Inhibition of human telomerase by 2′-O-methyl-RNA (original) (raw)
Abstract
Telomerase, a ribonucleoprotein up-regulated in many types of cancers, possesses an RNA template necessary to bind and extend telomere ends. The intrinsic accessibility of telomerase to incoming nucleic acids makes the RNA template an ideal target for inhibition by oligonucleotides. We report here that 2′-_O_-methyl-RNA (2′-_O_-meRNA), an oligonucleotide chemistry known to exert sequence-specific effects in cell culture and animals, inhibits telomerase with potencies superior to those possessed by analogous peptide nucleic acids (PNAs). Potent inhibition relative to PNAs is surprising, because the binding affinity of 2′-_O_-meRNAs for complementary RNA is low relative to analogous PNAs. A 2′-_O_-meRNA oligomer with terminal phosphorothioate substitutions inhibits telomerase sequence-selectively within human-tumor-derived DU145 cells when delivered with cationic lipids. In contrast to the ability of 2′-_O_-meRNA oligomers to inhibit telomerase, the binding of a 2′-_O_-meRNA to an inverted repeat within plasmid DNA was not detectable, whereas binding of PNA was efficient, suggesting that the relative accessibility of the telomerase RNA template is essential for inhibition by 2′-_O_-meRNA. Inhibition of telomerase by 2′-_O_-meRNA will facilitate probing the link between telomerase activity and sustained cell proliferation and may provide a basis for the development of chemopreventive and chemotherapeutic agents.
Human telomerase is a ribonucleoprotein that adds repeated units of TTAGGG to the ends of chromosomes (1). Telomerase activity is not detectable in somatic cells, with the exception of proliferative cells of renewal tissues, but is present in most immortal cell lines and primary human tumors (2–4). High telomerase activity has been correlated with an unfavorable prognosis for neuroblastoma, and low telomerase activity has been associated with a likelihood for spontaneous regression (5). These observations have led to the hypothesis that telomerase activation is necessary for the sustained growth of most tumors and that telomerase inhibition is a valid strategy for limiting long-term cell proliferation and metastasis (6,7). The link between telomerase and sustained growth of most tumors is in dispute, because other mechanisms can maintain telomere length in yeast (8, 9). In addition, telomerase activity is not detectable in some human tumors and immortal cell lines (10–12), and mice lacking the gene for telomerase RNA can survive, reproduce, and develop tumors (13), though this latter result may reflect mouse rather than human physiology (14). A more definitive evaluation of the role of telomerase would be facilitated by the development of highly selective inhibitors of the human enzyme that can modulate telomerase activity in varied genetic backgrounds and tumor types (15). Recent experiments have shown that expression of the reverse-transcriptase-like component of telomerase leads to the extension of cellular life spans (16), suggesting that expression of telomerase alone may be sufficient for cell immortalization and reinforcing the belief that its inhibition may return cells to a mortal state.
The RNA component (17) of telomerase base-pairs with the terminal nucleotides of telomeres and acts as a template for the addition of TTAGGG repeats by the reverse-transcriptase domain (18, 19). These dual functions make telomerase an ideal target for inhibition by oligonucleotides, because the template RNA is intrinsically accessible to nucleic acids and is critical for the maintenance of telomere length. To be useful in investigating the intracellular role of telomerase, oligonucleotides must (i) bind with high affinity per base pair to minimize overall size and take advantage of the limited number of accessible template bases, (ii) bind with high selectivity to telomerase so that changes in cell proliferation can be attributed directly to telomerase inhibition, and (iii) cross the cell membrane. We have shown that 8- to 13-base peptide nucleic acids (PNAs) complementary to the telomerase template are potent and sequence-selective inhibitors in vitro (20, 21), fulfilling the first two of these criteria. However, while we and others have developed methods for passively delivering PNAs within cells (22, 23), current protocols have not yet established sequence-specific intracellular effects.
Simultaneously with our efforts to improve intracellular delivery of PNAs, we have explored oligonucleotide chemistries that have already shown sequence-specific effects within cells in culture and in animals. We have established that phosphorothioate-substituted DNA oligonucleotides, an oligomer motif currently being evaluated in several clinical trials including phase three trials for cytomegalovirus retinitis (24, 25), inhibit telomerase (20). Inhibition of telomerase by phosphorothioate-modified DNA, however, has poor sequence selectivity, presumably because of interactions between the phosphorothioate backbone and the telomerase reverse-transcriptase domain. This led us to search for other oligonucleotide chemistries that would be better suited to probing definitively the link between telomerase inhibition and cell proliferation.
2′-_O_-Alkyl-RNA, a second-generation class of oligonucleotide, binds complementary sequences with high affinity relative to analogous DNA or RNA oligonucleotides (26–30). The clinical application of 2′-_O_-meRNA with a phosphorothioate backbone is being explored in a phase one trial directed against the R1-α subunit of protein kinase A in refractory solid tumors and a phase one/two trial directed against cytomegalovirus retinitis. Furthermore, Lingner and Cech (31) have used 2′-_O_-meRNA to purify telomerase from Euplotes aediculatus by affinity chromatography, showing that 2′-_O_-meRNA can recognize the RNA component of telomerase. We describe here 6- to 13-nucleotide 2′-_O_-meRNAs that inhibit human telomerase better than analogous PNAs, despite possessing substantially lower affinity for complementary RNA sequences and being unable to recognize a structured nucleic-acid target that is readily recognized by PNA. In addition, we show that 2′-_O_-meRNA-based oligomers can efficiently and sequence-selectively inhibit telomerase upon transfection of human-prostate tumor-derived DU145 cells by using cationic lipids.
MATERIALS AND METHODS
Oligomer Synthesis.
2′-_O_-meRNA and RNA oligonucleotides were purchased from Oligos Etc. (Wilsonville, OR), and purity was confirmed by gel electrophoresis. All oligomers used in these studies also yielded standard melting curves when hybridized to complementary DNA or RNA. DNA oligonucleotides were synthesized on an Applied Biosystems 451 DNA Synthesizer. PNAs were synthesized manually by tBoc solid-phase synthesis (32) with monomers from PerSeptive Biosystems (Framingham, MA), analyzed by matrix-assisted laser-desorption time-of-flight (MALDI-TOF) mass spectrometry, and purified by reverse-phase HPLC. All PNAs were readily soluble at the concentrations used in these assays.
Melting Temperature Determination.
DNA, 2′-_O_-meRNA, or PNA oligomers and equimolar complementary RNA were heated for 5 min at 90°C in a 1-ml solution of 0.1 M KH2PO4/K2HPO4(pH 7.6) and 1 mM MgCl2, then slowly cooled to room temperature over 20 min. Solutions were then placed into a capped quartz cuvette and heated from 15°C to 90°C with absorbance at 260 nm recorded over a 2-min equilibration time at increments of 3°C with an 8452A UV spectrophotometer and a HP 89090A Peltier temperature-control accessory (Hewlett–Packard). Absorbance values were normalized and plotted against temperature.
Telomerase Assays.
Telomerase activity from immortal human-prostate cell line DU145 was determined with the telomere repeat-amplification protocol (TRAP) (33) by using the TRAPeze telomerase detection kit (Oncor). Differing concentrations of the oligomer being tested for inhibition were incubated for 30 min at 23°C or 37°C with the lysate from 200 cells. The TRAPeze reaction mixture was then added to each tube and incubated at 23°C or 37°C for 30 min to allow the extension of radiolabeled primer by telomerase. Reactions were then amplified by PCR with a two-step cycle of 30 sec at 94°C and 30 sec at 60°C, repeated 27 times. Control experiments included monitoring telomerase activity (i) in the absence of inhibitor to establish the maximal level of product formation, (ii) with no cell lysate to ensure that spurious products were not being amplified, and (iii) with inhibitor added directly to cell lysate immediately before PCR amplification to ensure that the inhibitor was not interfering with primer hybridization or some other component of the amplification.
Reaction products were run on nondenaturing polyacrylamide gels, and telomerase activity was quantitated by PhosphorImager analysis (Molecular Dynamics; refs. 20 and 21). To estimate rates of association for 2′-_O_-meRNA inhibitors, a lysate from 200 cells was incubated with 333 nM (final concentration) inhibitor for 0–10 min before PCR components were added. The remainder of the assay was identical to that described above. To estimate the rate of dissociation of 2′-_O_-meRNA, 333 nM 2′-_O_-meRNA 1 was preincubated with a lysate from 200 cells for 30 min before the addition of a 50-fold excess of DNA oligonucleotide complementary to the inhibitory sequence.
Transfection of 2′-_O_-meRNA Oligomers into DU145 Cells.
DU145 cells were plated at 25,000 cells per well in a 24-well plate in DMEM supplemented with 10% fetal calf serum and 500 units/ml penicillin and 0.1 mg/ml streptomycin. After allowing cells to adhere, they were transfected with 3.5 μl LipofectAmine (Life Technologies, Gaithersburg, MD) and 1 μM oligomer in 200 μl Opti-Mem (Life Technologies) according to manufacturers’ directions. After 4 h at 37°C, the transfecting mixture was removed, and medium with serum was added. Cells were then harvested 12–15 h later after three washes with PBS and treatment with trypsin. Cells were counted and assayed for telomerase inhibition as described above.
Hybridization to pUC19.
Nondenatured plasmid pUC19 was incubated with an oligonucleotide–peptide conjugate consisting of an oligonucleotide 5′-(Cys)-GGATCTTCACCTAGATCCT-3′ complementary to bases 1544–1562 coupled by disulfide exchange to peptide KKAAKKAAKKAAKKAAC (34). A 2′-_O_-meRNA oligonucleotide of sequence 5′-TTCACCTAGATCCT-3′ and analogous PNA were added either before or after conjugate. Their effect on the hybridization of the oligonucleotide–peptide conjugate was evaluated by monitoring the extent of the elongation of the oligonucleotide domain of the conjugate by modified T7 DNA polymerase (Sequenase from United States Biochemical) as described (34). The DNA sequence produced by this elongation allowed the location of hybridization to be confirmed, and PhosphorImager analysis allowed the relative efficiency of hybridization to be determined.
RESULTS AND DISCUSSION
Effect of Oligomer Chemistry on Inhibition of Telomerase.
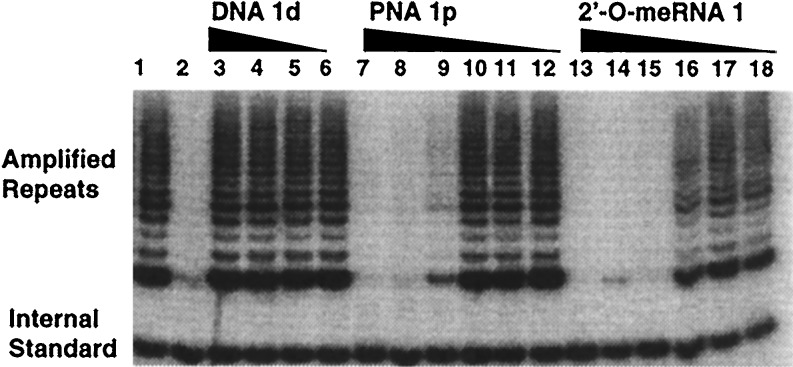
We used TRAP (33) to evaluate the inhibition of human telomerase by 2′-_O_-meRNA, DNA, PNA, and phosphorothioate-modified 2′-_O_-meRNA oligomers (Table1, Fig.1). In all cases, control experiments were performed as described in Materials and Methods to ensure that the observed inhibition was not caused by interference of amplification of elongation products. Reported values are the average of triplicate determinations. DNA 1dinhibited telomerase with an IC50 value of 12 μM (Table 1) when assayed at 23°C but showed no inhibition at 37°C. Both PNA 1p and 2′-_O_-meRNA 1were ≈1000-fold more potent inhibitors than 1d, with PNA1p possessing IC50 values of 10 nM at 23°C and 20 nM at 37°C. 2′-_O_-meRNA 1 had IC50 values of 2 nM at 23°C and 8 nM at 37°C. As we had established for PNAs (20, 21), inhibition by 2′-_O_-meRNA was characterized by a high level of sequence selectivity. 2′-_O_-meRNA 2, which contains two mismatches relative to the template sequence, possessed an IC50 value >16,500-fold less potent than that possessed by 1 (Table 1).
Table 1.
Inhibition of telomerase by 2′-_O_-methyl RNA, PNA, and DNA oligomers and melting temperatures to complementary RNA
| Sequence | IC50, μM | _T_m, °C | |
|---|---|---|---|
| 23°C | 37°C | ||
| 3′-GAGUCAAUCCCAAUCUG-5′ (RNA template) | |||
| 1 CAGUUAGGGUUAG | 0.002 | 0.008 | 58 |
| 1d CAGTTAGGGTTAG (DNA) | 12 | ND | 46 |
| 1p CAGTTAGGGTTAG (PNA) | 0.01 | 0.02 | 76 |
| 2 GACUUAGAAUUAG (mismatch) | >33 | ND | 38 |
| 3 GAGUUAGGG | 0.02 | 0.2 | 53 |
| 4 AGUUAGGG | 0.03 | 1.0 | 47 |
| 5 GUUAGGG | 0.3 | 0.7 | 35 |
| 8 UUAGGG | 0.3 | 11 | 28 |
| 7 CA GUUAGGGUU AG | 0.002 | 0.003 | 59 |
| 8 CA GUUAGAAUUAG(mismatch) | 0.6 | 3.0 | 34 |
| 9 CAGUUAGGGUUAG | 0.007 | 0.03 | 56 |
| 10 CAGUUAGAAUUAG (mismatch) | 0.07 | 0.2 | 29 |
| 11 UGACUGUGAUGGA (mixed) | 0.1 | 0.7 | ND |
Figure 1.
Relative inhibition of analogous DNA, PNA, and 2′-_O_-meRNA oligomers. Inhibition was monitored as a function of the concentration of DNA 1d, PNA 1p, and 2′-_O_-meRNA 1. Lane 1, no inhibitor added. Lane 2, no cell lysate added. Lanes 3–6, DNA 1d added at the following concentrations: 33.3 μM, 3.33 μM, 333 nM, and 33.3 nM. Lanes 7–12, PNA 1p added at the following concentrations: 33.3 μM, 3.33 μM, 333 nM, 33.3 nM, 3.33 nM, and 333 pM. Lanes 13–18, 2′-_O_-meRNA 1 added at the following concentrations: 33.3 μM, 3.33 μM, 333 nM, 33.3 nM, 3.33 nM, and 333 pM.
We also assayed 2′-_O_-meRNAs 3–6 to examine the inhibition of telomerase by shorter oligonucleotides (Table1). Nine- and eight-nucleotide 2′-_O_-meRNAs (designated3 and 4, respectively) inhibited telomerase at 23°C with IC50 values of 20 and 30 nM, respectively, with the potency of inhibition decreasing 10- to 30-fold upon assay at 37°C. The IC50 value reported for the PNA analogous in sequence to 2′-_O_-meRNA4 is 30 nM at 23°C (21). Seven- and six-nucleotide 2′-_O_-meRNAs (designated 5 and 6, respectively) were also inhibitors, but with weaker IC50 values (300 nM at 23°C; 700 nM and 11 μM, respectively, at 37°C). These results establish that even relatively short 2′-_O_-meRNA oligomers are better inhibitors of human telomerase than longer RNA or DNA oligonucleotides and are suitable lead compounds for the design of inhibitors aimed at combining maximal potency with minimal size.
Because phosphorothioate substitution confers enhanced stability to nuclease digestion (35), we assayed the sequence specificity and potency of telomerase inhibition by oligomers containing varied numbers of phosphorothioate linkages. Oligomer 7, which is analogous in sequence to 2′-_O_-meRNA 1 and possesses terminal phosphorothioate linkages, has IC50values similar to those of 1 (2 nM at 23°C and 3 nM at 37°C). Oligomer 8, with two mismatches compared with its RNA target, has IC50 values that are 500- to 1000-fold higher (600 nM at 23°C and 3 μM at 37°C) than those of7, showing that the inhibition of telomerase continues to be sequence-selective in spite of limited phosphorothioate substitution.
In contrast to the high degree of sequence selectivity of oligomers with terminal phosphorothioate substitutions, oligomers that were fully phosphorothioate-substituted were potent inhibitors of human telomerase regardless of sequence. Oligomer 9, in which every internucleotide linkage was phosphorothioate, possessed IC50 values of 7 nM at 23°C and 30 nM at 37°C, similar to those observed for 1 and 7. However, the analogous mismatch-containing oligomer 10inhibited telomerase with IC50 values only 10-fold higher (70 nM at 23°C and 200 nM at 37°C). Similarly, oligomer 11, a fully phosphorothioate-substituted 2′-_O_-meRNA oligomer containing a mixed sequence with minimal complementarity to the RNA template, inhibited telomerase with relatively potent IC50 values of 100 nM at 23°C and 700 nM at 37°C.
The lesser sequence selectivity that we observe for the inhibition of human telomerase by fully phosphorothioate-substituted oligomers does not necessarily imply that these oligomers cannot be used to control telomerase activity in vivo. Regardless of whether inhibition is sequence-specific, it may be sufficiently telomerase-specific to exert an antiproliferative effect through a telomerase-dependent mechanism. However, lack of sequence specificity complicates the use of fully phosphorothioate-substituted oligonucleotides by preventing full exploitation of mismatch-containing oligonucleotides as controls in long-term studies that relate telomere-length maintenance and cell senescence. Thus, it appears that 2′-_O_-meRNA oligomers with terminal phosphorothioate linkages are more promising candidates for initial studies.
Correlation of Telomerase Inhibition with Hybridization Affinities of DNA, PNA, 2′-_O_-meRNA, and Modified 2′-_O_-meRNA Oligomers.
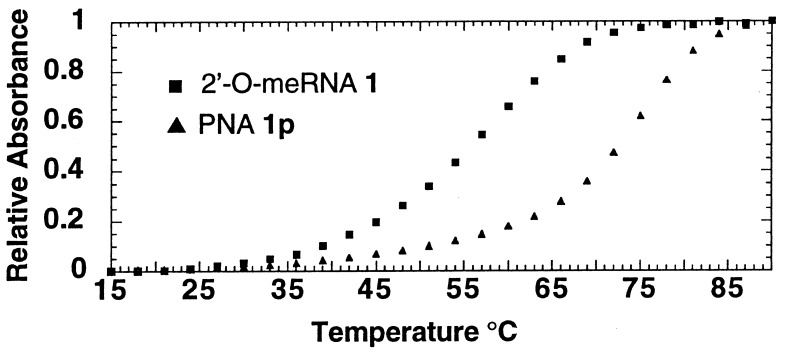
We measured melting temperatures for the hybridization of oligomers 1, 1d,1p, and 2–11 to RNA strands analogous in sequence to the template region of the telomerase RNA to correlate IC50 values for oligonucleotide-directed inhibition with hybridization affinity (Table 1, Fig.2). DNA 1d was the poorest inhibitor and also had a relatively low melting temperature of 46°C. However, for 2′-_O_-meRNA 1 and PNA1p, it was determined that, although 2′-_O_-meRNA1 was a better inhibitor of telomerase activity,1p had an 18°C higher melting temperature.
Figure 2.
Melting temperature determination for PNA1p and 2′-_O_-meRNA 1 hybridized to their complementary RNA sequence. Values of the y axis are normalized to correct for differing initial concentration values.
One explanation for the inconsistency between hybridization affinity and potency for 1 and 1p is that binding to the RNA template is influenced by the secondary structure of the template, by the rest of the RNA component, and by interactions with nearby amino acids of the reverse-transcriptase domain, considerations that do not exist for recognition of an isolated complementary RNA. The ribose and phosphodiester backbone of 2′-_O_-meRNA are chemically and sterically similar to DNA, the native substrate of telomerase. These similarities may allow 2′-_O_-meRNA to make favorable electrostatic contacts with the protein component of telomerase while avoiding unfavorable steric clashes that may be occurring with the amide-based PNA backbone.
We also note that the relatively short 2′-_O_-meRNAs3–6 inhibit telomerase with IC50values that are more potent than the IC50 values of DNA 1d, even though 3–6 possess melting temperatures (28–47°C) similar to or lower than the melting temperature of 1d (Table 1), suggesting that the 2′ modification plays a direct role in the stabilization of oligomer binding to telomerase independent of its role in increasing hybridization affinity. It appears, therefore, that of the three oligomer chemistries assayed, 2′-_O_-meRNA is best able to convert the intrinsic binding affinity to complementary sequences into the inhibition of telomerase.
Dynamics of Telomerase Inhibition by 2′-_O_-meRNA.
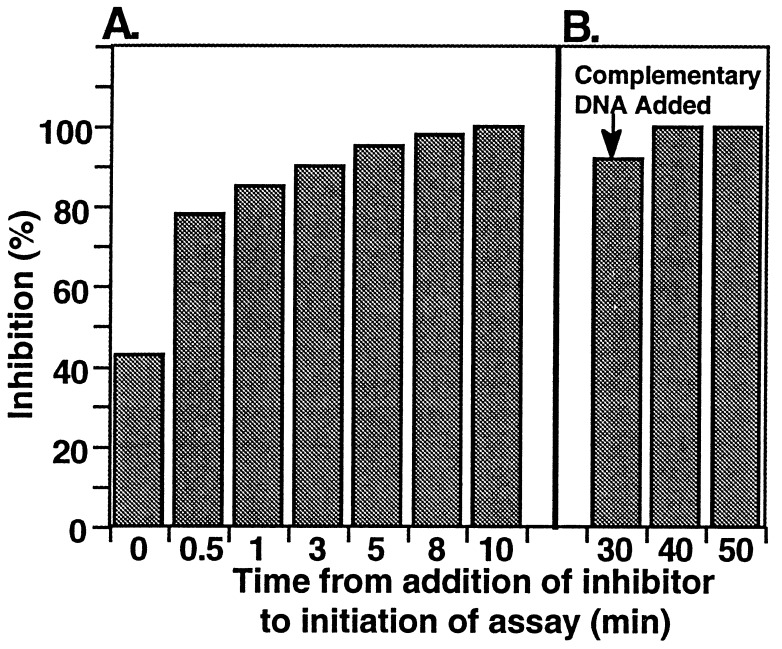
To characterize the inhibition of telomerase activity by 2′-_O_-meRNA, we monitored the association and dissociation of1 to and from the telomerase template. To monitor association, we examined inhibition as a function of time allowed for the incubation of 1 with telomerase. We observed that a 333 nM concentration of 1 produced almost total inhibition of telomerase activity after a 1-min preincubation with telomerase before initiation of strand elongation (Fig.3A), a result similar to that obtained with PNA 1p (results not shown). To monitor dissociation, we allowed the binding of inhibitor to occur under conditions that result in complete inhibition of telomerase activity and then added 50-fold excess of a DNA oligonucleotide complementary to the inhibitor so that any 2′-_O_-meRNA dissociating from the template would be captured, resulting in the release of inhibition over time. PNAs that were preincubated with complementary DNA before they were added to telomerase did not inhibit strand elongation (result not shown), establishing that they could be captured successfully and that the presence of excess DNA did not inhibit telomerase activity or product amplification.
Figure 3.
Association and dissociation of telomerase and 2′-_O_-meRNA 1. (A) Rate of telomerase inhibition upon addition of 2′-_O_-meRNA1. 2′-_O_-meRNA 1 (333 nM) was added to telomerase-containing cell extract for the time indicated (0, 0.5, 1, 3, 5, 8, or 10 min) before initiation of strand elongation. (B) Stability of inhibition by 2′-_O_-meRNA1. Telomerase was incubated with 100 nM 2′-_O_-meRNA 1 for 30 min to ensure complete inhibition. Competitor DNA (5.0 μM final concentration) complementary to 1 was then added for 0, 10, or 20 min before the assay for telomerase activity.
When the binding of 2′-_O_-meRNA 1 to telomerase was allowed to occur before the addition of complementary DNA, at both 23 (data not shown) and 37°C (Fig. 3B), inhibition was maintained 20 min after the addition of competing oligonucleotide, suggesting that 1 did not readily dissociate. Similar trends were observed for inhibition by PNA 1p (data not shown). These results show that the association to and dissociation from telomerase of 1 is similar to those of 1p in spite of the dramatic structural and electrostatic differences between 2′-_O_-meRNA and PNA and that relatively rapid association and relatively slow dissociation contribute to potent inhibition.
Inhibition of Human Telomerase Within Cells.
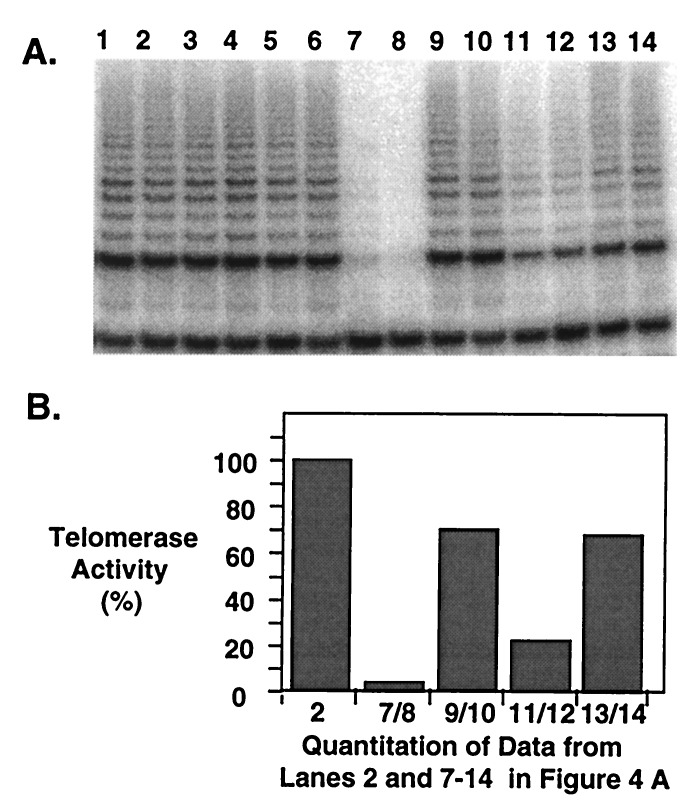
We chose oligomer7, a 2′-_O_-meRNA containing terminal phosphorothioate linkages, to examine intracellular inhibition of human telomerase. Oligomer 7 was chosen, because it combined potent inhibition and high sequence selectivity (Table 1) with enhanced serum stability when added to cells in culture (results not shown). Because oligonucleotides do not generally enter cells efficiently when added alone (36), we used LipofectAmine, a cationic lipid, to facilitate their uptake. We determined conditions for transfecting DU145 cells by varying the ratio of LipofectAmine to oligomer and observed that a narrow range of ratios resulted in efficient cell transfection, low toxicity, and telomerase inhibition. Every transfection experiment included control cells treated with serum-free media, LipofectAmine alone, or oligomer alone. These additions did not inhibit telomerase activity (Fig.4A), nor did the addition of 10 μM PNA 1p in conjunction with LipofectAmine (data not shown). This result was expected, because the neutral PNA backbone lacks the ability to interact with cationic lipid.
Figure 4.
(A) Inhibition of telomerase after transfection of human-prostate tumor-derived DU145 cells by 2′-_O_-meRNA oligomers. Lane 1, no oligomer or LipofectAmine added. Lane 2, 1 μM oligomer 7, no LipofectAmine. Lane 3, 1 μM oligomer 8, no LipofectAmine. Lane 4, 1 μM oligomer 1, no LipofectAmine. Lane 5, 1 μM oligomer 2, no LipofectAmine. Lane 6, 3.5 μl LipofectAmine, no oligonucleotide. Lanes 7 and 8, duplicate additions of 3.5 μl LipofectAmine and 1 μM oligomer 7. Lanes 9 and 10, duplicate additions of 3.5 μl LipofectAmine and 1 μM oligomer8. Lanes 11 and 12, duplicate additions of 3.5 μl LipofectAmine and 1 μM oligomer 1. Lanes 13 and 14, duplicate additions 3.5 μl LipofectAmine and 1 μM oligomer2. (B) Bar-graph representation of telomerase inhibition by 2′-_O_-meRNA oligomers from lanes 2 and 7–14 in A as quantitated by PhosphorImager analysis of telomerase products. The activity when no oligomer or LipofectAmine was added was defined as 100% activity, although 100% activity was also observed when oligonucleotide or LipofectAmine was added alone (see A, lanes 1–6). Bar 1, 1 μM oligomer7, no LipofectAmine. Bar 2, average of duplicate additions of 3.5 μl LipofectAmine and 1 μM oligomer 7. Bar 3, average of duplicate additions of 3.5 μl LipofectAmine and 1 μM oligomer 8. Bar 4, average of duplicate additions of 3.5 μl LipofectAmine and 1 μM oligomer 1. Bar 5, average of duplicate additions of 3.5 μl LipofectAmine and 1 μM oligomer2.
Transfection of DU145 cells with LipofectAmine and a 1 μM concentration of oligomer 7 reduced telomerase activity by 97% relative to treatment with LipofectAmine or oligonucleotide alone (Fig. 4) and persisted over incubations as long as 72 h (results not shown). The potency of inhibition was sequence-dependent, as is evident with oligomer 8 which contained two mismatched bases and inhibited telomerase only slightly. Inhibition was also influenced by the presence of terminal phosphorothioate substitutions; for instance, oligomer 1, which was analogous in sequence to7 but contained only phosphodiester linkages, inhibited telomerase activity less potently than 7. This difference in inhibitory potency emphasizes that the potential for stability in nuclease digestion is also important in determining the intracellular potency of oligonucleotide inhibitors and that the results from cell lysate do not necessarily correlate with those from cell culture. We have observed that 7 is more stable when added to cells than1 (results not shown). This observation supports the suggestion that enhanced stability conferred by terminal phosphorothioate linkages contributes to the observed difference in inhibitory potency.
Comparison of Hybridization of PNA and 2′-_O_-meRNA Oligomers to a Base-Paired Nucleic-Acid Target.
The RNA template of telomerase is an atypical target for oligonucleotide recognition because of its intrinsic accessibility to incoming nucleic acids and its intimate associate with a critical protein component. Therefore, the greater potency of telomerase inhibition by 2′-_O_-meRNA relative to PNA oligomers may not reflect a generally superior ability of 2′-_O_-meRNA to bind nucleic-acid targets. To compare hybridization by PNA and 2′-_O_-meRNA oligomers further and gain a broader perspective on their relative potencies of telomerase inhibition, we examined PNA and 2′-_O_-meRNA hybridization to an inverted repeat within plasmid pUC19, a site we have used as a model for hybridization to sequences containing base pairing (34).
We incubated plasmid pUC19 with analogous 2′-_O_-meRNA or PNA oligomers and examined their ability to compete for hybridization with an oligonucleotide–cationic-peptide conjugate targeted to the same sequence within the inverted repeat. Hybridization of the conjugate can be monitored by its ability to act as a primer for strand extension by modified T7 DNA polymerase and occurs with rapid-association and slow-dissociation kinetics relative to unmodified DNA (34). PNA and 2′-_O_-meRNA oligomers cannot act as primers for T7 DNA polymerase, but their hybridization can be monitored by their ability to inhibit priming by the conjugate. By using this assay, we found that the PNA oligomer hybridized readily, whereas the 2′-_O_-meRNA oligomer did not (results not shown). The inability of a 2′-_O_-meRNA to hybridize to an inverted repeat that is recognized by an analogous PNA suggests that the necessary accessibility of the telomerase RNA template is an important factor, allowing 2′-_O_-meRNA oligomers to be potent inhibitors of telomerase. The contrasting relative abilities of PNA and 2′-_O_-meRNA to hybridize to various targets also support the hypothesis (34, 37) that PNAs possess greater advantages for hybridization to sequences possessing a base-paired structure than to less structured sequences.
Advantages of Oligonucleotide-Directed Inhibition of Human Telomerase.
Substantiating the relevance of inhibiting telomerase activity as a therapy for various types of human tumors will require examination of the effects of its inhibition during the extended period necessary to allow telomeres to shorten to a critical length. To accomplish this, inhibitors must be highly specific and subject to rigorous controls to confirm that any decrease in cell proliferation is related to telomerase inhibition rather than interference with other components of the cellular apparatus that might affect telomere-length maintenance or cell growth. Oligonucleotides, with their potential for high sequence specificity and straightforward design of inhibitory and control oligomers, meet these criteria.
Of all the potential RNA targets for oligonucleotide binding, telomerase should be among the most susceptible because of its necessary accessibility to incoming nucleic acids. In addition, lowered viability in response to mutant telomeres has been reported for yeast containing a mutated telomerase RNA template (38), and stringent sequence requirements have been shown for the addition of human telomeric repeats (8, 39). Therefore, it is possible that cells may have difficulty mutating the telomerase RNA to gain resistance to template-directed inhibitors, because mutations may disrupt the cellular machinery that maintains telomere length. Finally, 2′-_O_-alkyl oligonucleotides possess favorable pharmacological properties (24–30), facilitating their application as antitelomerase agents in animal testing and therapeutic development.
Conclusion.
2′-_O_-meRNA oligomers are potent and sequence-selective inhibitors of human telomerase in spite of their structural similarity to DNA, an oligonucleotide chemistry that shows little potential for potent telomerase inhibition. Protocols for delivery of negatively charged oligonucleotides into varied cell lines are well developed (36). Delivery of 2′-_O_-meRNA 7into cells in conjunction with the cloned RNA and reverse-transcriptase domains will afford new options for investigating the role of telomerase in telomere biology and for examining the effects of telomerase inhibition in varied human-tumor cell lines. Oligonucleotide inhibitors should facilitate testing the link between telomerase and sustained tumor growth and help to determine whether telomerase is really a novel target for chemoprevention and chemotherapy.
This work also provides a direct comparison of two classes of oligonucleotides that bind with high affinity to complementary sequences, PNA and 2′-_O_-meRNA. Surprisingly, although 2′-_O_-meRNAs possessed lower affinity for complementary sequences than do analogous PNAs, inhibition of human telomerase activity by 2′-_O_-meRNAs was more potent than inhibition by analogous PNAs. This result shows that the exceptionally high affinity offered by PNA hybridization is not always sufficient to confer an advantage for hybridization to complementary sequences and that the highly accessible telomerase RNA template can bind avidly to either PNA or 2′-_O_-meRNA oligomers. Our contrasting observation that PNAs remain superior to 2′-_O_-meRNA for hybridization to a sequence within duplex DNA establishes that PNAs do possess important advantages relative to 2′-_O_-meRNA for hybridization to structured nucleic acids. This dichotomy emphasizes the long-term need for the development and characterization of a variety of nucleic-acid chemistries and nucleic-acid mimics for binding to the different types of nucleic acids and nucleic-acid topologies present within cells so that the biochemical and biophysical characteristics of individual classes of oligonucleotides can be matched with the demands of individual targets.
Acknowledgments
We thank the reviewers of this manuscript for their helpful comments and Carla Simmons and Scott Baker for their assistance. This work was support by Robert A. Welch Foundation Grant I-1244, National Institutes of Health Grant 1R01CA74908, and Texas Advanced Technology Program Grant 18603. A.E.P. was supported by National Institutes of Health Training Grant 5T32GM0706223. D.R.C. is an Assistant Investigator with the Howard Hughes Medical Institute.
ABBREVIATIONS
PNA
Peptide nucleic acid
TRAP
telomere repeat-amplification protocol
Footnotes
This paper was submitted directly (Track II) to the_Proceedings_ Office.
References
- Morin G B. Cell. 1989;59:521–529. doi: 10.1016/0092-8674(89)90035-4. [DOI] [PubMed] [Google Scholar]
- 2.Kim N W, Piatyszek M A, Prowse K R, Harley C B, West M D, Ho P L C, Coviello G M, Wright W E, Weinrich S L, Shay J W. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 3.Counter C M, Hirte H W, Bachetti S, Harley C B. Proc Natl Acad Sci USA. 1994;91:2900–2904. doi: 10.1073/pnas.91.8.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shay J W, Bachetti S. Eur J Cancer. 1997;33:787–791. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 5.Hiyama E, Hiyama K, Yokoyama T, Matsuura Y, Piatyszek M A, Shay J W. Nat Med. 1995;1:249–255. doi: 10.1038/nm0395-249. [DOI] [PubMed] [Google Scholar]
- 6.Holt S E, Shay J W, Wright W E. Nat Biotechnol. 1996;14:836–839. doi: 10.1038/nbt0796-836. [DOI] [PubMed] [Google Scholar]
- 7.Kim N W. Eur J Cancer. 1997;33:781–786. doi: 10.1016/S0959-8049(97)00057-9. [DOI] [PubMed] [Google Scholar]
- 8.McEachern M J, Blackburn E H. Nature (London) 1995;376:403–409. doi: 10.1038/376403a0. [DOI] [PubMed] [Google Scholar]
- 9.Singer M S, Gottschling D E. Science. 1994;266:404–409. doi: 10.1126/science.7545955. [DOI] [PubMed] [Google Scholar]
- 10.Broccoli D, Young J W, de Lange T. Proc Natl Acad Sci USA. 1995;92:9082–9086. doi: 10.1073/pnas.92.20.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bryan T M, Englezou A, Gupta J, Bachetti S, Reddel R R. EMBO J. 1995;14:4240–4248. doi: 10.1002/j.1460-2075.1995.tb00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bryan T M, Englezou A, Dalla-Pozza L, Dunham M A, Reddel R R. Nat Med. 1997;3:1271–1274. doi: 10.1038/nm1197-1271. [DOI] [PubMed] [Google Scholar]
- 13.Blasco M A, Lee H-W, Hand M P, Samper E, Lansdorp P M, DePinho R A, Greider C W. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 14.Wynford-Thomas S, Kipling D. Nature (London) 1997;389:551–552. doi: 10.1038/39207. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton S E, Corey D R. Chem Biol. 1996;3:863–867. doi: 10.1016/s1074-5521(96)90173-8. [DOI] [PubMed] [Google Scholar]
- 16.Bodnar A G, Ouellette M, Frolkis M, Holt S E, Chiu C-P, Morin G B, Harley C B, Shay J W, Lichsteiner S, Wright W E. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 17.Feng J, Funk W D, Wang S-S, Weinrich S L, Avilion A A, Chiu C-P, Adams R R, Chang E, Allsopp R C, Yu J, et al. Science. 1995;269:1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura T M, Morin G B, Chapman K B, Weinrich S L, Andrews W H, Lingner J, Harley C B, Cech T R. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- 19.Meyerson M, Counter C M, Eaton E N, Ellisen L W, Steiner P, et al. Cell. 1997;90:785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- 20.Norton J C, Piatyszek M A, Wright W E, Shay J W, Corey D R. Nat Biotechnol. 1996;14:615–619. doi: 10.1038/nbt0596-615. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton S E, Pitts A E, Katipally R R, Jia X, Davies B A, Rutter J P, Wright W R, Shay J W, Corey D R. Biochemistry. 1997;36:11873–11880. doi: 10.1021/bi970438k. [DOI] [PubMed] [Google Scholar]
- 22.Simmons C G, Pitts A E, Mayfield L D, Shay J W, Corey D R. Bioorg Med Chem Lett. 1997;7:3001–3007. [Google Scholar]
- 23.Basu S, Wickstrom E. Bioconjugate Chem. 1997;8:481–488. doi: 10.1021/bc9700650. [DOI] [PubMed] [Google Scholar]
- 24.Akhtar S, Agrawal S. Trends Pharmacol Sci. 1997;18:12–18. doi: 10.1016/s0165-6147(96)01002-4. [DOI] [PubMed] [Google Scholar]
- 25.Bennett C F. Biochem Pharmacol. 1998;55:9–19. doi: 10.1016/s0006-2952(97)00214-1. [DOI] [PubMed] [Google Scholar]
- 26.Monia B P, Lesnik E A, Gonzalez C, Lima W F, McGee D, Guinosso C J, Kawasaki A M, Cook P D, Freier S M. J Biol Chem. 1993;268:14514–14522. [PubMed] [Google Scholar]
- 27.Monia B P, Johnston J F, Sasmor H, Cummins L L. J Biol Chem. 1996;271:14533–14540. doi: 10.1074/jbc.271.24.14533. [DOI] [PubMed] [Google Scholar]
- 28.Baker B F, Lot S S, Condon T P, Cheng-Flournoy S, Lesnik E A, Sasmor H, Bennett C F. J Biol Chem. 1997;272:11994–12000. doi: 10.1074/jbc.272.18.11994. [DOI] [PubMed] [Google Scholar]
- 29.Yu D, Iyer R P, Shaw D R, Lisziewicz J, Li Y, Jiang Z, Roskey A, Agrawal S. Bioorg Med Chem. 1996;4:1685–1692. doi: 10.1016/0968-0896(96)00160-5. [DOI] [PubMed] [Google Scholar]
- 30.Agrawal S, Jiang Z, Zhao Q, Shaw D, Cai Q, Roskey A, Channavajjala L, Saxinger C, Zhang R. Proc Natl Acad Sci USA. 1997;94:2620–2625. doi: 10.1073/pnas.94.6.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lingner J, Cech T R. Proc Natl Acad Sci USA. 1996;93:10712–10717. doi: 10.1073/pnas.93.20.10712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norton J C, Waggenspack J H, Varnum E, Corey D R. Bioorg Med Chem. 1995;3:437–445. doi: 10.1016/0968-0896(95)00033-d. [DOI] [PubMed] [Google Scholar]
- 33.Holt S E, Norton J C, Wright W E, Shay J W. Methods Cell Sci. 1996;18:237–248. [Google Scholar]
- 34.Smulevitch S V, Simmons C G, Norton J C, Wise T W, Corey D R. Nat Biotechnol. 1996;14:1700–1705. doi: 10.1038/nbt1296-1700. [DOI] [PubMed] [Google Scholar]
- 35.Peyman A, Uhlmann E. Biol Chem Hoppe-Seyler. 1996;377:67–70. doi: 10.1515/bchm3.1996.377.1.67. [DOI] [PubMed] [Google Scholar]
- 36.Lewis J G, Lin K-Y, Kothavale A, Flanagan W M, Matteucci M D, DePrince R B, Mook R A, Hendren R W, Wagner R W. Proc Natl Acad Sci USA. 1996;93:3176–3181. doi: 10.1073/pnas.93.8.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corey D R. Trends Biotechnol. 1997;15:224–229. doi: 10.1016/S0167-7799(97)01037-8. [DOI] [PubMed] [Google Scholar]
- 38.McEachern, M. J. & Blackburn E. H. Genes Dev.10, 1822–1834. [DOI] [PubMed]
- 39.Hanish J P, Yanowitz J L, De Lange T. Proc Natl Acad Sci USA. 1994;91:8861–8865. doi: 10.1073/pnas.91.19.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]