Inhibition of Hepatitis B Virus Replication during Schistosoma mansoni Infection in Transgenic Mice (original) (raw)
Abstract
Although coinfection of hepatitis B virus (HBV) and Schistosoma mansoni is a frequent event in humans, little is known about the interactions between these two pathogens. S. mansoni infection induces T helper cell type 2 (Th2)–type cytokines in the liver of humans and mice. The intrahepatic induction of nitric oxide (NO) and Th1-type cytokines, such as interferon (IFN)-γ and IFN-α/β, inhibits HBV replication noncytopathically in the liver of transgenic mice. To examine whether S. mansoni infection and the accompanying induction of Th2-type cytokines could interfere with HBV replication in the liver, HBV transgenic mice were infected with S. mansoni. By 5 wk after infection, HBV replication disappeared concomitant with the intrahepatic induction of NO and Th1-type cytokines, and in the absence of Th2-type cytokines. By 6–8 wk after infection, HBV replication remained undetectable and this was associated with further induction of NO and Th1-type cytokines together with the appearance of Th2-type cytokines. The S. _mansoni_–dependent antiviral effect was partially blocked by genetically deleting IFN-γ, although it was unaffected by deletion of IFN-α/β. These results indicate that IFN-γ (probably via NO) mediates most of this antiviral activity and that Th2-type cytokines do not counteract the antiviral effect of IFN-γ. Similar events may suppress HBV replication during human S. mansoni infection.
Keywords: transgenic/knockout, infectious immunity virus, helminth parasites, Th1/Th2 cytokines, liver
Introduction
Hepatitis B virus (HBV) is a noncytopathic, double-stranded DNA virus that causes acute and chronic hepatitis and hepatocellular carcinoma in humans 1. It is widely believed that the cytotoxic T cell (CTL) response to the virus plays a critical role in viral clearance and liver disease. Indeed, the CTL response is strong, polyclonal, and multispecific during acute HBV infection, but is usually undetectable in patients with chronic hepatitis 1. Using a transgenic mouse model, we have shown recently that the antiviral potential of the CTLs is primarily mediated by noncytolytic mechanisms that involve the intrahepatic production of Th1-type cytokines such as IFN-γ 2 3. We also showed that nitric oxide (NO) mediates most of the antiviral activity of IFN-γ produced by the CTLs and that IFN-α/β produced in the liver during unrelated hepatotropic virus infections inhibits HBV replication via NO-independent pathways 3 4 5 6. Noncytopathic antiviral mechanisms like these can contribute to viral clearance during acute viral hepatitis in chimpanzees, thus confirming the transgenic mouse studies in a natural infection model 7. All these events occur in the liver in the absence of Th2-type cytokines such as IL-4, IL-5, and IL-10. Whether the outcome of HBV infection in humans (viral clearance versus viral persistence) is influenced by qualitative differences in the intrahepatic cytokine profile (Th1-type versus Th2-type) is not known.
Schistosoma mansoni is a helminth parasite (class Trematoda) that infects both humans and mice. Schistosomiasis affects more than 200 million people worldwide, particularly in Africa, Asia, and South America, areas in which chronic HBV infection is endemic. Schistosomiasis is largely dependent on the abundance of the snail intermediate host. Infection begins when cercariae shed into the water by infected snails penetrate the skin of individuals exposed to contaminated water. After penetration, the larvae enter the microcirculation and reach the hepatic portal system where they remain. After several weeks of infection, the female worm deposits a large number of eggs, leading to the formation of granulomas in the liver 8. The pathogenesis of egg granuloma formation involves a Th2-type cytokine response that begins few days after egg laying with production of increasing levels of IL-4, IL-5, and IL-10 by Th2 cells, eosinophils, and basophils 8 9. In experimentally infected mice, the Th2-type cytokine response peaks on week 8 after infection, and this process is thought to downregulate the secretion of Th1-type cytokines (including TNF-α and IFN-γ) that peaks few weeks earlier (weeks 4–5) 8 9. This shift in cytokine profile may initiate the chronic stage of infection and contribute to the amelioration of liver pathology by inhibiting the continuous production of potentially harmful inflammatory mediators 8 9.
Although coinfection of HBV and S. mansoni is a frequent event in several geographic areas of the world, very little is known about the possible interactions between these two pathogens. In this study we took advantage of transgenic mice that replicate HBV at high levels in the liver to directly examine whether S. mansoni infection and the accompanying induction of Th2-type cytokines could modulate HBV replication. Thus, we monitored HBV replication in HBV transgenic mice that were infected with S. mansoni and we compared these results with those obtained in S. _mansoni_–infected HBV transgenic mice genetically deficient for IFN-γ or IFN-α/β receptor.
Materials and Methods
HBV Transgenic Mice.
HBV transgenic mice from lineage 1.3.32 (inbred C57BL/6, official designation Tg[HBV 1.3 genome]Chi32) and lineage 1.3.46 (inbred B10D2, official designation Tg [HBV 1.3 genome]Chi46) were used in this study. Lineages 1.3.32 and 1.3.46 replicate high levels HBV in their livers without any evidence of cytopathology, as previously described 10. Lineage 1.3.46 was backcrossed against knockout mice that lack IFN-γ (IFN-γ2/−) 11 and the IFN-α/β receptor (IFN-α/βR−/−) 12, as previously described 3. The knockout mice were provided by Drs. Timothy Stewart (IFN-γ2/−) and Michel Aguet (IFN-α/βR−/−) at Genentech, South San Francisco, CA. The genetic background of the original parental lineages of IFN-γ2/− and IFN-α/βR−/− were 129/Sv/Ev/ × C57BL/6. The IFN-γ2/− mice were backcrossed four to five generations against BALBc and the IFN-α/βR−/− mice were backcrossed more than five generations against C57BL/6 before they were mated with lineage 1.3.46 (inbred B10D2). F1 progeny were interbred to yield hepatitis B e antigen-positive (HBeAg) F2 progeny that were either homozygous or heterozygous for the null mutation. In all experiments, the mice were matched for age (8 wk), sex (male), and HBeAg levels in their serum before experimental manipulations. All animals were housed in pathogen-free rooms under strict barrier conditions.
Infection of Mice with S. mansoni.
8-wk-old HBV transgenic male mice were infected by percutaneous exposure of tail skin for 60 min in water containing 20–25 cercariae, as previously described 13. Mice were killed at different weeks after infection and their livers were harvested for histological and histochemical analyses, or they were snap frozen in liquid nitrogen and stored at −80°C for subsequent molecular analyses (see below).
Tissue DNA and RNA Analyses.
Frozen liver tissue was mechanically pulverized under liquid nitrogen and total genomic DNA and RNA were isolated for Southern and Northern blot analyses exactly as previously described 10. Nylon membranes were analyzed for HBV DNA, 2′5′-oligoadenylate synthetase (2′5′-OAS) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as previously described 4. Quantitation of inducible NO synthase (iNOS), cytokine, and T lymphocyte, macrophage, and NK cell marker mRNAs was performed by RNase protection assay exactly as previously described 6. The relative abundance of specific DNA and RNA molecules was quantitated by phosphorimaging analysis, using the Optiquant™ image analysis software (Packard Instrument Co.).
Biochemical, Histological, and Immunohistochemical Analyses.
Measuring serum alanine aminotransferase (sALT) activity at multiple time points after infection with S. mansoni monitored the extent of hepatocellular injury. sALT activity was measured in a Paramax chemical analyzer (Baxter Diagnostics Inc.), exactly as previously described 14. For histological analysis, liver tissue samples were fixed in 10% zinc-buffered formalin (Anatech), embedded in paraffin, sectioned (3 μm), and stained with hematoxylin and eosin exactly as described elsewhere 14. The intracellular distribution of hepatitis B core antigen (HBcAg) was assessed by the Labeled Avidin-Biotin (LAB; Dako) detection procedure exactly as previously described 14.
Results and Discussion
Inhibition of HBV Replication during S. mansoni Infection.
To determine whether S. mansoni infection could modulate HBV replication in the liver of transgenic mice, four groups (six mice per group) of age- (8 wk), sex- (male), and serum HBeAg–matched transgenic mice from lineage 1.3.32 (inbred C57BL/6) were exposed to water containing cercariae and killed on weeks 5, 6, 7, and 8 after exposure. All animals were successfully infected as indicated by the presence of adult worms, eggs, and granulomas in the liver (see below).
Since each mouse in each group of six mice showed identical results, two representative mice per group are shown in Fig. 1. On week 5 after infection, HBV DNA replicative forms completely disappeared from the liver of transgenic mice when compared with saline-injected controls (Fig. 1). At this time point, the messages for the T cell markers CD3, CD4, and CD8, the macrophage marker F4-80, and the NK cell marker NK1.1 were induced, indicating that granulomas were already present in the liver, as confirmed by histological analysis (data not shown). Despite the presence of granulomas, no hepatocellular lysis was observed at this time, as underscored by the absence of serum ALT elevation (Fig. 1, bottom). The intrahepatic cytokine profile was markedly directed towards a type 1 response, as indicated by the induction of iNOS, TNF-α, IL-1-α, IFN-γ, IL-1-β, and TGF-β. Furthermore, the messages for Th2-type cytokines were either not (IL-4 and IL-5) or barely (IL-10) induced (Fig. 1).
Figure 1.
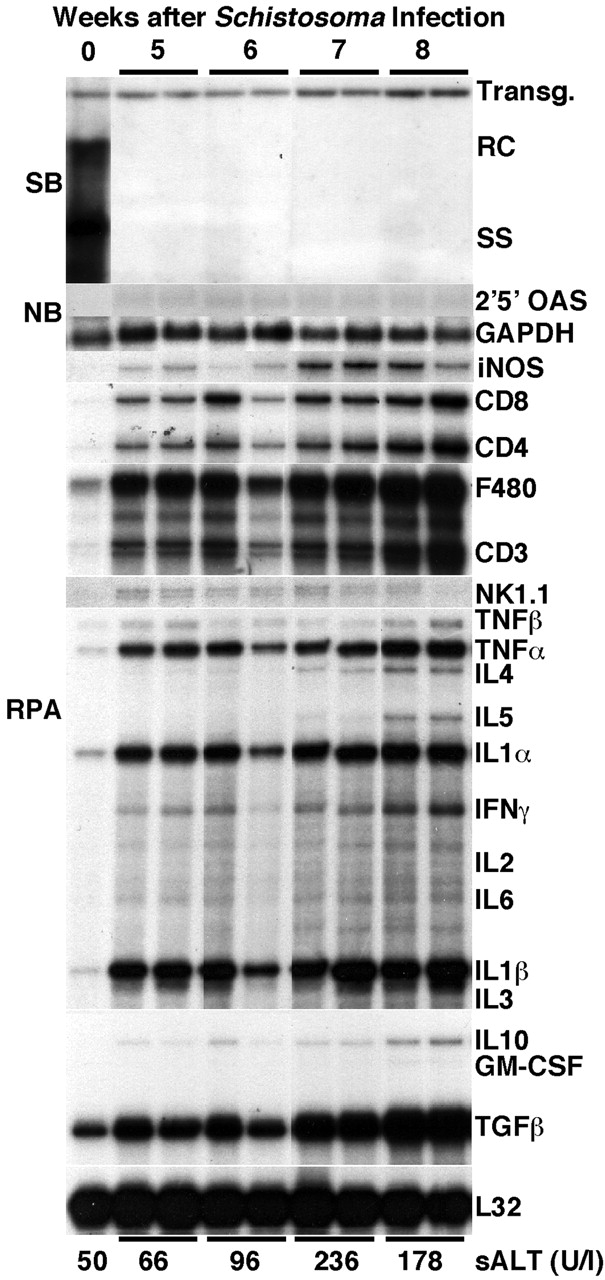
Inhibition of HBV replication during S. mansoni infection. Age-, sex-, and serum HBeAg–matched transgenic mice from lineage 1.3.32 were exposed to water containing cercariae and killed on weeks 5, 6, 7, and 8 after exposure. Total hepatic DNA was analyzed for HBV DNA by Southern blot (SB) analysis. All DNA samples were RNAse treated before gel electrophoresis. Bands corresponding to the integrated transgene (Transg.), relaxed-circular (RC), and single-stranded (SS) linear HBV DNA replicative forms are indicated. The integrated transgene can be used to normalize the amount of DNA bound to the membrane. The filter was hybridized with a 32P-labeled HBV-specific DNA probe. Total hepatic RNA was analyzed for the message of 2′5′ OAS and GAPDH by Northern blot (NB) analysis and for the message of iNOS, various cytokine transcripts, CD3, CD4, CD8, F480, and NK1.1 by RNase Protection assay (RPA), as indicated. The mRNA encoding the ribosomal protein L32 was used to normalize the amount of RNA loaded in each lane of the RPA assay. Results were compared with those observed in livers pooled from six age-, sex-, and serum HBeAg–matched, saline-injected transgenic controls (time 0). The mean sALT activity, measured at the time of autopsy, is indicated at the bottom for each group and is expressed in units per liter.
By weeks 6, 7, and 8 after infection, hepatic granulomas (Fig. 2 B) increased in number and size (data not shown) and HBV replication remained suppressed. Since viral replication occurs inside of HBcAg-positive nucleocapsid particles in the cytoplasm of centrilobular hepatocytes (Fig. 2 A) in these transgenic mice 10, it is not surprising that HBcAg disappeared from the cytoplasm of these cells (Fig. 2 B), along with the disappearance of HBV replicative forms (Fig. 1). The intrahepatic expression of iNOS, Th1-type cytokines, and T lymphocyte, macrophage, and NK cell markers progressively increased, reaching its peak by week 8 after infection. This was accompanied by the induction of Th2-type cytokines (IL-4, IL-5, and IL-10) that started by week 6 and also peaked by week 8 (Fig. 1). These results were somewhat surprising since others have reported that the peak of Th2-type cytokine response after S. mansoni infection in mice (week 8) coincides with the downregulation of Th1-type cytokines (for review see reference 8). Using a quantitative assay (RNase Protection) to measure the intrahepatic content of cytokine messages, we confirmed in this study that Th2-type cytokines peaked on week 8, but this did not coincide with any waning of the type 1 immune response in the liver, which also peaked by week 8 (Fig. 1).
Figure 2.
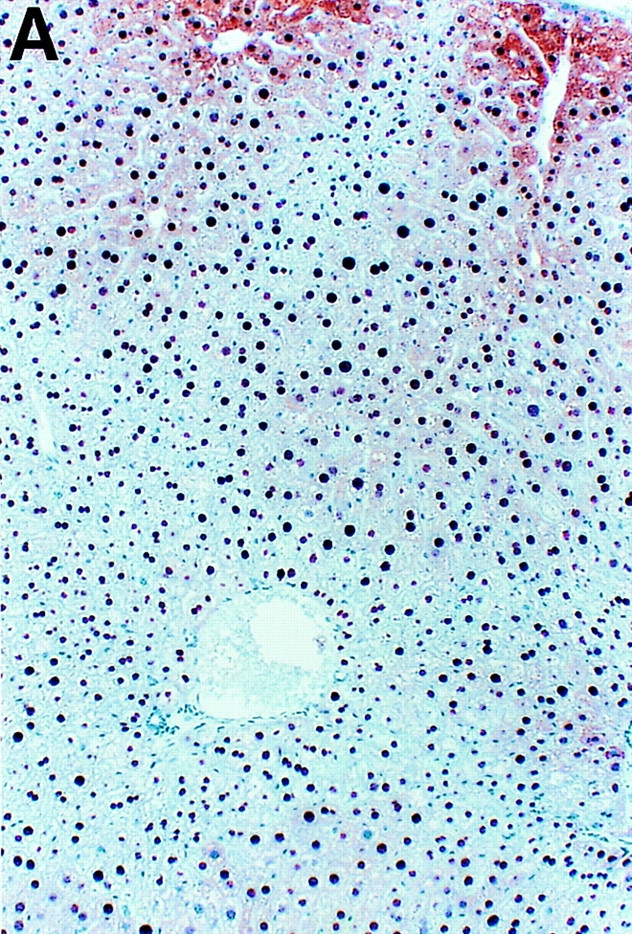
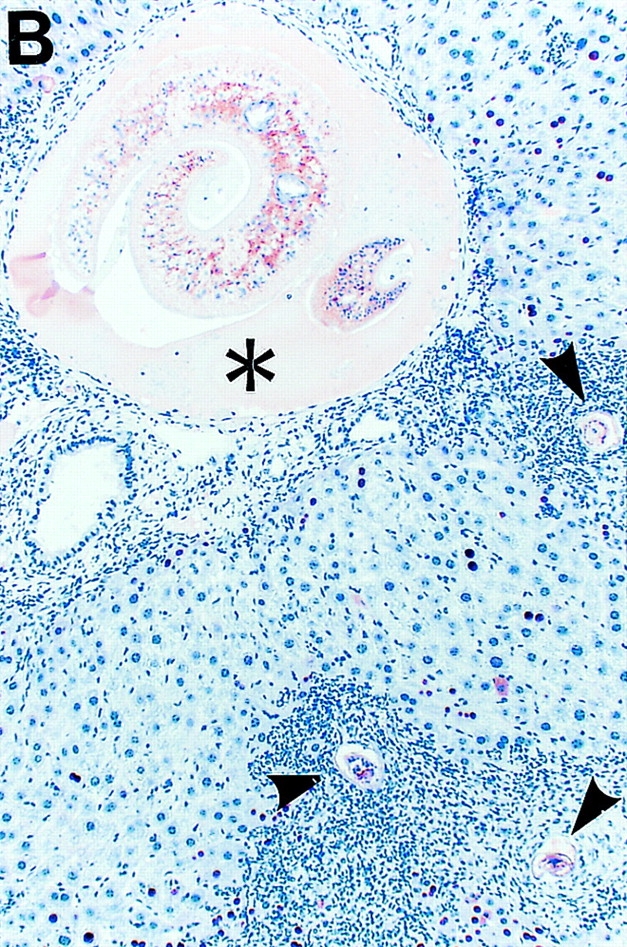
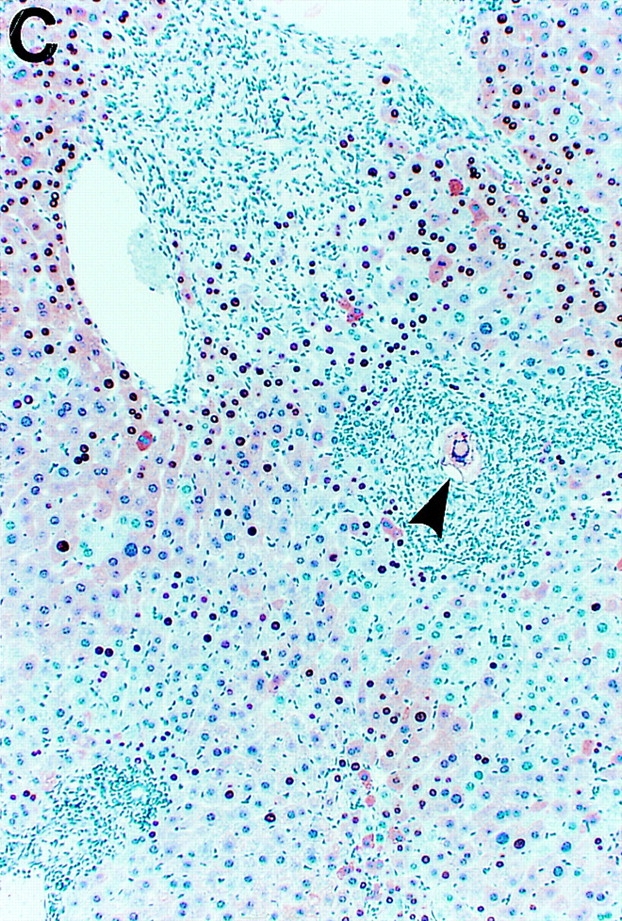
Intracellular localization of HBcAg in wild-type and IFN-γ–deficient HBV transgenic mice during S. mansoni infection. Age-, sex-, and serum HBeAg–matched transgenic mice from lineage 1.3.46 that were either heterozygous (+/−) or homozygous (−/−) for the IFN-γ null mutations were exposed to water containing cercariae and killed on week 8 after exposure. Liver sections obtained from uninfected HBV-IFN-γ+/− mice, S. _mansoni–_infected HBV–IFN-γ+/− or HBV–IFN-γ2/− mice were stained for the presence of HBcAg. Note that in uninfected HBV–IFN-γ+/− mice (A), cytoplasmic HBcAg is mainly detectable in centrilobular hepatocytes surrounding the central veins (top). These are the cells that sustain high levels of HBV replication. Also note that cytoplasmic HBcAg disappeared from centrilobular hepatocytes of S. _mansoni–_infected HBV-IFN-γ+/− (B) and was still detectable in the cytoplasm of centrilobular hepatocytes of S. _mansoni–_infected HBV–IFN-γ2/− mice (C). Adult worms (asterisk) and eggs (arrowheads) are indicated. Immunoperoxidase stain for HBcAg, original magnification: ×200.
No induction of 2′5′-OAS (a marker of IFN-α/β induction) was detected throughout the infection, indicating that IFN-α/β is not produced in the liver of S. _mansoni_–infected animals. sALT activity slightly increased by week 6, peaked at week 7, and started to decline by week 8 (Fig. 1, bottom). Given the relatively low levels of sALT, the extent of hepatocellular lysis in these animals was moderate throughout the infection.
Groups of HBV transgenic mice (three mice per group) were also killed during the chronic stage of infection (weeks 10 and 12). The number of hepatic granulomas decreased, Th1-type and Th2-type cytokines were still induced in the liver (although to lower levels compared with week 8), and HBV replication remained downregulated (data not shown). Finally, similar results were obtained when groups (four mice per group) of age- (8 wk), sex- (male), and serum HBeAg–matched transgenic mice from lineage 1.3.46 (inbred B10D2) were infected with S. mansoni and killed at 5, 6, 7, 8, and 12 wk after exposure (data not shown). This indicates that these results are highly reproducible and they are not affected by the genetic background of the mice.
Taken together, the data indicate that during S. mansoni infection the intrahepatic cytokine profile is initially of a Th1-type and this is associated with the noncytopathic inhibition of HBV replication. In the following weeks, despite the fact that both Th1- and Th-2 type cytokines are induced in the liver, HBV replication remains abolished.
Suppression of HBV Replication during S. mansoni Infection Is Mostly Mediated by IFN-γ.
Since we have previously identified IFN-γ and IFN-α/β as two cytokines capable of downregulating HBV replication noncytopathically in the liver of transgenic mice 3 4 14, we monitored the ability of S. mansoni infection to inhibit HBV replication in transgenic mice that were unable to produce IFN-γ or the receptor for IFN-α/β. Groups of age-, sex-, and HBeAg-matched transgenic mice (six mice per group) that were either homozygous (−/−) or heterozygous (+/−) for these null mutations were exposed to water containing cercariae and killed on week 8 after exposure.
As shown in Fig. 3 for three representative mice per group, HBV DNA replicative forms disappeared from the liver of all of the heterozygous mice, coinciding with induction of iNOS, Th1- and Th2-type cytokines, and T lymphocyte, macrophage, and NK cell markers. Similarly, HBV replication was abolished in the liver of IFN-α/βR−/− mice, indicating that IFN-α/β does not contribute to the antiviral effect of S. mansoni infection. This is compatible with the fact that 2′5′-OAS was not induced in the S. _mansoni_–infected livers (Fig. 1 and Fig. 3).
Figure 3.
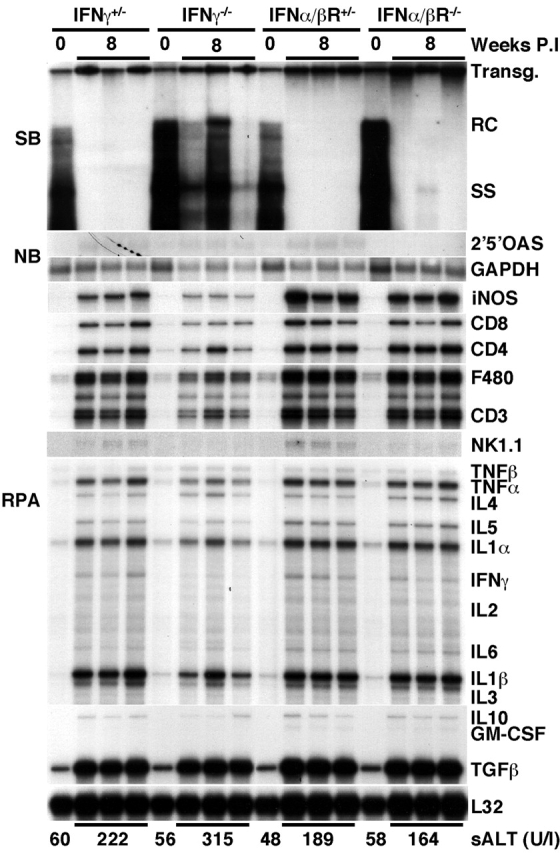
Suppression of HBV replication by S. mansoni infection is mostly mediated by IFN-γ. Age-, sex-, and serum HBeAg–matched transgenic mice from lineage 1.3.46 that were either heterozygous (+/−) or homozygous (−/−) for the IFN-γ and the IFN-α/β receptor null mutations were exposed to water containing cercariae and killed on week 8 after exposure. Total hepatic DNA was analyzed for HBV DNA by Southern blot (SB) analysis. All DNA samples were RNAse treated before gel electrophoresis. Bands corresponding to the integrated transgene (Transg.), relaxed-circular (RC), and single-stranded (SS) linear HBV DNA replicative forms are indicated. The integrated transgene can be used to normalize the amount of DNA bound to the membrane. The filter was hybridized with a 32P-labeled HBV-specific DNA probe. Total hepatic RNA was analyzed for the message of 2′5′ OAS and GAPDH by Northern blot (NB) analysis and for the message of iNOS, various cytokine transcripts, CD3, CD4, CD8, F480, and NK1.1 by RNase Protection assay (RPA), as indicated. The mRNA encoding the ribosomal protein L32 was used to normalize the amount of RNA loaded in each lane of the RPA assay. Results were compared with those observed in livers pooled from six age-, sex-, and serum HBeAg–matched, saline-injected transgenic controls (time 0). The mean sALT activity, measured at the time of autopsy, is indicated at the bottom for each group and is expressed in units per liter.
In contrast, HBV replication was only partially reduced in the IFN-γ knockout animals (Fig. 3), indicating that IFN-γ mediates most of the antiviral activity of S. mansoni infection. It is noteworthy that the hepatic levels of HBV replicative forms were quite variable among different individuals (the variability in HBV DNA content extended to three additional mice that are not shown in Fig. 3). The reason for this variability remains to be determined. Nevertheless, HBV DNA remained detectable in all IFN-γ2/− mice and, in keeping with this, HBcAg also remained detectable in the cytoplasm of hepatocytes, even in those that were directly adjacent to hepatic granulomas (Fig. 2 C).
Interestingly, the intrahepatic content of iNOS mRNA was markedly reduced in the liver of IFN-γ2/− mice (approximately fivefold, as measured by phosphorimaging analysis; data not shown), as compared with the liver of all the other S. _mansoni_–infected mice (Fig. 3). The fact that IFN-γ2/− mice showed reduced intrahepatic levels of iNOS at this time point suggests that NO may have contributed to the antiviral effect of IFN-γ in this system. This would be consistent with a previous report from our laboratory where it was shown that virus-specific CTLs can abolish HBV replication in the hepatocytes of transgenic mice by IFN-γ–dependent mechanisms that involve NO 6. Additionally, IFN-γ and NO may also exert a protective role during S. mansoni infection, since mice genetically deficient for IFN-γ showed the lowest levels of iNOS and the highest levels of sALT activity (Fig. 3). Similar findings have been reported recently in a study by Brunet et al., where treatment of S. _mansoni–_infected mice with iNOS inhibitors increased the severity of liver disease 15.
Very few (and contradictory) studies describing possible interactions between HBV and S. mansoni are available. Although some studies have shown little or no epidemiological association between S. mansoni infection and the presence of antibody to hepatitis B virus core antigen (anti-HBc) and hepatitis B surface antigen (HBsAg) 16 17 18 19, others have shown that concurrence of these two infections correlates with a more severe liver disease and a longer persistence of HBsAg 20 21. Based on the results of this report, it is possible that Th1-type cytokines may inhibit HBV replication during human S. mansoni infection, irrespective of the presence of Th2-type cytokines in the liver.
The results shown here also suggest that the presence of Th2-type cytokines do not counteract the antiviral activity of IFN-γ. This could be important for the pathogenesis of HBV infection in humans. It has been postulated that the outcome of HBV infection (viral clearance versus viral persistence) may be influenced by qualitative differences in the intrahepatic cytokine profile (Th1-type versus Th2-type) 22. Indirect evidence has suggested that persistence of HBV may be associated with a predominant Th2-type response. This is because the titer of antibody in the serum of chronically infected patients (in which the cellular immune response is absent or extremely weak) is higher than that of acutely infected patients who cleared the virus (in which cellular immune response is relatively strong) 22. Along these lines, it was recently shown that most liver-infiltrating T cells in chronic hepatitis B infection in humans have a Th0-like phenotype, since they are able to produce IFN-γ, IL-4, and IL-5 23. The results of this study indicate that IFN-γ inhibits HBV replication in the presence of IL-4, IL-5, and IL-10. Therefore, it is possible that persistence of HBV may primarily result from a quantitative deficiency of Th1-type responses rather than qualitative differences in the intrahepatic cytokine profile. Accordingly, it has been shown recently that the number of IFN-γ–producing antigen-specific T cells isolated from the peripheral blood of people chronically infected with HBV is much lower than that observed in the peripheral blood of people acutely infected with HBV 24.
Acknowledgments
We thank Alan Sher for helpful discussion; Fred Lewis for providing Schistosome life cycle stages supplied through National Institutes of Health–National Institute of Allergy and Infectious Diseases contract N01-AI-55270; Timothy Stewart and Michel Aguet for providing IFN-γ2/− and IFN-α/βR−/− mice, respectively; Ian Campbell for providing the iNOS probe, and Monte Hobbs for providing the cytokine gene and T cell marker probe sets used in the RNase Protection assays; and Margie Chadwell for excellent technical assistance.
This work was supported by grants AI-40696 (to L.G. Guidotti) and CA-40489 (to F.V. Chisari) from the National Institutes of Health. This is manuscript number 13090-MEM from the Scripps Research Institute.
References
- Chisari F.V., Ferrari C. Hepatitis B virus immunopathogenesis. Annu. Rev. Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- Guidotti L.G., Chisari F.V. Cytokine-induced viral purging--role in viral pathogenesis. Curr. Opin. Microbiol. 1999;2:388–391. doi: 10.1016/s1369-5274(99)80068-x. [DOI] [PubMed] [Google Scholar]
- McClary H., Koch R., Chisari F.V., Guidotti L.G. Relative sensitivity of hepatitis B virus and other hepatotropic viruses to the antiviral effects of cytokines. J. Virol. 2000;74:2255–2264. doi: 10.1128/jvi.74.5.2255-2264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti L.G., Borrow P., Hobbs M.V., Matzke B., Gresser I., Oldstone M.B.A., Chisari F.V. Viral cross talkintracellular inactivation of the hepatitis B virus during an unrelated viral infection of the liver. Proc. Natl. Acad. Sci. USA. 1996;93:4589–4594. doi: 10.1073/pnas.93.10.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh V.J., Guidotti L.G., Chisari F.V. Inhibition of hepatitis B virus replication during adenovirus and cytomegalovirus infections in HBV transgenic mice. J. Virol. 1998;72:2630–2637. doi: 10.1128/jvi.72.4.2630-2637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti L.G., McClary H., Moorhead Loudis J., Chisari F.V. Nitric oxide inhibits hepatitis B virus replication in the liver of transgenic mice. J. Exp. Med. 2000;191:1247–1252. doi: 10.1084/jem.191.7.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti L.G., Rochford R., Chung L., Shapiro M., Purcell R., Chisari F.V. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284:825–829. doi: 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]
- Fallon P.G. Immunopathology of schistosomiasisa cautionary tale of mice and men. Immunol. Today. 2000;21:29–35. doi: 10.1016/s0167-5699(99)01551-0. [DOI] [PubMed] [Google Scholar]
- Pearce E.J., La Flamme A., Sabin E., Brunet L.R. The initiation and function of Th2 responses during infection with Schistosoma mansoni . Adv. Exp. Med. Biol. 1998;452:67–73. doi: 10.1007/978-1-4615-5355-7_9. [DOI] [PubMed] [Google Scholar]
- Guidotti L.G., Matzke B., Schaller H., Chisari F.V. High level hepatitis B virus replication in transgenic mice. J. Virol. 1995;69:6158–6169. doi: 10.1128/jvi.69.10.6158-6169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton D.K., Pitts-Meek S., Keshav S., Figari I.S., Bradley A., Stewart T.A. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- Muller U., Steinhoff U., Reis L.F., Hemmi S., Pavlovic J., Zinkernagel R.M., Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;5167:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- Smithers S.R., Terry R.J. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology. 1965;55:695–700. doi: 10.1017/s0031182000086248. [DOI] [PubMed] [Google Scholar]
- Guidotti L.G., Ishikawa T., Hobbs M.V., Matzke B., Schreiber R., Chisari F.V. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- Brunet L.R., Beall M., Dunne D.W., Pearce E.J. Nitric oxide and the Th2 response combine to prevent severe hepatic damage during Schistosoma mansoni infection. J. Immunol. 1999;163:4976–4984. [PubMed] [Google Scholar]
- Ye X.P., Fu Y.L., Anderson R.M., Nokes D.J. Absence of relationship between Schistosoma japonicum and hepatitis B virus infection in the Dongting lake region, China. Epidemiol. Infect. 1998;121:193–195. doi: 10.1017/s0950268898008917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel M.A., Miller F.D., el Masry A.G., Zakaria S., Khattab M., Essmat G., Ghaffar Y.A. The epidemiology of Schistosoma mansoni, hepatitis B and hepatitis C infection in Egypt. Ann. Trop. Med. Parasitol. 1994;88:501–509. doi: 10.1080/00034983.1994.11812897. [DOI] [PubMed] [Google Scholar]
- Pereira L.M., Melo M.C., Lacerda C., Spinelli V., Domingues A.L., Massarolo P., Mies S., Saleh M.G., McFarlane I.G., Williams R. Hepatitis B virus infection in schistosomiasis mansoni. J. Med. Virol. 1994;42:203–206. doi: 10.1002/jmv.1890420219. [DOI] [PubMed] [Google Scholar]
- Larouze B., Dazza M.C., Gaudebout C., Habib M., Elamy M., Cline B. Absence of relationship between Schistosoma mansoni and hepatitis B virus infection in the Qalyub Governate, Egypt. Ann. Trop. Med. Parasitol. 1987;81:373–375. doi: 10.1080/00034983.1987.11812134. [DOI] [PubMed] [Google Scholar]
- Lyra L.G., Reboucas G., Andrade Z.A. Hepatitis B surface antigen carrier state in hepatosplenic schistosomiasis. Gastroenterology. 1976;71:641–645. [PubMed] [Google Scholar]
- Madwar M.A., Tahawy M.E., Strickland G.T. The relationship between uncomplicated schistosomiasis and hepatitis B infection. Trans. R. Soci. Trop. Med. Hyg. 1989;83:233–236. doi: 10.1016/0035-9203(89)90657-3. [DOI] [PubMed] [Google Scholar]
- Maruyama T., Schödel F., Iino S., Koike K., Yasuda K., Peterson D., Milich D.R. Distinguishing between acute and symptomatic chronic hepatitis B virus infection. Gastroenterology. 1994;106:1006–1015. doi: 10.1016/0016-5085(94)90761-7. [DOI] [PubMed] [Google Scholar]
- Bertoletti A., D'Elios M.M., Boni C., De Carli M., Zignego A.L., Durazzo M., Missale G., Penna A., Fiaccadori F., Del Prete G., Ferrari C. Different cytokine profiles of intraphepatic T cells in chronic hepatitis B and hepatitis C virus infections. Gastroenterology. 1997;112:193–199. doi: 10.1016/s0016-5085(97)70235-x. [DOI] [PubMed] [Google Scholar]
- Jung M.C., Hartmann B., Gerlach J.T., Diepolder H., Gruber R., Schraut W., Gruner N., Zachoval R., Hoffmann R., Santantonio T. Virus-specific lymphokine production differs quantitatively but not qualitatively in acute and chronic hepatitis B infection. Virology. 1999;261:165–172. doi: 10.1006/viro.1999.9833. [DOI] [PubMed] [Google Scholar]