The Drosophila melanogaster Cajal body (original) (raw)
Abstract
Cajal bodies (CBs) are nuclear organelles that are usually identified by the marker protein p80-coilin. Because no orthologue of coilin is known in Drosophila melanogaster, we identified D. melanogaster CBs using probes for other components that are relatively diagnostic for CBs in vertebrate cells. U85 small CB–specific RNA, U2 small nuclear RNA, the survival of motor neurons protein, and fibrillarin occur together in a nuclear body that is closely associated with the nucleolus. Based on its similarity to CBs in other organisms, we refer to this structure as the D. melanogaster CB. Surprisingly, the D. melanogaster U7 small nuclear RNP resides in a separate nuclear body, which we call the histone locus body (HLB). The HLB is invariably colocalized with the histone gene locus. Thus, canonical CB components are distributed into at least two nuclear bodies in D. melanogaster. The identification of these nuclear bodies now permits a broad range of questions to be asked about CB structure and function in a genetically tractable organism.
Introduction
The aim of this study was to identify the Drosophila melanogaster homologue of the vertebrate Cajal body (CB). CBs were discovered >100 yr ago by the Spanish neurobiologist Ramón y Cajal (Cajal, 1903), but their major molecular components have been described in only the past 15 yr, and few specific biochemical functions have been assigned to them. There is a general consensus that steps in the assembly and modification of the RNA processing machinery of the nucleus take place in vertebrate CBs, including the machinery for splicing, preribosomal RNA processing, and histone pre-mRNA processing (for reviews see Gall, 2000, 2003; Carmo-Fonseca, 2002; Matera, 2003; Cioce and Lamond, 2005). Cajal's original studies involved mammalian neurons, and even today the majority of studies on CBs make use of cultured mammalian cells. Nevertheless, CBs occur in a wide variety of other organisms, including amphibians, insects, plants, and probably budding yeast (for review see Gall, 2003).
The identification of CBs relies heavily on specific biochemical markers, of which the protein p80-coilin is the most widely used (Andrade et al., 1991; Raska et al., 1991). Orthologues of human coilin are known from several other vertebrates, including the mouse (Tucker et al., 2000), Xenopus laevis (Tuma et al., 1993), and Danio rerio (Tucker et al., 2000), as well as the plant Arabidopsis thaliana (Shaw, P.J., personal communication). However, the overall sequence of coilin is not highly conserved, and attempts to identify D. melanogaster coilin have so far been unsuccessful. Fortunately, four potentially specific D. melanogaster CB markers, two proteins and two RNAs, have recently been described. Three of these—dLsm10, dLsm11 (Pillai et al., 2001, 2003; Azzouz and Schümperli, 2003), and dU7 small nuclear RNA (snRNA; Dominski et al., 2003)—are components of the U7 snRNP, which is required for histone pre-mRNA maturation. In the amphibian oocyte nucleus (Wu and Gall, 1993) and in HeLa cells (Frey and Matera, 1995), U7 snRNA is localized almost exclusively in CBs. The fourth marker is dU85 (Jády and Kiss, 2001), which functions in the CB as a guide RNA for modifications on U5 snRNA (Jády et al., 2003). U85 and related RNAs have been called small CB-specific RNAs (scaRNAs) because of their high concentration in vertebrate CBs. Significantly, in situ hybridization of dU85 revealed a single small focus of label in the nuclei of D. melanogaster S2 cells, strongly suggesting that dU85 recognizes the D. melanogaster CB (Richard et al., 2003).
We began our study of D. melanogaster by examining the U7 snRNP on the assumption that U7 would be specific for D. melanogaster CBs, as it is in X. laevis and human cells. We identified a nuclear body that contains the U7 snRNP and showed that this body is physically associated with the histone gene locus. However, when we probed for four other CB components—dU85, dU2 snRNA, the D. melanogaster survival of motor neurons (SMN) protein (dSMN), and fibrillarin—we found them colocalized in a nuclear body separate from the body that contains the U7 snRNP. These findings pose both substantive and terminological questions. Based on its apparently greater complexity, we designate the dU85/dU2/dSMN/fibrillarin body as the D. melanogaster CB and the second nuclear body as the histone locus body (HLB). These two bodies are frequently close to one another or actually touching, although they may lie far apart in the nucleus. Our findings suggest that the D. melanogaster CB, like the CB in other organisms, is a composite structure whose subunits in some cases fuse together or reside next to each other but sometimes lie in separate parts of the nucleus.
Results
The D. melanogaster CB
dU85 scaRNA is a 316-nt nuclear RNA that contains sequences characteristic of both classes of small nucleolar RNAs (snoRNAs), the box C/D motif and the box H/ACA motif (Jády and Kiss, 2001). It is a guide RNA that simultaneously specifies the modification of two bases in dU5 snRNA, methylation at C46 and pseudouridylation at U47. Human U85 scaRNA was shown by in situ hybridization and biochemical fractionation to be localized exclusively in the CB, hence the name small CB-specific RNA (Darzacq et al., 2002; Richard et al., 2003). D. melanogaster U85 scaRNA has a sequence similar to that of human U85 and was localized by in situ hybridization to a discrete focus within cultured D. melanogaster S2 cells (Richard et al., 2003).
In our experiments, we used a full-length antisense RNA probe to detect dU85 scaRNA. In situ hybridization was performed on a variety of tissues, including brains and salivary glands of third instar larvae and nurse and follicle cells from adult ovaries. dU85 was detected in sharply defined foci with only background levels of label elsewhere in the nucleus or cytoplasm. Most nuclei in the brain, salivary glands, and follicle cells displayed a single focus of label (Fig. 1 B). This was also true of most nurse cell nuclei up to about stage 4, but nurse cell nuclei from larger egg chambers often contained two or three foci (see Fig. 6 E). Rarely did a large nurse cell nucleus contain as many as 10 foci.
Figure 1.
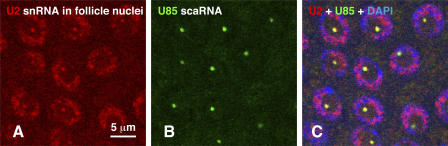
dU2 snRNA and dU85 scaRNA occur in the same nuclear body. (A) In situ hybridization of ovarian follicle nuclei with an antisense probe against dU2 snRNA (red). dU2 occurs diffusely throughout the nucleus with a single bright focus that is associated with the unlabeled nucleolus. (B) dU85 scaRNA is detected with an antisense probe in a single discrete focus (green). (C) The overlay shows precise colocalization of dU2 snRNA (red) and dU85 scaRNA (green). DNA labeled with DAPI (blue).
Figure 6.
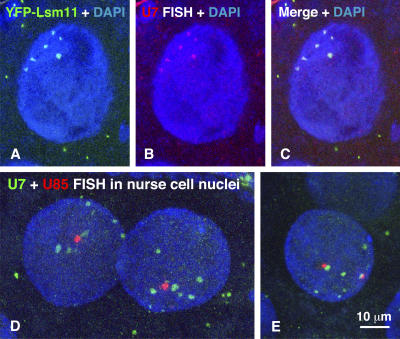
The distribution of dU7 snRNA and dU85 scaRNA in nurse cell nuclei. (A) YFP-dLsm11 (green) is concentrated in multiple bodies in the large polyploid/polytene nuclei of nurse cells. (B) In situ hybridization with an antisense probe against dU7 snRNA (red) reveals a similar pattern of multiple nuclear bodies. (C) Overlay of YFP-dLsm11 (green) and dU7 snRNA (red) images shows precise colocalization. (D) In situ hybridization with an antisense probe against dU7 (green) labels multiple nuclear bodies. Simultaneous in situ hybridization with an antisense probe against dU85 (red) shows a single focus of dU85 scaRNA, which lies near one of the larger dU7 nuclear bodies. (E) In this nucleus, there are two dU85 foci (red), both of which are next to dU7 nuclear bodies (green).
In situ hybridization was performed for dU2 snRNA in larval brains, salivary glands, and adult ovaries. In keeping with findings from vertebrate cells (Carmo-Fonseca et al., 1991; Huang and Spector, 1992; Matera and Ward, 1993), dU2 snRNA exhibited a speckled pattern superimposed on a more diffuse nuclear distribution. When double in situ hybridization was performed with dU2 and dU85, the most prominent “speckle” overlapped the dU85 signal (Fig. 1 C). However, the speckle was usually less discrete than the dU85 focus, and in some nuclei there were other equally prominent speckles that were not associated with dU85 (Fig. 1 A). In nurse cell nuclei, the strongest dU2 signals always coincided with dU85 signals (unpublished data). Richard et al. (2003) published an image of a D. melanogaster S2 cell after double in situ hybridization for dU85 and dU2. In this case, the dU85 signal was more discrete than the dU2 signal and there was a moderate level of dU2 signal throughout the nucleus.
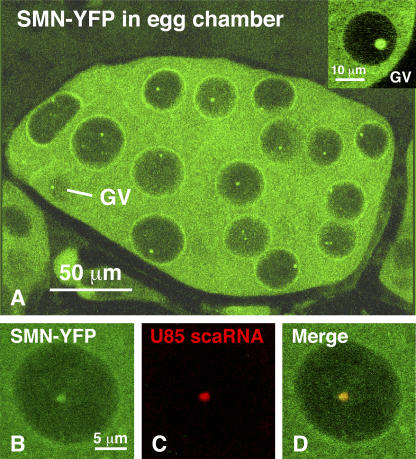
In vertebrate cells, SMN is a ubiquitous cytoplasmic protein involved in the assembly of the Sm snRNPs (Liu and Dreyfuss, 1996; Meister and Fischer, 2002; Pellizzoni et al., 2002). A small amount of SMN is found in the nucleus, where it is concentrated in CBs or in bodies closely associated with CBs, called gems (Liu and Dreyfuss, 1996). The distribution of dSMN has been examined with antibodies in cultured cells (Ilangovan et al., 2003), but its distribution in tissues of the fly has not been reported. Our observations were made on transgenic flies that express dSMN-YFP under the control of GAL4. Although we have not performed extensive genetic studies with these flies, we know that the transgene rescues smn hypomorphic and null mutations (Matera, A.G., personal communication). In keeping with the known distribution of the SMN protein in vertebrate (Liu and Dreyfuss, 1996) and D. melanogaster cells (Chan et al., 2003; Ilangovan et al., 2003), dSMN-YFP is strongly expressed in the cytoplasm of all cells examined, including larval salivary glands and brains and adult ovaries (Fig. 2, A and B). Although there is a low level of dSMN expression in most somatic nuclei, we have seen discrete foci of protein only in follicle cell nuclei of the ovary and in nurse cell nuclei and the germinal vesicle (GV). The GV always has a single bright focus up to about stage 9; the nurse cell nuclei usually have a single focus, but two or even three foci are common (Fig. 2 A). Immunostaining with an antibody against dSMN reveals a similar pattern in wild-type flies (unpublished data).
Figure 2.
dSMN-YFP expression in ovarian nurse cells and the oocyte. (A) As in other organisms, dSMN is primarily a cytoplasmic protein. Each of the 15 nurse cell nuclei contains one or occasionally two or three discrete foci of dSMN-YFP. Because this image is a projection of multiple sections, the relatively small GV is obscured by material lying above and below it. The inset shows a single section through the GV from another egg chamber. The GV from young egg chambers, up to about stage 9, invariably displays a single brightly labeled nuclear body. (B–D) dU85 scaRNA colocalizes with dSMN-YFP in nurse cell nuclei. (B) A nurse cell nucleus from a fly expressing dSMN-YFP (green), showing a single positive nuclear body. (C) In situ hybridization with an antisense probe against dU85 scaRNA (red). (D) dSMN-YFP and dU85 scaRNA precisely colocalize in the nurse cell nucleus.
To determine whether the dSMN foci in the germline are related to the similar dU85 foci seen in nurse cell nuclei, we performed in situ hybridization for dU85 in ovaries from flies that expressed dSMN-YFP. There was precise colocalization of the dU85 and dSMN signal in the nurse cell nuclei (Fig. 2, B–D).
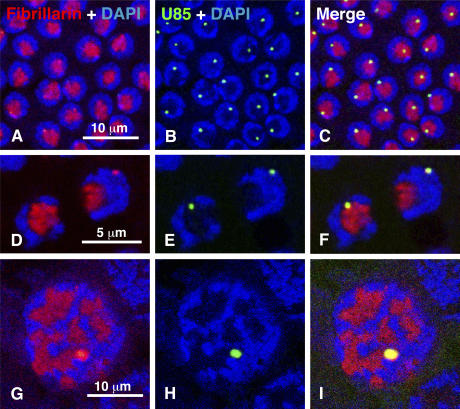
Fibrillarin is the methyl transferase that carries out 2′-_O_-methylation of ribose moieties using box C/D snoRNAs as guides (Galardi et al., 2002). Although its name derives from its localization in the fibrillar part of the nucleolus (Ochs et al., 1985), fibrillarin is also found in CBs and in fact was one of the first proteins demonstrated in CBs by immunofluorescent staining (Raska et al., 1991). The sequence of fibrillarin is highly conserved evolutionarily, and antibodies exist that react with many species. We used mAb 72B9 to detect fibrillarin in various tissues of D. melanogaster, including brain and salivary gland cells of third instar larvae and follicle and nurse cells of the ovary. In cells double stained with mAb 72B9 for fibrillarin and DAPI for DNA, one usually sees only a single contiguous area of stain within the nucleolus (Fig. 3, A–C). We made this observation early in our investigation and were puzzled that we did not see a separate focus of fibrillarin corresponding to a CB. Only when cells were simultaneously labeled by in situ hybridization for dU85 did it become clear that the CB (U85 body) is always colocalized with fibrillarin stain. In most cases, the CB touches or lies within the nucleolus so that its fibrillarin stain is contiguous with that in the fibrillar zone of the nucleolus. Sometimes the CB is completely independent of the nucleolus, and in these cases there is a separate focus of fibrillarin stain (Fig. 3, D–F). The situation in the giant nurse cell nuclei can be equally confusing. In these nuclei, the nucleolus expands enormously and takes on an irregular lobulated form. The CB lies somewhere within the giant nucleus, generally surrounded by nucleolar material. The CB often stains more intensely than the nucleolus with mAb 72B9 and can be recognized without an additional probe. However, reliable identification of the CB requires in situ hybridization for dU85, in which case one can see that the CB always stains with the antibody against fibrillarin (Fig. 3, G–I).
Figure 3.
Fibrillarin occurs primarily in the nucleolus but also in the CB in ovarian follicle cells and nurse cells. (A) In follicle cells, fibrillarin (red) is detected by mAb 72B9 in the fibrillar part of the nucleolus. DNA in the rest of the nucleus appears blue after staining with DAPI. (B) In situ hybridization with an antisense probe against dU85 (green) reveals a single CB in each nucleus. (C) Merge of the fibrillarin (red) and dU85 (green) images shows that the majority of CBs are at the periphery of the fibrillar part of the nucleolus. Some CBs appear to be inside the nucleolus, although it is difficult to be sure if they are simply above or below the fibrillar region. (D) Two follicle cell nuclei at higher magnification, stained for fibrillarin. The nucleus on the right displays a large patch of stain in the fibrillar region of the nucleolus and a smaller focus near the periphery of the nucleus. (E) In situ hybridization with dU85 reveals a CB in each nucleus. (F) The merge shows that the extra focus of fibrillarin in the right nucleus colocalizes with dU85 in the CB. (G) In this nurse cell nucleus, mAb 72B9 (red) stains the fibrillar region of the highly lobulated nucleolus, as well as a single, more intense focus at the periphery of one lobe. (H) In situ hybridization for dU85 (green) identifies a single CB. (I) The merged image clearly shows that the CB overlaps the most intense focus of the fibrillarin stain. DNA appears blue after DAPI staining.
The D. melanogaster HLB
In the amphibian oocyte (Wu and Gall, 1993) and in HeLa cells (Frey and Matera, 1995), the U7 snRNP colocalizes with coilin in the CB. Some of these CBs are associated with the histone genes. In D. melanogaster, the situation is somewhat different. Here the U7 snRNP resides in a separate body that is invariably located next to the histone genes.
The U7 snRNP consists of U7 snRNA associated with a ring of seven core Sm proteins. Five of the proteins are identical to the B/B', D3, E, F, and G proteins found in the U1, U2, U4, and U5 splicing snRNPs (for review see Kambach et al., 1999). The other two, Lsm10 and Lsm11, take the place of D1 and D2 in the ring and, so far as is known, are unique to the U7 snRNP (Pillai et al., 2001, 2003; Azzouz and Schümperli, 2003). Our first observations of the D. melanogaster U7 snRNP were based on the expression of YFP-dLsm11 in transgenic flies. To obtain expression, we crossed flies homozygous for a YFP-dLsm11 insert with flies homozygous for daGAL4, which carry the yeast GAL4 gene on the third chromosome under control of the daughterless (da) promoter. The da promoter supports the constitutive expression of the GAL4 protein in many cells of most tissues. Four different inserts gave similar expression. Two of these were on the X chromosome and had YFP fused to the carboxy terminus of dLsm11 and two were on the second chromosome with YFP at the amino terminus.
Brains and salivary glands.
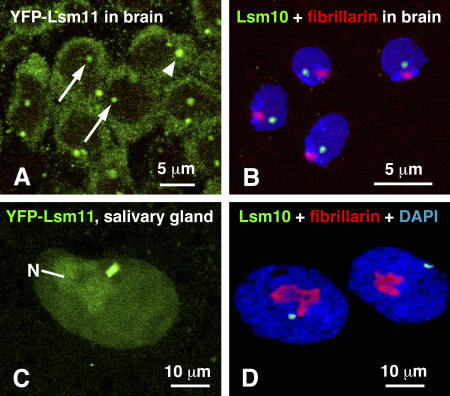
Brains and salivary glands from third instar larvae that expressed YFP-dLsm11 were dissected and gently squashed in PBS, OR2 saline, or Grace's medium. They were observed directly in the fluorescence microscope or were fixed and stained with Alexa 488–labeled antibody against GFP (Fig. 4, A and C). Fixed and stained tissue was brighter and more resistant to photobleaching than fresh tissue, but the overall pattern of fluorescence was similar. Most nuclei contained a single focus of green YFP-dLsm11, often near the nuclear envelope. In many salivary gland nuclei, the HLB was angular or distinctly rectangular in outline, suggesting that it might be coextensive with a segment of a polytene chromosome. Subsequent observations identified dLsm10 (Fig. 4, B and D) and dU7 snRNA (not depicted) in this focus, suggesting that it contained the entire dU7 snRNP. Cells that had a green HLB in the nucleus usually exhibited diffuse green fluorescence in the cytoplasm. In addition, many expressing cells contained an intense focus of YFP-dLsm11 in the cytoplasm, which was often brighter than the HLB in the nucleus (Fig. 4 A). The brain contained cells that did not express YFP-dLsm11 and therefore failed to show green fluorescence. Patches of expressing and nonexpressing cells were intermingled.
Figure 4.
dLsm10 and dLsm11 in larval brain and salivary gland cells. (A) Cells in the brain of a third instar larva expressing YFP-dLsm11. In each nucleus, YFP-dLsm11 (green) is restricted to a single nuclear body (arrows). It is also expressed throughout the cytoplasm, with a single very bright focus in many cells (arrowhead). (B) Brain cell nuclei stained with a polyclonal serum against dLsm10 (green), mAb 72B9 against fibrillarin (red), and DAPI (blue). The focus of dLsm10 often lies near the fibrillar zone of the nucleolus. (C) A single confocal section through a nucleus in an intact, unfixed salivary gland from a third instar larva. The highest concentration of YFP-dLsm11 (green) occurs in the polygonal body near the nuclear envelope. The next highest concentration is in the large nucleolus (N), with detectable levels throughout the rest of the nucleus. (D) Salivary gland nucleus stained with a polyclonal serum against dLsm10 (green), mAb 72B9 against the nucleolar protein fibrillarin (red), and DAPI (blue). As in the smaller brain cells shown in B, the focus of dLsm10 often lies near the fibrillar part of the nucleolus (left nucleus), but not invariably so (right nucleus).
Embryos.
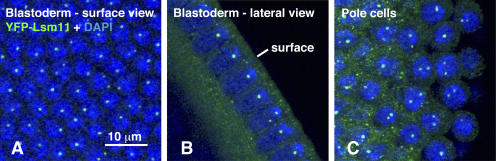
We examined eggs laid by females that expressed YFP-dLsm11 in their ovaries. HLBs first appear in embryos at cell cycle 11, after the majority of nuclei have migrated to the surface to form the blastoderm. All interphase nuclei during the blastoderm stages display one or two HLBs (Fig. 5 A). During cell cycle 14, as the nuclei elongate perpendicular to the surface of the embryo, the HLBs reside in close proximity to the heterochromatin, which occupies the end of the nucleus nearest the surface (Fig. 5 B). Pole cells (primordial germ cells) at the posterior end of the embryo display one or two HLBs in their nuclei (Fig. 5 C). Nuclei from older embryos also express YFP-dLsm11, but we have not yet studied the pattern of expression in detail.
Figure 5.
YFP-dLsm11 foci during the blastoderm stage of embryogenesis. (A) Surface view of blastoderm during the interphase after the 14th cell cycle. Most nuclei contain a single nuclear body, whereas a few have two. (B) Lateral view of the blastoderm from an embryo of the same stage. Note that the single nuclear body is located near the apical end of the nucleus. One nucleus has two nuclear bodies. (C) Pole cells at the posterior end of an embryo, at the time when they first begin to migrate dorsally. Unlike the blastoderm nuclei in the same embryo, many of the pole cells contain two nuclear bodies. Embryos were fixed in formaldehyde and stained with a polyclonal rabbit anti-GFP for YFP-dLsm11 (green) and DAPI for DNA (blue). These images are projections of several individual confocal sections taken through the entire thickness of the nuclei.
Ovary.
The ovary was studied as whole mounts or as squashes. The most prominent accumulations of YFP-dLsm11 in the ovary occur in the cytoplasm of the nurse cells. Often there is a single intense focus of fluorescence in each nurse cell. Nuclei in the smallest nurse cells have a single HLB, but as the nurse cells grow and their nuclei polyploidize, the number of HLBs increases (Fig. 6, A–C). In the largest cells, there may be as many as 30 per nucleus. Expression of YFP-dLsm11 in follicle cells of the ovary was variable and often in patches in a given egg chamber. Follicle cells that expressed YFP-dLsm11 generally exhibited a single HLB in the nucleus, with or without a bright focus of fluorescence in the cytoplasm.
dU7 snRNA colocalizes with YFP-dLsm11.
dU7 snRNA was detected by in situ hybridization with an antisense RNA probe labeled with Alexa 488 or 546. Because U7 snRNA in the nucleus exists in the form of the U7 snRNP, which contains both Lsm10 and Lsm11, we anticipated that the nuclear distribution of dU7 would be coincident with that of YFP-dLsm11. This was found to be the case. In the nuclei of all cells examined (larval brain and salivary glands and nurse and follicle cells from adult ovaries), dU7 snRNA displayed a pattern identical to that of YFP-dLsm11; namely, a single focus in most cells but multiple foci in the larger nurse cell nuclei (Fig. 6 and see Fig. 8). A strong dU7 signal also occurred in the bright cytoplasmic YFP-dLsm11 foci in nurse cells (unpublished data), suggesting that these foci might be sites of snRNP assembly rather than simple aggregates of overexpressed protein.
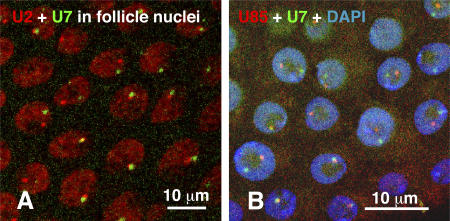
Figure 8.
Associations between CBs and HLBs. (A) Double in situ hybridization with antisense probes against dU2 (red) and dU7 (green) snRNA. dU2 has a diffuse nuclear distribution with a single prominent focus associated with the nucleolus (unstained area). dU7 is limited to a single discrete focus in each nucleus. dU2 and dU7 foci are often adjacent to each other but can be widely separated. (B) Double in situ hybridization with antisense probes against dU85 scaRNA (red) and dU7 snRNA (green). Each probe is limited to a single discrete focus. The dU85 and dU7 foci, like those of dU2 and dU7, are often close together but may be distantly separated.
Antibody staining.
After we completed our initial study on the distribution of YFP-dLsm11 in various tissues, we obtained polyclonal rabbit sera against D. melanogaster dLsm10 and dLsm11. Both antibodies stained bodies in the nucleus in the same pattern as YFP-dLsm11 expression in all tissues examined (Fig. 4, B and D). This was true of wild-type flies as well as those expressing YFP-dLsm11. It is noteworthy that every nucleus in all larval and adult tissues displays one or more HLBs after antibody staining, including nonexpressing cells in the transgenic flies.
Association of the HLB with the histone gene cluster
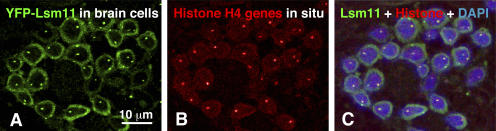
Many interphase nuclei of D. melanogaster maintain the so-called Rabl orientation, in which the centromeres and associated heterochromatin are on one side of the nucleus and the free chromosome ends are on the opposite side. This orientation is particularly evident in blastoderm nuclei after cellularization (Hiraoka et al., 1993; Fung et al., 1998). Early in our observations it became clear that the HLB often lies at the periphery of the nucleus near the heterochromatin, suggesting a specific chromosomal association (Fig. 4, B–D; and Fig. 5 B). Because of the well-known association of CBs with the histone genes in both amphibian (Gall et al., 1981; Callan et al., 1991) and human nuclei (Frey and Matera, 1995), we postulated that the HLB is also associated with these genes, which are located in polytene chromosome bands 39D2-3 to 39E1-2 near the centromeric heterochromatin of chromosome 2L (Pardue et al., 1977). This hypothesis was verified by in situ hybridization with a probe against D. melanogaster histone H4. Hybridization was performed on squashes and whole mounts of various tissues from flies that expressed YFP-dLsm11. In larval brain cells, there was essentially complete colocalization of histone genes with the YFP-dLsm11 signal (Fig. 7). The same was true in the giant salivary gland nuclei and ovarian nurse cells. In all cells of all tissues, the HLB was invariably associated with histone genes. This situation is in striking contrast to that of amphibian oocytes and mammalian tissue culture cells, where a minority of CBs is associated with histone genes.
Figure 7.
YFP-dLsm11 is localized at the histone gene loci in brain cell nuclei. (A) YFP-dLsm11 stained with a rabbit polyclonal antibody against GFP (green). A single nuclear body is evident in each nucleus. (B) In situ hybridization of the same cells with a probe against D. melanogaster histone H4 (red). (C) Merge of YFP-dLsm11 (green) and histone genes (red), also stained with DAPI to show DNA (blue). Precise colocalization of histone genes and YFP-dLsm11 foci in the nucleus is evident. There is a single histone gene cluster on chromosome 2L. Although there are two clusters in a diploid nucleus, there is only a single focus of in situ hybridization, which is presumably due to somatic pairing of homologous chromosomes.
Association of the CB and the HLB
Various tissues were examined that had been probed simultaneously for a CB component and an HLB component. In most cases, the CB and HLB lay close to each other or were in contact, but they never completely overlapped (Fig. 8, A and B). We made quantitative measurements on follicle cell nuclei that had been simultaneously hybridized with probes against dU7 and dU85. Multiple confocal images through individual nuclei were projected onto a single plane for analysis. The dU7 and dU85 bodies were said to be touching if their center-to-center distance was less than their diameter (0.5–1.0 μm for both bodies). They were said to be close if the center-to-center distance was greater than one but less than three diameters, and they were considered far away if the center-to-center distance was greater than three diameters. In a sample of 163 follicle nuclei, the two bodies were touching in 58% of the cases (94/163), close in 28% (45/163), and far away in 15% (24/163). These numbers overestimate the degree of association because they do not take into account the three-dimensional nature of the nucleus. In the larger nurse cell nuclei, there are multiple HLBs but only 1–3 CBs. Nevertheless, in these cells as well, most CBs lie close to or touch an HLB (Fig. 6, D and E).
Discussion
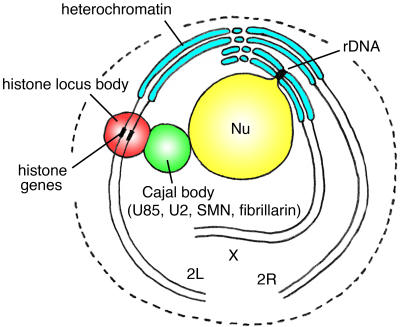
CBs in vertebrate cells are usually identified by immunostaining with antibodies against the marker protein p80-coilin (Andrade et al., 1991; Raska et al., 1991). Because an orthologue of coilin has not yet been recognized in D. melanogaster, we used other markers to search for the D. melanogaster CB. CBs in vertebrate cells contain many components involved in RNA processing. These include snRNAs and related factors that carry out pre-mRNA splicing; snoRNAs, fibrillarin, and other factors involved in preribosomal RNA processing; and the U7 snRNP and the stem-loop binding protein that are required for 3′-end cleavage of histone pre-mRNA. We examined the intranuclear distribution of proteins and RNAs from each of these categories: pre-mRNA processing (dU2, dU85, and dSMN), preribosomal RNA processing (fibrillarin), and histone pre-mRNA 3′-end cleavage (dU7, dLsm10, and dLsm11). We find that components in the first two categories reside in the same body, but components of the U7 snRNP are found in a separate nuclear body. In many nuclei, the two bodies appear to touch at the resolution of the light microscope. Furthermore, the body that contains the U7 snRNP is invariably associated with the histone genes. These relationships are shown diagrammatically in Fig. 9.
Figure 9.
A diagram summarizing the distribution of CB components and other features of a “typical” D. melanogaster nucleus. The HLB, here identified by dLsm10, dLsm11, and dU7 snRNA, is present in all nuclei and is invariably associated with the histone gene locus. The CB is identified by dU85 scaRNA, dU2 snRNA, dSMN, and fibrillarin. It is frequently, although not invariably, associated with the HLB. Both the CB and the HLB lie near the heterochromatin—the HLB because the histone gene cluster is at the base of chromosome 2L near the euchromatin/heterochromatin boundary, and the CB because it is associated with the nucleolus, which itself lies in the middle of the heterochromatin of the X chromosome (and the short arm of the Y). The diagram includes “Rabl orientation” of the chromosomes (centromeres clustered at one point on the nuclear periphery) and somatic pairing of homologues, features common to many D. melanogaster nuclei.
The D. melanogaster CB was identified in three earlier publications. The first was an electron microscope study by Mahowald and Tiefert (1970), who described a nearly spherical structure in the GV of the early oocyte, which they called the endobody. The CB, identified by dSMN-YFP expression (Fig. 2 A, inset), has the same size and relationship to the condensed chromatin as the endobody and occurs in the same early stages of the oocyte. Endobody is the English translation of the German Binnenkörper, a term originally applied to spherical, nonnucleolar structures in various insect oocytes, including flies, by Bier et al. (1967). We previously showed by immunostaining that the endobody of the cricket Acheta domesticus is the oocyte equivalent of the somatic CB (Gall et al., 1995).
The second study, by Yannoni and White (1997), concerned the localization of a neuron-specific protein encoded by the gene embryonic lethal, abnormal vision (elav). The ELAV protein was detected by immunostaining in a small structure inside the nuclei of larval brain cells. This structure was also stained by a polyclonal serum (R288) raised against the carboxy terminus of human coilin (Andrade et al., 1993), from which the authors concluded that they had identified the D. melanogaster CB (then referred to as the coiled body). Further experiments are needed to determine whether the ELAV-positive body corresponds to either of the nuclear bodies we describe here and what epitope is recognized by the antibody against human coilin.
The third report, by Richard et al. (2003), concerned the localization of dU85 scaRNA in cultured D. melanogaster cells (S2 cells). These authors reported a sharply defined focus in the nucleus after in situ hybridization with a probe against dU85. Simultaneous hybridization with a dU2 snRNA probe showed a major concentration of U2 in the U85 focus, with additional diffuse labeling throughout the nucleus. Richard et al. (2003) called this focus the D. melanogaster CB, based on the fact that human U85 is limited to the CB in HeLa nuclei. There is little question that the CB we describe in somatic and germline tissues of the fly corresponds to the CB in S2 cells, as our identification also relies on U85 and U2 probes.
The HLB was first described in a study that dealt with chorion gene amplification (Calvi et al., 1998). “A subnuclear sphere of unknown identity” was seen in follicle cell nuclei after staining with mAb MPM-2, a relatively nonspecific antibody against phosphorylated proteins (Davis et al., 1983). Staining of MPM-2 spheres depended strongly on cyclin E/Cdk2 levels in the cell. Subsequently, cyclin E and Cdk2 were shown to associate with vertebrate CBs in a cell cycle–dependent manner (Liu et al., 2000; Ma et al., 2000), suggesting that D. melanogaster MPM-2 bodies might be related to vertebrate CBs. Double-label experiments now show precise colocalization of MPM-2 and dLsm11 staining in blastoderm cells, thereby demonstrating identity of the MPM-2 body and the HLB (White, A., B. Calvi, W.F. Marzluff, and R.J. Duronio, personal communication).
The existence of two separate nuclear bodies in D. melanogaster that each contain canonical CB components emphasizes the composite nature of the CB. A similar composite nature is well documented in earlier studies on other organisms. One of the best-known examples concerns SMN and its associated gemin proteins, which can reside in separate nuclear bodies, the gems or Gemini of the CB (Liu and Dreyfuss, 1996). In most mammalian cultured cells and adult tissues, SMN colocalizes precisely with coilin in the CB, but in fetal tissues and in the line of HeLa cells (PV) in which gems were first described, gems exist either as completely separate bodies or as close partners with CBs (Matera and Frey, 1998; Young et al., 2000, 2001). The degree of association depends in part on the extent to which coilin is methylated (Hebert et al., 2001, 2002). In D. melanogaster, we find that SMN is colocalized with U2 snRNA and U85 scaRNA in CBs in the female germline and in follicle cell nuclei. A diffuse nuclear distribution of endogenous SMN was previously demonstrated in S2 cells after staining with an antibody against dSMN. Cells transfected with dSMN also gave a diffuse distribution, whereas some cells transfected with human SMN had dotlike structures in their nuclei (Ilangovan et al., 2003). Colocalization of these dots with other CB markers was not tested.
A second partner of CBs is the cleavage body, so called because it contains pre-mRNA cleavage factors, such as the 64-kD cleavage stimulation factor (CstF64) and the 100-kD cleavage and polyadenylation specificity factor (CPSF100). Cleavage bodies reside next to or overlap with CBs. When transcription was inhibited with α-amanitin or 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole, cleavage factors redistributed and colocalized with coilin in the CB (Schul et al., 1996, 1998, 1999). Cleavage factors have not yet been looked for specifically in D. melanogaster.
Coilin knockout mice provide an especially informative case of CB heterogeneity. In the original description of fibroblasts derived from coilin −/− embryos (Tucker et al., 2001), the authors described “residual CBs,” which contained fibrillarin and Nopp 140 but no splicing snRNPs. Some of these cells also displayed separate bodies that contained SMN. In a subsequent study of coilin −/− fibroblasts (Jády et al., 2003), a third nuclear body was found, which contained scaRNAs U85 and U93, along with their cognate substrates snRNAs U5 and U2. In mouse cells, coilin clearly plays an important role in bringing CB components together into a single nuclear body. The same conclusion was reached in a study of CBs in X. laevis pronuclei (Bauer and Gall, 1997). CBs in pronuclei contain coilin, fibrillarin, and splicing snRNAs, but when coilin was depleted from the extract in which pronuclei were formed, snRNAs no longer accumulated in the residual CBs.
The existence of the HLB provides a fortuitous opportunity to study the histone mRNA processing machinery separate from other CB components. For instance, the HLB should make it easier to examine molecules that are shared with other complexes, such as the Sm proteins that also occur in the splicing machinery and the recently discovered shared components of the cleavage and polyadenylation machinery (Dominski et al., 2005; Kolev and Steitz, 2005). A separate HLB also permits insight into the relationship of the body to the histone genes themselves. In amphibian oocytes (Gall et al., 1981; Callan et al., 1991) and HeLa cells (Frey and Matera, 1995; Shopland et al., 2001), a minor fraction of CBs is associated with the histone genes, whereas in D. melanogaster, HLBs are invariably present at the histone locus. One obvious result of this association is that the processing machinery is brought into proximity to the site of transcription of histone pre-mRNA. However, histone transcripts are synthesized and processed only during the relatively short S phase. Why, then, are HLBs present throughout the interphase period? Part of the answer may be that the processing machinery is simply stored where it will be used. Of greater interest, however, is the possibility that steps in the assembly or modification of the histone processing machinery occur in the HLB and that these events take place throughout the interphase. It is known that assembly and modification of the splicing machinery occur in CBs (Jády et al., 2003; Schaffert et al., 2004; Stanek and Neugebauer, 2004). It may well be that the HLB plays multiple roles in assembly, modification, storage, and delivery of the histone mRNA processing machinery.
Efforts to find a D. melanogaster orthologue of coilin have so far proved unsuccessful. A coilin gene could reside in an unsequenced heterochromatic region, or its sequence could be so divergent that it is not recognizable by the usual similarity comparisons. However, if coilin is indeed absent, D. melanogaster cells may lack an important component of the “glue” that holds CBs together in vertebrate cells. It will be particularly instructive to examine transgenic D. melanogaster lines that express a vertebrate coilin gene. Such lines could provide important clues concerning the molecular interactions of coilin as well as its role in the physical organization of CBs.
Materials and methods
Transgenic flies
Based on sequences published by Azzouz and Schümperli (2003), we constructed plasmids that contained dLsm10 and dLsm11 genes tagged with the HA epitope or with a modified YFP called Venus (Nagai et al., 2002). Transcripts from these constructs were injected into the cytoplasm of X. laevis oocytes, and the newly translated proteins were identified in the oocyte nucleus by Western blotting and immunostaining. YFP-labeled dLsm11 was properly translated and efficiently targeted to CBs in the oocyte nucleus, whether the YFP tag was at the amino or carboxy terminus of the protein. YFP-labeled dLsm10 was properly translated but not well targeted to CBs in these preliminary experiments. Therefore, we concentrated on YFP-labeled dLsm11 in subsequent studies on D. melanogaster. We made two P element constructs of dLsm11, with YFP at either the amino or carboxy terminus of the protein. The P element was pUASp, in which the cloned protein is under control of the yeast upstream activating sequence (UAS; Rorth, 1998). pUASp is a modified version of pUASt (Brand and Perrimon, 1993) and was used because it shows enhanced expression in the ovary. P element transformation was performed by standard procedures. Four different homozygous viable lines were obtained, two with YFP at the amino terminus and two at the carboxy terminus. The precise positions of the inserts were determined by sequencing (Singer and Burke, 2003) and in situ hybridization as follows: for VW-1 and VW-5, single P elements on chromosome 2 at 5,981,114 and 7,576,630, respectively; for WV-1, two P elements on the X chromosome at 11,567,054 and 19,472,038; and for WV-2, a single P element on the X chromosome at 11,567,054. We also constructed a pUASp plasmid that contained the D. melanogaster smn gene upstream of the YFP tag. Two different homozygous viable lines were isolated, one with smn-YFP on chromosome 2 and one on chromosome 3.
Tissue preparation
Brains and salivary glands from third instar larvae and ovaries from adult flies were examined as whole mounts or as squashes. Fresh tissues were isolated in OR2 medium (Wallace et al., 1973) or Grace's insect medium (Grace, 1962). Whole mount samples were fixed in 2% paraformaldehyde and 0.1% Triton X-100 in OR2 or Grace's medium for 5–30 min, rinsed in PBS (135 mM NaCl, 2.5 mM KCl, 4.3 mM Na2HPO4, and 1.5 mM KH2PO4, pH 7.2) and stained. Squashes were prepared essentially as described previously (Hulsebos et al., 1984; Gall, 1998). Small pieces of fresh tissue were transferred to an 8-μl drop of medium in the middle of an 18-mm2 coverslip (in some cases siliconized). The coverslip was inverted over a 3- × 1-inch glass slide, and gentle pressure was applied. The slide was submerged in liquid nitrogen until bubbling ceased, the coverslip was flipped off with a razor blade, and the still-frozen preparation was placed immediately in the fixative. Tissues were fixed 5–30 min in 95% ethanol, 2% paraformaldehyde in 86% ethanol, or 2% paraformaldehyde in PBS. Slides were then washed in PBS and stained. Eggs and embryos were fixed after removal of the chorion and vitelline membrane as previously described (Patel, 1994) with the following modifications. Washes were done with PBS + 0.1% Tween 20, the chorion was removed in 50% commercial bleach for 5 min, and fixation was performed with 4% paraformaldehyde in OR2 buffer.
Antibodies
Rabbits were injected with GST-tagged fragments of dLsm10 and dLsm11 that had been expressed in Escherichia coli. The fragments consisted of amino acids 61–142 for Lsm10 and 1–123 for Lsm11. Crude sera from the second or third bleed were diluted 1:1,000 for immunostaining. Other primary antibodies were as follows: mAb Y12 against the Sm epitope (Lerner et al., 1981; provided by J. Steitz, Yale University, New Haven, CT), mAb 72B9 against mouse fibrillarin (Reimer et al., 1987; provided by K.M. Pollard, the Scripps Research Institute, La Jolla, CA), affinity-purified rabbit polyclonal against dSMN (Ilangovan et al., 2003; provided by J. Zhou, University of Massachusetts Medical School, Worcester, MA), and rabbit polyclonal anti-GFP (Torrey Pines BioLabs). Secondary antibodies were goat anti–mouse IgG or goat anti–rabbit IgG labeled with Alexa 488, 546, or 594 (Invitrogen).
Immunostaining
Tissue squashes were blocked with 10% horse serum for 10 min, stained with a primary antibody for 1–2 h, rinsed in PBS, and stained with a secondary antibody for 1–2 h. Whole ovaries or other tissues were blocked for 10 min or longer, stained with primary antibody overnight, washed extensively with PBS, and stained with secondary antibody for 2 h or longer. To facilitate penetration of reagents into whole tissues, 0.1% Triton X-100 was included in all solutions. Most specimens were also stained for a few minutes with 0.1–0.5 μg/ml of the DNA-specific dye DAPI for easy recognition of nuclei.
In situ hybridization
Tissues for in situ hybridization were prepared essentially as described for immunostaining, with the following modifications. After a tissue sample was squashed and frozen and the coverslip was removed, it was fixed in 4% paraformaldehyde in PBS for 15 min. The slide was washed in PBS, transferred to 95% ethanol and then acetone, and dried in air. About 7 μl of probe was applied to the specimen, an 18-mm2 coverslip was added, and the edges were sealed with rubber cement. The preparation was incubated at 42–52°C for several hours or overnight, depending on the probe. The coverslip was removed under 2× SSC (1× SSC is 150 mM NaCl and 15 mM Na citrate, pH 7.0), and the specimen was stained with DAPI before being mounted in 50% glycerol + 1 mg/ml p-phenylenediamine. For greater permanence, some preparations were sealed with nail polish. Tissues for whole mounts were fixed in 4% paraformaldehyde in OR2 or Grace's medium for 15 min. They were then washed in excess medium and placed directly in hybridization mix for several hours or overnight at 42–52°C. Subsequent washing, staining with DAPI, and mounting were performed essentially as for squashes. Hybridization probes were diluted in the following hybridization mix: 50% formamide, 5× SSC, 10 mM citric acid, 50 μg/ml heparin, 500 μg/ml yeast tRNA, and 0.1% Tween 20. Sense and antisense RNA probes were made by in vitro transcription from DNA clones or PCR products as described previously (Gall et al., 1999).
Clones
Part of the D. melanogaster U2 gene was cloned in pUC19 as an EcoRI–XbaI fragment that consisted of the T3 phage promoter, the first 53 bases of U2, and the T7 phage promoter. The fragment was generated by PCR from D. melanogaster genomic DNA, based on the published sequence of the dU2 gene (Alonso et al., 1983). The dU85 clone was constructed in the same way from genomic DNA, based on the published sequence of dU85 scaRNA (Jády and Kiss, 2001). The clone contains the entire dU85 sequence. The D. melanogaster histone H4 clone consisted of the entire 312-nt coding region, generated by PCR from D. melanogaster clone GH10208 (Rubin et al., 2000). The D. melanogaster U7 clone was prepared by H. Gao (Carnegie Institute of Washington, Baltimore, MD), based on the published sequence of dU7 (Dominski et al., 2003). It was constructed by annealing two partially overlapping deoxyoligonucleotides, one of which included a PvuII site, the T7 promoter, and part of the dU7 sequence, whereas the other contained an HpaI site, the T3 promoter, and the rest of the dU7 sequence. The single-stranded ends were filled in by Klenow enzyme, and the product was cloned into pUC19 at the restriction sites. The inserts of all clones were fully sequenced.
Microscopy
Confocal images were taken with a 40× (NA 1.25) or a 63× (NA 1.40) Plan Apo objective on laser-scanning confocal microscopes (NT or SP2; Leica). Images were taken with the laser intensity and photomultiplier gain was adjusted so that pixels in the region of interest were not saturated (“glow-over” display). In most cases, contrast and relative intensities of the green (Alexa 488), red (Alexa 546 or 594), and blue (DAPI) images were adjusted with Photoshop (Adobe).
Acknowledgments
We thank the following individuals for antibodies: Joan Steitz (mAb Y12), Michael Pollard (mAb 72B9), and Jianhua Zhou (anti-dSMN). We thank Hongjuan Gao for the dU7 construct.
This work was supported in part by Research Grant GM 33397 from the National Institute of General Medical Sciences of the National Institutes of Health (to J.G. Gall) and American Cancer Society Grant PF-04-022-01-CSM (to M. Buszczak). J.G. Gall is an American Cancer Society Professor of Developmental Genetics.
Abbreviations used in this paper: CB, Cajal body; dSMN, Drosophila melanogaster SMN protein; GV, germinal vesicle; HLB, histone locus body; scaRNA, small CB-specific RNA; SMN, survival of motor neurons; snRNA, small nuclear RNA; snoRNA, small nucleolar RNA; UAS, upstream activating sequence.
References
- Alonso, A., J. Jorcano, E. Beck, and E. Spiess. 1983. Isolation and characterization of Drosophila melanogaster U2 small nuclear RNA genes. J. Mol. Biol. 169:691–705. [DOI] [PubMed] [Google Scholar]
- Andrade, L.E.C., E.K.L. Chan, I. Raska, C.L. Peebles, G. Roos, and E.M. Tan. 1991. Human autoantibody to a novel protein of the nuclear coiled body: immunological characterization and cDNA cloning of p80-coilin. J. Exp. Med. 173:1407–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade, L.E.C., E.M. Tan, and E.K.L. Chan. 1993. Immunocytochemical analysis of the coiled body in the cell cycle and during cell proliferation. Proc. Natl. Acad. Sci. USA. 90:1947–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzouz, T.N., and D. Schümperli. 2003. Evolutionary conservation of the U7 small nuclear ribonucleoprotein in Drosophila melanogaster. RNA. 9:1532–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer, D.W., and J.G. Gall. 1997. Coiled bodies without coilin. Mol. Biol. Cell. 8:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bier, K., W. Kunz, and D. Ribbert. 1967. Struktur und Funktion der Oocytenchromosomen und Nukleolen sowie der Extra-DNS während der Oogenese panoistischer und meroistischer Insekten. Chromosoma. 23:214–254. [DOI] [PubMed] [Google Scholar]
- Brand, A.H., and N. Perrimon. 1993. Targeted gene expression as a means of altering fates and generating dominant phenotypes. Development. 118:401–415. [DOI] [PubMed] [Google Scholar]
- Cajal, S.R.y. 1903. Un sencillo metodo de coloracion seletiva del reticulo protoplasmatico y sus efectos en los diversos organos nerviosos de vertebrados e invertebrados. Trabajos del Laboratorio de Investigaciones Biologicas de la Universidad de Madrid. 2:129–221. [Google Scholar]
- Callan, H.G., J.G. Gall, and C. Murphy. 1991. Histone genes are located at the sphere loci of Xenopus lampbrush chromosomes. Chromosoma. 101:245–251. [DOI] [PubMed] [Google Scholar]
- Calvi, B.R., M.A. Lilly, and A.C. Spradling. 1998. Cell cycle control of chorion gene amplification. Genes Dev. 12:734–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Fonseca, M. 2002. New clues to the function of the Cajal body. EMBO Rep. 3:726–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Fonseca, M., D. Tollervey, S.M.L. Barabino, A. Merdes, C. Brunner, P.D. Zamore, M.R. Green, E. Hurt, and A.I. Lamond. 1991. Mammalian nuclei contain foci which are highly enriched in components of the pre-mRNA splicing machinery. EMBO J. 10:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, Y.B., I. Miguel-Aliaga, C. Franks, N. Thomas, B. Trulzsch, D.B. Sattelle, K.E. Davies, and M. van den Heuvel. 2003. Neuromuscular defects in a Drosophila survival motor neuron gene mutant. Hum. Mol. Genet. 12:1367–1376. [DOI] [PubMed] [Google Scholar]
- Cioce, M., and A. Lamond. 2005. Cajal bodies: a long history of discovery. Annu. Rev. Cell Dev. Biol. 21:105–131. [DOI] [PubMed] [Google Scholar]
- Darzacq, X., B.E. Jády, C. Verheggen, A.M. Kiss, E. Bertrand, and T. Kiss. 2002. Cajal body-specific small nuclear RNAs: a novel class of 2′-_O_-methylation and pseudouridylation guide RNAs. EMBO J. 21:2746–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, F., T.Y. Tsao, S.K. Fowler, and P.N. Rao. 1983. Monoclonal antibodies to mitotic cells. Proc. Natl. Acad. Sci. USA. 80:2926–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominski, Z., X.-C. Yang, M. Purdy, and W.F. Marzluff. 2003. Cloning and characterization of the Drosophila U7 small nuclear RNA. Proc. Natl. Acad. Sci. USA. 100:9422–9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominski, Z., X.-C. Yang, and W.F. Marzluff. 2005. The polyadenylation factor CPSF-73 is involved in histone-pre-mRNA processing. Cell. 123:37–48. [DOI] [PubMed] [Google Scholar]
- Frey, M.R., and A.G. Matera. 1995. Coiled bodies contain U7 small nuclear RNA and associate with specific DNA sequences in interphase human cells. Proc. Natl. Acad. Sci. USA. 92:5915–5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung, J.C., W.F. Marshall, A.F. Dernburg, D.A. Agard, and J.W. Sedat. 1998. Homologous chromosome pairing in Drosophila melanogaster proceeds through multiple independent initiations. J. Cell Biol. 141:5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galardi, S., A. Fatica, A. Bachi, A. Scaloni, C. Presutti, and I. Bozzoni. 2002. Purified box C/D snoRNPs are able to reproduce site-specific 2′-_O_- methylation of target RNA in vitro. Mol. Cell. Biol. 22:6663–6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall, J.G. 1998. In situ hybridization of DNA and nuclear RNA in tissue squashes using 3H-labeled probes. In Cells: A Laboratory Manual, vol. 3. D. Spector, R. Goldman, and L. Leinwand, editors. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 114.1–114.11.
- Gall, J.G. 2000. Cajal bodies: the first 100 years. Annu. Rev. Cell Dev. Biol. 16:273–300. [DOI] [PubMed] [Google Scholar]
- Gall, J.G. 2003. The centennial of the Cajal body. Nat. Rev. Mol. Cell Biol. 4:975–980. [DOI] [PubMed] [Google Scholar]
- Gall, J.G., E.C. Stephenson, H.P. Erba, M.O. Diaz, and G. Barsacchi-Pilone. 1981. Histone genes are located at the sphere loci of newt lampbrush chromosomes. Chromosoma. 84:159–171. [DOI] [PubMed] [Google Scholar]
- Gall, J.G., A. Tsvetkov, Z. Wu, and C. Murphy. 1995. Is the sphere organelle/coiled body a universal nuclear component? Dev. Genet. 16:25–35. [DOI] [PubMed] [Google Scholar]
- Gall, J.G., M. Bellini, Z. Wu, and C. Murphy. 1999. Assembly of the nuclear transcription and processing machinery: Cajal bodies (coiled bodies) and transcriptosomes. Mol. Biol. Cell. 10:4385–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace, T.D. 1962. Establishment of four strains of cells from insect tissues grown in vitro. Nature. 195:788–789. [DOI] [PubMed] [Google Scholar]
- Hebert, M.D., P.W. Szymczyk, K.B. Shpargel, and A.G. Matera. 2001. Coilin forms the bridge between Cajal bodies and SMN, the spinal muscular atrophy protein. Genes Dev. 15:2720–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert, M.D., K.B. Shpargel, J.K. Ospina, K.E. Tucker, and A.G. Matera. 2002. Coilin methylation regulates nuclear body formation. Dev. Cell. 3:329–337. [DOI] [PubMed] [Google Scholar]
- Hiraoka, Y., A.F. Dernburg, S.J. Parmalee, M.C. Rykowski, D.A. Agard, and J.W. Sedat. 1993. The onset of homologous chromosome pairing during Drosophila melanogaster embryogenesis. J. Cell Biol. 120:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, S., and D.L. Spector. 1992. U1 and U2 small nuclear RNAs are present in nuclear speckles. Proc. Natl. Acad. Sci. USA. 89:305–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsebos, T.J.M., J.H.P. Hackstein, and W. Hennig. 1984. Lampbrush loop-specific protein of Drosophila hydei. Proc. Natl. Acad. Sci. USA. 81:3404–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilangovan, R., W.L. Marshall, Y. Hua, and J. Zhou. 2003. Inhibition of apoptosis by Z-VAD-fmk in SMN-depleted S2 cells. J. Biol. Chem. 278:30993–30999. [DOI] [PubMed] [Google Scholar]
- Jády, B.E., and T. Kiss. 2001. A small nucleolar guide RNA functions both in 2′-_O_-ribose methylation and pseudouridylation of the U5 spliceosomal RNA. EMBO J. 20:541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jády, B.E., X. Darzacq, K.E. Tucker, A.G. Matera, E. Bertrand, and T. Kiss. 2003. Modification of Sm small nuclear RNAs occurs in the nucleoplasmic Cajal body following import from the cytoplasm. EMBO J. 22:1878–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambach, C., S. Walke, and K. Nagai. 1999. Structure and assembly of the spliceosomal small nuclear ribonucleoprotein particles. Curr. Opin. Struct. Biol. 9:222–230. [DOI] [PubMed] [Google Scholar]
- Kolev, N.G., and J.A. Steitz. 2005. Symplekin and multiple other polyadenylation factors participate in 3′-end maturation of histone mRNAs. Genes Dev. 19:2583–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner, E.A., M.R. Lerner, C.A. Janeway, and J.A. Steitz. 1981. Monoclonal antibodies to nucleic acid-containing cellular constituents: Probes for molecular biology and autoimmune disease. Proc. Natl. Acad. Sci. USA. 78:2737–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J.-L., M.D. Hebert, Y. Ye, D.J. Templeton, H.-J. Kung, and A.G. Matera. 2000. Cell cycle-dependent localization of the CDK2-cyclin E complex in Cajal (coiled) bodies. J. Cell Sci. 113:1543–1552. [DOI] [PubMed] [Google Scholar]
- Liu, Q., and G. Dreyfuss. 1996. A novel nuclear structure containing the survival of motor neurons protein. EMBO J. 15:3555–3565. [PMC free article] [PubMed] [Google Scholar]
- Ma, T., B.A. Van Tine, Y. Wei, M.D. Garrett, D. Nelson, P.D. Adams, J. Wang, J. Qin, L.T. Chow, and J.W. Harper. 2000. Cell cycle-regulated phosphorylation of p220NPAT by cyclin E/Cdk2 in Cajal bodies promotes histone gene transcription. Genes Dev. 14:2298–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahowald, A.P., and M. Tiefert. 1970. Fine structural changes in the Drosophila oocyte nucleus during a short period of RNA synthesis. Wilhelm Roux' Archiv für Entwicklungsmechanik Der Organismen. 165:8–25. [DOI] [PubMed] [Google Scholar]
- Matera, A.G. 2003. Cajal bodies. Curr. Biol. 13:R503. [DOI] [PubMed] [Google Scholar]
- Matera, A.G., and D.C. Ward. 1993. Nucleoplasmic organization of small nuclear ribonucleoproteins in cultured human cells. J. Cell Biol. 121:715–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matera, A.G., and M.R. Frey. 1998. Coiled bodies and gems: Janus or Gemini? Am. J. Hum. Genet. 63:317–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister, G., and U. Fischer. 2002. Assisted RNP assembly: SMN and PRMT5 complexes cooperate in the formation of spliceosomal UsnRNPs. EMBO J. 21:5853–5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai, T., K. Ibata, E.S. Park, M. Kubota, K. Mikoshiba, and A. Miyawaki. 2002. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 20:87–90. [DOI] [PubMed] [Google Scholar]
- Ochs, R.L., M.A. Lischwe, W.H. Spohn, and H. Busch. 1985. Fibrillarin: a new protein of the nucleolus identified by autoimmune sera. Biol. Cell. 54:123–134. [DOI] [PubMed] [Google Scholar]
- Pardue, M.L., L.H. Kedes, E.S. Weinberg, and M.L. Birnstiel. 1977. Localization of sequences coding for histone messenger RNA in the chromosomes of Drosophila melanogaster. Chromosoma. 63:135–151. [DOI] [PubMed] [Google Scholar]
- Patel, N. 1994. Imaging neuronal subsets and other cell types in whole-mount Drosophila embryos and larvae using antibody probes. In Drosophila melanogaster: Practical Uses in Cell and Molecular Biology. L.S.B. Goldstein and E.A. Fyrberg, editors. Academic Press, San Diego, CA. 445–487. [DOI] [PubMed]
- Pellizzoni, L., J. Yong, and G. Dreyfuss. 2002. Essential role for the SMN complex in the specificity of snRNP assembly. Science. 298:1775–1779. [DOI] [PubMed] [Google Scholar]
- Pillai, R.S., C.L. Will, R. Lührmann, D. Schümperli, and B. Muller. 2001. Purified U7 snRNPs lack the Sm proteins D1 and D2 but contain Lsm10, a new 14 kDa Sm D1-like protein. EMBO J. 20:5470–5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai, R.S., M. Grimmler, G. Meister, C.L. Will, R. Lührmann, U. Fischer, and D. Schümperli. 2003. Unique Sm core structure of U7 snRNPs: assembly by a specialized SMN complex and the role of a new component, Lsm11, in histone RNA processing. Genes Dev. 17:2321–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raska, I., L.E.C. Andrade, R.L. Ochs, E.K.L. Chan, C.-M. Chang, G. Roos, and E.M. Tan. 1991. Immunological and ultrastructural studies of the nuclear coiled body with autoimmune antibodies. Exp. Cell Res. 195:27–37. [DOI] [PubMed] [Google Scholar]
- Reimer, G., K.M. Pollard, C.A. Penning, R.L. Ochs, M.A. Lischwe, H. Busch, and E.M. Tan. 1987. Monoclonal autoantibody from a (New Zealand black x New Zealand white) F1 mouse and some human scleroderma sera target an Mr 34,000 nucleolar protein of the U3 RNP particle. Arthritis Rheum. 30:793–800. [DOI] [PubMed] [Google Scholar]
- Richard, P., X. Darzacq, E. Bertrand, B.E. Jády, C. Verheggen, and T. Kiss. 2003. A common sequence motif determines the Cajal body-specific localisation of box H/ACA scaRNAs. EMBO J. 22:4283–4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorth, P. 1998. Gal4 in the Drosophila female germline. Mech. Dev. 78:113–118. [DOI] [PubMed] [Google Scholar]
- Rubin, G.M., L. Hong, P. Brokstein, M. Evans-Holm, E. Frise, M. Stapleton, and D.A. Harvey. 2000. A Drosophila complementary DNA resource. Science. 287:2222–2224. [DOI] [PubMed] [Google Scholar]
- Schaffert, N., M. Hossbach, R. Heintzmann, T. Achsel, and R. Lührmann. 2004. RNAi knockdown of hPrp31 leads to an accumulation of U4/U6 di-snRNPs in Cajal bodies. EMBO J. 23:3000–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schul, W., B. Groenhaut, K. Koberna, Y. Takagaki, A. Jenny, E.M.M. Manders, I. Raska, R. van Driel, and L. de Jong. 1996. The RNA 3′ cleavage factors CstF 64 kDa and CPSF 100 kDa are concentrated in nuclear domains closely associated with coiled bodies and newly synthesized RNA. EMBO J. 15:2883–2892. [PMC free article] [PubMed] [Google Scholar]
- Schul, W., L. de Jong, and R. van Driel. 1998. Nuclear neighbours: the spatial and functional organization of genes and nuclear domains. J. Cell. Biochem. 70:159–171. [DOI] [PubMed] [Google Scholar]
- Schul, W., I. van der Kraan, A.G. Matera, R. van Driel, and L. de Jong. 1999. Nuclear domains enriched in RNA 3′ processing factors associate with coiled bodies and histone genes in a cell cycle-dependent fashion. Mol. Biol. Cell. 10:3815–3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shopland, L.S., M. Byron, J.L. Stein, J.B. Lian, G.S. Stein, and J.B. Lawrence. 2001. Replication-dependent histone gene expression is related to Cajal body (CB) association but does not require sustained CB contact. Mol. Biol. Cell. 12:565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer, T., and E. Burke. 2003. High-throughput TAIL-PCR as a tool to identify DNA flanking insertions. Methods Mol. Biol. 236:241–271. [DOI] [PubMed] [Google Scholar]
- Stanek, D., and K.M. Neugebauer. 2004. Detection of snRNP assembly intermediates in Cajal bodies by fluorescence resonance energy transfer. J. Cell Biol. 166:1015–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker, K.E., M.T. Berciano, E.Y. Jacobs, D.F. LePage, K.B. Shpargel, J.J. Rossire, E.K.L. Chan, M. Lafarga, R.A. Conlon, and A.G. Matera. 2001. Residual Cajal bodies in coilin knockout mice fail to recruit Sm snRNPs and SMN, the spinal muscular atrophy gene product. J. Cell Biol. 154:293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker, K.E., L.K. Massello, L. Gao, T.J. Barber, M.D. Hebert, E.K.L. Chan, and A.G. Matera. 2000. Structure and characterization of the murine p80 coilin gene, Coil. J. Struct. Biol. 129:269–277. [DOI] [PubMed] [Google Scholar]
- Tuma, R.S., J.A. Stolk, and M.B. Roth. 1993. Identification and characterization of a sphere organelle protein. J. Cell Biol. 122:767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace, R.A., D.W. Jared, J.N. Dumont, and M.W. Sega. 1973. Protein incorporation by isolated amphibian oocytes: III. Optimum incubation conditions. J. Exp. Zool. 184:321–333. [DOI] [PubMed] [Google Scholar]
- Wu, C.-H.H., and J.G. Gall. 1993. U7 small nuclear RNA in C snurposomes of the Xenopus germinal vesicle. Proc. Natl. Acad. Sci. USA. 90:6257–6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannoni, Y.M., and K. White. 1997. Association of the neuron-specific RNA binding domain-containing protein ELAV with the coiled body in Drosophila neurons. Chromosoma. 105:332–341. [DOI] [PubMed] [Google Scholar]
- Young, P.J., T.T. Le, N.T. Man, A.H.M. Burghes, and G.E. Morris. 2000. The relationship between SMN, the spinal muscular atrophy protein, and nuclear coiled bodies in differentiated tissues and cultured cells. Exp. Cell Res. 256:365–374. [DOI] [PubMed] [Google Scholar]
- Young, P.J., T.T. Le, M. Dunckley, N.T. Man, A.H.M. Burghes, and G.E. Morris. 2001. Nuclear gems and Cajal (coiled) bodies in fetal tissues: nucleolar distribution of the spinal muscular atrophy protein, SMN. Exp. Cell Res. 265:252–261. [DOI] [PubMed] [Google Scholar]