A dual-kinase mechanism for Wnt coreceptor phosphorylation and activation (original) (raw)
. Author manuscript; available in PMC: 2007 Dec 3.
Published in final edited form as: Nature. 2005 Dec 8;438(7069):873–877. doi: 10.1038/nature04185
Abstract
Signalling by the Wnt family of secreted lipoproteins plays essential roles in development and disease1. The canonical Wnt/β-catenin pathway requires a single-span transmembrane receptor, _L_DL receptor _r_elated _p_rotein 6 (LRP6)2-4, whose phosphorylation at multiple PPPSP motifs is induced upon Wnt stimulation and critical for signal transduction5. The kinase responsible for LRP6 phosphorylation has not been identified. Here we provide biochemical and genetic evidence for a ‘dual-kinase’ mechanism for LRP6 phosphorylation and activation. Surprisingly, glycogen synthase kinase 3 (GSK3), which is known for its inhibitory role in Wnt signalling via promoting β-catenin phosphorylation and degradation, mediates LRP6 phosphorylation and activation. We demonstrate that Wnt induces sequential phosphorylation of LRP6 by GSK3 and casein kinase 1 (CK1), and this dual-phosphorylation promotes the engagement of LRP6 with the scaffolding protein Axin. We further show that a membrane-associated form of GSK3, contrary to cytosolic GSK3, stimulates Wnt signalling and Xenopus axis duplication. Our results identify two key kinases mediating Wnt coreceptor activation, reveal an unexpected and intricate logic of Wnt/β-catenin signalling, and illustrate GSK3 as a bona fide switch dictating both on and off states of a pivotal regulatory pathway.
Canonical Wnt signalling operates through regulating phosphorylation and degradation of the transcription co-activator β–catenin1,6. Without Wnt stimulation, β–catenin is assembled into the Axin complex, in which β–catenin is sequentially phosphorylated by CK1 and GSK3 and earmarked for degradation7-9. Wnt stimulation leads to inhibition of β–catenin phosphorylation/degradation. This signal transduction is initiated at the plasma membrane by two distinct receptors, a Frizzled serpentine receptor and LRP6 or LRP5, which together may form a Wnt-induced Frizzled-LRP6 (or LRP5) complex3,6,10-12. While the mechanism by which this receptor pair initiates signalling remains to be understood, Wnt-induced LRP6 phosphorylation at a PPPSP motif, which is reiterated five times in LRP5/6 cytoplasmic domain (Fig. 1a), plays a critical role5. (For simplicity we use PPPSP to represent PPPSP or PPPTP). Indeed, LRP6 mutants lacking these motifs or harbouring substitutions at the S/T residues are inactive in signalling5,13. Conversely, a single PPPSP motif upon transfer to a heterologous receptor is sufficient to initiate β–catenin signalling5. As the phosphorylated PPPSP motif mediates LRP5/6-Axin interaction5,14, we proposed a model5 in which Wnt-induced LRP6 phosphorylation promotes Axin recruitment by LRP6, thereby permitting the Wnt receptor complex to directly regulate β–catenin phosphorylation14.
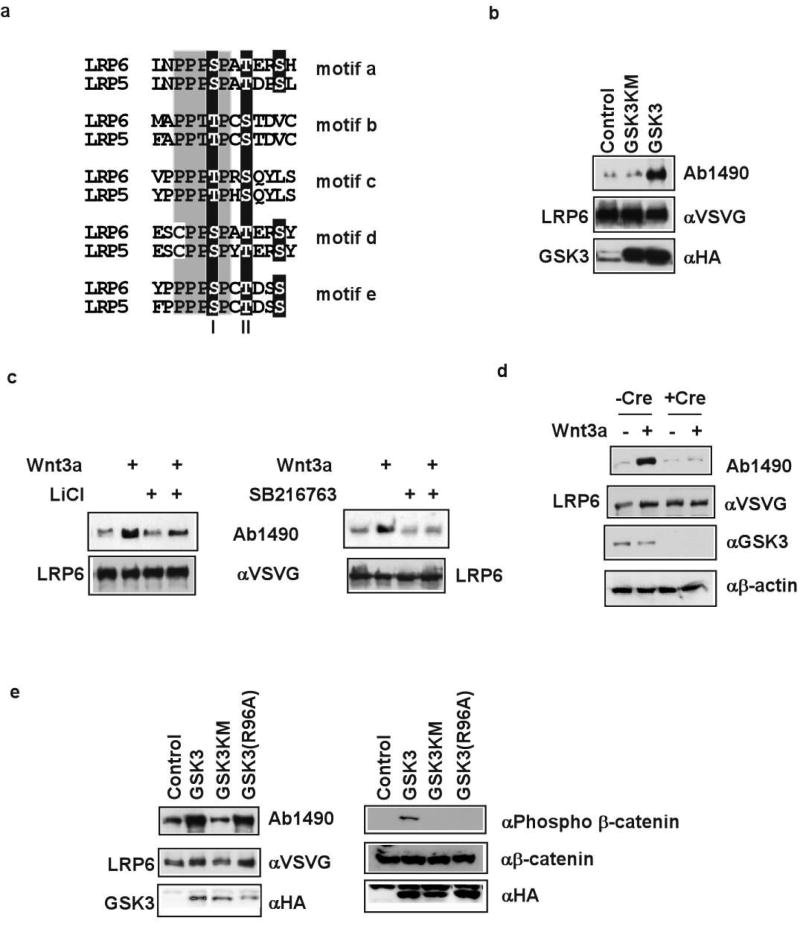
Figure 1. GSK3 involvement in PPPSP phosphorylation.
a. Five PPPSPxS motifs (motifs a-e) in LRP5/6. Site I, site II and another potential phosphorylation site are highlighted. b. GSK3β, but not the kinase-dead GSK3βKM, promoted LRP6 phosphorylation. LRP6 PPPSP phosphorylation in vivo was examined in 293T cells or MEFs that stably express LRP6 (VSVG-tagged)5 in this and other figures. c. Wnt3a-induced LRP6 PPPSP phosphorylation in MEFs was inhibited by LiCl (50 mM) or SB216763 (3 or 30 μM). d. Wnt3a-induced LRP6 PPPSP phosphorylation was abolished in MEFs that lack Gsk3α and Gsk3β. Gsk3α alleles were deleted via Cre expression in Gsk3β(−/−); Gsk3α(flox/flox) MEFs. β-actin: loading control. e. GSK3β(R96A) phosphorylated LRP6 PPPSP motif, but not β–catenin S33/S37/T41, detected via a phosphorylation-specific antibody7. GSK3βKM phosphorylated neither LRP6 nor β–catenin.
We tested whether the PPPSP kinase might associate with LRP6 and/or Axin, since Axin overexpression enhanced LRP6 PPPSP phosphorylation5. GST-LRP6C, a fusion protein between glutathione-S transferase (GST) and the LRP6 cytoplasmic domain, and Axin each coprecipitated from 293T cell extracts an associated PPPSP kinase (Supplementary Figs. 1 and 2). We suspected a role of GSK3. First, we isolated GSK3α in a yeast two-hybrid screen using LRP6C as bait and confirmed this interaction in yeast (not shown), implying that GSK3 may bind LRP6. Second, GSK3 associates with Axin. Third, GSK3 is a proline-directed kinase that prefers (S/T)P for phosphorylation, and was predicted by Scansite 2.0 (ref. 15) to phosphorylate the PPPSP motif. Finally, GSK3 overexpression promotes LRP5-Axin interaction14, consistent with the possibility that GSK3 may do so via phosphorylating LRP5/6. Indeed, the PPPSP kinase associated with GST-LRP6C or Axin was inhibited in vitro by LiCl or SB216763, two GSK3 inhibitors16,17 (Supplementary Figs. 1 and 2). In vivo GSK3 overexpression promoted LRP6 PPPSP phosphorylation (Fig. 1b) whereas LiCl and SB216763 prevented Wnt-induced LRP6 PPPSP phosphorylation (Fig. 1c). Importantly, Wnt-induced LRP6 phosphorylation was abolished in mouse embryo fibroblasts (MEFs) that harbour genetic deletions of Gsk3α and Gsk3β genes (Fig. 1d). These data show that GSK3 is involved in LRP6 PPPSP phosphorylation.
GSK3 phosphorylation of many substrates requires a priming kinase17, such as CK1 in the case of β–catenin7-9. None of the S/T residues in LRP6 PPPSP motifs and flanking regions (Fig. 1a) conforms to the priming phosphorylation consensus7,17, implying a priming-independent GSK3 phosphorylation. We tested this possibility using GSK3β(R96A), which has an alanine substitution of arginine 96 and is incapable of recognizing priming phosphorylation18-21. GSK3β(R96A) was inactive in β–catenin phosphorylation21, but active in LRP6 PPPSP phosphorylation (Fig. 1e). Therefore GSK3 phosphorylates LRP6 and β–catenin via distinct mechanisms.
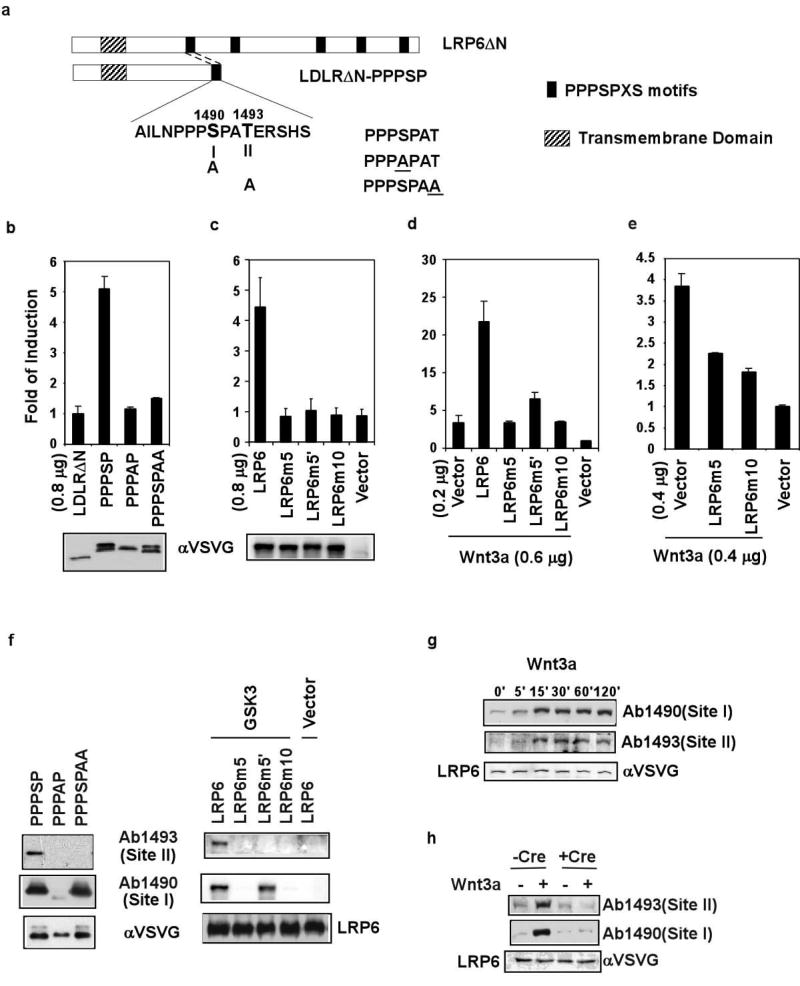
A single PPPSP motif upon transfer to a truncated LDL receptor (LDLRΔN) is sufficient to activate the Wnt pathway5. During characterization of this motif, we noticed a second S/T (site II) three amino acid residues downstream of the PPPSP phosphorylation site (site I, Fig. 2a). Site II is present in all PPPSP motifs (Fig. 1a) and conserved in Drosophila Arrow4, the ortholog of LRP5/6, and was included in the flanking residues in the LDLRΔN-PPPSP construct5 (Fig. 2a). Replacing the site II threonine to alanine in LDLRΔN-PPPSP, like replacing the site I serine to alanine, diminished its signalling activity in a TCF/β-catenin reporter assay (Fig. 2b). We demonstrated the importance of site II S/T residues in the full length LRP6. LRP6m5′, which harbours alanine substitution of all five site II S/T residues, was much less active than LRP6, alone or in synergy with Wnt3a, in the TCF/β-catenin reporter assay (Fig. 2c and 2d). LRP6m5 and LRP6m10, which have alanine substitution of five site I S/T residues5 and of five site I plus five site II S/T residues, respectively, were inactive alone or in synergy with Wnt3a (Fig. 2d). When expressed at higher amounts they each behaved as dominant negative mutants and suppressed Wnt3a signalling (Fig. 2e). Therefore site I is essential for, and site II augments, Wnt/LRP6 signalling.
Figure 2. Sequential phosphorylation of the PPPSPxS motif is induced by Wnt3a and required for LRP6 signalling.
a. Scheme of five PPPSPxS motifs in LRP6 and of LDLRΔN-PPPSP. Site I, site II, and their Alanine substitutions are illustrated. b-e. TCF/β–catenin reporter assays. b, c. Alanine substitution of site II or site I diminished the activity of LDLRΔN-PPPSP (b) and LRP6 (c). Protein expression level was examined via the VSVG tag. The site II mutation did not affect the mobility shift5, indicating normal site I phosphorylation (b). d, e. LRP6m5 and LRP6m10 did not synergize with Wnt3a (d) but inhibited signalling by Wnt3a (e) or by Wnt8 in Xenopus embryos5 (not shown). LRP6m5′ had diminished ability to synergize with Wnt3a (d) but did not inhibit Wnt3a signalling. f. Sequential phosphorylation of site I and site II in LDLRΔN-PPPSP and LRP6 (see Supplementary Fig. 4). Left: LDLRΔN-PPPSP/derivatives. Ab1490 and Ab1493 specifically recognized the phosphorylated/slower-migrating band. Right: GSK3β promoted LRP6 phosphorylation at site I and site II. g, h. LRP6 phosphorylation at site I and site II was induced by Wnt3a CM in MEFs (g), but not in MEFs lacking Gsk3α and Gsk3β (h).
Interestingly, site II, like site I, was also phosphorylated (Fig. 2f and 2g), as detected via a phospho-specific antibody (Ab1493, for phosphorylated T1493 in LRP6) (Supplementary Fig. 3). LDLRΔN-PPPSP, which is constitutively active, showed constitutive phosphorylation at site I and site II (Fig. 2f). Importantly, Wnt3a induced rapid LRP6 phosphorylation at site I and site II (Fig. 2g). Thus phosphorylation of the extended PPPSPxS motif correlates fully with Wnt/LRP6 signalling.
In both LDLRΔN-PPPSP and LRP6, site I mutation abolished phosphorylation at both site I and site II, whereas site II mutation had no effect on site I phosphorylation (Fig. 2f). The simplest explanation is that GSK3 phosphorylation of site I primes and is required for site II phosphorylation. Indeed, GSK3 overexpression increased LRP6 phosphorylation at site I and site II (Fig. 2f), whereas a lack of Gsk3 prevented Wnt-induced LRP6 phosphorylation at either site I or site II (Fig. 2h).
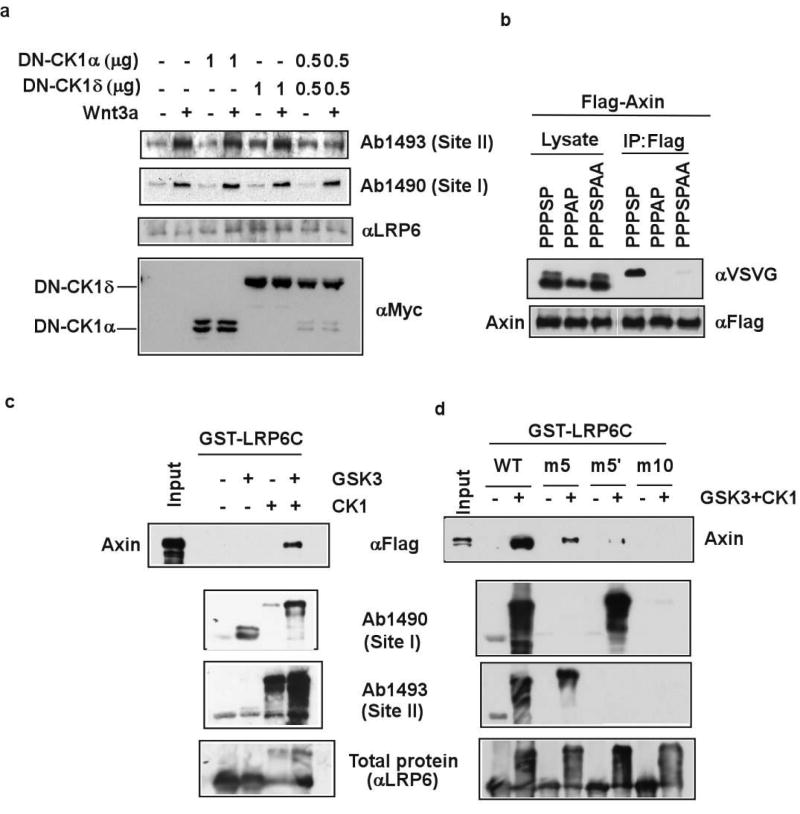
We considered the site II kinase distinct from GSK3 because the order and spacing between site II and site I phosphorylation are not what GSK3 requires. In fact site II resembles CK1 phosphorylation consensus (S/T)*xx(S/T), in which (S/T)* is a priming phosphorylated residue and xx is any two-amino acid combination22. Seven mammalian CK1 members, α, α2, δ, ε, γ1, γ2, and γ3, exist. CK1α, δ and ε have been implicated in Wnt/β–catenin signalling with apparently opposing roles: CK1α is a priming kinase for β–catenin phosphorylation/degradation7, whereas CK1δ and ε activate β–catenin signaling23,24 (also see ref. 8) via mechanisms that are to be fully understood. CK1γ3 overexpression can also stimulate, modestly, Wnt/β–catenin signalling25. Because of the multiplicity of the CK1 family, we employed specific dominant negative (DN) CK1 mutants, DN-CK1α and DN-CK1δ. DN-CK1α inhibits CK1α but not CK1δ/ε, whereas DN-CK1δ inhibits CK1δ/ε but not CK1α (S. L. and X. H., in preparation). DN-CK1α plus DN-CK1δ, but neither alone, prevented Wnt-induced site II, but not site I, phosphorylation (Fig. 3a). Thus CK1 likely phosphorylates site II following the priming site I phosphorylation by GSK3, and CK1α (and CK1α2) and CK1δ/ε may have redundant roles in the process.
Figure 3. Site I and site II phosphorylation by GSK3 and CK1 promotes LRP6 recruitment of Axin.
a. DN-CK1α plus DN-CK1δ, but neither alone, prevented Wnt3a-induced site II, but not site I, phosphorylation. Ck1ε-/- MEFs (Supplementary Fig. 5) were used. b. Alanine substitution at site I or site II in the PPPSPxS motif prevented Axin-binding. Axin was co-expressed with LDLRΔN-PPPSP or mutants. Axin co-precipitated the phosphorylated motif, but neither the unphosphorylated one (fast migrating) nor the site I or site II mutant, which had normal site I phosphorylation. c. GST-LRP6C binding to Axin in vitro required phosphorylation by recombinant GSK3β plus CK1ε. Phosphorylated GST-LRP6C was examined for phosphorylation and used to precipitate cell extracts expressing Flag-tagged Axin. d. Axin binding to phosphorylated GST-LRP6C required site I and site II. Axin exhibited much diminished binding to GST-LRP6Cm5, GST-LRP6Cm5′, or GST-LRP6Cm10.
We showed that Axin preferentially binds to phosphorylated PPPSP motif in LDLRΔN-PPPSP and LRP6 (ref. 5), which are also phosphorylated at site II (Fig. 2f and 2g). The site II mutation diminished, whereas the site I mutation abolished, Axin binding to the PPPSPxS motif (Fig. 3b). Thus phosphorylation at site I and site II is required for Axin recognition in vivo. We reconstituted GST-LRP6C phosphorylation in vitro via recombinant GSK3 and/or CK1 (Fig. 3c). GSK3 and CK1 phosphorylated site I and site II, respectively, and GSK3 phosphorylation of site I enhanced CK1 phosphorylation of site II (Fig. 3c). Remarkably, GST-LRP6C phosphorylated by GSK3 plus CK1, but by neither GSK3 nor CK1 alone, exhibited strong Axin-binding capability (Fig. 3c). This Axin-binding required the intact PPPSPxS motifs, because GST-LRP6Cm5 (site I mutated in all five motifs), GST-LRP6Cm5′(site II mutated in all five motifs), and GST-LRP6Cm10 (site I plus site II mutated in all five motifs) were defective in Axin interaction (Fig. 3d). Therefore phosphorylation of the PPPSPxS motif by GSK3 and CK1 is essential for LRP6 recruitment of Axin.
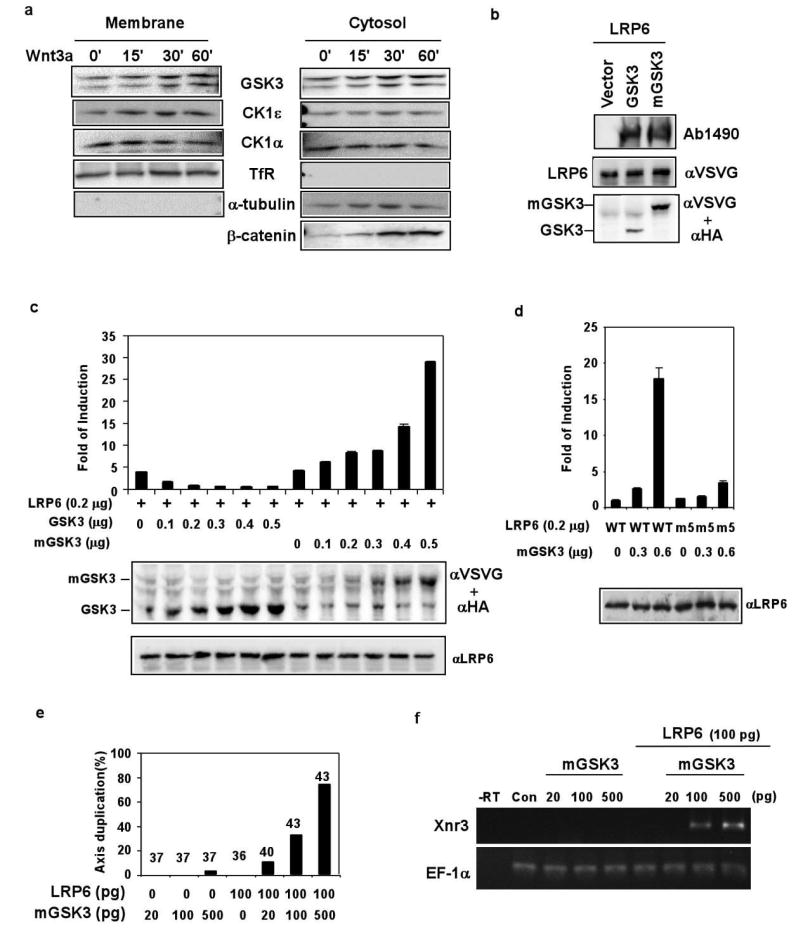
CK1 phosphorylation of LRP6 offers a mechanism to account, in part, for a documented activating role of CK1 in Wnt/β–catenin signalling. However, our results that GSK3 is required for LRP6 phosphorylation/activation depart drastically from the dogma that GSK3 is solely an inhibitor of Wnt signalling via its role in β–catenin phosphorylation/degradation. We hypothesized that different pools of GSK3 have distinct functions in the Wnt pathway. In this scenario, cytosolic GSK3 phosphorylates β–catenin together with CK1 thus antagonizes Wnt/β–catenin signalling, whereas plasma membrane-associated GSK3 phosphorylates LRP6 together with CK1 in response to Wnt stimulation and activates Wnt/β–catenin signalling. We found significant amounts of membrane-associated GSK3 and CK1, which did not change appreciably with/without Wnt stimulation (Fig. 4a). We then generated mGSK3, a GSK3 mutant that targets GSK3 to the plasma membrane via a mini transmembrane unit, LDLRΔN5. mGSK3, like GSK3, stimulated LRP6 phosphorylation (Fig. 4b). However in sharp contrast to GSK3, which antagonized LRP6 signalling, mGSK3 synergized with LRP6 in activating the TCF/β–catenin reporter (Fig. 4c). mGSK3 did not synergize with LRP6m5 (Fig. 4d), thus depended on the PPPSP motif for its stimulatory activity. In Xenopus embryos mGSK3 plus LRP6 induced axis duplication and the expression of Xnr3 (Fig. 4e and 4f), a TCF/β–catenin target. Therefore GSK3 in the proximity of the plasma membrane mediates Wnt/LRP6 signalling via phosphorylating the PPPSP motif.
Figure 4. Membrane-associated GSK3 phosphorylates LRP6 and activates LRP6 and TCF/β-catenin signalling.
a. Membrane-associated GSK3α/β and CK1α/ε is independent of Wnt3a stimulation. 10% GSK3α/β and 20% CK1α/ε were detected in the membrane fraction, which was marked by the transferrin receptor (TfR) and a lack of α-tubulin. Wnt3a stabilized cytosolic β-catenin. b. mGSK3β, like GSK3β, promoted LRP6 PPPSP phosphorylation. mGSK3β (VSVG-tagged) and GSK3β (HA-tagged) were detected via the two antibodies. c, d. TCF/β–catenin reporter assays. mGSK3β activated, whereas GSK3β inhibited, LRP6 signalling (c). mGSK3β did not synergize with LRP6m5; the slight stimulation seen might be due to the endogenous LRP5/6 (d). e, f. mGSK3β synergized with LRP6 to induce axis duplication (e) and Xnr3 expression (f) in Xenopus embryos. RNA doses injected are per embryo. e. The number above each bar indicates embryos injected. f. RT-PCR assay. EF-1α: loading control; -RT: without reverse transcriptase; con: un-injected embryos.
We provided biochemical/genetic/functional evidence that GSK3 and CK1 sequentially phosphorylate PPPSPxS motifs in LRP6 upon Wnt stimulation, and this dual-kinase phosphorylation underlies LRP6 activation via promoting Axin recruitment through phosphorylated LRP6. The critical function of GSK3 in mediating Wnt/β–catenin signalling is likely performed by membrane associated GSK3, which acts to antagonize β-catenin phosphorylation/degradation by cytosolic GSK3. These opposing GSK3 functions in a single pathway are unprecedented, but may be separated spatially and temporally. How Wnt induces LRP6 phosphorylation by GSK3 and CK1 remains unknown. As GSK3 and CK1 exhibit membrane association regardless of Wnt stimulation, Wnt may control the access of GSK3 to LRP6. Or, Wnt may inhibit a phosphatase that counteracts the constitutive LRP6 phosphorylation by GSK3 and CK1. Our study further implies that in Drosophila Arrow is probably activated by GSK3/Zeste-White 3/Shaggy or another GSK3-related protein (see ref. 11, 26, 27).
Sequential phosphorylation of LRP6 and β–catenin mirror each other, i.e., they are regulated by Wnt in the opposite way and employ GSK3 and CK1 as the mutual priming kinase. In both cases however, GSK3 phosphorylation appears to be the main regulatory step while CK1 phosphorylation may be constitutive. Of interest GSK3 employs distinct mechanisms to phosphorylate β-catenin (a primed substrate) and LRP6 (a non-primed substrate). Our findings also uncover further striking parallels between Wnt and Hedgehog (Hh) pathways. In the absence of Hh, protein kinase A (PKA) and CK1 (and GSK3) sequentially phosphorylate transcription factor Ci for processing Ci into a repressor, thus suppress Hh signalling. Upon Hh stimulation, PKA and CK1 sequentially phosphorylate the cytoplasmic domain of the Smoothened receptor, leading to inhibition of Ci phosphorylation thus activation of Hh signaling28-30.
Methods
Plasmids
VSVG-tagged LRP6, LRP6m5, LDLRΔN-PPPSP, and LDLRΔN-PPPAP, Flag-tagged Axin, HA-tagged GSK3β and GSK3βKM in pCS2+ were described5,7. VSVG-tagged LRP6m5′ and LRP6m10 were generated via sequential mutagenesis from VSVG-LRP6 in pCS2+ using the QuickChange Kit (Stratagene). The HA-GSK3β(R96A) was generated by site directed mutagenesis of HA-GSK3β in pCS2+. mGSK3 was generated by replacing the last 11 amino acid (aa) residues of VSVG- tagged LDLRΔN5 with the coding sequence of GSK3β. Myc-tagged DN-CKIα and DN-CKIδ will be described elsewhere (S.L., and X.H., in preparation). GST-LRP6C and GST-LRP6N1 are in pGEX with the GST coding region fused in frame to the entire (aa1397-1613) and a partial (aa1397-1534) cytoplasmic domain of LRP6, respectively. Details of the plasmids are available upon request.
Antibodies
The polyclonal Ab1490 was described5. Polyclonal Ab1493 was generated similarly except that the synthetic phospho-peptide CLNPPPSPAT*ERSH (T*: phospho-T) was used as the immunogen. Other antibodies were used according to manufacturers' instructions: anti-β-catenin (C2206, Sigma), anti-phospho-β–catenin S33/S37/T41 (Cell Signaling), anti-VSVG (V4888, Sigma), anti-α-tubulin (sc8053, Santa Cruz), anti-HA (Sc7392, Santa Cruz), anti-Myc (A14, Santa Cruz), anti-Flag (M2, Sigma), anti-GSK3 (4G-1E, Upstate), anti–transferrin receptor (13-6800, Zymed), anti-CKIε(610445, BD Transduction Lab), anti-CKIα (sc-6477, Santa Cruz).
Cell culture, Wnt induction, transfections and reporter assays
293T cells were used unless MEFs were specifically mentioned. 293T cells or MEFs stably expressing VSVG-LRP6 or Flag-Axin were established via puromycin selection after cotransfection of LRP6 or Axin with the pBABE puro plasmid. These lines have essentially the same Wnt response as parental cells (not shown). MEFs and 293T cells were maintained in D-MEM media supplemented with 10% FBS and 1% Penicillin-Streptomycin-Glutamine. Transfections were carried out with Fugene6 (Roche). Luciferase assays were performed in 293T cells in duplicates as previously described5, using 0.3 μg TCF-luciferase reporter plasmid plus 50 ng Renilla luciferase plasmid (internal control)/per well in the 12-well plate. Each experiment was repeated at least three times. Luciferase activity, presented in fold induction versus the control level, was shown as mean +/− standard deviation. For induction of LRP6 phosphorylation, cells were treated with Wnt3a CM (collected from mouse L cells stably expressing Wnt3a, from ATCC) for 1 hr unless indicated otherwise.
Cre-mediated deletion of Gsk3a alleles in Gsk3β(−/−);Gsk3α(flox/flox) MEFs
Gsk3α(-/-);Gsk3β(-/-) MEFs failed to survive after several passages. Gsk3β(−/−);Gsk3α(flox/flox) MEFs (Supplementary Information) were freshly infected with an adenoviral Cre expression system to delete both alleles of the Gsk3α gene and were maintained for additional 3 days for maximal turnover of the residual Gsk3α protein before each experiment. These MEFs after Cre expression exhibit normal response to EGF, and show high levels of β–catenin protein as expected (not shown). Control MEFs infected with the Cre expression system exhibited normal Wnt3a induction of LRP6 phosphorylation (not shown).
Xenopus laevis embryo manipulations
Synthetic RNAs were transcribed in vitro using MessageMachine (Ambion), and were injected in the animal region at 2-cell stage for Xnr3 induction in animal pole explants or ventrally at 4-cell stage for axis duplication. Procedures for embryo staging, injection and animal pole explants were described3.
Supplementary Material
Supplemental Methods
Supplemental Figure 1
Supplemental Figure 2
Supplemental Figure 3
Supplemental Figure 4
Supplemental Figure 5
Acknowledgments
We thank M. Semenov and B. MacDonald for suggestions/discussion. This work is supported by grants from NIH to X. H., who is a W. M. Keck Foundation Distinguished Young Scholar and a Leukemia and Lymphoma Society Scholar. X.Z., K.T., H.H., and R. H. are/were in part supported by postdoctoral fellowships from the Children's Hospital Boston, Uehara Memorial Foundation (Japan), and CIHR (Canada), and a training grant from NIH, respectively. B.D. and J.W. are supported by CIHR. J.W. is an HHMI International Scholar. H.O. was in part supported by a NIH grant to Anjana Rao.
Footnotes
Competing interests statement
The authors declare that they have no competing financial interests.
References
- 1.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 2.Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–8. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- 3.Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–5. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- 4.Wehrli M, Dougan ST, Caldwell K, O'Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, Tomlinson A, DiNardo S. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature. 2000;407:527–30. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- 5.Tamai K, Zeng X, Liu C, Zhang X, Harada Y, Chang Z, He X. A mechanism for Wnt coreceptor activation. Mol Cell. 2004;13:149–56. doi: 10.1016/s1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- 6.He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131:1663–77. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- 7.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–47. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 8.Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, Mann M, Ben-Neriah Y, Alkalay I. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 2002;16:1066–76. doi: 10.1101/gad.230302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yanagawa S, Matsuda Y, Lee JS, Matsubayashi H, Sese S, Kadowaki T, Ishimoto A. Casein kinase I phosphorylates the Armadillo protein and induces its degradation in Drosophila. EMBO J. 2002;21:1733–42. doi: 10.1093/emboj/21.7.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11:951–61. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 11.Tolwinski NS, Wehrli M, Rives A, Erdeniz N, DiNardo S, Wieschaus E. Wg/Wnt signal can be transmitted through arrow/LRP5,6 and Axin independently of Zw3/Gsk3beta activity. Dev Cell. 2003;4:407–18. doi: 10.1016/s1534-5807(03)00063-7. [DOI] [PubMed] [Google Scholar]
- 12.Cong F, Schweizer L, Varmus H. Wnt signals across the plasma membrane to activate the beta-catenin pathway by forming oligomers containing its receptors, Frizzled and LRP. Development. 2004;131:5103–15. doi: 10.1242/dev.01318. [DOI] [PubMed] [Google Scholar]
- 13.Brennan K, Gonzalez-Sancho JM, Castelo-Soccio LA, Howe LR, Brown AM. Truncated mutants of the putative Wnt receptor LRP6/Arrow can stabilize beta-catenin independently of Frizzled proteins. Oncogene. 2004;23:4873–84. doi: 10.1038/sj.onc.1207642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mao J, Wang J, Liu B, Pan W, Farr GH, 3rd, Flynn C, Yuan H, Takada S, Kimelman D, Li L, Wu D. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell. 2001;7:801–9. doi: 10.1016/s1097-2765(01)00224-6. [DOI] [PubMed] [Google Scholar]
- 15.Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: Proteome-wide prediction of cell signaling interactions using short sequence motifs. Nuc Acids Res. 2003;31:3635–41. doi: 10.1093/nar/gkg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ, Rausch OL, Murphy GJ, Carter PS, Roxbee Cox L, Mills D, Brown MJ, Haigh D, Ward RW, Smith DG, Murray KJ, Reith AD, Holder JC. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol. 2000;7:793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- 17.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–86. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frame S, Cohen P, Biondi RM. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol Cell. 2001;7:1321–7. doi: 10.1016/s1097-2765(01)00253-2. [DOI] [PubMed] [Google Scholar]
- 19.Dajani R, Fraser E, Roe SM, Young N, Good V, Dale TC, Pearl LH. Crystal structure of glycogen synthase kinase 3 beta: structural basis for phosphate-primed substrate specificity and autoinhibition. Cell. 2001;105:721–32. doi: 10.1016/s0092-8674(01)00374-9. [DOI] [PubMed] [Google Scholar]
- 20.ter Haar E, Coll JT, Austen DA, Hsiao HM, Swenson L, Jain J. Structure of GSK3beta reveals a primed phosphorylation mechanism. Nature Structural Biol. 2001;8:593–6. doi: 10.1038/89624. [DOI] [PubMed] [Google Scholar]
- 21.Hagen T, Di Daniel E, Culbert AA, Reith AD. Expression and characterization of GSK-3 mutants and their effect on beta-catenin phosphorylation in intact cells. J Biol Chem. 2002;277:23330–5. doi: 10.1074/jbc.M201364200. [DOI] [PubMed] [Google Scholar]
- 22.Marin O, Bustos VH, Cesaro L, Meggio F, Pagano MA, Antonelli M, Allende CC, Pinna LA, Allende JE. A noncanonical sequence phosphorylated by casein kinase 1 in beta-catenin may play a role in casein kinase 1 targeting of important signaling proteins. Proc Natl Acad Sci USA. 2003;100:10193–200. doi: 10.1073/pnas.1733909100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peters JM, McKay RM, McKay JP, Graff JM. Casein kinase I transduces Wnt signals. Nature. 1999;401:345–50. doi: 10.1038/43830. [DOI] [PubMed] [Google Scholar]
- 24.Sakanaka C, Leong P, Xu L, Harrison SD, Williams LT. Casein kinase iepsilon in the wnt pathway: regulation of beta-catenin function. Proc Natl Acad Sci USA. 1999;96:12548–52. doi: 10.1073/pnas.96.22.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKay RM, Peters JM, Graff JM. The casein kinase I family in Wnt signaling. Dev Biol. 2001;235:388–96. doi: 10.1006/dbio.2001.0308. [DOI] [PubMed] [Google Scholar]
- 26.Cliffe A, Hamada F, Bienz M. A Role of Dishevelled in Relocating Axin to the Plasma Membrane during Wingless Signaling. Curr Biol. 2003;13:960–6. doi: 10.1016/s0960-9822(03)00370-1. [DOI] [PubMed] [Google Scholar]
- 27.Ruel L, Pantesco V, Lutz Y, Simpson P, Bourouis M. Functional significance of a family of protein kinases encoded at the shaggy locus in Drosophila. EMBO J. 1993;12:1657–69. doi: 10.1002/j.1460-2075.1993.tb05811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia J, Tong C, Wang B, Luo L, Jiang J. Hedgehog signalling activity of Smoothened requires phosphorylation by protein kinase A and casein kinase I. Nature. 2004;432:1045–50. doi: 10.1038/nature03179. [DOI] [PubMed] [Google Scholar]
- 29.Zhang C, Williams EH, Guo Y, Lum L, Beachy PA. Extensive phosphorylation of Smoothened in Hedgehog pathway activation. Proc Natl Acad Sci USA. 2004;101:17900–7. doi: 10.1073/pnas.0408093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Apionishev S, Katanayeva NM, Marks SA, Kalderon D, Tomlinson A. Drosophila Smoothened phosphorylation sites essential for Hedgehog signal transduction. Nature Cell Bio. 2005;7:86–92. doi: 10.1038/ncb1210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Methods
Supplemental Figure 1
Supplemental Figure 2
Supplemental Figure 3
Supplemental Figure 4
Supplemental Figure 5