AP-1 and ARF1 Control Endosomal Dynamics at Sites of FcR–mediated Phagocytosis (original) (raw)
Abstract
Phagocytosis, the mechanism of ingestion of large material and microorganisms, relies on actin polymerization and on the focal delivery of intracellular endocytic compartments. The molecular mechanisms involved in the formation and delivery of the endocytic vesicles that are recruited at sites of phagocytosis are not well characterized. Here we show that adaptor protein (AP)-1 but not AP-2 clathrin adaptor complexes are recruited early below the sites of particle attachment and are required for efficient receptor-mediated phagocytosis in murine macrophages. Clathrin, however, is not recruited with the AP complexes. We further show that the recruitment of AP-1–positive structures at sites of phagocytosis is regulated by the GTP-binding protein ARF1 but is not sensitive to brefeldin A. Furthermore, AP-1 depletion leads to increased surface levels of TNF-α, a cargo known to traffic through the endosomes to the plasma membrane upon stimulation of the macrophages. Together, our results support a clathrin-independent role for AP complexes in endosomal dynamics in macrophages by retaining some cargo proteins, a process important for membrane remodeling during phagocytosis.
INTRODUCTION
Phagocytosis is a mechanism of internalization that allows the ingestion of large particulate material and is used by amoeba to feed on bacteria. Specialized immune cells, such as macrophages, polymorphonuclear granulocytes, and dendritic cells, also use phagocytosis to internalize, degrade, and eventually present antigens derived from large foreign particles and microorganisms, thereby contributing to immune responses (Aderem and Underhill, 1999; Blander and Medzhitov, 2006). The engulfment of particles is initiated by the interaction between ligands exposed on the particle and receptors present on the surface of the phagocyte. A large number of receptors have been implicated in this process, including mannose receptors, scavenger receptors, and Toll-like receptors (TLRs), together with receptors for opsonins, namely receptors for the Fc portion of immunoglobulins (Ig; FcRs) and receptors for complement C3bi (CR3). Phagocytosis induced by the FcRs has been best characterized. It activates a signaling cascade that involves small GTP-binding proteins of the Rho and Arf families and eventually leads to actin polymerization, plasma membrane remodeling, and extension of pseudopods around the particle (Greenberg and Grinstein, 2002; Underhill and Ozinsky, 2002; Niedergang and Chavrier, 2004).
Plasma membrane extension around large particles is supported by a process of internal membrane delivery that involves the fusion machinery relying on vesicle-associated, soluble _N_-ethylmaleimide–sensitive factor attachment protein receptors (v-SNAREs; Booth et al., 2001; Aderem, 2002; Braun and Niedergang, 2006; Stow et al., 2006). The recycling endosomes bearing the SNARE protein VAMP3/Cellubrevin (Bajno et al., 2000; Niedergang et al., 2003) and a subpopulation of late endosomes bearing the SNARE protein VAMP7/TI-VAMP (Braun et al., 2004) have been shown to undergo focal exocytosis at the site of phagocytosis and to be required for efficient phagocytosis. In addition, the endoplasmic reticulum and the SNARE protein ERS24/Sec22b have also been implicated in the phagocytosis of large particles (Gagnon et al., 2002; Becker et al., 2005). Of note, the major part of the membrane forming a phagosome is of plasmalemmal origin (Touret et al., 2005b) and different intracellular compartments might contribute differentially depending on the cell type, the size and the nature of the particles engulfed and the receptors engaged (for reviews, see Booth et al., 2001; Gagnon et al., 2005; Touret et al., 2005a; Braun and Niedergang, 2006). Of interest, the Golgi apparatus is considered not to contribute to early steps of phagosome formation, because FcR-mediated phagocytosis proceeds normally in the presence of brefeldin A (BFA; Zhang et al., 1998; Becker et al., 2005). This fungal metabolite induces profound perturbation of the Golgi apparatus structure by stabilizing a complex between some guanine-nucleotide exchange factors (GEF) and the GDP-loaded ARF protein and therefore inhibiting its GTP loading. As a consequence, ARF1, COPI, and clathrin coat adaptor proteins rapidly dissociate from Golgi membranes (Jackson and Casanova, 2000; D'Souza-Schorey and Chavrier, 2006). Therefore, ARF1 was also considered not to be involved in phagocytosis until a recent study reported that ARF1 was activated in macrophages at sites of FcR-mediated phagocytosis (Beemiller et al., 2006). As a consequence, ARF1 could regulate Golgi-independent trafficking events during phagocytosis, as reported for the related ARF6 protein, which controls the focal delivery of VAMP3-positive endocytic compartments (Zhang et al., 1998; Uchida et al., 2001; Niedergang et al., 2003).
Although some of the endosomal markers and some proteins of the fusion machinery involved in membrane fusion at sites of phagocytosis are identified, the mechanisms underlying the budding and transport of vesicles to the plasma membrane remain poorly characterized. Formation of transport vesicles carrying cargo proteins between the plasma membrane, the _trans_-Golgi network (TGN) and endosomes relies in part on coats containing clathrin and adaptor protein (AP) complexes. The AP family contains four members, AP-1 to -4, each composed of two large subunits (γ/β1, α/β2, δ/β3, or ε/β4), a “medium” subunit (μ1-4), and a “small” subunit (σ1-4). AP-2 is located specifically at the plasma membrane. AP-1 is predominantly found at the TGN but also on endosomes and specialized granules, depending on the cell type. AP-3 is present on endosomal compartments and is involved in the trafficking of cargo proteins to lysosomes and lysosome-related organelles (for reviews, see Bonifacino and Glick, 2004; Robinson, 2004; Raposo et al., 2007). The association of AP-1 and -3, but not AP-2, with the membrane of endosomes or the TGN is mediated by ARF1 (D'Souza-Schorey and Chavrier, 2006). So far, only AP-1 has been connected to the phagocytic process. The implication of AP-1 has been reported in Dictyostelium discoideum, where AP-1 was shown to be necessary for efficient phagocytosis (Lefkir et al., 2004). The efficiency of uptake in the mu1 mutants was dependent on the size of the particles to internalize, indicating that the defect of phagocytosis in the mu1 mutant cells was at the stage of pseudopod extension. Nevertheless, the precise function of the AP-1 complexes at the onset of phagocytosis remains elusive.
Here, we investigated the role of APs during FcR-mediated phagocytosis in murine macrophages, and we observed that AP-1, but not -2, is recruited and accumulates in the cytoplasm of macrophages at sites of phagocytosis. RNA interference (RNAi) experiments showed that AP-1 and -3 are necessary for efficient phagocytosis. In addition, we showed that the recruitment of AP-1 under phagocytic cups is controlled by ARF1, but unaffected by BFA. Interestingly, clathrin was not recruited together with AP-1 at phagocytic sites. Furthermore, we observed that AP-1 depletion lead to an increased surface delivery of tumor necrosis factor (TNF)-α upon lipopolysaccharide (LPS) stimulation of the macrophages. Altogether, these results suggest that, in macrophages, AP-1 could function without clathrin to retain cargo proteins rather than playing a role in their sorting through a budding process.
MATERIALS AND METHODS
Plasmids and Reagents
The pEGFP-expressing (enhanced green fluorescent protein) plasmid (Clontech, Palo Alto, CA) and pEGFP-VAMP3/Cellubrevin plasmids (Braun et al., 2004) have been described previously. The pSRα-ARF1T31N-HA and pSRα-ARF6T27N-HA plasmids have been described previously (Dubois et al., 2005). The Clathrin-DsRed–expressing plasmid was a kind gift from Dr. Thomas Kirchhausen (Harvard Medical School, Boston, MA) (Rappoport et al., 2003).
BFA and LPS were from Sigma (St. Louis, MO). TNF-α protease inhibitor-1 (TAPI-1) is from Calbiochem (La Jolla, CA). The following antibodies were used: anti-clathrin heavy chain (clone 23), anti-γ-adaptin (clone 88), anti-GM130 (clone 35), anti-mouse TNF-α (rat monoclonal, clone MP6-XT22; Becton Dickinson, Mountain View, CA); anti-clathrin heavy chain (mouse monoclonal, clone X22; ABR Affinity BioReagents, Golden, CO); anti-mouse TNF-α (goat polyclonal, R&D Systems, Minneapolis, MN); Cy2-, Cy3-, and Cy5-labeled F(ab′)2 (Ig fragment after digestion with the enzyme papain) anti-mouse or anti-rabbit IgG, R-phycoerythrin (RPE)-labeled F(ab′)2 anti-rat IgG and fluorescein isothiocyanate (FITC)-labeled anti-goat IgG (Jackson ImmunoResearch, West Grove, PA); horseradish peroxidase–labeled anti-mouse IgG (GE Healthcare, Waukesha, WI). Alexa633- or Alexa488-conjugated transferrin and Alexa350-coupled phalloidin were from Molecular Probes (Eugene, OR). Anti-TGN38 was provided by Dr. George Banting (Department of Biochemistry, University of Bristol, Bristol, United Kingdom). Anti-α-adaptin (clone AP6) was from Dr. Frances Brodsky (UCSF, San Francisco, CA). Anti-δ-adaptin (rabbit serum used for Western blots and mouse monoclonal, clone KF4, used for immunofluorescence) were a kind gift from Dr. Andrew Peden (Genentech, San Francisco, CA).
Cell Culture, Transfection, and siRNA Treatment
RAW264.7 macrophages were grown and transfected as described (Niedergang et al., 2003). Bone marrow–derived macrophages were prepared as described (Braun et al., 2004).
Human monocytes were isolated from blood of healthy volunteers (Etablissement Français du Sang, Rungis, France) by density gradient sedimentation in Ficoll (GE Healthcare), followed by two washes in 1× phosphate-buffered saline (PBS) supplemented with 2 mM EDTA and 1 mg/ml bovine serum albumin and a negative selection with magnetic beads (Monocyte negative isolation kit, Dynal, Lake Success, NY).
HeLa cells (a gift from A. Dautry, Institut Pasteur, Paris) were grown in the same medium as macrophages (RPMI 1640 supplemented with 10% fetal calf serum [FCS], 2 mM glutamine, 10 mM HEPES, 1 mM sodium pyruvate, and 50 μM β-mercaptoethanol; all from Invitrogen, Carlsbad, CA).
RAW264.7 macrophages were transfected with small interfering RNA (siRNA) duplex (Eurogentec, Serain, Belgium or Proligo, Kyoto, Japan) and Lipofectamine 2000 according to the manufacturer's instructions (Invitrogen; Elbashir et al., 2001). GFP siRNA duplex used as control was described (Braun et al., 2004). The sequence of the siRNA specific for the γ subunit of the AP-1 complex (γ-adaptin) was 5′-AGCUAUGAAUGAUAUAUUATT-3′, which is similar to the sequence used in Dugast et al. (2005). The sequence of the siRNA specific for the δ subunit of the AP-3 complex (δ-adaptin) was 5′-CAUCAAGAUCAUCAAGCUG-3′. After 24 and 48 h, cell lysates were analyzed by Western blotting as described (Braun et al., 2004).
Transferrin (Tf) internalization was performed as described previously (Niedergang et al., 2003).
Phagocytosis Assays
When indicated, cells were pretreated with BFA at 10 μg/ml for 30 min at 37°C or with the same volume of methanol. Phagocytosis was performed as described (Niedergang et al., 2003; Braun et al., 2004) except that BFA or methanol was kept in the phagocytosis medium.
To quantitate phagocytosis, the number of internalized sheep red blood cells (SRBCs) was counted in 50 cells randomly chosen on the coverslips, and the phagocytic index, i.e., the mean number of phagocytosed SRBCs per cell, was calculated. The index obtained for transfected cells was divided by the index obtained for control nontransfected cells and expressed as a percentage of control cells. We also counted the number of cell-associated (bound + internalized) SRBCs, calculated the association index (mean number of associated SRBCs per cell), and expressed it as percentage of control nontransfected cells. The index obtained for the siRNA-treated cells was divided by the index obtained for control (siRNA GFP) cells and expressed as a percentage of the latter.
To quantitate polymerized actin recruitment, we scored the presence or absence of F-actin around particles in 50 cells randomly chosen on the coverslips and calculated an accumulation index, i.e., the mean number of accumulations per cell.
To quantitate AP-1 recruitment, we scored the presence or absence of AP-1 accumulations as estimated by eye around particles in 50 cells randomly chosen on the coverslips and calculated an accumulation index, i.e., the mean number of accumulations per cell. In Figure 2, quantitation was performed from immunofluorescent images (see below).
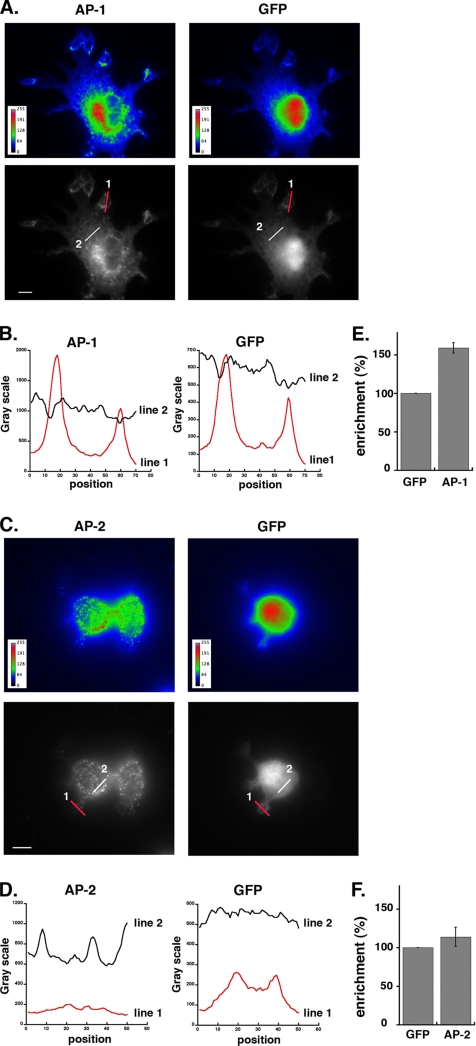
Figure 2.
Quantitation of AP-1 enrichment at the phagocytic sites. (A) pEGFP transfected RAW264.7 cells were incubated for 3 min at 37°C with IgG-SRBCs, then fixed, permeabilized, and stained with anti-γ-adaptin (AP-1; left panels) followed by Cy3-anti-mouse F(ab′)2 and Alexa350-phalloidin to detect phagocytic sites (not shown). GFP fluorescence is shown in the right panels. Images are shown using the Rainbow2 (top panels) or Grayscale (bottom panels) look-up table. Values on the color scales in the left corners of the top panels indicate the fluorescence intensities. Scale bar, 5 μm. (B) The profiles of γ-adaptin (left) and GFP (right) fluorescence intensities along the lines drawn at the phagocytic site (line 1) and in the cell body (line 2) are shown. (C) As described in A, except that GFP-expressing RAW264.7 cells were stained for α-adaptin (AP-2) after incubation for 3 min at 37°C with IgG-SRBCs (left panels). GFP fluorescence is shown in the right panels. A section on the dorsal side of the cells has been analyzed to better detect AP-2 staining at the plasma membrane. (D) The profiles of α-adaptin (left) and GFP (right) fluorescence intensities along the lines drawn at the phagocytic site (line 1) and in the cell body (line 2) are shown. (E and F) The fluorescence intensities measured in the phagocytic cups were background subtracted and expressed as a percentage of the fluorescence intensities in the cell body. Data obtained for γ-adaptin (E) or for α-adaptin (F) were compared with and expressed as a percentage of values obtained for GFP in the same regions of interest. Data are the mean + SEM of 15 independent measurements. Accumulation of AP-1 at phagocytic sites was significantly higher than that of GFP (p < 0.0001), but AP-2 was not enriched in nascent phagosomes as compared with GFP (p > 0.1).
Immunofluorescence Analysis
Cells were fixed in 4% paraformaldehyde/PBS and labeled (Niedergang et al., 2003). Cells were examined under a motorized upright wide-field microscope (DMRA2, Leica, Deerfield, IL) equipped for image deconvolution as described (Braun et al., 2004). Acquisition was performed using an oil immersion objective (100× PL APO HCX, 1.4 NA) and a cooled CCD camera (Roper CoolSnap HQ, Tucson, AZ). Z-positioning was accomplished by a piezo-electric motor and deconvolution was performed (see Figures 1A and 5, A and C, and Supplementary Figure S2A). Alternatively, acquisitions were also performed using a motorized upright wide-field microscope (DMRXA2, Leica) equipped with oil immersion objective (63× PL APO HCX, 1.32 NA) and a cooled CCD camera (Roper CoolSnap HQ; see Figure 4, A and C). Acquisitions were also performed using an inverted wide-field microscope (CTR 6000, Leica) equipped with oil immersion objective (100× PL APO HCX, 1.4 NA) and a cooled CCD camera (Micromax, Princeton Instruments Research Instruments, Princeton, NJ; see Figures 2, A and B, and 5B, and Supplementary Figure S1, A and B). Z-positioning was accomplished by a piezo-electric motor and deconvolution was then performed (see Figures 5B and 6B). Image analysis was performed using the Image J (NIH) and Adobe Photoshop software (San Jose, CA).
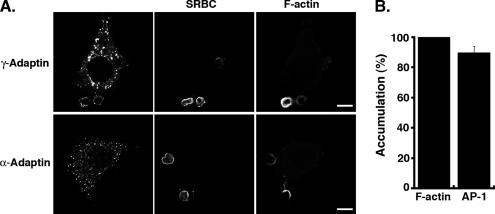
Figure 1.
AP-1 is recruited at sites of FcR-mediated phagocytosis but AP-2 is not. (A) RAW264.7 cells were incubated for 3 min with IgG-SRBCs, fixed, and stained with Cy2-anti-rabbit IgG to reveal external SRBCs (middle panels). The cells were then permeabilized and labeled with anti-γ-adaptin (top panels) or anti-α-adaptin (bottom panels) followed by Cy3-anti-mouse IgG to detect AP-1 and -2 complexes, respectively (left panels). Polymerized actin was labeled with Alexa350-phalloidin (right panels). Cells were analyzed by wide-field microscopy with deconvolution. Medial optical sections are shown. Bar, 5 μm. (B) The number of accumulations of γ-adaptin and F-actin in nascent phagosomes was scored for 50 cells randomly chosen on coverslips. Results are expressed as percentage and are the mean ± SEM of three independent experiments. Differences between F-actin and AP-1 accumulations were not significant (p > 0.05).
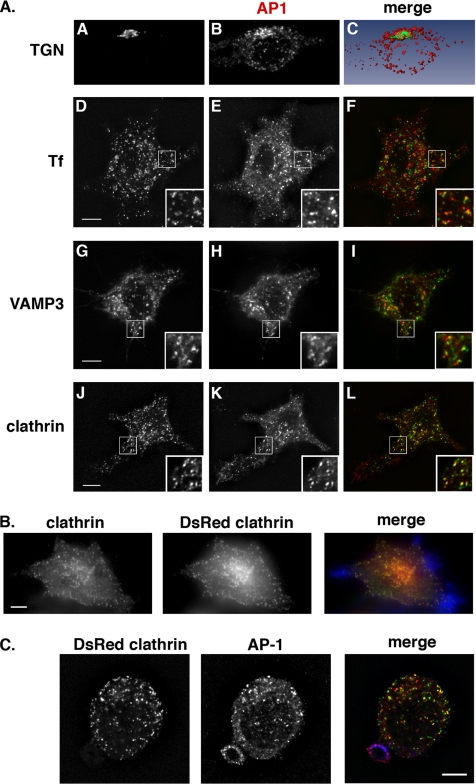
Figure 5.
Colocalization of AP-1 with subcellular markers in RAW264.7 macrophages. (A) When indicated, RAW264.7 cells were incubated with Alexa488-coupled transferrin internalized for 10 min at 37°C (D–F) or transiently transfected to express GFP-VAMP3 (G–I). The cells were then fixed and stained with anti-γ-adaptin followed by Cy3 anti-mouse IgG (B, E, H, and K) together with anti-TGN (A) or anti-clathrin (J) antibodies. Cells were analyzed by wide-field microscopy with deconvolution. Medial optical sections are shown. Combined images are presented (C, F, I, and L). Insets are shown in D–L. Images were converted numerically using the AMIRA software to calculate the volume rending and visualize the colocalization between the TGN (green) and γ -adaptin (red; C). Bar, 5 μm. (B) RAW264.7 cells transiently expressing clathrin-DsRed were incubated for 3 min with IgG-SRBCs, fixed, permeabilized, and labeled with anti-clathrin heavy chain followed by Cy2 anti-mouse F(ab′)2 (green in merged image) and Alexa350-phalloidin (blue in merged image) to detect polymerized actin at phagocytic sites. Cells were analyzed by wide-field microscopy with deconvolution. Combined image (right panel) shows no recruitment of clathrin at phagocytic sites. Bar, 5 μm. (C) RAW264.7 cells transiently expressing Clathrin-DsRed were incubated for 3 min with IgG-SRBCs (blue in merge), fixed, permeabilized, and labeled with anti-γ-adaptin followed by Cy3 anti-mouse F(ab′)2 to detect AP-1 complexes (middle panel, red in merge). Cells were analyzed by wide-field microscopy with deconvolution. Although AP-1 is recruited in the phagocytic site, clathrin is not, as shown in combined image (right panel). Bar, 5 μm.
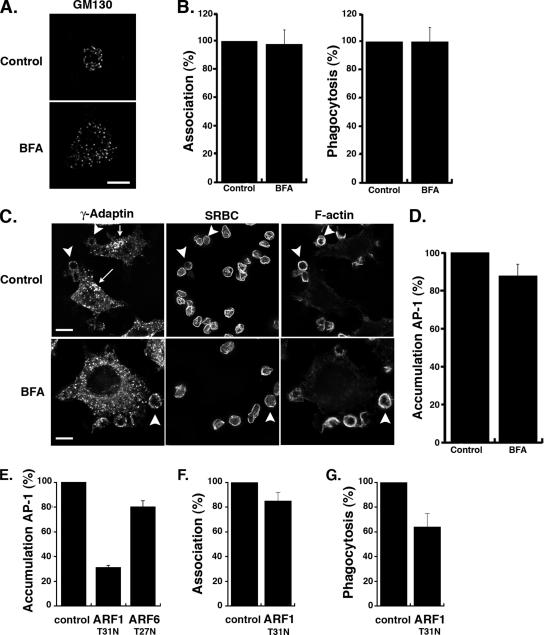
Figure 4.
Recruitment of γ-adaptin at sites of phagocytosis is BFA-insensitive and controlled by ARF1. (A) RAW264.7 cells were incubated in the presence BFA (bottom panel) at 10 μg/ml or the equivalent volume of methanol (top panel) for 30 min at 37°C, fixed, and stained with an anti-GM130 antibody followed by Cy3 anti-mouse-IgG. Cells were analyzed by wide-field microscopy with deconvolution. Medial optical sections are shown. Bar, 5 μm. (B) Cells pretreated or not with BFA were incubated for 60 min at 37°C with IgG-SRBCs, then fixed, and stained with Cy2-anti-rabbit IgG. The efficiencies of association (left) and phagocytosis (right) were determined as described in Figure 2. The means ± SEM of three independent experiments are plotted. (C) γ-adaptin recruitment during phagocytosis was evaluated in the presence of brefeldin A (BFA, bottom panels) or in the presence of the equivalent volume of methanol (control, top panels). RAW264.7 cells were fixed after 3 min of contact with IgG-SRBCs and stained with Cy2 anti-rabbit IgG to detect external SRBCs (middle panels). The cells were then permeabilized and labeled with anti-γ-adaptin followed by Cy3 anti-mouse IgG (left panels) and Alexa350-phalloidin (right panels). Cells were analyzed by wide-field microscopy with deconvolution. Medial optical sections are shown. γ-adaptin exhibits a perinuclear localization in control cells (arrow), which is lost in BFA-treated cells. By contrast, γ-adaptin is enriched in phagocytic cups (arrowheads) in control as well as in BFA-treated cells. Bar, 5 μm. (D) BFA-treated and control cells were scored for γ-adaptin accumulations around bound particles. Results are expressed as a percentage of control cells. Means ± SEM of n = 7 independent experiments are plotted. (E) RAW264.7 cells transiently transfected to express ARF1T31N or ARF6T26N were incubated with IgG-SRBCs for 3 min at 37°C. Accumulation of γ-adaptin at sites of particles attachment was scored in 50 ARF1T31N-positive cells or 50 ARF6T26N-positive cells as well as 50 negative control cells on the same coverslips. Results are expressed as a percentage of control cells. Means ± SEM of n = 6 independent experiments are plotted. Both ARF1T31N and ARF6T26N significantly inhibited AP-1 accumulations at phagocytic sites (p < 0.005). (F and G) RAW264.7 cells transfected with ARF1T31N were incubated for 60 min at 37°C with IgG-SRBCs, then fixed, and stained with Cy2-anti-rabbit F(ab′)2 to detect external SRBCs. The efficiencies of association (F) and phagocytosis (G) were calculated as described in _Material and Methods_ in 50 transfected or control cells (control). Results are expressed as a percentage of control cells. The means ± SEM are the result of four independent experiments. ARF1T31N significantly inhibited phagocytosis (p < 0.05) but not association of particles (p > 0.05).
Figure 6.
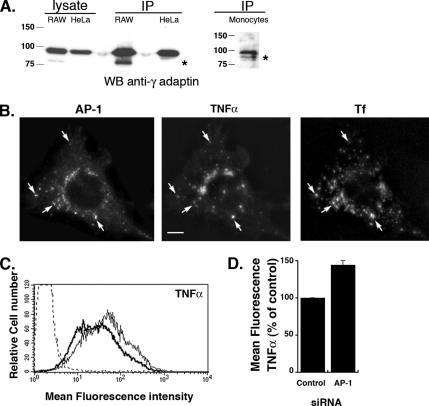
AP-1 is cleaved in macrophages, and its depletion leads to increased surface detection of TNF-α. (A) HeLa cells, RAW264.7 macrophages and freshly isolated human monocytes were lysed, immunoprecipitated with anti-γ-adaptin antibody, run onto SDS-PAGE, and analyzed by Western blotting with anti-γ-adaptin antibodies. For HeLa and RAW264.7 cells an aliquot of the total cell lysate was also analyzed. (B) RAW264.7 cells were incubated with Alexa633-coupled transferrin internalized for 30 min at 37°C. The cells were then fixed, permeabilized, and stained with anti-γ-adaptin and anti-TNF-α followed, respectively, by Cy3 anti-mouse F(ab′)2 and Cy2 anti-rat F(ab′)2. Cells were analyzed by wide-field microscope with deconvolution. A Z projection of eight planes is shown. Bar, 5 μm. (C) Surface TNF-α was detected by flow cytometry in AP-1–depleted cells (thin line) and compared with control siRNA-depleted cells (bold line). Cells stained with the secondary antibodies alone were used as a control (dotted line). (D) Mean fluorescence intensities of surface TNF-α detection in AP-1–depleted cells were expressed as a percentage of control siRNA-depleted cells. Data are the means ± SEM of four experiments. Depletion of AP-1 lead to a significant increase in the surface TNF-α on macrophages compared with control siRNA-treated cells (p < 0.0005).
Fluorescence Quantitation
Quantitation of fluorescence was performed using Metamorph software (Universal Imaging, West Chester, PA) on selected linear regions in one selected plane of the 16-bit stack acquired as described above with an inverted wide-field microscope (CTR6000, Leica) equipped with oil immersion objective (100× PL APO HCX, 1.4 NA) and a cooled CCD camera (Micromax, Princeton Instruments). Primary fluorescence intensities of selected regions were background corrected by subtracting the mean value from a cell-free region. Ratio values obtained by dividing the fluorescence intensities in the phagocytic cups by fluorescence intensities in the cell body were calculated for AP proteins and for GFP, used as a free cytosolic probe. Results are expressed as a percentage of the cup/cell body ratio obtained for GFP.
Flow Cytometry Analysis
To measure surface TNF-α expression, when indicated, RAW 264.7 cells were incubated for 4 h with 100 ng/ml LPS and 10 μM TAPI-1 at 37°C as described in Murray et al. (2005). TAPI-1 is an inhibitor of TACE, the enzyme that cleaves the transmembrane TNF-α protein and releases TNF-α in the extracellular medium. After incubation, cells were recovered from wells, incubated 1 h at 4°C with 10 μg/ml anti-mouse TNF-α in 100 μl of PBS supplemented with 2% FCS, and washed two times with 0.5 ml of PBS FCS 2%. The cells were then incubated 45 min at 4°C with RPE-anti-rat IgG and washed once with 0.5 ml of PBS FCS 2% and once with 0.5 ml of PBS to be finally resuspended in 0.5 ml of PBS. Flow cytometry was carried out in a FACScalibur flow cytometer (Becton Dickinson). At least 10,000 live cells were acquired and the median RPE fluorescence was calculated. We could not select the depleted cells population. Therefore, the acquisition was performed on a mixed population of depleted and nondepleted cells. The values obtained for specific siRNA-depleted cells were expressed as a percentage of the value measured for control siRNA-depleted cells.
Immunoprecipitation
RAW264.7 cells or HeLa cells were grown until subconfluence. RAW264.7 cells were scrapped and HeLa cells detached with trypsin to be collected and washed once with prechilled PBS. Cells were then lysed on ice with 0.5 ml of lysis buffer (0.5% NP40, 20 mM Tris, pH 7.4, 150 mM NaCl, Complete Mini EDTA-free protease inhibitor cocktail [Roche, Alameda, CA], 1 mM sodium orthovanadate, and 50 mM sodium fluoride). As a preclearing step, lysates were incubated 30 min with protein G-Sepharose (4 Fast Flow, GE Healthcare). Lysates were then centrifuged and the supernatants were incubated for 4 h at 4°C with 1 μg of anti-γ adaptin antibody and protein G-Sepharose. After four washes in cold wash buffer (0.5% NP40, 20 mM Tris, pH 7.4, 150 mM NaCl), the samples were analyzed by SDS-PAGE and Western blotting as described (Braun et al., 2004).
Statistics
The statistical significance of the data were tested with an unpaired Student's t test, and calculated goodness-of-fit value (p value) is indicated in the figures legends.
Online Supplementary Materials
The plasmid encoding α-adaptin-GFP was a kind gift of Lois Greene (NIH, Bethesda, MD) and was described in Wu et al. (2003).
RESULTS
AP-1 Complex Is Recruited to the Phagosome at Early Stages of Phagocytosis
To investigate the role of APs during phagocytosis, we first analyzed the localization of AP-1 and -2 during FcR-mediated phagocytosis in RAW264.7 macrophages as well as in bone marrow–derived macrophages. Macrophages were incubated with IgG-SRBCs for 5 or 10 min at 37°C and then fixed and stained to detect external SRBCs and intracellular AP-1 (γ-adaptin) or AP-2 (α-adaptin; Figure 1A). The AP-1 staining revealed a punctate pattern scattered in the cytoplasm of macrophages and concentrated in the perinuclear region (Figures 1 and 4C). We observed that AP-1 accumulated in the cytoplasmic regions located under the sites of phagosome formation, whereas AP-2 did not in the same conditions (Figure 1A). The lack of recruitment of AP-2 could be due to a defective recognition of the complex by the anti-α-adaptin antibodies, as a consequence of a potential cleavage of α or β2 AP-2 large subunits, as described for AP-1 (see below and Figure 6A; Santambrogio et al., 2005; Austin et al., 2006). We therefore expressed an α-adaptin-GFP construct in RAW264.7 macrophages. The construct was properly localized in structures reminiscent of coated pits at the plasma membrane, but AP-2 was not found accumulated at sites of phagosome formation (Supplemental Figure S1A). Because actin polymerization is a rapid and transient event concomitant to phagosome formation (Swanson et al., 1999; Coppolino et al., 2002; Araki et al., 2003; Henry et al., 2004), we then scored the number of AP-1 recruitments around external particles and compared it to F-actin accumulations (Figure 1B). We observed that 90 ± 4% of actin cups also showed AP-1 cytoplasmic accumulations. To better estimate the enrichment of endogenous AP-1 in phagocytic cups, fluorescent intensities around the particles and in the cell body were quantified and compared with the intensities obtained for GFP, used as a freely diffusible cytosolic marker (Figure 2). AP-1 fluorescent intensities exhibited up to a twofold increase around the particles compared with the cell body. Compared with GFP, AP-1 was specifically enriched in the extensions around the particles (Figure 2, B and E), whereas a similar quantitative analysis confirmed that AP-2 was not accumulated at sites of phagosome formation (Figure 2, D and F). Taken together, these results show that AP-1–positive structures are specifically recruited at sites of phagosome formation.
AP-1 and -3 Are Required for Efficient FcR-mediated Phagocytosis in RAW264.7 Cells
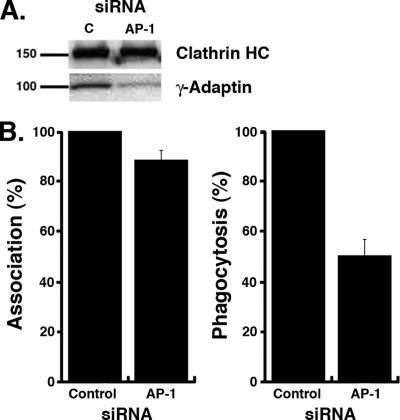
We next assessed whether AP-1 is required for efficient FcR-mediated phagocytosis in macrophages (Figure 3). For this, we inactivated the AP-1 complexes using RNAi against the γ-adaptin subunit (Dugast et al., 2005). Although the γ-adaptin siRNA only affects one subunit of the complex, previous reports have demonstrated that the depletion of one subunit of an AP complex decreases the stability of other subunits (Motley et al., 2003; Rose et al., 2005). We observed a diminished γ-adaptin expression in specific siRNA-treated cells compared with control cells treated with a nonspecific siRNA sequence (Figure 3A). The efficiency of particle binding and uptake via FcRs was monitored in AP-1–depleted cells identified by immmunostaining with anti-γ-adaptin antibodies. Although the association of opsonized particles was hardly affected in AP-1–depleted cells (93 ± 5% of control cells), particles internalization was reduced by 47 ± 7% (Figure 3B), indicating that AP-1 is required for efficient FcR-mediated phagocytosis.
Figure 3.
γ-adaptin is required for efficient FcR-mediated phagocytosis. (A) RAW264.7 cells were transfected either with γ-adaptin siRNA (AP-1) or with a nonspecific control siRNA (c). Twenty-four hours after transfection, cell lysates were analyzed by Western blot to detect γ-adaptin (bottom panel) and clathrin heavy chain (top panel). (B) γ-adaptin–depleted cells were incubated for 60 min at 37°C with IgG-SRBCs, then fixed, and stained with Cy2-anti-rabbit IgG to detect external SRBCs. The efficiencies of association (left) and phagocytosis (right) were calculated as described in Material and Methods in 50 γ-adaptin–depleted (AP-1) or control siRNA-treated cells (control). Results are expressed as a percentage of control cells. The means ± SEM of three independent experiments are plotted. Particle association (p < 0.05) and phagocytosis (p < 0.005) were significantly different between control siRNA- and AP-1-siRNA–treated cells.
We also examined whether AP-3 could be involved in FcR-mediated phagocytosis. First, we stained phagocytosing cells with antibodies against the δ-adaptin subunit of AP-3 and observed that, although it was present in the cytoplasm under phagocytic sites during FcR-mediated phagocytosis in RAW264.7 macrophages (Supplementary Figure S2A), it was not quantitatively enriched (Supplementary Figure S2B). Using RNAi against the δ-adaptin subunit, we next assessed whether AP-3 was required for efficient FcγR-mediated phagocytosis in macrophages (Supplementary Figure S2C). Expression of δ-adaptin was decreased in specific siRNA-treated cells compared with cells treated with a nonspecific siRNA sequence. We then measured the efficiencies of particles binding and uptake via FcRs in AP-3–depleted cells and compared them to control cells (Supplementary Figure S2D). We observed that in AP-3–depleted cells, particles internalization was reduced to 52 ± 10% of control cells, whereas the association of opsonized particles was not significantly affected. These results indicate that AP-3 is also important for efficient phagocytosis mediated by FcRs, although in contrast to AP-1 it is not accumulated in phagocytic cups.
AP-1 Recruitment is BFA Insensitive and Positively Regulated by ARF1
To get further insight into the function of AP-1 during phagocytosis in macrophages, we analyzed whether the recruitment of AP-1 in phagocytic cups occurred under the control of small GTP-binding proteins of the Arf family. For this, we treated the cells with BFA. As already described (Orci et al., 1991; Donaldson et al., 1992; Robinson and Kreis, 1992; Traub et al., 1993), treatment of RAW264.7 macrophages with BFA for 30 min led to disassembly of the Golgi apparatus and scattering of the Golgi marker GM130 in the cytoplasm (Figure 4A). By contrast, BFA treatment did not affect association or phagocytosis of opsonized particles in macrophages (Figure 4B and Zhang et al., 1998). Interestingly, the recruitment of AP-1 under phagocytic cups was unaffected by BFA treatment in contrast to its perinuclear (TGN) localization that was lost upon BFA treatment (Figure 4, C and D). We then tested if ARF1 and ARF6 could play a role in the accumulation of AP-1–positive structures at sites of phagosome formation. For this, we analyzed γ-adaptin recruitment in RAW264.7 macrophages transiently expressing dominant negative mutants of ARF6 or ARF1, ARF6T27N or ARF1T31N, respectively. Dominant negative ARF1T31N expression inhibited AP-1 recruitment under opsonized particles by 69 ± 1.7%, whereas ARF6T27N expression only affected the AP-1 localization during phagocytosis by 20 ± 5% (Figure 4E), although it inhibited particle uptake (Bajno et al., 2000; Niedergang et al., 2003). As reported recently (Beemiller et al., 2006), dominant negative ARF1T31N expression lead to a decreased phagocytic activity of macrophages (64 ± 11% of control cells).
Taken together, these results indicate that AP-1 recruitment under bound IgG-opsonized particles is controlled by ARF1, although it is BFA insensitive. Because Golgi-associated ARF1-GEFs are inhibited by BFA (Jackson and Casanova, 2000; D'Souza-Schorey and Chavrier, 2006), these results also suggest that the pool of AP-1 that is present at sites of particle attachment does not originate from the Golgi compartment and could therefore derive from other structures.
AP-1 Is Associated with Endosomal Structures and Accumulated without Clathrin under the Particles
To address the origin of AP-1 recruited at the phagocytic cup, we first examined the steady-state localization of AP-1 in RAW 264.7 macrophages by immunofluorescence. In macrophages, AP-1 exhibited a punctate staining localized in a perinuclear region as well as scattered throughout the cytoplasm (Figures 1 and 5). The perinuclear staining colocalized with a TGN marker, TGN38 (Figure 5) and was BFA sensitive (Figure 4C). The cytoplasmic AP-1–positive structures partially colocalized with internalized Tf, a marker of early and recycling endosomes (Dautry-Varsat et al., 1983) and with cellubrevin/VAMP3, a marker of recycling endosomes (Daro et al., 1996; Bajno et al., 2000; Niedergang et al., 2003). The AP-1–positive structures also exhibited extensive colocalization with clathrin-DsRed (Figure 5).
These observations therefore tend to identify the AP-1–positive structures as part of the TGN and endosomal compartments labeled by clathrin in macrophages.
We then examined the localization of AP-1 in phagocytosing macrophages (Figure 5C). Surprisingly, although AP-1 accumulation was observed at sites of FcR-mediated phagocytosis (Figures 1A and 5C), clathrin was never detected in the same location (Figure 5C). Because antibodies against clathrin and AP-1 are mouse monoclonal antibodies, we could not use them to detect endogenous clathrin and AP-1 at the same time in phagocytosing cells and therefore used the clathrin-DsRed construct for colocalization experiments. The lack of detection of clathrin was not due to a potential processing of the protein and/or an impaired recognition by antibodies, because clathrin expressed as a fluorescent fusion protein or detected by specific antibodies labeled the same structures and were not recruited under the sites of particles attachment (Figure 5B). This suggests that the pool of AP-1 that is present around nascent phagosomes is not associated with clathrin.
These observations indicate that γ-adaptin, and thus the AP-1 complex, is present predominantly on endosomal compartments in macrophages. Although colocalization with clathrin could be observed in the cytoplasm, clathrin could not be detected at sites of phagocytosis together with AP-1.
AP-1 Controls the Surface Delivery of TNF-α
The observation that AP-1 is necessary for efficient phagosome formation and that AP-1–positive vesicles present in phagocytic cups are devoid of clathrin led us to investigate further the role of AP-1 in membrane trafficking during phagocytosis. It has recently been shown that AP-1 complexes undergo a caspase-dependent cleavage in their hinge regions in immature dendritic cells, resulting in the removal of domains responsible for the interaction with clathrin and other endocytic effectors (Santambrogio et al., 2005). It was therefore possible that a similar cleavage of AP-1 existed in macrophages. Western blot analysis after γ-adaptin immunoprecipitation in RAW264.7 cells revealed the presence of two bands for AP-1: a high-molecular-weight form presumably corresponding to noncleaved γ-adaptin and a lower molecular weight product (Figure 6A). Of note, we never observed such degradation products in HeLa cells in the same conditions, but freshly isolated human monocytes expressed a lower molecular weight form as well (Figure 6A).
These observations indicate that a fraction of γ-adaptin is cleaved in macrophages as observed in dendritic cells and that truncated AP-1 complexes could play a role in organizing endosomal sorting by interacting with cargos rather than participating in the formation of clathrin buds. To test this hypothesis, we set out to determine if the surface delivery of cargos could be altered in AP-1–depleted cells. For this, we chose to follow the export of TNF-α, which was recently shown to be trafficked from the Golgi apparatus to the recycling endosomes before being delivered to the plasma membrane in a VAMP3-dependent manner (Murray et al., 2005). Microscopic analysis showed an induction of TNF-α after activation of macrophages with LPS (not shown), as reported (Murray et al., 2005). In our hands, LPS was more potent than phagocytosis to trigger the surface delivery of TNF-α. We observed a partial colocalization of TNF-α with AP-1 in the perinuclear region as well as in cytoplasmic vesicles close to the plasma membrane, where the staining sometimes coincided with fluorescent internalized Tf that labels endosomes (Figure 6B). Therefore, we followed by flow cytometry the surface expression of TNF-α after LPS stimulation of macrophages in cells where AP-1 was depleted and compared it to control siRNA-treated cells. Interestingly, although we could not specifically select the AP-1–depleted cell population, knocking down AP-1 lead to an increased TNF-α surface delivery (144 ± 6%, n = 4) compared with control cells (Figure 6, C and D). This observation indicates that AP-1 could play a role in retention of cargo proteins such as TNF-α at the level of endosomes.
DISCUSSION
In this work, we show that the adaptor complexes AP-1 but not -2 is recruited below phagocytic sites and is necessary for efficient phagocytosis mediated by FcRs in murine macrophages. Recruitment of AP-1 is controlled by ARF1 in a BFA-independent manner, suggesting that activation of ARF1 does not occur at the level of Golgi membranes. AP-1 complexes were indeed present on endosomal structures but clathrin was not accumulated together with AP-1 under phagocytosed particles. In addition, depletion of AP-1 lead to an increase in the surface delivery of TNF-α, which trafficks from the TGN to the plasma membrane via endosomes. These results support a role of AP complexes in cargo sorting at the level of endosomal compartments.
Control of AP-1 Recruitment by ARF1
We show here that AP-1 recruitment in phagocytic cups is positively regulated by ARF1, but not by ARF6. This result was somehow unexpected because ARF6 controls endosomal membrane delivery at the onset of phagocytosis (Niedergang et al., 2003), whereas ARF1 was supposed not to be involved in phagocytosis, because phagocytosis is not impaired by BFA (Zhang et al., 1998; Becker et al., 2005). A recent report using elegant in vivo fluorescence microscopy however shows that ARF1 and ARF6 are indeed activated early during phagosome formation and proposes that both ARF proteins coordinate different functions at the onset of phagocytosis (Beemiller et al., 2006). Given that ARF1, but not ARF6, controls the recruitment of AP-1–positive structures in phagocytic cups (Figure 4) and given that ARF6 is implicated in the control of membrane tethering and fusion steps (Donaldson, 2003; D'Souza-Schorey and Chavrier, 2006), our results are consistent with subsequent roles of ARF1 and ARF6: ARF1, which is not activated in a particular region under the nascent phagosome (Beemiller et al., 2006), would control sorting at the level of endosomes located in the cytoplasm underneath attached particles, whereas ARF6, which is specifically activated at the tips of the pseudopods (Beemiller et al., 2006), would control a later step at the leading edge of the phagocytic cup (see Figure 7).
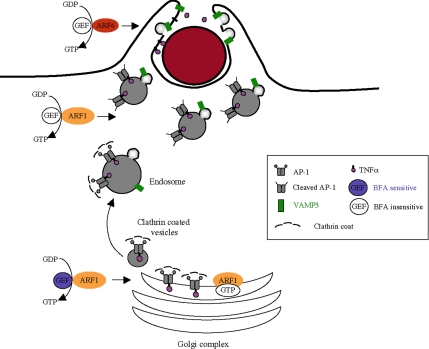
Figure 7.
Schematic representation of the possible role of adaptors during phagocytosis. This model proposes that AP-1 complexes exist as two pools in macrophages. The uncleaved proteins would be recruited on Golgi membranes after activation of ARF1 by a BFA-sensitive GEF, whereas the cleaved proteins would be recruited on endosomal membranes close to sites of particle attachment after activation of ARF1 by a BFA-insensitive GEF. In addition, no clathrin is associated with the AP-1 complexes in the cytoplasm under the sites of phagosome formation. The cleaved complexes without clathrin would help segregate proteins away from recycling to the plasma membrane during phagocytosis, a process that is necessary for phagocytosis to be efficiently completed. ARF6 would be important for later steps of VAMP3-dependent membrane fusion and pseudopod extension.
Nature of the AP-1–positive Vesicles and Absence of Clathrin
Association of ARF1 and ARF6 with membranes is stabilized by GDP/GTP exchange, and this step is catalyzed by GEFs. We observed that phagocytosis and AP-1 recruitment under the sites of phagocytosis were unaffected by the presence of BFA. Because BFA inhibits Golgi-associated ARF1-GEFs, the activation of ARF1 during phagocytosis most likely involves GEFs of the ARNO family associated with non-Golgi compartments (Jackson and Casanova, 2000; D'Souza-Schorey and Chavrier, 2006). Interestingly, a recent study shows that activated GTP-bound ARF6 recruits ARNO/cytohesin GEFs at the plasma membrane by binding to their pleckstrin homology domain. This in turn activates ARF1 (Cohen et al., 2007). In addition, our fluorescent and ultrastructural analyses demonstrate that at steady state AP-1 is localized on endosomal structures scattered throughout the cytoplasm in macrophages. In other cell types, AP-1 strongly accumulates in a perinuclear region corresponding to the TGN, but it has also been found on endosomes and specialized granules (Dittie et al., 1996; Le Borgne et al., 1996; Stoorvogel et al., 1996; Futter et al., 1998; Klumperman et al., 1998; Mallard et al., 1998; Theos et al., 2005; Popoff et al., 2007).
Interestingly, although AP-1 and clathrin were colocalized in resting cells, we found AP-1 but not clathrin under the sites of particles attachment. In addition, AP-1 was found on punctate endosomal structures in the cytoplasm underneath the forming phagosome, but did not decorate the plasma membrane or the membrane of the phagosome. This is consistent with the localization of AP-1 in mammalian phagocytes as reported previously in Lefkir et al. (2004) and is different from the situation in Dictyostelium, where AP-1 was found on phagosomal membranes and could be identified on purified phagosomal fractions. The pattern of accumulation we observed below phagocytosed particles in murine macrophages is very different from the pattern of VAMP3 recruitment on forming phagosomes (Bajno et al., 2000; Niedergang et al., 2003), which indicates that the AP-1–positive membranes do not fuse with the plasma membrane, whereas VAMP3-positive membranes do so (see Figure 7).
We did not observe enriched clathrin or AP-2 under the sites of attachment of IgG-opsonized SRBCs. These observations were made when we stained the cells with antibodies against clathrin or AP-2 or when we expressed clathrin-DsRed or alpha-adaptin-GFP, which rules out a problem of detection by the antibodies used. This observation differs from some studies reporting the presence of clathrin in phagocytic cups (Aggeler and Werb, 1982; Takemura et al., 1986; Perry et al., 1999; Castellano et al., 2000; Lefkir et al., 2004). Many differences in the experimental systems might explain these discrepancies. In Dictyostelium, clathrin was found associated with purified phagosomes together with AP-1 (Lefkir et al., 2004). Another difference is the size of the particles. Indeed, particles internalized in Aggeler et al. were latex beads of 0.1 μm only. These beads may therefore be internalized by a classical clathrin-coated pit. FcR clustering on coverslips during frustrated phagocytosis might also favor the accumulation of clathrin-coated regions (Takemura et al., 1986). Coated pits were also observed in phagosomes induced by a system mimicking receptor-mediated phagocytosis by direct recruitment of active Rac1 under the plasma membrane (Castellano et al., 2000). Because ligation of receptors connected to active Rac1 was induced at 37°C before beads addition, and because active Rac1 was shown to block clathrin-mediated endocytosis (Lamaze et al., 1996), this system might be prone to accumulate clathrin-coated pits. However, in our experimental system, the lack of accumulation of AP-2 and clathrin in phagocytic cups is consistent with data showing that specific inhibition of clathrin-mediated endocytosis by a dominant negative Eps15 construct (Niedergang et al., 2003) or by RNAi against clathrin (Tse et al., 2003) does not impair FcR-mediated phagocytosis. Therefore, in the case of FcR-mediated phagocytosis, clathrin is not required for internalization to proceed. This suggests another role for AP-1 at the onset of phagocytosis than promoting clathrin coat formation and vesicle budding.
AP-1 Cleavage and Role for APs without Clathrin in Cargo Sorting
The absence of clathrin associated with AP-1 was correlated with the presence of two forms of the γ-adaptin subunit of AP-1 in macrophages: a higher and a lower molecular weight protein of a size compatible with cleavage of the ear domain, as reported previously in dendritic cells (DCs; Santambrogio et al., 2005). Such a cleavage of the ear domain was proposed to modify the properties of the AP-1 complex, which would be still recruited efficiently onto membranes but, because of a lack of interaction with clathrin and some accessory proteins, would be unable to promote the budding of a vesicle (Santambrogio et al., 2005). In immature DCs, cleaved AP-1 would retain proteins such as the major histocompatibility complex proteins in endosomes, whereas in mature cells, intact AP-1 would allow vesicle budding and surface delivery of the proteins. Cleavage of large subunits of AP-1 in immature DCs relies on a caspase-dependent mechanism (Wong et al., 2004; Santambrogio et al., 2005). We therefore tested if the mechanism was similar in macrophages, but treatment of macrophages with a pan-caspase inhibitor did not prevent the cleavage of the γ-adaptin subunit. In addition, this cleavage was not regulated during phagocytosis (data not shown). Therefore, it seems that two pools of AP-1 complexes coexist in macrophages. Based on the lack of clathrin recruitment at sites of particles attachment, we propose that the complexes associated with the vesicles present under phagocytic cups are the cleaved complexes. We could not directly test this hypothesis because we had no possibility to differentially purify the AP-1–positive vesicles located below the forming phagosomes. We therefore speculate that these complexes could serve as a platform of sorting and retention for some cargos (see Figure 7). In line with this hypothesis, we observed an increase in TNF-α surface expression when AP-1 γ-adaptin was depleted. Similarly, the surface delivery of Lamp-1 was augmented in cells depleted for AP-3 (Dell'Angelica et al., 1999; Peden et al., 2002 and our unpublished observations). Membrane delivery at sites of phagosome formation would therefore involve subdomains of endosomes devoid of AP-1 but bearing the SNARE proteins such as VAMP3 (Bajno et al., 2000; Niedergang et al., 2003; Figure 7).
Because AP-1 and -3 complexes are required for efficient phagocytosis to proceed, our results lead us to suggest that AP complexes, by acting as retention platforms at the level of endosomes, control the outcome of a trafficking event that is the hallmark of specialized cells, phagocytosis. Whether AP complexes use similar mechanisms to modulate intracellular recycling pathways that are crucial for cell growth, migration, or spreading will be of major interest for future studies.
Supplementary Material
[Supplemental Materials]
ACKNOWLEDGMENTS
We thank Julie Mazzolini (Institut Cochin, Paris) for purifying of human monocytes, Jean-François Alkombre (INRA, Centre de Jouy-en Josas) for collecting samples of sheep blood, Pierre Bourdoncle (Institut Cochin) and Dr. Vincent Fraisier (Institut Curie) for expert help with fluorescence imaging. This work was supported by grants from Fondation pour la Recherche Médicale (INE20041102865), Centre National de la Recherche Scientifique (CNRS; ATIP Program), and Ville de Paris to F.N. and from the CNRS, Institut Curie, Fondation BNP-Paribas, and La Ligue Nationale contre le Cancer (“équipe labelisée”) to P.C. V.B. was supported by a doctoral Allocation de Recherche du Ministèrede l'Enseignement Supérieur et de la Recherche and then by a fellowship from ARC (Association pour la Recherche contre le Cancer). C.D. is supported by a postdoctoral fellowship from the CNRS (“chercheur associé”).
Abbreviations used:
AP
adaptor protein
ARF
ADP-ribosylation factor
BFA
brefeldin A
FcR
crystallizable fragment receptor
Lamp1
lysosomal-associated membrane protein 1
LPS
lipopolysaccharide
RPE
R-phycoerythrin
SRBC
sheep red blood cell
TAPI-1
TNF-α protease inhibitor-1
TGN
_trans_-Golgi network
TNF-α
tumor necrosis factor alpha.
Footnotes
REFERENCES
- Aderem A. How to eat something bigger than your head. Cell. 2002;110:5–8. doi: 10.1016/s0092-8674(02)00819-x. [DOI] [PubMed] [Google Scholar]
- Aderem A., Underhill D. M. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- Aggeler J., Werb Z. Initial events during phagocytosis by macrophages viewed from outside and inside the cell: membrane-particle interactions and clathrin. J. Cell Biol. 1982;94:613–623. doi: 10.1083/jcb.94.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki N., Hatae T., Furukawa A., Swanson J. A. Phosphoinositide-3-kinase-independent contractile activities associated with Fcgamma-receptor-mediated phagocytosis and macropinocytosis in macrophages. J. Cell Sci. 2003;116:247–257. doi: 10.1242/jcs.00235. [DOI] [PubMed] [Google Scholar]
- Austin C. D., et al. Death-receptor activation halts clathrin-dependent endocytosis. Proc. Natl. Acad. Sci. USA. 2006;103:10283–10288. doi: 10.1073/pnas.0604044103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajno L., Peng X. R., Schreiber A. D., Moore H. P., Trimble W. S., Grinstein S. Focal exocytosis of VAMP3-containing vesicles at sites of phagosome formation. J. Cell Biol. 2000;149:697–706. doi: 10.1083/jcb.149.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker T., Volchuk A., Rothman J. E. Differential use of endoplasmic reticulum membrane for phagocytosis in J774 macrophages. Proc. Natl. Acad. Sci. USA. 2005;102:4022–4026. doi: 10.1073/pnas.0409219102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemiller P., Hoppe A. D., Swanson J. A. A phosphatidylinositol-3-kinase-dependent signal transition regulates ARF1 and ARF6 during Fcgamma receptor-mediated phagocytosis. PLoS Biol. 2006;4:e162. doi: 10.1371/journal.pbio.0040162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander J. M., Medzhitov R. On regulation of phagosome maturation and antigen presentation. Nat. Immunol. 2006;7:1029–1035. doi: 10.1038/ni1006-1029. [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S., Glick B. S. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. doi: 10.1016/s0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- Booth J. W., Trimble W. S., Grinstein S. Membrane dynamics in phagocytosis. Semin. Immunol. 2001;13:357–364. doi: 10.1006/smim.2001.0332. [DOI] [PubMed] [Google Scholar]
- Braun V., Fraisier V., Raposo G., Hurbain I., Sibarita J. B., Chavrier P., Galli T., Niedergang F. TI-VAMP/VAMP7 is required for optimal phagocytosis of opsonised particles in macrophages. EMBO J. 2004;23:4166–4176. doi: 10.1038/sj.emboj.7600427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Niedergang F. Linking exocytosis and endocytosis during phagocytosis. Biol. Cell. 2006;98:195–201. doi: 10.1042/BC20050021. [DOI] [PubMed] [Google Scholar]
- Castellano F., Montcourrier P., Chavrier P. Membrane recruitment of Rac1 triggers phagocytosis. J. Cell Sci. 2000;113(Pt 17):2955–2961. doi: 10.1242/jcs.113.17.2955. [DOI] [PubMed] [Google Scholar]
- Cohen L. A., Honda A., Varnai P., Brown F. D., Balla T., Donaldson J. G. Active Arf6 recruits ARNO/cytohesin GEFs to the PM by binding their PH domains. Mol. Biol. Cell. 2007;18:2244–2253. doi: 10.1091/mbc.E06-11-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppolino M. G., Dierckman R., Loijens J., Collins R. F., Pouladi M., Jongstra-Bilen J., Schreiber A. D., Trimble W. S., Anderson R., Grinstein S. Inhibition of phosphatidylinositol-4-phosphate 5-kinase Iα impairs localized actin remodeling and suppresses phagocytosis. J. Biol. Chem. 2002;277:43849–43857. doi: 10.1074/jbc.M209046200. [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey C., Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat. Rev. Mol. Cell Biol. 2006;7:347–358. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- Daro E., van der Sluijs P., Galli T., Mellman I. Rab4 and cellubrevin define different early endosome populations on the pathway of transferrin receptor recycling. Proc. Natl. Acad. Sci. USA. 1996;93:9559–9564. doi: 10.1073/pnas.93.18.9559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautry-Varsat A., Ciechanover A., Lodish H. F. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc. Natl. Acad. Sci. USA. 1983;80:2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Angelica E. C., Shotelersuk V., Aguilar R. C., Gahl W. A., Bonifacino J. S. Altered trafficking of lysosomal proteins in Hermansky-Pudlak syndrome due to mutations in the beta 3A subunit of the AP-3 adaptor. Mol. Cell. 1999;3:11–21. doi: 10.1016/s1097-2765(00)80170-7. [DOI] [PubMed] [Google Scholar]
- Dittie A. S., Hajibagheri N., Tooze S. A. The AP-1 adaptor complex binds to immature secretory granules from PC12 cells, and is regulated by ADP-ribosylation factor. J. Cell Biol. 1996;132:523–536. doi: 10.1083/jcb.132.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson J. G. Multiple roles for Arf 6, sorting, structuring, and signaling at the plasma membrane. J. Biol. Chem. 2003;278:41573–41576. doi: 10.1074/jbc.R300026200. [DOI] [PubMed] [Google Scholar]
- Donaldson J. G., Finazzi D., Klausner R. D. Brefeldin A inhibits Golgi membrane-catalysed exchange of guanine nucleotide onto ARF protein. Nature. 1992;360:350–352. doi: 10.1038/360350a0. [DOI] [PubMed] [Google Scholar]
- Dubois T., Paleotti O., Mironov A. A., Fraisier V., Stradal T. E., De Matteis M. A., Franco M., Chavrier P. Golgi-localized GAP for Cdc42 functions downstream of ARF1 to control Arp2/3 complex and F-actin dynamics. Nat. Cell Biol. 2005;7:353–364. doi: 10.1038/ncb1244. [DOI] [PubMed] [Google Scholar]
- Dugast M., Toussaint H., Dousset C., Benaroch P. AP2 clathrin adaptor complex, but not AP1, controls the access of the major histocompatibility complex (MHC) class II to endosomes. J. Biol. Chem. 2005;280:19656–19664. doi: 10.1074/jbc.M501357200. [DOI] [PubMed] [Google Scholar]
- Elbashir S. M., Lendeckel W., Tuschl T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 2001;15:188–200. doi: 10.1101/gad.862301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futter C. E., Gibson A., Allchin E. H., Maxwell S., Ruddock L. J., Odorizzi G., Domingo D., Trowbridge I. S., Hopkins C. R. In polarized MDCK cells basolateral vesicles arise from clathrin-gamma-adaptin-coated domains on endosomal tubules. J. Cell Biol. 1998;141:611–623. doi: 10.1083/jcb.141.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon E., Bergeron J. J., Desjardins M. ER-mediated phagocytosis: myth or reality? J. Leukoc. Biol. 2005;77:843–845. doi: 10.1189/jlb.0305129. [DOI] [PubMed] [Google Scholar]
- Gagnon E., Duclos S., Rondeau C., Chevet E., Cameron P. H., Steele-Mortimer O., Paiement J., Bergeron J. J., Desjardins M. Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell. 2002;110:119–131. doi: 10.1016/s0092-8674(02)00797-3. [DOI] [PubMed] [Google Scholar]
- Greenberg S., Grinstein S. Phagocytosis and innate immunity. Curr. Opin. Immunol. 2002;14:136–145. doi: 10.1016/s0952-7915(01)00309-0. [DOI] [PubMed] [Google Scholar]
- Henry R. M., Hoppe A. D., Joshi N., Swanson J. A. The uniformity of phagosome maturation in macrophages. J. Cell Biol. 2004;164:185–194. doi: 10.1083/jcb.200307080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C. L., Casanova J. E. Turning on ARF: the Sec7 family of guanine-nucleotide-exchange factors. Trends Cell Biol. 2000;10:60–67. doi: 10.1016/s0962-8924(99)01699-2. [DOI] [PubMed] [Google Scholar]
- Klumperman J., Kuliawat R., Griffith J. M., Geuze H. J., Arvan P. Mannose 6-phosphate receptors are sorted from immature secretory granules via adaptor protein AP-1, clathrin, and syntaxin 6-positive vesicles. J. Cell Biol. 1998;141:359–371. doi: 10.1083/jcb.141.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamaze C., Chuang T. H., Terlecky L. J., Bokoch G. M., Schmid S. L. Regulation of receptor-mediated endocytosis by Rho and Rac. Nature. 1996;382:177–179. doi: 10.1038/382177a0. [DOI] [PubMed] [Google Scholar]
- Le Borgne R., Griffiths G., Hoflack B. Mannose 6-phosphate receptors and ADP-ribosylation factors cooperate for high affinity interaction of the AP-1 Golgi assembly proteins with membranes. J. Biol. Chem. 1996;271:2162–2170. doi: 10.1074/jbc.271.4.2162. [DOI] [PubMed] [Google Scholar]
- Lefkir Y., Malbouyres M., Gotthardt D., Ozinsky A., Cornillon S., Bruckert F., Aderem A. A., Soldati T., Cosson P., Letourneur F. Involvement of the AP-1 adaptor complex in early steps of phagocytosis and macropinocytosis. Mol. Biol. Cell. 2004;15:861–869. doi: 10.1091/mbc.E03-06-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard F., Antony C., Tenza D., Salamero J., Goud B., Johannes L. Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of shiga toxin B-fragment transport. J. Cell Biol. 1998;143:973–990. doi: 10.1083/jcb.143.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motley A., Bright N. A., Seaman M. N., Robinson M. S. Clathrin-mediated endocytosis in AP-2-depleted cells. J. Cell Biol. 2003;162:909–918. doi: 10.1083/jcb.200305145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R. Z., Kay J. G., Sangermani D. G., Stow J. L. A role for the phagosome in cytokine secretion. Science. 2005;310:1492–1495. doi: 10.1126/science.1120225. [DOI] [PubMed] [Google Scholar]
- Niedergang F., Chavrier P. Signaling and membrane dynamics during phagocytosis: many roads lead to the phagos(R)ome. Curr. Op. Cell Biol. 2004;16:422–428. doi: 10.1016/j.ceb.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Niedergang F., Colucci-Guyon E., Dubois T., Raposo G., Chavrier P. ADP ribosylation factor 6 is activated and controls membrane delivery during phagocytosis in macrophages. J. Cell Biol. 2003;161:1143–1150. doi: 10.1083/jcb.200210069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Tagaya M., Amherdt M., Perrelet A., Donaldson J. G., Lippincott-Schwartz J., Klausner R. D., Rothman J. E. Brefeldin A, a drug that blocks secretion, prevents the assembly of non-clathrin-coated buds on Golgi cisternae. Cell. 1991;64:1183–1195. doi: 10.1016/0092-8674(91)90273-2. [DOI] [PubMed] [Google Scholar]
- Peden A. A., Rudge R. E., Lui W. W., Robinson M. S. Assembly and function of AP-3 complexes in cells expressing mutant subunits. J. Cell Biol. 2002;156:327–336. doi: 10.1083/jcb.200107140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D. G., Daugherty G. L., Martin W. J., II Clathrin-coated pit-associated proteins are required for alveolar macrophage phagocytosis. J. Immunol. 1999;162:380–386. [PubMed] [Google Scholar]
- Popoff V., Mardones G. A., Tenza D., Rojas R., Lamaze C., Bonifacino J. S., Raposo G., Johannes L. The retromer complex and clathrin define an early endosomal retrograde exit site. J. Cell Sci. 2007;120:2022–2031. doi: 10.1242/jcs.003020. [DOI] [PubMed] [Google Scholar]
- Raposo G., Marks M., Cutler D. Lysosome-related organelles: driving post-Golgi compartments into specialisation. Curr. Opin. Cell Biol. 2007;19:394–401. doi: 10.1016/j.ceb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappoport J. Z., Taha B. W., Lemeer S., Benmerah A., Simon S. M. The AP-2 complex is excluded from the dynamic population of plasma membrane-associated clathrin. J. Biol. Chem. 2003;278:47357–47360. doi: 10.1074/jbc.C300390200. [DOI] [PubMed] [Google Scholar]
- Robinson M. S. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004;14:167–174. doi: 10.1016/j.tcb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Robinson M. S., Kreis T. E. Recruitment of coat proteins onto Golgi membranes in intact and permeabilized cells: effects of brefeldin A and G protein activators. Cell. 1992;69:129–138. doi: 10.1016/0092-8674(92)90124-u. [DOI] [PubMed] [Google Scholar]
- Rose J. J., Janvier K., Chandrasekhar S., Sekaly R. P., Bonifacino J. S., Venkatesan S. CD4 down-regulation by HIV-1 and simian immunodeficiency virus (SIV) Nef proteins involves both internalization and intracellular retention mechanisms. J. Biol. Chem. 2005;280:7413–7426. doi: 10.1074/jbc.M409420200. [DOI] [PubMed] [Google Scholar]
- Santambrogio L., Potolicchio I., Fessler S. P., Wong S. H., Raposo G., Strominger J. L. Involvement of caspase-cleaved and intact adaptor protein 1 complex in endosomal remodeling in maturing dendritic cells. Nat. Immunol. 2005;6:1020–1028. doi: 10.1038/ni1250. [DOI] [PubMed] [Google Scholar]
- Stoorvogel W., Oorschot V., Geuze H. J. A novel class of clathrin-coated vesicles budding from endosomes. J. Cell Biol. 1996;132:21–33. doi: 10.1083/jcb.132.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow J. L., Manderson A. P., Murray R. Z. SNAREing immunity: the role of SNAREs in the immune system. Nat. Rev. Immunol. 2006;6:919–929. doi: 10.1038/nri1980. [DOI] [PubMed] [Google Scholar]
- Swanson J. A., Johnson M. T., Beningo K., Post P., Mooseker M., Araki N. A contractile activity that closes phagosomes in macrophages. J. Cell Sci. 1999;112(Pt 3):307–316. doi: 10.1242/jcs.112.3.307. [DOI] [PubMed] [Google Scholar]
- Takemura R., Stenberg P. E., Bainton D. F., Werb Z. Rapid redistribution of clathrin onto macrophage plasma membranes in response to Fc receptor-ligand interaction during frustrated phagocytosis. J. Cell Biol. 1986;102:55–69. doi: 10.1083/jcb.102.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theos A. C., et al. Functions of adaptor protein (AP)-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes. Mol. Biol. Cell. 2005;16:5356–5372. doi: 10.1091/mbc.E05-07-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touret N., Paroutis P., Grinstein S. The nature of the phagosomal membrane: endoplasmic reticulum versus plasmalemma. J. Leukoc. Biol. 2005a;77:878–885. doi: 10.1189/jlb.1104630. [DOI] [PubMed] [Google Scholar]
- Touret N., et al. Quantitative and dynamic assessment of the contribution of the ER to phagosome formation. Cell. 2005b;123:157–170. doi: 10.1016/j.cell.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Traub L. M., Ostrom J. A., Kornfeld S. Biochemical dissection of AP-1 recruitment onto Golgi membranes. J. Cell Biol. 1993;123:561–573. doi: 10.1083/jcb.123.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse S.M.L., Furuya W., Gold E. S., Schreiber A. D., Sandvig K., Inman R. D., Grinstein S. Differential role of actin, clathrin, and dynamin in Fcγ receptor-mediated endocytosis and phagocytosis. J. Biol. Chem. 2003;278:3331–3338. doi: 10.1074/jbc.M207966200. [DOI] [PubMed] [Google Scholar]
- Uchida H., Kondo A., Yoshimura Y., Mazaki Y., Sabe H. PAG3/Papalpha/KIAA0400, a GTPase-activating protein for ADP-ribosylation factor (ARF), regulates ARF6 in Fcgamma receptor-mediated phagocytosis of macrophages. J. Exp. Med. 2001;193:955–966. doi: 10.1084/jem.193.8.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill D. M., Ozinsky A. Phagocytosis of microbes: complexity in action. Annu. Rev. Immunol. 2002;20:825–852. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- Wong S. H., Santambrogio L., Strominger J. L. Caspases and nitric oxide broadly regulate dendritic cell maturation and surface expression of class II MHC proteins. Proc. Natl. Acad. Sci. USA. 2004;101:17783–17788. doi: 10.1073/pnas.0408229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Zhao X., Puertollano R., Bonifacino J. S., Eisenberg E., Greene L. E. Adaptor and clathrin exchange at the plasma membrane and trans-Golgi network. Mol. Biol. Cell. 2003;14:516–528. doi: 10.1091/mbc.E02-06-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Cox D., Tseng C. C., Donaldson J. G., Greenberg S. A requirement for ARF6 in Fcgamma receptor-mediated phagocytosis in macrophages. J. Biol. Chem. 1998;273:19977–19981. doi: 10.1074/jbc.273.32.19977. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental Materials]