A crucial role for interleukin (IL)-1 in the induction of IL-17–producing T cells that mediate autoimmune encephalomyelitis (original) (raw)
Abstract
It was recently demonstrated that interleukin (IL)-23–driven IL-17–producing (ThIL-17) T cells mediate inflammatory pathology in certain autoimmune diseases. We show that the induction of antigen-specific ThIL-17 cells, but not T helper (Th)1 or Th2 cells, by immunization with antigens and adjuvants is abrogated in IL-1 receptor type I–deficient (IL-1RI−/−) mice. Furthermore, the incidence of experimental autoimmune encephalomyelitis (EAE) was significantly lower in IL-1RI−/− compared with wild-type mice, and this correlated with a failure to induce autoantigen-specific ThIL-17 cells, whereas induction of Th1 and Th2 responses was not substantially different. However, EAE was induced in IL-1RI−/− mice by adoptive transfer of autoantigen-specific cells from wild-type mice with EAE. IL-23 alone did not induce IL-17 production by T cells from IL-1RI−/− mice, and IL-23–induced IL-17 production was substantially enhanced by IL-1α or IL-1β, even in the absence of T cell receptor stimulation. We demonstrate essential roles for phosphatidylinositol 3-kinase, nuclear factor κB, and novel protein kinase C isoforms in IL-1– and IL-23–mediated IL-17 production. Tumor necrosis factor α also synergized with IL-23 to enhance IL-17 production, and this was IL-1 dependent. Our findings demonstrate that IL-1 functions upstream of IL-17 to promote pathogenic ThIL-17 cells in EAE.
T cells play a pathogenic role in many autoimmune diseases, including rheumatoid arthritis, type 1 diabetes, and multiple sclerosis (MS). MS and the mouse model, experimental autoimmune encephalomyelitis (EAE), are chronic inflammatory diseases of the central nervous system (1), which until recently were assumed to be mediated by Th1 cells (2). However, IFN-γ KO mice have an increased susceptibility to EAE (3), and disease is exacerbated in mice deficient in the Th1-polarizing cytokine IL-12 (4). In contrast, induction of disease is blocked in mice deficient in the novel IL-12 family member, IL-23 (4), which promotes the differentiation of IL-17–producing (ThIL-17) cells (5, 6). The demonstration that adoptive transfer of autoantigen-specific ThIL-17 cells, but not Th1 cells, induced EAE (6), as well as studies in collagen-induced arthritis (7), has established a definitive pathogenic role for these cells in T cell–mediated autoimmune diseases.
Recent reports have demonstrated that ThIL-17 cells are a distinct subtype from Th1 and Th2 cells and do not share a precursor intermediate (8, 9). Indeed, their differentiation process is inhibited by the signature Th1 and Th2 cytokines, IFN-γ and IL-4 (8). IL-23 can act on effector and memory CD4+ T cells to induce T cell proliferation and IL-17 secretion (5), but it can also promote the differentiation of naive T cells into ThIL-17 cells (8, 9). ThIL-17 cells may function in autoimmunity by promoting the induction of IL-1β and TNF-α. It has been shown that IL-1β and TNF-α can mediate inflammatory pathology in many autoimmune diseases, and antibodies or receptor antagonists of these inflammatory cytokines are effective therapeutics. In rheumatoid arthritis, IL-17 is thought to play a major role upstream of IL-1 (10, 11) in promoting autoimmune joint destruction. Furthermore, studies with IL-1RI−/− mice have established a role for IL-1 in the induction of EAE (12). In addition, IL-1β is present in MS lesions and families with a high IL-1β to IL-1 receptor antagonist (IL-1Ra) production ratio are at a greater risk of having a relative with relapse-onset MS than those with a low ratio (13). However, the precise role of IL-1 in the pathogenesis of EAE is unclear.
We demonstrate that IL-23 alone is a poor stimulus for T cell IL-17 production and that IL-1 is essential for its induction. Immunization with foreign or self-antigens and a Toll-like receptor ligand or CFA demonstrated that IL-1 plays a vital and selective role in promoting antigen-specific ThIL-17 cells in vivo. Furthermore, a failure to induce EAE in IL-1RI−/− mice was associated with a failure to generate ThIL-17 cells. IL-1 acted directly on T cells in synergy with IL-23 to promote IL-17 secretion. TNF-α also synergized with IL-23 to induce IL-17, but this was dependant on IL-1. Our findings demonstrate a central role for IL-1 upstream of IL-17 in promoting autoimmunity in EAE by driving the differentiation of pathogenic ThIL-17 cells.
RESULTS AND DISCUSSION
IL-1RI is required for the induction of ThIL-17 cells
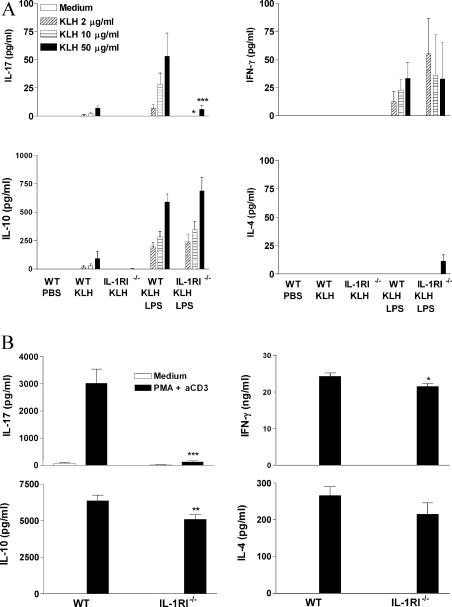
In a study on the role of IL-1RI in the adjuvant effect of the Toll-like receptor agonist LPS, we discovered that IL-1 is selectively required for induction of ThIL-17 cells. Parenteral immunization of C57BL/6 mice with KLH in the presence of LPS induced antigen-specific T cells that secreted IFN-γ, IL-10, and IL-17, whereas immunization with KLH in the absence of adjuvant induced little or no antigen-specific cytokine production (Fig. 1 A). Immunization of IL-1RI−/− mice with KLH and LPS also induced antigen-specific IFN-γ and IL-10 production. However, antigen-specific IL-17 production was significantly lower in IL-1RI−/− than in wild-type C57BL/6 mice. Antigen- specific proliferation was not significantly different between the groups (not depicted). IL-17 concentrations induced by PMA and anti-CD3 were also significantly lower in cells from IL-1RI−/− mice (Fig. 1 B). Thus, IL-1 is selectively required for antigen or anti-CD3 and PMA-induced IL-17 production.
Figure 1.
IL-1RI is required for induction or activation of antigen-specific ThIL-17 T cells. (A) C57BL/6 or IL-1RI−/− mice were immunized s.c. with 10 μg KLH either alone or with 10 μg LPS. Inguinal lymph nodes were harvested after 7 d and cells were restimulated with medium or 2, 10, or 50 μg/ml KLH. (B) Lymph node cells were restimulated with medium or 20 ng/ml PMA and 1 μg/ml anti-CD3. Supernatants were collected after 3 d, and IL-4, IL-10, IL-17, and IFN-γ concentrations were determined by ELISA. C57BL/6 versus IL-1RI−/−: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
IL-1RI–deficient mice are resistant to EAE, and this correlates with reduced autoantigen-specific IL-17 production
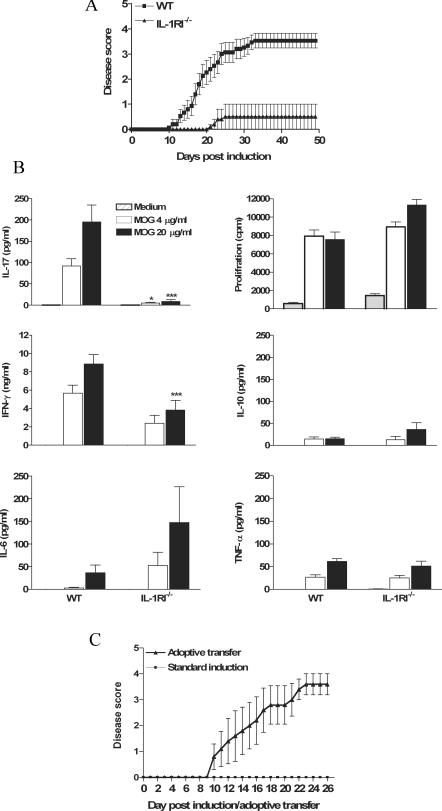
We next examined the possibility that IL-1 may function in the pathology of EAE by promoting pathogenic autoantigen-specific ThIL-17 cells. Wild-type C57BL/6 mice developed severe EAE after immunization with myelin oligodendrocyte glycoprotein (MOG) and CFA. In contrast, the incidence of EAE (2/15 IL-1RI−/− vs. 13/15 in C57BL/6) was considerably lower in IL-1RI−/− mice (Fig. 2 A). This is consistent with studies indicating an important role for IL-1 in EAE (12, 14). However, we demonstrate that the resistance of IL-1RI−/− mice to the development of EAE correlates with a specific defect in activating autoantigen-specific ThIL-17 cells (Fig. 2 B). MOG-specific IL-17 production was selectively reduced in IL-1RI−/− mice in comparison with wild-type mice (Fig. 2 B). MOG-specific proliferation and secretion of IL-6, IL-10, and TNF-α were comparable in IL-1RI−/− and wild-type mice. Production of IFN-γ was reduced, but to a modest extent (twofold reduction compared with a 22-fold reduction in IL-17 production).
Figure 2.
The induction of EAE and autoantigen-specific ThIL-17 cells is dependent on functional IL-1RI. C57BL/6 or IL-1RI−/− mice were injected with 150 μg MOG in 200 μl CFA and 500 ng PT followed 2 d later with a second dose of PT. Mice were observed for clinical symptoms of EAE daily for 48 d. A separate group of mice were killed on d 21, and spleen cells were restimulated with MOG peptide. After 3 d, supernatants were collected and cytokine concentrations were determined by ELISA. Cell proliferation was determined on day 4 of culture by [3H]thymidine incorporation. (A) Disease scores (n = 15) ± SD. (B) Antigen-specific cytokine production and proliferation. C57BL/6 versus IL-1RI−/−: *, P < 0.05; **, P < 0.01; ***, P < 0.001. (C) IL-1RI−/− mice (n = 7) were injected with MOG in CFA followed by PT as described above or with MOG-specific T cells (9 × 106 cells/mouse) generated from the spleens of C57BL/6 mice with EAE. Disease scores (n = 5) ± SD.
We examined the ability of MOG-specific T cells from wild-type mice to induce EAE in IL-1RI−/− mice and compared this with standard EAE induction. Transfer of a MOG-specific T cell line from C57BL/6 mice with advanced EAE into IL-1RI−/− mice induced EAE in five out of five mice, whereas mice injected directly with MOG and CFA did not develop disease (Fig. 2 C), demonstrating that IL-1RI is dispensable in the development of EAE after induction of the autoantigen-specific ThIL-17 T cells. Our data support findings that neuroantigen-specific ThIL-17 cells can transfer EAE (6) and suggest that the major role of IL-1 in the development of EAE is in promoting pathogenic ThIL-17 cells. The clinical symptoms in EAE are ameliorated by blocking IL-17 (9, 15), and injection of anti–IL-17 delays the onset of disease and prevents the characteristic mononuclear cell infiltrate in spinal cord white matter (9). Our data place IL-1 upstream of IL-17 and suggest that the efficacy of blocking IL-1 as a therapy for EAE may reflect a failure to activate ThIL-17 cells.
IL-1 synergizes with IL-23 to promote T cell IL-17 production
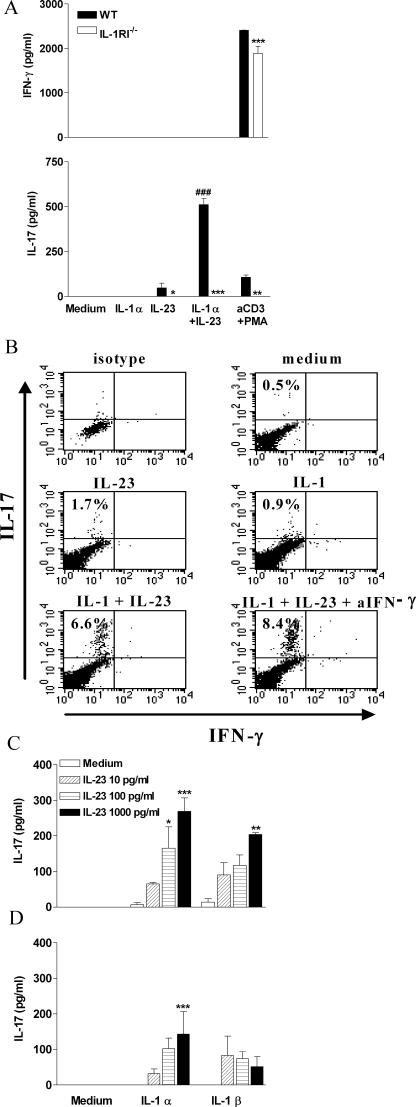
Because our data pointed to a selective effect of IL-1 in promoting ThIL-17 cells, we determined whether IL-1 directly induced IL-17 secretion by T cells in vitro. Stimulation with rIL-23 induced IL-17 production by spleen cells from C57BL/6 but not from IL-1RI−/−mice (Fig. 3 A). However, production of IL-17 in response to IL-23 alone was relatively low. Furthermore, IL-1 alone did not induce IL-17 secretion by spleen cells but significantly augmented IL-23–induced IL-17 production. Intracellular cytokine staining revealed that IL-17 was produced by CD3+ T cells (Fig. 3 B). Furthermore, the addition of anti–IFN-γ increased the frequency of IL-17–secreting T cells after stimulation with IL-1 and IL-23, consistent with a negative effect of IFN-γ on IL-17 production (9).
Figure 3.
IL-1 synergizes with IL-23 to promote IL-17 production by spleen cells and purified CD4+ and CD8+ T cells. (A) Spleen cells (106 cells/ml) from C57BL/6 or IL-1RI−/− mice were cultured with 100 pg/ml IL-23 alone or together with 1 ng/ml IL-1α, and supernatants were collected after 3 d for analysis of IL-17 and IFN-γ by ELISA. C57BL/6 versus IL-1RI−/−: *, P < 0.05; **, P < 0.01; ***, P < 0.001. IL-1α plus IL-23 versus IL-23; #, P < 0.001. (B) Spleen cells (2.25 × 106 cells/ml) from C57BL/6 mice were cultured for 48 h with medium or cytokines and for the last 4 h in the presence of 10 μg/ml brefeldin A. Intracellular staining for IL-17 and IFN-γ was performed. Percentages refer to IL-17 production by gated CD3+ T cells. Splenic CD4+ (C) and CD8+ (D) T cells (5 × 105cells/ml) from C57BL/6 mice were incubated with 10 U/ml IL-2 and 1 μg/ml anti-CD3 antibody alone or together with 0.1–1 ng/ml IL-23 and/or 1 ng/ml IL-1α or IL-1β. IL-17 concentrations were measured by ELISA in supernatants collected after 3 d. IL-23 plus IL-1 versus IL-23 only: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To determine whether the synergistic effect of IL-1 on IL-23–induced IL-17 production was mediated directly on T cells or required accessory cells, we assessed the effects of IL-1 and IL-23 on purified T cells. CD4+ and CD8+ T cells from C57BL/6 mice were stimulated with IL-23 in the presence or absence of IL-1. IL-23 at concentrations of up to 1 ng/ml did not induce significant IL-17 production (Fig. 3 C). However, the addition of IL-1α or IL-1β resulted in a dramatic synergistic effect with IL-23 in promoting IL-17 secretion. Both CD4+ and CD8+ T cells have been implicated in the pathology of MS (16) and EAE, and ThIL-17 T cells have been shown to induce EAE (6). Collectively with our data, these findings suggest that the requirement for IL-1RI to promote ThIL-17 cells and EAE reflects the need for synergy between IL-1 and IL-23 to induce IL-17 production.
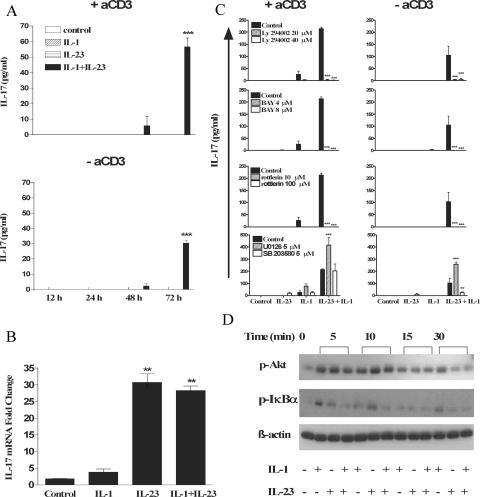
To address whether the effects of IL-23 and IL-1 involved amplification of a TCR-activated pathway, we examined the effects of IL-1 and IL-23 in the presence or absence of anti-CD3. IL-1– and IL-23–induced IL-17 production was retained in the absence of anti-CD3 (Fig. 4 A). Recent studies have indicated that IL-23 acts by promoting the survival and expansion of ThIL-17 T cells (17). Analysis of IL-17 mRNA by real-time PCR revealed that IL-23 or IL-1 and IL-23, but not IL-1 alone, induced potent IL-17 gene transcription (Fig. 4 B). Therefore, our data suggest that IL-23 is vital for induction of IL-17 mRNA expression. The recently documented ability of IL-6 and TGF-β to promote the differentiation of ThIL-17 cells (17–19) may be dependant on IL-1. We have found that IL-6 can synergize with IL-23 to promote IL-17 production, but this effect was abrogated in cells from IL-1RI−/− mice (not depicted). Alternatively, IL-1 and IL-23 may act independently of IL-6 and TGF-β to promote IL-17 production from T cells. However, the relative roles of IL-23, IL-6, TGF-β, and IL-1 in the differentiation and survival of ThIL-17 cells require further investigation.
Figure 4.
IL-1 and IL-23 promote IL-17 production in the absence of TCR stimulation via stimulation of PI3K, NF-κB, and PKCθ. (A) Splenic CD3+ cells (5 × 105 cells/ml) from C57BL/6 mice were incubated with 10 ng/ml IL-1β, 10 ng/ml IL-23, IL-1 and IL-23, or medium only in the presence or absence of anti-CD3, and supernatants were collected at 12, 24, 48, or 72 h for analysis by ELISA. (B) Splenic CD3+ cells from C57BL/6 mice were incubated with 1 μg/ml anti-CD3 for 2 h before stimulation with IL-1, IL-23, IL-1 and IL-23, or medium. IL-17 mRNA expression was evaluated by real-time PCR 48 h after stimulation, and the data represent the fold change for IL-17 mRNA in relation to 18S rRNA. IL-23 plus IL-1 versus IL-23 only: *, P < 0.05; **, P < 0.01; ***, P < 0.001. (C) CD3+ cells (5 × 105 cells/ml) were stimulated with medium (control) or with IL-23, IL-1, or IL-23 and IL-1 with or without anti-CD3 in the presence or absence of (30-min pretreatment) LY294002 (PI3K inhibitor at 20 and 40 μM), BAY 11-7082 (NF-κB inhibitor at 4 and 8 μM), rottlerin (novel PKC inhibitor at 10 and 100 μM), UO126 (ERK inhibitor at 5 μM), or SB203580 (p38 inhibitor at 5 μM). Supernatants were collected 3 d later for analysis of IL-17 production by ELISA. IL-23 plus IL-1 versus IL-23 plus IL-1 plus inhibitor: *, P < 0.05; **, P < 0.01; ***, P < 0.001. (D) CD3+ cells were incubated with medium only or with IL-1, IL-23, or IL-1 and IL-23. Cell lysates were analyzed by Western blotting with antibodies specific for phosphorylated Akt and IκB-α or β-actin as a loading control.
Our data suggest that in addition to roles for IL-23, as well as CD28 and ICOS costimulation (9), in promoting the differentiation of naive T cells into ThIL-17 cells, there is an additional and central requirement for IL-1.
Signaling pathways involved in IL-1– and IL-23–induced IL-17 production
Using specific inhibitors of intracellular signaling pathways, we found that novel isoforms of protein kinase C (PKC), NF-κB, and PI3K are essential for IL-1 and IL-23 to promote IL-17 production by T cells (Fig. 4 C). These inhibitors completely blocked IL-1– and IL-23–induced IL-17 production, which was observed in the presence or absence of TCR stimulation, indicating that the cytokines can activate signaling pathways that directly promote IL-17 production.
PKCθ-deficient mice are resistant to EAE and this correlates with reduced IL-17 in the central nervous systems of these mice (20). Our data support an important role for PKCθ (21) in IL-1– and IL-23–mediated induction of T cell IL-17 production. We also demonstrate that PI3K and NF-κB are important in the induction of IL-17 (22) and that phosphorylation of Akt (a readout for PI3K activation) and IκB-α were induced independently by IL-1 and IL-23 (Fig. 4 D). It was reported that MAP kinase pathways are involved in IL-17 transcriptional regulation in human T cells (23). We demonstrate that in murine cells, inhibitors of ERK (UO126) and p38 (SB203580) did not inhibit IL-17 and, notably, ERK inhibition enhanced IL-17 production (Fig. 4 C). However, in the absence of TCR stimulation, inhibition of p38 reduced IL-17 production in response to IL-1 and IL-23.
TNF-α enhances IL-23–induced IL-17 by an IL-1–dependent mechanism
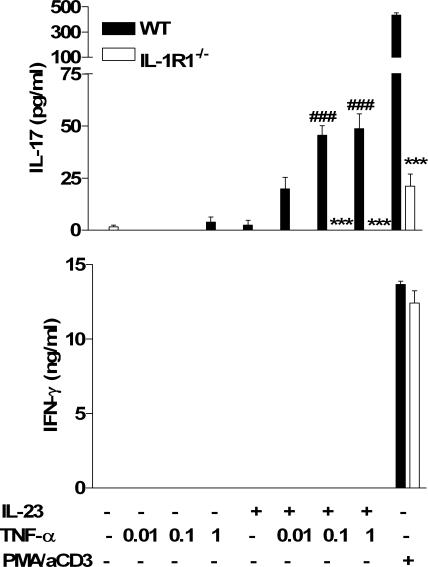
In addition to IL-1, TNF-α has been shown to play a major role in the inflammatory pathology in T cell–mediated autoimmune diseases and other chronic inflammatory conditions. Drugs targeting either IL-1 or TNF-α are effective therapeutics for these diseases. It has been assumed that TNF-α and IL-1 mediate inflammatory reactions downstream of the effects of T cells. However, the spontaneous arthritis that develops in IL-1Ra−/− mice does not occur in T cell–deficient mice (24) or mice deficient in IL-17 (25), whereas IL-17 is enhanced in IL-1Ra−/− mice (25). We found that TNF-α alone did not induce IL-17 production by spleen cells but did synergize with IL-23 to promote IL-17 production (Fig. 5). This effect was dependent on IL-1, which is consistent with reports that TNF-α can promote IL-1 production and stromal cell IL-1RI expression (26) and that IL-1 was inhibited by treatment of psoriasis patients with a soluble TNF receptor (27). We found that TNF-α, but not IL-1 or IL-23, increased expression of IL-1RI on both CD4+ and CD8+ T cells (not depicted), which may enhance their responsiveness to IL-1.
Figure 5.
TNF-α promotes IL-23–induced IL-17 production in an IL-1–dependent manner. Spleen cells (106 cells/ml) from C57BL/6 or IL-1RI−/− were cultured with 1 ng/ml IL-23, 0.01–1 ng/ml TNF-α, 0.01–1 ng/ml IL-23 and TNF-α, medium, or PMA and anti-CD3. Supernatants were collected after 3 d for analysis of IL-17 and IFN-γ production by ELISA. C57BL/6 versus IL-1RI−/−: *, P < 0.05; **, P < 0.01; ***, P < 0.001. TNF-α plus IL-23 versus IL-23: #, P < 0.05; ##, P < 0.01; ###, P < 0.001.
Because there are complex cytokine networks operating at sites of inflammation, several cytokines, including IL-23, IL-1, and TNF-α, may contribute to amplify IL-17 production. For example, IL-17 can synergize with TNF-α and IL-1 to promote inflammatory gene expression (28). Our demonstration that IL-1 acts upstream of IL-17 in EAE, combined with findings that IL-17 can promote IL-1, indicates that there may be a “vicious circle” with IL-1 and IL-17 promoting each other and amplifying the autoimmune response.
Our findings provide further evidence for a role of innate immune responses in directing adaptive immunity. Specifically, in addition to the established proinflammatory role of IL-1, our findings reveal a hitherto unrecognized regulatory role for IL-1α and IL-1β in selectively promoting pathogenic ThIL-17 cells that mediate autoimmunity.
MATERIALS AND METHODS
Mice.
C57BL/6 and IL-1RI−/− mice were obtained from Harlan Ltd. and bred at Trinity College Dublin under specific pathogen-free conditions. Mice were maintained according to the regulations of the European Union and the Irish Department of Health.
Immunization experiments.
Mice were immunized s.c. in the flank with KLH (10 μg per mouse; endotoxin-free; Calbiochem) alone or together with LPS (10 μg per mouse; Escherichia coli, serotype R515; Qbiogene), or with PBS. Mice were killed after 7 d and inguinal lymph nodes were removed.
Induction and assessment of EAE.
C57BL/6 and IL-1RI−/− mice were immunized s.c. with 150 μg MOG peptide 35-55 (Cambridge BioSciences) emulsified in CFA supplemented with 5 mg/ml killed Mycobacterium tuberculosis (Chondrex). For the adoptive transfer studies, EAE was induced in C57BL/6 mice and when mice reached an average disease score of 4, spleens were harvested and cultured with 16 μg/ml MOG, 10 ng/ml IL-1, and 10 ng/ml IL-23 for 3 d. Cells were washed and injected i.v. (9 × 106 per mouse) into IL-1RI−/− mice. Mice were injected i.p. with 500 ng pertussis toxin (PT; Sigma-Aldrich) on days 0 and 2. From day 2, mice were observed daily for signs of clinical disease. Disease severity was recorded as follows: grade 0, normal; grade 1, limp tail; grade 2, wobbly gait; grade 3, hind limb weakness; grade 4, hind limb paralysis; and grade 5, tetraparalysis/death.
Antigen-specific proliferation and cytokine production.
Lymph node cells (106 cells/ml) were stimulated with 2–50 μg/ml KLH, 20 ng/ml PMA (Sigma-Genosys), and 1 μg/ml anti–mouse CD3 (BD Biosciences), or medium only. Alternatively, spleen cells (2 × 106 cells/ml) from mice with EAE were cultured with MOG (4 or 20 μg/ml), or medium only. Supernatants were collected after 72 h and cytokine concentrations were determined by ELISA using antibody pairs specific for IL-4, IFN-γ, IL-6 (BD Biosciences), IL-17, TNF-α, or IL-10 (Duo-Set; R&D Systems.). On day 4, 1 μCi/well [3H]thymidine (GE Healthcare) was added and the cells were cultured for an additional 6 h, after which cells were harvested and proliferation was assessed by [3H]thymidine incorporation (1450 Microbeta; PerkinElmer).
T cell purification.
Mouse T cell CD4 and CD8 subset column kits (R&D Systems) were used to purify CD4+ and CD8+ cells from the spleens of C57BL/6 mice. T cell purity was routinely >95%. T cells and spleen cells were incubated with IL-1α, IL-1β, TNF-α, and IL-23 (endotoxin content was <1 EU/μg protein in all cases; R&D Systems) alone or in combination. In the studies on intracellular signaling pathways, all inhibitors were obtained from Calbiochem. For experiments on purified T cells, IL-2 (Hoffman-La Roche Inc.) and anti-CD3 were also included except where stated.
Intracellular cytokine staining.
Spleen cells from C57BL/6 mice were incubated for 72 h with medium or cytokines in the presence of 10 μg/ml brefeldin A (for the last 4 h) before extracellular staining with anti-CD3. Cells were washed, blocked with 1 μg/ml of Fcγ blocker (BD Biosciences), fixed, permeabilized (Fix and Perm cell permeabilization kit; Caltag), and stained with anti–IL-17, anti–IFN-γ antibody, or the appropriate isotype control. Flow cytometric analysis was performed using a FACSCalibur flow cytometer (Becton Dickinson).
Western blotting.
CD3+ cells (106 cells/ml) were purified from the spleens of C57BL/6 mice and incubated with 10 ng/ml IL-1, 10 ng/ml IL-23, or IL-1 and IL-23 in the presence or absence of 1 μg/ml anti-CD3. Cell lysates were prepared and resolved on 12% SDS-PAGE gels, transferred onto nitrocellulose membranes, and blocked. Membranes were incubated with antibodies specific for phospho-Akt and phospho-IκBα (Cell Signaling Technology) and β-actin (Sigma-Aldrich). After washing, the membranes were incubated with horseradish peroxidase–labeled secondary antibodies for 1 h at room temperature and developed by chemiluminescence (LiteAblot; Euroclone).
Real-time PCR.
CD3+ T cells (3.5 × 106 cells/ml) were stimulated with 1 μg/ml anti-CD3 and 10 ng/ml IL-1, 10 ng/ml IL-23, or IL-1 and IL-23. RNA was harvested from the cells 48 h later using an RNEasy isolation kit (QIAGEN) followed by reverse transcription using a QuantiTect Reverse transcription kit (QIAGEN). Real-time PCR for the detection of IL-17 mRNA was performed using sense and antisense primers for IL-17A (Mm00439619_m1; Applied Biosystems) and a FAM-labeled MGB Taqman probe. 18S ribosomal RNA was used as an endogenous control. Samples were assayed on an Applied Biosystems 7300 PCR machine.
Statistical analysis.
Cytokine levels were compared by one-way ANOVA. Where significant differences were found, the Tukey-Kramer multiple comparisons test was used to identify differences between individual groups. Significance of the results from real-time PCR was determined using Newman-Keul's test.
Acknowledgments
We would like to thank Dr. Thomas Connor, Department of Physiology, Trinity College Dublin, for help with real-time PCR.
This work was supported by a Science Foundation Ireland Principal Investigator Award (no. 00/PI.I/B045 to K.H.G. Mills).
K.H.G. Mills is a founder, director, and shareholder in Opsona Therapeutics, a University campus company involved in the development of antiinflammatory therapeutics. The authors declare no other potential conflicts of interest.
K.H.G. Mills and E.C. Lavelle contributed equally to this work.
B. Keogh's present address is Opsona Therapeutics Ltd., Institute for Molecular Medicine, Trinity College, Dublin 2, Ireland.
References
- 1.Steinman, L. 2001. Multiple sclerosis: a two-stage disease. Nat. Immunol. 2:762–764. [DOI] [PubMed] [Google Scholar]
- 2.McKenzie, B.S., R.A. Kastelein, and D.J. Cua. 2006. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 27:17–23. [DOI] [PubMed] [Google Scholar]
- 3.Krakowski, M., and T. Owens. 1996. Interferon-gamma confers resistance to experimental allergic encephalomyelitis. Eur. J. Immunol. 26:1641–1646. [DOI] [PubMed] [Google Scholar]
- 4.Cua, D.J., J. Sherlock, Y. Chen, C.A. Murphy, B. Joyce, B. Seymour, L. Lucian, W. To, S. Kwan, T. Churakova, et al. 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 421:744–748. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal, S., N. Ghilardi, M.H. Xie, F.J. de Sauvage, and A.L. Gurney. 2003. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278:1910–1914. [DOI] [PubMed] [Google Scholar]
- 6.Langrish, C.L., Y. Chen, W.M. Blumenschein, J. Mattson, B. Basham, J.D. Sedgwick, T. McClanahan, R.A. Kastelein, and D.J. Cua. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy, C.A., C.L. Langrish, Y. Chen, W. Blumenschein, T. McClanahan, R.A. Kastelein, J.D. Sedgwick, and D.J. Cua. 2003. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 198:1951–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrington, L.E., R.D. Hatton, P.R. Mangan, H. Turner, T.L. Murphy, K.M. Murphy, and C.T. Weaver. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6:1123–1132. [DOI] [PubMed] [Google Scholar]
- 9.Park, H., Z. Li, X.O. Yang, S.H. Chang, R. Nurieva, Y.H. Wang, Y. Wang, L. Hood, Z. Zhu, Q. Tian, and C. Dong. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6:1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koenders, M.I., J.K. Kolls, B. Oppers-Walgreen, L. van den Bersselaar, L.A. Joosten, J.R. Schurr, P. Schwarzenberger, W.B. van den Berg, and E. Lubberts. 2005. Interleukin-17 receptor deficiency results in impaired synovial expression of interleukin-1 and matrix metalloproteinases 3, 9, and 13 and prevents cartilage destruction during chronic reactivated streptococcal cell wall-induced arthritis. Arthritis Rheum. 52:3239–3247. [DOI] [PubMed] [Google Scholar]
- 11.Koenders, M.I., E. Lubberts, B. Oppers-Walgreen, L. van den Bersselaar, M.M. Helsen, F.E. Di Padova, A.M. Boots, H. Gram, L.A. Joosten, and W.B. van den Berg. 2005. Blocking of interleukin-17 during reactivation of experimental arthritis prevents joint inflammation and bone erosion by decreasing RANKL and interleukin-1. Am. J. Pathol. 167:141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schiffenbauer, J., W.J. Streit, E. Butfiloski, M. LaBow, C. Edwards III, and L.L. Moldawer. 2000. The induction of EAE is only partially dependent on TNF receptor signaling but requires the IL-1 type I receptor. Clin. Immunol. 95:117–123. [DOI] [PubMed] [Google Scholar]
- 13.de Jong, B.A., T.W. Huizinga, E.L. Bollen, B.M. Uitdehaag, G.P. Bosma, M.A. van Buchem, E.J. Remarque, A.C. Burgmans, N.F. Kalkers, C.H. Polman, and R.G. Westendorp. 2002. Production of IL-1beta and IL-1Ra as risk factors for susceptibility and progression of relapse-onset multiple sclerosis. J. Neuroimmunol. 126:172–179. [DOI] [PubMed] [Google Scholar]
- 14.Matsuki, T., S. Nakae, K. Sudo, R. Horai, and Y. Iwakura. 2006. Abnormal T cell activation caused by the imbalance of the IL-1/IL-1R antagonist system is responsible for the development of experimental autoimmune encephalomyelitis. Int. Immunol. 18:399–407. [DOI] [PubMed] [Google Scholar]
- 15.Hofstetter, H.H., S.M. Ibrahim, D. Koczan, N. Kruse, A. Weishaupt, K.V. Toyka, and R. Gold. 2005. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell. Immunol. 237:123–130. [DOI] [PubMed] [Google Scholar]
- 16.Sospedra, M., and R. Martin. 2005. Immunology of multiple sclerosis. Annu. Rev. Immunol. 23:683–747. [DOI] [PubMed] [Google Scholar]
- 17.Veldhoen, M., R.J. Hocking, C.J. Atkins, R.M. Locksley, and B. Stockinger. 2006. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 24:179–189. [DOI] [PubMed] [Google Scholar]
- 18.Mangan, P.R., L.E. Harrington, B. O'Quinn, W.S. Helms, D.C. Bullard, C.O. Elson, R.D. Hatton, S.M. Wahl, T.R. Schoeb, and C.T. Weaver. 2006. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 441:231–234. [DOI] [PubMed] [Google Scholar]
- 19.Bettelli, E., Y. Carrier, W. Gao, T. Korn, T.B. Strom, M. Oukka, H.L. Weiner, and V.K. Kuchroo. 2006. Reciprocal developmental pathways for the generation of pathogenic effector T(H)17 and regulatory T cells. Nature. 441:235–238. [DOI] [PubMed] [Google Scholar]
- 20.Tan, S.L., J. Zhao, C. Bi, X.C. Chen, D.L. Hepburn, J. Wang, J.D. Sedgwick, S.R. Chintalacharuvu, and S. Na. 2006. Resistance to experimental autoimmune encephalomyelitis and impaired IL-17 production in protein kinase C theta-deficient mice. J. Immunol. 176:2872–2879. [DOI] [PubMed] [Google Scholar]
- 21.Solomou, E.E., Y.T. Juang, and G.C. Tsokos. 2001. Protein kinase C-theta participates in the activation of cyclic AMP-responsive element-binding protein and its subsequent binding to the -180 site of the IL-2 promoter in normal human T lymphocytes. J. Immunol. 166:5665–5674. [DOI] [PubMed] [Google Scholar]
- 22.Kim, K.W., M.L. Cho, M.K. Park, C.H. Yoon, S.H. Park, S.H. Lee, and H.Y. Kim. 2005. Increased interleukin-17 production via a phosphoinositide 3-kinase/Akt and nuclear factor kappaB-dependent pathway in patients with rheumatoid arthritis. Arthritis Res. Ther. 7:R139–R148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu, X.K., X. Lin, and S.L. Gaffen. 2004. Crucial role for nuclear factor of activated T cells in T cell receptor-mediated regulation of human interleukin-17. J. Biol. Chem. 279:52762–52771. [DOI] [PubMed] [Google Scholar]
- 24.Horai, R., A. Nakajima, K. Habiro, M. Kotani, S. Nakae, T. Matsuki, A. Nambu, S. Saijo, H. Kotaki, K. Sudo, et al. 2004. TNF-alpha is crucial for the development of autoimmune arthritis in IL-1 receptor antagonist-deficient mice. J. Clin. Invest. 114:1603–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakae, S., S. Saijo, R. Horai, K. Sudo, S. Mori, and Y. Iwakura. 2003. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc. Natl. Acad. Sci. USA. 100:5986–5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei, S., H. Kitaura, P. Zhou, F.P. Ross, and S.L. Teitelbaum. 2005. IL-1 mediates TNF-induced osteoclastogenesis. J. Clin. Invest. 115:282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gottlieb, A.B., F. Chamian, S. Masud, I. Cardinale, M.V. Abello, M.A. Lowes, F. Chen, M. Magliocco, and J.G. Krueger. 2005. TNF inhibition rapidly down-regulates multiple proinflammatory pathways in psoriasis plaques. J. Immunol. 175:2721–2729. [DOI] [PubMed] [Google Scholar]
- 28.Ruddy, M.J., G.C. Wong, X.K. Liu, H. Yamamoto, S. Kasayama, K.L. Kirkwood, and S.L. Gaffen. 2004. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J. Biol. Chem. 279:2559–2567. [DOI] [PubMed] [Google Scholar]