Identification of an IL-17–producing NK1.1neg iNKT cell population involved in airway neutrophilia (original) (raw)
Abstract
Invariant natural killer T (iNKT) cells are an important source of both T helper type 1 (Th1) and Th2 cytokines, through which they can exert beneficial, as well as deleterious, effects in a variety of inflammatory diseases. This functional heterogeneity raises the question of how far phenotypically distinct subpopulations are responsible for such contrasting activities. In this study, we identify a particular set of iNKT cells that lack the NK1.1 marker (NK1.1neg) and secrete high amounts of interleukin (IL)-17 and low levels of interferon (IFN)-γ and IL-4. NK1.1neg iNKT cells produce IL-17 upon synthetic (α-galactosylceramide [α-GalCer] or PBS-57), as well as natural (lipopolysaccharides or glycolipids derived from Sphingomonas wittichii and Borrelia burgdorferi), ligand stimulation. NK1.1neg iNKT cells are more frequent in the lung, which is consistent with a role in the natural immunity to inhaled antigens. Indeed, airway neutrophilia induced by α-GalCer or lipopolysaccharide instillation was significantly reduced in iNKT-cell–deficient Jα18−/− mice, which produced significantly less IL-17 in their bronchoalveolar lavage fluid than wild-type controls. Furthermore, airway neutrophilia was abolished by a single treatment with neutralizing monoclonal antibody against IL-17 before α-GalCer administration. Collectively, our findings reveal that NK1.1neg iNKT lymphocytes represent a new population of IL-17–producing cells that can contribute to neutrophil recruitment through preferential IL-17 secretion.
Invariant natural killer T (iNKT) cells constitute a distinctive population of mature T lymphocytes that coexpress a highly restricted TCR repertoire composed of a single invariant Vα14Jα18 chain in mice and a Vα24Jα18 chain in humans, preferentially paired with limited TCR Vβ chains (1–4). This semiinvariant TCR reflects a positive selection by glycolipid antigens presented by the nonpolymorphic MHC class I–like molecule CD1d (5). iNKT cells are well known for their prompt production of cytokines, such as IL-4, IFN-γ, TNF-α, IL-3, and GM-CSF, in response to the exogenous CD1d-bound glycolipid α-galactosylceramide (α-GalCer), the most commonly used stimulant, which was originally isolated from a marine sponge (2, 4–8). Recently, more physiological ligands have also been identified, i.e., the endogenous lysosomal glycosphingolipid isoglobotrihexosyl-ceramide (iGb3), exogenous glycosylceramides from the cell wall of Sphingomonas wittichii, and diacylglycerol antigens from pathogenic bacteria (9–12).
Because of their large cytokine spectrum, iNKT cells can interact with a variety of cells from the innate immune system and, consequently, affect the outcome of many inflammatory responses against pathogens (8, 13–15). In this line of evidence, it has been reported that they provide an early host protection against Streptococcus pneumonia by promoting the trafficking of neutrophils into airways (16). Moreover, we have previously demonstrated that a single injection of α-GalCer induces mobilization of myeloid progenitors (CFU cells) and neutrophils from the bone marrow to the periphery (8). Yet, it is still not clear how iNKT cells promote neutrophil recruitment to inflammatory sites and what mediators are involved.
The newly described cytokine IL-17 is a likely candidate for this task because it has already been implicated in airway neutrophilia induced by endotoxin exposure (17, 18). Furthermore, it has been documented that in IL-17 receptor– deficient mice, the host defense against lung bacterial infection is impaired (19).
Based in these data, we set out to examine whether stimulated iNKT cells were able to produce IL-17, and whether this cytokine mediated the neutrophil recruitment. We found that a small subset of iNKT cells lacking the NK1.1 marker generated high amounts of IL-17, together with low IL-4 and IFN-γ levels, in response to several iNKT cell ligands, namely, α-GalCer or its analogue PBS-57, as well as glycolipids derived from S. wittichii and Borrelia burgdorferi. This NK1.1neg iNKT cell subset was more frequent among lung iNKT cells, which is in accordance with a potential contribution to the airway neutrophilia elicited by intranasal (i.n.) exposure to α-GalCer, PBS-57, or LPS.
RESULTS AND DISCUSSION
α-GalCer stimulation induces IL-17 production
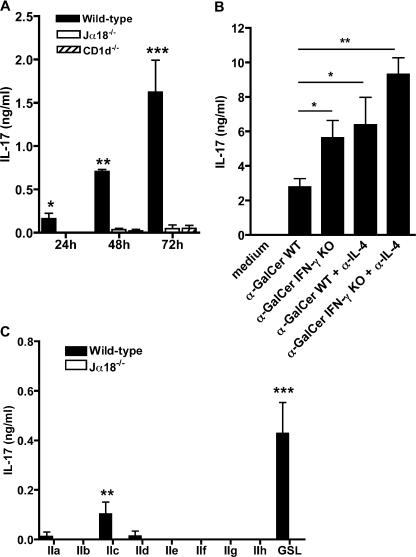
iNKT cells are plausible candidates for IL-17 production (20–24), considering that their biological activities overlap with most of those ascribed to this proinflammatory mediator. We tested this hypothesis using mononuclear cells (MNCs) isolated from the liver, where iNKT cells are more abundant than in other organs, and compared IL-17 production by total hepatic MNCs from wild-type C57BL/6 and iNKT cell-deficient (Jα18−/−) mice in response to the iNKT cell–specific antigen ligand α-GalCer. As shown in Fig. 1 A, IL-17 was easily detected in cell supernatants from wild- type mice and accumulated during the 72-h incubation period. In contrast, it failed to be produced by MNCs from Jα18−/− or from CD1d−/− mice (Fig. 1 A), which are both iNKT-cell deficient.
Figure 1.
iNKT cell ligands induce IL-17 production by liver MNCs. Liver MNCs from wild-type, Jα18−/−, and CD1d−/− mice were stimulated in vitro by α-GalCer (A) or synthetic B. burgdorferi glycolipids (BbGL-II [IIa–IIh]) or GalA-GSL (GSL) (C). (B) Liver MNCs from wild-type (WT) or IFN-γ−/− mice were stimulated with α-GalCer in the presence or absence of anti–IL-4 mAb. In all experiments, IL-17 levels were measured in supernatants. The addition of isotype controls did not modify IL-17 production by α-GalCer–stimulated liver MNCs and no cytokine were detected without ligand stimulation (not depicted). Data represent the mean ± the SD of two to seven individual mice. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We further addressed the question of whether the capacity to induce IL-17 production was shared by more physiological ligands of iNKT cells, such as glycosphingolipids from Sphingomonas sp and diacylglycerol antigens from B. burgdorferi, which causes Lyme disease (11, 12). We found that liver cells from wild-type, but not from Jα18−/−, mice produced IL-17 in response to the galacturonic acid–containing S. wittichii glycosphingolipid (GalA-GSL) and, to a lesser extent, to some synthetic variants of BbGLII from B. burgdorferi (Fig. 1 C). Our results concord with previous studies identifying BbGLIIc as the best BbGLII variant for iNKT cell activation (12) and prove that ligands with more physiological relevance than α-GalCer can also induce IL-17 production.
It has been widely documented that iNKT cells produce large amounts of both IFN-γ and IL-4 in response to α-GalCer (1–4). Knowing that both cytokines are potent inhibitors of IL-17 production (22, 23), we examined how this activity was affected when endogenous IFN-γ and/or IL-4 production was abolished in genetically modified IFN-γ−/− mice and/or in the presence of neutralizing anti–IL-4 mAbs. The lack of either cytokine resulted in a clear increase of IL-17 secretion after α-GalCer activation (Fig. 1 B), which was further enhanced in the absence of both, indicating that IL-4 and IFN-γ are produced endogenously and contribute similarly to the inhibition.
The iNKT NK1.1neg subset is the major source of IL-17 after α-GalCer stimulation
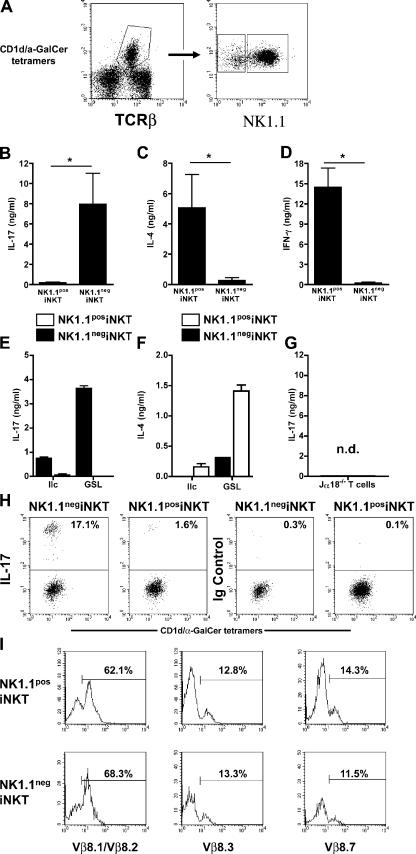
It is well established that α-GalCer acts specifically on iNKT cells (5). However, other cells could be secondarily stimulated and potentially produce IL-17 in our experimental model. To confirm the direct involvement of iNKT cells in IL-17 production, we gated the tetramer CD1d/α-GalCer+ population from hepatic MNCs and sorted them into two subsets according to their NK1.1 expression (Fig. 2 A). Upon stimulation with α-GalCer, IL-17 was only detected in supernatants of NK1.1neg iNKT cells (Fig. 2 B), along with very low amounts of IL-4 and IFN-γ (Fig. 2, C and D). In contrast, the NK1.1pos subset produced high levels of the latter two cytokines (Fig. 2, C and D), but little IL-17 (Fig. 2 B), proving that it responded normally to α-GalCer stimulation. GalA-GSL and BbGLIIc ligands also induced IL-17 production by sorted NK1.1neg, but not NK1.1pos, iNKT cells (Fig. 2 E). NK1.1pos iNKT cells were activated by these ligands because they produced IL-4 (Fig. 2 F). No detectable IL-17 production was observed when sorted T cells from Jα18−/− mice were stimulated with α-GalCer (Fig. 2 G). The conclusion that the NK1.1neg subset is the main source of IL-17 among iNKT cells was confirmed by intracellular cytokine staining, as shown in Fig. 2 H.
Figure 2.
NK1.1neg iNKT cells are the major iNKT subset producing IL-17. Liver MNCs from wild-type mice were stained with CD1d/α-GalCer tetramers, anti-TCRβ, and NK1.1 before sorting. (A) Representative FACS profiles obtained before (left) and after (right) sorting of CD1d/α-GalCer tetramers +NK1.1neg (NK1.1neg iNKT) and CD1d/α-GalCer tetramers +NK1.1pos (NK1.1pos iNKT) liver iNKT cells. (B–F) Sorted NK1.1neg iNKT and NK1.1pos iNKT liver MNCs were stimulated with α-GalCer (B–D) or synthetic B. burgdorferi glycolipids (BbGL-II [IIc]) or GalA-GSL (GSL; E and F) plus irradiated liver MNCs from Jα18−/− mice as APCs. Sorted CD4+CD62L+ T cells from Jα18−/− mice were stimulated with α-GalCer plus irradiated liver MNCs from Jα18−/− mice as APCs (G). 3 d later, IL-17 (B, E, and G), IL-4 (C and F), and IFN-γ (D) were measured in the supernatants. No cytokine was detected in the absence of α-GalCer stimulation, in the absence of APCs or when APCs alone were stimulated with α-GalCer (not depicted). Data represent the mean ± the SD of two to three individual experiments. *, P < 0.05. (H) Intracellular IL-17 staining was performed after in vitro stimulation of liver MNCs and analyzed among gated CD1d/α-GalCer tetramers +NK1.1neg or CD1d/α-GalCer tetramers +NK1.1pos by flow cytometry. The percentage of IL-17+ and Ig control+ cells is indicated in each graph. (I) Representative FACS profile of Vβ expression by gated NK1.1neg and NK1.1pos iNKT cells. Data (H and I) are representative of three independent experiments. nd, not detected.
With the exception of IL-17, which is produced by NK1.1neg iNKT cells, and IL-4, IFN-γ, and IL-3, which are produced more efficiently by NK1.1pos than NK1.1neg iNKT cells, the cytokine profile generated by the two subsets in response to α-GalCer was essentially the same, as assessed by a protein array detecting 32 different cytokines (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20061551/DC1). Moreover, NK1.1neg and NK1.1pos iNKT cells were undistinguishable by the expression of major iNKT cell markers, such as CD4, CD44, CD62L, CD69, Ly49A, and Ly49C, which occurred at similar levels (Fig. S2). Furthermore, both populations shared the Vβ bias that is typical for NK1.1pos iNKT cells (Fig. 2 I).
NK1.1neg and NK1.1pos iNKT cell subsets are functionally distinct
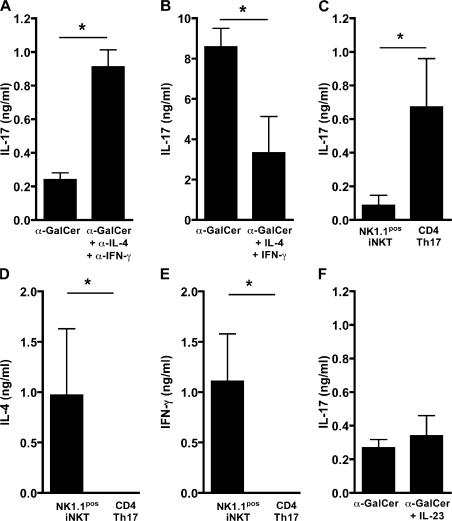
The preferential production of IL-17 by NK1.1neg iNKT cells raised the question of whether their NK1.1pos counterparts were unable to produce the same amount because of the inhibition exerted by endogenous IL-4 and IFN-γ. To address this issue, we blocked both cytokines by the corresponding neutralizing mAbs before stimulation with α-GalCer. Even though approximately fourfold more IL-17 was produced by NK1.1pos cells in these conditions (Fig. 3 A), the concentrations remained eight times lower than those generated by the NK1.1neg subset (Fig. 3 B), indicating that the two populations are functionally distinct. Nonetheless, the IL-17 production by the NK1.1neg population remained sensitive to down-regulation by IL-4 and IFN-γ, as assessed by the strong inhibitory effect of exogenous cytokines (Fig. 3 B).
Figure 3.
Inhibition of IL-17 production by iNKT cells in the presence of IL-4 and IFN-γ. (A and B) Sorted liver NK1.1pos iNKT (A and F) and NK1.1neg iNKT (B) cells were cocultured with irradiated liver MNCs from Jα18−/− mice as APCs and stimulated with α-GalCer in the presence or absence of anti–IL-4 and anti–IFN-γ mAb (A) of IL-4 and IFN-γ (B), or IL-23 (F). (C–E) Sorted NK1.1pos iNKT and naive conventional T cells were cocultured with anti-CD3, anti-CD28, TGFβ, IL-1α, IL-6 and TNF-α. 3 d later, IL-17 (C), IL-4 (D), and IFN-γ (E) were measured in all supernatants. Data represent the mean ± the SD of two to three individual experiments. *, P < 0.05.
Recent studies reported that TGF-β and IL-6 are required for driving the differentiation of naive CD4 T cells into Th17 cells (25), thus prompting us to verify whether NK1.1pos iNKT cells become more efficient IL-17 producers in these conditions. Fig. 3 C clearly shows that this is true for naive conventional T cells, but not for NK1.1pos iNKT cells, even though they retained their ability to produce both IL-4 and IFN-γ (Fig. 3, D and E), which proves responsiveness to stimulation. In addition, we tested the effect of IL-23 on NK1.1pos iNKT cells, knowing that it enhances IL-17 production by conventional T cells (26). Yet, once again, this treatment did not increase IL-17 secretion by NK1.1pos iNKT (Fig. 3 F), suggesting that NK1.1neg and NK1.1pos cells are, indeed, functionally distinct iNKT cell subsets.
Physiological relevance of NK1.1neg iNKT and IL-17 in early host defense to airborne antigens
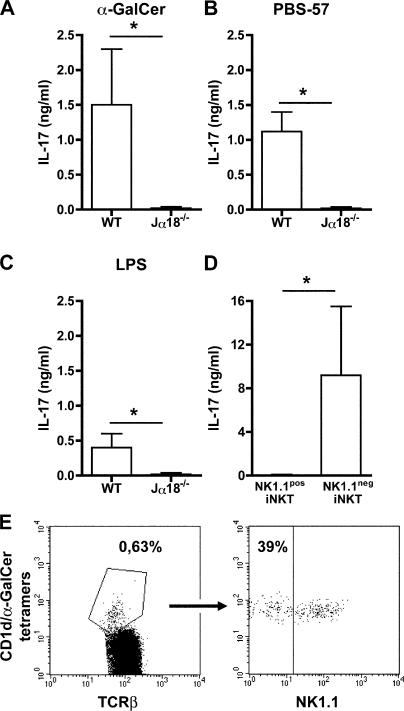
Because of their constant exposure to foreign antigens, airways and lungs depend on a competent immune response to avoid deleterious inflammatory responses caused by inefficient clearance of pathogens. Having established that iNKT cells are potent IL-17 producers, we addressed the question of their participation in airway neutrophilia resulting from exposure to α-GalCer, PBS-57, which is another iNKT cell ligand (27), or LPS. We first verified that pulmonary iNKT cells could produce IL-17 upon activation, which was actually the case for MNCs from wild-type, but not from Jα18−/−, mice after exposure to these ligands (Fig. 4, A–C). We next sorted NKT cells from pulmonary MNCs and found once again that only the NK1.1neg subset responded to α-GalCer stimulation in terms of IL-17 production (Fig. 4 D). Remarkably, this subpopulation turned out to be much more frequent in the lung than in the liver (Fig. 2 A) or in the spleen (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20061551/DC1) because it comprises up to 40% of pulmonary iNKT cells in naive mice (Fig. 4 E).
Figure 4.
IL-17 production by lung MNCs stimulated with α-GaCer, PBS-57, or LPS requires iNKT cells. Total (A–C) or sorted (D) NK1.1pos iNKT and NK1.1neg iNKT cells from lung MNCs from wild-type (A–D) and Jα18−/− (A–C) mice were stimulated in vitro with α-GalCer (A–D), PBS-57 (B), or LPS (C). 3 d later, supernatants were recovered and IL-17 was measured by ELISA. Data represent the mean ± the SEM of four individual mice. No cytokine was detected without stimulation (not depicted). *, P < 0.05. (E) Representative FACS profiles showing the higher percentage of NK1.1neg iNKT cells among gated TCRβ+ iNKT cells from lung.
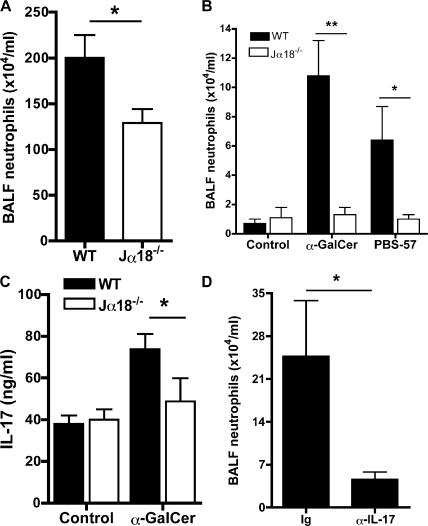
NK1.1neg iNKT cells display a tissue distribution and a capacity to produce IL-17 that is consistent with their potential role in pulmonary neutrophil recruitment. The physiological relevance of our data was also supported by the observation that in iNKT cell–deficient Jα18−/− mice, airway neutrophilia in response to LPS instillation was significantly decreased relative to wild-type controls (Fig. 5 A). Because in vivo treatment with LPS activates several cell populations besides iNKT cells, we delivered α-GalCer or PBS-57 by the same i.n. route to target iNKT cells specifically. In these conditions, neutrophilia occurred only in the lung of wild-type, but not of Jα18−/−, mice (Fig. 5 B). Furthermore, higher IL-17 levels were observed in bronchoalveolar lavage fluid (BALF; Fig. 5 C), and the neutralization of endogenously produced IL-17 by anti–IL-17 mAb administered before specific iNKT cell activation with α-GalCer resulted in a net decrease in airway neutrophilia (Fig. 5 D), providing an additional argument for the role of IL-17 as an important mediator of neutrophil recruitment.
Figure 5.
α-GalCer–, PBS-57–, or LPS-induced neutrophil recruitment to airways implicates iNKT cells. (A–D) Wild-type and Jα18−/− mice received a single i.n. dose of 10 μg LPS (A), 2 μg PBS-57 (B), or 2 μg α-GalCer (B–D) 24 h before sacrifice. The number of neutrophils recruited in BALF (A and B) and the concentration of IL-17 (C) is represented. (D) Mice were treated with anti–IL-17 mAb 24 h before i.n. exposure to α-GalCer. The number of neutrophils recruited in BALF was determined 24 h later. The injection of control mAb did not modify neutrophil recruitment (not depicted). Data represent the mean ± the SEM of 5–10 individual mice. *, P < 0.05; **, P < 0.01.
In conclusion, our study has revealed a new iNKT subset phenotypically characterized by the lack of the NK1.1 surface marker. NK1.1neg iNKT cells are functionally distinct from their NK1.1pos counterpart because of their high production of IL-17 and low secretion of IFN-γ and IL-4. The existence of distinctive subpopulations provides a possible explanation for the contrasting effects exerted by iNKT cells. In support of this idea, a recent report demonstrates that liver CD4neg NKT cells are unique in their capacity to confer an antitumor response (28). Our findings provide the first evidence for a particular NK1.1neg iNKT subset endowed with a preferential IL-17–producing profile induced by various antigens that we propose to name iNKT17 cells. The fact that this phenotype is more abundant in the lung than in liver or spleen (Fig. S3) supports the notion that specialized iNKT cell subsets may reside in different organs.
MATERIALS AND METHODS
Animals.
7–9-wk-old C57BL/6 mice were purchased from Janvier. Jα18−/−–, CD1d−/−–, and IFN-γ−/−–deficient mice (29, 30) were bred in our own facilities. All mice were kept in well-controlled animal housing facilities and had free access to tap water and pellet food. Animal experiments were performed according to the French Institutional Committee.
Cell preparation.
Lymphocytes were isolated from the liver, spleen, or lung, as previously described (15, 30).
FACS analysis and sorting of iNKT cells and conventional, naive T cells.
MNCs were stained with anti-Vβ8.1/8.2 (clone MR5-2), anti-Vβ8.3 (clone 1B3.3), anti-Vβ7 (clone TR310; mAb provided by S. Latour [Institut National de la Santé et de la Recherche Médicale, Paris France] and J.C. Bories [EA3963, Paris, France]), or anti-NK1.1 (clone PK136) mAb and CD1d/α-GalCer tetramers (plasmids containing CD1d and b2m genes were provided by M. Kronenberg, La Jolla Institute for Allergy and Immunology, San Diego, CA). NK1.1pos iNKT (tetramerspos) and NK1.1neg iNKT (tetramerspos) cells were then sorted. In parallel, splenocytes were stained with anti-CD4 (clone RM4-5) and anti-CD62L (clone Mel14) antibodies before sorting of conventional naive CD4+CD62L+ T cells. All cells were sorted using a FACSVantage cell sorter (Becton Dickinson).
Cell culture.
A final concentration of 106 liver or lung MNCs or 2.5 × 105 sorted iNKT cells per milliliter were cultured with or without irradiated liver MNCs (5 Gy) from Jα18−/− or CD1d−/− mice as APCs, at a ratio of 1:2. Cells were cultured in RPMI 1640 medium containing antibiotics, 10% FCS, 4 mg/ml β-mercaptoethanol, and 200 mM glutamine (all from Invitrogen) incubated at 37°C with 100 ng/ml α-GalCer solution, 100 ng/ml PBS-57, 10 μg/ml BbGL compounds, or 1 μg/ml LPS. 10 μg/ml of blocking anti–IL-4 (clone 11B11) and/or 10 μg/ml anti–IFN-γ (clone R46A2), as well as 1 μg/ml of coated anti-CD3 and 10 μg/ml anti-CD28 antibodies, or their respective isotype controls, were used in some experiments. In some conditions, exogenous cytokines, such as 4 ng/ml mouse IL-4, 10 ng/ml IFN-γ, 1 ng/ml TGF-β, 40 ng/ml IL-1α, 5 ng/ml IL-6, 10 ng/ml IL-23, and 20 ng/ml TNF-α (all from R&D Systems), were also added. All culture supernatants were harvested and stored at −80°C.
Determination of cytokines.
The levels of IL-17A (R&D Systems), IL-4, and IFN-γ were assessed by ELISA, as previously described (28, 29). Cytokine protein array II was purchased from Ray Biotech and used for analyzing supernatants from α-GalCer–stimulated sorted NK1.1pos and NK1.1neg iNKT cells according to the manufacturer's instructions.
Intracellular cytokine staining.
Liver MNCs were stimulated for 4 h with 10−8 M PMA (Sigma-Aldrich), 10−6 M ionomycin, and 10 μg/ml brefeldin A. Cells were then washed and incubated with CD1d-α-GalCer tetramer-APC, anti-NK1.1 PerCP-Cy-5.5, and anti–TCRβ-FITC. For intracellular staining, cells were fixed with 4% PFA, washed, and permeabilized with 0.5% saponin (Sigma-Aldrich), and then further incubated with anti–IL-17-PE or isotype control (BD Biosciences). The cells were washed and analyzed on a FACSCalibur (Becton Dickinson) using CellQuest software (BD Biosciences).
In vivo treatment.
Mice received a single i.n. administration of 2 μg α-GalCer (Kirin Brewery Co., Ltd), 2 μg PBS-57 (Sigma-Aldrich), or 10 μg LPS (Sigma-Aldrich) 24 h before sacrifice. In some experiments, mice received 100 μg of anti–IL-17 mAb (R&D Systems) or control Ig (Sigma-Aldrich) i.p. 24 h before ligand administration. Differential cell counts were determined in BALF 24 h after ligand instillation, as previously described (30).
Statistical analysis.
A nonparametric Mann-Whitney test was used to calculate significance levels for all measurements. P values <0.05 were considered statistically significant.
Online supplemental material.
Fig. S1 shows cytokine profile of NK1.1pos and NK1.1neg liver iNKT cells stimulated with α-GalCer for 3 d. 32 different cytokines were analyzed using mouse cytokine array II membranes. Fig. S2 shows that NK1.1neg iNKT and NK1.1pos iNKT cells express similar levels of CD4, CD69, CD44, CD62L, Ly49A, and Ly49C markers. All antibodies used were obtained from Becton Dickinson. Fig. S3 shows the percentage of NK1.1pos iNKT and NK1.1neg iNKT cells among gated CD1d/α-GalCer tetramers +TCRβ+ iNKT splenocytes.
Supplemental Material
[Supplemental Material Index]
Acknowledgments
We are grateful to André Herbelin for helpful discussions and to Séverine Diem for technical assistance. We are especially indebted to Pharmaceutical Research Laboratory, Kirin Brewery Co., Ltd. for providing α-GalCer, to Sylvain Latour and Jean-Christophe Bories for kindly giving us reagents, and to Mitchell Kronenberg and P. Van Endert for providing plasmid containing CD1d and β2m genes and helping with CD1d/α-GalCer-tetramer preparation. We are grateful to Corinne Garcia-Cordier and Jérôme Mégret (Necker Institut) for cell sorting.
This work was supported by institute funds from the Centre National de la Recherche Scientifique, Université René Descartes - Paris V, and the Fondation pour la Recherche Médicale (Equipe FRM/Jeune Investigateur en allergologie) to M.C. Leite-de-Moraes. M.L. Michel is the recipient of a doctoral fellowship from the Ministère de l'Education Nationale de la Recherche et Technique, and A.C. Keller is the recipient of a postdoctoral fellowship from the FRM and Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq).
The authors have no conflicting financial interests.
References
- 1.Taniguchi, M., M. Harada, S. Kojo, T. Nakayama, and H. Wakao. 2003. The regulatory role of Vα14 NKT cells in innate and acquired immune response. Annu. Rev. Immunol. 21:483–513. [DOI] [PubMed] [Google Scholar]
- 2.Kronenberg, M. 2005. Toward an understanding of NKT cell biology: progress and paradoxes. Annu. Rev. Immunol. 23:877–900. [DOI] [PubMed] [Google Scholar]
- 3.Benlagha, K., D.G. Wei, J. Veiga, L. Teyton, and A. Bendelac. 2005. Characterization of the early stages of thymic NKT cell development. J. Exp. Med. 202:485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bendelac, A., P.B. Savage, and L. Teyton. 2007. The biology of NKT cells. Annu. Rev. Immunol. 25:297–336. [DOI] [PubMed] [Google Scholar]
- 5.Kawano, T., J. Cui, Y. Koezuka, I. Toura, Y. Kaneko, K. Motoki, H. Ueno, R. Nakagawa, H. Sato, E. Kondo, et al. 1997. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 278:1626–1629. [DOI] [PubMed] [Google Scholar]
- 6.Leite-de-Moraes, M.C., G. Moreau, A. Arnould, F. Machavoine, C. Garcia, M. Papiernik, and M. Dy. 1998. IL-4-producing NK T cells are biased towards IFN-gamma production by IL-12. Influence of the microenvironment on the functional capacities of NK T cells. Eur. J. Immunol. 28:1507–1515. [DOI] [PubMed] [Google Scholar]
- 7.Leite-de-Moraes, M.C., A. Hameg, M. Pacilio, Y. Koezuka, M. Taniguchi, L. Van Kaer, E. Schneider, M. Dy, and A. Herbelin. 2001. IL-18 enhances IL-4 production by ligand-activated NKT lymphocytes: a pro-Th2 effect of IL-18 exerted through NKT cells. J. Immunol. 166:945–951. [DOI] [PubMed] [Google Scholar]
- 8.Leite-de-Moraes, M.C., M. Lisbonne, A. Arnould, F. Machavoine, A. Herbelin, M. Dy, and E. Schneider. 2002. Ligand-activated natural killer T lymphocytes promptly produce IL-3 and GM-CSF in vivo: relevance to peripheral myeloid recruitment. Eur. J. Immunol. 32:1897–1904. [DOI] [PubMed] [Google Scholar]
- 9.Zhou, D., J. Mattner, C. Cantu III, N. Schrantz, N. Yin, Y. Gao, Y. Sagiv, K. Hudspeth, Y.P. Wu, T. Yamashita, et al. 2004. Lysosomal glycosphingolipid recognition by NKT cells. Science. 306:1786–1789. [DOI] [PubMed] [Google Scholar]
- 10.Mattner, J., K.L. Debord, N. Ismail, R.D. Goff, C. Cantu III, D. Zhou, P. Saint-Mezard, V. Wang, Y. Gao, N. Yin, et al. 2005. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 434:525–529. [DOI] [PubMed] [Google Scholar]
- 11.Kinjo, Y., D. Wu, G. Kim, G.W. Xing, M.A. Poles, D.D. Ho, M. Tsuji, K. Kawahara, C.H. Wong, and M. Kronenberg. 2005. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 434:520–525. [DOI] [PubMed] [Google Scholar]
- 12.Kinjo, Y., E. Tupin, D. Wu, M. Fujio, R. Garcia-Navarro, M.R. Benhnia, D.M. Zajonc, G. Ben-Menachem, G.D. Ainge, G.F. Painter, et al. 2006. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat. Immunol. 7:978–986. [DOI] [PubMed] [Google Scholar]
- 13.Ronet, C., S. Darche, M. Leite de Moraes, S. Miyake, T. Yamamura, J.A. Louis, L.H. Kasper, and D. Buzoni-Gatel. 2005. NKT cells are critical for the initiation of an inflammatory bowel response against _Toxoplasma gondii._J. Immunol. 175:899–908. [DOI] [PubMed] [Google Scholar]
- 14.Ranson, T., S. Bregenholt, A. Lehuen, O. Gaillot, M.C. Leite- de-Moraes, A. Herbelin, P. Berche, and J.P. Di Santo. 2005. Invariant V alpha 14+ NKT cells participate in the early response to enteric Listeria monocytogenes infection. J. Immunol. 175:1137–1144. [DOI] [PubMed] [Google Scholar]
- 15.Mallevaey, T., J.P. Zanetta, C. Faveeuw, J. Fontaine, E. Maes, F. Platt, M. Capron, M.C. Leite-de-Moraes, and F. Trottein. 2006. Activation of invariant NKT cells by the helminth parasite _Schistosoma manson_i. J. Immunol. 176:2476–2485. [DOI] [PubMed] [Google Scholar]
- 16.Kawakami, K., N. Yamamoto, Y. Kinjo, K. Miyagi, C. Nakasone, K. Uezu, T. Kinjo, T. Nakayama, M. Taniguchi, and A. Saito. 2003. Critical role of Valpha14+ natural killer T cells in the innate phase of host protection against Streptococcus pneumoniae infection. Eur. J. Immunol. 33:3322–3330. [DOI] [PubMed] [Google Scholar]
- 17.Miyamoto, M., O. Prause, M. Sjostrand, M. Laan, J. Lotvall, and A. Lindén. 2003. Endogenous IL-17 as a mediator of neutrophil recruitment caused by endotoxin exposure in mouse airways. J. Immunol. 170:4665–4672. [DOI] [PubMed] [Google Scholar]
- 18.Ferretti, S., O. Bonneau, G.R. Dubois, C.E. Jones, and A. Trifilieff. 2003. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J. Immunol. 170:2106–2112. [DOI] [PubMed] [Google Scholar]
- 19.Ye, P., F.H. Rodriguez, S. Kanaly, K.L. Stocking, J. Schurr, P. Schwarzenberger, P. Oliver, W. Huang, P. Zhang, J. Zhang, et al. 2001. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 194:519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawaguchi, M., M. Adachi, N. Oda, F. Kokubu, and S.-K. Huang. 2004. IL-17 cytokine family. J. Allergy Clin. Immunol. 114:1265–1273. [DOI] [PubMed] [Google Scholar]
- 21.Kolls, J.K., and A. Lindén. 2004. Interleukin-17 family members and inflammation. Immunity. 21:467–476. [DOI] [PubMed] [Google Scholar]
- 22.Harrington, L.E., R.D. Hatton, P.R. Mangan, H. Turner, T.L. Murphy, K.M. Murphy, and C.T. Weaver. 2005. Interleukin 17- producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6:1123–1132. [DOI] [PubMed] [Google Scholar]
- 23.Park, H., Z. Li, X.O. Yang, S.H. Chang, R. Nurieva, Y.H. Wang, Y. Wang, L. Hood, Z. Zhu, Q. Tian, and C. Dong. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6:1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakae, S., Y. Komiyama, A. Nambu, K. Sudo, M. Iwase, I. Homma, K. Sekikawa, M. Asano, and Y. Iwakura. 2002. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 17:357–387. [DOI] [PubMed] [Google Scholar]
- 25.Veldhoen, M., R.J. Hocking, C.J. Atkins, R.M. Locksley, and B. Stockinger. 2006. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 24:179–189. [DOI] [PubMed] [Google Scholar]
- 26.Oppmann, B., R. Lesley, B. Blom, J.C. Timans, Y. Xu, B. Hunte, F. Vega, N. Yu, J. Wang, K. Singh, et al. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 13:715–725. [DOI] [PubMed] [Google Scholar]
- 27.Liu, Y., R.D. Goff, D. Zhou, J. Mattner, B.A. Sullivan, A. Khurana, C. Cantu III, E.V. Ravkov, C.C. Ibegbu, J.D. Altman, et al. 2006. A modified alpha-galactosyl ceramide for staining and stimulating Natural Killer T cells. J. Immunol. Methods. 312:34–39. [DOI] [PubMed] [Google Scholar]
- 28.Crowe, N.Y., J.M. Coquet, S.P. Berzins, K. Kyparissoudis, D.G. Pellicci, Y. Hayakawa, D.I. Godfrey, and M.J. Smyth. 2005. Differential antitumor immunity mediated by NKT cell subsets in vivo. J. Exp. Med. 202:1279–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lisbonne, M., S. Diem, A. de Castro Keller, J. Lefort, L.M. Araujo, P. Hachem, J.M. Fourneau, S. Sidobre, M. Kronenberg, M. Taniguchi, et al. 2003. Cutting edge: invariant V alpha 14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J. Immunol. 171:1637–1641. [DOI] [PubMed] [Google Scholar]
- 30.Hachem, P., M. Lisbonne, M.L. Michel, S. Diem, S. Roongapinun, J. Lefort, G. Marchal, A. Herbelin, P.W. Askenase, M. Dy, and M.C. Leite-de-Moraes. 2005. alpha-Galactosylceramide-induced iNKT cells suppress experimental allergic asthma in sensitized mice: role of IFN-gamma. Eur. J. Immunol. 35:2793–2802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental Material Index]