Expression of lymphotoxin-αβ on antigen-specific T cells is required for DC function (original) (raw)
Abstract
During an immune response, activated antigen (Ag)-specific T cells condition dendritic cells (DCs) to enhance DC function and survival within the inflamed draining lymph node (LN). It has been difficult to ascertain the role of the tumor necrosis factor (TNF) superfamily member lymphotoxin-αβ (LTαβ) in this process because signaling through the LTβ-receptor (LTβR) controls multiple aspects of lymphoid tissue organization. To resolve this, we have used an in vivo system where the expression of TNF family ligands is manipulated only on the Ag-specific T cells that interact with and condition Ag-bearing DCs. We report that LTαβ is a critical participant required for optimal DC function, independent of its described role in maintaining lymphoid tissue organization. In the absence of LTαβ or CD40L on Ag-specific T cells, DC dysfunction could be rescued in vivo via CD40 or LTβR stimulation, respectively, suggesting that these two pathways cooperate for optimal DC conditioning.
Efficient initiation of an immune response relies on the interaction of APCs that have acquired antigen (Ag) and rare Ag-specific T cells. DCs are widely accepted as the most potent APC because of their optimal positioning as sentinels in the periphery, their rapid migration to the draining LNs, their ability to acquire and present Ag on MHC class II, and their expression of the costimulatory molecules CD80 and CD86. Once in the draining LN, Ag-bearing DCs make intimate contacts with Ag-specific CD4+ T cells, so that the TCR is triggered by MHC class II and peptide (“signal 1”) and CD28 is engaged by CD80/CD86 expressed on the APC (“signal 2”), resulting in clonal expansion of Ag-specific T cells (1). In addition to signals 1 and 2 being delivered by APC to T cells, T cells also provide signals to “condition” cognate APC. This process is exemplified by engagement of DC-expressed CD40 by the TNF family member CD40L, which is induced on activated T cells (2). Conditioning, in combination with TLR signaling, provokes an inflammatory response from the DCs, including the up-regulation of CD80/CD86 and the secretion of IL-12p70 (3–5).
To achieve this mutually activating interaction between DCs and rare Ag-specific T cells, the spatial and temporal coordination of these encounters is facilitated by the existence of chemokine gradients, which guide DCs into the appropriate “niches” of the draining LN and recruit naive T cells via the high endothelial venules (HEVs) (6). In the spleen, the organization of lymphocytes and the expression of these chemokines are governed in part by the lymphotoxin (LT) pathway (7). The LT-β receptor (LTβR) is a TNF family receptor expressed on a variety of cell types, including follicular dendritic cells, HEVs, DCs, and macrophages. The expression of its ligand, LTαβ, is restricted to activated lymphocytes, NK cells, and a subset of follicular B cells. Complicating this picture, an additional LTβR ligand, LIGHT (which is homologous to LTs, exhibits inducible expression, and competes with herpes simplex virus glycoprotein D for herpes virus entry mediator (HVEM), a receptor expressed by T lymphocytes), is expressed by activated T cells and binds to another TNF family receptor, HVEM (8). Studies examining mice treated with a soluble decoy receptor (LTβR-Ig) to inhibit the LT pathway, as well as LTα, LTβ, or LTβR-deficient mice, have shown that LTβR signaling is required for DC homeostasis, the maintenance of a marginal zone, the appearance of follicular dendritic cells in the primary and secondary follicles, and the formation of germinal centers within the spleen (9).
Many rodent models of autoimmunity that are initiated by LN-resident T cells are sensitive to LTβR-Ig treatment (9). In these cases, the draining LN has somehow lost its ability to support effective T cell responses. This could be because LTβR-Ig treatment has been shown to modulate peripheral LN addressin (PNAd) expression on the HEV, thus, impairing naive lymphocyte trafficking to the LN (10). Another possibility is that Ag-presenting DCs fail to migrate to or persist within the draining LN (11). Signaling of LTβR on DCs was shown to be important for DC homeostasis in both the spleen and the LNs (12). In an inflammatory setting, however, it is not known whether signals delivered to LTβR on DCs are relevant for their ability to prime T cells.
In this study, we have used two methods for inhibiting LTβR signaling. In one set of experiments, we have examined the effects of inhibiting the LT–LIGHT pathways by administering LTβR-Ig to wild-type animals. In a second set of experiments, we have made use of an adoptive transfer approach where LTβR ligands are absent only on Ag-specific CD4+ T cells in the context of an animal with intact LTβR signaling and normal lymphoid architecture. Using this comparative approach, we show that global LTβR signaling is required for maximal expression of CD86 on Ag-bearing DCs and for efficient priming of CD4 and CD8 T cells. However, to our surprise, we found that in the context of a wild-type mouse with normal LTαβ expression and intact lymphoid microarchitecture, expression of LTαβ on Ag-specific T cells is required to condition DCs for CD4 and CD8 T cell priming ex vivo. Moreover, LTβ-deficient Ag-specific T cells exhibit delayed proliferation and reduced cytokine secretion in vivo. In the absence of LTβ on Ag-specific T cells, DC function can be recovered by administration of anti-CD40 agonistic antibody; likewise, the DC defect in the absence of CD40L on Ag-specific T cells can be overcome by administration of anti-LTβR agonistic antibody. Therefore, we propose that CD40 and LTβR signals can cooperate to promote full conditioning of DCs during the immune response to protein Ag.
RESULTS
LTβR signaling controls DC function ex vivo
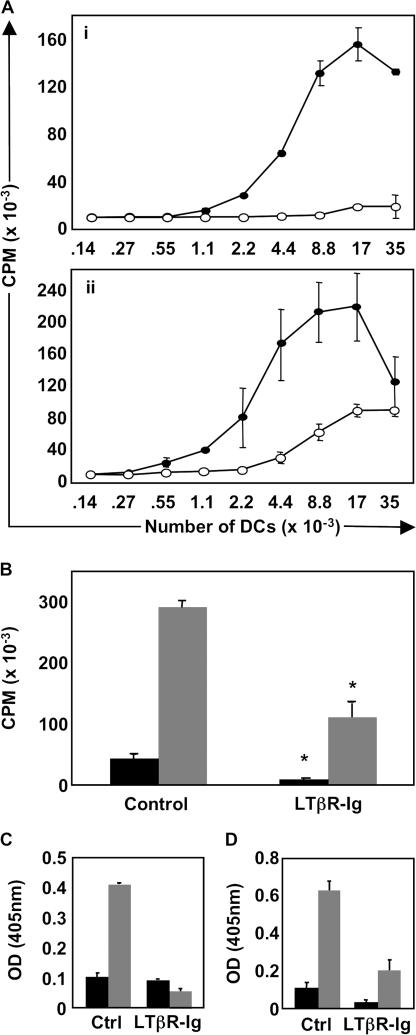
Given that LTβR signaling is required to maintain the organization of immune cells in the spleen, we evaluated the effects of global LTβR signaling inhibition on the stimulatory capacity of LN DCs during an immune response. To do this, we designed a system to recapitulate T cell–DC interactions during a brief 36-h time period in vivo. Specifically, mice were first treated with LTβR-Ig or control huIgG, followed by an adoptive transfer of OVA-specific Thy1.1+ OTII T cells and subsequent subcutaneous immunization with OVA protein and LPS. Draining LNs were isolated 36 h after immunization, and from them DCs were purified. To read-out DC function, we cocultured equal numbers of DCs derived from the LNs that drain OVA-immunized sites from control versus LTβR-Ig–treated mice with naive OTII T cells in vitro in the absence of exogenous Ag. DCs derived from control-treated OVA+LPS-immunized mice stimulated robust T cell proliferation in the absence of any exogenously added OVA. In contrast, however, DCs derived from LTβR-Ig–treated mice failed to stimulate naive T cell proliferation ex vivo, and these DCs were virtually inert (Fig. 1 A, i). To eliminate the possibility that DCs derived from LTβR-Ig–treated mice were simply dead/dying, we fed exogenous OVA323-339 peptide into our DC-OTII cocultures so that Ag is directly loaded onto MHC class II on the surface of DCs in vitro to induce potent OTII T cell proliferation (Fig. 1 A, ii). As expected, in vitro addition of OVA323-339 peptide to OTII T cells cocultured with DCs from either control Ig– or LTβR-Ig–treated mice resulted in OTII proliferation, although there was a two- to fourfold reduction in OTII proliferation if DCs were derived from LTβR-Ig–treated mice, suggesting that DCs from LTβR-Ig–treated mice are intrinsically not as potent as DCs from control-treated mice. These data demonstrate that DCs that acquire OVA in vivo absolutely require LTβR signaling for their function ex vivo, although DC function can be partially restored with addition of a strong agonistic peptide in vitro.
Figure 1.
Signals mediated by the LTβR control DC function. (A) The stimulating capacity of DCs from OVA-immunized mice treated with either control Ig or LTβR-Ig was evaluated ex vivo. Draining LN DCs were harvested from each huIgG control-treated (filled circles) and LTβR-Ig–treated (empty circles) mice and plated with naive OTII responder T cells either without (i) or with (ii) exogenous OVA peptide. (B) The experiment was repeated as in A, but using naive OTI responder T cells. Black bars, wells with 5,000 DCs; gray bars, 20,000 DCs. IFNγ secretion by responder OTII cells (C) or OTI cells (D) in the absence of added OVA peptide was quantified by measuring IFNγ levels in cultured supernatants by ELISA (gray bars, 20,000 input DCs; black bars, 140 input DCs). These experiments were performed three times on five mice per group with similar results.
DCs derived from LTβR-Ig–treated mice were also poorly stimulatory when cocultured with naive OVA-specific CD8 OTI T cells ex vivo, with OTI responder T cells exhibiting a significant reduction in proliferation (P < 0.005 at 20,000 DCs; P < 0.02 at 5,000 DCs; Fig. 1 B). As a measure of effector function, IFNγ secretion from both OTII (Fig. 1 C) and OTI T cells (Fig. 1 D) was quantified by ELISA. As expected, in the case of DCs derived from control-treated animals, high levels of T cell proliferation correlated with production of IFNγ from OTII (IFNγ = 0.88 ± 0.13 ng/ml) and OTI T cells (IFNγ = 1.933 ± 0.35 ng/ml), whereas DCs derived from LTβR-Ig–treated mice failed to induce secretion of detectable IFNγ from either OTII or OTI responder T cells (OD did not register in the linear portion of the standard curve), despite residual OTI proliferation in this assay (Fig. 1 B). Collectively, these data demonstrate that LTβR signaling is required for DCs to stimulate proliferation and IFNγ production by naive T cells ex vivo.
Antigen processing is independent of LTβR signaling
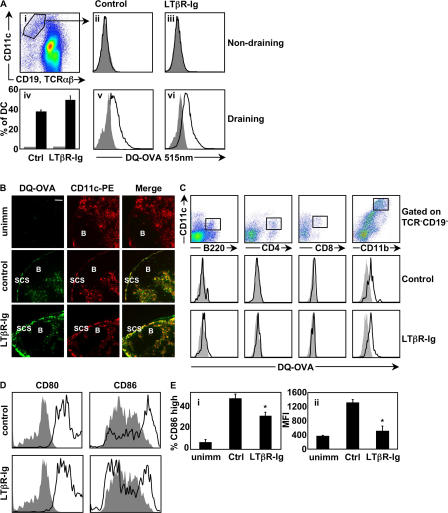
In these experiments, the in vitro activation of naive T cells by DCs relies entirely on Ag acquired in vivo. One explanation for the impaired function of DCs from LTβR-Ig–treated animals is that they have failed to acquire Ag, either because DCs at the site of immunization (skin) do not migrate to the draining LNs, or because Ag uptake and processing is impaired. To evaluate these possibilities, we immunized mice with DQ-OVA, which is a self-stimulating OVA conjugate that emits a fluorescent signal upon proteolytic processing, such as that which occurs during Ag processing (13). Therefore, we immunized control- or LTβR-Ig–treated C57BL/6 mice that had received adoptively transferred WT-OTII T cells with DQ-OVA+LPS s.c. Mice were killed 18 h after immunization, and draining and nondraining LN DCs were identified as CD11c+TCRβ−CD19− cells (Fig. 2 A, i). DCs in the nondraining inguinal LNs of both control (Fig. 2 A, ii) and LTβR-Ig–treated (Fig. 2 A, iii) mice had background fluorescence equivalent to DCs from unimmunized animals, suggesting that they had not taken up and processed DQ-OVA Ag. As expected, draining popliteal LN DCs from control-treated (Fig. 2 A, v) mice acquired and processed DQ-OVA; however, there was no defect in the capacity of DCs from LTβR-Ig–treated animals to acquire Ag (Fig. 2 A, iv and vi), suggesting that LTβR-Ig treatment does not impact Ag acquisition by DCs. DQ-OVA–derived fluorescence was localized predominantly to the T–B interface, and the subcapsular sinus of the draining LNs in both control and LTβR-Ig–treated animals, and DQ-OVA staining colocalized with CD11c staining (Fig. 2 B). In addition, the DC subset that acquired DQ-OVA was found to be a CD11c+CD11b+CD8−CD4−B220− DC subset in both control and LTβR-Ig–treated mice (Fig. 2 C). Collectively, acquisition of OVA by CD11b+ DC is not impaired in LTβR-Ig–treated mice.
Figure 2.
Expression of some DC maturation markers, but not antigen processing by DCs, is dependent on LTβR signaling. (A) Control or LTβR-Ig–treated mice received OTII T cells, were immunized with DQ-OVA+LPS, and DCs from draining (v and vi) and nondraining (ii and iii) LNs were gated as indicated (i) and analyzed for their processed DQ-OVA content by evaluating the fluorescence emitted at 515 nm. Gray-filled histograms are DCs from unimmunized mice, and empty histograms are DCs from DQ-OVA–immunized mice. Results are summarized in iv as a frequency of DCs that have acquired DQ-OVA, with gray bars representing unimmunized mice and black bars representing DQ-OVA–immunized mice. (B) Cryosections of popliteal LNs from either control-treated unimmunized or control versus LTβR-Ig–treated immunized mice were visualized with a 20× objective (Leica) for localization of proteolytically digested DQ-OVA and colocalization with CD11c+ DCs. B-cell follicles and the subcapsular sinus (SCS) are identified. (C) DC subsets were analyzed for DQ-OVA content by first eliminating TCR+CD19+ lymphocytes from analysis and gating on CD11c+ cells, as in A (i). Note that the CD11c Ab used in analyzing the CD11b+ DC subset was conjugated to a different fluorochrome than for the other FACS cocktails. (D) CD11c+ DQ-OVA+ DC were analyzed for the expression of CD80 and CD86. Percentage of CD86-high DC was tabulated, as well as the mean fluorescence intensity (MFI) (E). The flow cytometry experiment was performed twice on a total of 10 mice per group. The immunohistochemistry images are representative examples of six different mice per group. Bar, 100 μm.
Expression of surface markers on DCs from LTβR-Ig–treated mice
Using the DQ-OVA reagent, we sought to determine if Ag-bearing DCs from LTβR-Ig–treated mice exhibited any difference in accessory molecules that could account for their defective function ex vivo. We found that the level of expression of LTβR itself on DCs is stable and unaltered during inflammation in either treatment group (unpublished data). In addition, expression of MHCII and OX40-ligand were unaltered on DQ-OVA+ DCs from either treatment group, and we could not detect expression of CD70 at this early time point on DQ-OVA+ DCs in the draining LN (unpublished data). Ligation of CD40 has been shown to be a potent inducer of the expression of CD80/CD86, even more so than LPS (4). Therefore, we evaluated if LTβR signaling was likewise required for up-regulation of these costimulatory molecules by measuring the expression of CD80/CD86 on DQ-OVA+ DC from control versus LTβR-Ig–treated mice. Although CD80 levels were found to be normal, CD86 levels were substantially decreased (Fig. 2, D and E; P < 0.02 for E, i; P < 0.0007 for E, ii). Therefore, signaling through LTβR is required for full induction of CD86 on Ag-bearing DCs.
OTII T cells up-regulate LTβR ligands in response to Ag
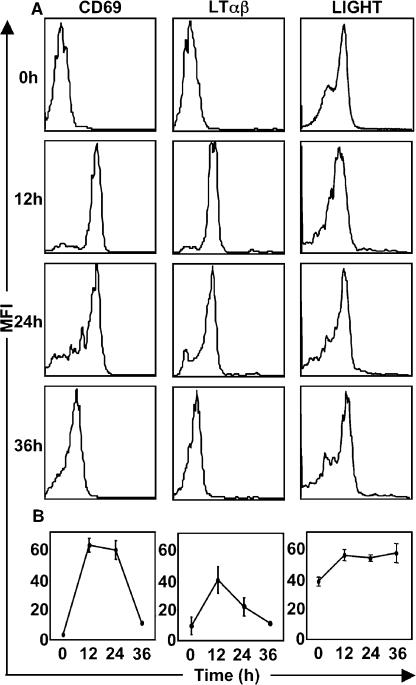
We next evaluated whether the expression of LTβR ligands (LIGHT, LTαβ) on Ag-activated T cells may be required for effective T cell–DC communication. The expression kinetics of CD40L on Ag-specific T cells in vivo has been shown to be different compared with what has been measured after in vitro stimulation (14). Because the kinetics of expression of LTαβ and LIGHT on Ag-specific T cells in vivo has not been previously evaluated, we assessed the expression of LTαβ and LIGHT during the time window relevant to our DC function experiments by evaluating the expression of LTαβ and LIGHT on adoptively transferred Thy1.1+ OTII-CD4+ T cells in vivo. Expression of the early activation markers CD69 and CD25 (not depicted) was used to identify Ag-activated T cells and, as expected, they were not expressed on naive CD4+ OTII T cells, but they were up-regulated by 12 h and continued to be expressed 24 h after immunization with OVA (Fig. 3 A). Elevated expression of LTαβ and LIGHT was observed by 12 h (Fig. 3 A) and sustained to varying degrees during the 36-h time course (Fig. 3 B). LIGHT was found to be more modestly up-regulated on OTII T cells in vivo, and the relatively low expression of LIGHT may be accounted for by rapid cleavage by matrix metalloproteinases (15). Interestingly, the peak expression of LTαβ coincides with that of CD69 (12 h), which corresponds with a period of intense contact between DCs and Ag-specific T cells (16). Because CD40L has also been shown to be up-regulated on OVA-specific CD4+ T cells during the same 36-h period (14), we hypothesized that in addition to CD40L, LTβR ligands may also play a role in DC conditioning.
Figure 3.
Expression of LTβR ligands on OTII CD4+ T cells in response to OVA antigen. Purified OTII T cells were adoptively transferred into C57BL/6 mice, which were then immunized with OVA. Thy1.1+ OTII T cells in the draining LNs were analyzed for the expression of CD69, LTαβ, and LIGHT. The expression of LTαβ and LIGHT on WT OTII T cells is demonstrated with representative histograms from five mice (A), and a kinetic analysis of expression of CD69, LTαβ, and LIGHT was generated (B). The experiment was performed twice with similar results. Expression on respective knockout T cells was insignificant over background (not depicted).
Adoptive transfers of T cells from LTβ−/−-, LIGHT−/−-, and WT-OTII mice
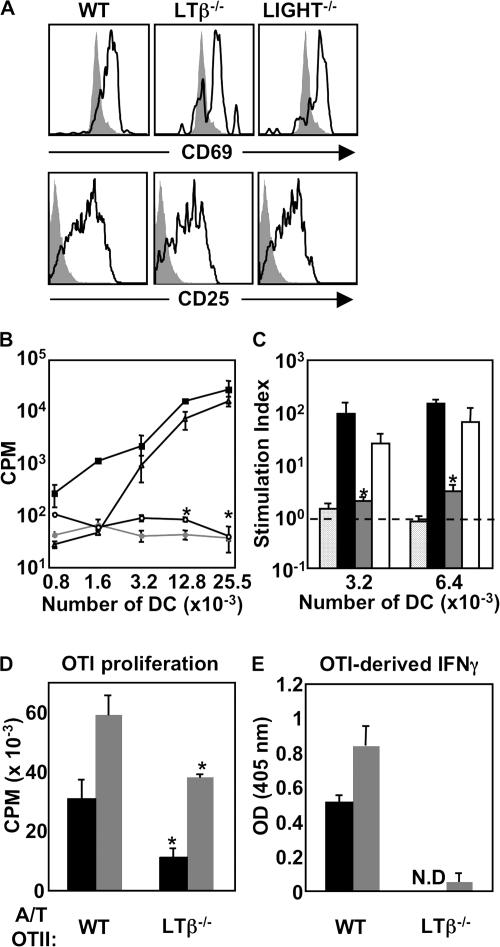
To identify a contribution of T cell–derived LTβR ligands to DC function, we generated LTβ−/−- and LIGHT−/−-OTII mice as a source of ligand-deficient, Ag-specific T cells. These knockout OTII T cells were then used as “DC-conditioning” T cells in adoptive transfer experiments. This experimental approach was imperative to dissect the role of LT pathway in DC activation, independent of the effects of LT pathway on the lymphoid microenvironment. Therefore, we first assessed whether LTβ−/−- or LIGHT−/−-OTII T cells could become activated in response to OVA Ag in vivo by using an adoptive transfer and immunization scheme whereby donor CFSE-labeled WT, LTβ−/−-, and LIGHT−/−-OTII T cells were transferred into C57BL/6 recipient mice 24 h before immunization. Mice were immunized subcutaneously with OVA protein and LPS, and 36 h after immunization, draining LNs were harvested for IHC or flow-cytometric analysis. In response to OVA immunization, WT, LTβ−/−-, and LIGHT−/−-OTII T cells each up-regulated CD25 and CD69 (Fig. 4 A), confirming normal early activation of LTβ−/−- and LIGHT−/−-OTII T cells in vivo.
Figure 4.
Function of DCs conditioned by adoptively transferred WT-, LTβ−/−-, or LIGHT−/−-OTII T cells. (A) C57BL/6 mice received WT-, LTβ−/−-, or LIGHT−/−-OTII T cells and were immunized, and LN cell suspensions were gated on CD4 and Thy1.1 and analyzed for CD69 and CD25 at 36 h after immunization, with filled histograms representing Thy1.1 T cells transferred into WT-unimmunized mice. (B) C57BL/6 mice received OVA-specific WT- (filled squares, filled circles), LTβ−/−- (empty circles), or LIGHT−/−-OTII T cells (empty triangles), or were immunized or left unimmunized (filled circles). At 36 h after immunization, draining LN DCs were plated with OTII responder T cells and incubated at 37°C for 72 h. (C) Proliferation results from B are represented as a SI. CPM derived from OTII T cells cocultured with DCs was divided by CPM derived from OTII T cells cocultured with the same number of DC-depleted cells at the same cell input number for each individual group (internally controlled). Groups are responder OTII T cells stimulated by DCs conditioned in vivo by WT- (black bars), LTβ−/−- (gray bars), or LIGHT−/−-OTII T cells (open bars) and compared with DCs from unimmunized mice that received WT-OTII T cells (speckled bars). (D) A similar experiment was performed using DCs conditioned by WT-OTII versus LTβ−/−-OTII to stimulate naive responder OTI T cells using 15,000 or 30,000 DCs per well (black and gray bars, respectively). (E) IFNγ secretion by OTI CD8+ T cells from was evaluated by ELISA using 15,000 or 30,000 DCs per well (black and gray bars, respectively). OTII responder experiments were performed four times using DCs pooled from seven individual animals. OTI responder experiments were performed two times using DCs pooled from seven individual animals.
LTαβ expressed on Ag-specific T cells is required for DC function ex vivo
To address the relative DC-conditioning potential of T cell–expressed LTαβ and LIGHT, we compared DC functionality from the draining LN of OVA-immunized C57BL/6 mice that received WT, LTβ−/−-, or LIGHT−/−-OTII T cells. In contrast to the experiments in Fig. 1, in this case, recipient mice have normal lymphoid microarchitecture, equivalent DC accumulation in the draining LN, and normal expression of LTβR ligands on all hematopoietic cells with the exception of the small transferred population of OVA-specific T cells. DCs from each group of mice were harvested, purified, serially diluted, and plated with naive OTII and OTI responder cells to measure DC stimulatory capacity as in Fig. 1 (A and B, respectively). In agreement with our findings from Fig. 1 A, DCs that interacted with WT-OTII T cells in vivo stimulated robust CD4 T cell proliferation (Fig. 4 B), with a stimulation index (SI) over background flow-through controls of 331.7 ± 10.9 in the wells containing the highest number of DCs (Fig. 4 C). DCs that interacted with LIGHT−/−-OTII T cells in vivo stimulated robust CD4 T cell proliferation roughly equivalent to DCs conditioned by WT-OTII T cells, with a SI of 201.1 ± 26.3; however, at lower numbers of DCs, there was a modest decrease in proliferation (Fig. 4 B; not statistically significant). Strikingly, DCs that interacted with T cells lacking LTαβ completely failed to stimulate naive CD4+ T cell proliferation (P < 0.03 for 3,200 DCs; P < 0.008 for 6,400 DCs). Indeed, DCs derived from OVA-immunized C57BL/6 mice that received LTβ−/−-OTII T cells were no better at stimulating naive responder OTII T cells in vitro than DCs derived from unimmunized mice (SI of 1.7 ± 1.1 for DCs conditioned by LTβ−/−-OTII T cells versus 1.0 ± 0.2 for DCs from unimmunized mice; P = 0.54). Likewise, in agreement with the data from Fig. 1 B, DCs that interacted with LTβ−/−-OTII T cells stimulated OTI T cells poorly ex vivo (P < 0.008 for 15,000 DCs; P < 0.03 for 30,000 DCs; Fig. 4 D). This poor proliferation correlated with undetectable levels of IFNγ in culture supernatants (OD did not register in the linear portion of the standard curve) compared with robust levels of IFNγ in culture supernatants of OTI T cells cocultured with DCs from mice that received WT-OTII T cells (5.19 ± 1.97 ng/ml; Fig. 4 E). These results identify a requirement for LTαβ on activated T cells for proper DC function in the context of a mouse with normal lymphoid microarchitecture.
LTβ–LTβR interactions are required for optimal priming of Ag-specific CD4 T cells in vivo
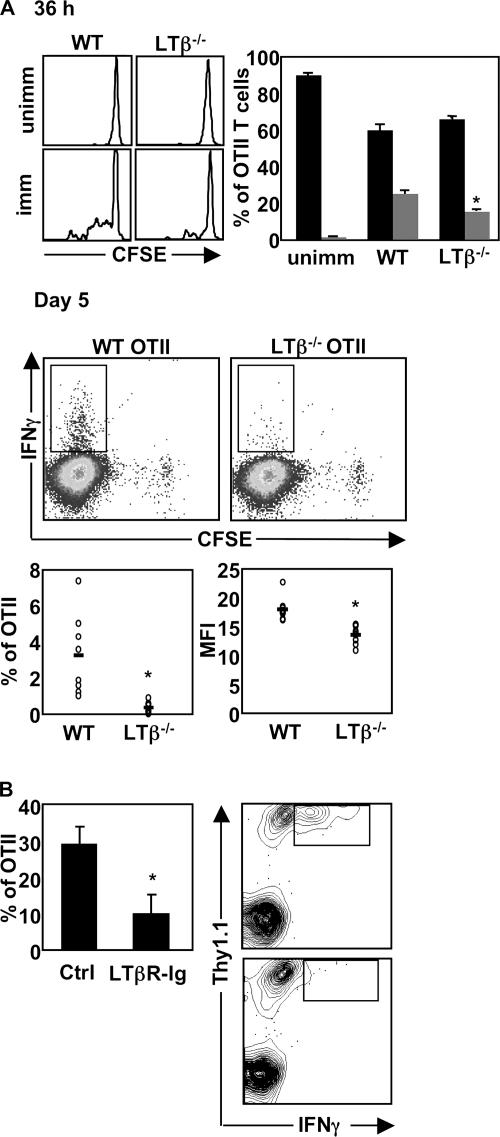
Using naive OTI and OTII T cells as “responder cells” in vitro, we have shown that DCs conditioned by LTβ−/− Ag- specific T cells in vivo have impaired function at 36 h after immunization. We next sought to determine if the DC defect observed in vitro could be manifested in vivo. We did not observe any differences in CD69 or CD25 up-regulation on WT versus LTβ−/− OVA-specific T cells at 36 h (Fig. 4 A), which is reminiscent of Ag-specific T cells primed in the absence of CD40 signaling where there are no obvious defects in delivery of signal 1 or 2 to OVA-specific T cells (Fig. 6 A and [reference 17]). However, close examination of cell division, as measured by loss of CFSE labeling, revealed a statistically significant reduction in the proportion of LTβ−/−-OTII T cells that divided compared with WT-OTII T cells in the draining LNs of OVA-immunized mice (Fig. 5 A; P < 0.02). To examine if this lag in cell division translates into impaired development of effector function, we evaluated the frequency of IFNγ+ OTII T cells at day 5 after immunization. We found a significant reduction in IFNγ+ LTβ−/−-OTII T cells compared with their WT counterparts at this time-point (Fig. 5 A; P < 0.008). In addition, the level of IFNγ secreted by LTβ−/−-OTII T cells was significantly reduced (Fig. 5 A; P < 0.0003).
Figure 6.
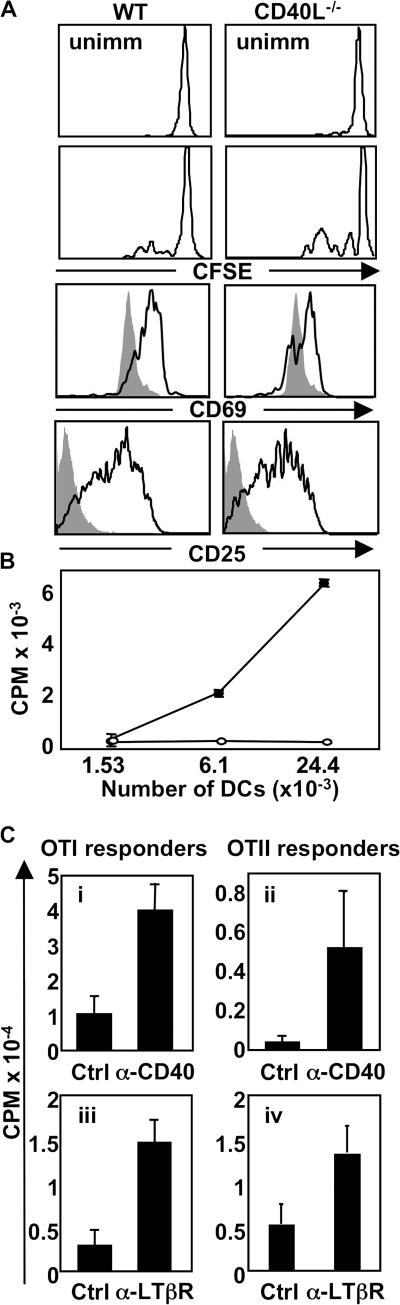
Expression of CD40L on OTII T cells is required for DC function, and agonistic anti-CD40 or -LTβR treatment can rescue DCs conditioned by LTβ−/−-OTII or CD40L−/−-OTII T cells, respectively. (A) C57BL/6 mice received CFSE-labeled WT- or CD40L−/−-OTII T cells, were immunized with OVA-LPS s.c., or were left unimmunized. CFSE content and the expression of CD69 and CD25 were measured by FACS at 36 h after immunization. (B) Draining LN DCs from mice immunized as in A were plated with OTII responder T cells and incubated at 37°C for 72 h. Filled circles, WT-OTII T cells; empty circles, CD40L−/−-OTII T cells. (C) C57BL/6 mice received LTβ−/−- (i and ii) or CD40L−/−-OTII T cells (iii and iv), were immunized with OVA-LPS s.c., and were treated with the indicated agonistic Ab or control Ab. Purified draining LN DCs (20,000 DCs for i and ii; 10,000 DCs for iii and iv) were plated with OTI and OTII responder T cells and incubated at 37°C for 72 h. The experiments were performed two times with seven mice per group, except the experiment in C (i–ii), which was performed three times with seven mice per group.
Figure 5.
LTαβ–LTβR interactions are required for optimal CD4 priming in vivo. (A) C57BL/6 mice received CFSE-labeled, OVA-specific WT- or LTβ−/−-OTII T cells, were immunized with OVA+LPS, or were left unimmunized. At 36 h after immunization, OTII CFSE content was assessed, comparing baseline CFSE content for each individual genotype. At day 5 after immunization, secretion of IFNγ from OTII T cells was assessed by intracellular FACS. (B) C57BL/6 mice received OVA-specific WT-OTII T cells, were treated with huIgG or LTβR-Ig, and were immunized with OVA+LPS. At day 7, the frequency of IFNγ+ OTII T cells was assessed. The experiments were performed twice with three to five mice per group in each experiment.
Although in the first 36 h after immunization, endogenous WT helper cells are present at very low frequencies, endogenous LTαβ+ OVA-specific T cells will become primed and expand at later time points in the immune response, potentially rescuing DC function in vivo. Therefore, we reassessed if LTβR signaling was important for CD4 T cell effector function using the LTβR-Ig inhibitor to block any LTβR signaling triggered by LTαβ on endogenous OVA-specific T cells. In the context of global LTβR signal inhibition, we found that WT OTII T cells also produced significantly less IFNγ (Fig. 5 B; P < 0.05), which is in agreement with the observed reduction in IFNγ production by LTβ−/−-OTII T cells shown in Fig. 5 A. Therefore, consistent with a DC defect manifested in vitro, LTβ−/−-OTII T cells exhibit suboptimal proliferation and effector function in vivo.
CD40 signals are also required for DC conditioning and can rescue poorly conditioned DCs
It is well accepted that CD40 signaling on DCs is required for full expression of costimulatory molecules and conditions DCs for optimal T cell priming (3–5). To confirm that this is the case in our system, we generated CD40L−/−-OTII mice and performed adoptive transfer experiments identical to those in Fig. 4. Similar to LTβ−/−-OTII T cells, we observed no defect in the up-regulation of early activation markers CD69 and CD25 on CD40L−/−-OTII T cells in response to OVA immunization, and there did not appear to be any defect in CD40L−/−-OTII T cell division, as measured by CFSE dilution (Fig. 6 A). However, DCs that were conditioned by CD40L−/−-OTII T cells in vivo were profoundly impaired in their ability to induce naive OTII T cell proliferation ex vivo (Fig. 6 B; P < 0.008 at both 6.3 × 103 and 24.4 × 103 input DCs). Therefore, in our system, expression of CD40L and LTαβ on Ag-specific T cells are both required for optimal DC conditioning in vivo.
Because both LTβ−/−- and CD40L−/− -OTII T cells fail to adequately condition DCs in vivo, CD40L and LTαβ presumably exert nonredundant functions for DC conditioning. Nonetheless, it is possible that very strong signals delivered through CD40 may be able to rescue defective DC function in mice that received LTβ−/−-OTII T cells. To test this, we coinjected either control Ab or agonistic anti-CD40 Ab with adoptively transferred LTβ−/−-OTII T cells and compared DC function ex vivo. In contrast to DCs derived from control Ab-treated mice, DCs from mice that received LTβ−/− -OTII T cells and were concomitantly treated with anti-CD40 agonistic Ab in vivo could stimulate the proliferation of both OTI and OTII T cells ex vivo (Fig. 6 C, i and ii). Likewise, strong signals delivered through the LTβR by administration of agonistic anti-LTβR Ab were able to recover DC function from mice that received CD40L−/−-OTII T cells as measured by the ability to provoke OTI and OTII T cell proliferation ex vivo (Fig. 6 C, iii and iv). Therefore, although both LTαβ and CD40L expression on Ag-specific T cells are both required for optimal DC conditioning in vivo, DC function can be recovered by enforced and sustained signaling through either CD40 or LTβR in vivo, suggesting potential cooperation between both pathways.
DISCUSSION
We have shown that global inhibition of LTβR signaling impairs DC function. Our initial hypothesis was that LT pathway controls DC function by virtue of its overarching organization of the DCs within the LN stroma. However, in an experimental system uncomplicated by the myriad effects exerted by LTβR-Ig treatment, expression of LTαβ on Ag-specific T cells is required for DC function ex vivo.
Before this study, there were some hints in the literature of a role for LTβR signaling in DC function independent of LIGHT. For example, DCs derived from LTβ−/− mice exhibit reduced IL-12 secretion in response to LPS stimulation ex vivo (18), and DC numbers in the spleen are sustained by LTαβ expression (11, 19). Furthermore, ligation of LTβR with agonistic antibodies in combination with GM-CSF induces the maturation of bone marrow–derived DCs (12). In parallel with these collective hints, we have now shown for the first time that activated Ag-specific T cell must express LTαβ to condition DCs in vivo using CD4 proliferation/IFNγ secretion as readouts. Importantly, we have been able to distinguish the effects of LTβR signaling during the immune response independent of how these signals control the organization of the lymphoid microenvironment. We also observed that OTI CD8+ T cell proliferation was impaired when cocultured with DCs from LTβR-Ig–treated mice or mice that received LTβ−/−-OTII T cells, and the capacity of OTI responder T cells to secrete IFNγ was ablated. Finally, we have shown that signaling through LTβR is required for the maximal up-regulation of CD86 on Ag-bearing DCs, demonstrating that signals mediated by LTβR are critical at the earliest stages of DC maturation.
Several groups have demonstrated a role for LIGHT in mediating CD8 T cell responses, particularly alloresponses (20, 21). These defects in alloresponses in the absence of LIGHT-mediated signaling have prompted examinations into the role of LIGHT in DC-driven function. In agreement, Morel et al. found that LIGHT could enhance CD40L-delivered signals to induce a mature DC phenotype, although they did not confirm whether the LIGHT- mediated signals were through HVEM or LTβR on the DCs (22). We found that although expression of LIGHT on OTII T cells is not absolutely required for DC function, there was a modest, nonsignificant reduction in DC function when DCs were plated at lower numbers. Therefore, we hypothesize that, in the context of protein Ag, LIGHT may play a secondary role in DC function, and can perhaps be compensated for by other stimulatory signals in vivo, such as CD40L.
We have found that DQ-OVA–bearing DCs from control versus LTβR-Ig–treated mice express equivalent levels of MHC class II, CD80, and OX40-ligand. This could explain why we found that LTβ−/−-OTII T cells exhibit relatively normal early T cell activation. This is similar to what has been observed in CD40−/− mice (17), and in agreement, we find that CD40L−/−-OTII T cells also exhibit normal early T cell activation (Fig. 6). As opposed to an in vitro system consisting of only DCs and Ag-specific T cells, the context of a highly inflamed LN is presumably adequate for inducing initial T cell activation in vivo in cases where CD40 or LTβR signals on DCs are absent. However, this in vivo context may not necessarily promote competent T cell immunity. Indeed, when we carefully examined CFSE dilution of LTβ−/−-OTII T cells we observed a lag in cell division. Moreover, a significantly lower frequency of LTβ−/−-OTII T cells develop the capacity to secrete IFNγ in vivo, which is consistent with previously reported global reductions in serum IFNγ levels in LTβR-Ig–treated, collagen-immunized mice (23). Curiously, the CD4 response in LCMV-infected CD40L−/− mice was found to be relatively intact early on, but activated CD4 T cells were found to decrease in frequency 5 d after immunization (24). Therefore, subtle problems in T cell–DC interactions caused by the absence of LTβR and/or CD40 signaling on DCs may result in more compounded defects in CD4 T cell function later in the immune response.
There are several putative mechanisms that could explain the DC defect resulting from impaired LTβR signaling. Our experiments with DQ-OVA rule out a role for LTβR signaling in mediating Ag uptake and processing, although we cannot eliminate Ag-presentation as a possible defect. Interestingly, Ag-bearing DCs from LTβR-Ig–treated mice exhibited a significant decrease in the expression of CD86 that could lead to selective engagement of CTLA-4, rather than CD28, at the immunological synapse (25). In addition, we found that enforced signaling through CD40 with an agonistic Ab could restore DC function in mice that had received LTβ−/−-OTII T cells, suggesting that the two pathways may be complimentary. It should be noted that anti-CD40 treatment can induce LTαβ on resting B cells in vitro (26, 27) and in vivo (unpublished data). Thus, the effects of anti-CD40 Ab may have been to provoke LTβR signaling on DCs by provision of LTαβ in trans. This, combined with our observation that anti-LTβR agonistic Ab can rescue DC function in mice that had received CD40L−/−-OTII T cells suggests that LTβR signaling in DCs may provide important DC maturation signals downstream of CD40 activation. However, it should be noted that treatment with agonistic Abs over the entire 36-h time course of our experiment will result in signaling of other receptor-positive cells, including stromal cells, which could induce the secretion of unidentified factors that enhance DC function. Nonetheless, under circumstances where agonistic Abs are not administered, our data show that CD40L expression on Ag-activated T cells cannot compensate for the absence of LTαβ expression and vice versa.
This nonredundant role for CD40 versus LTβR signaling in DC function is curious, and whether this is caused by an altered “signalsome” assembled by CD40 versus LTβR warrants examination. Signals delivered by CD40 have been shown to use TNF receptor–associated factor 6 (TRAF6) for their exerted effects in DCs (28). However, a TRAF6 consensus-binding site (PXEXX) is lacking in LTβR, and LTβR does not coimmunoprecipitate with TRAF6 (28), thus, revealing a potential difference between these two TNF family receptors. In addition, although both CD40 and LTβR activate the alternative and canonical NFκB pathways, one could imagine a scenario where CD40 and LTβR exert qualitatively and temporally distinct signaling cascades in maturing DCs, resulting in the expression of different sets of genes that shape the inflammatory response. It is also possible that LTβR signaling has a particular propensity to activate the alternative NFκB pathway in DCs (29), and this may be highly relevant to DC function in vivo.
Our data provide a potential explanation for the efficacy of LTβR-Ig treatment in many autoimmune disease models and suggest that this efficacy may be caused by poor DC function. This could, in turn, result in the generation of “tolerogenic” DCs. For example, although LTβR-Ig treatment has no effect on the initiation of relapsing-remitting EAE (R-EAE), this treatment prevents subsequent relapses, suggesting that encephalogenic T cells have acquired a tolerized phenotype in this setting (30). Collectively, we hypothesize that T cells primed by DCs that do not receive signals through the LTβR ultimately fail to acquire effector function; thus, LTβR may be providing unique signals to DCs that ultimately shape inflammatory T cell responses.
MATERIALS AND METHODS
Mice.
WT C57BL/6 mice were either bred in-house or obtained from The Jackson Laboratory. C57BL/6 OTII Thy1.1+ transgenic mice (31) were obtained from the Jackson Laboratory and were bred in-house. LTβ−/− mice were generated using C57BL/6 embryonic stem cells (32), and were purchased from B&K Universal. LIGHT−/− mice were generated by K. Pfeffer (21) and were backcrossed to C57BL/6 for at least seven generations. The C57BL/6 background for LIGHT−/− mice was confirmed by performing a tail skin graft experiment with LIGHT−/− tail tissue transplanted to C57BL/6 tails (unpublished data). No rejection was observed during a 6-wk period, indicating that for the LIGHT−/− mice, genes governing graft rejection were derived from the C57BL/6 background. Each LT_β_−/− and LIGHT−/− mouse was crossed with OTII Thy1.1 males. The LT pathway has been implicated in the maintenance of mature thymic medullary epithelial cells, as well as thymic expression of the autoimmune regulator gene (33). We evaluated thymic development of T cells in the ligand knockout TCR transgenic mice and noted normal thymic cellularity and unaltered frequency of all thymic subsets in each genotype (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20061968/DC1). All animals were housed in specific pathogen-free conditions. In all cases, 6–10-wk-old age-matched male mice were used for comparative studies. All experiments were performed according to the University of Toronto approved animal use protocols.
T cell purifications and adoptive transfers.
Single-cell suspensions of LN and spleen from WT and knockout OTII donor mice (spleen only from LTβ−/−-OTII mice), as well as OTI donor mice, were prepared. CD4+ and CD8+ T cells were enriched using CD4+ T-cell negative selection and CD8+ T-cell negative selection magnetic beads, respectively (Stem Cell Technologies). After purification, 5 × 107 OTII T cells/ml were stained in 2.5 nM CFSE (Invitrogen) in PBS for 10 min at 37°C. Enrichment for T cells was confirmed by flow cytometry and was typically 85–95% pure. 1–3 × 106 CD4+ T cells were injected i.v. in the tail vein of each mouse 1 d before immunization (day –1).
Immunizations and treatments.
At the time of adoptive transfer, mice were treated with 100 μg of either LTβR-Ig fusion protein versus human Ig control protein (a gift from J. Browning, Biogen Idec, Inc., Cambridge, MA). 24 h later, mice were immunized s.c. in the back (day 0) with a total of 1 mg of OVA protein and 20 μg of LPS (Sigma-Aldrich) dissolved in a volume of 200 μl of PBS. Importantly, we have found in our system that ex vivo DC function was poor in the absence of LPS coadministration (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20061968/DC1). For i.p. immunizations, mice were immunized with a total of 1 mg of OVA protein and 20 μg of LPS. For in vivo experiments using agonistic anti-CD40 (a gift from R. Mittler, Emory University, Atlanta, GA) or anti-LTβR antibodies (a gift from J. Browning), mice were treated with 100 μg of agonistic antibody or hamster Ab control at the time of immunization, and received adoptive transfers and were immunized as for s.c. immunization.
Immunohistochemistry.
Immunohistochemistry was performed as previously described (34). In brief, 36 h after immunization with OVA, mice were killed and immediately perfused with 30 ml of cold PBS. Draining popliteal LNs were collected and embedded in OCT compound (Tissue-Tek) and flash frozen in a bath of 2-methylbutane chilled on dry ice. 5-μm-thick cryostat sections were stained with antibodies to CD11c.
Antibodies and staining.
Antibodies to Vβ5.1, Thy1.1, CD11c, and CD40L (MR1) were obtained from BD Biosciences. Antibodies to CD4, TCRβ, CD25, and CD19, as well as all streptavidin fluorescent conjugates, were obtained from eBioscience. Anti-LTβ hamster monoclonal antibody BBF6 and hamster antibody control HA4/8 were gifts from J. Browning and have been previously described (30). Antibodies to CD69 and CD11c were both gifts from T. Watts (University of Toronto, Toronto, Canada). T cell purity after magnetic bead isolation was based on the percentage of CD4+Thy1.1+Vβ5.1+ cells, and DC purity was based on the percentage of CD11c+MHC II+TCRβ−CD19− cells. Ligand expression on OVA-specific OTII cells was determined by staining with anti-Thy1.1, anti-CD4, anti-CD69, anti-CD25, and the respective ligand-specific antibody cocktails. For LIGHT staining we first blocked with anti-LTβ BBF6, followed by LTβR-Ig and PE-conjugated anti–human Ig (Jackson ImmunoResearch Laboratories); for LTαβ staining we stained with anti-LTβ BBF6, followed by FITC-conjugated anti–hamster Ig (Jackson ImmunoResearch Laboratories); for anti-CD40L staining, we stained with biotinylated MR1, followed by fluorochrome-conjugated streptavidin.
For intracellular IFNγ staining, cells were incubated for 5 h with OVA323-339 ex vivo (Hospital for Sick Children Peptide Facility, Toronto, ON) and subsequently stained with Ab against CD4, CD8, and Thy1.1 on ice for 30 min. Cells were washed three times and incubated in 100 μl of Cytofix/Cytoperm (BD Biosciences) for 20 min on ice. Cells were washed with Perm/Wash (BD Biosciences), and incubated with a PE- or APC-conjugated anti-IFNγ antibody (eBioscience) diluted in Perm/Wash for 30 min on ice. Cells were washed twice and resuspended in Perm/Wash.
DC purifications.
LNs were suspended in Hank's Buffered Salt Solution (Invitrogen), 10 mM Hepes, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, and 1.8 mM CaCl2 supplemented with 1 mM collagenase D (Roche) and 60 μg/ml DNase I. LNs were mashed with glass slides, and the suspensions were incubated at 37°C with 5% CO2 for 30 min. At this time, tissues were disrupted by pipetting up and down, and suspensions were further incubated at 37°C with 5% CO2 for 10 min. EDTA was added to a final concentration of 1 mM, and the cell suspension was incubated at room temperature for 10 min. Cells were filtered (70 μm), spun, and resuspended in PBS/1 mM EDTA/2% FBS. DCs were enriched using CD11c+ positive selection kit (Stem Cell Technologies). The LN-derived, DC-enriched suspension typically comprised 80% CD11c+MHCII+TCRβ−CD19− DC, whereas the DC-depleted “flow-through” fraction did not contain detectable numbers of DCs (unpublished data).
DC stimulatory capacity assays.
36 h after s.c. immunization with OVA, mice were killed and DCs were purified from the draining LNs (axillary, inguinal, and brachial) by enriching DCs using CD11c+ positive selection kit. As a control, we plated the DC-depleted fraction with responder T cells and confirmed that DCs were the relevant, most potent APCs in our system (unpublished data). The DCs and flow-through fractions were irradiated (2,000 rads) and plated in quadruplicate wells in serial dilutions in RPMI-1640 medium (Sigma-Aldrich), 10% FBS/1% β-mercaptoethanol, 1% l-glutamine, 1% penicillin-streptavidin.
For responder cells, naive WT CD4+ OTII T cells and CD8+ OTI T cells were purified using negative selection beads (StemCell Technologies). They were plated with DCs or flow-through cells at 3 × 104 cells/well. In some cases, exogenous OVA323-339 peptide was added to half of the wells at 2 μg/ml. DC–T-cell cocultures were incubated at 37°C with 5% CO2 for 72 h. 100 μl of supernatant was removed for IFNγ quantification, and was replaced by 100 μl of fresh media with [3H]Thymidine ([3H]Td) at 0.1 μCi/ml (GE Healthcare); cultures were incubated for an additional 18 h and [3H]Td incorporation was measured. To calculate the SI, counts derived from DC cocultures were compared internally with the stimulation potential of the flow-through, DC-depleted fraction for each individual group.
ELISA.
96-well, round-bottom MaxiSorp immunoplates (Nunc) were coated overnight with anti–mouse IFNγ capture antibody (BD Biosciences). Nonspecific binding was blocked using PBS/1% BSA before adding supernatants. IFNγ was detected using biotinylated anti-IFNγ detection antibody (BD Biosciences), followed by HRP-conjugated SA (eBioscience), 2,2’-Amino-bis-3-ethylbenzthiazoline-6-sulfonic acid substrate (Sigma-Aldrich) and H2O2. Optical density was read at 405 nm. The concentration of IFNγ was extrapolated from an IFNγ standard (BD Biosciences) using the linear portion of the titration curve.
Tracers.
DQ-OVA was purchased from Invitrogen and diluted to 5 mg/ml in PBS (PBS). 100 μg of DQ-OVA was injected s.c. into the hind paw. 18 h later, draining popliteal LNs were harvested and DCs were analyzed for DQ-OVA content by detecting emissions of 515 nm by flow cytometry.
Online supplemental material.
Fig. S1 shows the thymic profiles of WT, LTβ−/−, LIGHT−/−, and CD40L−/− OTII mice. Fig. S2 shows that DCs require OVA+LPS in vivo to activate naive OTII T cells ex vivo.
Supplemental Material
[Supplemental Material index]
Acknowledgments
The authors wish to acknowledge Ms. Cheryl Smith, Mr. Edwin Young, Dr. Alberto Martin, Dr. Heinrich Korner, and Dr. Tania Watts for technical advice and reagents. We wish to also acknowledge Dr. Jeff Browning for providing LTβR-Ig, AFH6 and BBF6 reagents, Dr. Robert Mittler for anti-CD40 hybridoma, and Dr. Michael Ratcliffe for critical reading of the manuscript.
This work was supported by an operating grant from the Canadian Institutes of Health Research (CIHR/IRSC), a salary award to J.L. Gommerman from the CIHR, and a studentship award for L. Summers-deLuca from the Multiple Sclerosis Society of Canada. A Canadian Foundation for Innovation and Ontario Research Fund grant supported the acquisition of a FACS-ARIA for some of our analyses.
The authors have no conflicting financial interests.
Abbreviations used: FDC, follicular dendritic cell; HEV, high endothelial venule; HVEM, herpes virus entry mediator; LT, lymphotoxin; LTαβ, LT-αβ; LTβR, LTβ-receptor; SI, stimulation index; TRAF, TNF receptor–associated factor.
References
- 1.Mellman, I., and R.M. Steinman. 2001. Dendritic cells: specialized and regulated antigen processing machines. Cell. 106:255–258. [DOI] [PubMed] [Google Scholar]
- 2.Quezada, S.A., L.Z. Jarvinen, E.F. Lind, and R.J. Noelle. 2004. CD40/CD154 interactions at the interface of tolerance and immunity. Annu. Rev. Immunol. 22:307–328. [DOI] [PubMed] [Google Scholar]
- 3.Koch, F., U. Stanzl, P. Jennewein, K. Janke, C. Heufler, E. Kampgen, N. Romani, and G. Schuler. 1996. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J. Exp. Med. 184:741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cella, M., D. Scheidegger, K. Palmer-Lehmann, P. Lane, A. Lanzavecchia, and G. Alber. 1996. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 184:747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schulz, O., A.D. Edwards, M. Schito, J. Aliberti, S. Manickasingham, A. Sher, and C. Reis e Sousa. 2000. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 13:453–462. [DOI] [PubMed] [Google Scholar]
- 6.von Andrian, U.H., and T.R. Mempel. 2003. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 3:867–878. [DOI] [PubMed] [Google Scholar]
- 7.Ngo, V.N., H. Korner, M.D. Gunn, K.N. Schmidt, D. Sean Riminton, M.D. Cooper, J.L. Browning, J.D. Sedgwick, and J.G. Cyster. 1999. Lymphotoxin α/β and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J. Exp. Med. 189:403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mauri, D.N., R. Ebner, R.I. Montgomery, K.D. Kochel, T.C. Cheung, G.L. Yu, S. Ruben, M. Murphy, R.J. Eisenberg, G.H. Cohen, et al. 1998. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity. 8:21–30. [DOI] [PubMed] [Google Scholar]
- 9.Gommerman, J.L., and J.L. Browning. 2003. Lymphotoxin/LIGHT, lymphoid microenvironments and autoimmune disease. Nat. Rev. Immunol. 3:642–655. [DOI] [PubMed] [Google Scholar]
- 10.Browning, J.L., N. Allaire, A. Ngam-ek, E. Notidis, J. Hunt, S. Perrin, and R.A. Fava. 2005. Lymphotoxin-beta receptor signaling is required for the homeostatic control of HEV differentiation and function. Immunity. In press. [DOI] [PubMed]
- 11.Wu, Q., Y. Wang, J. Wang, E.O. Hedgeman, J.L. Browning, and Y.X. Fu. 1999. The requirement of membrane lymphotoxin for the presence of dendritic cells in lymphoid tissues. J. Exp. Med. 190:629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kabashima, K., T.A. Banks, K.M. Ansel, T.T. Lu, C.F. Ware, and J.G. Cyster. 2005. Intrinsic lymphotoxin-beta receptor requirement for homeostasis of lymphoid tissue dendritic cells. Immunity. 22:439–450. [DOI] [PubMed] [Google Scholar]
- 13.Sixt, M., N. Kanazawa, M. Selg, T. Samson, G. Roos, D.P. Reinhardt, R. Pabst, M.B. Lutz, and L. Sorokin. 2005. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 22:19–29. [DOI] [PubMed] [Google Scholar]
- 14.Hochweller, K., and S.M. Anderton. 2005. Kinetics of costimulatory molecule expression by T cells and dendritic cells during the induction of tolerance versus immunity in vivo. Eur. J. Immunol. 35:1086–1096. [DOI] [PubMed] [Google Scholar]
- 15.Morel, Y., J.M. Schiano de Colella, J. Harrop, K.C. Deen, S.D. Holmes, T.A. Wattam, S.S. Khandekar, A. Truneh, R.W. Sweet, J.A. Gastaut, et al. 2000. Reciprocal expression of the TNF family receptor herpes virus entry mediator and its ligand LIGHT on activated T cells: LIGHT down-regulates its own receptor. J. Immunol. 165:4397–4404. [DOI] [PubMed] [Google Scholar]
- 16.Mempel, T.R., S.E. Henrickson, and U.H. Von Andrian. 2004. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 427:154–159. [DOI] [PubMed] [Google Scholar]
- 17.Fujii, S., K. Liu, C. Smith, A.J. Bonito, and R.M. Steinman. 2004. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J. Exp. Med. 199:1607–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berger, D.P., D. Naniche, M.T. Crowley, P.A. Koni, R.A. Flavell, and M.B. Oldstone. 1999. Lymphotoxin-beta-deficient mice show defective antiviral immunity. Virology. 260:136–147. [DOI] [PubMed] [Google Scholar]
- 19.Abe, K., F.O. Yarovinsky, T. Murakami, A.N. Shakhov, A.V. Tumanov, D. Ito, L.N. Drutskaya, K. Pfeffer, D.V. Kuprash, K.L. Komschlies, and S.A. Nedospasov. 2003. Distinct contributions of TNF and LT cytokines to the development of dendritic cells in vitro and their recruitment in vivo. Blood. 101:1477–1483. [DOI] [PubMed] [Google Scholar]
- 20.Tamada, K., H. Tamura, D. Flies, Y.X. Fu, E. Celis, L.R. Pease, B.R. Blazar, and L. Chen. 2002. Blockade of LIGHT/LTbeta and CD40 signaling induces allospecific T cell anergy, preventing graft-versus-host disease. J. Clin. Invest. 109:549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheu, S., J. Alferink, T. Potzel, W. Barchet, U. Kalinke, and K. Pfeffer. 2002. Targeted disruption of LIGHT causes defects in costimulatory T cell activation and reveals cooperation with lymphotoxin β in mesenteric lymph node genesis. J. Exp. Med. 195:1613–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morel, Y., A. Truneh, R.W. Sweet, D. Olive, and R.T. Costello. 2001. The TNF superfamily members LIGHT and CD154 (CD40 ligand) costimulate induction of dendritic cell maturation and elicit specific CTL activity. J. Immunol. 167:2479–2486. [DOI] [PubMed] [Google Scholar]
- 23.Fava, R.A., E. Notidis, J. Hunt, V. Szanya, N. Ratcliffe, A. Ngam-Ek, A.R. De Fougerolles, A. Sprague, and J.L. Browning. 2003. A role for the lymphotoxin/LIGHT axis in the pathogenesis of murine collagen-induced arthritis. J. Immunol. 171:115–126. [DOI] [PubMed] [Google Scholar]
- 24.Borrow, P., D.F. Tough, D. Eto, A. Tishon, I.S. Grewal, J. Sprent, R.A. Flavell, and M.B. Oldstone. 1998. CD40 ligand-mediated interactions are involved in the generation of memory CD8(+) cytotoxic T lymphocytes (CTL) but are not required for the maintenance of CTL memory following virus infection. J. Virol. 72:7440–7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pentcheva-Hoang, T., J.G. Egen, K. Wojnoonski, and J.P. Allison. 2004. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 21:401–413. [DOI] [PubMed] [Google Scholar]
- 26.Worm, M., and R.S. Geha. 1994. CD40 ligation induces lymphotoxin alpha gene expression in human B cells. Int. Immunol. 6:1883–1890. [DOI] [PubMed] [Google Scholar]
- 27.Ansel, K.M., V.N. Ngo, P.L. Hyman, S.A. Luther, R. Forster, J.D. Sedgwick, J.L. Browning, M. Lipp, and J.G. Cyster. 2000. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 406:309–314. [DOI] [PubMed] [Google Scholar]
- 28.Ishida, T., S. Mizushima, S. Azuma, N. Kobayashi, T. Tojo, K. Suzuki, S. Aizawa, T. Watanabe, G. Mosialos, E. Kieff, et al. 1996. Identification of TRAF6, a novel tumor necrosis factor receptor-associated factor protein that mediates signaling from an amino-terminal domain of the CD40 cytoplasmic region. J. Biol. Chem. 271:28745–28748. [DOI] [PubMed] [Google Scholar]
- 29.Moore, F., S. Buonocore, E. Aksoy, N. Ouled-Haddou, S. Goriely, E. Lazarova, F. Paulart, C. Heirman, E. Vaeremans, K. Thielemans, et al. 2007. An alternative pathway of NF-{kappa}B activation results in maturation and T cell priming activity of dendritic cells overexpressing a mutated I{kappa}B{alpha}. J. Immunol. 178:1301–1311. [DOI] [PubMed] [Google Scholar]
- 30.Gommerman, J.L., K. Giza, S. Perper, I. Sizing, A. Ngam-ek, C. Nickerson-Nutter, and J.L. Browning. 2003. A role for surface lymphotoxin in experimental autoimmune encephalomyelitis independent of LIGHT. J. Clin. Invest. 112:755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnden, M.J., J. Allison, W.R. Heath, and F.R. Carbone. 1998. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 76:34–40. [DOI] [PubMed] [Google Scholar]
- 32.Wilhelm, P., D.S. Riminton, U. Ritter, F.A. Lemckert, C. Scheidig, R. Hoek, J.D. Sedgwick, and H. Korner. 2002. Membrane lymphotoxin contributes to anti-leishmanial immunity by controlling structural integrity of lymphoid organs. Eur. J. Immunol. 32:1993–2003. [DOI] [PubMed] [Google Scholar]
- 33.Boehm, T., S. Scheu, K. Pfeffer, and C.C. Bleul. 2003. Thymic medullary epithelial cell differentiation, thymocyte emigration, and the control of autoimmunity require lympho-epithelial cross talk via LTβR. J. Exp. Med. 5:757–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCarthy, D.D., S. Chiu, Y. Gao, L.E. Summers-deLuca, and J.L. Gommerman. 2006. BAFF induces a hyper-IgA syndrome in the intestinal lamina propria concomitant with IgA deposition in the kidney independent of LIGHT. Cell. Immunol. 241:85–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental Material index]