high-energy thioester bond between its catalytic cysteine residue and the C terminus of Ub. Activated Ub is subsequently passed to a distinct Ub-conjugating enzyme (E2) by transthiolation. Finally, Ub is transferred to the ε-amino group of an internal lysine residue of a target protein by a Ub ligase (E3). Polyubiquitin chains can be assembled when additional Ub molecules are transferred, one at a time, to a lysine residue in the substrate-bound Ub molecule via an isopeptide bond linkage. In addition, with the assistance of an E3 ligase, certain Ub-conjugating enzymes can form Lys-48-linked Ub chains linked to its catalytic cysteine before transferring the assembled Ub chains to a substrate (2, 3).
The essential components involved in the degradation of certain short-lived proteins have been identified in Saccharomyces cerevisiae by using a model proteasomal substrate consisting of a Ub moiety fused to the N terminus of a reporter protein. These components, which were designated as UFD1–5, represent the so-called UFD (Ub fusion degradation) pathway (4). Interestingly, with the exception of Ufd5p, a transcriptional regulator of the proteasome (5), and of Ufd4p, a HECT (homologous to E6-associated protein C terminus) domain Ub ligase (6), the other UFD proteins all interact with a conserved AAA ATPase (ATPase associated with various activities) named Cdc48p in yeast or p97 in mammals to regulate its activities in a subset of proteasome-dependent degradation pathways (7). The role of Cdc48p/p97 in protein turnover is best characterized for the degradation of misfolded endoplasmic reticulum (ER) proteins, which occurs through a pathway termed ER-associated protein degradation or retrotranslocation. During this process, Cdc48p/p97 associates with the ER membrane to extract its client proteins (misfolded polypeptides undergoing retrotranslocation) out of the ER membrane and subsequently target them for degradation by the proteasome. The Cdc48p/p97-dependent retrotranslocation requires the function of at least two UFD proteins, Ufd1p and Ufd2p. Ufd1p associates with Npl4p to form a heterodimeric cofactor complex, which promotes substrate recognition by Cdc48p/p97 (8–10). Ufd2p appears to act downstream of Cdc48p/p97 to facilitate the transfer of ER-associated protein degradation substrates to Rad23p, a proteasome-associated Ub receptor (11). Although Ufd2p is not essential for cell viability under normal conditions, its activity becomes critical under stress conditions in yeast (6). Likewise, the Ufd2a+/− mice lacking one functional copy of the Ufd2a gene, a homologue of Ufd2, develop a neurological disorder as a result of axonal dystrophy induced by ER stress. Mice deficient in UFD2a die in utero because of marked apoptosis in the developing heart (12). Taken together, the major function of Ufd2 is probably to cooperate with Cdc48/p97 to maintain a stress-free environment for cells.
Ufd2p contains a U-box domain that is structurally related to the RING finger domain that is found in certain E3 Ub ligases (13, 14). Ufd2 is capable of elongating Ub chains, an activity that is essential for its function in ER-associated protein degradation. In the presence of an E1, Ubc4p (E2), and Ufd4p (E3), a short Ub chain attached to a substrate can be further extended by Ufd2p. For this reason, Ufd2p is often referred to as an E4 enzyme (6). It is unclear how Ufd2p promotes Ub chain elongation in conjunction with the HECT domain containing E3 ligase Ufd4p. A HECT domain E3 ligase usually accepts Ub from its cognate E2 and forms a thioester-linked Ub–E3 intermediate before transferring the attached Ub to a substrate. In contrast, RING finger E3 ligases usually associate directly with cognate E2 enzymes to promote the transfer of Ub directly from E2 to a substrate. RING finger E3 ligases can also mediate the discharge of Ub from the E2 active cysteine in the absence of a substrate. When excess free Ub molecules are present, the released Ub molecules can be linked to a lysine residue in these free Ub molecules, forming an isopeptide bond-linked diubiquitin molecule (15). Whether Ufd2p also contains these activities is unknown.
Here we present the crystal structure of the full-length yeast Ufd2p enzyme. The structure has an elongated shape with a flexible U-box domain attached to it. The core region of Ufd2p consists of multiple irregular Armadillo-like repeats with two pronounced helical hairpin protrusions. The core region of Ufd2p is structurally reminiscent of importin α, a nuclear transport protein. Based on the CHIP/E2 complex crystal structure (16), we built a model of the Ufd2p/E2 complex that predicts interactions between E2 and the U-box domain as well as the core of Ufd2p. Biochemical experiments confirmed that Ufd2p binds directly to the E2 enzyme Ubc4p and furthermore revealed that its U-box domain is involved in the transfer of an E2-conjugated Ub to a free Ub and to Ufd2p itself. Thus, Ufd2p has the characteristics of a typical RING finger E3 ligase.
Results
Structure Determination.
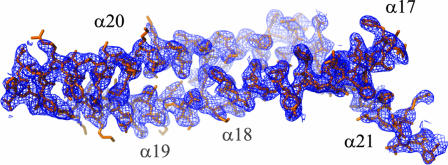
Crystals of the yeast Ufd2p, a 110-kDa protein consisting of 961 residues, were formed in space group _P_212121 containing one molecule per asymmetric unit. The structure was solved by multiple-wavelength anomalous dispersion (MAD) phasing to _d_min = 3.0 Å using selenomethionine substitution. Density modification and phase extension to _d_min = 2.74 Å using data set Native2(Se) (Tables 1 and 2) resulted in a readily interpretable electron density map (Fig. 1). Iterative model building and refinement produced a model with good statistics and geometry (Table 1). A higher-resolution data set was subsequently collected on a different crystal form in the same space group, but with a significantly different c cell axis (Tables 1 and 2, Native1). The final refined model comprising 933 residues produced _R_free = 27.6% and R = 22.1% to _d_min = 2.56 Å. A representative 2_F_o − _F_c σA-weighted electron density map contoured at 1 σ is shown in supporting information (SI) Fig. 10. The quality of the electron density map was excellent except for residues 27–35 and residues 707–718.
Table 1.
X-ray data collection and refinement
| Single wavelength | MAD (Se) | ||||
|---|---|---|---|---|---|
| Native1 | Native2 (Se) | Inflection | Peak | Remote | |
| Crystallographic data | |||||
| Beamline | SSRL/11-1 | ALS/8.2.2 | SSRL/11-1 | SSRL/11-1 | SSRL/11-1 |
| Wavelength, Å | 0.97945 | 1.00000 | 0.97920 | 0.97895 | 0.91837 |
| a, b, c, Å | 65.5, 123.0, 176.4 | 65.8, 122.8, 178.9 | 65.4, 122.7, 178.0 | 65.4, 122.7, 178.0 | 65.4, 122.7, 178.0 |
| Resolution, Å | 2.56 | 2.74 | 3.0 | 3.0 | 3.0 |
| Unique reflections | 46,444 | 37,483 | 55,241 | 55,152 | 55,389 |
| Redundancy | 4.2 | 3.8 | 4.1 | 4.1 | 4.2 |
| Completeness, % | 99.2 (95.4) | 96.3 (74.2) | 99.2 (94.5) | 99.1 (94.0) | 99.6 (98.2) |
| I/σ | 21.6 (2.1) | 19.0 (2.5) | 18.2 (1.7) | 19.1 (2.0) | 17.4 (1.9) |
| _R_sym,* % | 5.8 (59.3) | 5.6 (39.8) | 6.7 (55.6) | 6.8 (55.3) | 7.1 (57.0) |
| Refinement | |||||
| Resolution, Å | 50–2.56 | 50–2.65 | |||
| _R_cryt/_R_free† | 22.1/27.6 | 23.4/26.9 | |||
| Bond length deviation, Å | 0.007 | 0.008 | |||
| Bond angle deviation, ° | 1.2 | 1.4 | |||
| Average B factor, Å2 | 63.0 | 77.7 | |||
| Minimum B factor, Å2 | 23.6 | 31.4 | |||
| Maximum B factor, Å2 | 138.7 | 156.0 | |||
| Residues in core ϕ–ψ region, % | 87.0 | 84.4 | |||
| Residues in disallowed regions, % | 0.0 | 0.0 |
Table 2.
Phasing power and figure of merit
| Phasing power | Figure of merit | |
|---|---|---|
| 48.67–2.99 Å | 1.75 | 0.54 |
| 3.13–2.99 Å | 0.44 | 0.14 |
Fig. 1.
Representative region of the experimental electron density map of Ufd2p. The map was subjected to phase extension and density modification at 2.74 Å and contoured at 1 σ. Shown are helices α17 to α21.
Overall Structure.
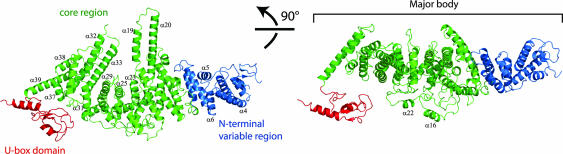
Ufd2p (residues 1–879) has an elongated shape, ≈146 Å in length, ≈84 Å in height, and ≈70 Å in width (Fig. 2). Based on sequence conservation (SI Fig. 11), the major body of Ufd2p can be approximately divided into two regions, consisting of residues 1–187 and 188–879, respectively. The highly variable N-terminal region consists of a short β-hairpin and eight α-helices. Helices α1 to α4 form a four-helix bundle, whereas helices α5 and α6 interact with α3 and α4 through hydrophobic contacts that are partly mediated by their connecting loops. The more conserved core region, residues 188–879, consists of a series of interacting helices with two pronounced helical hairpin protrusions (Fig. 2).
Fig. 2.
Overall structure of Ufd2p. The two views are related by a 90° rotation as specified. The N-terminal region is colored blue, the core region is colored green, and the U-box domain is colored red. Selected α-helices are labeled.
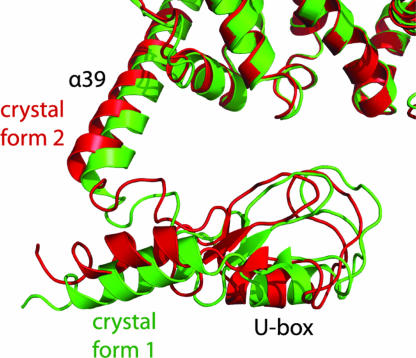
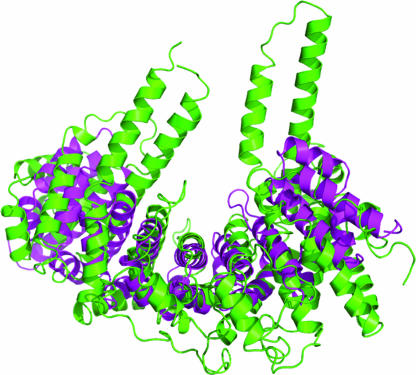
The C-terminal residues (884–947) form a U-box domain consisting of a short three-stranded β-sheet and two α-helices (Fig. 2), which is connected to the major body by a five-residue loop. The structure of this domain superimposes well on that of the U-box of CHIP (16) with a rmsd of 1.1 Å out of 63 equivalent Cα atoms. The U-box domain forms no direct contact with the major body of Ufd2p. We observed two conformations of the U-box domain in the two different crystal forms (Fig. 3). Whereas the majority of the atomic coordinates between the two crystal forms are very similar (rmsd = 0.6 Å for 829 Cα atoms within residues 1–850), the U-box domain and helix α39 have rotated with a maximum displacement of 4.2 Å between equivalent Cα atoms, indicating flexibility of the linker connecting the U-box domain to the core region. Clearly, the movement of the U-box domain is restricted by crystal packing contacts, so we expect an increased amount of conformational variability in solution.
Fig. 3.
Conformational variability of the U-box domain. The structures from the two crystal forms were superimposed by using the atoms of the major body. Green, structure from Native1; red, structure from Native2(Se).
The Conserved Core Region.
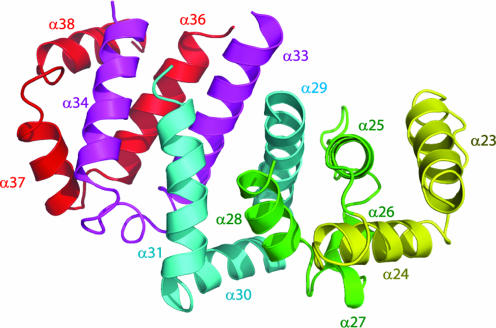
The core region of Ufd2p contains 31 α-helices of variable lengths that are connected by loops of different size (Fig. 2). Most of these helices form a compact unit that resembles a shallow-grooved, right-handed spiral, apart from the α19/20 and α32/33 helical hairpin protrusions, and helices α16 and α22. The helical packing pattern of the compact unit consists of five structurally repeating units that resemble tandem Armadillo (ARM) repeats, each of which comprises three α-helices formed by ≈40 aa (α23–α24, α25–α28, α29–α31, α33–α34, and α36–α38) (Fig. 4). However, the Ufd2p repeating units are highly irregular, comprising multiple short helices and/or loops whose structural equivalent in ARM motif would be composed of a single helix.
Fig. 4.
Repeating folding motifs of the core region of Ufd2p. Each repeating motif is colored differently. For clarity, helices α32 and α35 and part of helix α33 are not shown because they are not involved in the folding motifs.
Conserved Residues and Surface Features.
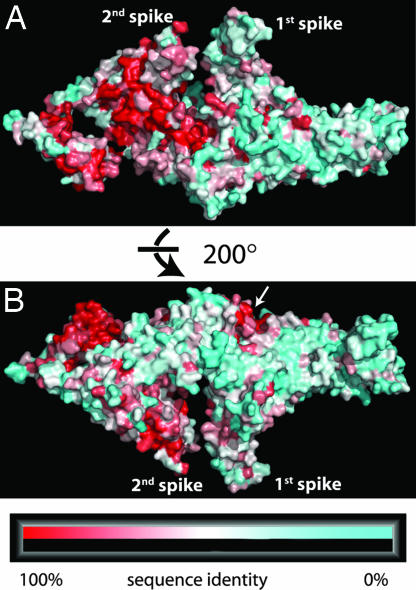
As mentioned above, the N-terminal region of Ufd2p exhibits primary sequence variability, indicating that other Ufd2p homologues may adopt different structures in that region. The side of Ufd2p from which the U-box domain protrudes is more conserved compared with the other side (Fig. 5). There is a large continuous patch of conserved residues covering one side of the core region and part of one of the helical hairpins (α32/α33). In contrast, the surface residues of the N-terminal region of Ufd2p are not conserved apart from the spot that is formed by the termini of helices α14 and α17 and by the loop region around residue Lys-333 (white arrow in Fig. 5B).
Fig. 5.
Molecular surface conservation of Ufd2p. Ufd2p homologues from 12 species (S. cerevisiae, Schizosaccharomyces pombe, Aspergillus oryzae, Drosophila melanogaster, Apis mellifera, Mus musculus, Rattus norvegicus, Homo sapiens, Danio rerio, Arabidopsis thaliana, Dictyostelium discoideum, and Caenorhabditis elegans) were aligned by using ClustalW (17). Residues were each assigned a conservation score by using the ConSurf server 3.0 (http://consurf.tau.ac.il), and scores were mapped onto the surface of the molecule. The colors range from 100% (red) to 50% (white) to 0% (cyan) sequence identity. Two views are shown. (A) View of the side of Ufd2p from where the U-box domain protrudes. (B) The view in A is rotated 200° away from the reader. The white arrow marks the only conserved spot in the region of residues 1–380.
One of the two sides of Ufd2p is mostly covered by negatively charged amino acids, especially around the linker region between the U-box and the core region (SI Fig. 12 Upper). The other side has a mostly neutral charge distribution, except for two positively charged regions: the abovementioned conserved spot near Lys-333 and the area between the two helical hairpins (SI Fig. 12 Lower).
Structural Comparisons with Other Proteins.
A search for structurally related proteins of Ufd2p was performed with DALI (18). The core region of Ufd2p shares significant structural similarity with the nuclear import factor importin α, which recognizes proteins that carry a nuclear localization signals to mediate their transport from the cytosol into the nucleus (19, 20). However, in contrast to Ufd2p's irregular ARM-like motifs, importin α consists of a series of regular ARM repeats. These repeating units form a superhelical structure with a shallow groove on one side that binds a nuclear localization signal-containing substrate (21). Residues 196–857 of the core region of Ufd2p superimpose with the eight ARM repeats of importin α (residues 141–508) with a rmsd deviation of 5.2 Å for 276 aligned Cα atoms and a Z score of 7.9 (Fig. 6). The structural similarity between Ufd2p and importin α raises the interesting possibility that the core region of Ufd2p is interacting with substrates or cofactors, such as Ub and Cdc48p.
Fig. 6.
Superposition of residues 196–857 of the Ufd2p core region (green) and residues 141–509 of importin α (Protein Data Bank ID code 1EE4) (magenta) using the overlay matrix determined by DALI.
E2–Ufd2p Interactions and Ub Ligase Activities.
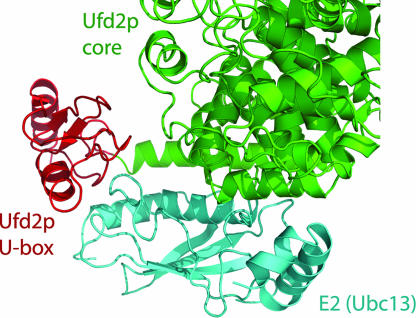
We predicted a model of the complex between Ufd2p and its cognate E2 by superimposing the structure of the U-box domain of Ufd2p on that of the CHIP–E2 (Ubc13) complex (16). CHIP is a U-box-containing Ub ligase that mediates the ubiquitination of a variety of substrates (22, 23). Upon superposition of the U-box domains, the E2 of the CHIP–E2 complex fits well underneath a cleft located between the Ufd2p core region and its U-box, forming contacts with both the U-box and the core domain without steric clashes (Fig. 7).
Fig. 7.
A model of the Ufd2p/E2 complex. The Ufd2p core region is shown in green, its U-box domain is shown in red, and the docked E2 (Ubc13) is shown in cyan. For clarity, the U-box domain of the CHIP–E2 complex is not shown.
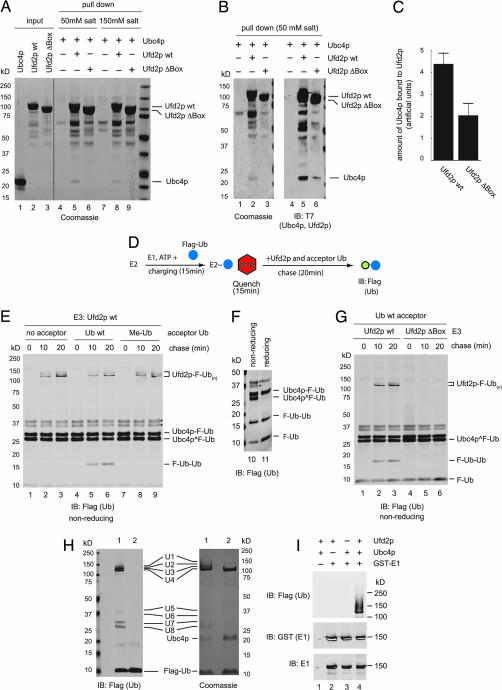
We tested the model of the Ufd2p/E2 complex by demonstrating a direct interaction between Ufd2p and its cognate E2 Ubc4p (Fig. 8 A and B) and a reduced interaction between the U-box-deleted Ufd2p mutant and Ubc4p (Fig. 8C). Thus, Ufd2p may act similarly to an E3 Ub ligase to mediate the transfer of Ub from Ubc4p to a substrate. To test this hypothesis, we developed a Ub turnover assay to monitor the single-round transfer of Ub from the active cysteine in Ubc4p to free Ub acceptors (Fig. 8D). His-tagged Ubc4p purified from Escherichia coli was incubated with a low concentration of Flag-Ub in the presence of E1 and ATP. This charging reaction generated Ubc4p carrying a Flag-Ub moiety on its catalytic cysteine residue (Fig. 8E, labeled Ubc4p ^F-Ub; confirmed by mass spectrometry, Fig. 8H, lane 1, labeled U8) as well as some Ubc4p molecules that are linked to Ub via a lysine residue (Fig. 8E, labeled Ubc4b-F-Ub; confirmed by mass spectrometry, Fig. 8H, lane 1, labeled U7; note that this species is resistant to reducing agent; see Fig. 8F). Mass spectrometry experiments showed that the higher-molecular-weight species in the range of 35–37 kDa (marked by asterisks) were also ubiquitinated Ubc4p that contain extra Ub molecules (Fig. 8H, lane 1, labeled U5 and U6) attached to either a lysine or a noncatalytic cysteine residue, which was likely generated by Ubc4p autoubiquitination. The charging reaction was quenched by addition of EDTA and _N_-ethylmaleimide to prevent additional rounds of charging. The quenched reaction was further incubated in the presence of Ufd2p and excess untagged acceptor Ub. After incubation, a fraction of Flag-Ub molecules attached to the catalytic cysteine in Ubc4p was transferred to the untagged Ub acceptor, leading to the formation of unanchored diubiquitin molecules (Fig. 8E, lanes 5 and 6, labeled F-Ub-Ub). The two Ub molecules are likely linked via an isopeptide bond because the F-Ub-Ub band is resistant to treatment by a reducing agent (Fig. 8F). As expected, no such diubiquitin molecules were formed in the absence of Ub acceptor or when a methylated Ub mutant was used as acceptor (Fig. 8E). Furthermore, the Ufd2p ΔBox mutant did not yield the formation of unanchored diubiquitin (Fig. 8G, lanes 4–6). These data suggest that the U-box of Ufd2p promotes the discharge of Ub from the catalytic cysteine of Ubc4p, a property shared by RING domain E3 ligases.
Fig. 8.
Ufd2p has E3 ligase activities. (A) Association of Ubc4p with Ufd2p. Biotinylated Ufd2p or the Ufd2pΔbox mutant immobilized on streptavidin-coated beads was incubated with purified Ubc4p. The precipitated material was analyzed by SDS/PAGE and Coomassie staining. Two salt conditions were used for binding: either 50 mM sodium chloride (lanes 4–6) or 150 mM potassium chloride (lanes 7–9). Each input protein was also analyzed directly (lanes 1–3). (B) Another independent binding experiment using the 50 mM salt condition as in A. Ten percent of the reaction was also probed by an anti-T7 antibody immunoblotting (lanes 4–6). (C) Amount of Ubc4p bound to Ufd2p or to Ufd2pΔBox quantified from Western blots of three independent binding experiments (using the 50 mM salt condition). Results were normalized based on the amount of Ufd2p or Ufd2pΔbox present in the precipitated materials. Error bars show the standard deviation. (D) Schematic representation of the single-round turnover experiment. (E) Transfer of Ub from Ubc4p to an acceptor Ub. Ubc4p charged with Flag-Ub was incubated with excess untagged Ub in the presence of Ufd2p. Where indicated, the reaction was carried out with no Ub acceptor or with methylated Ub (Me-Ub). The double bands marked by asterisks are Ubc4p molecules conjugated with two Ub molecules with the upper one likely containing lysine-linked Ub and the bottom band carrying only cysteine-linked Ub. (F) The sample in E, lane 6, was analyzed under both reducing and nonreducing conditions. (G) The formation of diubiquitin molecules and Ufd2p autoubiquitination require the Ufd2p U-box. Experimental details are as in E. Where indicated, the Ufd2Δbox mutant was used. (H) Gel for mass spectrometry analysis. Experimental details similar to E, except that the reaction time was 1 h (lane 1) and the acceptor Ub was omitted from the reaction. Lane 2 was a mock reaction that contained everything, but not E1. The reactions were incubated with glutathione beads to remove most GST-E1. Ninety-five percent of the reaction was analyzed by Coomassie staining (Right), whereas the rest was analyzed by immunoblotting (IB) (Left). The bands labeled as U1–U8 were subjected to mass spectrometry analyses. (I) Experimental details are as in H. The Western blots show that GST-E1 is not ubiquitinated.
Interestingly, a fraction of Ubc4p-conjugated Flag-Ub was transferred to a protein of ≈110 kDa to form a Ub ladder (Fig. 8 E and G, labeled Ufd2p-F-Ub(n); Fig. 8H, lane 1, labeled U1–U4). Because E1 and Ufd2p were the only two proteins that had the molecular mass of ≈110 kDa, we performed immunoblotting experiments to rule out that E1 was ubiquitinated (Fig. 8I). Mass spectrometry analyses further confirmed that the Ub molecules were indeed conjugated to Ufd2p (data not shown). As expected, Ufd2p autoubiquitination required its U-box (Fig. 8G). Taken together, we conclude that Ufd2p has several hallmarks of a RING E3 ligase: direct association with its cognate E2, promotion of the transfer of E2-conjugated Ub to a free Ub molecule, and autoubiquitination.
Prediction of Interactions Between Ufd2, Cdc48p, and Rad23p.
Biochemical studies suggest that Ufd2p binds Cdc48p via charged residues because the interaction between the two molecules is highly sensitive to salt concentration (24). Yeast two-hybrid analyses showed that a segment between Thr-808 and Lys-856 in Ufd2p may be critical for its interaction with Cdc48p (11). Within that region there are several conserved surface side chains: Asp-826, Glu-827, and Arg-828; Arg-844; and Glu-855 (SI Fig. 13). These residues are located near the C terminus of the core region of Ufd2p. The two proximal residues Arg-844 and Glu-855 are likely candidates for binding to Cdc48p because they are unobstructed by other domains and fully exposed, which may be required to accommodate the interaction with the 600-kDa Cdc48p.
The region in Ufd2p that is involved in Rad23p binding (1–380) (11) is less conserved. However, a conserved and positively charged spot stands out as a potential binding site for Rad23p, which includes residues Phe-326, Asp-328, Lys-333, Asn-380, and Phe-381 (white arrow in Fig. 5B).
Discussion
Ufd2p is the first discovered member of the U-box domain-containing protein family that is involved in polyubiquitination (22). Ufd2p was initially termed E4 because it can cooperate with an E3 to extend a Ub chain on an oligoubiquitinated artificial substrate. Subsequent studies showed that Ufd2p may be also involved in escorting ubiquitinated proteins from Cdc48p to the proteasome for degradation (11). During this process, Ufd2p appears to interact first with Cdc48p to upload polyubiquitinated substrates. Subsequently, the Ufd2p–substrate complex may dissociate from Cdc48p, allowing Ufd2p to bind and hand over substrates to Rad23p, a proteasome-associated factor.
The structure of Ufd2p provides some clues on how it can have such distinct functions. The large elongated body of Ufd2p is well suited for interactions with multiple binding partners. Furthermore, we have found an unexpected structural similarity between the core region of Ufd2p and the nuclear transporter protein, importin α, which may imply a transport function for Ufd2p. With respect to the polyubiquitination function of Ufd2p, Ufd2p also contains an U-box domain that is both structurally and functionally related to the RING domain in certain Ub E3 ligases. Based on the crystal structure of the complex between U-box domain of CHIP and E2 Ubc13 (16), we predicted a complex between Ufd2p and its cognate E2 (Fig. 7). The modeled interaction between the U-box domain and E2 is similar to that between a RING domain and an E2 (25); in both cases a hydrophobic ridge on the surface of E2 inserts into a hydrophobic groove formed by a short α-helix and the tips of two hairpin turns on the U-box or RING finger surface.
The ability of Ufd2p to bind to Ubc4p (Fig. 8 A and B) and the structural similarity between the U-box and RING domains suggested that Ufd2p can act as an E3 Ub ligase. We tested this hypothesis by a single-round Ub turnover assay and found that Ufd2p functions by a mechanism similar to a RING domain Ub ligase. In particular, Ufd2p catalyzes the formation of diubiquitin by transferring Ub from an E2 to free Ub acceptors, a hallmark of a RING domain-containing E3 ligase. Furthermore, we found that Ufd2p can ubiquitinate itself in the presence of just E1 and E2. This is consistent with previous findings that mammalian UFD2 and other U-box proteins can catalyze the assembly of long polyubiquitination chains on themselves and heterologous substrates (26). We therefore propose that Ufd2p can function as a bona fide E3 ligase.
In the work of Koegl et al. (6), Ufd2p was classified as an E4 enzyme because Ufd2p cannot cooperate directly with an E2 in the ubiquitination reaction of a particular substrate: its ability to ubiquitinate a model UFD substrate (Ub-ProtA) requires the presence of a HECT domain E3. Our results do not contradict their conclusion because they only examined the fate of Ub-ProtA instead of following the flow of the Ub molecules. Thus, autoubiquitination of Ufd2p may have escaped detection. It is likely that the nature of the substrate prevents Ufd2p from directly using E2 to ubiquitinate Ub-ProtA. For example, the Ufd2p may have a low affinity to this substrate and thus would require another E3 to initiate the ubiquitination process.
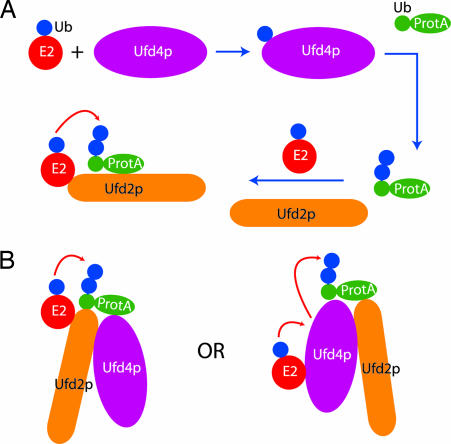
How Ufd2p assists Ufd4p, a HECT domain E3, to extend oligoubiquitin chains remains to be determined. Based on our data we propose the following two possibilities. First, Ufd2p may act downstream of Ufd4p (sequential model in Fig. 9A). In this model, Ub would be first transferred via Ubc4p to the HECT domain of Ufd4p before being transferred to a substrate. Once a few Ub moieties have been added to the substrate, Ufd4p may become inactive and its role is taken over by Ufd2p. Ufd2p would then transfer Ub directly from an E2 to the substrate to extend the polyubiquitin chain. However, this sequential model does not explain the observation that Ufd2p combined with an E2 is unable to polyubiquitinate a purified oligoubiquitinated substrate Ub-ProtA (6). In alternative models (cooperative models in Fig. 9B), Ufd2p would work in conjunction with Ufd4p. The two proteins may form a complex together with the E2 Ubc4p. Within this complex, Ufd2p may promote the transfer of Ub from Ubc4p to a substrate protein directly or via Ufd4p. Alternatively, Ufd4p may serve as a cofactor to activate Ufd2p, allowing it to efficiently polymerize Ub molecules on a substrate once a few Ub molecules have been added to it by Ufd4p. Our crystal structure of the yeast Ufd2p provides a platform for further dissecting the mechanism of Ufd2p-mediated polyubiquitination and its role in ER-associated protein degradation.
Fig. 9.
Models of how Ufd2p and Ufd4p can cooperate to multiubiquitinate substrate Ub-ProtA. (A) Sequential model. Transfer of oligoubiquitins (blue disks) to substrate (Ub-ProtA) is performed by Ufd4p. Subsequently, Ufd2p takes over to directly transfer additional Ub from E2 to substrate. The red arrow indicates movement of Ub. (B) Cooperative models. Ufd2p and Ufd4p cooperate in the transfer of Ub from E2 to substrate. In Left, Ufd2p promotes direct Ub transfer from E2 to substrate. In Right, Ub is transferred first to Ufd4p, then from Ufd4p to substrate. Ufd2p may promote the release of Ub from E2 or facilitate the transfer of Ub from Ufd4p to the substrate.
Materials and Methods
Protein Preparation.
Ufd2p was expressed as N-terminal hexahistidine-tagged fusion protein in pET28a vector as previously described (11). Nucleotide sequencing showed two substitutions in the expression vector as compared with the yeast genome sequence, Ser102Leu, and Asp677Val, which agrees with the originally published sequence of Ufd2p when it was first cloned (4). The plasmid was introduced into Rosetta (DE3) cells (Novagen) and grown in Terrific Broth at 37°C to an optical density of 0.8. The temperature of the culture was then shifted to 20°C, and cells were induced with 0.25 mM isopropyl β-d-thiogalactoside for 10 h. Cells were harvested by centrifugation and resuspended in lysis buffer (50 mM Tris, pH 7.4/300 mM NaCl/10 mM imidazole) with 1 mM PMSF. After lysis by sonication and high-speed centrifugation of lysate, the supernatant was incubated with Ni-NTA resin (Qiagen) for 3 h at 4°C. The resin was washed, and the protein was eluted by using the lysis buffer supplemented with 250 mM imidazole. Bovine thrombin (Haematologic Technologies) was added to remove the hexahistidine tag, and the protein was dialyzed against thrombin digest buffer (50 mM Hepes, pH 7.4/150 mM NaCl/5 mM DTT) overnight at 4°C. After cleavage, Ufd2p was depleted of thrombin by anion-exchange chromatography at pH 7.0. Ammonium acetate (150 mM) was added to prevent Ufd2p from precipitation during column loading under low-salt conditions. The purified protein was dialyzed against protein buffer (20 mM Hepes, pH 7.0/100 mM NaCl), concentrated to 10 mg/ml, and stored at −80°C. The protein is a monomer in solution as determined by size-exclusion chromatography coupled to multiangle laser light scattering (Wyatt Technology). A circular dichroism temperature melting experiment revealed a sharp unfolding transition at 52°C (Aviv Biomedical). Selenomethioine (SeMet) protein was obtained as above, except for the following: SeMet was incorporated by metabolic inhibition (27), 20 mM 2-mercaptoethanol was added in lysis buffer, and after the Ni-NTA elution step all buffers contained 10 mM DTT.
Crystallization, Data Collection, and Processing.
Initial screens of crystallization condition were carried out by using 96-well format Index and Crystal Screen HT kits (Hampton Research) on a Phoenix robot (Art Robbins Instruments). Automatic plate scanning by a CrystalPro 2 imaging system (TriTek) identified initial hits. After optimization, crystals of Ufd2p were grown by using hanging-drop vapor diffusion at 20°C. A total of 10 mg/ml native protein was mixed with an equal volume of mother liquor consisting of 19% (wt/vol) PEG-3350, 500 mM sodium malonate (pH 7.0), and 5 mM DTT and equilibrated against 1 ml of mother liquor. Crystals grew to full size (≈0.2 mm × 0.2 mm × 0.1 mm) within 24 h. However, SeMet protein crystals grown under this condition invariably produced multiple lattices that could not be processed. A new condition was found for SeMet-substituted protein at 20% PEG-3350, 300 mM triammonium citrate (pH 7.2), 100 mM NaCl, and 10 mM DTT that produced a single crystal lattice. SeMet crystals were cryoprotected by increasing the PEG-3350 concentration to 30%. Combination of 30% PEG-3350 and 300 mM triammonium citrate quickly produced phase separation when drops were exposed to air at room temperature. Therefore, crystals were first transferred into 40 μl of mother liquor on microbridges (Hampton Research), moved to 4°C for 24 h, and then slowly transferred at 4°C into the final condition of 30% PEG-3350, 300 mM triammonium citrate (pH 7.2) and 10 mM DTT, followed by flash-freezing in liquid nitrogen. MAD data were collected at three wavelengths in 20° wedges by using inverse beam geometry. The diffraction pattern generated by Ufd2p SeMet crystals exhibited significant anisotropy. The data set Native2 was collected on a SeMet crystal, which had strong diffraction to _d_min = 2.7 Å along b* and c*, but along a* the diffraction became relatively weak beyond 3.0 Å. The data set Native1 was collected on a native Ufd2p protein crystal grown under a third condition consisting of 20% PEG-3350, 200 mM tripotassium citrate, and 5 mM DTT. This crystal was cryoprotected as described above, was diffracted to _d_min = 2.5 Å, and was less anisotropic. Integration, scaling, and merging of the diffraction data were performed with HKL2000 (28). A summary of the data collection statistics is given in Table 1. Crystals of Ufd2p formed in space group _P_212121 with one molecule per asymmetric unit and 62% solvent content.
Structure Determination.
The Se substructure was found by using dual-space direct methods with Patterson seeding as implemented in SHELXD (29). The structure factors, FA, of the substructure were calculated with SHELXC. The correlation coefficient between the signed anomalous difference Δ_F_ at the peak and high energy remote wavelengths fell below 25% past 3.5-Å resolution; hence, the MAD data were truncated at that resolution. The number of trials was set to 10, and seven of them produced solutions with correlation coefficients >63.0%. The top solution (correlation coefficient = 64.0) produced 18 selenium sites, and there was a large drop in occupancy for an additional site (0.31 vs. 0.17). The correct hand was determined by observing a much higher contrast value between the protein and solvent densities compared with that of the wrong hand (0.766 vs. 0.145) after density modification in SHELXE. All subsequent phasing, density modification, and refinement calculations were performed by using CNS 1.2 (30). Experimental MAD phase probability distributions were obtained from the Se substructure. The positions, thermal factors, and anomalous f′ and f″ form factors were refined individually for each atom and wavelength. Phases were further improved by density modification and extended to _d_min = 2.74 Å by using data set Native2.
Model Building and Refinement.
The initial model was built by using the program COOT (31). The density-modified, phase-extended experimental map had clear electron density for most helices, loops, and side chains (Fig. 1). Thirty polyalanine α-helices were automatically placed into the electron density map by using the “Place Helix Here” feature in COOT. Guided by the 18 selenium sites, it was relatively straightforward to trace the loops and remaining helices and assign the entire sequence of the protein. The progress of model refinement was monitored by using the free R value computed from a randomly omitted set of 10% of the observed diffraction data at 50- to 2.65-Å resolution. The refinement consisted of alternating rounds of torsion angle molecular dynamics simulated annealing, individual restrained thermal factor refinement, and model building in COOT. All model refinement used the maximum likelihood target function using amplitudes and experimental phase probability distribution (option MLHL in CNS). The bulk solvent model refined to an electron density level ρsol = 0.3 e/Å3 with _B_sol = 42.9 Å2. The final model of data set Native2(Se) consists of residues 1–25, 34–706, and 719–951 and 94 water molecules.
The higher-resolution data set Native1 was solved by molecular replacement starting with the previous model using Phaser (32). Initially the entire refined structure of data set Native2(Se) was used as the search model. The electron density map calculated from molecular replacement phases showed that the C-terminal U-box had moved significantly. Consequently the structure was split into two fragments, residues 1–879 and 880–951 (U-box), which were then used as separate groups in rigid body refinement. The resulting model was subjected to molecular dynamics simulated annealing and restrained thermal factor refinement. Refinement used the maximum likelihood target function (option MLF in CNS) using amplitudes of Native1. The bulk solvent refined to an electron density level ρsol = 0.3 e/Å3 with _B_sol = 35.0 Å2. The final model consists of residues 1–27, 35–707, and 718–954, 145 water molecules, and one potassium ion. Final model statistics are shown in Tables 1 and 2. The atomic coordinates and diffraction data have been deposited in the Protein Data Bank [ID codes 2QIZ (Native1) and 2QJ0 (Native2)].
Biochemical Experiments.
Binding assay.
The biotinylation labeling was performed by using EZ-link sulfo-NHS-Biotin (Pierce) according to the manufacturer's instruction. The E2 binding experiment was performed at 4°C in 300 μl of low-salt buffer (50 mM Tris, pH 7.4/50 mM sodium chloride/2.5 mM magnesium chloride/0.1% Triton X-100/1 mg/ml BSA) or a higher-salt buffer (50 mM Hepes, pH 7.3/150 mM potassium chloride/2.5 mM magnesium chloride/5% glycerol/0.1% Triton X-100/1 mg/ml BSA). Ubc4p (3 μg) was incubated with 5 μg of biotinylated Ufd2p or the Ufd2p mutant lacking the U-box that were immobilized on streptavidin beads. The precipitated complex was analyzed by either Coomassie blue staining or immunoblotting. The immunoblotting was performed by using a fluorescent dye-labeled secondary antibody (A21058; Invitrogen), and the blots were scanned by an Odyssey infrared imager. The data were quantified by Odyssey software. The level of Ubc4p coprecipitated was normalized on the basis of the amount of Ufd2p or Ufd2pΔBox bound to the beads.
Single-round turnover assay.
To monitor the single-round Ub turnover, 62.5 nM E1, 800 nM Ubc4p, and 400 nM Flag-Ub were incubated at 37°C for 15 min in a buffer containing 25 mM Tris·HCl (pH 7.4) and 2 mM magnesium/ATP. The reaction was quenched by addition of 50 mM EDTA and 10 mM NEM. Subsequently, Ufd2p or the U-box-deleted mutant was added at 278 nM. The reaction was further incubated in the presence of 150 μM untagged Ub or methylated Ub. The formation of diubiquitin was analyzed by immunoblotting with anti-Flag antibody.
Mass spectrometry analysis.
To generate a sufficient amount of material for mass spectrometry analysis, the autoubiquitination of Ufd2p was done in a modified condition based on that of the single-round turnover assay described above: the concentrations of E2 and Ub were 6 μM and 17 μM, respectively; Ufd2p was added at 1 μM; no acceptor Ub was added during the chase reaction, and the reaction time was increased to 1 h. The bands labeled as U1–U8 in Fig. 8H were subjected to an in-gel trypsin digest and then analyzed by LC tandem mass spectrometry. The resulting data show that (i) U1–U4 consists of Ufd2p and Ub, (ii) U5–U8 consists of Ubc4p and Ub, and (iii) the U1 band also contains GST-E1, which comigrates with some of the ubiquitinylated Ufd2p species. Mass spectrometry analyses were performed by the Taplin Biological Mass Spectrometry Facility at Harvard Medical School (Boston, MA) and the Vincent Coates Foundation Mass Spectrometry Laboratory at Stanford University.
Figure Preparation.
Figs. 1–7 and SI Figs. 10, 12, and 13 were prepared by using PyMol (33).
Supplementary Material
Supporting Figures
Acknowledgments
We thank Drs. Ron Kopito and Timothy Fenn for critical reading of the manuscript, Stefan Jentsch (Max Planck Institute of Biochemistry, Munich, Germany) for the Ufd2p plasmid, Rongsheng Jin for assistance with data collection, and Corie Ralston (Advanced Light Source Beamline 8.2.2) and Irimpan Mathews (Stanford Synchrotron Radiation Laboratory Beamline 11-1) for advice during data collection. The Stanford Synchrotron Radiation Laboratory is a national user facility operated by Stanford University on behalf of the U.S. Department of Energy (Office of Basic Energy Sciences). The Stanford Synchrotron Radiation Laboratory Beamline Structural Molecular Biology Program is supported by the Department of Energy (Office of Biological and Environmental Research) and by the National Institutes of Health (National Center for Research Resources, Biomedical Technology Program), and the National Institutes of General Medical Sciences. The Advanced Light Source is supported by the Office of Energy Research (Office of Basic Energy Sciences, Material Sciences Division) of the U.S. Department of Energy at Lawrence Berkeley National Laboratory. This work was supported by the Howard Hughes Medical Institute (A.T.B.) and by an intramural research program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (Y.Y.).
Abbreviations
Ub
ubiquitin
ER
endoplasmic reticulum
SeMet
selenomethioine
MAD
multiple-wavelength anomalous dispersion.
Footnotes
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on May 3, 2005.
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. B.A.S. is a guest editor invited by the Editorial Board.
Data deposition: The atomic coordinates and diffraction data have been deposited in the Protein Data Bank, www.pdb.org [PDB ID codes 2QIZ (Native1) and 2QJ0 (Native2)].
References
- 1.Hochstrasser M. Annu Rev Genet. 1996;30:405–439. doi: 10.1146/annurev.genet.30.1.405. [DOI] [PubMed] [Google Scholar]
- 2.Li W, Tu D, Brunger AT, Ye Y. Nature. 2007;446:333–337. doi: 10.1038/nature05542. [DOI] [PubMed] [Google Scholar]
- 3.Ravid T, Hochstrasser M. Nat Cell Biol. 2007;9:422–427. doi: 10.1038/ncb1558. [DOI] [PubMed] [Google Scholar]
- 4.Johnson ES, Ma PC, Ota IM, Varshavsky A. J Biol Chem. 1995;270:17442–17456. doi: 10.1074/jbc.270.29.17442. [DOI] [PubMed] [Google Scholar]
- 5.Xie Y, Varshavsky A. Proc Natl Acad Sci USA. 2001;98:3056–3061. doi: 10.1073/pnas.071022298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koegl M, Hoppe T, Schlenker S, Ulrich HD, Mayer TU, Jentsch S. Cell. 1999;96:635–644. doi: 10.1016/s0092-8674(00)80574-7. [DOI] [PubMed] [Google Scholar]
- 7.Ye Y. J Struct Biol. 2006;156:29–40. doi: 10.1016/j.jsb.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Meyer HH, Wang Y, Warren G. EMBO J. 2002;21:5645–5652. doi: 10.1093/emboj/cdf579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rape M, Hoppe T, Gorr I, Kalocay M, Richly H, Jentsch S. Cell. 2001;107:667–677. doi: 10.1016/s0092-8674(01)00595-5. [DOI] [PubMed] [Google Scholar]
- 10.Ye Y, Meyer HH, Rapoport TA. J Cell Biol. 2003;162:71–84. doi: 10.1083/jcb.200302169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richly H, Rape M, Braun S, Rumpf S, Hoege C, Jentsch S. Cell. 2005;120:73–84. doi: 10.1016/j.cell.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Kaneko-Oshikawa C, Nakagawa T, Yamada M, Yoshikawa H, Matsumoto M, Yada M, Hatakeyama S, Nakayama K, Nakayama KI. Mol Cell Biol. 2005;25:10953–10964. doi: 10.1128/MCB.25.24.10953-10964.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aravind L, Koonin EV. Curr Biol. 2000;10:R132–R134. doi: 10.1016/s0960-9822(00)00398-5. [DOI] [PubMed] [Google Scholar]
- 14.Ohi MD, Vander Kooi CW, Rosenberg JA, Chazin WJ, Gould KL. Nat Struct Biol. 2003;10:250–255. doi: 10.1038/nsb906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petroski MD, Deshaies RJ. Cell. 2005;123:1107–1120. doi: 10.1016/j.cell.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 16.Zhang M, Windheim M, Roe SM, Peggie M, Cohen P, Prodromou C, Pearl LH. Mol Cell. 2005;20:525–538. doi: 10.1016/j.molcel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 17.Thompson JD, Higgins DG, Gibson TJ. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holm L, Sander C. J Mol Biol. 1993;233:123–138. doi: 10.1006/jmbi.1993.1489. [DOI] [PubMed] [Google Scholar]
- 19.Enenkel C, Blobel G, Rexach M. J Biol Chem. 1995;270:16499–16502. doi: 10.1074/jbc.270.28.16499. [DOI] [PubMed] [Google Scholar]
- 20.Gorlich D, Henklein P, Laskey RA, Hartmann E. EMBO J. 1996;15:1810–1817. [PMC free article] [PubMed] [Google Scholar]
- 21.Conti E, Kuriyan J. Structure (London) 2000;8:329–338. doi: 10.1016/s0969-2126(00)00107-6. [DOI] [PubMed] [Google Scholar]
- 22.Cyr DM, Hohfeld J, Patterson C. Trends Biochem Sci. 2002;27:368–375. doi: 10.1016/s0968-0004(02)02125-4. [DOI] [PubMed] [Google Scholar]
- 23.Murata S, Chiba T, Tanaka K. Int J Biochem Cell Biol. 2003;35:572–578. doi: 10.1016/s1357-2725(02)00394-1. [DOI] [PubMed] [Google Scholar]
- 24.Saeki Y, Tayama Y, Toh-e A, Yokosawa H. Biochem Biophys Res Commun. 2004;320:840–845. doi: 10.1016/j.bbrc.2004.05.216. [DOI] [PubMed] [Google Scholar]
- 25.Zheng N, Wang P, Jeffrey PD, Pavletich NP. Cell. 2000;102:533–539. doi: 10.1016/s0092-8674(00)00057-x. [DOI] [PubMed] [Google Scholar]
- 26.Hatakeyama S, Yada M, Matsumoto M, Ishida N, Nakayama KI. J Biol Chem. 2001;276:33111–33120. doi: 10.1074/jbc.M102755200. [DOI] [PubMed] [Google Scholar]
- 27.Van Duyne GD, Standaert RF, Karplus PA, Schreiber SL, Clardy J. J Mol Biol. 1993;229:105–124. doi: 10.1006/jmbi.1993.1012. [DOI] [PubMed] [Google Scholar]
- 28.Otwinowski Z, Minor W. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 29.Schneider TR, Sheldrick GM. Acta Crystallogr D. 2002;58:1772–1779. doi: 10.1107/s0907444902011678. [DOI] [PubMed] [Google Scholar]
- 30.Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, et al. Acta Crystallogr D. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 31.Emsley P, Cowtan K. Acta Crystallogr D. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 32.McCoy AJ, Grosse-Kunstleve RW, Storoni LC, Read RJ. Acta Crystallogr D. 2005;61:458–464. doi: 10.1107/S0907444905001617. [DOI] [PubMed] [Google Scholar]
- 33.DeLano WL. The PyMol Molecular Graphics System. San Carlos, CA: DeLano Scientific; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Figures