Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1(−/−) mice (original) (raw)
Abstract
Agonist-induced Ca2+ entry via store-operated Ca2+ (SOC) channels is suggested to regulate a wide variety of cellular functions, including salivary gland fluid secretion. However, the molecular components of these channels and their physiological function(s) are largely unknown. Here we report that attenuation of SOC current underlies salivary gland dysfunction in mice lacking transient receptor potential 1 (TRPC1). Neurotransmitter-regulated salivary gland fluid secretion in TRPC1-deficient TRPC1(−/−) mice was severely decreased (by 70%). Further, agonist- and thapsigargin-stimulated SOC channel activity was significantly reduced in salivary gland acinar cells isolated from TRPC1(−/−) mice. Deletion of TRPC1 also eliminated sustained Ca2+-dependent potassium channel activity, which depends on Ca2+ entry and is required for fluid secretion. Expression of key proteins involved in fluid secretion and Ca2+ signaling, including STIM1 and other TRPC channels, was not altered. Together, these data demonstrate that reduced SOC entry accounts for the severe loss of salivary gland fluid secretion in TRPC1(−/−) mice. Thus, TRPC1 is a critical component of the SOC channel in salivary gland acinar cells and is essential for neurotransmitter-regulation of fluid secretion.
Keywords: transient receptor potential, canonical, Ca2+ entry, acinar cells, muscarinic receptor
Store-operated calcium (SOC) entry (SOCE) was first described almost two decades ago as a Ca2+ entry mechanism in the plasma membrane that is activated by the depletion of Ca2+ in the internal Ca2+ store (1) and has been suggested to regulate of a wide variety of cellular functions (1, 2). Typically, SOCE is initiated in response to stimulation of membrane receptors that leads to the hydrolysis of PIP2, IP3 generation, and IP3-mediated Ca2+ release from the endoplasmic reticulum (ER). Two major problems in understanding the molecular basis of SOCE have been: (i) identifying the signal that relays the status of ER-[Ca2+] to the plasma membrane to regulate channel activity and (ii) identifying the components of the SOC channel that mediates SOCE. This has been further confounded by the observations that distinct SOC channels are present in different cell types (2–8). The physiological basis for these diverse channels is not yet known and their molecular components have not been established.
Members of the transient receptor potential canonical (TRPC) family of ion channel proteins are activated by receptor-stimulated PIP2 hydrolysis and have been proposed as components of the SOC channel, although only some TRPC channels appear to be activated by intracellular Ca2+ store depletion (2, 9–16). The characteristics of SOC channels generated by TRPCs are distinct from those of calcium release-activated calcium (CRAC) channels, which mediate SOCE in T lymphocytes, mast cells, and other hematopoietic cells (6–8, 11, 12, 17–21). A large number of studies demonstrate a role for TRPC1 in SOCE. Although heterologous expression of TRPC1 has not always resulted in increased SOCE, knockdown of endogenous TRPC1 has more consistently decreased SOCE and related cell function in several different cell types (2, 3, 9, 11, 13, 14, 16). Furthermore, directed mutagenesis of negatively charged amino acid residues in the putative pore region of TRPC1 results in a decrease in the Ca2+ permeability of SOC channel, with a corresponding decrease in SOCE (12). The majority of previous studies, with one exception (16), have used cell lines or primary cell cultures, and thus the physiological function of TRPC1 in its native environment has not yet been assessed.
Two proteins have been recently identified as molecular components of CRAC channels. STIM1, a Ca2+-binding ER protein, is suggested to be the ER-Ca2+ sensor protein regulating SOCE in a number of different cell types (6–8, 18, 22, 23). STIM1 also interacts with TRPC-SOC channels and determines their function (3, 8, 19, 24, 25). The second protein, Orai1, is an essential component of CRAC channel in T lymphocytes and mutation in Orai1 result in defects in T lymphocyte function (26, 27). Overexpression of Orai1 and STIM1 together in HEK-293 cells generates ICRAC, whereas mutations in specific amino acid residues of Orai1 alter the Ca2+ permeability of the CRAC channel (28–32). Thus, it is proposed that STIM1 and Orai1 together are sufficient for generating the CRAC channel. However, the exact molecular interaction(s) involved in the regulation of Orai1 by STIM1 is not yet clear. It is interesting that the R91W mutation in Orai1 completely blocks CRAC in T cells but only partially in EBV-transformed B cells, and a patient carrying this mutation did not show widespread deficits in extraimmune organs that are functionally dependent on Ca2+ entry and known to express SOCs channel (26). Thus, presently the function of Orai1 in extraimmune tissues is not known. The presence of non-SOCE mechanisms or tissue-specific SOC subtypes could account for these observations. Further, although Orai2 and Orai3 proteins have been demonstrated to form channels distinct from the Orai1 + STIM1 CRAC channels, their physiological function remains to be established (28, 33).
In this study, we have assessed the role of TRPC1 in salivary gland function by using targeted disruption of TRPC1 expression in mice. Neurotransmitter-stimulated fluid secretion from salivary gland was shown almost three decades ago to critically depend on Ca2+ entry (34, 35). Although this Ca2+ entry is suggested to be mediated by a SOCE mechanism, this has not yet been established. The molecular components of this Ca2+ entry pathway have also not been identified. We report here that TRPC1-deficient, TRPC1(−/−) mice display severe reduction in neurotransmitter-stimulated saliva secretion, and submandibular gland (SMG) acinar cells isolated from these mice demonstrate substantial attenuation of SOCE and SOC channel function. Together, the data reveal that TRPC1 is a key component of SOC channels in salivary gland cells, and TRPC1-mediated SOCE is critical for salivary fluid secretion. Thus, this study provides definitive genetic evidence for the role of TRPC1 in SOCE, its contribution to SOC channel, and its physiological function in salivary gland cells.
Results
Neurotransmitter-Stimulated Salivary Gland Fluid Secretion in TRPC1(−/−) mice.
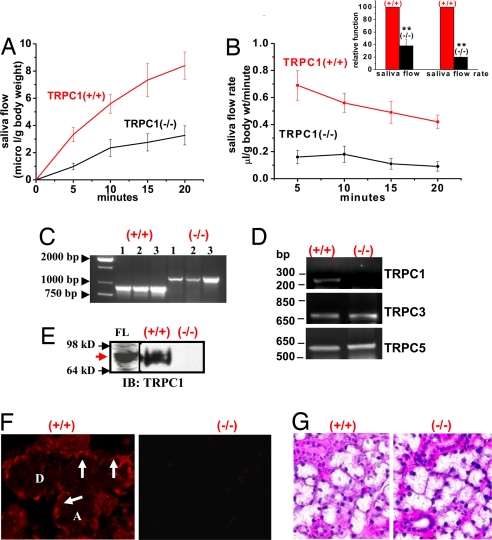
Saliva secretion, measured after parasympathetic stimulation of the mice with pilocarpine, was severely reduced in TRPC1(−/−) mice compared with that in TRPC1(+/+) animals (Fig. 1A, P < 0.02, _n_ = >20 in each set, average data shown in Inset). The rate of saliva flow (Fig. 1B; also see Inset) was similarly reduced by ≈60–70% in TRPC1(−/−) mice. Control mice displayed typical high rates of secretion immediately after stimulation that decreased with time, whereas the rate in (−/−) mice was consistently low. Ion composition and osmolarity of the saliva were altered in mice lacking TRPC1 [supporting information (SI) Fig. 6]. Loss of secretion was not due to changes in salivary gland weight. Weights of SMG, a major salivary gland contributing to saliva secretion, from (+/+) and (−/−) mice, were similar (0.20 ± 0.1 and 0.19 ± 0.1 g; n = 12 and 14, respectively). Further, 12- to 16-week-old mice, used for all experiments, appeared to be healthy and maintained normal weight and growth [body weight 25.5 ± 0.8 g (+/+), n = 18 vs. 31 ± 1.2 g (−/−), n = 19)].
Fig. 1.
Salivary gland function in TRPC1(−/−) mice. (A) Pilocarpine-stimulated saliva flow in TRPC1(+/+) and TRPC1(−/−) mice. (B) Rate of saliva flow at the various time points in TRPC1(+/+) and (−/−) mice (average data shown in Inset; ** indicates significant difference, P < 0.01). (C) PCR amplification of mouse genomic DNA; 893-bp PCR fragment (lane 2–4) was detected in (+/+) samples, whereas 1,193 bp (lane 5–7) was detected in (−/−) samples. (D) RT-PCR of TRPC1, TRPC3, and TRPC5 from SMG of TRPC1(+/+) and (−/−) mice. (E) Detection of TRPC1 (red arrow) in crude-membrane preparations from FLAG-TRPC1-expressing HSG cells (FL), TRPC1(+/+), and TRPC1(−/−) SMG (IB with anti-TRPC1 antibody; FLAG-TRPC1 was also detected by using anti-FLAG antibody; not shown). (F) Immunofluorescence of TRPC1 in SMG sections from TRPC1(+/+) and (−/−) mice (details are provided in Materials and Methods). TRPC1 was localized basolaterally (arrows) in (+/+) but not in (−/−) SMG [acini (A) and ducts (D)]. (G) Morphology of SMG sections from (+/+) and (−/−) mice.
Expression and Localization of TRPC1 in SMG.
Fig. 1C shows the PCR products obtained by using genomic DNA samples from TRPC1(+/+) and (−/−) mice (three from each group) that confirm insertion of the PGK-neocassette in the (−/−) group (SI Fig. 7). Further, we examined TRPC1 transcripts in SMG from (+/+) and (−/−) mice. The RT-PCR products shown in Fig. 1D confirm the PCR data and indicate a change in TRPC1, but not TRPC3 or TRPC5, transcripts (see details in Materials and Methods). Western blotting (Fig. 1E and SI Fig. 8_A_) by using crude membranes isolated from SMG (FLAG-TRPC1, FL, also detected by anti-FLAG antibody, was used as a control) showed that TRPC1 was present in samples from TRPC1(+/+) but not in (−/−) mice. This was confirmed by immunofluorescence. TRPC1 was detected in the basolateral plasma membrane region of acinar cells (Fig. 1F Left,arrow; “A” and “D” indicate acini and ducts, respectively) but not in samples obtained from TRPC1(−/−) mice (Fig. 1A Right; this signal is similar to that seen when primary antibody is not added; data not shown). Similar results were obtained by using another anti-TRPC1 antibody (targeted to the TRPC1 sequence EVMALKDVREVKEENT[C]) that was generated as described for the anti-TRPC1 antibody, T1E3 (ref. 36; data not shown).
Deletion of TRPC1 expression did not alter the morphology of the SMG (Fig. 1G); the pattern of hematoxylin/eosin staining in SMG sections from TRPC1(−/−) and (+/+) mice was similar (note that SMG was used for all subsequent experiments). Localization and expression of AQP5, the water channel mediating fluid flow, as well as NKCC1, a key ion transporter for fluid secretion (34, 35), were similar in SMG from TRPC1(+/+) and TRPC1(−/−) mice (SI Fig. 8_Bi_; arrows indicate apical localization of AQP5 and basolateral localization of NKCC1). Also levels of IP3R3, AQP5, TRPC3, caveolin-1, actin, and STIM1 in (+/+) and (−/−) SMG were similar (SI Fig. 8_Bii_). Thus, loss of saliva flow in TRPC1(−/−) mice is not due to gross changes in salivary gland morphology (polarization) or expression of key proteins involved in fluid secretion and Ca2+ signaling. Transcripts for Orai1, -2, and -3 were detected in mouse SMG from TRPC1(+/+) and (−/−) mice at the same levels (SI Fig. 8_C_).
SOC Entry in SMG Acinar Cells from TRPC1(−/−) Mice.
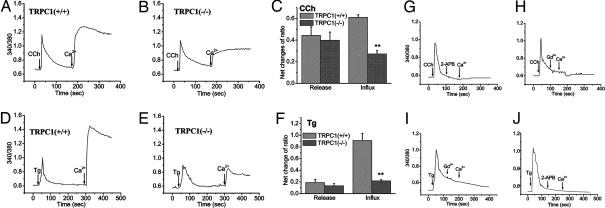
Ca2+ entry in SMG acinar cells stimulated with either carbachol (CCh) (10 μM; Fig. 2 A–C) or thapsigargin (Tg) (1 μM; Fig. 2 D–F) was greatly reduced (by >60%, P < 0.01, n = 90–120 cells obtained from three to four different experiments; see average data in Fig. 2 C and F) in TRPC1(−/−) as compared with TRPC1(+/+) cells. The pattern and amplitude of [Ca2+]i increase with either CCh or thapsigargin (Tg) in Ca2+ free medium were not significantly different in the two preparations. Thus, reduced Ca2+ entry is not a consequence of changes in the intracellular Ca2+ store status or CCh-induced upstream signaling events, including IP3-induced internal Ca2+ release. Further, unlike in TRPC1(−/−) DT-40 cells, where a small percentage of cells displayed normal levels of SOCE, Ca2+ entry was consistently decreased in all TRPC1(−/−) cells (SI Fig. 9_A_) (37). Both CCh- and Tg-induced Ca2+ entry were completely blocked by 2-APB and 1 μM gadolinium (Gd)3+ (Fig. 2 G–J), demonstrating that CCh and Tg primarily stimulate SOCE in SMG acini. In aggregate, these data demonstrate that deletion of TRPC1 severely decreases SOCE in SMG acini. SOCE was also decreased in ductal cells prepared from TRPC1(−/−) mice (SI Fig. 9_B_); the variation of Ca2+ entry in TRPC1(−/−) SMG ductal cells was similar to that seen in acinar cells (data not shown). The latter data suggest that ductal function is also altered by deletion of TRPC1, which most likely accounts for the altered ionic composition of the saliva in TRPC1(−/−) mice.
Fig. 2.
CCh- and Tg-stimulated Ca2+ entry in SMG acini. Fura2 fluorescence (traces represent average from at least 25 cells) measured in acinar cells stimulated with 10 μM CCh (A–C, G, and H) or 1 μM TG (D–F, I, and J) in Ca2+-free media (internal Ca2+ release). A 1 mM concentration of Ca2+ was added to the medium to detect Ca2+ entry. (C and F) Average data from three to four separate preparations (one to two mice per preparation). ** indicates a statistically difference, P < 0.05, _n_ = >150 cells. Additions of 20 μM 2-APB or 1 μM Gd3+ to acini from control mice are indicated in G and I and H and J, respectively.
Characterization of Store-Operated Cation Current in SMG Acinar Cells.
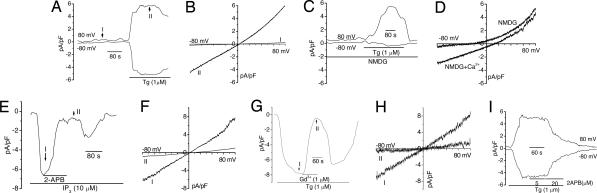
The role of TRPC1 in SOCE was further examined by measuring store-operated currents in single SMG acinar cells by using methods described previously (see Materials and Methods for details). The characteristics of the current were first established because there are no previous reports describing SOC current in this cell type. A linear current (with _E_rev = 3 ± 2 mV) was generated in cells perfused with Na+ + Ca2+-containing external medium and stimulated with Tg (Fig. 3 A and B), CCh (SI Fig. 10_A_), or when IP3 was included in the pipette solution (Fig. 3 E and F). When cells were stimulated in _N_-methyl-d-glutamine (NMDG)-containing external medium (Fig. 3 C and D), there was >80% reduction in the inward current at negative membrane potentials, whereas the outward current was not changed significantly (5.8 ± 1.2 and 5.1 ± 1.1 pA/pF in Na++Ca2+ and NMDG media, respectively), and _E_rev was left-shifted, to −35 ± 5 mV. Inclusion of Ca2+ with NMDG caused an increase in inward currents (pA/pF = 1.1 ± 0.25 and 2.8 ± 0.5 in NMDG and NMDG + Ca2+ media, respectively) and a right shift in _E_rev to 13 ± 2 mV (Fig. 4 C and D). These data demonstrate that a Ca2+-permeable but relatively nonselective cation channel is activated by store depletion in mouse SMG acini. The I–V characteristics of this current in Na+ + Ca2+ medium are different from the relatively inwardly rectifying SOC channel-mediated current (ISOC) we have reported in human submandibular gland (HSG) cells but more like the ISOC in human parotid gland (HSY) cells (5, 11, 12, 19). This variation in ISOC in different salivary epithelial cells can be due to heteromeric interaction of TRPC1 with other TRPCs (3, 8, 11, 38). As in HSG cells, the current was not induced by the presence of 10 mM EGTA [or 1,2-bis(2-aminophenoxy)ethane-_N,N,N′,N′_-tetraacetate; data not shown] alone in the pipette solution (Fig. 3 A and B). In contrast, ICRAC is activated under these conditions (17, 20, 21). Consistent with the data shown in Fig. 2, the current induced by Tg, IP3, or CCh was blocked by 2-aminoethoxydiphenyl borate (2-APB) and 1–5 μM Gd2+ (Fig. 3 E–H; also see SI Fig. 10 B and C), suggesting it is activated by Ca2+ store depletion. The current stimulated by Tg, CCh, or IP3 in SMG acini was not affected by buffering the [Ca2+] in the pipette solution to 100 nM or addition of niflumic acid in the bath solution (SI Fig. 11 A and B; data with IP3 are not shown). ISOC was not detected if Gd3+ was included in the external medium for the duration of the experiment (SI Fig. 11_C_). We have not yet established whether this SOC channel displays anomalous mol fraction behavior, although substitution of normal medium with divalent cation free (DVF) increased the current ≈2-fold (see Fig. 4 E and F). Together, these data suggest that ISOC in SMG acini, consistent with our previous findings with HSG and HSY cells (5, 11), is different from ICRAC in rat basophilic leukemia (RBL) cells and T lymphocytes (20, 21). This is further demonstrated in Fig. 3I; low concentrations of 2-APB that increase ICRAC (26, 39) did not increase ISOC (lack of effect of low [2APB] on Ca2+ entry is shown in SI Fig. 12_A_).
Fig. 3.
Characterization of store-operated cation current in SMG acinar cells. Whole-cell patch–clamp measurements (see Materials and Methods for details) in single acinar cells perfused with external Na+ + Ca2+ medium or other media in E and F (pipette solution contained 10 μM IP3 as indicated). (A) Current obtained with Tg added to external medium. (B) I–V relationship of the current at the time points I and II in A. (C) Current activated by Tg in NMDG external medium. (D) I–V relationship of the current in NMDG or NMDG + Ca2+ medium, as indicated. (E and G) A 1 μM concentration of Gd3+ or 20 μM 2-APB was included in the external medium in Tg- (G) or IP3− (E) stimulated cells. (F and H) I–V curves of current at time points shown in E and G, respectively. (I) Current in cells stimulated by Tg in Na+ + Ca2+ medium; 5 or 20 μM 2-APB was included in the external medium as shown. All traces represent results obtained with more than five cells in each case.
Fig. 4.
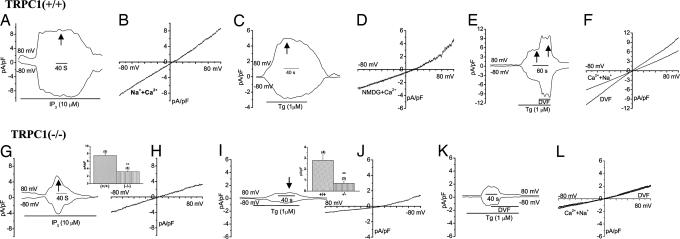
SOC channel function is decreased in SMG acini from TRPC1(−/−) mice. Store-operated current (ISOC) stimulated by inclusion of IP3 in the pipette solution in TRPC1(+/+) (A and B) and TRPC1(−/−) (G and H) cells. A and G show inward and outward currents, whereas B and H show the I–V characteristics of the maximum currents shown in A and C. Average data are shown in H Inset; ** denotes significant difference, P < 0.05 from unmarked value, (+/+) cells and (−/−)cells, “n” for each experiment is indicated. Tg-stimulated currents in NMDG + Ca2+-containing external medium in TRPC1(+/+) (C) and (−/−) (I) cells, I–V curves of the currents at the time points indicated are shown in D and J, respectively (J Inset shows average of data, as described above). (E) TRPC1(+/+) or K. (−/−) cells were stimulated with Tg in Na+ + Ca2+ medium, which was substituted with DVF medium where indicated. F and L show I–V curves of the current in the two conditions in both sets of cells.
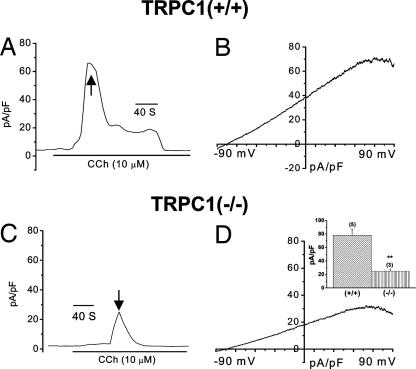
SOC Channel Activity Is Decreased in SMG Acini from TRPC1(−/−) Mice.
ISOC was dramatically decreased in TRPC1(−/−) SMG acini. Both inward and outward currents detected in a Na++Ca2+ medium with IP3 in the pipette solution were activated slowly, greatly reduced (≥75%), and transient in TRPC1(−/−) compared with that in TRPC1(+/+) cells (compare Fig. 4 A and B with G and H; average data of peak current are shown in Inset). A similar decreased current was detected in TRPC1(−/−) cells treated with Tg (compare Fig. 4 E and F with K and L) or CCh (SI Fig. 10_D_). The current in NMDG + Ca2+ medium was also significantly decreased (Fig. 4 C, D, I, J, and Inset). Further, similar to ISOC in TRPC1(+/+) cells, the residual current in TRPC1(−/−) cells was not increased by low [2APB] (data not shown) (also see SI Fig. 11_B_). Although the I–V curve of the residual transient current in TRPC1(−/−) cells is generally similar to that in TRPC1(+/+) cells, it is distinct from ISOC. Substitution of Na++Ca2+ medium with DVF medium in TRPC1(+/+) cells increased ISOC ≈2-fold (Fig. 4 E and F) but not the residual current in TRPC1(−/−) cells (see Fig. 4 K and L). Although the molecular basis for this residual transient current in TRPC1(−/−) cells, and whether it is present in TRPC1(+/+) cells or is generated in response to TRPC1 deletion, have yet to be determined, our findings clearly demonstrate this transient Ca2+ entry is independent of TRPC1 and is not sufficient for pilocarpine-stimulated saliva flow in TRPC1(−/−) mice, which requires sustained Ca2+ entry. Also, although both TRPC1(+/+) as well as (−/−) cells express similar levels of STIM1 protein and Orai transcripts, the currents in TRPC1(+/+) as well as TRPC1(−/−) cells are not similar to ICRAC. More importantly, our data indicate that Orai1 and STIM1 cannot support fluid secretion in the absence of TRPC1. Previous studies show that Orai1 contributes to ISOC in HSG cells (19) and alters the function of TRPC channels (C3 and C6) when overexpressed together in HEK-293 cells (25). Thus, it is possible that Orai1 might also regulate in TRPC1-SOC in mouse SMG acinar cells and further studies are required to demonstrate this.
Ca2+-Activated K+ Channel Activity in SMG Acinar Cells from TRPC1(−/−) Mice.
To confirm that knockdown of TRPC1 results in reduced Ca2+ entry, we examined Ca2+-activated K+ channel activity (KCa), which is critical for maintaining fluid secretion and has been used previously as a readout for [Ca2+]i (34, 35, 40). Compared with the sustained KCa activity in acini from TRPC1(+/+) mice (Fig. 5 A and B), a transient and attenuated (75% reduction) current was seen in SMG acini isolated from TRPC1(−/−) mice (Fig. 5 C, D, and Inset). This transient activation of the K+ current is likely due to internal Ca2+ release or the small residual transient Ca2+ entry seen in TRPC1(−/−) cells (seen in Figs. 2 and 4). Based on previous studies reported by us and others (13, 35, 40), we conclude that lack of sustained K+-channel activity in CCh-stimulated cells is due to loss of sustained Ca2+ entry in TRPC1(−/−) cells and can account for the decreased in stimulated fluid secretion in TRPC1(−/−) mice.
Fig. 5.
Ca2+-activated maxi K+ channel currents in TRPC1 (+/+) and (−/−) SMG acini. (A and B) Currents recorded under conditions described in Materials and Methods. Cells in (+/+) and (−/−) acini, respectively. I–V curves for the time points shown by arrows are shown in B and D. Inset shows average data. All traces are representative of data obtained in three to five cells.
Discussion
The data presented above demonstrate that targeted disruption of TRPC1 significantly impairs salivary gland fluid secretion without any change in the morphology of SMG or expression of key proteins involved in fluid secretion and Ca2+ signaling. Further, lack of TRPC1 induced similar reductions in Tg-, CCh, and IP3-stimulated SOCE. We have shown that IP3, Tg, and CCh activate a Ca2+-permeable SOC channel in SMG acinar cells that is distinct from CRAC channels in T lymphocytes and mast cells (6, 17, 20, 21). Deletion of TRPC1 resulted in severe attenuation (≥75%) of ISOC. A residual transiently increased current was seen in TRPC1(−/−) cells that was distinct from ISOC in TRPC1(+/+) cells, because it was not increased in a DVF medium. This current was also distinct from ICRAC. The molecular basis and functional significance of this residual current are not yet known. Additionally, and consistent with the decreased Ca2+ entry, CCh-activated KCa current was also greatly reduced transient which can account for the decreased fluid secretion. Together, these data suggest that defective SOCE underlies the salivary gland dysfunction induced by targeted deletion of TRPC1. The findings of this study lead to two major conclusions: (i) TRPC1 is required for generation of SOC channel in salivary gland cells, and (ii) TRPC1-mediated SOCE is required for neurotransmitter stimulated saliva secretion in SMG.
It has been recently reported that STIM1 and Orai1 are sufficient for generation of CRAC channels, which mediate SOCE in rat basophilic leukemia (RBL) cells and T lymphocytes (23, 26, 30–32, 41). Although it is clear that internal Ca2+ store depletion induces relocalization of STIM1 into punctae in the plasma membrane region of the cells, the exact molecular interaction(s) that determine regulation of Orai1 by STIM1 in response to store depletion has not yet been established. STIM1 also regulates TRPC1-dependent SOCE channels (3, 8, 19, 24, 38, 42), and Orai1 is suggested to have a role in TRPC1- and TRPC3-dependent SOCE (19, 25). The physiological function of STIM1 and the Orai proteins, as well as their possible role in regulation TRPC1-dependent SOCE channels, in salivary gland acini has yet to be determined. Based on the following observations: (i) there is no apparent compensation for loss of SOCE in TRPC1(−/−) mice, and (ii) neither ISOC in WT cells nor the residual transient current in TRPC1(−/−) cells display ICRAC-like properties, it appears that Orai1 and STIM1 do not generate detectable CRAC channels in these cells. Note that STIM1 as well as Orai1, -2, and -3 are expressed at similar levels in TRPC1 (−/−) and (+/+) SMG. Further, we have recorded ICRAC in RBL-2H3 cells (5, 19) and in Orai1 + STIM1 expressing HEK-293 cells (X.L., K.T.C., and I.S.A., unpublished observations) by using the same experimental conditions used here. We also cannot rule out the possibility that other as-yet-unidentified proteins might interact with and regulate TRPC1 function. Thus, detailed studies are required to fully understand the mechanism by which TRPC1 is activated in response to store depletion. Based on the data we have shown here, we suggest that deletion of TRPC1 results in a sustained loss of SOCE in salivary gland cells, which underlies the secretory dysfunction seen in TRPC1(−/−) mice. It should be noted that TRPC4, which has been strongly implicated in SOC channels is not expressed in salivary glands (43). Further, although other TRPCs, e.g., TRPC3, that have been suggested to mediate SOCE are expressed in SMG (44), their involvement in SOC channels is likely to depend on TRPC1 (8, 11).
In conclusion, we report here that defective SOCE underlies the salivary gland phenotype induced by deletion of TRPC1. Salivary gland fluid secretion is coordinately regulated by activation of Ca2+ signaling events, which are coupled downstream to regulation of multiple water and ion flux proteins. Na+/H+ exchanger, Na+K+2Cl− cotransporter, maxi and intermediate Ca2+-activated K+ channels, Cl−/HCO3− exchanger, and AQP5 all contribute to varying extents to fluid secretion as seen by the impact of their deletion on saliva flow and its ionic composition (35). Most importantly, regulation of all these key transporter and channel proteins, and consequently saliva secretion, depends on a sustained increase in [Ca2+]i. Although both internal Ca2+ release and Ca2+ entry contribute to the initial increase in [Ca2+]i seen in agonist-stimulated cells, sustained fluid secretion is determined by Ca2+ entry (34, 35). Knockout of the M3 receptors demonstrates that it is involved upstream in parasympathetic control of saliva secretion as well as CCh-stimulated Ca2+ signaling in the salivary gland cells (45). Simultaneous knockout of the IP3R2 and IP3R3 in mice induced loss of internal Ca2+ release as well as Ca2+ entry in acini and these mice also displayed significant attenuation of neurotransmitter-stimulated fluid secretion (46). Despite significant developmental defects in these mice, this previous study confirmed that agonist stimulation of Ca2+ entry that supports fluid secretion depends on Ca2+ release via IP3Rs (34, 35). Further, and consistent with our data, these previous findings indicate that CCh does not stimulate any non-SOCE pathway, i.e., independent of IP3-induced Ca2+ release. Major findings of the present study are that SOCE is the primary determinant for neurotransmitter-regulation of fluid secretion and that TRPC1 is a critical component of the SOC channel-mediating SOCE in SMG cells. Thus, the data presented here provide definitive genetic evidence for the physiological function for TRPC1 in the SOCE mechanism in salivary gland cells and in the regulation of salivary fluid secretion. We suggest that TRPC1 is a potentially useful target molecule for treatment of salivary gland dysfunction.
Materials and Methods
Generation of TRPC1(−/−).
The targeting vector was designed to insert a PGK-neo selection marker into the EcoRI site of exon 8 with creation of a Stop codon by replacing the EcoRI (Exon 8)-BstEII (Intron 8) fragment of genomic DNA cloned from a 129/OLA genomic P1 library (Genome Systems, Berkeley, CA) and used to target EK-CCE embryonic stem (ES) cells (47); also see SI Fig. 7. Heterozygous ES clone (3G12), identified by Southern blotting, was devoid of vector (pBS) or PGK-Neo DNA elsewhere in the genome with integration of the PGK-Neo sequence at the correct site (screened with a 5′ external probe, a 3′ internal probe and a Neo probe). Germ-line chimeras were obtained by injection of the correctly targeted ES cell clone 3G12 into C57BL/6J blastocysts and subsequent mating of chimeras to 129Sv mice. TRPC1(+/−) mice were phenotypically wild-type (tested for saliva secretion) and used to generate TRPC1(−/−) and TRPC1(+/+) mice. Genomic DNA extracted from mouse tail biopsy samples of offspring was analyzed by PCR by using appropriate primers. Analysis of RNA obtained from tissues of mice testing as TRPC1(−/−) revealed presence of mRNA originating from the TRPC1 gene by direct splicing of exon 7 to (out of frame) exon 9 (not shown). All mice were maintained and treated according to guidelines approved by the National Institute of Dental and Craniofacial Research and National Institutes of Health Animal Care and Use Committee.
RT-PCR Analysis.
RT-PCR analysis for TRPC1 transcripts was done with primers (Up-5′ GCAACCTTTGCCCTCAAAGTG and Dn-5′ GGAGGAACATT-CCCAGAAATTTCC) from the eighth and ninth exons, after the EcoRI site. RT-PCR for TRPC3 and TRPC5 transcripts were as described (48). Products were confirmed by sequencing and restriction analysis.
Isolation of Genomic DNA.
PCR was performed with genomic DNA by using the following primers: C1wtF, 5′-AAAGATCTTCACCCAAAAGCTGTT-3′ and C1R 5′-CCAACATGCTG-CACACTTTTGA-3′ (SI Fig. 7) and C1NeoF, 5-TGCCGAGAAAGTATCCATCAT-3′ and C1R to detect PGK-neo insert in the knockout mice.
Measurement of Saliva Secretion.
Mice were anesthetized, whole saliva was collected after stimulation with 0.5 mg of pilocarpine/kg body weight and osmolality, and ion concentrations were measured (49).
Dispersed SMG Cell Preparation.
Dispersed SMG cells were prepared after excision and cleaning of tissue (16, 49). Cells were maintained at 37°C with frequent gassing until use.
Electrophysiological Recording.
The patch pipette (3–5 megaohms resistance) containing standard intracellular solution; 145 mM cesium methane-sulfonate/8 mM NaCl/10 mM MgCl2/10 mM Hepes/10 mM EGTA, pH 7.2 (CsOH). External solutions were as follows: Ca2+ and Na+ solution, 145 mM NaCl/5 mM CsCl/1 mM MgCl2/10 mM CaCl2/10 mM Hepes/10 mM glucose, pH 7.4 (NaOH); NMDG solution, Na+ was replaced with equivalent concentration of NMDG; DVF-Na+ solution, 165 mM NaCl/5 mM CsCl/10 mM EDTA/10 mM Hepes/10 mM glucose, pH 7.4 (NaOH). Osmolarity for all solutions was adjusted with d-mannitol to 305 ± 5 mmol/kg. For KCa measurements, pipette solution contained 150 mM KCl, 2 mM MgCl2, 1 mM Mg-ATP, 5 mM Hepes, 0.1 mM EGTA and pH 7.2, potassium hydroxide. Measurements were done in the tight-seal whole-cell configuration at room temperature (22–25°C) by using an Axopatch 200B amplifier (Axon Instruments, Sunnyvale, CA) (5, 19). Voltage ramps ranging from −90 to 90 mV over a period of 1 s were imposed every 4 s from a holding potential of 0 mV and digitized at a rate of 1 kHz. A liquid–junction potential of <8 mV was not corrected, and capacitative currents and series resistance were determined and minimized. For analysis, the first ramp was used for leak subtraction for the subsequent current records.
Measurement of Intracellular [Ca2+].
Cells were loaded with fura-2 and were allowed to attach to a glass bottom dish (Matek, Ashland, OR) Acinar cells were identified by morphology and used for fluorescence measurements (19). Fluorescence traces show 340-/380-nm ratio (representing [Ca2+]i) averaged from >150 cells obtained in at least three to four individual experiments (see SI Fig. 9).
SDS/PAGE and Western Blotting.
Crude membranes were prepared from mouse salivary gland as described earlier and subjected to SDS/PAGE and Western blotting (11, 19, 44). Primary antibodies used are indicated in the figure legends. Proteins were detected by using appropriate secondary antibodies and ECL reactions (Amersham Pharmacia Biotechnology, Uppsala, Sweden).
Immunocytochemistry and Immunofluorescence.
SMG were fixed, embedded in paraffin, and used for preparing 5- to 10-μm-thick sections. Immunocytochemistry was performed after dewaxing, rehydration, and permeabilization by using the DAB Histostain kit (Zymed, San Francisco, CA) (13, 16, 44) and anti-NKCC1 and anti-AQP5 (1:200 and 1:100 dilutions, respectively). In control sections, rabbit IgG was used instead of primary antibody. TRPC1 was detected by immunofluorescence by using anti-TRPC1 antibody at 1:100 dilution and TRITC-tagged anti-rabbit secondary antibody. Images were collected by using a Leica Confocal microscope and MetaMorph software (Molecular Devices, Sunnyvale, CA).
Supplementary Material
Supporting Figures
Acknowledgments
We thank Drs. James Melvin and Tetsuji Nakamoto for assistance with the measurement of the saliva ion composition and Dr. Klaus Groshner for helpful comments and suggestions. B.B.S. and B.P. were partially supported by National Institutes of Health/National Institute of Dental and Craniofacial Research Grant DE 017102. This study was supported by the Intramural Research Programs of the National Institute of Dental and Craniofacial Research (I.A.) and the National Institute of Environmental Health Sciences (L.B.).
Abbreviations
SOC
store-operated calcium
SOCE
SOC entry
ER
endoplasmic reticulum
TRPC
transient receptor potential canonical
CRAC
calcium release-activated calcium
SMG
submandibular glands
CCh
carbachol
Tg
thapsigargin
NMDG
_N_-methyl-d-glutamine
HSG
human submandibular gland
2-APB
aminoethoxydiphenyl borate
Gd
gadolinium
DVF
divalent cation free
ISOC
SOC channel-mediated current.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Putney JW., Jr Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 2.Parekh AB, Putney JW., Jr Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 3.Ambudkar IS, Ong HL, Liu X, Bandyopadhyay B, Cheng KT. Cell Calcium. 2007;42:213–223. doi: 10.1016/j.ceca.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Bolotina VM. Sci STKE. 2004;2004:pe34. doi: 10.1126/stke.2432004pe34. [DOI] [PubMed] [Google Scholar]
- 5.Liu X, Groschner K, Ambudkar IS. J Membr Biol. 2004;200:93–104. doi: 10.1007/s00232-004-0698-3. [DOI] [PubMed] [Google Scholar]
- 6.Parekh AB. Cell Calcium. 2007 in press. [Google Scholar]
- 7.Smyth JT, Dehaven WI, Jones BF, Mercer JC, Trebak M, Vazquez G, Putney JW., Jr Biochim Biophys Acta. 2006;1763:1147–1160. doi: 10.1016/j.bbamcr.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 8.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. Nat Cell Biol. 2007;9:636–645. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beech DJ. Pflügers Arch. 2005;451:53–60. doi: 10.1007/s00424-005-1441-3. [DOI] [PubMed] [Google Scholar]
- 10.Birnbaumer L, Zhu X, Jiang M, Boulay G, Peyton M, Vannier B, Brown D, Platano D, Sadeghi H, Stefani E, et al. Proc Natl Acad Sci USA. 1996;93:15195–15202. doi: 10.1073/pnas.93.26.15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X, Bandyopadhyay BC, Singh BB, Groschner K, Ambudkar IS. J Biol Chem. 2005;280:21600–21606. doi: 10.1074/jbc.C400492200. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Singh BB, Ambudkar IS. J Biol Chem. 2003;278:11337–11343. doi: 10.1074/jbc.M213271200. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Wang W, Singh BB, Lockwich T, Jadlowiec J, O'Connell B, Wellner R, Zhu MX, Ambudkar IS. J Biol Chem. 2000;275:3403–3411. doi: 10.1074/jbc.275.5.3403. [DOI] [PubMed] [Google Scholar]
- 14.Montell C. Sci STKE. 2005;2005:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- 15.Freichel M, Suh SH, Pfeifer A, Schweig U, Trost C, Weissgerber P, Biel M, Philipp S, Freise D, Droogmans G, et al. Nat Cell Biol. 2001;3:121–127. doi: 10.1038/35055019. [DOI] [PubMed] [Google Scholar]
- 16.Singh BB, Zheng C, Liu X, Lockwich T, Liao D, Zhu MX, Birnbaumer L, Ambudkar IS. FASEB J. 2001;15:1652–1654. doi: 10.1096/fj.00-0749fje. [DOI] [PubMed] [Google Scholar]
- 17.Hoth M, Penner R. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 18.Lewis RS. Nature. 2007;446:284–287. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- 19.Ong HL, Cheng KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh BB, et al. J Biol Chem. 2007;282:9105–9116. doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parekh AB, Penner R. J Physiol. 1995;489:377–382. doi: 10.1113/jphysiol.1995.sp021058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prakriya M, Lewis RS. J Physiol. 2001;536:3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, et al. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 25.Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong DL, Birnbaumer L. Proc Natl Acad Sci USA. 2007;104:4682–4687. doi: 10.1073/pnas.0611692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 27.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, et al. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gwack Y, Srikanth S, Feske S, Cruz-Guilloty F, Oh-Hora M, Neems DS, Hogan PG, Rao A. J Biol Chem. 2007;282:16232–16243. doi: 10.1074/jbc.M609630200. [DOI] [PubMed] [Google Scholar]
- 29.Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJ, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet JP. Nat Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 31.Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, Koomoa DL, Soboloff J, Gill DL, Fleig A, et al. Curr Biol. 2006;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lis A, Peinelt C, Beck A, Parvez S, Monteilh-Zoller M, Fleig A, Penner R. Curr Biol. 2007;17:794–800. doi: 10.1016/j.cub.2007.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ambudkar IS. Crit Rev Oral Biol Med. 2000;11:4–25. doi: 10.1177/10454411000110010301. [DOI] [PubMed] [Google Scholar]
- 35.Melvin JE, Yule D, Shuttleworth T, Begenisich T. Annu Rev Physiol. 2005;67:445–469. doi: 10.1146/annurev.physiol.67.041703.084745. [DOI] [PubMed] [Google Scholar]
- 36.Xu SZ, Beech DJ. Circ Res. 2001;88:84–87. doi: 10.1161/01.res.88.1.84. [DOI] [PubMed] [Google Scholar]
- 37.Mori Y, Wakamori M, Miyakawa T, Hermosura M, Hara Y, Nishida M, Hirose K, Mizushima A, Kurosaki M, Mori E, et al. J Exp Med. 2002;195:673–681. doi: 10.1084/jem.20011758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Worley PF, Zeng W, Huang GN, Yuan JP, Kim JY, Lee MG, Muallem S. Cell Calcium. 2007;42:363–371. doi: 10.1016/j.ceca.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mercer JC, Dehaven WI, Smyth JT, Wedel B, Boyles RR, Bird GS, Putney JW., Jr J Biol Chem. 2006;281:24979–24990. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X, Rojas E, Ambudkar IS. Am J Physiol. 1998;275:C571–C580. doi: 10.1152/ajpcell.1998.275.2.C571. [DOI] [PubMed] [Google Scholar]
- 41.Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopez JJ, Salido GM, Pariente JA, Rosado JA. J Biol Chem. 2006;281:28254–28264. doi: 10.1074/jbc.M604272200. [DOI] [PubMed] [Google Scholar]
- 43.McKay RR, Szymeczek-Seay CL, Lievremont JP, Bird GS, Zitt C, Jungling E, Luckhoff A, Putney JW., Jr Biochem J. 2000;351:735–746. [PMC free article] [PubMed] [Google Scholar]
- 44.Bandyopadhyay BC, Swaim WD, Liu X, Redman RS, Patterson RL, Ambudkar IS. J Biol Chem. 2005;280:12908–12916. doi: 10.1074/jbc.M410013200. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura T, Matsui M, Uchida K, Futatsugi A, Kusakawa S, Matsumoto N, Nakamura K, Manabe T, Taketo MM, Mikoshiba K. J Physiol. 2004;558:561–575. doi: 10.1113/jphysiol.2004.064626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Futatsugi A, Nakamura T, Yamada MK, Ebisui E, Nakamura K, Uchida K, Kitaguchi T, Takahashi-Iwanaga H, Noda T, Aruga J, et al. Science. 2005;309:2232–2234. doi: 10.1126/science.1114110. [DOI] [PubMed] [Google Scholar]
- 47.Dietrich A, Mederos YSM, Gollasch M, Gross V, Storch U, Dubrovska G, Obst M, Yildirim E, Salanova B, Kalwa H, et al. Mol Cell Biol. 2005;25:6980–6989. doi: 10.1128/MCB.25.16.6980-6989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bollimuntha S, Singh BB, Shavali S, Sharma SK, Ebadi M. J Biol Chem. 2005;280:2132–2140. doi: 10.1074/jbc.M407384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nehrke K, Arreola J, Nguyen HV, Pilato J, Richardson L, Okunade G, Baggs R, Shull GE, Melvin JE. J Biol Chem. 2002;277:23604–23611. doi: 10.1074/jbc.M202900200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Figures