Influence of Polymorphism in the Genes for the Interleukin (IL)-1 Receptor Antagonist and IL-1β on Tuberculosis (original) (raw)
Abstract
Several lines of evidence suggest that host genetic factors controlling the immune response influence infection by Mycobacterium tuberculosis. The proinflammatory cytokine interleukin (IL)-1β and its antagonist, IL-1Ra (IL-1 receptor agonist), are strongly induced by M. tuberculosis and are encoded by polymorphic genes. The induction of both IL-1Ra mRNA and secreted protein by M. tuberculosis in IL-1Ra allele A2–positive (IL-1Ra A2+) healthy subjects was 1.9-fold higher than in IL-1Ra A2− subjects. The M. _tuberculosis–_induced expression of mRNA for IL-1β was higher in subjects of the IL-1β (+3953) A1+ haplotype (P = 0.04). The molar ratio of IL-1Ra/IL-1β induced by M. tuberculosis was markedly higher in IL-1Ra A2+ individuals (P < 0.05), with minor overlap between the groups, reflecting linkage between the IL-1Ra A2 and IL-1β (+3953) A2 alleles. In M. _tuberculosis_–stimulated peripheral blood mononuclear cells, the addition of IL-4 increased IL-1Ra secretion, whereas interferon γ increased and IL-10 decreased IL-1β production, indicative of a differential influence on the IL-1Ra/IL-1β ratio by cytokines. In a study of 114 healthy purified protein derivative–reactive subjects and 89 patients with tuberculosis, the frequency of allelic variants at two positions (−511 and +3953) in the IL-1β and IL-1Ra genes did not differ between the groups. However, the proinflammatory IL-1Ra A2−/IL-1β (+3953) A1+ haplotype was unevenly distributed, being more common in patients with tuberculous pleurisy (92%) in comparison with healthy M. _tuberculosis_–sensitized control subjects or patients with other disease forms (57%, P = 0.028 and 56%, P = 0.024, respectively). Furthermore, the IL-1Ra A2+ haplotype was associated with a reduced Mantoux response to purified protein derivative of M. tuberculosis: 60% of tuberculin-nonreactive patients were of this type. Thus, the polymorphism at the IL-1 locus influences the cytokine response and may be a determinant of delayed-type hypersensitivity and disease expression in human tuberculosis.
Keywords: interleukin 1 receptor; tuberculosis; susceptibility, disease; hypersensitivity, delayed; granuloma
By comparison with other pathogens, widely distributed isolates of Mycobacterium tuberculosis show a striking lack of antigenic variation (1). The occurrence of tuberculosis epidemics in populations previously unexposed to M. tuberculosis (2, 3) and the twofold risk of disease in identical, compared with nonidentical, twins (4) indicates a genetic component in susceptibility. Rare susceptibility to recurrent atypical intracellular infection is proved to be conferred by mutation in the genes for the IFN-γ receptor (5– 7), the IL-12 receptor (8, 9), or IL-12 (10). However, the extent to which these severe defects contribute to susceptibility in populations is unknown. In a recent large case-control study, disease susceptibility in West Africans was conferred by variants of the human Nramp1 and vitamin D receptor genes (11, 12). The possibility also exists that disease expression, as well as susceptibility to tuberculosis per se, is influenced by the host response. A single genetic isolate of M. tuberculosis associated with a disease outbreak caused highly varied disease manifestations (13), and in earlier population-based studies, severe pulmonary tuberculosis has been associated with both HLA-DR15 and haptoglobin 2-2 (for review, see reference 14).
A key element in the inflammatory response is the prompt production of proinflammatory cytokines such as IL-1β and TNF-α, required to control infection by M. tuberculosis (15, 16). To terminate the immune response and limit the potential for immunopathology, the proinflammatory response is in turn downregulated by cytokines such as TGF-β, IL-10, and, specifically in the case of IL-1β, the IL-1 receptor antagonist (IL-1Ra), a pure antagonist of the IL-1 type 1 receptor (IL-1RI) (17). The genes coding for both IL-1β and the IL-1Ra gene are on chromosome 2q. Two biallelic polymorphisms in the IL-1β gene at positions −511 and +3953 relative to the transcriptional start codon have been described (18, 19). Allele 1 of the +3953 polymorphism (IL-1β +3953 A1+) is associated with moderately increased IL-1β production in response to LPS (19). The IL-1Ra gene is also polymorphic due to a variable number (2–6) of tandem repeats of 86 bp (VNTR) within its second intron (20). This polymorphism has been shown to be unambiguously functional at the level of secreted protein, as monocytes from individuals homo- or heterozygous for allele 2 (IL-1Ra A2+, IL-1RN*2, 2 repeats) produce significantly more IL-1Ra in response to GM-CSF (21) and also have higher plasma levels (22). Serum IL-1Ra is known to be elevated in patients with tuberculosis (23). In addition, the ratio of IL-1Ra to IL-1β is elevated in the cerebrospinal fluid of cases of tuberculous, as compared with pyogenic, meningitis (24). These data suggest that the expression of IL-1Ra may impact on disease expression. However, the effect of M. tuberculosis infection on the secretion of IL-1β and IL-1Ra in vitro has not been related to these polymorphisms nor has the relevance of the latter to tuberculosis been investigated.
In this study, we found that M. _tuberculosis–_induced IL-1Ra mRNA and protein secretion in healthy IL-1Ra A2+ subjects was approximately twofold that of IL-1Ra A2− individuals. In addition, the molar ratio of IL-1Ra/IL-1β was strikingly higher in IL-1Ra A2+ individuals. In M. _tuberculosis_–stimulated PBMC, the addition of IL-4 increased IL-1Ra secretion, whereas IFN-γ increased, and IL-10 decreased, IL-1β production, indicative of a differential influence on the IL-1Ra/IL-1β ratio by cytokines. In a pilot case-control analysis, the IL-1β and IL-1Ra allele frequencies were not different between patients with tuberculosis and purified protein derivative (PPD) skin test (Mantoux)– reactive control subjects. However, the proinflammatory IL-1Ra A2−/IL-1β (+3953) A1+ haplotype was unevenly distributed, being more common in patients with pleural tuberculosis and less common in extrapulmonary disease. Furthermore, and consistent with the in vitro observations, the IL-1Ra A2+ haplotype was associated with a reduced Mantoux response: 60% of tuberculin-nonreactive patients were of this type. Thus, the polymorphism at the IL-1 locus influences the cytokine response to, and may be a determinant of, delayed-type hypersensitivity (DTH)1 and disease expression in human tuberculosis.
Materials and Methods
Study Populations.
For cell culture, healthy, PPD skin test– negative donors from the laboratory staff at Case Western Reserve University were bled and genotyped as below. In the pilot case-control analysis, a different population of 89 unselected patients and 114 control subjects who were Hindu, residents of London, and identified as being of Gujarati origin were recruited from Northwick Park Hospital, Harrow, England. The peak migration of Gujaratis to west London followed political change in East Africa in the decade 1970–1980. There is a high incidence of tuberculosis amongst Gujaratis in Harrow of ∼128/100,000 (25), with an unusual excess of extrapulmonary disease in females. Within this community, 35–65% of marriages are prearranged, marriage to non-Gujarati Hindus is rare, and marriage to non-Hindus is exceptional (Patel, P., and R.J. Wilkinson, unpublished observations). 62% of subjects in this study were bacille Calmette-Guérin vaccinated. All 89 patients (average age 42.3 ± 1.7 yr; 56 females and 33 males) had culture- or biopsy-proven tuberculosis. All patients had free access to optimal medical care. The median duration of symptoms at diagnosis was 31 d (21 and 90 d being the 25th and 75th quartile values), thereby minimizing the effect of chronicity on clinical presentation. The definition of clinical phenotype was based on the International Classification of Disease 9 classification, and the overwhelming majority of patients were judged to have delayed postprimary (reactivation) disease. Patients known to be immunosuppressed (e.g., by HIV infection or corticosteroid therapy) were excluded. Mantoux testing was performed by the intradermal injection of one tuberculin unit of PPD (Evans Medical). The resultant diameter of transverse induration was recorded after 48 h. This low dose of tuberculin is routinely used in the United Kingdom to avoid necrotic reactions. All 114 nonconsanguineous (spouses of patients where possible) healthy controls (average age 42.9 ± 1.2 yr; 54 females and 60 males) were recruited from the tuberculosis contact clinic at the same hospital and had documented contact with tuberculosis (often multiple). All were PPD skin test–positive, asymptomatic, and had normal chest radiographs. 10/114 (8.7%) received chemoprophylaxis. These subjects were recruited between June 1995 and May 1998; in June 1998, all remained disease free. Ethical permission for this case-control analysis was obtained from the Harrow local research ethical committee (EC1646).
IL-1Ra and IL-1β Genotypes.
The genotypes were determined as previously described (20, 22). DNA was isolated by phenol-chloroform extraction, and 5 ng was used in the PCR amplification of the IL-1Ra VNTR region, using 0.05 μM of the following primers: 5′-TCC TGG TCT GCA GGT AA-3′ and 5′-CTC AGC AAC ACT CCT AT-3′. The mixture was heated to 96°C for 1 min, followed by 30 cycles of 94°C for 1 min, 60°C for 1 min, 70°C for 1 min, and then a final 7 min at 70°C. Products were run on an ethidium bromide–stained, 1.5% agarose MR gel (Boehringer Mannheim) and visualized directly. A 304-bp fragment of the IL-1β gene from −702 to −398 was amplified using the following primers: 5′-TGG CAT TGA TCT GGT TCA TC-3′ and 5′-GTT TAG GAA TCT TCC CAC TT-3′, using the same cycling conditions as above. The products were digested overnight at 37°C with 5 U Ava 1 and run on a 2.5% gel as above, generating the following patterns: single band of 304 bp, A2/A2 homozygote; two bands at 190 and 114 bp, A1/A1 homozygote; all three bands, heterozygote. A 249-bp fragment of the IL-1β exon 5 was amplified using the following primers: 5′-GTT GTC ATC AGA CTT TGA CC-3′ and 5′-TTC AGT TCA TAT GGA CCA GA-3′. The mixture was heated for three cycles of 94°C for 2 min, 55°C for 2 min, 74°C for 1 min, then 32 cycles of 94°C for 1 min, 55°C for 1 min, 74°C for 1 min, and then a final 10 min at 70°C. The products were digested overnight at 65°C with 2.5 U Taq 1 and run on a 3% gel, generating the following patterns: single band of 249 bp, A2/A2 homozygote; two bands at 135 and 114 bp, A1/A1 homozygote; all three bands, heterozygote.
Cell Culture.
PBMCs were separated over a Ficoll (Pharmacia Biotech) gradient. Preliminary experiments established that conventional separation of monocytes by adherence to plastic, harvesting, and replating led to spontaneous release of IL-1Ra. To reduce such activation, freshly isolated PBMCs were cultured at 2.5 × 106 /ml in 24-well plates in RPMI 1640 (Biowhittaker) without antibiotics in the presence of 2% autologous serum. In each experiment, the number of monocytes present in PBMCs was determined by washing off nonadherent cells (×3) in a duplicate well and then detaching the adherent cells using ice cold PBS and a cell scraper. Monocyte counts were generally ∼10% of the total PBMC numbers. Preliminary experiments showed that IL-1Ra production under these conditions was detectable by 4 h and reached a plateau by 10–12 h, with no further significant increase during the next 12 h. There was no significant difference in production between experiments in which the nonadherent cells had been removed by washing and wells containing unseparated PBMC, indicating that the adherent cells were responsible for the IL-1Ra secretion. We therefore collected, and froze at −70°C, PBMC supernates after 10 h of culture. In some cases, cell lysates were prepared by adding an equal volume of PBS and then freeze-thawing once. In this way we established that the ratio of IL-1Ra secreted into the supernate to that remaining in cell lysates was consistently >10:1, irrespective of time point, stimulus, and genotype.
Reagents.
M. tuberculosis H37Ra and H37Rv was prepared and aliquotted as previously described (26). Aliquots were vortexed for 15–20 min before use at an infection ratio of 0.1 or 1 M. tuberculosis bacilli/1 PBMC (corresponding to ∼1:1 and 10:1 per monocyte). PPD of M. tuberculosis was the gift of Lederle Labs. (American Cyanamid Co.) and used at 0.1–100 μg/ml. Recombinant TGF-β, IL-4, and IFN-γ, and the neutralizing antibodies to IL-1β (mouse IgG1), IL-6 (polyclonal goat IgG), TGF-β (polyclonal chicken IgY), and TNF-α (mouse IgG1), and appropriate isotype control antibodies were purchased from R & D Systems, Inc. All recombinant cytokines, PPD, M. tuberculosis, and neutralizing antibodies used were tested for endotoxin contamination by the Limulus amebocyte assay (Biowhittaker) and were either free or contained very small levels (always <2ng/mg) of endotoxin.
Cytokine ELISA.
Maxisorp (Nunc, Inc.) plates were coated overnight at 4°C with 100 μl of the following coating antibodies in PBS: 2 μg/ml anti–human IL-1β mAb or 5 μg/ml of anti– human IL-1Ra mAb (both from R & D Systems, Inc.). After washing in PBS/0.05% Tween 20 (×3), the plates were blocked for 1 h at room temperature (rt) using 300 μl 1% BSA/5% sucrose/ 0.05% NaN3 in PBS. After three further washes, duplicate 100-μl samples and dilutions of standard cytokines were then incubated for 2 h at rt. After washing (×4), 100 μl of the following biotinylated detection antibodies were added in diluent (0.1% BSA, 0.05% Tween 20 in TBS, pH 7.3): 100 ng/ml anti–human IL-1β antibody or 20 ng/ml anti–human IL-1Ra antibody (both from R & D Systems, Inc.). After 2 h at rt, the plates were washed (×5) and 100 μl streptavidin horseradish peroxidase (Jackson Immunoresearch) at 1:5000 in diluent was added. After 20 min, six final washes were followed by the addition of 100 μl of 3,3′,5,5′-tetramethylbenzidine hydrochloride solution in perborate (Sigma Chemical Co.) to each well. The reaction was stopped by adding 50 μl/well 0.5 N H2SO4, and the plates were read at 450 nm in an ELISA reader. The sensitivity of each cytokine ELISA was as follows: IL-1β, <1 pg/ml and IL-1Ra, 0.05 ng/ml.
Ribonuclease Protection Assay.
5 × 107 freshly isolated PBMCs were used to obtain ∼5 × 106 adherent cells. This population of cells is up to 90% monocytes by cytostaining and is 99% viable (27). After resting overnight, the adherent cells were infected as above with M. tuberculosis at 1:1. After 4 h, the cells were harvested, and total RNA was extracted using guanidinium isothiocyanate, CsCl2 density gradient centrifugation, and ethanol precipitation. 2 μg of the resultant RNA was hybridized overnight according to the manufacturer's instructions to a cocktail of [32P]UTP (Du Pont)-labeled complimentary RNA probes (PharMingen) for IL-1α, IL-1β, IL-1Ra, IL-6, IL-10, IL-12 p40 and p35, TNF-α and -β, TGF-β1–3, LT-β, and the housekeeping genes L32 and GAPDH at 56°C. Single-stranded RNA was digested by incubation with RNase for 45 min at 37°C and the protected fragments reextracted by ethanol precipitation. The products were electrophoresed on a 5% denaturing polyacrylamide gel; a negative control RNA and the unhybridized radioactive probe were run in each experiment. The gel was exposed overnight using a Biorad Geldoc 1000. The identity of the protected bands was confirmed by reference to the unhybridized probes and quantitated by reference to bands for the housekeeping genes L32 and GAPDH.
CFU Assay for the Intracellular Growth of M. tuberculosis.
This assay was performed as previously described with minor modifications (26). In brief, adherent cells were plated in triplicate wells in 96-U microtiter plates (Corning Glass Works) and readhered for 2 h. Cells were infected with M. tuberculosis H37Ra at 1:1, 10:1, and 100:1 (bacillus/cell) in 30% autologous serum. After 2 h, noningested bacteria were removed by washing gently (×3) with prewarmed RPMI 1640. Each well then received RPMI 1640 containing 2% autologous serum, and the plates were cultured in a humidified incubator at 37°C in the presence of 5% CO2 for as little as 1 h (time 0 sample) up to 10 d. Duplicate wells contained 2 μg/ml of neutralizing anti–IL-1Ra (goat IgG; R & D Systems, Inc.) or the same amount of isotype control antibody. At the end of the culture period, supernates were aspirated, and the plates containing the infected adherent cells were frozen at −70°C. To determine the number of intracellular bacteria in the CFU assay, the plates were thawed and cells lysed with 0.25% SDS in PBS for 12 min and then neutralized using 20% BSA. The lysates were then 10-fold serially diluted with 7H9 broth (Difco Labs., Inc.), and three 10-μl aliquots of each dilution were plated on Middlebrook 7H10 agar (Difco Labs., Inc.). The plates were then incubated for 19 d at 37°C in humidified air with 5% CO2. At the end of this culture period, the number of CFUs in each of the three replicate spots was enumerated for at least two consecutive dilutions using a stereomicroscope and averaged. Using this technique, extracellular growth of mycobacteria as assessed by culture of the supernates is consistently >1 log lower than intracellular growth (26). The rate of intracellular growth expressed as doubling time was determined by reference to the logarithmic growth from the cultures.
Statistical Analysis.
Values throughout are quoted or shown as the mean ± SE. Normally distributed variables were analyzed by paired or unpaired t test. P values reflect two-tailed values of t. Unpaired nonparametric variables were analyzed by the Mann-Whitney U test. Contingency analysis was performed using Fisher's exact test of probability.
Results
Polymorphism in the IL-1Ra Gene Associates with the Stimulated Production of IL-1Ra.
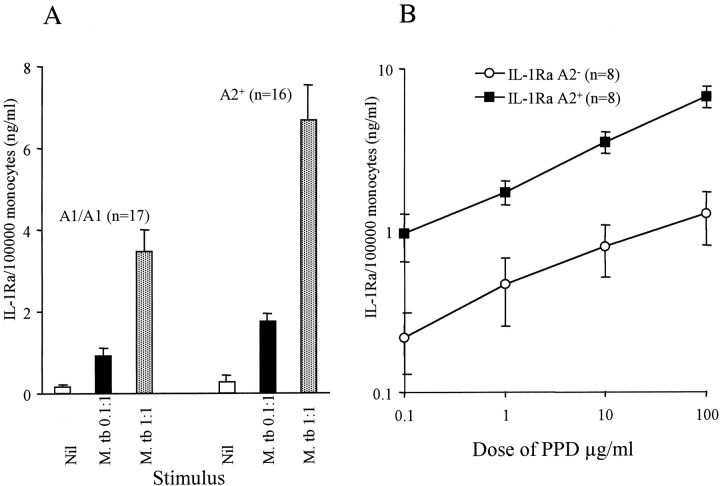
First, we examined the M. _tuberculosis_–stimulated production of IL-1Ra by culture of 2.5 × 106 PBMCs for 10 h. Culture supernates were assayed for IL-1Ra content, and the results were normalized to the number of monocytes in culture. The relationship between polymorphism in IL-1RN in 17 donors homozygous for the A1 allele (IL-1Ra A2−) and 16 donors at least heterozygous for A2 (3 A2/A2, 13 A1/A2; IL-1Ra A2+) and the M. _tuberculosis–_induced secretion of IL-1Ra was determined. The other alleles of IL-1RN were very rare and therefore could not be assessed. The M. _tuberculosis–_stimulated IL-1Ra response of A2/A2 homozygotes did not differ from A1/A2 (data not shown), confirming the previous finding that IL-1Ra A2 is codominant (21). The unstimulated production of IL-1Ra was slightly, but not significantly, higher in the IL-1Ra A2+ group (Fig. 1 A). Stimulation by M. tuberculosis (0.1 and 1:1 bacillus/cell) caused a dose-dependent increase in IL-1Ra secretion irrespective of genotype. However, the median response of the IL-1Ra A2+ group was 1.9 times greater at both doses of M. tuberculosis tested (P = 0.02 at 1:1). In a subset of 16 healthy subjects, the dose response of IL-1Ra induction to PPD was also determined (Fig. 1 B). Although IL-1Ra A2− individuals showed a dose-dependent increase in IL-1Ra secretion, this did not become statistically significant until the dose of PPD was 100 μg/ml. The response of IL-1Ra A2+ individuals was 2.1–3.6 times higher, depending on the dose. In contrast, induction of IL-1Ra in IL-1Ra A2+ donors was significant at 1 μg/ml. Thus, IL-1Ra A2+ donors appeared more sensitive to PPD stimulation. The median production of IL-1Ra in response to LPS (10 μg/ml) was also 1.82 times greater in the IL-1Ra A2+ donors (6.6 ± 1.3 vs. 3.6 ± 0.5 ng/ml/105 monocytes, P = 0.012).
Figure 1.
Association between IL-1Ra genotype and the monocyte production of IL-1Ra in response to M. tuberculosis and PPD. The amount of IL-1Ra produced by PBMCs from healthy, PPD-nonreactive donors during a 10-h coculture with either M. tuberculosis (A) or PPD (B) was determined by ELISA. Values were normalized to the number of monocytes present in the culture. The response of IL-1Ra A2+ individuals was higher at all doses of either stimuli.
Relationship between Polymorphisms and the Production of IL-1β.
We next determined the level of IL-1β in the same culture supernates used for the analysis of IL-1Ra. In contrast to the IL-1Ra polymorphism, the two polymorphisms in the IL-1β gene did not correlate with the M. _tuberculosis_–stimulated production of IL-1β to the same extent. The median M. tuberculosis (at 1:1)-stimulated production of IL-1β in subjects positive for the −511 A2 (n = 20) was 635 ± 119 pg/ml and 404 ± 261 pg/ml in A1/A1 homozygotes (n = 8). The corresponding figures for the +3953 polymorphism were 404 ± 84 pg/ml (A2+, n = 12) and 643 ± 171 pg/ml (A1/A1 homozygotes, n = 16). IL-1β production did tend to be higher in IL-1Ra A2− subjects, but only significantly so in response to M. tuberculosis at 0.1:1 (P = 0.01) (data not shown).
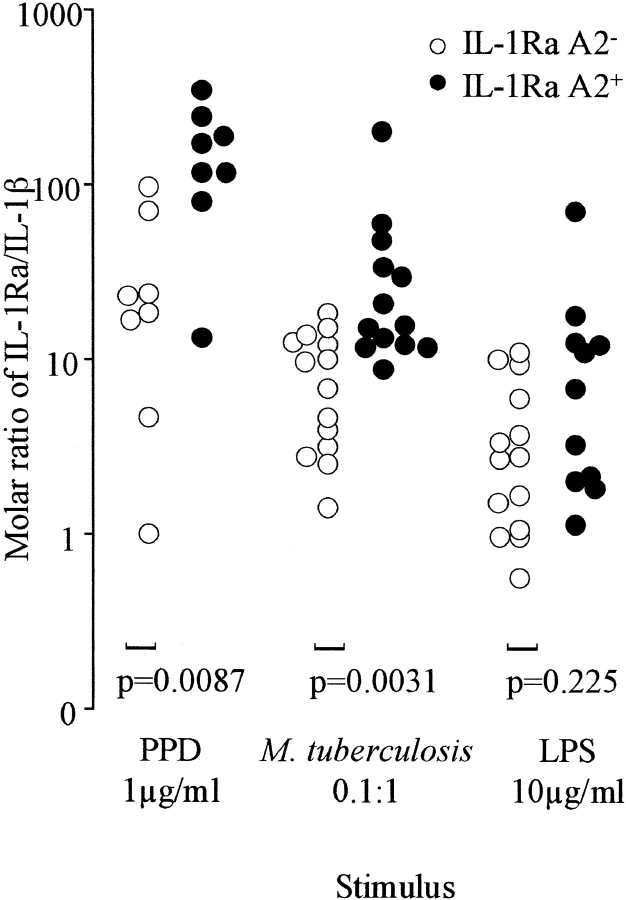
As a pure antagonist of IL-1, IL-1Ra competes for occupancy of IL-1RI, and it has been estimated that IL-1Ra needs to be present in a large molar excess (25–50×) to antagonize IL-1β significantly (28). Therefore, the ratio of IL-1Ra/ IL-1β is likely to be more relevant to regulation of the inflammatory response than the absolute value of either cytokine. The molar ratio of IL-1Ra/IL-1β was therefore calculated for each supernate and was significantly higher in IL-1Ra A2+ individuals (P ≤ 0.05) in response to doses of both M. tuberculosis and PPD at 1, 10, and 100 μg/ml (Fig. 2 B), in some cases with only minor overlap between the groups. By contrast, the response to LPS did not differ significantly between the groups. Fig. 2 B shows that the highest ratios likely to result in antagonism of the IL-1β response to PPD and M. tuberculosis stimulation (especially at lower doses likely to be relevant to M. _tuberculosis_–infected foci) were observed in the majority of IL-1Ra A2+ individuals but only in a minority of IL-1Ra A2− subjects. The bulk of the experiments were performed using attenuated M. tuberculosis H37Ra. Therefore, parallel determination of IL-1Ra and IL-1β secretion using the same doses of M. tuberculosis H37Rv in three donors (one A1/A1 and two A1/A2) was also performed. The level of each cytokine was very similar, such that at an infection multiplicity of 1:1 the IL-1Ra/IL-1β ratio when stimulated by H37Rv was 4.1, 16.8, and 12.6 for the three donors and 6.2, 17.3, and 8.0, respectively when stimulated by H37Ra. We thus have no reason to suspect that the findings using M. tuberculosis H37Ra would not apply to virulent clinical isolates.
Figure 2.
Relationship between polymorphism in IL-1Ra and the IL-1Ra/IL-1β ratio. The IL-1β content of the supernates shown in Fig. 1 was also assayed by ELISA. The molar ratio of IL-1Ra/IL-1β was calculated. This ratio is selectively increased in IL-1Ra A2+ individuals in response to PPD and M. tuberculosis, accentuated by the finding that IL-1Ra A2+ subjects tended to produce less IL-1β in response to M. tuberculosis. By comparison, the ratio in response to LPS was not different between IL-1Ra A2+ and A2− subjects. Response to PPD is data from eight individuals.
Cytokine Gene Expression by Ribonuclease Protection Assay.
We next sought to investigate association between the polymorphisms and the expression of mRNA. Ribonuclease protection assay was performed on RNA from 13 donors, all of different genotypes. The spontaneous expression of IL-1Ra and IL-1β transcript was low. There was no constitutive expression of any other monocyte cytokine, indicating that this low expression was unlikely to have been due to a nonspecific effect of cellular activation during isolation. Within 1 h, M. tuberculosis induced IL-1Ra gene expression in all individuals, irrespective of genotype, together with the mRNAs for IL-1β and TNF-α and followed slightly later (2 h) by IL-1α and IL-6. At hour 4, there was higher induction of IL-1Ra in the IL-1Ra A2+ subjects consistent with the protein data, although the difference was not statistically significant (Table I). The IL-1β +3953 allele A2 was associated with significantly lower production of IL-1β transcript (P = 0.04). Taken together, we interpret these observations to indicate that the alleles are associated with differences in transcription, but the dissociation between induction and secretion, particularly in the case of IL-1β, indicates that posttranscriptional mechanisms also influence cytokine secretion.
Table I.
Mean Fold Induction of the IL-1Rα and IL-1β Genes in Response to M. tuberculosis
| Haplotype | Number | Fold induction* | Range |
|---|---|---|---|
| IL-1Ra | |||
| A2− | 5 | 5.7 | 1.7–8.6 |
| A2+ | 8 | 10.0 | 2.6–29.5 |
| IL-1β (−511) | |||
| A2− | 3 | 25.9 | 11.3–51.0 |
| A2+ | 10 | 46.5 | 23.7–84.7 |
| IL-1β (+3953) | |||
| A2− | 7 | 52.3 | 15.5–84.7 |
| A2+ | 6 | 29.3 | 11.3–47.5 |
Effect of Monocyte Cytokines on the Production of IL-1Ra in Response to M. tuberculosis.
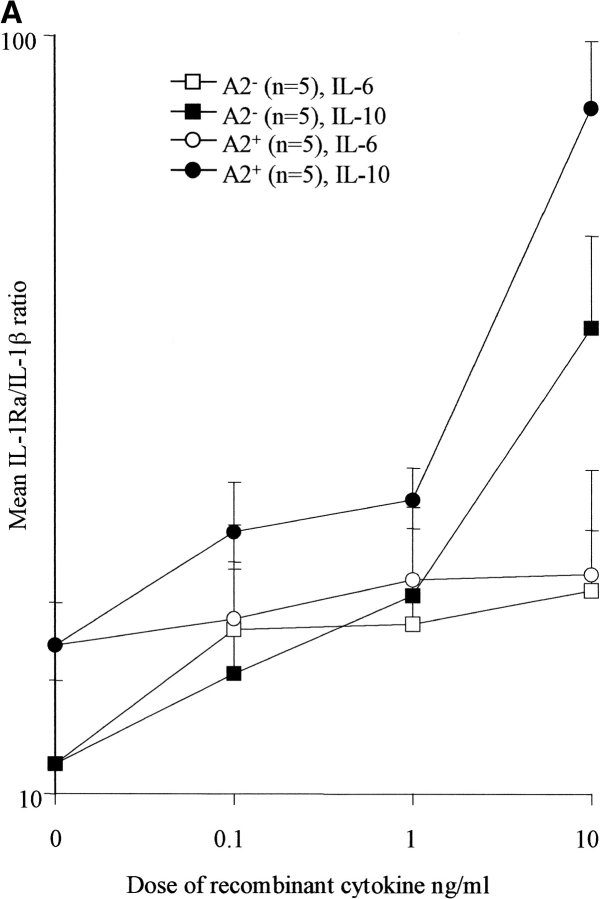
The results so far showed that in response to M. tuberculosis or its PPD, IL-1Ra gene expression is induced within 1 h, large quantities of protein are secreted within 10 h, and differences between individuals could be related to their genotypes. However, an indirect modulating influence of M. tuberculosis via increased translation of preexisting IL-1Ra mRNA or an effect of other cytokines (such as TGF-β, TNF-α, IL-1β, and IL-6) produced by monocytes early in response to infection is also possible. We investigated this possibility by assessing the ability of antibodies known to neutralize the biological effects of TGF-β, TNF-α, IL-1β, and IL-6 on M. _tuberculosis–_stimulated production of IL-1Ra. Control wells received isotype-matched antibodies. No consistent effect on constitutive or stimulated IL-1Ra secretion was seen, irrespective of genotype, cytokine, or dose of antibody used (up to 1,000-fold the ED50 concentrations). TGF-β modulates the human response to tuberculosis (29, 30) and has also been reported to increase IL-1Ra in some (31) but not all studies (32). We therefore also evaluated the effect of rTGF-β (0.1–10 ng/ml) on both M. _tuberculosis_–stimulated and –unstimulated IL-1Ra production in 12 individuals (6 IL-1Ra A2− and 6 IL-1Ra A2+). No significant enhancement of the early secretion of IL-1Ra was seen (data not shown). However, rIL-10 (0.1–10 ng/ml) caused a significant dose-dependent increase in the M. _tuberculosis_– stimulated IL-1Ra/IL-1β ratio in IL-1Ra A2+ and IL-1Ra A2− donors at all doses tested (P < 0.02), an effect largely due to the suppression of IL-1β production (Fig. 3 A). The addition of rhIL-6, however, caused no significant change in the IL-1Ra/IL-1β ratio in either group.
Figure 3.
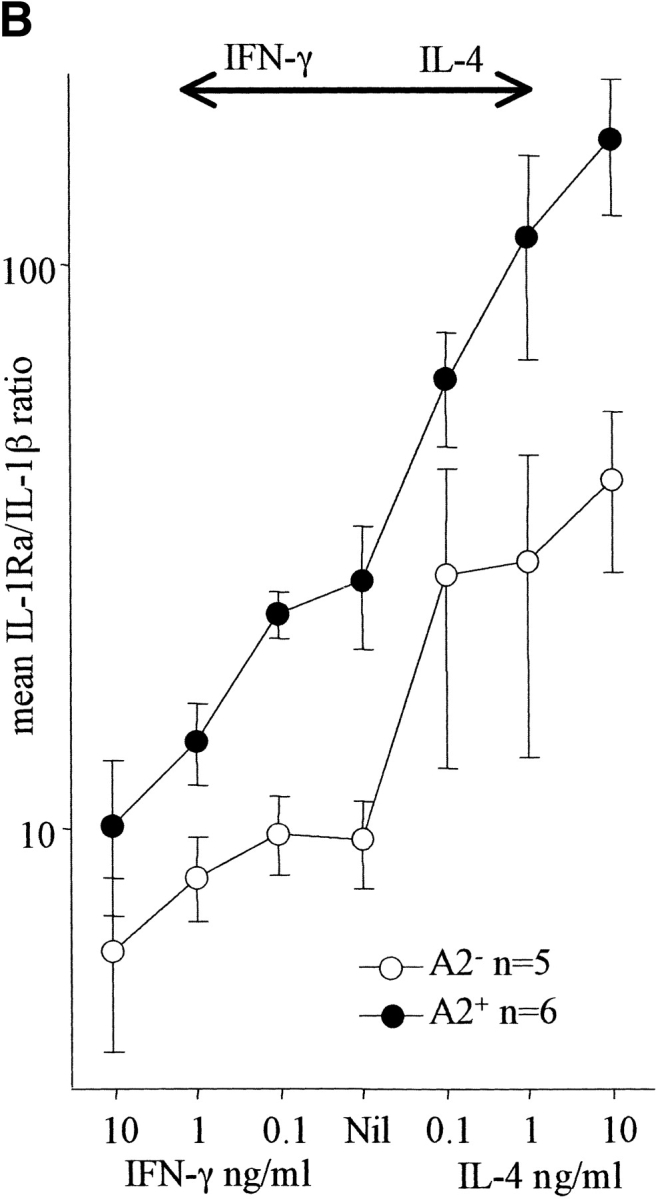
(A) Effect of IL-6 and IL-10 on the IL-1Ra/IL-1β ratio. PBMCs were cultured with M. tuberculosis in the presence or absence of rhIL-6 or rhIL-10 over a dose range of 0.1–10 ng/ml. Culture supernates were assayed for IL-1Ra and IL-1β and the molar ratio calculated. IL-10 increased the ratio significantly even at the lowest dose (P < 0.02), whereas rhIL-6 had no significant effect. (B) Effect of IFN-γ and IL-4 on the IL-1Ra/IL-1β ratio. PBMCs were cultured with M. tuberculosis in the presence or absence of rhIFN-γ or rhIL-4 over a dose range of 0.1–10 ng/ml. Culture supernates were assayed for IL-1Ra and IL-1β and the molar ratio calculated. IL-4 increased the ratio markedly even at the lowest dose (P < 0.01), whereas higher doses of IFN-γ were required to cause a significant reduction.
Effect of T Cell Cytokines on the Production of IL-1Ra and IL-1β in Response to M. tuberculosis.
It has also been shown that the lymphocyte production of IFN-γ and IL-4 can differentially modulate IL-1β and IL-1Ra production (33). Our coculture system excluded the possibility of an obscuring effect of T cell cytokines by the sole use of PBMCs from PPD− individuals and a short culture duration. In fact, the production of IFN-γ was negligible in the M. _tuberculosis–_stimulated cultures (20 pg/ml) from these subjects. To investigate the possibility that T cell cytokines modulate M. _tuberculosis–_induced IL-1Ra and IL-1β secretion, rhIFN-γ or rhIL-4 were added (0.1–10 ng/ml) to cultures. IL-4 caused a dose-dependent increase in both unstimulated and M. _tuberculosis_–stimulated IL-1Ra production, which was most significant in the M. _tuberculosis_–stimulated IL-1Ra A2+ group (P = 0.002 at 10 ng/ml). Furthermore, IL-4 also significantly decreased IL-1β production in M. _tuberculosis_–stimulated cells from both genotypes (P < 0.05 at 10 ng/ml). By comparison, IFN-γ led to a dose-dependent increase in M. _tuberculosis_–stimulated IL-1β production that was most marked in the IL-1Ra A2+ group (P = 0.052 at 10 ng/ml). Thus, IFN-γ tended to increase IL-1β production in M. _tuberculosis_–stimulated cells without affecting IL-1Ra production, whereas IL-4 increased IL-1Ra production irrespective of genotype and also depressed IL-1β secretion. This differential effect was reflected in the mean M. _tuberculosis_–stimulated IL-1Ra/IL-1β ratio, which increased in response to IL-4 even at the lowest dose of 0.1 ng/ml (P < 0.01, both groups combined). By comparison, higher doses of IFN-γ (1–10 ng/ml) were required to reduce the IL-1Ra/IL-1β ratio significantly (Fig. 3 B).
Relationship between Polymorphism in IL-1Ra and the Intracellular Growth of M. tuberculosis.
We next investigated the effect of IL-1Ra polymorphism on the rate of intracellular replication of M. tuberculosis. Monocytes from 22 donors (12 IL-1Ra A2− and 10 IL-1Ra A2+) were infected with M. tuberculosis at various multiplicities (1:1, 10:1, and 100:1 bacillus/cell) and then cultured in vitro for up to 240 h. Cell lysates were set up for M. tuberculosis CFU assay at 0, 24, 96, 168, and 240 h. Although there was interindividual variation in the establishment of initial infection, there was no significant difference between the IL-1Ra A2− and IL-1Ra A2+ groups. Logarithmic growth was established in 8 donors. The remainder showed either minimal or linear intracellular growth of mycobacteria only, with no difference between IL-1Ra A2− and IL-1Ra A2+ donors. In those donors in whom logarithmic growth did occur (5 IL-1Ra A2− and 3 IL-1Ra A2+), the doubling time of M. tuberculosis was estimated from the growth curve. Data from these individuals is shown in Table II. Intra- and interindividual differences did not appear to be related to the presence or absence of the IL-1Ra A2 allele. These data therefore contrast with the readily demonstrable increase in IL-1Ra secretion conferred by the A2 allele in the same donors (shown in parentheses in Table II). In each experiment, triplicate wells were also included to assess the effect of 2 μg/ml neutralizing antibody to IL-1Ra (and goat IgG isotype control). No consistent effect of these antibodies on intracellular growth was seen (data not shown).
Table II.
Lack of Relationship between IL-1Ra Polymorphism and the Intracellular Growth of M. tuberculosis In Vitro
| Donor | Genotype | Culture duration | Doubling times at various multiplicities of infection by M. tuberculosis (h) | ||
|---|---|---|---|---|---|
| 1 | 10 | 100 | |||
| h | |||||
| 1 | A1/A1 | 96 | 24 (2.47) | 22 | 14 |
| 2 | A1/A1 | 168 | − | − | 22 |
| 3 | A1/A1 | 240 | 39 (2.56) | 32 | − |
| 4 | A1/A1 | 240 | 38 (1.45) | − | − |
| 5 | A1/A1 | 240 | 42 | 28 | |
| 6 | A1/A2 | 96 | 27 (10.65) | 15 | 13 |
| 7 | A1/A2 | 168 | 38 | 25 | 27 |
| 8 | A1/A2 | 240 | 53 (10.67) | 29 | − |
IL-1β and IL-1Ra Genotype and Allele Frequency in Tuberculosis Patients and Control Subjects.
We next sought in vivo correlates by determination of the frequency of the IL-1β and IL-1Ra polymorphisms in patients with tuberculosis and healthy PPD-reactive control subjects in a pilot case-control analysis of Gujarati asians in west London. This population is distinct and has a high incidence of tuberculosis with an excess of extrapulmonary forms. Individual alleles at each locus were in Hardy-Weinberg equilibrium. The IL-1β (−511) allele 1 was in linkage disequilibrium with IL-1β (+3953) allele 2 and vice-versa (P < 0.03). In addition, there was weaker linkage between IL-1Ra A2 and IL-1β (+3953) A2. No allele or genotype, singly or in combination, was associated with an increased risk of tuberculosis (Table III). We concluded that, in this population, these polymorphisms have little effect on susceptibility to tuberculosis per se.
Table III.
IL-1Ra and IL-1β Allele and Genotype Frequencies in Tuberculosis Patients and Tuberculin-reactive Healthy Control Subjects
| Gene | Position | Genotype or allele frequency | Patients | Controls |
|---|---|---|---|---|
| IL-1Ra | N/A | A1 | 0.713 | 0.719 |
| A2 | 0.236 | 0.241 | ||
| A3 | 0.045 | 0.039 | ||
| A4 | 0.006 | 0 | ||
| A1/A1 | 51 (57%) | 65 (57%) | ||
| A1/A2 | 21 (24%) | 30 (26%) | ||
| A2/A2 | 9 (10%) | 10 (9%) | ||
| Others* | 8 (9%) | 9 (8%) | ||
| IL-1β | −511 | A1 | 0.438 | 0.404 |
| A2 | 0.562 | 0.596 | ||
| A1/A1 | 20 (22%) | 18 (16%) | ||
| A1/A2 | 38 (43%) | 56 (49%) | ||
| A2/A2 | 31 (35%) | 40 (35%) | ||
| IL-1β | +3953 | A1 | 0.837 | 0.794 |
| A2 | 0.163 | 0.206 | ||
| A1/A1 | 64 (72%) | 76 (67%) | ||
| A1/A2 | 21 (24%) | 29 (25%) | ||
| A2/A2 | 4 (4%) | 9 (8%) |
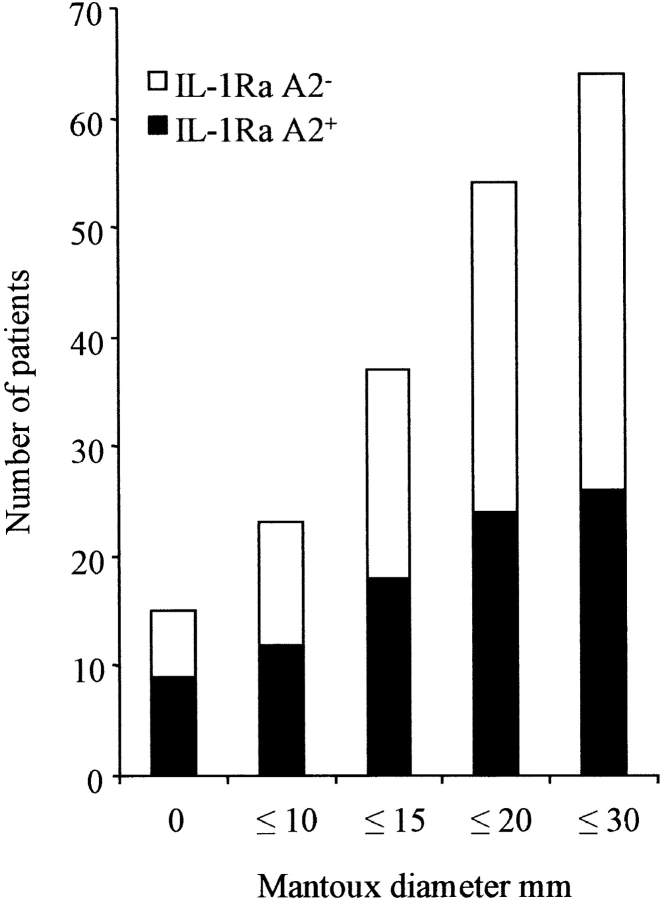
The in vitro data indicated that the IL-1Ra A2−/IL-1β (+3953) A1+ haplotype was associated with low IL-1Ra protein and gene expression and higher corresponding IL-1β values, implying a proinflammatory phenotype. We therefore examined association between the gene polymorphisms and the presenting form of post-primary tuberculosis (Table IV). This proinflammatory haplotype was more common in patients with pleural disease (P = 0.028 by comparison with control subjects). Pleural tuberculosis represents a contained disease phenotype associated with a high DTH response and marked proinflammatory cytokine responses at the site of disease (34, 35). By comparison, the IL-1Ra A2 was more common in patients with extrapulmonary disease (P = 0.009, by comparison with pleural disease). A similar reduction in DTH as manifested by cutaneous reactivity to PPD was also associated with the presence of the IL-1Ra A2 allele: the proportion of IL-1Ra A2+ individuals progressively decreased at higher grades of Mantoux (Table IV, Fig. 4).
Table IV.
Relationship between IL-1Ra/IL-1β Haplotype and Disease Phenotype
| Groups | IL-1Ra A2−/IL-1β (+3953) A1+ | Other haplotypes* | P |
|---|---|---|---|
| Total controls | 65 (57%) | 49 (43%) | |
| Total patients | 54 (61%) | 35 (39%) | 0.67 |
| Pleural | 11 (92%) | 1 (8%) | 0.028 |
| Pulmonary | 16 (62%) | 10 (38%) | 0.83 |
| Miliary | 6 (60%) | 4 (40%) | 1 |
| Lymphadenopathic | 12 (60%) | 8 (40%) | 1 |
| Extrapulmonary‡ | 9 (43%) | 12 (57%) | 0.24 |
| Median Mantoux/mm | 15.5 ± 1.4 | 11.5 ± 1.8 | 0.068 |
Figure 4.
Association between IL-1Ra haplotype and the cutaneous response to PPD of M. tuberculosis. The mean diameter of induration in response to one tuberculin unit of PPD in tuberculosis patients of various genotypes is shown. The proportion of IL-1Ra A2+ was highest (60%) in patients with an absent response and lowest in the category 21–30 mm (25%), falling gradually to its overall frequency (41%) as successively higher grades of Mantoux were considered.
Discussion
We have investigated the effect of polymorphisms in the IL-1β and IL-1Ra genes on M. _tuberculosis_–stimulated cytokine production in vitro and their relevance in patients with tuberculosis. When compared with healthy IL-1Ra A2− subjects, A2+ subjects as a group secreted nearly twice as much IL-1Ra in response to both laboratory-adapted and virulent M. tuberculosis, PPD, or LPS. The mean fold induction of IL-1Ra mRNA was also nearly twice that of IL-1Ra A2− subjects. The two polymorphisms in the IL-1β gene were not clearly associated with the level of M. _tuberculosis_–stimulated IL-1β production in vitro, although the IL-1β (+3953) A1+ haplotype was associated with significantly increased M. _tuberculosis–_induced expression of the IL-1β gene. The individual molar ratios of IL-1Ra/IL-1β, which determine the net effect of these cytokines in response to PPD and M. tuberculosis, were clearly higher in IL-1Ra A2+ subjects. Furthermore, the IL-1Ra/IL-1β ratios were affected by cytokines, as IL-4 upregulated IL-1Ra production and downregulated IL-1β production. IL-10 greatly suppressed and IFN-γ moderately enhanced the production of IL-1β. In patients with tuberculosis, the proinflammatory IL-1Ra A2−/IL-1β (+3953) A1+ haplotype was unevenly distributed, being more common in patients with pleural disease and less common in those with extrapulmonary disease. A further finding, consistent with the in vitro observations, was that the IL-1Ra A2+ haplotype was associated with a reduced Mantoux response to PPD of M. tuberculosis: 60% of tuberculin-nonreactive patients were of this type.
Our study of IL-1RN gene expression indicates the early induction by M. tuberculosis of its mRNA together with IL-1β, IL-1α, TNF-α, and IL-6. Although IL-1Ra A2 was associated with an increased induction of the IL-1RN gene, the exact mechanism of increased IL-1Ra production requires further elucidation. Whereas the fold induction of IL-1β mRNA was higher than that of IL-1Ra and could also be related to both IL-1β polymorphisms, the amount of secreted IL-1β protein was much less. In addition, the IL-1β polymorphisms could not so readily be related to protein secretion. This observation is consistent with other data (36) and indicates a dominant influence of both posttranscriptional and posttranslational events on the secretion of IL-1β. Many cytokines can upregulate IL-1Ra expression in vitro (17). The production of IL-1Ra, however, was unaffected by antibody neutralization of IL-1β, IL-6, TGF-β, and TNF-α, suggesting that M. tuberculosis or its products induce the early production of large quantities of IL-1Ra by a direct mechanism.
IL-1β is involved in the early recruitment of inflammatory cells to M. tuberculosis– or PPD-induced granulomas (37–41). Submaximal occupancy of IL-1RIs can mediate the full biological effects of IL-1β, and as a consequence, it has been postulated that IL-1Ra needs to be present in a large molar excess in order to exert its antagonism (28). In tuberculosis, this condition would be best fulfilled in IL-1Ra A2+ subjects (Fig. 2); the IL-1Ra A2 allele was associated with reduced DTH (Fig. 4) and was lower in frequency in patients with pleural tuberculosis, consistent with the in vitro data and suggestive of biological significance. Antigen-specific lymphocytes are also necessary for the DTH reaction to proceed. In our experiments, IL-4 increased IL-1Ra secretion, particularly in stimulated cultures from IL-1Ra A2+ subjects (Fig. 3 B). The production of IL-4 in tuberculosis has been best demonstrated in T cell clones (42), but one study has also documented small amounts of antigen-specific secretion of IL-4 by PBMCs (43). As cell-associated IL-4 is a stimulus for IL-1Ra, there is the possibility that relatively small amounts of IL-4 may greatly affect the IL-1Ra response (33). IFN-γ decreased and IL-10 increased the IL-1Ra/IL-1β ratio mainly through an effect on IL-1β secretion. Both IFN-γ and IL-10 are produced by PBMCs and at disease sites in patients with tuberculosis (29, 44, 45). Our data therefore suggests that the polymorphism in the IL-1Ra gene may exert regulatory influence on cytokine circuits beyond its direct effect on IL-1Ra production.
There is both epidemiological and experimental evidence of a dissociation between DTH and protection from tuberculosis (46, 47). Our finding that IL-1Ra appears to influence DTH with minimal effect on either the intracellular growth of M. tuberculosis in vitro or disease susceptibility in the case-control study further suggests a basis for the dissociation between DTH and susceptibility. In addition to disease susceptibility, the degree of cutaneous reactivity to PPD after bacille Calmette-Guérin vaccination in both mono- and dizygotic twins and in siblings is also heritable (48, 49). Our in vitro data (Figs. 1 and 2) clearly suggest a functional basis for the observed association between reduced DTH and A2 of the IL-1RN gene. Although our case-control analysis was modest in size, there was a distinct difference in IL-1Ra A2+ frequency between patients with pleural and extrapulmonary tuberculosis, and this preliminary data encourages us to determine in larger studies whether this association is generalizable to other populations. As our data also support a heritable component in the quantitative skin response to PPD, another appropriate strategy would be to perform a genome-wide search, which may not only confirm the involvement of the IL-1 locus but also potentially identify loci of relevance to other infectious processes as well (50). As the frequency of the IL-1Ra A2 allele is approximately six times lower in Gambia (51) and also in Kenya (Wilkinson, R.J., and P.A. Zimmerman, unpublished observations), perhaps this gene has been subject to natural selection by different major infectious diseases in India or Africa. It would also be interesting to determine whether a high IL-1Ra allele A2 frequency is present in populations with a high degree of PPD “anergy” (3).
The association between the IL-1Ra genotype and disease expression supports the hitherto unproven concept that host genes can influence disease phenotype in tuberculosis (52). We propose that the early recruitment and activation of inflammatory cells by IL-1β to foci of tuberculous infection is in turn downregulated by IL-1Ra that, under polymorphic host control, acts to limit the resultant DTH. This hypothesis could be readily tested in IL-1 and IL-1Ra gene knockout mice (53, 54). Reduction of DTH by targeted immunotherapy with either IL-1Ra or other engineered antagonists of IL-1RI (55) may also be a possible approach to modulation of immunopathologic cytokine circuits in tuberculosis.
Abbreviations used in this paper
DTH
delayed-type hypersensitivity
PPD
purified protein derivative
rt
room temperature
Footnotes
Dr. C.L. King (Case Western Reserve University) is thanked for providing DNA samples from Kenya. We are grateful to Drs. P.A. Zimmerman and J.J. Ellner for their critical review of the manuscript. We are grateful to the clinical, microbiological, and secretarial staff of Northwick Park Hospital, particularly Dr. R.A. Wall, Dr. M.G. Harries, Dr. M. Latif, and Miss Dina Shah. Manijeh Phillips and Beverly Hamilton are thanked for technical assistance. Dr. Carlos Moreno of King's College Hospital Medical School, London is thanked for encouraging this project in its early stages.
R.J. Wilkinson is a Wellcome Trust Fellow in Clinical Tropical Medicine. Additional support was provided by the National Institutes of Health (grant AI-18471), to P. Patel by an MSc studentship from Imperial College of Science, Technology and Medicine, and by the Medical Research Council of the United Kingdom.
References
- 1.Sreevastsan S, Pan X, Stockbauer KE, Connell ND, Kreiswirth BN, Whittam TS, Musser JM. Restricted structural gene polymorphism in the Mycobacterium tuberculosiscomplex indicates evolutionarily recent global dissemination. Proc Natl Acad Sci USA. 1997;94:9869–9874. doi: 10.1073/pnas.94.18.9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stead W. Genetics and resistance to tuberculosis: could resistance be enhanced by genetic engineering? . Ann Intern Med. 1992;116:937–941. doi: 10.7326/0003-4819-116-11-937. [DOI] [PubMed] [Google Scholar]
- 3.Sousa AO, Salem JI, Lee FK, Verçosa MC, Cruaud P, Bloom BR, Lagrange PH, David HL. An epidemic of tuberculosis with a high rate of tuberculin anergy among a population previously unexposed to tuberculosis, the Yanomami Indians of the Brazilian Amazon. Proc Natl Acad Sci USA. 1997;94:13227–13232. doi: 10.1073/pnas.94.24.13227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Comstock GW. Tuberculosis in twins: a re-analysis of the Prophit survey. Am Rev Respir Dis. 1978;117:621–624. doi: 10.1164/arrd.1978.117.4.621. [DOI] [PubMed] [Google Scholar]
- 5.Newport MJ, Huxley CM, Huston S, Hawrylowicz CM, Oostra BA, Williamson R, Levin M. A mutation in the interferon-γ receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1996;335:1941–1949. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- 6.Jouanguy E, Altare F, Lamhamedi S, Revy P, Emile JF, Newport M, Levin M, Blanche S, Seboun E, Fischer A, et al. Interferon-gamma-receptor deficiency in an infant with fatal Bacille Calmette-Guérin infection. N Engl J Med. 1996;335:1956–1961. doi: 10.1056/NEJM199612263352604. [DOI] [PubMed] [Google Scholar]
- 7.Dorman SE, Holland SM. Mutation in the signal-transducing chain of the interferon γ receptor and susceptibility to mycobacterial infection. J Clin Invest. 1998;101:2364–2369. doi: 10.1172/JCI2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jong R, Altare F, Haagen IA, Elferink DG, Boer T, van Breda PJC, Vriesman, Kabel PJ, Draaisma JMT, van Dissel JT, Kroon FP, et al. Severe mycobacterial and salmonella infections in interleukin-12 receptor-deficient patients. Science. 1998;280:1435–1438. doi: 10.1126/science.280.5368.1435. [DOI] [PubMed] [Google Scholar]
- 9.Altare F, Durandy A, Lammas D, Emile JF, Lamhamedi S, Le Deist F, Drysdale P, Jouanguy E, Doffinger R, Bernaudin F, et al. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280:1432–1435. doi: 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- 10.Altare F, Lammas D, Revy P, Jouanguy E, Doffinger R, Lamhamedi S, Drysdale P, Scheel-Toellner D, Girdlestone J, Darbyshire P, et al. Inherited interleukin 12 deficiency in a child with bacille Calmette-Guerin and Salmonella enteritidis–disseminated infection. J Clin Invest. 1998;102:2035–2040. doi: 10.1172/JCI4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellamy R, Ruwende C, Corrah T, McAdam KP, Whittle HC, Hill AV. Variations in the NRAMP1 gene and susceptibility to tuberculosis in West Africans. N Engl J Med. 1998;338:640–644. doi: 10.1056/NEJM199803053381002. [DOI] [PubMed] [Google Scholar]
- 12.Bellamy R, Ruwende C, Corrah T, McAdam KP, Thursz M, Whittle HC, Hill AV. Tuberculosis and chronic Hepatitis B virus infection in Africans and variation in the vitamin D receptor gene. J Infect Dis. 1999;179:721–724. doi: 10.1086/314614. [DOI] [PubMed] [Google Scholar]
- 13.Valway SE, Sanchez MP, Shinnick TF, Orme I, Agerton T, Hoy D, Jones JS, Westmoreland H, Onorato IM. An outbreak involving extensive transmission of a virulent strain of Mycobacterium tuberculosis. . N Engl J Med. 1998;338:633–639. doi: 10.1056/NEJM199803053381001. [DOI] [PubMed] [Google Scholar]
- 14.Hill AV. Immunogenetics of human infectious diseases. Annu Rev Immunol. 1998;16:593–617. doi: 10.1146/annurev.immunol.16.1.593. [DOI] [PubMed] [Google Scholar]
- 15.Sugisaki K, Dannenberg AM, Jr, Abe Y, Tsuruta J, Su WJ, Said W, Feng L, Yoshimura T, Converse PJ, Mounts P. Nonspecific and immune-specific up-regulation of cytokines in rabbit dermal tuberculous (BCG) lesions. J Leukoc Biol. 1998;63:440–450. doi: 10.1002/jlb.63.4.440. [DOI] [PubMed] [Google Scholar]
- 16.Kindler V, Sappino AP, Grau GE, Piguet PF, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 17.Arend WP, Malyak M, Guthridge CJ, Gabay C. Interleukin 1 receptor antagonist: role in biology. Annu Rev Immunol. 1998;16:27–55. doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]
- 18.di Giovine FS, Takhsh E, Blakemore AIF, Duff GW. Single base polymorphism at −511 in the human interleukin-1β gene. Hum Mol Genet. 1993;1:450. doi: 10.1093/hmg/1.6.450. [DOI] [PubMed] [Google Scholar]
- 19.Pociot F, Molvig J, Wogensen L, Worsaae H, Nerup J. A TaqI polymorphism in the human interleukin-1 beta (IL-1 beta) gene correlates with IL-1 beta secretion in vitro. Eur J Clin Invest. 1992;22:396–402. doi: 10.1111/j.1365-2362.1992.tb01480.x. [DOI] [PubMed] [Google Scholar]
- 20.Tarlow JK, Blakemore AIF, Lennard A, Solari R, Hughes HN, Steinkasserer A, Duff GW. Polymorphism in human IL-1 receptor antagonist gene intron 2 is caused by variable numbers of an 86-bp tandem repeat. Hum Genet. 1993;91:403–404. doi: 10.1007/BF00217368. [DOI] [PubMed] [Google Scholar]
- 21.Danis VA, Millington M, Hyland VJ, Grennan D. Cytokine production by normal human monocytes: inter-subject variation and relationship to an IL-1 receptor antagonist (IL-1Ra) gene polymorphism. Clin Exp Immunol. 1995;99:303–310. doi: 10.1111/j.1365-2249.1995.tb05549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurme M, Santilla S. IL-1 receptor antagonist (IL-1Ra) plasma levels are co-ordinately regulated by both IL-1Ra and IL-1β genes. Eur J Immunol. 1998;28:2598–2602. doi: 10.1002/(SICI)1521-4141(199808)28:08<2598::AID-IMMU2598>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 23.Juffermans N, Verbon A, van Deventer S, van Deutekom H, Speelman P, van der Poll T. Tumor necrosis factor and interleukin-1 inhibitors as markers of disease activity of tuberculosis. Am J Respir Crit Care Med. 1998;157:1328–1331. doi: 10.1164/ajrccm.157.4.9709126. [DOI] [PubMed] [Google Scholar]
- 24.Akalin H, Akdis AC, Mistik R, Helvaci S, Kilicturgay K. Cerebrospinal fluid interleukin-1 beta/interleukin-1 receptor antagonist balance and tumor necrosis factor-alpha concentrations in tuberculous, viral and acute bacterial meningitis. Scand J Infect Dis. 1994;26:667–674. doi: 10.3109/00365549409008634. [DOI] [PubMed] [Google Scholar]
- 25.Ormerod L, Charlett A, Gilham C, Darbyshire J, Watson J. Geographical distribution of tuberculosis notifications in national surveys of England and Wales in 1988 and 1993: report of the public health laboratory service/ British Thoracic Society/Department of Health collaborative group. Thorax. 1998;53:176–181. doi: 10.1136/thx.53.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirsch CS, Yoneda T, Averill L, Ellner JJ, Toossi Z. Enhancement of intracellular growth of Mycobacterium tuberculosisin human monocytes by transforming growth factor-beta 1. J Infect Dis. 1994;170:1229–1237. doi: 10.1093/infdis/170.5.1229. [DOI] [PubMed] [Google Scholar]
- 27.Toossi Z, Hirsch CS, Hamilton BD, Knuth CK, Friedlander MA, Rich EA. Decreased production of TGF-β1 by human alveolar macrophages compared with blood monocytes. J Immunol. 1996;156:3461–3468. [PubMed] [Google Scholar]
- 28.Arend WP. Interleukin-1 receptor antagonist. Adv Immunol. 1993;54:167–227. doi: 10.1016/s0065-2776(08)60535-0. [DOI] [PubMed] [Google Scholar]
- 29.Hirsch CS, Hussain R, Toossi Z, Dawood G, Shahid F, Ellner JJ. Cross-modulation by transforming growth factor beta in human tuberculosis: suppression of antigen-driven blastogenesis and interferon gamma production. Proc Natl Acad Sci USA. 1996;93:3193–3198. doi: 10.1073/pnas.93.8.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirsch CS, Ellner JJ, Blinkhorn R, Toossi Z. In vitro restoration of T cell responses in tuberculosis and augmentation of monocyte effector function against Mycobacterium tuberculosisby natural inhibitors of transforming growth factor beta. Proc Natl Acad Sci USA. 1997;94:3926–3931. doi: 10.1073/pnas.94.8.3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muzio M, Sironi M, Polentarutti N, Mantovani A, Colotta F. Induction by transforming growth factor-beta 1 of the interleukin-1 receptor antagonist and of its intracellular form in human polymorphonuclear cells. Eur J Immunol. 1994;24:3194–3198. doi: 10.1002/eji.1830241242. [DOI] [PubMed] [Google Scholar]
- 32.Jenkins JK, Arend WP. Interleukin 1 receptor antagonist production in human monocytes is induced by IL-1 alpha, IL-3, IL-4 and GM-CSF. Cytokines. 1993;5:407–415. doi: 10.1016/1043-4666(93)90030-9. [DOI] [PubMed] [Google Scholar]
- 33.Chizzolini C, Chicheportiche R, Burger D, Dayer JM. Human Th1 cells preferentially induce interleukin (IL)-1β while Th2 cells induce IL-1 receptor antagonist production upon cell/cell contact with monocytes. Eur J Immunol. 1997;27:171–177. doi: 10.1002/eji.1830270125. [DOI] [PubMed] [Google Scholar]
- 34.Barnes P, Fong S, Brennan P, Twomey P, Mazumder A, Modlin R. Local production of tumor necrosis factor and IFN-γ in tuberculous pleuritis. J Immunol. 1990;145:149–154. [PubMed] [Google Scholar]
- 35.Barnes PF, Mistry SD, Cooper CL, Pirmez C, Rea TH, Modlin RL. Compartmentalization of a CD4+T lymphocyte subpopulation in tuberculous pleuritis. J Immunol. 1989;142:1114–1119. [PubMed] [Google Scholar]
- 36.Santtila S, Savinainen K, Hurme M. Presence of the IL-1RA allele 2 (IL1RN*2)is associated with enhanced IL-1beta production in vitro. Scand J Immunol. 1998;47:195–198. doi: 10.1046/j.1365-3083.1998.00300.x. [DOI] [PubMed] [Google Scholar]
- 37.Denis M, Ghadirian E. Interleukin-1 is involved in mouse resistance to Mycobacterium avium. . Infect Immun. 1994;62:457–461. doi: 10.1128/iai.62.2.457-461.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marshall BG, Wangoo A, Cook HT, Shaw RJ. Increased inflammatory cytokines and new collagen formation in cutaneous tuberculosis and sarcoidosis. Thorax. 1996;51:1253–1261. doi: 10.1136/thx.51.12.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Broser M, Cohen H, Bodkin M, Law K, Reibman J, Rom WN. Enhanced interleukin-8 release and gene expression in macrophages after exposure to Mycobacterium tuberculosisand its components. J Clin Invest. 1995;95:586–592. doi: 10.1172/JCI117702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gordon S, Keshav S, Stein M. BCG-induced granuloma formation in murine tissues. Immunobiology. 1994;191:369–377. doi: 10.1016/S0171-2985(11)80442-0. [DOI] [PubMed] [Google Scholar]
- 41.Bergeron A, Bonay M, Kambouchner M, Lecossier D, Riquet M, Soler P, Hance A, Tazi A. Cytokine patterns in tuberculous and sarcoid granulomas: correlations with histopathologic features of the granulomatous response. J Immunol. 1997;159:3034–3043. [PubMed] [Google Scholar]
- 42.Agrewala JN, Wilkinson RJ. Differential regulation of Th1 and Th2 cells by p91-110 and p21-40 peptides of the 16 kDa α-crystallin antigen of Mycobacterium tuberculosis. . Clin Exp Immunol. 1998;104:392–397. doi: 10.1046/j.1365-2249.1998.00724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Surcel H-M, Troye-Blomberg M, Paulie S, Andersson G, Moreno C, Pasvol G, Ivanyi J. Th1/Th2 profiles in tuberculosis based on proliferation and cytokine response of blood lymphocytes to mycobacterial antigens. Immunology. 1994;81:171–176. [PMC free article] [PubMed] [Google Scholar]
- 44.Wilkinson RJ, Vordermeier HM, Wilkinson KA, Sjölund A, Moreno C, Pasvol G, Ivanyi J. Peptide specific response to M. tuberculosis: clinical spectrum, compartmentalization, and effect of chemotherapy. J Infect Dis. 1998;178:760–768. doi: 10.1086/515336. [DOI] [PubMed] [Google Scholar]
- 45.Barnes P, Lu S, Abrams J, Wang E, Yamamura M, Modlin R. Cytokine production at the site of disease in human tuberculosis. Infect Immun. 1993;61:3482–3489. doi: 10.1128/iai.61.8.3482-3489.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson CM, Cooper AM, Frank AA, Orme IM. Adequate expression of protective immunity in the absence of granuloma formation in Mycobacterium tuberculosis- infected mice with a disruption in the intracellular adhesion molecule 1 gene. Infect Immun. 1998;66:1666–1670. doi: 10.1128/iai.66.4.1666-1670.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bloom, B.R., and P.E.M. Fine. 1994. The BCG experience: implications for future vaccines against tuberculosis. In Tuberculosis: Pathogenesis, Protection and Control. B.R. Bloom, editor. American Society for Microbiology, Washington, DC. 531–557.
- 48.Sepulveda RL, Heiba IM, Navarrete C, Elston RC, Gonzalez B, Sorensen RU. Tuberculin reactivity after newborn BCG immunisation in mono- and dizygotic twins. Tuber Lung Dis. 1994;75:138–143. doi: 10.1016/0962-8479(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 49.Sepulveda RL, Heiba IM, King A, Gonzalez B, Elston RC, Sorensen RU. Evaluation for tuberculin reactivity in BCG immunised siblings. Am J Respir Crit Care Med. 1994;149:620–624. doi: 10.1164/ajrccm.149.3.8118628. [DOI] [PubMed] [Google Scholar]
- 50.Abel L, Dessein AJ. Genetic epidemiology of infectious diseases in humans: design of population based studies. Emerg Infect Dis. 1998;4:593–603. doi: 10.3201/eid0404.980409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bellamy R, Ruwende C, Corrah T, McAdam KPWJ, Whittle HC, Hill AV. Assessment of the interleukin 1 gene cluster and other candidate gene polymorphisms in host susceptibility to tuberculosis. Tuberc Lung Dis. 1998;79:83–90. doi: 10.1054/tuld.1998.0009. [DOI] [PubMed] [Google Scholar]
- 52.Daniel, T.M. 1997. Captain of Death: The Story of Tuberculosis. University of Rochester Press, Rochester, NY. 121–130.
- 53.Hirsch E, Irikura VM, Paul SM, Hirsh D. Functions of interleukin 1 receptor antagonist in gene knockout and overproducing mice. Proc Natl Acad Sci USA. 1996;93:11008–11013. doi: 10.1073/pnas.93.20.11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horai R, Asano M, Sudo K, Kanuka H, Suzuki M, Nishihara M, Takahashi M, Iwakura Y. Production of mice deficient in genes for interleukin (IL)-1α, IL-1β, IL-1α/β, and IL-1 receptor antagonist shows that IL-1β is crucial in turpentine-induced fever development and glucocorticoid secretion. J Exp Med. 1998;187:1463–1475. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akeson AL, Woods CW, Hsieh LC, Bohnke RA, Ackermann BL, Chan KY, Robinson JL, Yanofsky SD, Jacobs JW, Barrett RW, et al. AF12198, a novel low molecular weight antagonist, selectively binds the human type I interleukin (IL)-1 receptor and blocks in vivo responses to IL-1. J Biol Chem. 1996;271:30517–30523. doi: 10.1074/jbc.271.48.30517. [DOI] [PubMed] [Google Scholar]