Cholera Toxin Suppresses Interleukin (IL)-12 Production and IL-12 Receptor β1 and β2 Chain Expression (original) (raw)
Abstract
Cholera toxin (CT) is a potent mucosal vaccine adjuvant, which has been shown to induce T helper cell type 2 (Th2) responses in systemic and mucosal tissues. We report that CT inhibits the production of interleukin (IL)-12, a major Th2 counterregulatory cytokine. IL-12 p70 production by stimulated human monocytes was inhibited by CT in a dose-dependent manner. This suppression occurred at the level of gene transcription, was maximal at low concentrations of CT, and was dependent on the A subunit of the toxin, since purified CT B subunit had minimal effect. CT also inhibited the production of IL-12 p70 by monocyte-derived dendritic cells, as well as the production of tumor necrosis factor α, but not IL-10, IL-6, or transforming growth factor (TGF)-β1, by stimulated monocytes. The effects of CT were not due to autocrine production of IL-10, TGF-β1, or prostaglandin E2. CT inhibited the production of IFN-γ by anti-CD3-stimulated human peripheral blood mononuclear cell, due in part to suppression of IL-12 production, but also to the inhibition of expression of the β1 and β2 chains of the IL-12 receptor on T cells. In vivo, mice given CT before systemic challenge with lipopolysaccharide had markedly reduced serum levels of IL-12 p40 and interferon γ. These data demonstrate two novel mechanisms by which CT can inhibit Th1 immune responses, and help explain the ability of mucosally administered CT to enhance Th2-dependent immune responses.
Keywords: interleukin 12, monocytes, dendritic cells, Th1 and Th2 cells, cholera toxin
Immunization at mucosal surfaces can result in the induction of a broad range of immune responses. The application of soluble protein antigens applied to the nasal or intestinal mucosa in the absence of adjuvants normally results in systemic tolerance due either to clonal T cell anergy and deletion, or to the induction of antigen-specific T cells that secrete suppressive cytokines such as TGF-β1 and IL-10 (for review see references 1, 2). B cell responses are also suppressed primarily due to a lack of T cell help (3, 4). In animal studies, oral (mucosal) tolerance has been determined either (a) by demonstrating suppressed delayed-type hypersensitivity (DTH)1 reactions, reduced systemic antibody responses, and inhibited in vitro T cell proliferation and cytokine production after antigen feeding and subsequent systemic antigenic challenge in complete Freund's adjuvant, or (b) by resistance to the induction of autoimmune diseases, such as experimental autoimmune encephalomyelitis, in animals fed self-antigens. In contrast to the induction of tolerance, the mucosal administration of protein antigens together with adjuvants, such as those normally expressed by infecting microbes, vaccine vectors, or in the form of ADP-ribosylating bacterial toxins such as cholera toxin (CT), results in the abrogation of oral tolerance, the induction of both local secretory IgA (sIgA) and systemic (IgG) humoral immunity, and the induction of cytotoxic T cell responses (for review see references 5–7).
The ability of CT to prevent oral tolerance and induce strong systemic immune responses to mucosal antigens is shared by the genetically and structurally similar heat-labile toxin (LT) from Escherichia coli (8). These bacterial toxins are composed of a monomeric “A” and a pentameric “B” subunit. The B subunit binds to cell surface gangliosides and facilitates entry of the A subunit into the cell. Once inside the cell, the A subunit acts to catalyze the ADP-ribosylation of the intracellular G protein Gsα. Subsequently, the covalently modified Gsα dissociates from the Gsβγ dimer and activates adenylate cyclase, causing an increase in intracellular cAMP. During human infection with Vibrio cholera, or enterotoxigenic E. coli, the induction of cAMP in intestinal epithelial cells by CT or LT results in active fluid secretion and diarrhea.
The mechanisms by which CT or LT act as mucosal adjuvants, including the extent to which this adjuvanticity relies on their ability to induce cAMP, are not fully understood, primarily because these toxins have broad effects on both immune and nonimmune cells (5–7). Some of the reported effects of CT that have potential relevance to its adjuvanticity are the ability to enhance antigen transport across the epithelium (9), the production of IL-6 and IL-1 from epithelial cells (10, 11), the expression of T cell costimulatory molecules, such as B7-2 (CD86) (12), by APCs, and the promotion of B cell differentiation (13, 14). In vivo, CT has also been shown to augment T cell activation and differentiation. In this regard, several studies of restimulated lymphoid cells, isolated from mice mucosally immunized with CT as an adjuvant, have demonstrated a Th2-dominant T cell response with increased production of IL-4, IL-5, and IL-10 (15–18). Consistent with this finding, mucosal (17–21), as well as systemic (16) immunization with a protein and CT results in serum levels of antigen-specific IgG1 that are consistently higher (10– 100-fold) than the levels of IgG2a; in several studies, high levels of IgG1 and no IgG2a were found (16, 17, 22). In addition, oral immunization with CT can induce the production of IL-4–dependent antigen-specific IgE (16–18, 20, 23), which can result in IgE-mediated anaphylaxis on secondary antigenic challenge (17, 20). Th2 responses are also important for the optimal induction of sIgA (21, 24); therefore, it is logical that CT-driven Th2 responses are important for the ability of CT to induce mucosal humoral immunity to a coadministered oral antigen. Consistent with this possibility is the fact that IL-4−/− mice are resistent to the adjuvant effects of CT, including the induction of IgA at mucosal surfaces (17, 21).
Currently it is not clear why immunization with CT results in a Th2-dominated T cell response. To explore this issue, we initially determined whether CT could inhibit the production of IL-12, a major counterregulatory cytokine for the induction of Th2 T cell responses (for review see references 25, 26). This possibility was suggested by studies demonstrating that administration of exogenous IL-12 redirects Th2 to Th1 responses after oral immunization with CT (18). We found that CT, as well as the related LT, but not purified CT B subunit (CT-B), inhibited IL-12 p70 production by both human monocytes and human monocyte-derived dendritic cells (DCs) in response to a variety of stimuli. This suppression by CT was dose dependent, probably acted at the level of gene transcription, and was selective. We next determined whether CT could suppress the responsiveness of lymphocytes to IL-12. We found that CT could inhibit the expression of the β1 and β2 chains of IL-12R on human T cells stimulated in vitro with anti-CD3. Finally, we demonstrated the relevance of these effects by showing that CT can inhibit IFN-γ production both in vitro, in cultures of human PBMCs, and in vivo, in mice challenged systemically with LPS.
Materials and Methods
Reagents.
CT and purified CT-B were purchased from List Biological Laboratories. E. coli LT, LPS (E. coli serotype O127: B8), and indomethacin were obtained from Sigma Chemical Co. Staphylococcus aureus, Cowan's strain I (SAC), was supplied by Calbiochem. Recombinant human IFN-γ, IL-4, and GM-CSF were purchased from Genzyme Diagnostics. Recombinant human IL-12, neutralizing antibody to human TGF-β1 (polyclonal chicken Ig) and control antibody (normal chicken Ig), neutralizing antibody to human IL-10 (clone 23738.11), and isotype control (clone 20116.11), as well as neutralizing antibody to human IL-12 (polyclonal goat IgG), were obtained from R&D Systems. Recombinant trimerized human CD40L (CD154) was provided by Immunex Corp.
Isolation and Stimulation of Human Monocytes and Dendritic Cells.
Human monocytes were obtained from normal healthy donors (total n = 25) by standard leukaphoresis, purified by counterflow centrifugation (elutriation), which yielded cells of uniform forward/side scatter that were 95–99% CD14+ by flow cytometry. Cells were cultured at a density of 2 × 106 cells/ml in 1 ml of RPMI 1640 (Biofluids Inc.) supplemented with 10% FCS (Biofluids Inc.), 100 μg/ml penicillin, 100 μg/ml streptomycin, 50 μg/ml gentamicin, 5% NCTC-109 media (Biofluids Inc.), 15 mM Hepes, and 200 mM glutamine (cRPMI) at 37°C and 6% CO2 unless otherwise noted. For measurement of cytokine production, human monocytes were preincubated with media alone or with varying concentrations of CT, CT-B, or LT, for 1 h at 37°C before stimulation with SAC (0.01% wt/vol) and IFN-γ (100 ng/ml), LPS (1 μg/ml) and IFN-γ (100 ng/ml), or CD40L (3 μg/ml) and IFN-γ (100 ng/ml) for 24 h, after which culture supernatants were collected and stored at −20°C until assayed for cytokines.
Dendritic cells were derived from elutriated monocytes as previously described (27). In brief, monocytes were cultured for 7 d in cRPMI supplemented every other day with IL-4 (100 ng/ml) and GM-CSF (100 ng/ml). Nonadherent cells were harvested by gentle washing, and the majority (70–90%) were demonstrated by flow cytometry to express high levels of CD1a (PharMingen) and low levels of CD83 (PharMingen; data not shown), consistent with prior reports (27). Greater than 95% of the cells excluded trypan blue, and demonstrated characteristic dendrite formation on examination with phase-contrast, light microscopy. The DCs were resuspended at a density of 106 cells/ml in cRPMI, and treated with CT (10 ng/ml), CT-B (10 ng/ml), or media alone for 1 h before stimulation with either SAC (0.01%) and IFN-γ (100 ng/ml), or CD40L (3 μg/ml) and IFN-γ (100 ng/ml). Supernatants were collected after 24 h of culture and stored at −20°C until assayed for cytokines.
To determine the role of autocrine inhibitors of IL-12 in the monocyte cultures, elutriated monocytes were preincubated for 1 h with CT (10 ng/ml) or media alone and one of the following: neutralizing antibody to TGF-β1 (10 μg/ml) or control antibody (10 μg/ml), neutralizing antibody to IL-10 (2.5 μg/ml) or isotype matched control antibody (2.5 μg/ml), or indomethacin (10−5 M). SAC (0.01%) and IFN-γ (100 ng/ml) were then added, and after 24 h of culture, supernatants were harvested and stored at −20°C until assayed for IL-12 p70 production.
Cytokine ELISAs.
Cell culture supernatants were assayed for cytokines by ELISA using matched antibody pairs according to the manufacturer's suggestions. ELISA reagents for human IL-12 p70 were purchased from R&D Systems, and for IL-10, TNF-α, and IFN-γ from Biosource International; the respective capture and detection antibodies were as follows: IL-12 p70 clone 24945.11 and polyclonal goat IgG, IL-10 clones AHC8102 and AHC7109, TNF-α clones AHC3712 and AHC3419, and IFN-γ clones AHC4432 and AHC4539. In brief, the capture antibody was bound to 96-well ELISA plates (Immulon 4™; Dynex) in the appropriate buffer overnight at 4°C; capture antibody for IL-12 p70 was used at a concentration of 4 μg/ml diluted in PBS; IL-10, TNF-α, and IFN-γ capture antibodies were used in a concentration of 2 μg/ml diluted in bicarbonate buffer (pH 9.6). The plates were then washed (three times) with PBS with 0.05% Tween 20, and blocked for 2 h at room temperature; 1% BSA, 5% sucrose, and 0.05% sodium azide in PBS (pH 7.3) was used to block the IL-12 p70 plates, and 3% BSA in PBS was used for the remaining cytokines. Cytokine standards and supernatants were diluted as necessary; 20 mM Tris-HCl, 150 mM NaCl, 0.05% Tween 20, and 0.1% BSA was used as the diluent for the IL-12 p70 assay, the IL-10, TNF-α, and IFN-γ ELISAs used 3% BSA in PBS as a diluent. The ELISA plates were then incubated overnight at 4°C. The plates were washed, and bound cytokine was revealed with a biotin-labeled detecting antibody (2 h at room temperature). IL-12 p70 detection antibody was diluted 1:333, whereas IL-10, TNF-α, and IFN-γ detection antibodies were diluted 1:1,000. This was followed by horseradish peroxidase–conjugated streptavidin (Zymed; 1:1,000 for 30 min at room temperature), and the substrate _o_-phenylenediamine dihydrochloride (OPD; Sigma Chemical Co.) at 0.5 mg/ml in phosphate-citrate buffer (pH 5.0) and 0.03% H2O2. Optical density of the individual wells was determined at 450 nm using an automated ELISA reader (Dynex). The lower limit of sensitivity of the assays was 32 pg/ml for IL-12 p70, IL-10, and IFN-γ, and 64 pg/ml for TNF-α. Assays for IL-6 and TGF-β1 were performed using ELISA kits from R&D systems and Genzyme Diagnostics, respectively, according to the manufacturers' instructions. TGF-β1 levels were measured after acidification, and therefore reflect both the active and latent forms of TGF-β1.
Reverse Transcriptase PCR.
Total RNA was obtained from 107 elutriated monocytes using STAT-60 (TEL-TEST Inc.) according to the manufacturer's instructions. RNA concentrations were determined by measuring the optical density at 260 nm. mRNA for each experimental condition was reverse transcribed with oligo (dT) priming to first strand cDNA using Superscript II™ reverse transcriptase (GIBCO BRL). In brief, 1 μg of total RNA suspended in RNAse free water was added to 16 μl of a reverse transcription reaction mixture consisting of 20 mM Tris-HCL, 2.5 mM MgCl2, 50 mM KCl, 10 mM dithiothreitol, and 1 mM dNTP. After an initial incubation for 5 min at 42°C, 200 U of Superscript II™ reverse transcriptase was added and the reaction was continued for 50 min at 42°C, and then terminated at 70°C for 10 min. The samples were then chilled on ice for 15 min. RNAse H (2 U) was then added, and the was reaction incubated for 20 min at 37°C. The first strand cDNA was then stored at −80°C before PCR amplification.
PCR amplification was performed using 2 μl of cDNA template, and 50 μl of a reaction mixture consisting of 20 mM Tris-HCl, 50 mM KCl, 0.2 mM dNTP, 1.25 mM MgCl2, and 2.5 U Platinum _Taq_™ DNA polymerase (GIBCO BRL). The following primer pairs were used at a concentration of 1 μM: IL-6, sense 5′-ATGAACTCCTTCTCCACAAGCGC-3′, antisense 5′-GAAGAGCCCTCAGGCTGGACTG-3′; IL-12 p40, sense 5′-AGAGGCTCTTCTGACCCCCAG-3′, antisense 5′-CTCTTGCTCTTGCCCTGGACCTG-3′; IL-12 p35, sense 5′-TCAGCAACATGCTCCAGAAGGC-3′, antisense 5′-TGCATTCATGGTCTTGAACTCCACC-3′; and GAPDH, sense 5′-TGAAGGTCGGAGTCAACGGATTTGGT-3′, antisense 5′-CATGTGGGCCATGAGGTCCACCAC-3′. PCR amplification was performed under the following conditions: initial denaturation at 94°C for 2 min, followed by cycles of 94°C for 45 s, 60°C for 60 s, and 70°C for 90 s, and a final elongation step of 70°C for 5 min. The number of amplification cycles was determined by prior experiments to ensure linear phase amplification of cDNA template (data not shown). GAPDH was amplified 25 cycles, IL-6 and IL-12 p40 30 cycles, and IL-12 p35 32 cycles. Amplified PCR products were resolved by gel electrophoresis on a 1.5% agarose gel, and stained with ethidium bromide.
Isolation and Stimulation of Human PBMCs.
PBMCs were obtained from three healthy donors by centrifugation of whole blood of over Ficoll-sodium diatrizoate (LSM® Lymphocyte Separation Medium; Organon Teknika Corp.) using the recommended standard procedures (28). Cells were extensively washed and cultured at a density of 106 cells/ml cRPMI in the presence or absence of CT (10 ng/ml) for 1 h. The PBMCs were then stimulated with nothing (controls), soluble anti-CD3 alone (1 μg/ml), rIL-12 alone (1 ng/ml), anti-CD3 and anti-IL-12 (1 μg/ml), or anti-CD3 and rIL-12. After 72 h, cell supernatants were harvested and stored at −20°C until assayed for cytokines. For cell proliferation studies, identical conditions were used; however, cells were plated in triplicate in 96-well U-bottomed microtiter plates with a total cell culture volume of 100 μl/well. After 72 h in culture, the plates were pulsed for 6 h with [3H]thymidine (1 μCi/well) and frozen overnight at −20°C. The plates were thawed, and 3H-incorporation from cell lysates was determined using a Betaplate™ cell harvester and liquid scintillation counter (Wallac Inc.).
Flow Cytometry for IL-12Rβ1 and IL-12Rβ2 Chain Expression.
PBMCs were obtained as above from two healthy donors on two separate occasions and cultured at a density of 106 cells/ml cRPMI in the presence or absence of CT (10 ng/ml) for 1 h, and then stimulated with soluble anti-CD3 (1 μg/ml). Controls were cultured without stimulation. The cells were harvested after 72 h of culture. 106 cells in 100 μl staining buffer (PBS containing 0.2% BSA and 0.1% sodium azide) were sequentially incubated with rat anti–human mAb for the IL-12Rβ1 chain or IL-12Rβ2 chain (29) (clones 2B10 and LM-5.2B6, respectively, provided by Dr. David Presky, Hoffmann-La Roche, Nutley, NJ) for 30 min, followed by biotinylated anti–rat Ig F(ab′)2 fragments (Boehringer Mannheim Corp.) for 30 min, and finally with PE-labeled streptavidin (PharMingen) for 20 min. All incubations were performed at 4°C in staining buffer, and cells were washed twice with staining buffer between incubations. The stained cells were analyzed on a FACScan® flow cytometer using CellQuest® software (Becton Dickinson).
In Vivo Production of IL-12 and IFN-γ after Systemic LPS Challenge.
6–10-wk-old BALB/c mice housed under standard conditions were treated intraperitoneally with 250 μl of PBS alone or 250 μl of PBS containing 10 μg of CT. 1 h later, 250 μg LPS in 100 μl PBS, or PBS alone was injected intraperitoneally. 4 h later the mice were anesthetized and then bled by cardiac puncture. After clotting the blood for 30 min at 37°C, serum was isolated by centrifugation. Serum cytokine levels were measured by ELISA using murine IL-12 p40 (R&D Systems) and IL-12 p70 (Genzyme) ELISA kits according to the manufacturers' instructions, and antibody pairs against murine IFN-γ (PharMingen) as described above. Serum samples for measurement of IL-12 p70 were used neat, for IL-12 p40 were diluted 1:125, and for IFN-γ diluted 1:5. The lower limit of sensitivity of the murine IL-12 p40, IL-12 p70, and IFN-γ ELISAs were 2 pg/ml, 50 pg/ml, and 1 U/ml, respectively.
Statistics.
Statistical analysis was performed by paired t testing using SigmaStat™ software (Jandel Corp.).
Results
Inhibition of IL-12 p70 Production from Human Monocytes and Dendritic Cells.
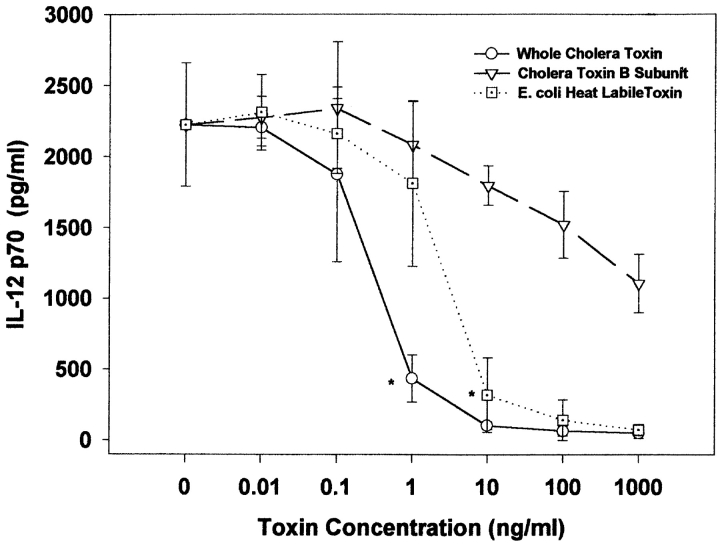
We initially sought to determine whether CT had direct effects on IL-12 production by purified human mononuclear cells. For these assays we pretreated elutriated monocytes from random healthy donors with varying concentrations of CT, LT, or CT-B before stimulation with SAC and IFN-γ. As shown in Fig. 1, CT and LT, but not CT-B, inhibited the production of IL-12 p70 in a dose-dependent fashion. Inhibition was seen with doses as low as 1 ng/ml, and were maximal (97% reduction) at 10 ng/ml for both toxins. At high concentrations (>100 ng/ml), CT-B had minimal suppressive effects (<50% reduction) that were not statistically significant. As shown in Fig. 2 A, this inhibition was not dependent on the cell stimulus, as the effects of CT were similar with two other potent IL-12 stimuli, LPS and IFN-γ, and CD40L and IFN-γ.
Figure 1.
(A) CT and E. coli LT, but not CT-B, suppressed IL-12 p70 production in a dose-dependent manner. Elutriated human monocytes (2 × 106 cells/ml) were pretreated with varying concentrations of toxin for 1 h, followed by stimulation with SAC (0.01%) and IFN-γ (100 ng/ml). 24 h after stimulation, cell supernatants were harvested and analyzed by ELISA for IL-12 p70. Data are mean values ± SD from three donors per experimental condition. *P < 0.05 for given concentration, and all higher concentrations.
Figure 2.
(A) Suppression of IL-12 p70 by CT was independent of stimuli. Human monocytes (2 × 106 cells/ml) were pretreated for 1 h with CT (10 ng/ml), and then stimulated with SAC (0.01%), LPS (10 ng/ml), or CD40L (3 μg/ml) and IFN-γ (100 ng/ml). Values are mean ± SD from three donors per experimental condition. (B) CT markedly suppressed IL-12 p70 production in human DCs. DCs were derived from human monocytes cultured for 1 wk with IL-4 and GM-CSF. After phenotypic confirmation, cells (106 cells/ml) were treated with either SAC (0.01%) and IFN-γ (100 ng/ml) or CD40L (3 μg/ml) and IFN-γ (100 ng/ml) with or without CT (10 ng/ml) pretreatment. Data are mean values ± SD of four separate experiments using DCs derived from four different donors. IL-12 production was analyzed by ELISA. **P < 0.01; *P < 0.05.
We then determined whether this inhibitory effect was also seen with DCs. DCs have been shown to migrate to T cell regions of lymphoid tissues and present antigen to naive T cells more efficiently than monocyte/macrophages, and thus are more likely to play a role in directing initial T cell differentiation (for review see references 30, 31). For these assays, we established DC populations in vitro from elutriated human monocytes cultured for 7 d with GM-CSF and IL-4. These cells were shown to be typical of DCs in an intermediate stage of differentiation (27), as they had moderate but consistent dendrite formation by phase-contrast light microscopy, and expressed both high levels of CD1a and low levels of CD83 by flow cytometry (data not shown). As shown in Fig. 2 B, stimulation of this population with CD40L and IFN-γ or SAC and IFN-γ resulted in dramatic production of IL-12 p70 (>3,500 pg/ml). Although this was inhibited by >90% with CT (10 ng/ml) pretreatment, CT-B (10 ng/ml) had minimal inhibitory effects (<10% suppression) on IL-12 p70 production from DCs (data not shown).
Inhibition of IL-12 Production by Monocytes Is Selective and Not Due to Cell Death.
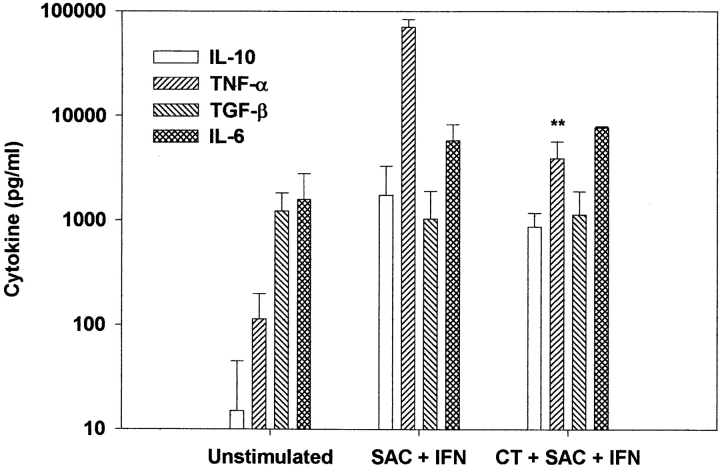
We next determined whether the suppression of cytokine production from monocytes by CT was selective for IL-12 p70. As shown in Fig. 3, we found that, similar to other known inhibitors of IL-12 (32–38), production of IL-10, TGF-β1, and IL-6 were not significantly affected by CT. However, TNF-α was also suppressed (by 90%) by treatment with CT. The suppression of TNF-α by CT is distinct from that seen with other inhibitors of IL-12, which typically do not affect TNF-α synthesis (34–38). Although the selective suppression of IL-12 and TNF-α by CT suggested that this inhibition was not due to cell death, we confirmed this by determining cell viability at the end of the 24-h culture period. We found that the percentage of cells excluding trypan blue by visualization with light microscopy correlated well with the percentage that stained minimally with propidium iodide as detected with flow cytometry. In both control cultures and cultures pretreated with either CT or LT at concentrations of <1 μg/ml, 70–80% of all monocytes were viable at 24 h. In addition, the total numbers of cells collected at 24 h from the cultures with and without CT were similar, suggesting that in vitro cells were not dying in the presence of CT. CT concentrations >1 μg/ml resulted in enhanced cell death (data not shown).
Figure 3.
CT treatment selectively suppressed IL-12 p70 and TNF-α, but not IL-10, TGF-β1, or IL-6. Human monocytes (2 × 106 cell/ml) were stimulated with SAC (0.01%) and IFN-γ (100 ng/ml) in the presence or absence of CT (10 ng/ml). Cytokine production was measured by ELISA 24 h after stimulation. Data are mean values ± SD of four separate experiments using four different donors. **P < 0.01.
Inhibition of IL-12 Production Is Not Due to Known Autocrine Inhibitors.
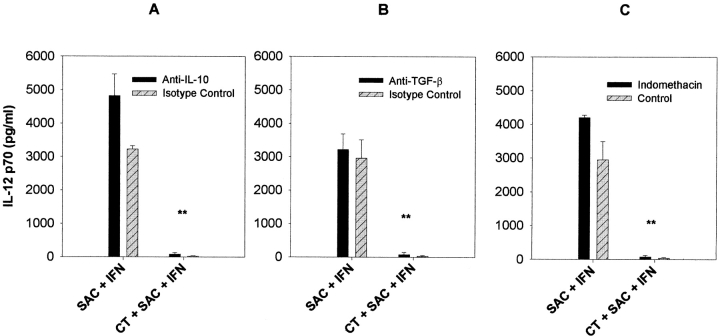
To determine whether the inhibition of IL-12 p70 production could be due to the induction of several known autocrine IL-12 inhibitors, we pretreated monocytes with indomethacin (to block the production of prostaglandins, specifically PGE2), anti-IL-10, or anti-TGF-β1, together with CT before stimulation with SAC and IFN-γ. As shown in Fig. 4, the inhibition of IL-12 by CT (10 ng/ml) was not reversed by any of these treatments. These data are consistent with the fact that the levels of IL-10 and TGF-β1 as measured in monocyte cultures were unaffected by CT (Fig. 3).
Figure 4.
CT-mediated suppression of IL-12 p70 was independent of known autocrine inhibitors of IL-12. Elutriated human monocytes (2 × 106 cells/ml) with or without CT (10 ng/ml) were incubated with neutralizing antibody to IL-10 (A), neutralizing antibody to TGF-β1 (B) indomethacin (C) to inhibit PGE2 production, and then stimulated with SAC (0.01%) and IFN-γ (100 ng/ml). IL-12 p70 production was measured 24 h later by ELISA. Data are mean values ± SD from three separate experiments using three different donors. **P < 0.01.
CT Inhibits mRNA for IL-12 p35 and p40 Chains.
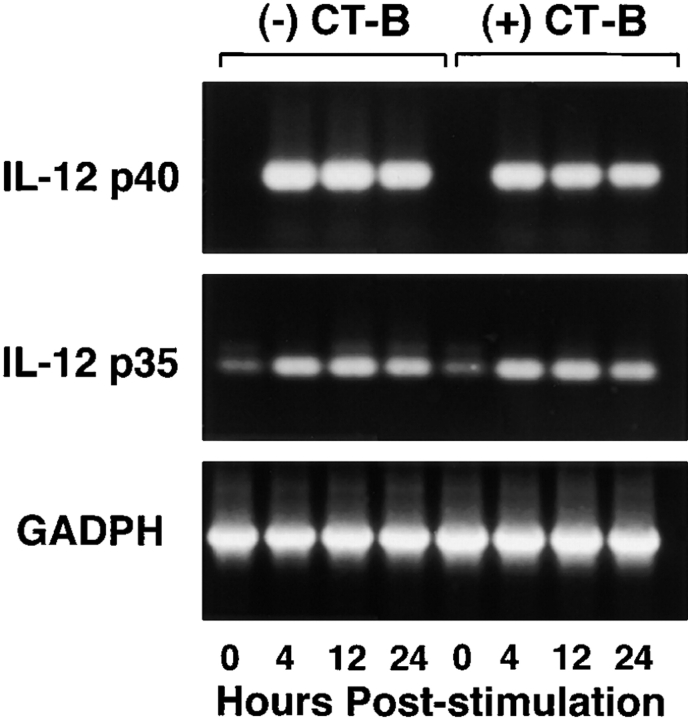
We next sought to determine the level at which CT mediates its suppressive effects. For these studies we used semiquantitative reverse transcription PCR to analyze the levels of IL-12 p35 and p40 mRNA present in monocytes stimulated with SAC and IFN-γ in the presence and absence of CT. Stimulation of cells with SAC and IFN-γ resulted in increased mRNA for both p35 and p40, as well as IL-6 (Fig. 5). Pretreatment of monocytes with CT (10 ng/ml) resulted in suppression of SAC and IFN-γ–stimulated mRNA levels for IL-12 p35 and p40, but did not affect levels of mRNA for IL-6. IL-12 p40 and p35 mRNA expression in CT-B–treated (10 ng/ml) monocytes did not differ from SAC and IFN-γ–stimulated controls (Fig. 6). Since previous studies have shown that IL-12 is primarily regulated at the level of transcription (39, 40), the data presented here suggest that CT is acting to suppress IL-12 p70 production by preventing transcription of the genes for both chains of IL-12.
Figure 5.
Analysis by reverse transcription PCR demonstrates significantly reduced levels of mRNA for IL-12 p40 and p35, but not IL-6, in CT-treated cells after stimulation. mRNA was obtained at intervals from human monocytes (107 cells) after stimulation with SAC (0.01%) and IFN-γ (100 ng/ml) in the presence or absence of CT (10 ng/ml).
Figure 6.
Analysis by reverse transcription PCR demonstrates no significant difference in levels of mRNA for IL-12 p40 and p35 in CT-B–treated cells after stimulation. mRNA was obtained at intervals from human monocytes (107 cells) after stimulation with SAC (0.01%) and IFN-γ (100 ng/ml) in the presence or absence of CT-B (10 ng/ml).
CT Inhibits Proliferation and IFN-γ Production by PBMCs Stimulated with Anti-CD3.
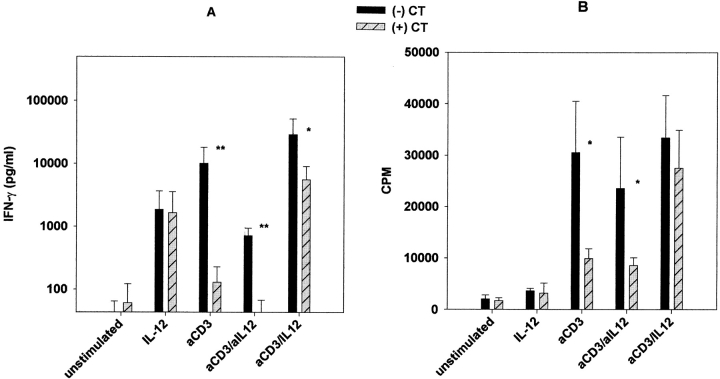
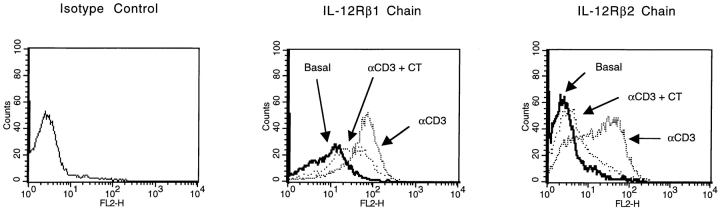
To determine the functional relevance of the effects of CT on IL-12 production, we initially examined the ability of CT to inhibit IFN-γ production by PBMCs stimulated with soluble anti-CD3. Under such stimulation conditions, T cells are activated to express CD40L, which stimulates CD40-dependent production of IL-12 by both monocytes and DCs. In turn, IL-12 drives IFN-γ production by circulating NK and T cells, and augments CD3-mediated proliferation of T cells. As shown in Fig. 7, PBMCs stimulated with anti-CD3 produced IFN-γ and proliferated in an IL-12 dependent fashion, as anti–IL-12 abrogated this response. The addition of CT inhibited IFN-γ production as well as proliferation; however, such inhibition was only partially reversed by the addition of exogenous IL-12 to the cultures. These latter findings suggested that CT may also inhibit the responsiveness of T or NK cells to IL-12. To examine this possibility, we stimulated PBMCs with anti-CD3 in the presence or absence of CT. After 72 h in culture, the level of expression of the two chains of IL-12R on T cells was determined by flow cytometry. As shown in Fig. 8, expression of both the β1 and β2 chains of IL-12R were significantly upregulated by stimulation with anti-CD3, and this enhancement was blocked by treatment with CT. Thus, CT appears to act by at least two distinct mechanisms to suppress IFN-γ production in cultured PBMCs.
Figure 7.
(A) IFN-γ production by cultured PBMCs was significantly reduced by CT treatment. PBMCs (106 cells/ml) were cultured for 72 h in the presence or absence of CT (10 ng/ml) with nothing (unstimulated), rIL-12 (1 ng/ml), anti-CD3 (1 μg/ml), anti-CD3 and anti-IL-12 (1 μg/ml), or anti-CD3 and rIL-12. Supernatants were then harvested and analyzed for IFN-γ production by ELISA. Data are mean values ± SD from a total of seven donors from three separate experiments. **P < 0.01; *P < 0.05. (B) CT treatment also inhibited anti-CD3-mediated cell proliferation. PBMCs were cultured as noted above for 72 h, then pulsed for 6 h with [3H]thymidine, harvested, and analyzed. Data are mean values ± SD of triplicate samples from three different donors. *P < 0.05.
Figure 8.
CT reduced cell surface expression of IL-12Rβ1 and IL-12Rβ2 chains in anti-CD3-stimulated PBMCs. Flow cytometry was performed on 106 PBMCs after 72 h in culture on cells that were unstimulated (basal expression), cells stimulated with anti-CD3 (1 μg/ml), or cells stimulated with anti-CD3 in the presence of CT (10 ng/ml). Data are representative of flow cytometric analysis of two separate experiments each with two different donors. On average, anti-CD3 stimulation increased β1 chain mean fluorescence by 6.5-fold, and β2 fluorescence by 5.1-fold. In the presence of CT, the average mean fluorescence of the β1 chain increased by only 3.5-fold, and the β2 chain by only 1.9-fold. This difference was statistically significant (P < 0.05).
CT Inhibits IFN-γ and IL-12 p40 Production in Mice Challenged Systemically with LPS.
Finally, to demonstrate the inhibitory effect of CT in an established in vivo model of IL-12 production, we examined the ability of CT to inhibit IFN-γ and IL-12 p40 in the serum of mice challenged systemically with LPS (41, 42). IFN-γ production in this model has been shown to depend on IL-12, as anti–IL-12 inhibits this response. For these studies, mice were given CT (10 μg/mouse) intraperitoneally 1 h before systemic challenge with 250 μg of LPS. 4 h later, serum levels of IL-12 p70, IL-12 p40, and IFN-γ were determined by ELISA. IL-12 p70 was found in the serum of control-stimulated mice at or near the levels of detection of our ELISA (50–100 pg/ml). Therefore, this measurement was not helpful for determining levels of IL-12 suppression in vivo. However, as shown in Table I, we found that prior treatment with CT resulted in an 80% reduction in serum levels of IL-12 p40. In addition, in vivo production of IFN-γ was completely suppressed by CT pretreatment. Since IFN-γ production in this model has been shown by several groups to be IL-12 dependent (41–45), these findings strongly suggest that bioactive IL-12 was suppressed by CT administration.
Table I.
Serum Cytokine Concentrations in 6-wk-old BALB/c Mice
| Condition | IL-12p40 | IFN-γ |
|---|---|---|
| pg/ml | U/ml | |
| Unstimulated | <250 | <5 |
| CT | <250 | <5 |
| LPS | 15,167 (± 4,018)** | 158.3 (± 65.4)* |
| LPS/CT | 3,625 (± 2,080) | <5 |
Discussion
CT is a potent mucosal vaccine adjuvant that can augment the production of secretory IgA and prevent the induction of oral tolerance to soluble proteins. These adjuvant effects are likely due in large measure to its ability to augment T cell activation and differentiation in vivo. In this regard, studies of cytokines produced after in vitro restimulation of lymphoid cells isolated from mice mucosally immunized with CT alone, or with CT as an adjuvant, have generally demonstrated one of two responses: (a) one clearly dominated by Th2 cytokines, including IL-4, IL-5, and IL-10 (15–18), or (b) a mixed Th1 and Th2 cytokine response showing enhanced production of IFN-γ as well IL-4 (15, 46, 47). However, studies of immunoglobulin isotypes in CT immunized mice are more consistent in demonstrating that a Th2 response predominates in vivo (16–23). In this report, we provide two mechanisms for the ability of CT to drive Th2 cell differentiation after mucosal immunization. We demonstrate that the production of IL-12, a major counterregulatory cytokine for the development of Th2 responses, by human monocytes and DCs is potently suppressed by CT. In addition, we demonstrate that T cell responsiveness to IL-12 is also inhibited by CT, and that this is due to a decreased expression of the β1 and β2 chains of IL-12R. Finally, we demonstrate the ability of CT to inhibit IL-12 and/or Th1 responses both in vitro, by anti-CD3-stimulated human PBMCs, and in vivo, in a mouse model of septic shock.
IL-12 p70 production by human monocytes in response to a variety of stimuli (SAC, LPS, or CD40L) in combination with IFN-γ was suppressed by CT in a dose dependent manner (Figs. 1 and 2 A). The CT-related LT from E. coli similarly suppressed IL-12 p70 production by monocytes stimulated with SAC and IFN-γ. In contrast, purified CT-B had only minimal effects on IL-12 p70 production and only at concentrations 100–1,000-fold higher than suppressive doses of the holotoxin. These findings indicate that the A subunit of CT, and probably of LT, is of primary importance for the ability of these toxins to suppress IL-12 p70 production. In fact, the minimal suppressive activity seen here with CT-B, although possibly related to direct effects of CT-B binding, is most likely due to residual holotoxin in the CT-B preparations. For the effects seen here, this would require only 0.1% of active holotoxin, a level consistent with the reported 0.06–2.0% residual enzymatic activity of the purified CT-B from List Biologicals.
Whether CT and LT actually act to suppress IL-12 via the induction of cAMP is not clear. This appears to be the most likely mechanism, as others have shown that direct stimulation of cAMP pathways with forskolin, or dibutyryl-cAMP, can inhibit IL-12 p35 and p40 gene transcription (37). In addition, PGE2 (37) and β-adrenergic agonists (38) both activate adenylate cyclase and inhibit IL-12 production. However, other IL-12 inhibitors, such as iC3b (via complement receptor 3; references 34, 48), IgG (via Fc-receptors; reference 48), measles virus (via CD46; reference 35), TGF-β1, and IL-10, are not thought to suppress IL-12 via the induction of cAMP. In addition, a mutant CT with no detectable ADP-ribosylase activity, due to a point mutation introduced into the A subunit, was recently shown to have maintained its adjuvant effects as well as the ability to induce Th2 responses in vivo (16). These findings, together with reports of the induction of non-cAMP signaling pathways by CT, but not CT-B (49, 50), suggest that cAMP-independent, A subunit–dependent processes could be responsible for the suppression of IL-12 seen here. We attempted to address this issue by determining whether defined cAMP, or adenylate cyclase, inhibitors would prevent the suppression of IL-12 by CT. Thus far, we found these agents to be toxic to monocytes at the concentrations required to suppress cAMP. We are currently testing the ability of LT-mutants, which have far less ADP-ribosylase activity than normal LT, to suppress IL-12.
CT also inhibits IL-12 production from DCs stimulated with CD40L and IFN-γ (Fig. 2 B). Similar to our findings with monocytes, CT-B did not suppress the production of IL-12 p70 by DCs. Since DCs are the major APCs for the induction of primary T cell immune responses (31), this finding implies that induction of primary Th1 T cell responses can be inhibited directly by CT. Thus, CT could act at two stages of T cell development; it could inhibit Th1 priming in lymphoid organs that are more dependent on DC production of IL-12, and it could inhibit secondary stimulation of Th1 T cells in inflammatory sites, where they may be more dependent on macrophage production of IL-12 for their survival and function. The latter point is illustrated by the fact that the systemic administration of IL-12 antibodies to mice with ongoing Th1-mediated experimental inflammatory colitis results in reversal of the inflammation (51), due in large part to the induction of local T cell apoptosis (Fuss, I., and T. Marth, unpublished data).
The inhibition of IL-12 production and responsiveness are not the only mechanisms by which CT may drive Th2 T cell responses in vivo. Earlier studies have shown that CT has the capacity to suppress the production of IL-2 and IL-2 responsiveness in both resting and activated T cells (52–54). This effect has been confirmed in Th1 T cell clones (55). In contrast, CT has little effect on the proliferation of Th2 clones, or on their production of IL-4 (55), suggesting that CT may promote Th2 T cell development partially because of its differential direct suppressive effects on Th1 cells. Whether active holotoxin is required for these effects on T cells is not completely clear. In several earlier studies, CT-B was shown to suppress T cell proliferation and IL-2 production in vitro (at doses ranging from 100 ng/ml to 4 μg/ml) (54–57). An increase in cAMP was not discernible in cultures with these concentrations of CT-B, suggesting this suppression is not due to residual holotoxin activity (54). However, other studies demonstrate that the induction of cAMP in activated murine CD4+ T cells by treatment with CT, the combination of forskolin and phosphodiesterase inhibitors, or by direct stimulation of cAMP pathways with dibutyryl-cAMP, results in the enhanced production of IL-4 and IL-5, with concurrent suppression of IL-2 production (58). Regardless of the mechanisms involved, these studies, together with the results presented here, suggest that CT, by blocking IL-12 production and responsiveness in the inductive phase of T cell responses, and by suppressing the production of and responsiveness to IL-2 and IL-12, but not IL-4 on secondary T cell stimulation, results in the skewing of T cell responses to the Th2 phenotype.
These combined effects of CT may also explain some of the disparate findings in the literature with respect to cytokine responses by cells isolated from mice after CT immunization. In this regard, several studies showing a mixed Th1 and Th2 cytokine response were performed with cells isolated after a single immunization (15, 46, 47). Since it is now clear that naive T cells require multiple rounds of stimulation in the presence of IL-4 to acquire a stable Th2 phenotype (59, 60), it is possible that T cells isolated after only one immunization in vivo with CT are incompletely polarized to the Th2 phenotype, despite being induced in an environment that may have low levels of IL-12, as well as high levels of IL-4. Stimulation of these cells in vitro with activated APCs (taken from non-CT-treated mice) that produce high levels of IL-12 may result in IL-4 as well as IFN-γ production. In contrast, studies showing distinct Th2 responses in vitro after CT administration have primarily been performed with cells isolated from animals given multiple in vivo immunizations (15–17, 19). Under these conditions, the combined effects of CT noted above would result in a more stable Th2 phenotype after each repeated immunization. Since Th2 cells have been shown to lose expression of the IL-12Rβ2 chain (61), the more polarized Th2 populations isolated after multiple immunizations with CT as an adjuvant would not produce significant amounts of IFN-γ in vitro despite high levels of IL-12 produced by stimulating APCs.
However, the ability of CT to suppress IL-12 production and drive Th2 development cannot explain the findings by several groups that CT can augment cytotoxic T cell responses to antigens, including soluble peptides, applied to mucosal surfaces (62–64). In fact, IL-12 has long been known to be capable of directly augmenting CD8+ CTL responses (25, 65). Consistent with a similar role for IL-12 after mucosal immunization, it was recently shown that after intrarectal immunization with a peptide containing both helper and CTL epitopes of HIV gp120 using CT as an adjuvant, a CTL response was induced in wild-type mice, but not in IFN-γ−/− mice, nor in wild-type mice pretreated with anti–IL-12 antibody (62).
There are at least two possible explanations for these seemingly inconsistent findings. First, it is possible that the level of IL-12 required for the induction of CD8+ CTL is less than that required for the induction of CD4+ Th1 responses, and that CT does not suppress IL-12 below the level required for CTLs. Indeed, we found that CT does not completely suppress IL-12 p40 production in vivo (Table I). Support for the possibility that CD8+ CTLs and CD4+ Th1 cells have differential requirements for IL-12 is the finding that IFN-γ producing alloreactive CD8+ T cells are equivalently induced in wild-type and IL-12 p35−/− mice, which still produce IL-12 p40 that can signal CD8+ cells (66). In contrast, the induction of IFN-γ–producing CD4+ T cells after systemic immunization is substantially suppressed in IL-12 p35−/− mice (67, 68) that do not respond to IL-12 p40. The ability of CT to augment CTL induction may thus be due to its positive effects on antigen adsorption (9), antigen presentation (7, 10, 69, 70), and expression of other costimulatory molecules, such as B7-2 (CD86; reference 12) and IL-1 (11) in the setting of low but sufficient levels of IL-12. The fact that the administration of exogenous IL-12 has recently been shown to significantly enhance CT-induced CTL at mucosal surfaces in response to immunization with the HIV peptide vaccine discussed above (Belyakov, I., unpublished data), is also consistent with this hypothesis. Second, it is possible that CT has direct positive, rather than inhibitory, effects on CD8+ T cells that enhance their differentiation into CTL. However, this possibility seems less likely given a recent study demonstrating that CT and CT-B can inhibit CD8+ T cell activation in vitro (55). Future studies will need to address the mechanisms by which CT can drive the induction of CTL.
Also difficult to explain with the current data is the fact LT given to mice orally induces a mixed Th1 and Th2 response, with significant amounts of antigen-specific IgG2a, whereas CT induces a Th2 response with strong IgG1 and IgG2b antibody responses (71). One possible explanation is suggested by our preliminary observation that the potency of LT is less than that of CT with respect to IL-12 suppression (Fig. 1). Clearly these experiments will need to be repeated with a variety of toxin preparations to confirm this observation; however, a partial reduction of IL-12 by LT could potentially explain why this toxin does not induce T cell responses as strongly polarized in the Th2 direction as does CT. In addition, it has been suggested that since LT-B has higher affinity for galactose residues than has CT-B, it may have higher binding affinity for a broader range of gangliosides than CT (e.g., GM2 or asialo-GM1 in addition to GM1), thus affecting the cell types that are potentially activated by the toxins (6, 72–74). Such a difference could also potentially account for differences between CT and LT in vivo.
We found that CT also inhibited TNF-α production, but had no effect on TGF-β1, IL-6, or IL-10 production by stimulated monocytes (Fig. 3). Although the latter cytokines were not responsible for suppressing the production of IL-12 via an autocrine inhibitory circuit, they each are thought to be important for the differentiation of IgA B cells. TGF-β1 is an essential factor (75), and IL-10 and IL-6, in addition to IL-4, have additive, significant roles in IgA B cell switching and differentiation (for a recent review see reference 76). In fact, IL-10 and IL-6 have been implicated in the induction of the diminished but present IgA responses in IL-4−/− mice (22). In addition, both TGF-β1 and IL-10 can act in an autocrine fashion to induce the differentiation of naive T cells into cells producing each of these respective cytokines (77, 78). Thus, in addition to the direct effects of APC-produced IL-10 and TGF-β1 on B cells, the uninhibited capacity to produce these cytokines after treatment with CT could indirectly result in the induction of IL-10– and TGF-β1–producing T cells that could provide help for IgA B cell responses. Recently, we also demonstrated in mice that blocking the effect of IL-12 both in vitro and in vivo with anti–IL-12 antibodies results in enhanced priming for TGF-β1–producing T cells (77, 79, 80), suggesting that the direct inhibition of IL-12 production by CT could result in high levels of TGF-β1 production that may act in concert with local Th2 cells to drive high levels of IgA B cell switching and differentiation.
The primary paradox with respect to this reasoning, and with respect to the action of CT in general, is how CT can act to promote IgA responses, which are dependent on the production of TGF-β1, yet result in the abrogation of oral tolerance (81, 82), a response that can be mediated by T cells arising in the Peyer's patches that produce TGF-β1 and IL-10. Unfortunately, this issue has not been adequately addressed, in that all studies of the effect of CT on oral tolerance have been performed using high dose oral tolerance regimens that optimally induce T cell anergy and deletion, rather than with clearly defined low dose regimens that induce regulatory cells (83, 84). The most convincing data has shown that CT can prevent high dose oral tolerization of antibody responses (23, 82). Although CT can clearly prevent the deletion of Th2 T cells, studies showing prevention of tolerance of Th1-mediated responses, such as DTH, are contradictory (23, 85–87). In fact, there are clear examples of the ability of CT to prevent oral tolerance with respect to antibody responses, while concurrently having no effect on “tolerance” of DTH (85). One could argue that in these studies, the lack of abrogation of oral tolerance to DTH is actually the lack of induction of DTH due to deviation to the Th2 phenotype (85, 86). Finally, it has been shown that CT can directly induce B cell differentiation (13) as well as TGF-β1 production from B cells (14), which may drive B cell switching to IgA. Thus, CT could simultaneously drive T cells to differentiate into Th2 cells and induce TGF-β1 production from B cells, resulting in IgA production.
The physiologic relevance of the findings presented here were demonstrated by showing that CT completely inhibited the production of IFN-γ by anti-CD3–stimulated human PBMCs. This was partially restored by adding IL-12 back to the cultures (Fig. 7). The fact that IL-12 did not completely restore IFN-γ production in these cultures suggested that CT also acts to inhibit the responsiveness of T cells to IL-12. In support of this possibility, we demonstrated by flow cytometry that CT also inhibits the anti-CD3–stimulated expression of the β1 and β2 chains of IL-12R on T cells. It has been shown that both IL-12R chains are required for high affinity binding of IL-12 and optimal signal transduction (88, 89).
Finally, the effects of CT were demonstrated in vivo, by showing that the pretreatment of mice with CT (10 μg/ mouse given intraperitoneally) inhibited both serum IL-12 p40 (80% reduction) and IFN-γ (>95% reduction) responses to systemic challenge with LPS. Since earlier studies have shown that the induction of serum IFN-γ after exposure to LPS is IL-12 dependent (41–45), these data strongly suggest that CT inhibited the production of biologically active IL-12 in vivo. In support of this conclusion, an earlier study convincingly demonstrated that the Th2-dominated response after oral immunization with CT as an adjuvant was reversed when the mice were also treated with IL-12 (17).
In summary, our data demonstrates that CT can inhibit Th1 immune responses by its ability to suppress IL-12 production from both monocytes and DCs, as well as by its capacity to inhibit the expression of IL-12 receptors on T cells. These effects may help to explain the ability of mucosally administered CT to enhance Th2-dependent immune responses in vivo.
Abbreviations used in this paper
CT
cholera toxin
DTH
delayed type hypersensitivity
LT
heat-labile toxin
SAC
Staphylococcus aureus, Cowan's strain I
sIgA
secretory immunoglobulin A
References
- 1.Strober W, Kelsall B, Marth T. Oral tolerance. J Clin Immunol. 1998;18:1–30. doi: 10.1023/a:1023222003039. [DOI] [PubMed] [Google Scholar]
- 2.Weiner HL. Oral tolerance: immune mechanisms and treatment of autoimmune diseases. Immunol Today. 1997;18:335–343. doi: 10.1016/s0167-5699(97)01053-0. [DOI] [PubMed] [Google Scholar]
- 3.Reese RT, Cebra JJ. Anti-dinitrophenyl antibody production in strain 13 guinea pigs fed or sensitized with dinitrochlorobenzene. J Immunol. 1975;114:863–871. [PubMed] [Google Scholar]
- 4.Husby S, Mestecky J, Moldoveanu Z, Holland S, Elson CO. Oral tolerance in humans: T cell but not B cell tolerance after antigen feeding. J Immunol. 1994;152:4663–4670. [PubMed] [Google Scholar]
- 5.Elson, C.O. 1996. Cholera toxin as a mucosal adjuvant. In Mucosal Vaccines. Academic Press, San Diego. 59–72.
- 6.Snider DP. The mucosal adjuvant activities of ADP-ribosylating bacterial enterotoxins. Crit Rev Immunol. 1995;15:317–348. doi: 10.1615/critrevimmunol.v15.i3-4.70. [DOI] [PubMed] [Google Scholar]
- 7.Lycke N. The mechanism of cholera toxin adjuvanticity. Res Immunol. 1997;148:504–520. doi: 10.1016/s0923-2494(98)80144-2. [DOI] [PubMed] [Google Scholar]
- 8.Spangler BD. Structure and function of cholera toxin and the related Escherichia coliheat-labile enterotoxin. Microbiol Rev. 1992;56:622–647. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lycke N, Karlsson U, Sjolander A, Magnusson KE. The adjuvant action of cholera toxin is associated with an increased intestinal permeability for luminal antigens. Scand J Immunol. 1991;33:691–698. doi: 10.1111/j.1365-3083.1991.tb02542.x. [DOI] [PubMed] [Google Scholar]
- 10.Bromander AK, Kjerrulf M, Holmgren J, Lycke N. Cholera toxin enhances alloantigen presentation by cultured intestinal epithelial cells. Scand J Immunol. 1993;37:452–458. doi: 10.1111/j.1365-3083.1993.tb03318.x. [DOI] [PubMed] [Google Scholar]
- 11.Bromander A, Holmgren J, Lycke N. Cholera toxin stimulates IL-1 production and enhances antigen presentation by macrophages in vitro. J Immunol. 1991;146:2908–2914. [PubMed] [Google Scholar]
- 12.Cong Y, Weaver CT, Elson CO. The mucosal adjuvanticity of cholera toxin involves enhancement of costimulatory activity by selective up-regulation of B7.2 expression. J Immunol. 1997;159:5301–5308. [PubMed] [Google Scholar]
- 13.Lycke N, Strober W. Cholera toxin promotes B cell isotype differentiation. J Immunol. 1989;142:3781–3787. [PubMed] [Google Scholar]
- 14.Kim P, Eckman L, Lee WJ, Han W, Kagnoff MF. Cholera toxin and cholera toxin B subunit induce IgA switching through the action of TGF-β1. J Immunol. 1998;160:1198–1203. [PubMed] [Google Scholar]
- 15.Xu-Amano J, Kioyno H, Jackson RL, Staats HF, Fujihashi K, Burrows PD, Elson CO, Pillai S, McGhee JR. Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa- associated tissues. J Exp Med. 1993;178:1309–1320. doi: 10.1084/jem.178.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamamoto S, Kiyono H, Yamamoto M, Imaoka K, Fujihashi K, Van Ginkel FW, Noda M, Takeda Y, McGhee JR. A nontoxic mutant of cholera toxin elicits TH2-type responses for enhanced mucosal immunity. Proc Natl Acad Sci USA. 1997;94:5267–5272. doi: 10.1073/pnas.94.10.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marinaro M, Staats HF, Hiroi T, Jackson RJ, Coste M, Boyaka PN, Okahashi N, Yamamoto M, Kiyono H, Bluethmann H, et al. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J Immunol. 1995;155:4621–4629. [PubMed] [Google Scholar]
- 18.Marinaro M, Boyaka PN, Finkelman FD, Kiyono H, Jackson RJ, Jirillo E, McGhee JR. Oral but not parenteral interleukin (IL)-12 redirects T helper 2 (Th2)- type responses to an oral vaccine without altering mucosal IgA responses. J Exp Med. 1997;185:415–427. doi: 10.1084/jem.185.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kjerrulf M, Grdic D, Ekman L, Schon K, Vajdy M, Lycke NY. Interferon-γ receptor-deficient mice exhibit impaired gut mucosal immune responses but intact oral tolerance. Immunology. 1997;92:60–68. doi: 10.1046/j.1365-2567.1997.00312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snider DP, Marshall JS, Perdue MH, Liang H. Production of IgE antibody and allergic sensitization of intestinal and peripheral-tissues after oral immunization with protein Ag and cholera-toxin. J Immunol. 1994;153:647–657. [PubMed] [Google Scholar]
- 21.Vajdy M, Kosco-Vilbois MH, Kopf M, Kohler G, Lycke N. Impaired mucosal immune responses in interleukin 4–targeted mice. J Exp Med. 1995;181:41–53. doi: 10.1084/jem.181.1.41. [DOI] [PubMed] [Google Scholar]
- 22.Okahashi N, Yamamoto M, Vancott JL, Chatfield SN, Roberts M, Bluethmann H, Hiroi T, Kiyono H, McGhee JR. Oral immunization of interleukin-4 (IL-4) knockout mice with a recombinant Salmonellastrain or cholera toxin reveals that CD4(+) Th2 cells producing IL-6 and IL-10 are associated with mucosal immunoglobulin A responses. Infect Immun. 1996;64:1516–1525. doi: 10.1128/iai.64.5.1516-1525.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamura S, Shoji Y, Hasiguchi K, Aizawa C, Kurata T. Effects of cholera-toxin adjuvant on IgE antibody-response to orally or nasally administered ovalbumin. Vaccine. 1994;12:1238–1240. doi: 10.1016/0264-410x(94)90250-x. [DOI] [PubMed] [Google Scholar]
- 24.Kjerrulf M, Grdic D, Kopf M, Lycke N. Induction of gut mucosal immune responses: importance of genetic background and Th1/Th2 cross-regulation. Scand J Immunol. 1998;47:401–407. doi: 10.1046/j.1365-3083.1998.00316.x. [DOI] [PubMed] [Google Scholar]
- 25.Trinchieri G. Proinflammatory and immunoregulatory functions of interleukin-12. Int Rev Immunol. 1998;16:365–396. doi: 10.3109/08830189809043002. [DOI] [PubMed] [Google Scholar]
- 26.Trinchieri G. Immunobiology of interleukin-12. Immunol Res. 1998;17:269–278. doi: 10.1007/BF02786451. [DOI] [PubMed] [Google Scholar]
- 27.Bender A, Sapp M, Schuler G, Steinman RM, Bhardwaj N. Improved methods for the generation of dendritic cells from nonproliferating progenitors in human blood. J Immunol Methods. 1996;196:121–135. doi: 10.1016/0022-1759(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 28.Fotino M, Merson EJ, Allen FH. Instant lymphocytes. Vox Sang. 1971;21:469–470. [Google Scholar]
- 29.Rogge L, D'Ambrosio D, Biffi M, Penna G, Minetti LJ, Presky DH, Adorini L, Sinigaglia F. The role of Stat4 in species-specific regulation of T helper cell development by type 1 interferons. J Immunol. 1998;161:6567–6574. [PubMed] [Google Scholar]
- 30.Steinman RM, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev. 1997;156:25–37. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 31.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 32.Moller DR, Wysocka M, Greenlee BM, Ma X, Wahl L, Flockhart DA, Trinchieri G, Karp CL. Inhibition of IL-12 production by thalidomide. J Immunol. 1997;159:5157–5161. [PubMed] [Google Scholar]
- 33.Moller DR, Wysocka M, Greenlee BM, Ma X, Wahl L, Trinchieri G, Karp CL. Inhibition of human interleukin-12 production by pentoxifylline. Immunology. 1997;91:197–203. doi: 10.1046/j.1365-2567.1997.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marth T, Kelsall BL. Regulation of interleukin-12 by complement receptor 3 signaling. J Exp Med. 1997;185:1987–1995. doi: 10.1084/jem.185.11.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karp, C.L., M. Wysocka, L.M. Wahl, J.M. Ahearn, P.J. Cuomo, B. Sherry, G. Trinchieri, and D.E. Griffin. 1996. Mechanism of suppression of cell-mediated immunity by measles virus. Science. 273:228–231. (Published erratum appears 275:1053.) [DOI] [PubMed]
- 36.Sutterwala FS, Noel GJ, Clynes R, Mosser DM. Selective suppression of interleukin-12 induction after macrophage receptor ligation. J Exp Med. 1997;185:1977–1985. doi: 10.1084/jem.185.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Pouw Kraan, T.C., L.C. Boeije, R.J. Smeenk, J. Wijdenes, and L.A. Aarden. Prostaglandin-E2is a potent inhibitor of human interleukin 12 production. J Exp Med. 1995;181:775–779. doi: 10.1084/jem.181.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panina-Bordignon P, Mazzeo D, Lucia PD, D'Ambrosio D, Lang R, Fabbri L, Self C, Sinigaglia F. Beta2-agonists prevent Th1 development by selective inhibition of interleukin 12. J Clin Invest. 1997;100:1513–1519. doi: 10.1172/JCI119674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma X, Chow JM, Gri G, Carra G, Gerosa F, Wolf SF, Dzialo R, Trinchieri G. The interleukin 12 p40 gene promoter is primed by interferon γ in monocytic cells. J Exp Med. 1996;183:147–157. doi: 10.1084/jem.183.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aste-Amezaga M, Ma X, Sartori A, Trinchieri G. Molecular mechanisms of the induction of IL-12 and its inhibition by IL-10. J Immunol. 1998;160:5936–5944. [PubMed] [Google Scholar]
- 41.Heinzel FP, Rerko RM, Ling P, Hakimi J, Schoenhaut DS. Interleukin 12 is produced in vivo during endotoxemia and stimulates synthesis of gamma interferon. Infect Immun. 1994;62:4244–4249. doi: 10.1128/iai.62.10.4244-4249.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wysocka M, Kubin M, Vieira LQ, Ozmen L, Garotta G, Scott P, Trinchieri G. Interleukin-12 is required for interferon-gamma production and lethality in lipopolysaccharide-induced shock in mice. Eur J Immunol. 1995;25:672–676. doi: 10.1002/eji.1830250307. [DOI] [PubMed] [Google Scholar]
- 43.Heinzel FP, Hujer AM, Ahmed FN, Rerko RM. In vivo production and function of IL-12 p40 homodimers. J Immunol. 1997;158:4381–4388. [PubMed] [Google Scholar]
- 44.Heinzel FP, Rerko RM, Ahmed F, Hujer AM. IFN-γ-independent production of IL-12 during murine endotoxemia. J Immunol. 1996;157:4521–4528. [PubMed] [Google Scholar]
- 45.Mattner F, Ozmen L, Podlaski FJ, Wilkinson VL, Presky DH, Gately MK, Alber G. Treatment with homodimeric interleukin-12 (IL-12) p40 protects mice from IL-12 dependent but not from tumor necrosis factor alpha-dependent shock. Infect Immun. 1997;65:4734–4737. doi: 10.1128/iai.65.11.4734-4737.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roberts M, Bacon A, Rappuoli R, Pizza M, Cropley I, Douce G, Dougan G, Marinaro M, McGhee J, Chatfield S. A mutant pertussis toxin molecule that lacks ADP-ribosyltransferase activity, PT-9K/129G, is an effective mucosal adjuvant for intranasally delivered proteins. Infect Immun. 1995;63:2100–2108. doi: 10.1128/iai.63.6.2100-2108.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hornquist E, Lycke N. Cholera toxin adjuvant greatly promotes antigen priming of T cells. Eur J Immunol. 1993;23:2136–2143. doi: 10.1002/eji.1830230914. [DOI] [PubMed] [Google Scholar]
- 48.Sutterwala FS, Noel GJ, Salgame P, Mosser DM. Reversal of proinflammatory responses by ligating the macrophage Fc gamma receptor type I. J Exp Med. 1998;188:217–222. doi: 10.1084/jem.188.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Imboden JB, Shoback DM, Pattison G, Stobo JD. Cholera toxin inhibits the T-cell antigen receptor- mediated increases in inositol trisphosphate and cytoplasmic free calcium. Proc Natl Acad Sci USA. 1986;83:5673–5677. doi: 10.1073/pnas.83.15.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krakauer T. Evidence for protein kinase C pathway in the response of human peripheral blood mononuclear cells to cholera toxin. Cell Immunol. 1996;72:224–228. doi: 10.1006/cimm.1996.0236. [DOI] [PubMed] [Google Scholar]
- 51.Neurath MF, Fuss I, Kelsall BL, Stuber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anderson DL, Tsoukas CD. Cholera toxin inhibits resting human T cell activation via a cAMP-independent pathway. J Immunol. 1989;143:3647–3652. [PubMed] [Google Scholar]
- 53.Lycke N, Bromander AK, Ekman L, Karlsson U, Holmgren J. Cellular basis of immunomodulation by cholera toxin in vitro with possible association to the adjuvant function in vivo. . J Immunol. 1989;142:20–27. [PubMed] [Google Scholar]
- 54.Woogen SD, Ealding W, Elson CO. Inhibition of murine lymphocyte proliferation by the B subunit of cholera toxin. J Immunol. 1987;139:3764–3770. [PubMed] [Google Scholar]
- 55.Munoz E, Zubiaga AM, Merrow M, Sauter NP, Huber BT. Cholera toxin discriminates between T helper 1 and T helper 2 cells in T cell receptor mediated activation: role of cAMP in T cell proliferation. J Exp Med. 1990;172:95–103. doi: 10.1084/jem.172.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woogen SD, Turo K, Dieleman LA, Beagley KW, Elson CO. Inhibition of murine T cell activation by cholera toxin B subunit is not mediated through the phosphatidylinositol second messenger system. J Immunol. 1993;150:3274–3283. [PubMed] [Google Scholar]
- 57.Elson CO, Holland SP, Dertzbaugh MT, Cuff CF, Anderson AO. Morphologic and functional alterations of mucosal T cells by cholera toxin and its B subunit. J Immunol. 1995;154:1032–1040. [PubMed] [Google Scholar]
- 58.Lacour M, Arrighi JF, Muller KM, Carlberg C, Saurat JH, Hauser C. cAMP up-regulates IL-4 and IL-5 production from activated CD4+ T cells while decreasing IL-2 release and NF-AT induction. Int Immunol. 1994;6:1333–1343. doi: 10.1093/intimm/6.9.1333. [DOI] [PubMed] [Google Scholar]
- 59.Murphy E, Shibuya K, Hosken N, Openshaw P, Maino V, Davis K, Murphy K, O'Garra A. Reversibility of T helper 1 and 2 populations is lost after long-term stimulation. J Exp Med. 1996;183:901–913. doi: 10.1084/jem.183.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sornasse T, Larenas PV, Davis KA, de Vries VE, Yssel H. Differentiation and stability of T helper 1 and 2 cells derived from naive human neonatal CD4+T cells, analyzed at the single-cell level. J Exp Med. 1996;184:473–483. doi: 10.1084/jem.184.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Szabo SJ, Jacobson NG, Dighe AS, Gubler U, Murphy KM. Developmental commitment to the Th2 lineage by extinction of IL-12 signaling. Immunity. 1995;2:665–675. doi: 10.1016/1074-7613(95)90011-x. [DOI] [PubMed] [Google Scholar]
- 62.Belyakov IM, Derby MA, Ahlers JD, Kelsall BL, Earl P, Moss B, Strober W, Berzofsky JA. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc Natl Acad Sci USA. 1998;95:1709–1714. doi: 10.1073/pnas.95.4.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Porgador A, Staats HF, Faiola B, Gilboa E, Palker TJ. Intranasal immunization with CTL epitope peptides from HIV-1 or ovalbumin and the mucosal adjuvant cholera toxin induces peptide-specific CTLs and protection against tumor development in vivo. J Immunol. 1997;158:834–841. [PubMed] [Google Scholar]
- 64.Bowen JC, Nair SK, Reddy R, Rouse BT. Cholera toxin acts as a potent adjuvant for the induction of cytotoxic T-lymphocyte responses with non-replicating antigens. Immunology. 1994;81:338–342. [PMC free article] [PubMed] [Google Scholar]
- 65.Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008–4027. [PubMed] [Google Scholar]
- 66.Piccotti JR, Li K, Chan SY, Ferrante J, Magram J, Eichwald EJ, Bishop KD. Alloantigen-reactive Th1 development in IL-12-deficient mice. J Immunol. 1998;160:1132–1138. [PubMed] [Google Scholar]
- 67.Magram J, Sfarra J, Connaughton S, Faherty D, Warrier R, Carvajal D, Wu CY, Stewart C, Sarmiento U, Gately MK. IL-12-deficient mice are defective but not devoid of type 1 cytokine responses. Ann NY Acad Sci. 1996;795:60–70. doi: 10.1111/j.1749-6632.1996.tb52655.x. [DOI] [PubMed] [Google Scholar]
- 68.Magram J, Connaughton S, Warrier R, Carvajal D, Wu CY, Ferrante J, Stewart C, Sarmiento U, Faherty D, Gately MK. IL-12-deficient mice are defective in IFN-γ production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 69.Matousek MP, Nedrud JG, Harding CV. Distinct effects of recombinant cholera toxin B subunit and holotoxin on different stages of class II MHC antigen processing and presentation by macrophages. J Immunol. 1996;156:4137–4145. [PubMed] [Google Scholar]
- 70.Matousek MP, Nedrud JG, Cieplak WJ, Harding CV. Inhibition of class II major histocompatibility complex antigen processing by Escherichia coliheat-labile enterotoxin requires an enzymatically active A subunit. Infect Immun. 1998;66:3480–3484. doi: 10.1128/iai.66.7.3480-3484.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takahashi I, Marinaro M, Kiyono H, Jackson RJ, Nakagawa I, Fujihashi K, Hamada S, Clements JD, Bost KL, McGhee JR. Mechanisms for mucosal immunogenicity and adjuvancy of Escherichia colilabile enterotoxin. J Infect Dis. 1996;173:627–635. doi: 10.1093/infdis/173.3.627. [DOI] [PubMed] [Google Scholar]
- 72.Holmgren J, Lindblad M, Fredman P, Svennerholm L, Myrvold H. Comparison of receptors for cholera and Escherichia colienterotoxins in human intestine. Gastroenterology. 1985;89:27–35. doi: 10.1016/0016-5085(85)90741-3. [DOI] [PubMed] [Google Scholar]
- 73.Holmgren J, Fredman P, Lindblad M, Svennerholm AM, Svennerholm L. Rabbit intestinal glycoprotein receptor for Escherichia coliheat-labile enterotoxin lacking affinity for cholera toxin. Infect Immun. 1982;38:424–433. doi: 10.1128/iai.38.2.424-433.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Griffiths SL, Finkelstein RA, Critchley DR. Characterization of the receptor for cholera toxin and Escherichia coliheat-labile toxin in rabbit intestinal brush borders. Biochem J. 1986;238:313–322. doi: 10.1042/bj2380313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Coffman RL, Lebman DA, Shrader B. Transforming growth factor-β specifically enhances IgA production by lipopolysaccharide-stimulated murine B-lymphocytes. J Exp Med. 1989;170:1039–1044. doi: 10.1084/jem.170.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.MacIntyre, T., and W. Strober. 1999. Gut-associated lymphoid tissue: regulation of IgA B cell development. In Mucosal Immunology, Second Edition. P. Ogra, J. Mestecky, M. Lamm, W. Strober, J.R. McGhee, and J. Bienenstock, editors. Academic Press, San Diego. 319–356.
- 77.Seder RA, Marth T, Sieve MC, Strober W, Letterio JJ, Roberts AB, Kelsall B. Factors involved in the differentiation of TGF-beta1-producing cells from naive CD4+ T cells: IL-4 and IFN-gamma have opposing effects, while TGF-beta1positively regulates its own production. J Immunol. 1998;160:5719–5728. [PubMed] [Google Scholar]
- 78.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries VE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 79.Marth T, Strober W, Seder RA, Kelsall BL. Regulation of transforming growth factor-beta production by interleukin-12. Eur J Immunol. 1997;27:1213–1220. doi: 10.1002/eji.1830270524. [DOI] [PubMed] [Google Scholar]
- 80.Marth T, Strober W, Kelsall BL. High dose oral tolerance in ovalbumin TCR-transgenic mice: systemic neutralization of IL-12 augments TGF-beta secretion and T cell apoptosis. J Immunol. 1996;157:2348–2357. [PubMed] [Google Scholar]
- 81.Elson CO, Holland SP, Dertzbaugh MT, Cuff CF, Anderson AO. Morphologic and functional alterations of mucosal T cells by cholera toxin and its B subunit. J Immunol. 1995;154:1032–1040. [PubMed] [Google Scholar]
- 82.Elson CO, Ealding W. Cholera toxin feeding did not induce oral tolerance in mice and abrogated oral tolerance to an unrelated protein antigen. J Immunol. 1984;133:2892–2897. [PubMed] [Google Scholar]
- 83.Chen Y, Inobe J, Kuchroo VK, Baron JL, Janeway CJ, Weiner HL. Oral tolerance in myelin basic protein T-cell receptor transgenic mice: suppression of autoimmune encephalomyelitis and dose-dependent induction of regulatory cells. Proc Natl Acad Sci USA. 1996;93:388–391. doi: 10.1073/pnas.93.1.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Friedman A, Weiner HL. Induction of anergy or active suppression following oral tolerance is determined by antigen dosage. Proc Natl Acad Sci USA. 1994;91:6688–6692. doi: 10.1073/pnas.91.14.6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kay RA, Ferguson A. The immunological consequences of feeding cholera toxin. I. Feeding cholera toxin suppresses the induction of systemic delayed-type hypersensitivity but not humoral immunity. Immunology. 1989;66:410–415. [PMC free article] [PubMed] [Google Scholar]
- 86.Kay RA, Ferguson A. The immunological consequences of feeding cholera toxin. II. Mechanisms responsible for the induction of oral tolerance for DTH. Immunology. 1989;66:416–421. [PMC free article] [PubMed] [Google Scholar]
- 87.Sun JB, Holmgren J, Czerkinsky C. Cholera-toxin B-subunit: an efficient transmucosal carrier-delivery system for induction of peripheral immunological tolerance. Proc Natl Acad Sci USA. 1994;91:10795–10799. doi: 10.1073/pnas.91.23.10795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Presky DH, Minetti LJ, Gillessen S, Wilkinson VL, Wu CY, Gubler U, Chizzonite R, Gately MK. Analysis of the multiple interactions between IL-12 and the high affinity IL-12 receptor complex. J Immunol. 1998;160:2174–2179. [PubMed] [Google Scholar]
- 89.Wu C, Ferrante J, Gately MK, Magram J. Characterization of IL-12 receptor beta1 chain (IL-12Rβ1)- deficient mice: IL-12Rβ1 is an essential component of the functional mouse IL-12 receptor. J Immunol. 1997;159:1658–1665. [PubMed] [Google Scholar]