A Novel Gene Coding for a Fas Apoptosis Inhibitory Molecule (FAIM) Isolated from Inducibly Fas-resistant B Lymphocytes (original) (raw)
Abstract
The sensitivity of primary splenic B cells to Fas-mediated apoptosis is modulated in a receptor-specific fashion. Here we used a differential display strategy to detect cDNAs present in B cells rendered Fas resistant but absent in those rendered Fas sensitive. This led to the cloning and characterization of a novel 1.2-kb gene that encodes a Fas apoptosis inhibitory molecule (FAIM). _faim_-transfected BAL-17 B lymphoma cells were less sensitive by half or more to Fas-mediated apoptosis than were vector-transfected controls, using Fas ligand–bearing T cells or a cytotoxic anti-Fas antibody to trigger Fas, and this was associated with inhibition of Fas- induced poly-ADP ribose polymerase (PARP) cleavage. In primary B cells, the time course of faim mRNA and FAIM protein expression correlated with the induction of Fas resistance by surface (s)Ig engagement. Thus, FAIM is an inducible effector molecule that mediates Fas resistance produced by sIg engagement in B cells. However, faim is broadly expressed in various tissues and the faim sequence is highly conserved evolutionarily, suggesting that its role extends beyond lymphocyte homeostasis. As FAIM has no significant regions of homology to other gene products that modulate Fas killing, it appears to represent a distinct, new class of antiapoptotic protein.
Keywords: Fas, apoptosis, B lymphocytes, differential display, gene expression
The Fas/Fas ligand (FasL)1 system is one of an expanding family of receptor–ligand pairs involved in cell fate determination in a variety of cell types, notably including those of lymphoid origin (1). Fas engagement initiates an intracellular signaling cascade through oligomerization of caspase 8, mediated via the adaptor protein FADD, which contains both death and death effector homologous domains (2). Caspase 8 appears to be the most proximal of several ICE-like proteases whose sequential activation is responsible for the apoptotic demise of the cell (3–6). Fas engagement also triggers mitochondrial cytochrome c release that, in conjunction with apoptotic protease-activating factor (APAF)-1 and dATP, activates caspase 9 and downstream caspases (7–11). Fas signaling for cell death may be modulated by several distinct antiapoptotic proteins, which directly bind Fas, inhibit caspase activity or activation, or modulate mitochondrial events (9, 10, 12–21).
We and others have shown that the susceptibility of primary B cells to Fas-mediated apoptosis is regulated in a receptor-specific fashion (22–24). Engagement of CD40 upregulates Fas expression and renders B cells sensitive to Fas-mediated apoptosis, whereas concurrent or sequential signaling through the B cell antigen receptor induces a state of Fas resistance (22). Induction of Fas resistance develops progressively over a period of hours, and depends on macromolecular synthesis, suggesting that protection against Fas killing requires the induction and accumulation of one or more gene products (reference 25 and our unpublished observations). Indeed, we recently demonstrated that Bcl-xL expression is upregulated in primary B cells that have been rendered Fas resistant, and showed that overexpression of Bcl-xL diminishes Fas-mediated apoptosis in CD40L-stimulated B cells obtained from transgenic mice (26). However, a role for other gene products is suggested by three observations. First, Bcl-xL–overexpressing B cells were not completely protected from Fas killing (26). Second, treatment of Bcl-xL–overexpressing B cells with anti-Ig produced additional inhibition of Fas-mediated apoptosis (26). Third, Bcl-xL protein appeared in normal B cells after, not before, the first manifestations of anti-Ig–induced Fas resistance (25, 26). These results raise the possibility that additional gene products, induced by surface (s)Ig cross-linking, contribute to Fas resistance. Herein we describe the use of differential display to elucidate, in an unbiased fashion, inducible factors that modulate susceptibility to Fas-mediated apoptosis in B cells, and report the identification of a gene that, when overexpressed in a model B cell line, reduces Fas sensitivity and thus codes for a Fas apoptosis inhibitory molecule termed FAIM.
Materials and Methods
Mice.
Male Balb/cByJ mice at 8–14 wk of age were obtained from The Jackson Laboratory. Mice were housed at least 1 wk before experimentation. Mice were cared for and handled at all times in accordance with the National Institutes of Health and our institutional guidelines.
B Cell Purification.
Splenic B cells from 8–12-wk-old naive Balb/cByJ mice were purified and depleted of T cells and macrophages as previously described (22). RBCs and nonviable cells were removed by sedimentation over Lympholyte M (Cedarlane Labs., Canada). The resulting B cells were cultured at 37°C with 5% CO2 in RPMI 1640 medium (BioWhittaker) supplemented with 5% heat-inactivated fetal bovine serum (Sigma Chemical Co.), 10 mM Hepes (pH 7.2), 50 μM 2-ME, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin.
Differential Display.
Total RNA was prepared from primary murine splenic B cells stimulated with CD40L/CD8α fusion protein cross-linked with anti-CD8 antibody (CD40L) for 48 h, in the absence or presence of F(ab′)2 fragments of polyclonal goat anti–mouse IgM (anti-Ig) added for the final 6 h of the culture period, using Phenol/GITC (Sigma Chemical Co.; 27). Reverse transcription and differential display were performed as previously described (28) using the RNAImage Kit (GenHunter). Putatively differentially expressed cDNA fragments were excised from dried sequencing gels, eluted in dH2O, and reamplified using the same primer pair originally used in differential display. These fragments were tested for differential expression by Northern blot analysis; PCR products confirmed by this assay were subcloned into a TA cloning vector (Invitrogen). Plasmid DNA from individual clones was radiolabeled and used to probe additional Northern blots in order to identify the insert responsible for differential expression. Subcloned Northern blot–positive cDNAs were subject to automated fluorescent DNA sequencing (Applied Biosystems) and analyzed by comparison to standard sequencing databases in the public domain (BLAST; National Center for Biotechnology Information).
Northern Blotting.
Total RNA was prepared from primary murine splenic B cells using UltraSpec RNA isolation reagent (Biotecx Laboratories). Purified RNA was electrophoresed on a 1% agarose/formaldehyde gel, transferred to GeneScreen Plus (NEN) in 10× SSC and hybridized to a 234-bp radiolabeled fragment of faim generated by PCR, using the primers CTGGATGGCGAGGACCTGAG (5′) and GGTGTCACTGAGTGAGCTCTG (3′). Initial Northern probing to confirm differential expression was performed as above except that differential display primers were used and the annealing step of PCR was performed for 2 min at 40°C. Autoradiography was performed using intensifying screens at −80°C for 1–3 d. A multiple tissue Northern blot was obtained from OriGene Technologies.
cDNA Library Screening.
A radiolabeled probe generated as described above was used to screen a directional murine thymic cDNA library that was constructed in pBKCMV (Stratagene) and was provided by Dr. Adam Lerner (Boston University Medical Center, Boston, MA). Plaque lifts were performed using Protran membranes (Schleicher and Schuell). A number of individual clones from among 106 plaques were sequenced, leading to the isolation of a full-length clone as determined by an in-frame stop codon upstream of the start methionine. This clone encodes a novel 179–amino acid protein as discussed below.
Transfection.
Mid-log phase BAL-17 B lymphoma cells in suspension were transfected with 20 μg _faim_-containing plasmid or pBKCMV empty vector (plus 500 μg carrier salmon sperm DNA) by electroporation at 276 V and 550 μF using a Gene Pulser apparatus (Bio-Rad). Transfected cells were immediately plated to prewarmed medium and cultured at 37°C with 5% CO2 as above (29). After 2 d, transfected cells were split 1:15 into fresh medium containing 2 mg/ml G418 (Sigma Chemical Co.) to obtain pools of transfectants. Separately, individual stably transfected clones were isolated by limiting dilution in medium containing G418.
Fas-mediated Apoptosis.
BAL-17 transfectants stimulated for 24 h with CD40L were tested as targets in standard 4-h, lectin-dependent 51Cr-release assays with AE7 CD4+ Th1 effector cells at effector/target cell ratios of 0.3:1 to 9:1, as previously described (22, 25), or with Jo-2 anti-Fas antibody (PharMingen) at 50, 5, or 0.5 ng/ml. Alternatively, nuclei obtained from CD40L-stimulated BAL-17 transfectants were stained with propidium iodide and the level of subdiploid DNA was determined by flow cytometry, as described (30).
PARP Cleavage.
B cell protein lysates were resolved by 15% SDS-PAGE, transferred to Hybond nitrocellulose membranes (Amersham), and blocked with 5% nonfat dry milk in TBS-Tween 20 (TBS-T) for 1 h at room temperature. Membranes were probed with anti-PARP antibody 2-C-10 (Calbiochem) at 1:500 in TBS-T. After washing, blots were incubated with horseradish peroxidase–conjugated goat anti–mouse IgG and developed by enhanced chemiluminescence (NEN), as previously described (31).
Fas Expression.
B cells were stained with PE-conjugated Jo-2 Fas-specific antibody or anti-TNP isotype control antibody (PharMingen) in the presence of 2% normal rabbit serum and 2.4G2 (anti-FcR) antibody, as previously described (32). Relative fluorescence intensity was detected by flow cytometry with a FACScan® instrument (Becton Dickinson).
FAIM Expression.
Two peptides (amino acids 57–68 and 125–138) corresponding to predicted hydrophilic regions of the FAIM open reading frame (33) were synthesized by Research Genetics Corp. These peptides contain an NH2-terminal cysteine followed by an amino-caproic acid. Each peptide (2 mg) was separately coupled to KLH (Pierce Chemical Co.); the coupled peptides were combined and used to generate anti-FAIM peptide antibodies in chickens (Aves Labs.). B cell protein lysates were resolved by 15% SDS-PAGE, transferred to Hybond nitrocellulose membranes (Amersham), and blocked with 10% Blok-Hen blocking reagent (Aves Labs.) for 1 h at room temperature. The nitrocellulose filters were then probed with FAIM-specific antibodies diluted 1:1,000 in TBS-T containing 10% Blok-Hen for 1.5 h at room temperature. After washing, blots were incubated with horseradish peroxidase–conjugated goat anti–chicken IgY (Aves Labs.) for 1 h and developed by enhanced chemiluminescence (NEN) as described (31).
Reagents.
Affinity purified F(ab′)2 fragments of polyclonal goat anti–mouse IgM were obtained from Jackson ImmunoResearch Labs. and used at 10 μg/ml. Soluble rCD40L was obtained from transfected J558L cells that secrete a chimeric CD40L/ CD8α fusion protein (34), which was collected and dialyzed against 25,000 mol wt cut-off dialysis tubing, as previously described (35). A similarly dialyzed supernatant containing anti-CD8 antibody from the 53-6-72 hybridoma was used to cross-link the fusion protein. CD40L and anti-CD8 containing supernatants were used at final dilutions of 1:10 and 1:80, respectively. G418 was obtained from GIBCO BRL. An expressed sequence tag (EST) encompassing putative human FAIM was obtained from the I.M.A.G.E. consortium (36). 51Cr was obtained from NEN.
Results
In previous work we showed that B cell treatment with anti-Ig for only the final 1–12 h of a 48-h culture with CD40L produced a time-dependent increase in Fas resistance that was abrogated by cycloheximide (25). Additional experiments demonstrated that the induction of Fas resistance in CD40L-stimulated B cells by anti-Ig treatment for 6 h was completely blocked by the addition of actinomycin D (data not shown). These results strongly suggest that transcriptional activation and gene expression are required for the receptor-specific induction of the Fas-resistant state. For this reason, genes that oppose Fas-mediated apoptosis might be captured by identifying transcripts expressed uniquely in Fas-resistant B cells.
Differential Display Detects Transcripts Specific for B Cells Rendered Fas Resistant.
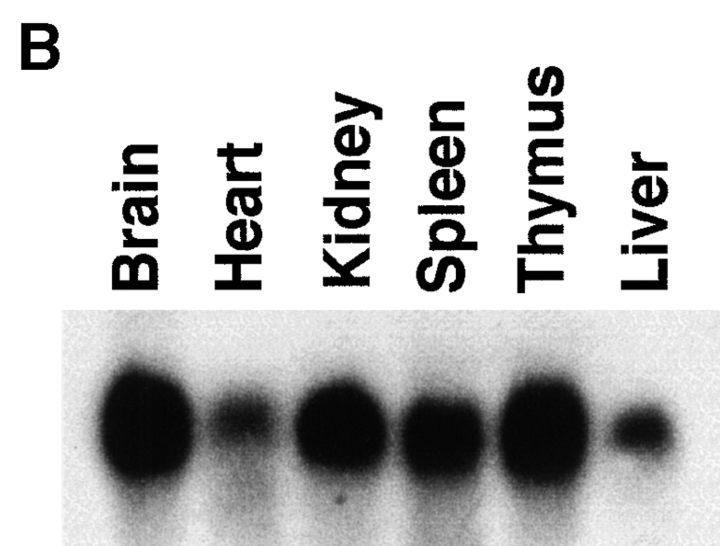
To identify genes expressed coordinately with the induction of Fas resistance, we used a differential display strategy (28). RNA was extracted from B cells stimulated with CD40L alone for 48 h (Fas-sensitive) and from B cells stimulated with CD40L (for 48 h) plus anti-IgM for the final 6 h of culture (Fas-resistant), and was reverse transcribed. Application of arbitrary decameric primer pairs to these cDNA populations permitted reproducible amplification of a number of transcripts present in Fas-resistant B cells but absent in their Fas-sensitive counterparts. These amplified gene fragments were excised and used as probes in Northern blots of RNA obtained from Fas-sensitive and Fas-resistant primary B cells to confirm differential expression (Fig. 1 A). Of 40 such fragments, 8 failed to reamplify. Of the remaining 32, 8 displayed differential expression by Northern blot analysis. One of these recognized an ∼1.2-kb transcript on Northern blot (Fig. 1 A) that was widely expressed in multiple tissues, with the highest levels present in murine brain, thymus, kidney, and spleen (Fig. 1 B). This transcript was chosen for further analysis.
Figure 1.
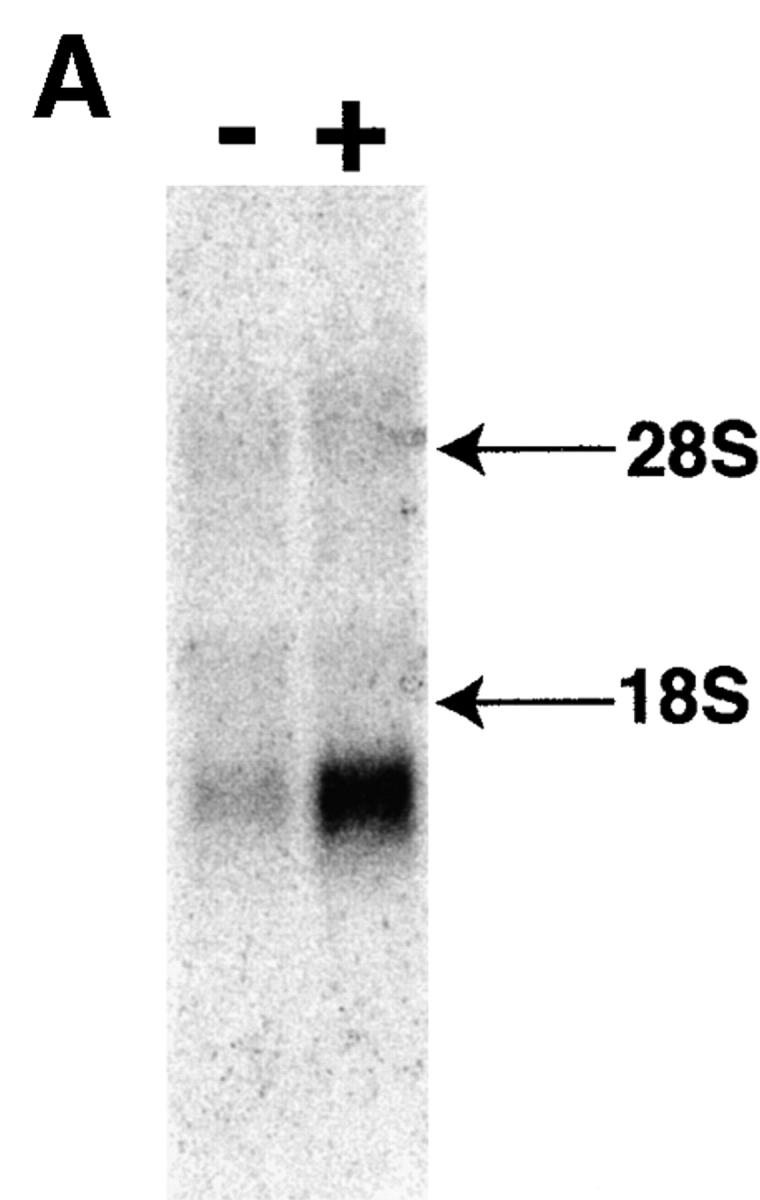
Expression of a gene identified by differential display. (A) Northern blot showing differential expression of an RNA species in Fas-sensitive primary B cells stimulated by CD40L alone (−) and in Fas-resistant B cells stimulated by CD40L plus anti-Ig (+), as described in Materials and Methods. Arrows indicate the location of ribosomal RNA. (B) Northern blot showing expression of an RNA species in various murine tissues, as indicated in figure.
A Differentially Expressed Gene Encodes a Novel Protein.
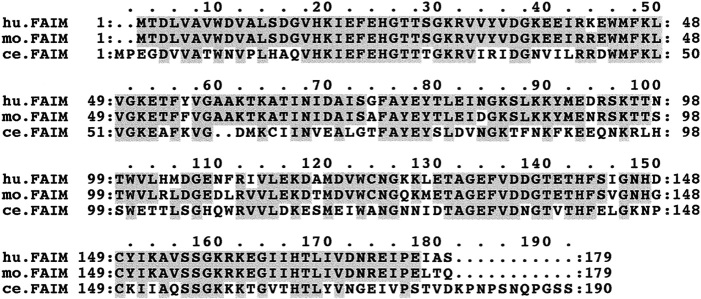
Using a radiolabeled probe generated by PCR, a murine thymic cDNA library was screened and the DNA from positive plaques was sequenced. A number of overlapping clones were identified whose consensus sequence was ∼1.2 kb, consistent with the expression data described above. Subsequently a full-length clone was identified that contained an in-frame STOP codon upstream of the START methionine, and possessed, in the 3′ UTR, an RNA instability motif, polyA+ consensus motifs and a polyA+ tail (37). This cDNA appeared to encode a novel 179–amino acid open reading frame (Fig. 2). Structural analysis predicted a β-strand–rich, stable, soluble protein with a slightly acidic pI (pH 5.4). No substantial regions of homology with any other sequence are present.
Figure 2.
Predicted amino acid sequence of a gene, differentially expressed in Fas-sensitive and Fas-resistant B cells, that encodes a Fas apoptosis inhibitory molecule (FAIM), and comparison of FAIM protein sequences from mouse, human, and C. elegans. Protein sequences were predicted from nucleotide sequences. Amino acids are numbered to the left and right of each sequence. Identical amino acids are shaded. Gaps incorporated into the C. _elegan_s sequence to optimize alignment are indicated by dots. Nucleotide sequence data are available from EMBL/GenBank/DDBJ under accession No. AF130367.
A Full Length Clone Functions as a Fas Apoptosis Inhibitory Molecule (FAIM).
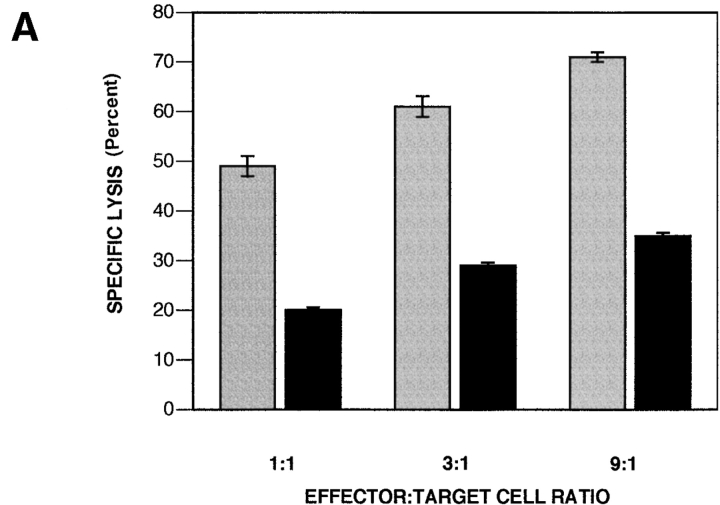
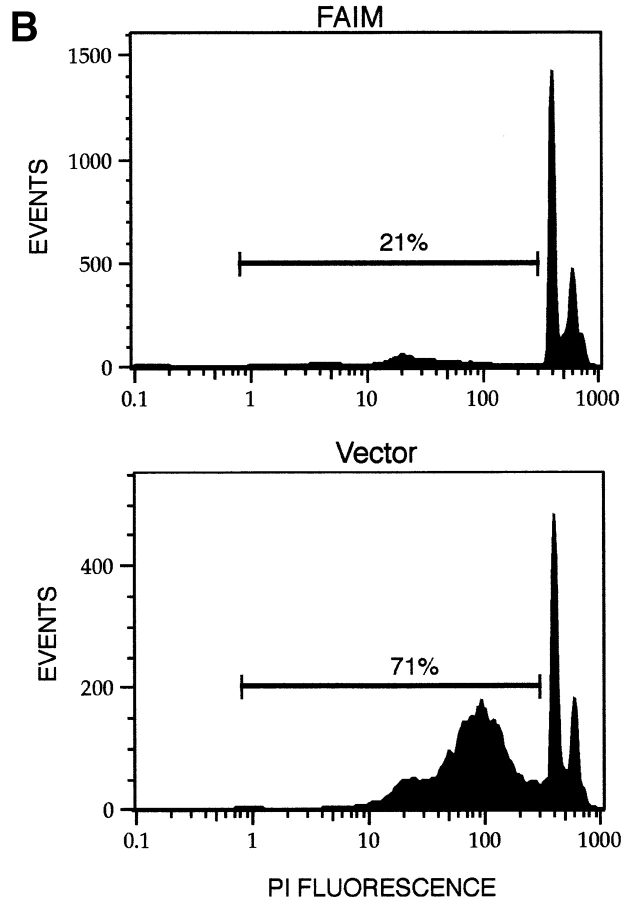
To determine the capacity of the isolated cDNA clone to produce resistance to Fas-mediated apoptosis, BAL-17 murine B lymphoma cells were transfected with the pBKCMV expression vector. BAL-17 cells were chosen because their activation responses mimic primary B cells in a variety of ways and they are readily transfectable (38–40). Like primary B cells, unstimulated BAL-17 B cells express little Fas, but treatment with CD40L induces Fas expression and sensitivity to Fas-mediated apoptosis (data not shown). After electroporation, pools of BAL-17 B cells stably transfected with either full-length cDNA or empty vector were selected in G418 for 2 wk. These two populations differed in their susceptibility to Fas killing induced by FasL-bearing Th1 effector cells: at each effector/target cell ratio tested, specific lysis of cDNA-transfected BAL-17 B cells, detected by Cr-release assay, was reduced by half or more in comparison to cells transfected with vector alone (Fig. 3 A), despite equivalent levels of surface Fas expression (data not shown). These results suggest a level of protection of ninefold or more, in terms of the effector/target ratio required to produce equivalent levels of apoptosis in _faim_- and vector-transfected BAL-17 B cells. The reduction in Fas killing was also apparent when cytotoxicity was induced by lytic Jo-2 anti-Fas antibody (data not shown). In addition, stably transfected clones were isolated by limiting dilution and tested for susceptibility to Fas killing. The results obtained with individual clones completely mimicked those obtained with G418-resistant pools in that Fas-mediated apoptosis (produced by Jo-2) was reduced by one-half to two-thirds in stably transfected cDNA-expressing BAL-17 B cells in comparison to BAL-17 cells transfected with empty vector, as detected by propidium iodide staining for subdiploid DNA (Fig. 3 B). These data indicate that the novel cDNA transcript initially identified in inducibly Fas-resistant B cells codes for a Fas apoptosis inhibitory molecule (FAIM) that counteracts Fas signaling for cell death when overexpressed.
Figure 3.
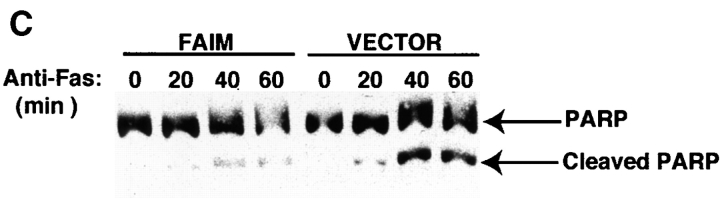
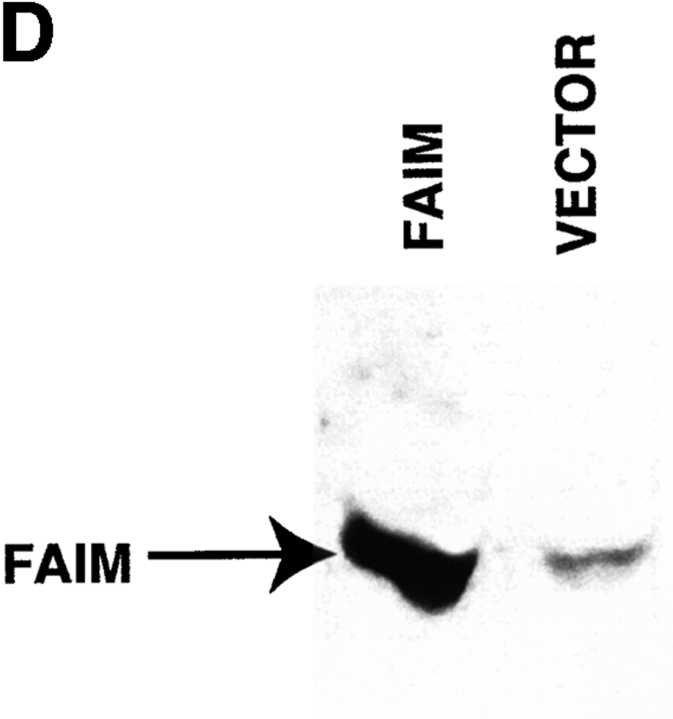
Modulation of Fas-mediated apoptosis in BAL-17 B cells transfected with faim. (A) Lysis of CD40L-stimulated _faim_- (black bars) and vector- (gray bars) transfected BAL-17 cells produced by FasL-bearing CD4+ Th1 AE7 effector cells at various effector/target cell ratios, as indicated. Levels of specific target cell lysis were assessed by 51Cr-release assay. One of five comparable experiments is shown. (B) DNA fragmentation in CD40L-stimulated _faim_- and vector-transfected BAL-17 cells produced by Jo-2 lytic anti-Fas antibody, as indicated. Relative levels of subdiploid DNA were assessed by propidium iodide staining and flow cytometric analysis. One of four comparable experiments is shown. (C) PARP cleavage in CD40L-stimulated _faim_- and vector-transfected BAL-17 cells produced by treatment by Jo-2 anti-Fas antibody for 20–60 min as indicated. PARP was detected by Western blotting size-separated whole cell lysates. Arrows indicate native PARP and cleaved PARP. One of three comparable experiments is shown. (D) FAIM expression in _faim_- and vector-transfected BAL-17 cells. FAIM was detected by Western blotting size-separated whole cell lysates. An arrow indicates the location of FAIM protein. One of three comparable experiments is shown.
FAIM Expression Blocks PARP Cleavage.
To characterize the nature of the FAIM-induced block in Fas signaling for cell death, the fate of PARP, a terminal caspase cleavage product, was examined by Western blot of size-separated whole cell extracts (41). Proteolytic fragments of PARP were readily detected when vector-transfected BAL-17 B cells were treated for 40 min with Jo-2 anti-Fas antibody. In contrast, there was little or no PARP cleavage in _faim_-transfected BAL-17 B cells up to 60 min after anti-Fas treatment (Fig. 3 C). Thus, FAIM blocks Fas apoptosis at a step proximal to the cleavage of the caspase substrate, PARP.
As a control for these experiments, FAIM expression in _faim_- and vector-transfected BAL-17 B cells was determined by Western blotting with polyclonal anti-FAIM antibody. Antibody was prepared by immunizing chickens with two relatively hydrophilic peptides derived from the FAIM sequence (amino acids 57–68, DGKEEIRREWMF; and 125–138, RLDGEDLRVVLEKD) coupled to KLH; the resultant antibody (purified from the IgY fraction of egg yolks) specifically recognized a protein of the expected size, ∼20 kD, on Western blot, whereas pre-immune IgY did not. Using this antibody, the expression of FAIM protein was found to be much increased in _faim_-transfected, as opposed to vector-transfected, BAL-17 B cells (Fig. 3 C).
faim Expression Correlates Well with Inducible Fas Resistance.
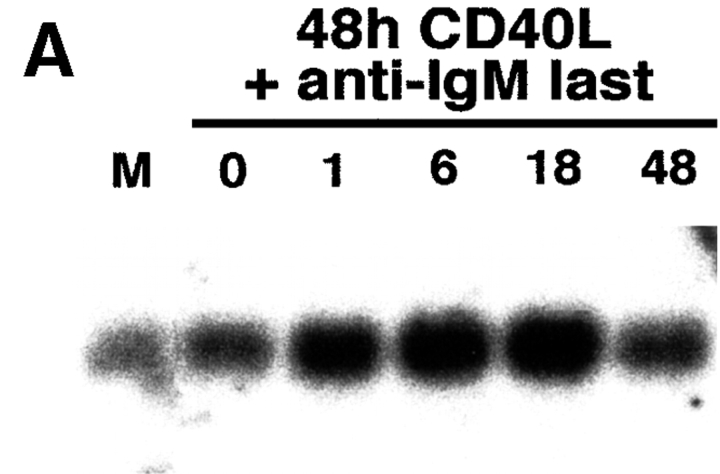
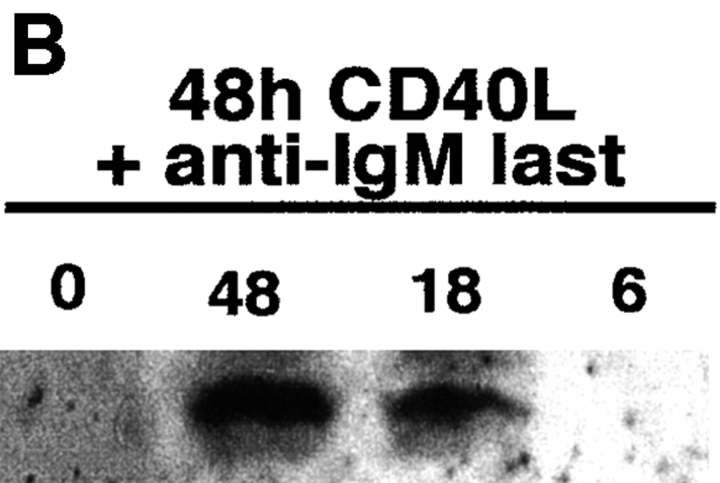
To further evaluate the association between FAIM expression and inducible Fas resistance, primary B cells were studied by Northern and Western blotting after stimulation with CD40L and anti-Ig. CD40L stimulation alone, which induces Fas expression and Fas sensitivity, elicited little or no increase in faim expression over the low basal level present in unstimulated B cells. However, addition of anti-IgM, which induces Fas resistance, to CD40L-stimulated B cells produced a marked, time-dependent increase in faim mRNA, beginning at 1 h and reaching a maximum after 6 h of anti-Ig treatment (Fig. 4 A). Similar results were obtained when the expression of FAIM protein was monitored. FAIM was absent in unstimulated B cells and B cells stimulated for 48 h with CD40L alone; however, addition of anti-IgM to CD40L-stimulated B cells produced a marked increase in FAIM protein, first seen after 18 h of anti-Ig treatment (Fig. 4 B). In some experiments FAIM protein expression was detected after 6 h of anti-Ig treatment (data not shown).
Figure 4.
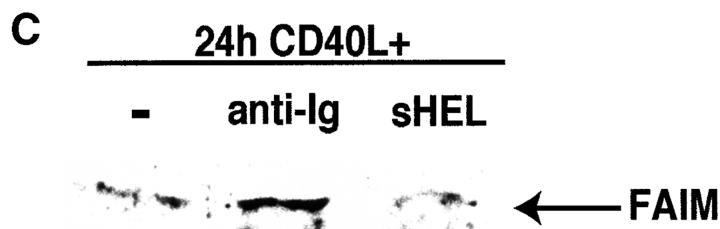
Expression of faim/ FAIM in Fas-resistant primary B cells. (A) Northern blot showing faim gene expression in primary B cells that were unstimulated (M), were stimulated by CD40L alone for 48 h (0), or were stimulated by CD40L for 48 h plus anti-Ig added for the last 1, 6, 18, or 48 h of culture, as indicated. One of four comparable experiments is shown. (B) Western blot showing FAIM protein expression in primary B cells that were stimulated by CD40L alone for 48 h (0), or were stimulated by CD40L for 48 h plus anti-Ig added for the last 6, 18, or 48 h of culture, as indicated. One of three comparable experiments is shown. (C) Western blot showing FAIM protein expression in primary B cells from double transgenic, anti-HEL/ HEL mice that were stimulated by CD40L alone for 24 h (−), or were stimulated by CD40L in combination with either anti-Ig or sHEL, as indicated. One of three comparable experiments is shown.
The correlation between sIg-induced FAIM expression and Fas resistance was tested further by examining tolerant, autoreactive B cells obtained from double transgenic, anti– hen egg lysozyme (HEL)/HEL mice. In these B cells, in vitro studies showed that specific antigen (soluble [s]HEL) is an insufficient stimulus to produce Fas resistance, whereas Fas resistance is induced by more extensive sIg cross-linking with anti-Ig (42). In keeping with this, sHEL failed to induce upregulation of FAIM protein expression in B cells drawn from double transgenic anti-HEL/HEL mice, whereas FAIM protein was induced by anti-Ig in these B cells (Fig. 4 C). In this situation, as with anti-Ig–treated B cells from normal mice, above, induction of FAIM expression correlates with production of Fas resistance.
faim Is Evolutionarily Conserved.
To evaluate the possibility that faim is phylogenetically conserved, public databases were searched for evidence of similar genes in other species. Human faim was obtained by identifying a consensus sequence from overlapping human ESTs with homology to mouse faim, followed by sequencing of a single EST clone that completely spanned putative human faim (36). The consensus/EST sequence was used to predict an amino acid sequence, which showed human FAIM to be 90% identical to the predicted amino acid sequence of mouse FAIM (see Fig. 2). These results are complemented by Southern blot analysis of genomic DNA showing hybridization by a mouse faim probe to all mammalian species tested (human, monkey, rat, mouse, dog, cow, and rabbit; data not shown).
C. elegans FAIM was obtained by amplifying cDNA with primers based on the predicted exon structure of a random genomic sequence of unknown function, and then sequencing the resultant DNA. The predicted amino acid sequence of this C. elegans FAIM is 50% identical to the predicted amino acid sequence of mouse FAIM (see Fig. 2). The extensive evolutionary conservation manifest in the sequences of human, mouse, and C. elegans faim strongly suggests that the faim gene product is a key apoptotic regulatory molecule that has been retained with minimal change throughout phylogeny.
Discussion
Fas engagement contributes to cell fate determination in a variety of cell types, and plays an important role in modulating immune responses and regulating autoreactive B lymphocytes (23, 42). The results reported here identify a novel gene, isolated by differential display (28) and termed faim, that opposes Fas-mediated apoptosis when overexpressed in a model B cell line, and whose expression in primary B cells is coordinately regulated with sIg signals that block Fas killing. Thus, FAIM is an inducible effector molecule that mediates Fas resistance produced in B cells by sIg engagement, and does so by blocking a step in the Fas signaling pathway before the activation of caspase 3, an effector caspase responsible for PARP cleavage (41). The influence of FAIM appears to be specific to Fas-induced cell death, because BAL-17 B cells stably transfected with faim are not more resistant to apoptosis produced by staurosporine or ultraviolet radiation than vector-transfected controls (data not shown). As FAIM bears no regions of homology with other gene products that modulate Fas killing, it appears to represent a distinct new class of antiapoptotic protein.
Overexpression of FAIM in stably transfected BAL-17 B cells produced substantial, but less than complete, resistance against Fas-mediated apoptosis, and was less effective in blocking Fas killing than optimal sIg signaling in primary B cells. This suggests that receptor-mediated Fas resistance in primary B cells cannot be explained on the basis of FAIM induction alone. Recently we reported that Bcl-xL is induced in primary B cells after sIg engagement that produces Fas resistance, and that the Fas sensitivity of CD40L-stimulated B cells from Bcl-xL overexpressing transgenic mice was substantially reduced compared with littermate control B cells (26). These results infer a key role for Bcl-xL in sIg-mediated Fas resistance. However, further inhibition of susceptibility to Fas-mediated apoptosis in Bcl-xL transgenic B cells was produced by anti-Ig treatment, implicating additional antiapoptotic factors (26). Thus, sIg-triggered Fas resistance may result, at least in part, from the combined effects of inducible FAIM and Bcl-xL expression. In support of this, Fas-resistant faim transfectants express no more Bcl-xL than do vector-transfected controls (data not shown), so there is no direct linkage between FAIM and Bcl-xL, the only other antiapoptotic gene presently implicated in B cell Fas resistance. Thus, inducible resistance against Fas is multigenic in nature. Although FAIM and Bcl-xL may inhibit separate and distinct intracellular pathways leading from Fas to terminal apoptotic events (43), the possibility that these antiapoptotic molecules interfere with the same death mediators has not been ruled out. Notably, the multigenicity of inducible protection against Fas killing suggests the intricacy of regulatory controls asserted over apoptotic death by environmental cues.
FAIM shares no regions of homology with known antiapoptotic proteins and contains no known protein–protein interaction motifs, implying that an as yet unidentified structure is responsible for its antiapoptotic function. Preliminary results suggest that the activity of this structure is broad and crosses species boundaries, in that human HeLa cells transiently transfected with mouse faim display Fas resistance in comparison to vector-transfected controls (data not shown). Moreover, FAIM-like genes are expressed in other species, including C. elegans. In fact, the degree of amino acid identity between C. elegans and mouse FAIM (50%) is much greater than that between C. elegans ced-3 and mouse ICE, and between C. elegans ced-9 and mouse bcl-2 (both <25%), two pairs of genes with accepted similar functions related to apoptosis (44, 45). This remarkably high degree of evolutionary conservation across a broad phylogenetic chasm suggests that FAIM plays a key role in cellular physiology. Furthermore, three specific regions of FAIM, encompassing 21, 17, and 17 amino acids, respectively, exceed 75% identity between mouse, human, and C. elegans FAIM; one or more of these regions may well be indispensable to the antiapoptotic function of FAIM.
Acknowledgments
This work was supported by United States Public Health Service grant AI40181 awarded by the National Institutes of Health, and by a grant from the Arthritis Foundation.
Abbreviations used in this paper
EST
expressed sequence tag
FAIM
Fas apoptosis inhibitory molecule
FasL
Fas ligand
HEL
hen egg lysozyme
PARP
poly-ADP ribose polymerase
sIg
surface Ig
References
- 1.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 2.Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:502–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 3.Boldin MP, Goncharov TM, Goltsev YV, Wallach D. Involvement of MACH, a novel MORT1/ FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 4.Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/ APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 5.Srinivasula SM, Ahmad M, Hernandes-Alnemri T, Litwack G, Alnemri ES. Molecular ordering of the Fas-apoptotic pathway: The Fas/APO-1 protease Mch5 is a CrmA-inhibitable protease that activates multiple Ced-3/ ICE-like cysteine proteases. Proc Natl Acad Sci USA. 1996;93:14486–14491. doi: 10.1073/pnas.93.25.14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medema JP, Scaffidi C, Kischkel FC, Shevchenko A, Mann M, Krammer PH, Peter ME. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC) EMBO (Eur Mol Biol Organ) J. 1997;16:2794–2804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 8.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 9.Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng T, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 10.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 11.Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegansCED-4, participates in cytochrome c-dependent activation of caspase 3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 12.Jaattela M, Benedict M, Tewari M, Shayman JA, Dixit VM. Bcl-x and Bcl-2 inhibit TNF and Fas- induced apoptosis and activation of phospholipase A2 in breast carcinoma cells. Oncogene. 1995;10:2297–2305. [PubMed] [Google Scholar]
- 13.Hu W, Vincenz C, Ni J, Gentz R, Dixit VM. I-FLICE, a novel inhibitor of tumor necrosis factor receptor-1- and CD95-induced apoptosis. J Biol Chem. 1997;272:17255–17257. doi: 10.1074/jbc.272.28.17255. [DOI] [PubMed] [Google Scholar]
- 14.Irmler M, Thome M, Hahne M, Schneider P, Hofman K, Steiner V, Bodmer JL, Schroter M, Burns K, Mattmann C, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 15.Okura T, Gong L, Kamitani T, Wada T, Okura I, Wei C-F, Chang H-M, Yeh ETH. Protection against Fas/APO-1- and tumor necrosis factor-mediated cell death by a novel protein, Sentrin. J Immunol. 1996;157:4277–4281. [PubMed] [Google Scholar]
- 16.Koseki T, Inohara N, Chen S, Nunez G. ARC, an inhibitor of apoptosis expressed in skeletal muscle and heart that interacts selectively with caspases. Proc Natl Acad Sci USA. 1998;95:5156–5160. doi: 10.1073/pnas.95.9.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider TJ, Grillot D, Foote LC, Nunez GE, Rothstein TL. Bcl-x protects primary B cells against Fas-mediated apoptosis. J Immunol. 1997;159:4834–4839. [PubMed] [Google Scholar]
- 18.Roy N, Deveraux QL, Takahashi R, Salvesen GS, Reed JC. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO (Eur Mol Biol Organ) J. 1997;16:6914–6925. doi: 10.1093/emboj/16.23.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi R, Deveraux Q, Tamm I, Welsh K, Assa-Munt N, Salvesen GS, Reed JC. A single BIR domain of XIAP sufficient for inhibiting caspases. J Biol Chem. 1998;273:7787–7790. doi: 10.1074/jbc.273.14.7787. [DOI] [PubMed] [Google Scholar]
- 20.Lacana E, Ganjei JK, Vito P, D'Adamio L. Dissociation of apoptosis and activation of IL-1β-converting enzyme/Ced-3 proteases by ALG-2 and the truncated Alzheimer's gene ALG-3. . J Immunol. 1997;158:5129–5135. [PubMed] [Google Scholar]
- 21.Yasumichi H, Lorens J, Kitada S-I, Fisher J, LaBarge M, Ring HZ, Francke U, Reed JC, Kioshita S, Nolan GP. Toso, a cell surface, specific regulator of Fas- induced apoptosis in T cells. Immunity. 1998;8:461–471. doi: 10.1016/s1074-7613(00)80551-8. [DOI] [PubMed] [Google Scholar]
- 22.Rothstein TL, Wang JKM, Panka DJ, Foote LC, Wang Z, Stanger B, Cui H, Ju S-T, Marshak-Rothstein A. Protection against Fas-dependent Th1-mediated apoptosis by antigen receptor engagement in B cells. Nature. 1995;374:163–165. doi: 10.1038/374163a0. [DOI] [PubMed] [Google Scholar]
- 23.Rathmell JC, Townsend SE, Xu JC, Flavell RA, Goodnow CC. Expansion or elimination of B cells in vivo: dual roles for CD40- and Fas (CD95)-ligands modulated by the B cell antigen receptor. Cell. 1996;87:319–329. doi: 10.1016/s0092-8674(00)81349-5. [DOI] [PubMed] [Google Scholar]
- 24.Lagresle C, Mondiere P, Bella C, Krammer PH, Defrance T. Concurrent engagement of CD40 and the antigen receptor protects naive and memory human B cells from APO-1/Fas-mediated apoptosis. J Exp Med. 1996;183:1377–1388. doi: 10.1084/jem.183.4.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foote LC, Schneider TJ, Fischer GM, Wang JKM, Rasmussen B, Campbell KA, Lynch DH, Ju S-T, Marshak-Rothstein A, Rothstein TL. Intracellular signaling for inducible antigen receptor-mediated Fas resistance in B cells. J Immunol. 1996;157:1878–1885. [PubMed] [Google Scholar]
- 26.Schneider TJ, Grillot D, Foote LC, Fischer GM, Nunez G, Rothstein TL. Bcl-x protects primary B cells against Fas-mediated apoptosis. J Immunol. 1997;159:4384–4389. [PubMed] [Google Scholar]
- 27.Xie W, Rothblum LI. Rapid, small-scale RNA isolation from tissue culture cells. Biotechniques. 1993;11:326–327. [PubMed] [Google Scholar]
- 28.Liang P, Pardee A. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992;257:967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- 29.Chu G, Hayakawa H, Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987;15:1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 31.Karras JG, Wang Z, Coniglio SJ, Frank DA, Rothstein TL. Antigen-receptor engagement in B cells induces nuclear expression of STAT5 and STAT6 proteins that bind and transactivate an IFN-γ activation site. J Immunol. 1996;157:39–47. [PubMed] [Google Scholar]
- 32.Foote LC, Howard RG, Marshak-Rothstein A, Rothstein TL. IL-4 induces Fas resistance in B cells. J Immunol. 1996;157:2749–2753. [PubMed] [Google Scholar]
- 33.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 34.Lane P, Brocker T, Hubele S, Padovan E, Lanzavecchia A, McConnell F. Soluble CD40 ligand can replace the normal T cell–derived CD40 ligand signal to B cells in T cell–dependent activation. J Exp Med. 1993;177:1209–1213. doi: 10.1084/jem.177.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Francis DA, Karras JG, Ke X-Y, Sen R, Rothstein TL. Induction of the transcription factors NF-κB, AP-1 and NF-AT during B cell stimulation through the CD40 receptor. Int Immunol. 1995;7:151–161. doi: 10.1093/intimm/7.2.151. [DOI] [PubMed] [Google Scholar]
- 36.Lennon GG, Auffray C, Polymeropoulos M, Soares MB. The I.M.A.G.E. consortium: an integrated molecular analysis of genomes and their expression. Genomics. 1996;33:151–152. doi: 10.1006/geno.1996.0177. [DOI] [PubMed] [Google Scholar]
- 37.Malter JS. Identification of an AUUUA-specific messenger RNA binding protein. Science. 1989;246:664–666. doi: 10.1126/science.2814487. [DOI] [PubMed] [Google Scholar]
- 38.Chiles TC, Liu J, Rothstein TL. Cross-linking of surface Ig receptors on murine B lymphocytes stimulates the expression of nuclear tetradecanoyl phorbol acetate- response element binding-proteins. J Immunol. 1991;146:1730–1735. [PubMed] [Google Scholar]
- 39.Mizuguchi J, Yong-Yong J, Nakabayaschi H, Huang K-P, Beaven MA, Chused T, Paul WE. Protein kinase C activation blocks anti-IgM-mediated signaling in BAL-17 B lymphoma cells. J Immunol. 1987;139:1054–1059. [PubMed] [Google Scholar]
- 40.Seyfert VL, McMahon S, Glenn W, Cao X, Sukhatme VP, Monroe JG. Egr-1 expression in surface Ig-mediated B cell activation: kinetics and association with protein kinase C activation. J Immunol. 1990;145:3547–3553. [PubMed] [Google Scholar]
- 41.Tewari M, Quan LT, O'Rourke K, Desnoyers S, Zeng Z, Beidler DR, Poirier GG, Salvesen GS, Dixit VM. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- 42.Foote LC, Marshak-Rothstein A, Rothstein TL. Tolerant B lymphocytes acquire resistance to Fas- mediated apoptosis after treatment with interleukin 4 but not after treatment with specific antigen unless a surface immunoglobulin threshold is exceeded. J Exp Med. 1998;6:847–853. doi: 10.1084/jem.187.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. EMBO (Eur Mol Biol Organ) J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan J, Shaham S, Ledoux S, Ellis HM, Horvitz HR. The C. eleganscell death gene ced-3 encodes a protein similar to mammalian interleukin-1β-converting enzyme. Cell. 1993;75:641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- 45.Hengartner MO, Horvitz HR. C. eleganscell survival gene ced-9 encodes a functional homolog of the mammalian proto-oncogene bcl-2. Cell. 1994;76:665–676. doi: 10.1016/0092-8674(94)90506-1. [DOI] [PubMed] [Google Scholar]