Identification of a radio-resistant and cycling dermal dendritic cell population in mice and men (original) (raw)
Abstract
In this study, we explored dermal dendritic cell (DC) homeostasis in mice and humans both in the steady state and after hematopoietic cell transplantation. We discovered that dermal DCs proliferate in situ in mice and human quiescent dermis. In parabiotic mice with separate organs but shared blood circulation, the majority of dermal DCs failed to be replaced by circulating precursors for >6 mo. In lethally irradiated mice injected with donor congenic bone marrow (BM) cells, a subset of recipient DCs remained in the dermis and proliferated locally throughout life. Consistent with these findings, a large proportion of recipient dermal DCs remained in patients' skin after allogeneic hematopoietic cell transplantation, despite complete donor BM chimerism. Collectively, our results oppose the traditional view that DCs are nondividing terminally differentiated cells maintained by circulating precursors and support the new paradigm that tissue DCs have local proliferative properties that control their homeostasis in the steady state. Given the role of residual host tissue DCs in transplant immune reactions, these results suggest that dermal DC homeostasis may contribute to the development of cutaneous graft-versus-host disease in clinical transplantation.
The skin represents an important interface between the external environment and the internal tissues, and one of its major roles is to provide immune function at this critical site (1). The most superficial layer of the skin, the epidermis, provides the first immune barrier against foreign invasion. The dermis, separated from the epidermis by the basement membrane, supports the vascular network that supplies the avascular epidermis with nutrients (1). Immune cells are distributed on both sides of the basement membrane and participate in the defense against pathogens through professional antigen-presenting cells. These include DCs in the epidermis (2), also called Langerhans cells (LCs), and DCs in the dermis (3–5). Dermal immune cells include dermal DCs, macrophages, mast cells, and T lymphocytes. Rare B lymphocytes and NK cells can also be found in the dermis, whereas plasmacytoid DCs and neutrophils are rarely present in the absence of cutaneous inflammatory reactions (6–8).
Dermal DCs can be distinguished from LCs by the absence of Langerin expression (4–7, 9) and from macrophages by their expression of MHC class II, CD11c, and CD205, and absence or poor expression of mMGL and cytoplasmic phagolysosomes. LCs and dermal DCs are both well equipped to capture environmental antigens, migrate to the draining lymph nodes, and initiate specific T cell immune responses playing a critical role in skin immunity (1, 10, 11). Because of their accessibility, LCs have been the most extensively studied DC population in the skin. In contrast, dermal DCs are more difficult to isolate and have been often overlooked in studies of skin immunity.
Recent studies demonstrating the critical role of dermal DCs in cutaneous immune responses have revived the interest in these cells. Indeed, two elegant murine models of inducible in vivo LC ablation showed that contact hypersensitivity can occur in the absence of LCs (12, 13). These results are consistent with previous results showing that contact hypersensitivity can develop in mice in the absence of the epidermis but is abolished if both the epidermis and the dermis are absent (14). Another recent study (15) using a constitutive in vivo LC ablation strategy found that contact hypersensitivity is amplified in the absence of LCs, further emphasizing the importance of dermal DCs in cutaneous immune responses. Consistent with these data, earlier studies on cutaneous DC turnover have previously noted that the considerable flux of DCs observed in skin-afferent lymphatics (16) contrasts with the slower turnover of epidermal LCs (17–19), suggesting that the majority of cutaneous DCs en route to the lymph nodes may not derive from epidermal LCs, but rather from the dermal DC population. Furthermore, recent studies using specific dyes to follow cutaneous DC migration to the draining lymph nodes after sensitization of the skin have revealed that dermal DCs leave the skin before LCs and segregate in separate areas of the draining lymph nodes (13). Collectively, these results emphasize the critical role of dermal DCs in cutaneous immunity and suggest the need for a better understanding and analysis of this cell population. We recently discovered that LCs are maintained by local radio-resistant precursors under steady-state conditions and are replaced by circulating precursors only during major skin injuries (19). In contrast, interstitial DCs present in peripheral nonlymphoid and vascularized tissues, such as kidney and liver (19), derived mostly from radio-sensitive precursors. We also demonstrated that recruitment of circulating BM-derived LC precursors during skin injury is a regulated process that depends on a cascade of inflammatory chemokines (19).
Regulation of DC homeostasis through local radio-resistant precursors has important implications in allogeneic hematopoietic cell transplantation (allo-HCT). Indeed, we and others have shown that the elimination of residual host DCs before donor T cell injection improves graft-versus-host disease (GVHD) and survival of recipient mice after allogeneic BM transplant (20–26). Our finding that epidermal LCs survive lethal doses of irradiation (24) suggests that conditioning regimens containing a radiation component may not be sufficient to eliminate host tissue DCs, and that novel therapies may be required to reduce residual allogeneic stimuli and improve GVHD outcome.
Skin, gut, and liver are the main tissues targeted by GVHD, with the skin being the most frequently affected organ (27). The recent emphasis on the critical role of dermal DCs in skin immunity suggests that in addition to LCs, dermal DC homeostasis can also affect cutaneous GVHD in clinical transplantation.
RESULTS
Characterization of murine dermal DCs
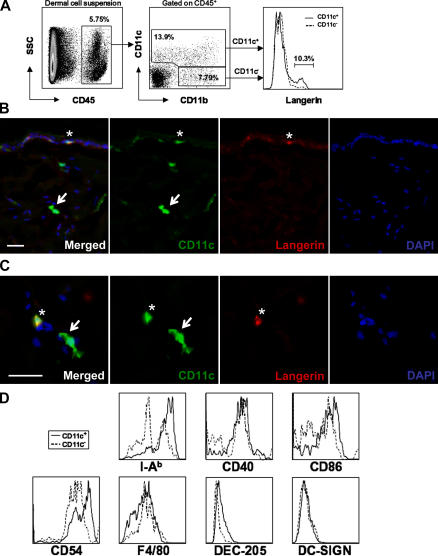
In this study, we used the hematopoietic marker CD45 and the integrin molecules CD11c and CD11b to identify dermal antigen-presenting cells in mice. In total dermal cell suspension, CD45+ hematopoietic cells could be divided into two populations based on CD11c and CD11b expression. These include CD11c+ and CD11c− CD11b+ cells (Fig. 1 A). The majority of CD45+ CD11c+ dermal cells was positive for CD11b and F4/80 (Fig. 1, A and D) and displayed phenotypic and morphological DC features (Fig. 1). In particular, CD11c+ cells expressed high levels MHC class II and were positive for CD205, CD86, and CD40 (Fig. 1 D), but negative for CD4, CD8, and B220 (not depicted). Consistent with previous studies (7), few dermal CD11c+ cells were positive for mMGL and a small fraction (10%) of CD11c+ CD11b+ dermal cells expressed the LC-specific marker langerin (9), likely corresponding to migrating LCs en route to draining lymph nodes (Fig. 1, B and C). Unlike human dermal DCs (8), murine CD11c+ cells did not express the murine analogue of human DC-SIGN (28), nor did any other murine dermal hematopoietic cell (Fig. 1 D). In contrast to CD11c+ dermal cells, CD11c− CD11b+ dermal cells were negative for CD205, expressed lower levels of MHC class II and costimulatory molecules, and were positive for F4/80 and mMGL. This subset most likely represents dermal macrophages (Fig. 1 D). Therefore, dermal CD45+ CD11c+ langerin− cells will be referred to as dermal DCs, whereas dermal CD45+ CD11c− CD11b+ cells will be referred to as dermal macrophages.
Figure 1.
Phenotype of murine dermal DCs. (A) A dermal cell suspension from naive C57BL/6 mice was stained with anti-CD45, CD11c, CD11b, and langerin mAbs and analyzed by flow cytometry. Dot plots show the percentage of dermal CD45+ cells positive for CD11c and CD11b, whereas the overlaid histogram demonstrates that only 10% of dermal CD45+ CD11c+ cells express langerin, likely corresponding to migrating LCs. (B and C) Back skin cross sections isolated from transgenic C57BL/6 mice expressing an enhanced yellow fluorescence protein reporter under the control of the CD11c promoter were stained with anti-langerin mAb. Nuclei were counterstained with DAPI. Image of Cy3 (langerin, red), YFP (CD11c, green), and DAPI (blue) channels show CD11c+ langerin− dermal DCs (arrows) and CD11c+ langerin+ LCs (asterisks). Bars, 10 μm. (D) Dermal cell suspensions were prepared as in A. Overlaid histograms show expression levels of I-Ab, CD40, CD86, CD54, F4/80, DEC-205, and DC-SIGN by CD45+ CD11c+ (solid line) and CD45+ CD11c− CD11b+ (dotted line) dermal cell subsets.
Turnover of dermal DCs under steady-state conditions
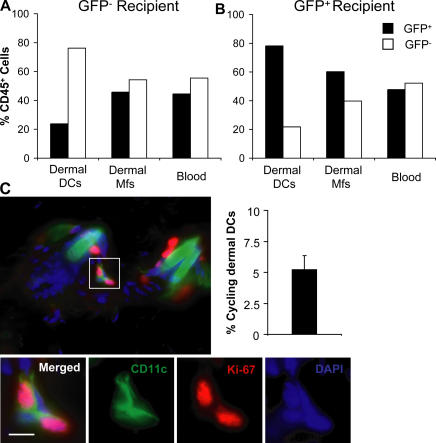
To explore the turnover of dermal DCs under steady-state conditions, we used parabiotic mice in which WT C57BL/6 mice were paired with GFP transgenic C57BL/6 mice so that they shared a single blood circulation but separate organs for prolonged periods of time. In these mice, GFP expression was used to trace the origin of the cells in each recipient. Each parabiont had complete mixing of GFP+ and GFP− leukocytes in the blood at the time of analysis (Fig. 2, A and B). In the skin of parabiotic mice, the mixing of macrophages was almost complete (45% donor cells) at 6 mo after parabiosis (Fig. 2, A and B). In contrast, dermal DCs failed to equilibrate in parabiotic partners up to 6 mo after parabiosis, with 80% of dermal DCs remaining of host origin in each partner (Fig. 2, A and B). These results suggest that, in contrast to dermal macrophages, a large subset of dermal DCs in quiescent skin is maintained independently of circulating precursors.
Figure 2.
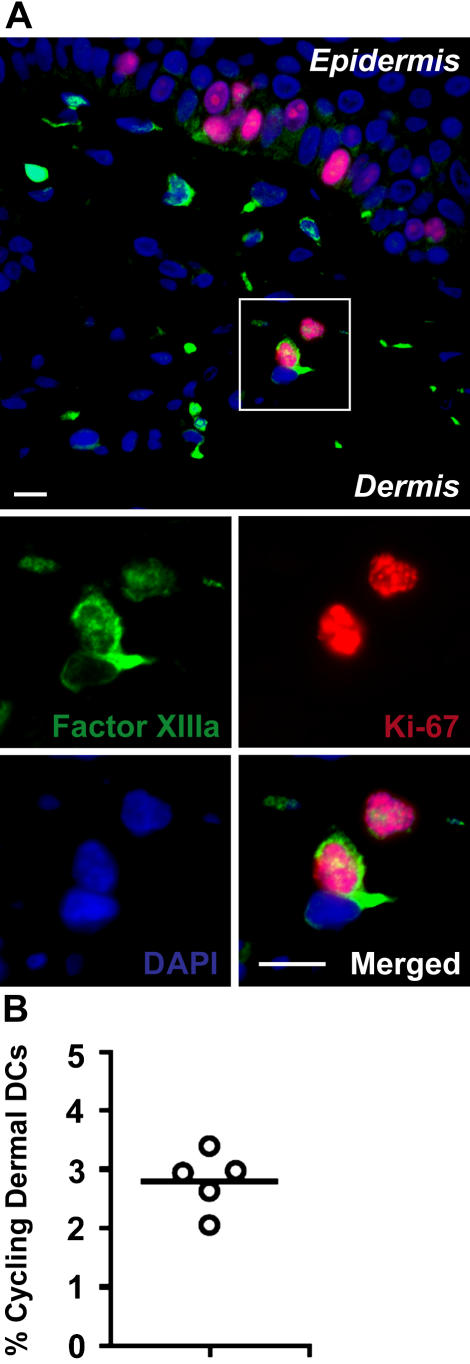
Homeostasis of dermal DCs in quiescent skin. (A and B) Dermal DC turnover in parabiotic mice. Each parabiotic pair consisted of one WT and one GFP+ transgenic mouse (both on a C57BL/6 background) sharing the same blood circulation. Chimerism of blood leukocytes and dermal DCs was analyzed at 6 mo after initiation of parabiosis. Bar graphs show the percentage of GFP+ (black bars) and GFP− (white bars) cells among CD45+ langerin− CD11c+ dermal DCs, CD45+ langerin− CD11c− CD11b+ dermal macrophages, and total blood leukocytes in the GFP− (A) and GFP+ (B) partner. One representative experiment out of two is shown. (C) A proportion of dermal DCs renews locally in quiescent skin. Back skin cross sections isolated from naive C57BL/6 mice were stained with a mAb against CD11c and the cell cycle protein Ki-67 and counterstained with DAPI. Overlaid images of Cy2 (CD11c, green), Cy3 (Ki-67, red), and DAPI (blue) channels are displayed and show that dermal CD11c+ cells coexpress the cell cycle protein Ki-67 in the dermis. Bars, 10 μm. Bar graph shows the percentage of Ki-67+ cells among total dermal DCs in three separate animals. An average of 150 dermal DCs was analyzed in each skin sample.
Dermal DCs proliferate locally in quiescent skin
To examine if dermal DC homeostasis relies on proliferative precursors in the skin, we searched for cycling dermal DCs in quiescent dermis. To detect these cells, we used the expression of Ki-67 antigen, a nuclear protein with a half-life of 60–90 min (29) expressed in late G1 and maintained through the S, G2, and M phase of the cell cycle (29–31). Thus, Ki-67 expression by dermal DCs should reflect an active proliferation state. As shown in Fig. 2 C, up to 5% of CD11c+ dermal DCs expressed Ki-67, whereas Ki-67+ langerin+ LCs were present exclusively in the epidermis (not depicted). These data suggest that local dermal DC proliferation plays an important role in dermal DC homeostasis in the steady state.
Turnover of dermal DCs after congenic BM transplantation
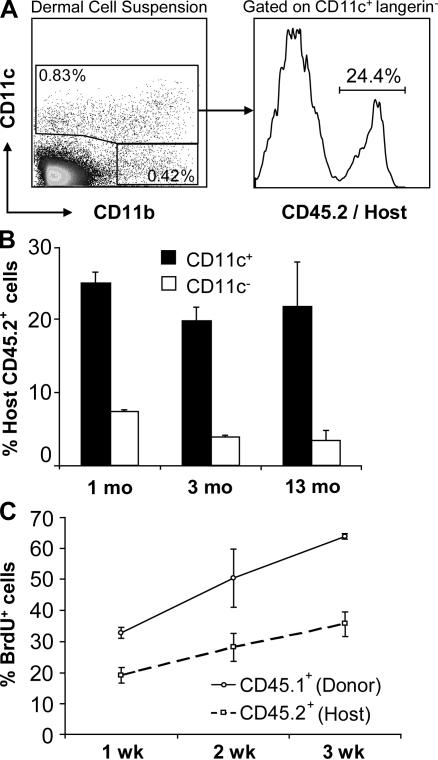
The results above demonstrate that in the absence of skin injury, interstitial dermal DCs are maintained through local proliferation with some participation of blood-derived precursors. Although blood-derived circulating precursors are likely to be sensitive to irradiation, we sought to explore the effect of a lethal conditioning regimen on local dermal DC proliferation. We reconstituted lethally irradiated CD45.2+ C57BL/6 mice with BM cells isolated from congenic CD45.1+ C57BL/6 donor mice and used expression of CD45 alleles to trace the origin of dermal DCs. 1 yr after reconstitution, 25% of total dermal DCs remained of host origin, whereas the majority of dermal macrophages was replaced by donor cells between 1 and 3 mo (Fig. 3, A and B), suggesting that a subset of dermal DCs is maintained by local radio-resistant precursors. To examine whether residual host dermal DCs corresponded to postmitotic long-lived cells or rather were actively proliferating in situ, we tested their ability to incorporate BrdU locally. We administered BrdU to lethally irradiated mice, which were reconstituted with congenic CD45.1+ BM cells 8 wk earlier, and followed BrdU incorporation in residual host (CD45.2+) dermal DCs and donor (CD45.1+) dermal DCs. As shown in Fig. 3 C, 3 wk after BrdU administration, 30% of residual host CD45.2+ dermal DCs incorporated BrdU in chimeric animals. At the time of BrdU administration, all mice had reached full donor (CD45.1) BM and blood chimerism; therefore, BrdU labeling of residual CD45.2+ dermal DCs must have been occurring in the skin. Because BrdU incorporation in the DNA occurs only in S phase, these results establish that a subset of dermal DCs derives from local radio-resistant proliferative cells.
Figure 3.
Homeostasis of dermal DCs after BM transplantation. (A and B) Dermal DC chimerism after congenic BM transplantation. Lethally irradiated 8-wk-old CD45.2+ C57BL/6 mice were reconstituted i.v. with BM cells isolated from congenic CD45.1+ C57BL/6 donor mice. Dermal cell suspensions were isolated at different time points after transplantation and stained with anti-CD45.1, CD45.2, CD11c, CD11b, and langerin antibodies. (A) Dot plot and histogram show the percentage host (CD45.2+) dermal DCs among total CD11c+ langerin− dermal DCs. (B) Bar graphs show the percentage of host CD45.2+ dermal CD11c+ (black bars) and CD11c− CD11b+ (white bars) cells in the dermis of chimeric mice at 1, 3, and 13 mo after BM transplantation. Results represent the mean of four independent experiments. (C) Residual host dermal DCs renew in the skin after BM transplantation. CD45.2+ mice were lethally irradiated and reconstituted with CD45.1+ BM cells. 6 wk after BM transplantation, chimeric mice received BrdU in their drinking water for 3 wk. Graphs show the percentage BrdU+ cells among gated donor CD45.1+ CD11c+ and host CD45.2+ CD11c+ cells in chimeric animals at different time points after BrdU administration. Each data point summarizes the results of three independent experiments.
Homeostasis of dermal DCs in inflamed skin
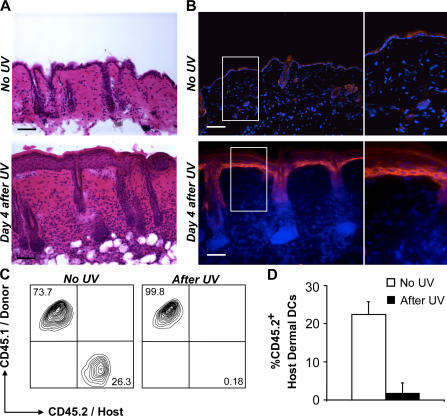
To examine the fate of radio-resistant skin-derived dermal DCs in response to a skin injury, we reconstituted lethally irradiated CD45.2+ mice with CD45.1+ BM and followed the turnover of skin-derived (CD45.2+) dermal DCs after cutaneous exposure to UV light. 4 d after exposure to UV light, we observed a thickening of the epidermis accompanied with a massive dermal leukocyte infiltration (Fig. 4 A) and secretion of inflammatory chemokines, including CCL-2 (Fig. 4 B) and CCL-7 (not depicted). These inflammatory changes correlated with a strong reduction of CD45.2+ dermal DCs and their replacement by donor-derived CD45.1+ dermal DCs (Fig. 4, C and D), suggesting that cutaneous inflammation leads to elimination of local dermal DCs and their replacement by blood-derived dermal DC precursors.
Figure 4.
Homeostasis of dermal DCs in inflamed skin. (A and B) UV-induced inflammatory changes in the skin. Backs of C57BL/6 mice were shaved (1cm2) and exposed to UV light. 4 d later, mice were killed to isolate the shaved back skin. (A) Hematoxylin and eosin–stained skin cross sections show large inflammatory infiltrates in mice exposed to UV light 4 d earlier (bottom) compared with unexposed skin (top). Bars, 100 μm. (B) Back skin cross sections (shown in A) were stained with anti-CCL2 mAb. Nuclei were counterstained with DAPI. Overlaid image of Cy3 (CCL2, red) and DAPI (blue) channels is displayed. Bars, 10 μm. (C and D) Exposure to UV light eliminates skin-resident dermal DCs. C57BL/6 CD45.2+ mice were lethally irradiated and reconstituted with CD45.1+ BM. 1 mo after BM transplantation, mice were exposed to UV light or left untreated. (C and D) Dot plots (C) and bar graph (D) show the percentage of host (CD45.2+) or donor (CD45.1+) cells among total CD11c+ dermal DCs in control animals or 6 wk after exposure to UV light. One representative experiment out of five is shown in C. In D, each bar is the result of five independent experiments.
Repopulation of dermal DCs in inflamed skin requires CCR2, but not CCR6
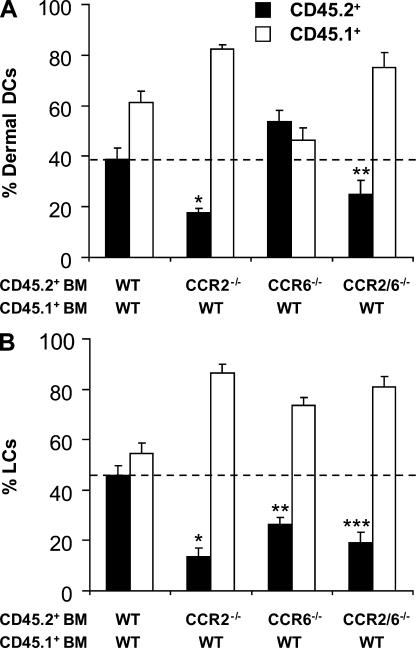
Numerous chemokines are secreted in inflamed skin, including RANTES (a ligand for CCR1 and CCR5; reference 32); MCP-1/CCL2 (a ligand for CCR2; reference 33); MDC/CCL22 and TARC/CCL17 (ligands for CCR4; references 34 and 35); MIG/CCL9, IP10/CCL10, and ITAC/CCL11 (ligands for CXCR3; references 36 and 37); and MIP-3α/CCL20 (the ligand for CCR6; references 38 and 39). In addition, several chemokines are made constitutively in normal skin, including SDF1/CXCL12 (40), CTACK/CCL27 (41), and BRAK/CXCL14 (42). Among these chemokines, we have previously found that CCR2 and CCR6 ligands were critical for LC repopulation in inflamed skin (19, 24). To explore whether CCR2 and CCR6 were also playing a role in the recruitment of dermal DCs to injured skin, we reconstituted lethally irradiated CD45.1+ C57BL/6 mice with a 1:1 mixture of WT CD45.1+ BM and CD45.2+ BM cells that either lacked CCR2 (CCR2−/−), CCR6 (CCR6−/−), or both CCR2 and CCR6 (CCR2/6−/−). 1 mo after reconstitution, we found that CCR2−/−, CCR6−/−, and CCR2/6−/− CD45.2+ and WT CD45.1+ BM gave rise to similar numbers of circulating B cells and neutrophils, suggesting that the absence of CCR2 and CCR6 did not affect BM engraftment (not depicted). In contrast, CCR2−/− and CCR2/6−/− CD45.2+ CD115+ circulating monocytes represented 30–50% of circulating CD45.2+ CCR6−/− or CD45.1+ WT monocytes (not depicted), confirming that the absence of CCR2 expression on hematopoietic progenitors affects monocyte repopulation in the blood (19, 24, 43). 1 mo after BM reconstitution, we exposed chimeric mice to UV light and followed the recruitment of mutant (CD45.2+) and WT (CD45.1+) dermal DCs to inflamed skin. 2 wk after exposure to UV light, CCR2−/− and CCR2/6−/− dermal DCs failed to repopulate inflamed skin (Fig. 5 A), whereas CCR6−/− dermal DCs were not affected (Fig. 5 A). In contrast, both CCR2 and CCR6 were critical for the recruitment of circulating LC precursors, as described previously (Fig. 5 B; references 19 and 44). These differences remained unchanged for at least 16 wk after UV light exposure (not depicted), establishing the essential role of CCR2 for the recruitment of dermal and epidermal DCs to inflamed skin, whereas the role of CCR6 seems to be restricted to the repopulation of epidermal LCs.
Figure 5.
Repopulation of dermal DCs in inflamed skin depends on CCR2, but not CCR6. C57BL/6 CD45.1+ mice were lethally irradiated and reconstituted with a 1:1 mixture of CD45.1+ WT BM and CD45.2+ BM cells isolated from WT animals or animals lacking CCR2 (CCR2−/−), CCR6 (CCR6−/−), or both CCR2 and CCR6 (CCR2/6−/−) as described in Materials and methods. 1 mo after BM transplantation, chimeric mice were exposed to UV light. Bar graphs show the percentage of WT CD45.1+ (white bars) and WT, CCR2−/−, CCR6−/−, or CCR2/CCR6−/− CD45.2+ cells (black bars) among total CD11c+ dermal DCs (A) and epidermal MHC class II+ LCs (B) 3 wk after UV exposure. Each bar summarizes the results of three independent experiments. (A) *, P = 0.0009; **, P = 0.009; (B) *, P = 0.00001; **, P = 0.0002; ***, P = 0.00007.
Human dermal DCs proliferate in situ under steady-state conditions
To determine whether human and murine dermal DC homeostasis are similarly regulated, we first sought to explore whether dermal DCs proliferate locally in human quiescent skin. In humans, dermal DCs are distinguished from LCs based on their expression of Factor XIIIa and DC-SIGN (1, 3). To measure local proliferation of dermal DCs in human skin, we costained paraffin-embedded skin sections, isolated from normal cadavers, with Factor XIIIa mAb to identify dermal DCs together with mAb to the cell cycle protein Ki-67. We chose to use Factor XIIIa mAb to detect dermal DCs because its staining intensity on paraffin sections was much brighter than that obtained with DC-SIGN mAb. We calculated the percentage of total Factor XIIIa+ dermal DCs coexpressing Ki-67 antigens and established that 2–3.5% of dermal DCs were actively cycling in situ (Fig. 6, A and B). These results are consistent with our data in murine skin (Fig. 2 C).
Figure 6.
Human dermal DCs proliferate in situ. Cross sections of paraffin-embedded normal human skin obtained from cadavers were stained with anti–Factor XIIIa and anti–Ki-67 mAbs as described in Materials and methods. Nuclei were counterstained with DAPI. (A) Overlaid images of Cy2 (Factor XIIIa, green), Cy3 (Ki-67, red), and DAPI (blue) channels are displayed. Bars, 10 μm. (B) A dot graph shows the percentage of Factor XIIIa/ Ki-67 double positive cells among total dermal Factor XIIIa+ cells in five separate donors. In each skin sample, an average of 600 Factor XIIIa+ cells was analyzed.
Homeostasis of human dermal DCs after allo-HCT
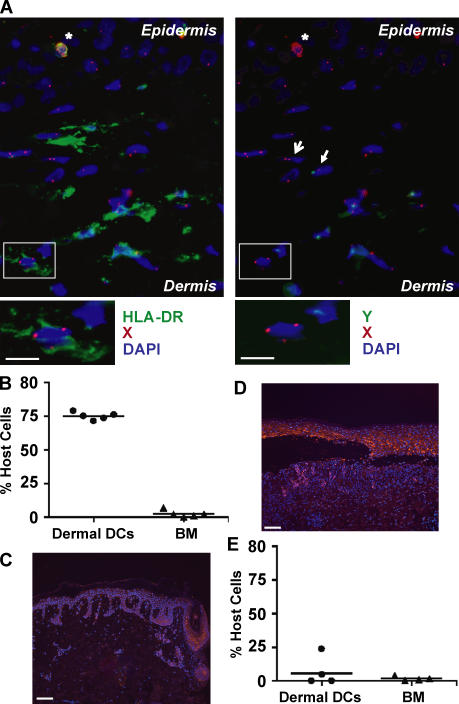
The results above established that a subset of dermal DCs persists in recipient mice for >1 yr despite radiation-conditioning regimen and BM transplantation. Because residual host DCs have been shown to be key initiators of tissue GVHD (20–26) after allo-HCT in mice, persistence of residual host dermal DCs in patients after allo-HCT may have a critical impact in clinical transplantation. Therefore, we sought to examine if host dermal DCs can also resist transplant-conditioning regimen and persist in the skin despite complete donor BM chimerism in patients who receive allo-HCT. We chose to analyze only patients who achieved complete donor BM and peripheral blood leukocyte chimerism, and focused on time points that preceded the occurrence of GVHD because cutaneous GVHD leads to inflammatory changes that could affect dermal DC chimerism. We analyzed only patients who received sex-mismatched allo-HCT and used a dual labeling technique associating fluorescence in situ hybridization (FISH) of X and Y chromosomes and immunostaining of dermal DCs to determine the origin (host vs. donor) of dermal DCs (Fig. 7 A). Dermal DCs were identified as HLA-DR+ langerin− cells because Factor XIIIa and DC-SIGN antigens were inactivated after FISH and could not be used for immunostaining. HLA-DR+ endothelial cells, present in inflamed dermis, were easily excluded based on their morphology, expression of Von Willebrand antigens, and levels of HLA-DR expression (not depicted). Although this technique may present a risk of sampling error, it has the major advantage of allowing analyses of dermal DCs directly in situ, avoiding the manipulation of small skin biopsy samples in vitro. Four out of five patients analyzed in this study received a nonmyeloablative regimen that included total body irradiation (TBI; 2 Gy), antithymocyte globulin (ATG), and fludarabine as described previously (Table I; reference 45), whereas one patient received conventional myeloablative therapy, including busulfan and cyclophosphamide (Table I). All patients received GVHD prophylaxis drugs that included cyclosporine and mycophenolate mofetil, and none of the patients received systemic or topical steroids. Consistent with our previous report (45), all patients achieved >93% myeloid and lymphoid donor BM chimerism by 1 mo after transplant. Compared with circulating hematopoietic cells, 70–75% of dermal DCs remained of host origin in all patients at 30 d after transplant (Fig. 7 B and Table I). These results demonstrate that recipient dermal DCs can survive reduced intensity conditioning for allo-HCT and persist in the skin independently of circulating precursors.
Figure 7.
The fate of human dermal DCs after allo-HCT. Noninflamed skin samples were obtained from patients who underwent reduced intensity (n = 4) or myeloablative conditioning (n = 1) for allo-HCT and did not develop GVHD (see Table I) (A–C). GVHD-affected skin samples were isolated from four patients who underwent myeloablative conditioning for allo-HCT (see Table II) and developed GVHD (D and E). (A) Cross sections of 2-mm skin biopsies obtained 1 mo after allo-HCT were stained with X (Cy3) and Y (Cy2) DNA probes, followed by staining with anti-langerin (Cy3) and HLA-DR (Cy5) antibodies and DAPI as described in Materials and methods. A representative image obtained from a female recipient injected with male hematopoietic cells is shown. Overlaid images of three out of four color channels in indicated combinations (HLA-DR, Y, and DAPI on the left, and X, Y, and DAPI on the right) show persistence of residual host XX+ HLA-DR+ langerin− cell dermal DCs. Langerin+ cells (asterisks) were excluded from the analysis. A female cell (XX; open arrow) and a male cell (XY; stealth arrow) are shown. Bars, 10 μm. (B) Dermal DC chimerism after allo-HCT in noninflamed skin. Dot graphs show the percentage of residual host cells among dermal DCs and total BM nucleated cells in each patient 30 d after allo-HCT. (C and E) CCL2 is highly up-regulated in GVHD-affected skin. Noninflamed skin from patient 1 (Table I) (C) and GVHD-affected skin from patient 1 (Table II) (D) were stained with anti–human CCL-2 mAb (Cy3). Nuclei were counterstained with DAPI. Overlaid images of Cy3 (CCL-2, red) and DAPI (blue) channels are displayed. Displayed images represent MCP-1 expression in one out of five samples in each patient group. Bars, 100 μm. (E) Dermal DC chimerism in GVHD-affected skin. Dot graph shows the percentage of remaining host dermal DCs and BM nucleated cells in patients who developed GVHD.
Table I.
The fate of dermal DCs after allo-HCT in the absence of cutaneous GVHD lesions
| Pt. | Host/donor | Conditioning regimen | Date of skin biopsy/timeafter transplant | No. of dermal DCsanalyzed | % Host dermalDCs | % Host BMcells |
|---|---|---|---|---|---|---|
| 1 | F/M | TBI/Fludarabine/ATG/MM/ Cyclosporine | 30 d | 102 | 73.5 | 2.4 |
| 2 | F/M | TBI/Fludarabine/ATG/MM/Cyclosporine | 30 d | 52 | 78.8 | 0.4 |
| 3 | F/M | TBI/Fludarabine/ATG/MM/Cyclosporine | 30 d | 40 | 75 | 1 |
| 4 | M/F | TBI/Fludarabine/ATG/MM/Cyclosporine | 30 d | 46 | 76.1 | 7 |
| 5 | F/M | Busufan/Cyclosphosphamide/MM/Cyclosporine | 30 d | 87 | 71.3 | 2.4 |
Dermal DC turnover in cutaneous GVHD lesions
We previously demonstrated that in mice, GVHD-like UV-induced cutaneous injuries lead to the elimination of skin-derived LCs and their replacement by circulating LC precursors in a CCR2- and CCR6-dependent manner (24). Consistent with these data, the results above showed that UV-induced cutaneous injuries eliminate skin-derived dermal DCs and induce the secretion of CCR2 ligands required for the recruitment of circulating dermal DC precursors and the establishment of dermal DC chimerism. To explore whether cutaneous GVHD lesions in patients also lead to the secretion of CCR2 ligands and dermal DC chimerism, we examined the skin of four patients who received sex-mismatched allo-HCT and developed cutaneous GVHD lesions (Table II). All patients received myeloablative regimen and developed GVHD 20–60 d after transplant (Table II). We found that the CCR2 ligand CCL2 was barely expressed in the skin of patients with no GVHD lesions (Fig. 7 C), whereas it was strongly up-regulated in the skin of all patients with cutaneous GVHD (Fig. 7 D and not depicted). In addition, residual host dermal DCs were absent and fully replaced by donor-derived dermal DC precursors in cutaneous GVHD lesions (Fig. 7 E), suggesting that inflammatory changes induced by cutaneous GVHD lead to the elimination of host dermal DCs and the recruitment of donor-derived precursors. Our data in mice showing that CCR2 ligands are required for dermal DC chimerism together with CCL2 up-regulation in cutaneous GVHD lesions in patients suggest that CCL2 may also play a role in the recruitment of donor-derived circulating dermal DC precursors after allo-HCT, thereby facilitating dermal DC chimerism.
Table II.
The fate of dermal DCs in cutaneous GVHD lesions
| Pt. | Host/donor | Conditioning regimen | Diagnosis of GVHD/timeafter tranplant | Date of skin biopsy/time after transplant | No. of DCsanalyzed | %Host dermalDCs | %HostBM cells |
|---|---|---|---|---|---|---|---|
| 1 | M/F | TBI/Cyclosphosphamide | 23 d | 40 d | 11 | 0 | 4 |
| 2 | M/F | TBI/Cyclosphosphamide | 30 d | 40 d | 14 | 0 | 0 |
| 3 | M/F | TBI/Cyclosphosphamide | 40 d | 80 d | 42 | 4.8 | 0.3 |
| 4 | F/M | TBI/Cyclosphosphamide | 60 d | 6 mo | 21 | 23.8 | 1 |
DISCUSSION
Our results established that in the absence of cutaneous inflammatory injuries, a proportion of DCs renews locally and independently of circulating precursors in both mouse and human dermis. This was demonstrated in several models. First, we discovered that up to 5% of dermal DCs are actively cycling in quiescent skin in mice. Second, in parabiotic mice with separate organs and shared blood circulation, the majority of dermal DCs failed to equilibrate in recipient mice for up to 6 mo after parabiosis despite the mixing of dermal macrophages and blood leukocytes. Third, in lethally irradiated mice reconstituted with congenic BM hematopoietic precursors, a substantial proportion of dermal DCs remained of host origin for >12 mo after transplant, whereas the majority of dermal macrophages was replaced by donor-circulating BM-derived precursors within 4 wk. Consistent with our results in mice, we found that 2–3% of dermal DCs are actively cycling in human quiescent skin. In addition, in patients who underwent reduced intensity conditioning for allo-HCT, the majority of recipient dermal DCs survived the conditioning regimen and remained in the skin despite full donor BM chimerism.
Parabiotic mice represent powerful tools to explore the physiological turnover of hematopoietic cells. The fact that 6 mo after parabiosis <20% of dermal DCs were recruited from the periphery together with our finding that 2–5% of dermal DCs in mice and humans are actively cycling in situ establish that local proliferation is critical to the maintenance of dermal DC homeostasis in the steady state.
The ability of dermal DCs to proliferate locally is not unique to dermal DC populations, as we have also identified cycling LCs in murine (19) and human skin (unpublished data) and proliferating DCs have also been detected in mice spleen in the steady state (46). We have not been able to identify obvious phenotypic differences between locally proliferating and blood-derived dermal DCs. In particular, none of these populations expressed early hematopoietic precursor markers, including flk2+/flt347, c-kit (47), CD34 (48), and Sca-1 (49; not depicted). Thus, it is possible that all dermal DCs have local proliferative properties or that a committed dermal DC precursor that has taken up residence in the skin controls local dermal DC homeostasis in the steady state. Identification of such a precursor will be the subject of further studies in the laboratory.
Our results also show that a subset of dermal DCs survives lethal dose of irradiation and maintains their proliferation potential. These findings recall our earlier observation that LCs are entirely maintained by local radio-resistant proliferative precursors (19). In contrast, spleen DCs, although able to proliferate locally (46), are eliminated after lethal irradiation (19). Therefore, it is possible that all tissue DC populations possess local proliferation properties but that only specific environments allow DCs to survive radiation injuries. The biological need for local DC proliferation is intriguing. This process may serve to maintain local DC homeostasis by providing a way to replace migratory DCs in the steady state and to repair locally damaged DCs induced by minor injuries. We are currently analyzing whether local proliferation properties extend to other DC populations in peripheral tissues. Collectively, our results oppose the traditional view that DCs are nondividing terminally differentiated cells that derive only from circulating committed precursors (50, 51) and support the new paradigm that tissue DCs have local proliferation properties that control their homeostasis in the steady state.
In contrast to DC homeostasis in noninflamed skin, UV-induced cutaneous injuries lead to the replacement of locally proliferating dermal DCs by circulating dermal DC precursors in a CCR2-dependent, CCR6-independent manner. These results contrast with our earlier findings demonstrating that both CCR2 and CCR6 were required for LC repopulation in inflamed skin (19, 24) and extend the role of CCR2 in the recruitment of leukocytes to inflamed skin to another population of cutaneous DCs. Consistent with our results in mice, expression of the CCR2 ligand CCL2 was strongly up-regulated in human inflamed skin and correlated with the disappearance of locally renewing dermal DCs and their replacement with circulating dermal DC precursors. This data suggests that CCR2 is also important for dermal DC repopulation in human inflamed skin.
Recently, we demonstrated that circulating CCR2+, but not CCR2−, monocytes migrate to inflamed skin and give rise to LCs in vivo (52). It will be interesting to examine whether CCR2+ monocytes are also the precursors for dermal DCs in the same setting. A recent study revealed that CCR2 regulates monocyte egress from the BM rather than regulating its recruitment to inflamed tissues (43). Consistent with this observation, we found that CCR2−/− BM progenitors give rise to reduced numbers of blood monocytes in vivo compared with CCR2+/+ BM progenitors (not depicted). However, induction of local CCR2 ligand gradients in murine and human injured skin is intriguing, and the transfer of purified CCR2−/− blood dermal DC precursors into WT mice exposed to UV light should help to determine whether CCR2 also acts at local cutaneous inflammatory sites.
Recipient DCs have been shown to be critical for the development of GVHD (20–26). Identification of radio-resistant and cycling dermal DCs in the murine dermis and of a large subset of remaining recipient dermal DCs in patients' skin after reduced intensity for allo-HCT shown in this study suggest that dermal DCs may also participate in the development of cutaneous GVHD.
The recent introduction of reduced intensity conditioning regimens (53) in clinical transplantation is likely to impact tissue DC turnover and increase the pool of residual host DCs in several tissues, including tissues like the spleen and the liver, where DCs are maintained by radio-sensitive precursors. A recent study compared the kinetics of LC chimerism in patients who received reduced intensity versus full intensity conditioning for allo-HCT (54). The reduced intensity regimen used a fludarabine and Melphalan combination (54), a regimen more cytotoxic than the one used in our study (45). Results from this study showed that a large pool of LCs survived both reduced and full intensity regimen. In addition, 40 d after transplant, 65% of LCs were still of host origin in patients receiving the reduced intensity regimen, although by day 100, all LCs were of donor origin in both groups (54). It is likely that conversion to donor LC chimerism correlates with the development of GVHD lesions in these patients.
Although we still lack sufficient perspective on the clinical impact of reduced intensity regimens, some studies have reported a delayed onset of acute GVHD in recipients of reduced intensity conditioning compared with patients treated with full intensity conditioning (55, 56). Our results suggest that although reduced intensity conditioning does not lead to DC activation to a level sufficient to induce donor T cell immunity at the time of transplant, residual recipient DCs that persist in these patients may still trigger delayed GVHD symptoms upon the right activating signal (i.e., infection or trauma). Another clinical setting where residual host DCs may be particularly relevant is in recipients of donor lymphocyte infusion (DLI). Early (or preemptive) DLI is used to improve blood chimerism after reduced intensity stem cell transplant (57, 58). These infusions are associated with high risk of acute GVHD (57) especially in the recipient of unrelated donors (58). Exploring ways to eliminate or reduce the pool of residual host DCs is likely to improve GVHD outcome after DLI.
It is important that DC studies after allo-HCT be performed before the onset of acute GVHD because the priming of donor T cells against host tissues would also lead to the elimination of recipient DCs, removing any proof of their participation in this process (24, 54). We have previously shown that although host residual LCs play a role in the development of cutaneous GVHD, they are eliminated once GVHD develops in the skin (24). In addition, the absence of residual host dermal DCs in patients with GVHD lesions found in this study together with results showing that large numbers of host LCs survive the conditioning regimen for allo-HCT but disappear from a patient's skin 40 d later (54) may reflect this process of elimination (27, 59).
Whether recipient DCs play a role in the initiation or as target of a GVHD reaction remains to be determined. These studies are critically needed as they should help to determine the need for novel conditioning therapies aimed at reducing the pool of residual recipient DCs as a means of improving GVHD outcome in clinical transplantation. However, when considering the negative impact of residual recipient DCs in GVHD, it is critical to keep in mind that these cells are likely to participate in the generation of potent antitumor immune responses and that their elimination may also reduce the graft-versus-tumor effect (60). Thus, it is conceivable that therapies targeted to tissues that are most affected by GVHD and where persistence of a large pool of residual recipient DCs has been demonstrated (i.e., the skin) may help improve local tissue damage caused by GVHD without hampering a systemic graft-versus-tumor effect. Targeted cutaneous therapies could include the use of UV light (24, 61), antibodies to cutaneous DCs, or electron beam therapy (62, 63).
MATERIALS AND METHODS
Animals.
5–8-wk-old WT C57BL/6 mice that express the CD45.2 or CD45.1 allele of CD45 were purchased from National Cancer Institute. CCR2−/− and CCR6−/− mice on the C57BL/6 background were generated as described previously (64, 65). C57BL/6 mice lacking CCR2 and CCR6 (CCR2−/−CCR6−/−) were generated by crossing CCR2−/− and CCR6−/− mice and maintained in our animal facility. Transgenic C57BL/6 mice expressing an enhanced yellow fluorescence protein reporter under the control of the CD11c promoter (66) were provided by M. Nussenweig (The Rockefeller University, New York, NY). All animal protocols were approved by the Mount Sinai Medical Center Institutional Animal Care and Use Committee.
Human skin biopsies.
To examine the presence of cycling human dermal DCs in the steady state, normal human split-thickness skin was obtained from the New York Firefighter's Skin Bank from cadavers within 24 h of death. Generally, dermatomes were ∼300-μm thick, including both epidermis and the dermis. To follow the fate of dermal DCs after allo-HCT, five patients who underwent sex-mismatch allo-HCT were included from the BM transplantation clinic affiliated with the Mount Sinai School of Medicine in New York under an institutional review board–approved research protocol. All but one patient underwent nonmyeloablative conditioning regimen that consisted of rabbit ATG (Atgam; Pharmacia-UpJohn), low dose TBI (2 Gy), and fludarabine as described previously (45). All patients received a GVHD prophylaxis drug that includes cyclosporine (6 mg/kg every 12 h) and oral mycophenolate mofetil (15 mg/kg every 12 h; CellCept; reference 45). One patient received a myeloablative regimen, including busulfan and cyclophosphamide. 2-mm skin biopsies were isolated at day 30 after transplant from the posterior iliac crest at the same time as routine BM aspiration to perform BM chimerism. Characteristics of these patients are included in Table I. None of the patients had clinical and histological signs of cutaneous GVHD. To analyze the fate of dermal DCs in inflamed skin after allo-HCT, formalin-fixed and paraffin-embedded biopsies of GVHD-affected skin from patients who received myeloablative sex-mismatch allo-HCT were obtained from the department of pathology at Mount Sinai School of Medicine. The diagnosis and scoring of acute GVHD was performed by a dermatopathologist. Characteristics of these patients are included in Table II.
Cell media.
Complete medium was prepared with RPMI (Cellgro), supplemented with 10% FBS (Sigma-Aldrich) and 1× penicillin/streptomycin (Cellgro). Staining of cell suspensions was done in PBS with 1% FBS and 2 mM EDTA.
Parabiosis.
Parabiotic mice were generated as described previously (67). Each pair of parabiotic mice consisted of a WT and a GFP-transgenic mouse on a C57BL/6 background. At 6 mo after initiation of parabiosis, mice were killed and subjected to tissue analysis. To confirm efficient blood mixing in parabiotic mice, the percentage of GFP+ and GFP− cells among blood CD45+ leukocytes was analyzed in each animal.
Preparation of dermal cell suspension.
Mouse ears were split in two (dorsal and ventral) parts and incubated for 45 min in PBS containing 0.5% trypsin with 5 mM EDTA (Invitrogen) to allow for separation of dermal and epidermal sheets. Dermal sheets were then cut in small pieces and incubated for 2.5 h in collagenase (Worthington) to obtain dermal cell suspension.
Flow cytometry.
mAbs against mouse I-Ab (clone AF6-120.1), CD11c (clone HL3), CD45 (clone 30-F11), CD45.1 (clone A2), CD45.2 (clone 104), CD86 (clone GL1), CD54 (clone 3E2), Gr-1 (Ly6C, clone 1A8), Gr-1 (Ly6C/G, clone RB6-8C5), DC-SIGN (clone 5H10), CD3 (clone 17A2), corresponding isotype controls, and secondary reagents (PE-Cy7–conjugated streptavidin) were purchased from BD Biosciences. mAbs against CD11b (clone M1/70), CD115 (clone AFS9), B220 (clone RA3-682), CD4 (clone L3T4), and CD8α (clone 53-6.7) were obtained from eBioscience. F480 (clone C1:A3-1) and CD205 (clone NLDC145) were purchased from Serotech. Anti-langerin antibody (goat polyclonal IgG) was purchased from Santa Cruz Biotechnology, Inc. Intracellular staining against langerin was performed with the BD Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer's protocol. Multi-parameter analyses of stained cell suspensions were performed on an LSR II (Becton Dickinson) and analyzed with FlowJo software (Tree Star).
Allogeneic BM transplantation in mice.
8-wk-old CD45.2+ C57BL/6 mice were lethally irradiated with 1,200 rad delivered in two doses of 600 rad each, 3 h apart, and injected i.v. with 5 × 105 BM cells obtained from congenic CD45.1+ C57BL/6 adult mice. To address the role of CCR2 and CCR6 in dermal DC repopulation in injured skin, lethally irradiated CD45.1+ mice were reconstituted with mixed BM cells that consisted of a 1:1 mixture of WT CD45.1+ BM cells and CD45.2+ BM isolated from WT, CCR2−/−, CCR6−/−, or CCR2/CCR6−/− mutant mice. Levels of blood donor chimerism were analyzed by measuring the percentage of CD45.1+ cells among total B220+ B cells, Ly6C/G+ CD115− granulocytes, and CD115+ monocytes in the blood 3 wk after transplantation.
Induction of cutaneous injury in BM chimeric mice.
Lethally irradiated mice were reconstituted with congenic BM cells and analyzed for blood donor chimerism 3 wk later. Fully donor BM chimeric mice were then exposed to UV light as described previously (19). Mouse ears were collected before and at various time points after UV exposure. When mentioned, back skin was also shaved and exposed to UV light.
BrdU labeling in vivo.
4 wk after transplantation, (CD45.1+ BM→CD45.2+ recipient) chimeric mice were injected i.p. with 1 mg BrdU (Sigma-Aldrich), to ensure its immediate availability, and given BrdU in 0.4 mg/ml of sterile drinking water that was changed daily for 3 wk. Dermal cell suspensions were prepared at different time points after initial BrdU administration. The percentage of BrdU+ cells among host (CD45.2+) and donor (CD45.1+) dermal DCs was analyzed using the BrdU Flow kit (BD Biosciences) according to the manufacturer's protocol.
Immunofluorescence analysis of murine skin.
For murine skin analysis, 8-μm histological sections of snap-frozen back skin were fixed in 100% acetone for 10 min and rinsed in PBS. Tissue sections were then stained with anti-langerin (goat IgG) and anti-CD11c (Armenian hamster IgG) antibodies for 1 h, washed in PBS, and incubated with secondary reagents Cy2-conjugated anti–goat IgG and biotinylated anti–hamster IgG followed by streptavidin-Cy3. To analyze local cell proliferation, tissue sections were stained with anti-langerin or CD11c antibody and costained with anti–mouse Ki-67 antibody (rat IgG, clone TEC-3; DakoCytomation), followed by Cy3 anti–rat IgG together with biotinylated anti–goat or anti–hamster IgG and completed by streptavidin-Cy2. To analyze CCL2 expression, slides were incubated with anti-murine CCL2 antibody (goat IgG; R&D Systems), followed by Cy3 anti–goat IgG. All secondary reagents were purchased from Jackson ImmunoResearch Laboratories.
Immunofluorescence analysis of human skin.
Human skin samples were fixed in 10% buffered formalin and embedded in paraffin. 6-μm skin cross sections were subjected to antigen retrieval using the antigen unmasking solution (Vector Laboratories). Tissue sections were stained with anti–human Factor XIIIa (mouse IgG; Vector Laboratories) and anti–human Ki-67 (rabbit IgG; Vector Laboratories) antibodies for 1 h, washed, and incubated with Cy2 anti–mouse IgG and biotinylated anti–rabbit IgG, followed by streptavidin-Cy3. Staining for CCL2 was performed using anti–human CCL2 antibody (mouse IgG, clone 24822.11; Sigma-Aldrich), followed by Cy3 anti–mouse IgG. Stainings with isotype controls were always performed in parallel. After completing the staining, slides were mounted with DAPI-containing Vectashield mounting medium (Vector Laboratories).
Fluorescence microscopy.
Low magnification images (20×) were acquired using a Leica DMRA2 fluorescence microscope with a Hamamatsu CCD digital camera and analyzed using Openlab software (Improvision). High magnification multicolored images (40 and 60×) were obtained with a configured for fluorescence imaging microscope (BX61WI; Olympus). Images were collected with a Coolsnap camera. A Dell workstation with SlideBook software (Intelligent Imaging Innovations) provided the synchronization of components, data acquisition, and image analysis.
Dual FISH and immunofluorescence analysis to determine dermal DC chimerism in patients after sex-mismatched allo-HCT.
2-mm skin biopsies were isolated from patients at the indicated time after transplant, fixed in formalin overnight, and embedded in paraffin. 6-μm paraffin- embedded skin sections were deparaffinized and antigen retrieval was performed by incubating slides in 1× sodium citrate buffer (Antigen Retrieval Solution; Vector Laboratories) for 16 min at 100°C. Slides were then washed three times in distilled water and incubated in 2× saline sodium citrate buffer (Vysis Inc.) for 30 min at 37°C. To expose genomic DNA, slides were treated with 0.5 mg/ml pepsin (Sigma-Aldrich) in 0.1 N HCl for 30 min at 37°C and washed in 2× saline sodium citrate buffer at room temperature for 10 min. After dehydration in ethanol, slides were dried out and subjected to FISH. X and Y chromosome–specific DNA probes provided in hybridization buffer (Vysis Inc.) were applied to the slides. Tissue DNA was denatured for 10 min at 72°C, followed by hybridization at 42°C overnight. Next, slides were washed in 0.4× saline sodium citrate at 73°C for 2 min, 0.1% of NP-40 in 2× saline sodium citrate for 1 min at room temperature, and in 2× saline sodium citrate for 5 min at room temperature. Slides were then stained with HLA-DR and anti-langerin antibody as described in Results, washed extensively in PBS, and mounted with DAPI-containing Vectashield mounting medium. Tissue sections were analyzed with a microscope (BX61WI; Olympus). Four filter sets were simultaneously used to picture each skin section in a Z-stack mode. Although we always acquired four different immunostainings, the available software only allowed the display of three separate color sets. HLA-DR+ (Cy5) langerin− (Cy3) dermal cells were analyzed for the presence of X- or Y-specific probes inside of their DAPI-stained nuclei. Only cells containing two dots were acquired for the statistical analysis. Normal skin biopsies of male and female origin were used as controls.
Analysis of BM chimerism in patients after allo-HCT.
BM chimerism was analyzed by interphase FISH using dual color XY probes as described previously (68). In brief, an aliquot of BM cells was applied on slides prepared for DNA hybridization with a dual X/Y probe mixed with hybridization buffer. The target and probe DNA was denatured for 10 min at 72°C, followed by hybridization at 42°C overnight. The slides were washed, dehydrated, and mounted the next day. Interphase nuclei were counterstained with DAPI, and the expression of X and Y chromosomes on DAPI+ nuclei was evaluated with a Zeiss Axioplan microscope. Only nuclei with two signals (XX or XY) were calculated. The percent chimerism was evaluated by two observers, each scoring 150 nuclei. At least six images were taken for documenting each hybridization signal pattern. To determine the accuracy and sensitivity of the probe hybridization, data were also obtained from male and female controls.
Statistical analysis.
Data are presented as mean ± standard deviation. The statistical significance of differences between group means was determined with the Student's t test. p-values of <0.05 were considered significant.
Acknowledgments
The authors would like to thank Drs. G. Randolph and M. Collin for their critical review of the manuscript.
This work was supported by grants from the National Institutes of Health (R01-CA112100) and the Leukemia Research Foundation (to M. Merad), the Sinsheimer scholar award (to M. Bogunovic), the Philippe Foundation Inc. (to F. Ginhoux), the Novo-Nordisk Transfusion Medicine Scholars Program (L. Lubrano), and the National Institutes of Health (R01-HL069438; to P.S. Frenette).
The authors have no conflicting financial interests.
Abbreviations used: allo-HCT, allogeneic hematopoietic cell transplantation; ATG, antithymocyte globulin; DLI, donor lymphocyte infusion; FISH, fluorescence in situ hybridization; GVHD, graft-versus-host disease; LC, Langerhans cell; TBI, total body irradiation.
References
- 1.Valladeau, J., and S. Saeland. 2005. Cutaneous dendritic cells. Semin. Immunol. 17:273–283. [DOI] [PubMed] [Google Scholar]
- 2.Schuler, G., F. Koch, C. Heufler, E. Kampgen, G. Topar, and N. Romani. 1993. Murine epidermal Langerhans cells as a model to study tissue dendritic cells. Adv. Exp. Med. Biol. 329:243–249. [DOI] [PubMed] [Google Scholar]
- 3.Cerio, R., C.E. Griffiths, K.D. Cooper, B.J. Nickoloff, and J.T. Headington. 1989. Characterization of factor XIIIa positive dermal dendritic cells in normal and inflamed skin. Br. J. Dermatol. 121:421–431. [DOI] [PubMed] [Google Scholar]
- 4.Nestle, F.O., L.A. Turka, and B.J. Nickoloff. 1994. Characterization of dermal dendritic cells in psoriasis. Autostimulation of T lymphocytes and induction of Th1 type cytokines. J. Clin. Invest. 94:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lenz, A., M. Heine, G. Schuler, and N. Romani. 1993. Human and murine dermis contain dendritic cells. Isolation by means of a novel method and phenotypical and functional characterization. J. Clin. Invest. 92:2587–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duraiswamy, N., Y. Tse, C. Hammerberg, S. Kang, and K.D. Cooper. 1994. Distinction of class II MHC+ Langerhans cell-like interstitial dendritic antigen-presenting cells in murine dermis from dermal macrophages. J. Invest. Dermatol. 103:678–683. [DOI] [PubMed] [Google Scholar]
- 7.Dupasquier, M., P. Stoitzner, A. van Oudenaren, N. Romani, and P.J. Leenen. 2004. Macrophages and dendritic cells constitute a major subpopulation of cells in the mouse dermis. J. Invest. Dermatol. 123:876–879. [DOI] [PubMed] [Google Scholar]
- 8.Ebner, S., Z. Ehammer, S. Holzmann, P. Schwingshackl, M. Forstner, P. Stoitzner, G.M. Huemer, P. Fritsch, and N. Romani. 2004. Expression of C-type lectin receptors by subsets of dendritic cells in human skin. Int. Immunol. 16:877–887. [DOI] [PubMed] [Google Scholar]
- 9.Valladeau, J., O. Ravel, C. Dezutter-Dambuyant, K. Moore, M. Kleijmeer, Y. Liu, V. Duvert-Frances, C. Vincent, D. Schmitt, J. Davoust, et al. 2000. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 12:71–81. [DOI] [PubMed] [Google Scholar]
- 10.Pope, M., M.G. Betjes, H. Hirmand, L. Hoffman, and R.M. Steinman. 1995. Both dendritic cells and memory T lymphocytes emigrate from organ cultures of human skin and form distinctive dendritic-T-cell conjugates. J. Invest. Dermatol. 104:11–17. [DOI] [PubMed] [Google Scholar]
- 11.Williams, I.R., and T.S. Kupper. 1996. Immunity at the surface: homeostatic mechanisms of the skin immune system. Life Sci. 58:1485–1507. [DOI] [PubMed] [Google Scholar]
- 12.Bennett, C.L., E. van Rijn, S. Jung, K. Inaba, R.M. Steinman, M.L. Kapsenberg, and B.E. Clausen. 2005. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J. Cell Biol. 169:569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kissenpfennig, A., S. Henri, B. Dubois, C. Laplace-Builhe, P. Perrin, N. Romani, C.H. Tripp, P. Douillard, L. Leserman, D. Kaiserlian, et al. 2005. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 22:643–654. [DOI] [PubMed] [Google Scholar]
- 14.Sreilein, J.W. 1989. Antigen-presenting cells in the induction of contact hypersentivity in mice: evidence that Langerhans cells are sufficient but not required. J. Invest. Dermatol. 93:443–448. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan, D.H., M.C. Jenison, S. Saeland, W.D. Shlomchik, and M.J. Shlomchik. 2005. Epidermal Langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 23:611–620. [DOI] [PubMed] [Google Scholar]
- 16.Fossum, S. 1989. The life history of dendritic leukocytes (DL). Curr. Top. Pathol. 79:101–124. [DOI] [PubMed] [Google Scholar]
- 17.Katz, S.I., K. Tamaki, and D.H. Sachs. 1979. Epidermal Langerhans cells are derived from cells originating in bone marrow. Nature. 282:324–326. [DOI] [PubMed] [Google Scholar]
- 18.Holt, P.G., S. Haining, D.J. Nelson, and J.D. Sedgwick. 1994. Origin and steady-state turnover of class II MHC-bearing dendritic cells in the epithelium of the conducting airways. J. Immunol. 153:256–261. [PubMed] [Google Scholar]
- 19.Merad, M., M.G. Manz, H. Karsunky, A. Wagers, W. Peters, I. Charo, I.L. Weissman, J.G. Cyster, and E.G. Engleman. 2002. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat. Immunol. 3:1135–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shlomchik, W.D., M.S. Couzens, C.B. Tang, J. McNiff, M.E. Robert, J. Liu, M.J. Shlomchik, and S.G. Emerson. 1999. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 285:412–415. [DOI] [PubMed] [Google Scholar]
- 21.Zhang, Y., J.P. Louboutin, J. Zhu, A.J. Rivera, and S.G. Emerson. 2002. Preterminal host dendritic cells in irradiated mice prime CD8+ T cell-mediated acute graft-versus-host disease. J. Clin. Invest. 109:1335–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang, Y., W.D. Shlomchik, G. Joe, J.P. Louboutin, J. Zhu, A. Rivera, D. Giannola, and S. Emerson. 2002. APCs in the liver and spleen recruit activated allogeneic CD8+ T cells to elicit hepatic graft-versus-host disease. J. Immunol. 169:7111–7118. [DOI] [PubMed] [Google Scholar]
- 23.Chan, G.W., G. Gorgun, K.B. Miller, and F.M. Foss. 2003. Persistence of host dendritic cells after transplantation is associated with graft-versus-host disease. Biol. Blood Marrow Transplant. 9:170–176. [DOI] [PubMed] [Google Scholar]
- 24.Merad, M., P. Hoffmann, E. Ranheim, S. Slaymaker, M.G. Manz, S.A. Lira, I. Charo, D.N. Cook, I.L. Weissman, S. Strober, and E.G. Engleman. 2004. Depletion of host Langerhans cells before transplantation of donor alloreactive T cells prevents skin graft-versus-host disease. Nat. Med. 10:510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duffner, U.A., Y. Maeda, K.R. Cooke, P. Reddy, R. Ordemann, C. Liu, J.L. Ferrara, and T. Teshima. 2004. Host dendritic cells alone are sufficient to initiate acute graft-versus-host disease. J. Immunol. 172:7393–7398. [DOI] [PubMed] [Google Scholar]
- 26.Anderson, B.E., J.M. McNiff, D. Jain, B.R. Blazar, W.D. Shlomchik, and M.J. Shlomchik. 2005. Distinct roles for donor- and host-derived antigen-presenting cells and costimulatory molecules in murine chronic graft-versus-host disease: requirements depend on target organ. Blood. 105:2227–2234. [DOI] [PubMed] [Google Scholar]
- 27.Vogelsang, G.B., L. Lee, and D.M. Bensen-Kennedy. 2003. Pathogenesis and treatment of graft-versus-host disease after bone marrow transplant. Annu. Rev. Med. 54:29–52. [DOI] [PubMed] [Google Scholar]
- 28.Figdor, C.G. 2003. Molecular characterization of dendritic cells operating at the interface of innate or acquired immunity. Pathol. Biol. (Paris). 51:61–63. [DOI] [PubMed] [Google Scholar]
- 29.Scholzen, T., and J. Gerdes. 2000. The Ki-67 protein: from the known and the unknown. J. Cell. Physiol. 182:311–322. [DOI] [PubMed] [Google Scholar]
- 30.Gerdes, J., H. Lemke, H. Baisch, H.H. Wacker, U. Schwab, and H. Stein. 1984. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 133:1710–1715. [PubMed] [Google Scholar]
- 31.Gerdes, J., L. Li, C. Schlueter, M. Duchrow, C. Wohlenberg, C. Gerlach, I. Stahmer, S. Kloth, E. Brandt, and H.D. Flad. 1991. Immunobiochemical and molecular biologic characterization of the cell proliferation-associated nuclear antigen that is defined by monoclonal antibody Ki-67. Am. J. Pathol. 138:867–873. [PMC free article] [PubMed] [Google Scholar]
- 32.Sebastiani, S., C. Albanesi, P.O. De, P. Puddu, A. Cavani, and G. Girolomoni. 2002. The role of chemokines in allergic contact dermatitis. Arch. Dermatol. Res. 293:552–559. [DOI] [PubMed] [Google Scholar]
- 33.Barker, J.N., M.L. Jones, C.L. Swenson, V. Sarma, R.S. Mitra, P.A. Ward, K.J. Johnson, J.C. Fantone, V.M. Dixit, and B.J. Nickoloff. 1991. Monocyte chemotaxis and activating factor production by keratinocytes in response to IFN-gamma. J. Immunol. 146:1192–1197. [PubMed] [Google Scholar]
- 34.Campbell, J.J., G. Haraldsen, J. Pan, J. Rottman, S. Qin, P. Ponath, D.P. Andrew, R. Warnke, N. Ruffing, N. Kassam, et al. 1999. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 400:776–780. [DOI] [PubMed] [Google Scholar]
- 35.Katou, F., H. Ohtani, T. Nakayama, K. Ono, K. Matsushima, A. Saaristo, H. Nagura, O. Yoshie, and K. Motegi. 2001. Macrophage-derived chemokine (MDC/CCL22) and CCR4 are involved in the formation of T lymphocyte-dendritic cell clusters in human inflamed skin and secondary lymphoid tissue. Am. J. Pathol. 158:1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tensen, C.P., J. Flier, E.M. Van Der Raaij-Helmer, S. Sampat-Sardjoepersad, R.C. Van Der Schors, R. Leurs, R.J. Scheper, D.M. Boorsma, and R. Willemze. 1999. Human IP-9: a keratinocyte- derived high affinity CXC-chemokine ligand for the IP-10/Mig receptor (CXCR3). J. Invest. Dermatol. 112:716–722. [DOI] [PubMed] [Google Scholar]
- 37.Flier, J., D.M. Boorsma, P.J. van Beek, C. Nieboer, T.J. Stoof, R. Willemze, and C.P. Tensen. 2001. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J. Pathol. 194:398–405. [DOI] [PubMed] [Google Scholar]
- 38.Dieu-Nosjean, M.C., C. Massacrier, B. Homey, B. Vanbervliet, J.J. Pin, A. Vicari, S. Lebecque, C. Dezutter-Dambuyant, D. Schmitt, A. Zlotnik, and C. Caux. 2000. Macrophage inflammatory protein 3α is expressed at inflamed epithelial surfaces and is the most potent chemokine known in attracting Langerhans cell precursors. J. Exp. Med. 192:705–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakayama, T., R. Fujisawa, H. Yamada, T. Horikawa, H. Kawasaki, K. Hieshima, D. Izawa, S. Fujiie, T. Tezuka, and O. Yoshie. 2001. Inducible expression of a CC chemokine liver- and activation-regulated chemokine (LARC)/macrophage inflammatory protein (MIP)-3 alpha/CCL20 by epidermal keratinocytes and its role in atopic dermatitis. Int. Immunol. 13:95–103. [DOI] [PubMed] [Google Scholar]
- 40.Pablos, J.L., A. Amara, A. Bouloc, B. Santiago, A. Caruz, M. Galindo, T. Delaunay, J.L. Virelizier, and F. Arenzana-Seisdedos. 1999. Stromal-cell derived factor is expressed by dendritic cells and endothelium in human skin. Am. J. Pathol. 155:1577–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morales, J., B. Homey, A.P. Vicari, S. Hudak, E. Oldham, J. Hedrick, R. Orozco, N.G. Copeland, N.A. Jenkins, L.M. McEvoy, and A. Zlotnik. 1999. CTACK, a skin-associated chemokine that preferentially attracts skin-homing memory T cells. Proc. Natl. Acad. Sci. USA. 96:14470–14475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schaerli, P., K. Willimann, L.M. Ebert, A. Walz, and B. Moser. 2005. Cutaneous CXCL14 targets blood precursors to epidermal niches for Langerhans cell differentiation. Immunity. 23:331–342. [DOI] [PubMed] [Google Scholar]
- 43.Serbina, N.V., and E.G.P. Am. 2006. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 7:311–317. [DOI] [PubMed] [Google Scholar]
- 44.Merad, M., T. Sugie, E.G. Engleman, and L. Fong. 2002. In vivo manipulation of dendritic cells to induce therapeutic immunity. Blood. 99:1676–1682. [DOI] [PubMed] [Google Scholar]
- 45.Grosskreutz, C., V. Ross, E. Scigliano, S. Fruchtman, and L. Isola. 2003. Low-dose total body irradiation, fludarabine, and antithymocyte globulin conditioning for nonmyeloablative allogeneic transplantation. Biol. Blood Marrow Transplant. 9:453–459. [DOI] [PubMed] [Google Scholar]
- 46.Kabashima, K., T.A. Banks, K.M. Ansel, T.T. Lu, C.F. Ware, and J.G. Cyster. 2005. Intrinsic lymphotoxin-beta receptor requirement for homeostasis of lymphoid tissue dendritic cells. Immunity. 22:439–450. [DOI] [PubMed] [Google Scholar]
- 47.Lyman, S.D., and S.E. Jacobsen. 1998. c-kit ligand and Flt3 ligand: stem/progenitor cell factors with overlapping yet distinct activities. Blood. 91:1101–1134. [PubMed] [Google Scholar]
- 48.Shizuru, J.A., R.S. Negrin, and I.L. Weissman. 2005. Hematopoietic stem and progenitor cells: clinical and preclinical regeneration of the hematolymphoid system. Annu. Rev. Med. 56:509–538. [DOI] [PubMed] [Google Scholar]
- 49.Ikuta, K., N. Uchida, J. Friedman, and I.L. Weissman. 1992. Lymphocyte development from stem cells. Annu. Rev. Immunol. 10:759–783. [DOI] [PubMed] [Google Scholar]
- 50.Kamath, A.T., J. Pooley, M.A. O'Keeffe, D. Vremec, Y. Zhan, A.M. Lew, A. D'Amico, L. Wu, D.F. Tough, and K. Shortman. 2000. The development, maturation, and turnover rate of mouse spleen dendritic cell populations. J. Immunol. 165:6762–6770. [DOI] [PubMed] [Google Scholar]
- 51.Kamath, A.T., S. Henri, F. Battye, D.F. Tough, and K. Shortman. 2002. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood. 100:1734–1741. [PubMed] [Google Scholar]
- 52.Ginhoux, F., F. Tacke, V. Angeli, M. Bogunovic, X.M. Dai, E. Stanley, G.J. Randolph, and M. Merad. 2006. Langerhans cells arise from monocytes in vivo. Nat. Immunol. 7:265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mielcarek, M., and R. Storb. 2003. Non-myeloablative hematopoietic cell transplantation as immunotherapy for hematologic malignancies. Cancer Treat. Rev. 29:283–290. [DOI] [PubMed] [Google Scholar]
- 54.Collin, M.P., D.N. Hart, G.H. Jackson, G. Cook, J. Cavet, S. Mackinnon, P.G. Middleton, and A.M. Dickinson. 2006. The fate of human Langerhans cells in hematopoietic stem cell transplantation. J. Exp. Med. 203:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mielcarek, M., and R. Storb. 2005. Graft-vs-host disease after non-myeloablative hematopoietic cell transplantation. Leuk. Lymphoma. 46:1251–1260. [DOI] [PubMed] [Google Scholar]
- 56.Mielcarek, M., P.J. Martin, W. Leisenring, M.E. Flowers, D.G. Maloney, B.M. Sandmaier, M.B. Maris, and R. Storb. 2003. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood. 102:756–762. [DOI] [PubMed] [Google Scholar]
- 57.Peggs, K.S., K. Thomson, D.P. Hart, J. Geary, E.C. Morris, K. Yong, A.H. Goldstone, D.C. Linch, and S. Mackinnon. 2004. Dose-escalated donor lymphocyte infusions following reduced intensity transplantation: toxicity, chimerism, and disease responses. Blood. 103:1548–1556. [DOI] [PubMed] [Google Scholar]
- 58.Marks, D.I., R. Lush, J. Cavenagh, D.W. Milligan, S. Schey, A. Parker, F.J. Clark, L. Hunt, J. Yin, S. Fuller, et al. 2002. The toxicity and efficacy of donor lymphocyte infusions given after reduced-intensity conditioning allogeneic stem cell transplantation. Blood. 100:3108–3114. [DOI] [PubMed] [Google Scholar]
- 59.Ferrara, J.L., and H.J. Deeg. 1991. Graft-versus-host disease. N. Engl. J. Med. 324:667–674. [DOI] [PubMed] [Google Scholar]
- 60.Mapara, M.Y., Y.M. Kim, S.P. Wang, R. Bronson, D.H. Sachs, and M. Sykes. 2002. Donor lymphocyte infusions mediate superior graft-versus-leukemia effects in mixed compared to fully allogeneic chimeras: a critical role for host antigen-presenting cells. Blood. 100:1903–1909. [DOI] [PubMed] [Google Scholar]
- 61.Vogelsang, G.B., D. Wolff, V. Altomonte, E. Farmer, W.L. Morison, R. Corio, and T. Horn. 1996. Treatment of chronic graft-versus-host disease with ultraviolet irradiation and psoralen (PUVA). Bone Marrow Transplant. 17:1061–1067. [PubMed] [Google Scholar]
- 62.Braverman, I.M., S. Klein, and A. Grant. 1987. Electron microscopic and immunolabeling studies of the lesional and normal skin of patients with mycosis fungoides treated by total body electron beam irradiation. J. Am. Acad. Dermatol. 16:61–74. [DOI] [PubMed] [Google Scholar]
- 63.McGregor, D.H., Q. Yang, F. Fan, R.L. Talley, and M. Topalovski. 2001. Scabies associated with radiation therapy for cutaneous T-cell lymphoma. Ann. Clin. Lab. Sci. 31:103–107. [PubMed] [Google Scholar]
- 64.Boring, L., J. Gosling, S.W. Chensue, S.L. Kunkel, R.V. Farese Jr., H.E. Broxmeyer, and I.F. Charo. 1997. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J. Clin. Invest. 100:2552–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cook, D.N., D.M. Prosser, R. Forster, J. Zhang, N.A. Kuklin, S.J. Abbondanzo, X.D. Niu, S.C. Chen, D.J. Manfra, M.T. Wiekowski, et al. 2000. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity. 12:495–503. [DOI] [PubMed] [Google Scholar]
- 66.Lindquist, R.L., G. Shakhar, D. Dudziak, H. Wardemann, T. Eisenreich, M.L. Dustin, and M.C. Nussenzweig. 2004. Visualizing dendritic cell networks in vivo. Nat. Immunol. 5:1243–1250. [DOI] [PubMed] [Google Scholar]
- 67.Wright, D.E., A.J. Wagers, A.P. Gulati, F.L. Johnson, and I.L. Weissman. 2001. Physiological migration of hematopoietic stem and progenitor cells. Science. 294:1933–1936. [DOI] [PubMed] [Google Scholar]
- 68.Najfeld, V., W. Burnett, A. Vlachos, E. Scigliano, L. Isola, and S. Fruchtman. 1997. Interphase FISH analysis of sex-mismatched BMT utilizing dual color XY probes. Bone Marrow Transplant. 19:829–834. [DOI] [PubMed] [Google Scholar]