Antigen-Specific Inhibition of Effector T Cell Function in Humans after Injection of Immature Dendritic Cells (original) (raw)
Abstract
Immunostimulatory properties of dendritic cells (DCs) are linked to their maturation state. Injection of mature DCs rapidly enhances antigen-specific CD4+ and CD8+ T cell immunity in humans. Here we describe the immune response to a single injection of immature DCs pulsed with influenza matrix peptide (MP) and keyhole limpet hemocyanin (KLH) in two healthy subjects. In contrast to prior findings using mature DCs, injection of immature DCs in both subjects led to the specific inhibition of MP-specific CD8+ T cell effector function in freshly isolated T cells and the appearance of MP-specific interleukin 10–producing cells. When pre- and postimmunization T cells were boosted in culture, there were greater numbers of MP-specific major histocompatibility complex tetramer-binding cells after immunization, but these had reduced interferon γ production and lacked killer activity. These data demonstrate the feasibility of antigen-specific inhibition of effector T cell function in vivo in humans and urge caution with the use of immature DCs when trying to enhance tumor or microbial immunity.
Keywords: dendritic cells, maturation, tolerance, CD8+ T cells, immunization
Introduction
Dendritic cells (DCs) are APCs specialized to regulate T cell immunity 1. DCs normally reside and traffic through nonlymphoid tissues in an immature form, poised for antigen capture. Inflammatory stimuli are necessary to switch DCs to an immunostimulatory mode. This process, termed “maturation,” is associated with changes in DC phenotype and function, including upregulation of costimulatory and adhesion molecules and expression of distinct chemokine receptors 2. Due to their potency as APCs, there is considerable interest in using these cells as adjuvants to enhance immunity against cancer and viral infections. We and others have previously shown that injection of antigen-bearing DCs derived from monocyte precursors and matured ex vivo using inflammatory stimuli leads to rapid enhancement of CD4+ and CD8+ T cell immunity in humans 3 4 5.
To examine whether the ex vivo maturation stimulus is essential for the immune efficacy of a DC vaccine, we initiated a clinical study comparing DCs cultured with or without such a stimulus. DCs were pulsed with KLH and, in the case of HLA A2.1+ subjects, additionally with HLA A*0201–restricted influenza matrix peptide (MP). Here we describe the findings on the first two study subjects injected with immature DCs.
Materials and Methods
Study Design.
The study was initiated as a randomized 2 × 2 factorial design, comparing a single injection of immature versus mature DCs administered subcutaneously versus intradermally. This brief report describes the inhibition of antigen-specific effector function in the first two subjects (Im1 and Im2) injected subcutaneously with immature DCs. Two additional subjects received an injection of mature DCs, either intradermally (M1) or subcutaneously (M2). Based on these findings, the study is now modified to only compare routes of administration of mature DCs in subsequent subjects.
Human Volunteers.
Normal healthy adult volunteers were recruited through advertisement. Eligibility and exclusion criteria were as in a prior study 4 and included age of 18–65 yr and no clinical evidence of malignancy, chronic infection, or autoimmunity. All subjects signed an informed consent, and the study was approved by the The Rockefeller University Institutional Review Board.
Baseline Studies.
All study participants were typed for HLA A2.1 status and initially followed for a 1–3-mo period, during which at least two baseline measurements of immune response were made. Laboratory tests at baseline to confirm eligibility included complete blood count, chemistry profile, hepatitis B and C and HIV serology, rheumatoid factor, antinuclear antibody (ANA), urinalysis, chest X-ray, pregnancy test, influenza serology, and anergy panel consisting of candida, mumps, and tetanus.
Generation and Injection of DCs.
DCs were generated from plastic adherent blood monocyte precursors after in vitro culture with GM-CSF and IL-4 as described 4 and pulsed with antigens on day 5 of culture. The antigens were: 10 μg/ml KLH (depyrogenated; Calbiochem) and 1 μg/ml influenza MP (manufactured by A. Houghton in the microchemistry facility of the Sloan-Kettering Institute, New York, NY). Autologous monocyte–conditioned medium (50% vol/vol) was added on day 5 of culture as a maturation stimulus for subjects receiving mature DCs (M1, M2; reference 4). Two million DCs were injected on day 6 (Im1) or day 7 (Im2, M1, M2) of culture (see Table ). On the day of injection, the DCs were washed free of antigen, resuspended in normal saline containing 5% autologous plasma in two 0.1–0.2-cm3 aliquots, and injected within 30 min of final reconstitution. The phenotype of the injected DCs was monitored by flow cytometry. All injected DC preparations tested negative for bacterial and fungal contamination.
Table 1.
Characteristics of DCs
| DC | Percentage large cells expressing: | |||||||
|---|---|---|---|---|---|---|---|---|
| ID | HLA A2.1 | Antigens | Maturity | Route | Purity | HLADR+ | CD83+ | CD14+ |
| % | ||||||||
| Im1 | + | KLH, MP | Immature | s.c. | 87 | 91 | 7 | 2 |
| Im2 | + | KLH, MP | Immature | s.c. | 76 | 100 | 3 | 1 |
| M1 | − | KLH | Mature | i.d. | 87 | 99 | 93 | 1 |
| M2 | − | KLH | Mature | s.c. | 96 | 100 | 92 | 0 |
Follow-up and Monitoring.
Immune responses were evaluated 1 wk after DC injection and at 1–3-mo intervals thereafter. Both subjects had a repeat hemogram, rheumatoid factor, ANA, and influenza serology 1 mo after DC injection.
Measurement of Immune Responses.
Measurement of immune response was performed on freshly isolated PBMCs. In addition, for most assays, cryopreserved pre- and postimmunization specimens were thawed, coded, and assayed together in a blinded fashion.
ELISPOT Assay for Antigen-specific Cytokine (IFN-γ, IL-4, IL-10)-secreting T Cells.
Antigen-specific T cells were quantified using a standard ELISPOT assay as described 4 after overnight culture in the presence or absence of antigens in plates precoated with anticytokine (IFN-γ, IL-4, or IL-10) antibodies (Mabtech). Antigens were 1 μg/ml HLA A*0201–restricted peptides from influenza MP (GILGFVFTL), HIV gag (gag; SLYNTVATL), and CMV pp65 (NLVPMVATV) as controls. The background reactivity with no peptide in this assay was low (mean ± SE: 1 ± 1 spot-forming cells [SFCs]/2 × 105 cells). For the detection of influenza-specific responses, PBMCs were infected with influenza virus at a multiplicity of infection of 2. For some assays, MP-pulsed mature DCs were used as APCs (PBMC/DC ratio 30:1). KLH (10 μg/ml) was also used as an antigen (no protein and superantigen as controls) in some assays. In additional assays, bulk T cells were depleted of CD4+ and CD8+ T cells using magnetic beads (Miltenyi Biotec) before use in the ELISPOT assays.
MHC Tetramer Binding Assays.
Soluble influenza MP–HLA A*0201 tetramers were prepared as described 6, and binding to tetramers was analyzed by FACS®. Frozen aliquots of PBMCs from before and after immunization were thawed together and stained as described 4 with A*0201–MP tetramer at 37°C, both directly before and after 7-d coculture with autologous MP-pulsed DCs (unpulsed DCs as controls).
Recall T Cell Assays.
For recall assays, pre-/postimmunization PBMCs were cocultured with freshly generated autologous mature DCs pulsed with MP (unpulsed DCs as control) at a PBMC/DC ratio of 30:1 for 7 d. At the end of 7-d culture, MP-specific T cells were quantified as described earlier 3 using (a) ELISPOT assay for antigen-specific cytokine–producing cells (after restimulation on day 7); (b) MHC tetramer binding assay; or (c) CTL assay. CTL activity was measured in a standard 5-h 51Cr-release assay at an E/T ratio of 20:1. Targets were T2 cells pulsed with 1 μg/ml MP or unpulsed T2 cells as controls. Excess cold K562 cell targets (80:1) were used to inhibit NK-mediated lysis.
Antigen-dependent Proliferation.
Antigen-dependent proliferation assays were performed as described, at two PBMC dose levels (3 × 104 cells per well and 105 cells per well) in the absence or presence of graded doses of KLH (0.1–10 μg/ml; reference 4). Tetanus toxoid and staphylococcal enterotoxin A (SEA) served as control antigens. For some assays, bulk T cells were depleted of CD4+ and CD8+ T cells using magnetic beads (Miltenyi Biotec) before use in proliferation assays.
Results
Inhibition of MP-specific Effector T Cell Function In Vivo after DC Injection.
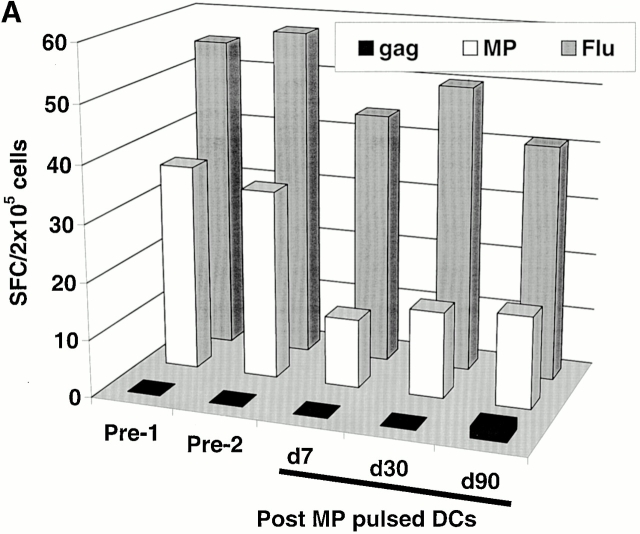
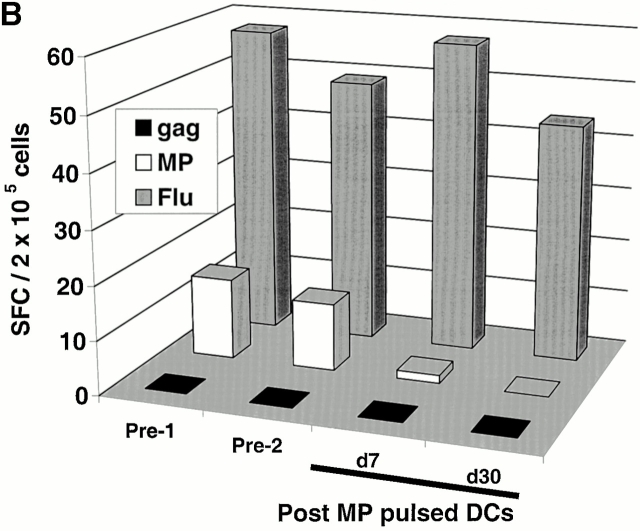
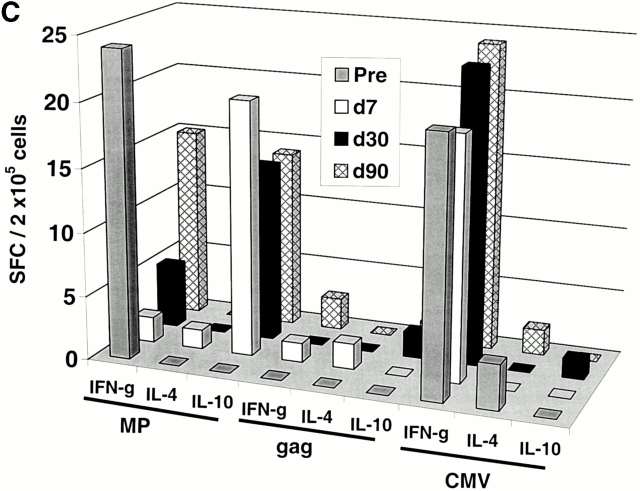
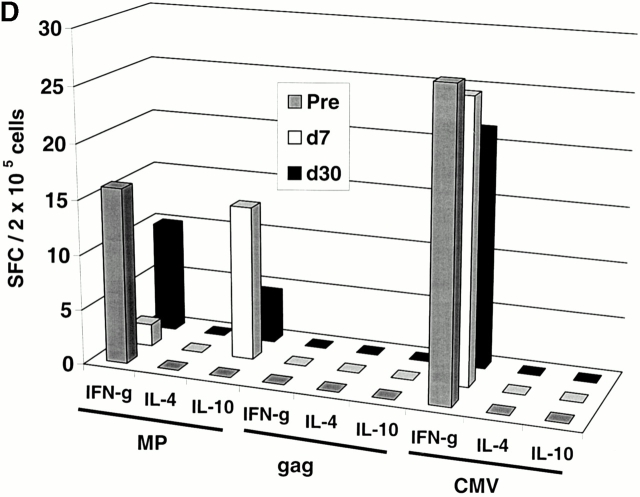
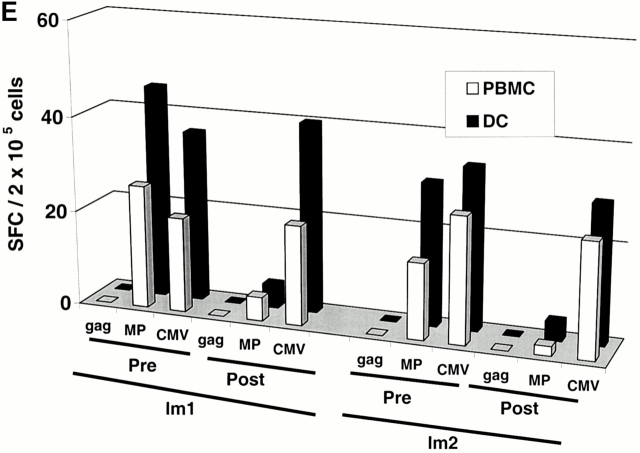
Two subjects (Im1 and Im2) received a single subcutaneous injection of 2 × 106 immature DCs pulsed with the influenza peptide, MP, and KLH (Table ). All DC injections in this study were well tolerated without any clinical toxicity and serologic/clinical evidence of autoimmunity. Before immunization, MP-specific IFN-γ–producing T cells were detectable in both subjects as expected, because most adults have been exposed to the influenza virus. However, after DC immunization, there was a decline in MP-specific IFN-γ–producing cells (Fig. 1a and Fig. b). The number of MP-specific effectors reached a nadir 7–30 d after immunization and improved thereafter. In contrast, there was little decline in total influenza effector T cell function, indicating that the decrease in MP effector function was specific for the immunizing peptide. Similar data were obtained when cryopreserved cells were assayed together (Fig. 1c and Fig. d), although the absolute reactivity was higher with fresh cells, as reported previously 4. As a control, no decline in antigen-specific IFN-γ–producing cells to HLA A*0201–restricted CMV peptide was observed. The loss of MP-specific IFN-γ–producing cells persisted, even when peptide-pulsed mature DCs were used as APCs in the ELISPOT (Fig. 1 E). In fact, the use of DCs increased the preimmunization measurements, so that the decrease in effectors after immature DC injection was even more striking.
Figure 1.
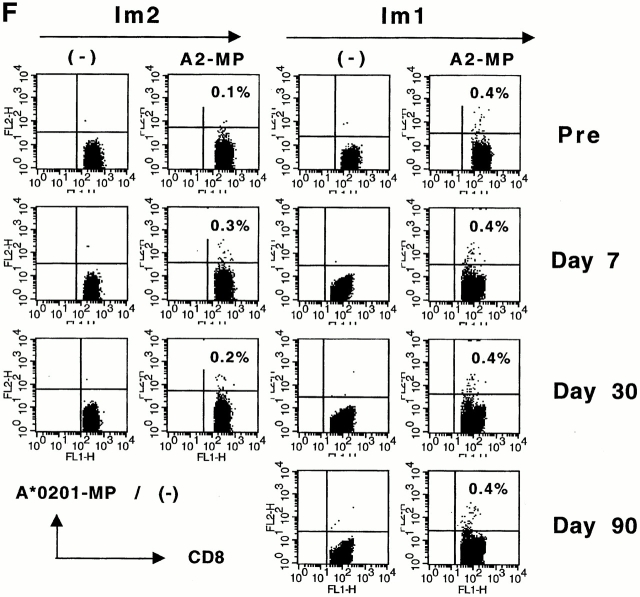
Immune responses in uncultured T cells. (A and B) MP-, gag-, and influenza (Flu)-specific IFN-γ–producing cells from before and after DC immunization were quantified in freshly isolated uncultured PBMCs using an ELISPOT assay. Data for influenza specific cells is per 105 cells. SEM for all measurements is <20%. (A) Im1; (B) Im2. (C and D). Pre- and postimmunization samples were thawed together and assayed for antigen-specific T cells secreting IFN-γ, IL-4, and IL-10 using a 16-h ELISPOT assay. Antigens were HLA A2.1–restricted peptides from influenza MP, HIV-gag (gag), and CMV pp65 (CMV). Positive controls for the assays included SEA for IFN-γ and IL-10 and PHA for IL-4 (not shown). SEM for all measurements is <20%. (C) Im1; (D) Im2. (E) Use of peptide-pulsed DCs as APCs in the ELISPOT. Pre- and postimmunization specimens were examined using peptide-pulsed mature DCs as APCs (PBMC/DC ratio 30:1) in the ELISPOT. SEM for all measurements is <20%. (F) Quantification of MP-specific T cells using MHC tetramers in uncultured cells. Pre-/postimmunization specimens were stained with A*0201–MP tetramers at 37°C and analyzed by flow cytometry. Data shown are gated for CD8+ T cells and expressed as percent CD8+ T cells binding A*0201–MP tetramer.
Induction of Antigen-specific IL-10–producing Cells In Vivo.
The decline in MP-specific IFN-γ–producing cells was associated with the appearance of MP-specific IL-10– but not IL-4–producing T cells (Fig. 1c and Fig. d). No induction of IL-4/IL-10–producing cells to the control antigen CMV was observed. The preimmunization IFN-γ secretors and postimmunization IL-10 producers were both CD8+CD4−, as indicated by magnetic bead depletion experiments (not shown). MP-specific CD8+ T cells elicited after mature DC injection in an earlier study 4 failed to produce IL-10 (not shown), so we propose that the induction of IL-10 producers is due to the use of immature DCs.
Decline of Effectors Is Not Due to Loss of Circulating MP-specific T Cells.
The decline in MP (but not CMV or influenza specific effectors) after immunization with MP-pulsed immature DCs raised several possible mechanisms: immature DCs could lead to the loss of circulating MP-specific T cells due to either cell death/deletion, or these cells may redistribute to tissues/nodes after activation in vivo. Alternatively, DC injection could lead to inhibition of effector T cell function or induction of anergy in an antigen-specific manner. Analysis of MP-specific T cells by MHC tetramer binding in uncultured PBMCs revealed either no change (Im1) or an increase (Im2) in MP-specific T cells after DC immunization (Fig. 1 F). Therefore, the loss of effector function after immunization with immature DCs is not due to a loss of circulating antigen-specific T cells.
Expansion of Memory T Cells with Defective Effector Function In Vivo.
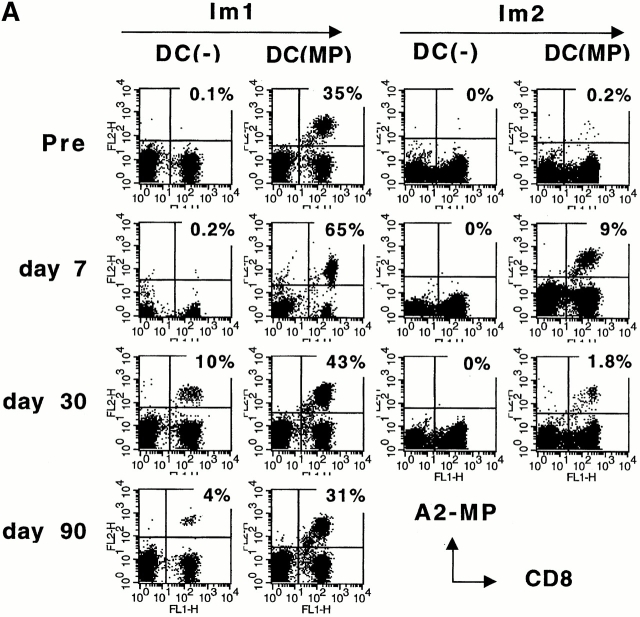
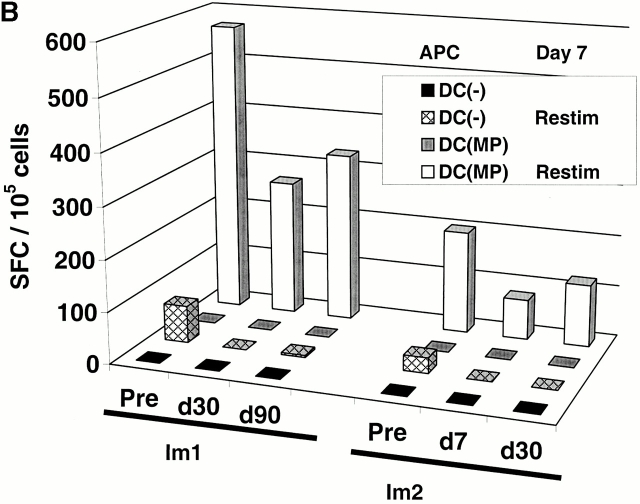
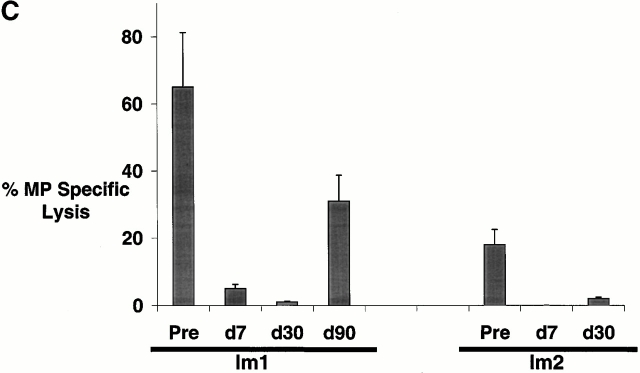
When pre- and postimmunization samples were thawed together and boosted in culture with MP-pulsed autologous mature DCs, there was a greater number of MP-specific MHC tetramer binding T cells in postimmunization cultures (Fig. 2 A). However, these specific antigen–binding T cells had reduced MP-specific IFN-γ producers in the ELISPOT assay (Fig. 2 B) and failed to kill peptide-pulsed targets (Fig. 2 C), even when they constituted up to 60% of all CD8+ T cells. There was no expansion of MP-specific IL-10– or IL-4–producing T cells in these cultures (not shown). We conclude that immunization with immature antigen-bearing DCs blunts the capacity of the corresponding antigen-specific CD8+ T cell to mount lytic function in vitro.
Figure 2.
(A–C) T cell recall assays in culture after DC immunization. Pre- and postimmunization specimens were thawed and cocultured with MP-pulsed DCs (unpulsed DCs as controls) for 7 d. After 7-d culture, MP-specific T cells were quantified by MHC tetramers (A), ELISPOT (B), and CTL assay (C). (A) MHC tetramer assay. Data are expressed as percent CD8+ T cells binding A*0201–MP tetramer. (B) ELISPOT assay. On day 7, cells were transferred to an ELISPOT plate and restimulated (Restim) overnight with specific antigen (10 μg/ml MP) or left without restimulation (as controls), and antigen-specific IFN-γ–producing cells were quantified using an ELISPOT. SEM for all measurements is <30%. (C) CTL assay. MP-specific lysis was measured using MP-pulsed T2 targets. Data shown are after subtracting lysis with control unpulsed T2 targets and from cells after expansion using unpulsed DCs.
Priming of KLH-specific T Cells In Vivo.
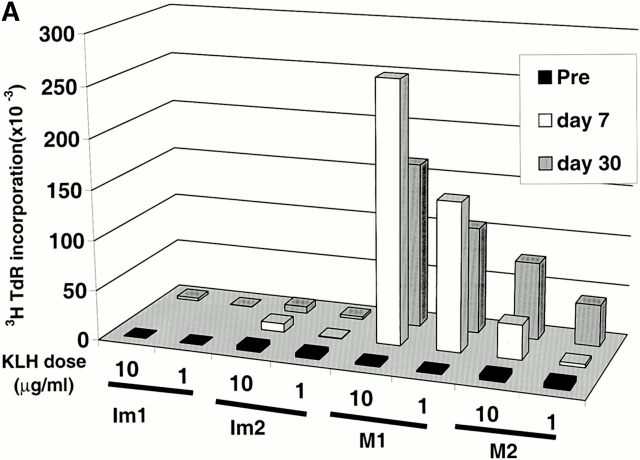
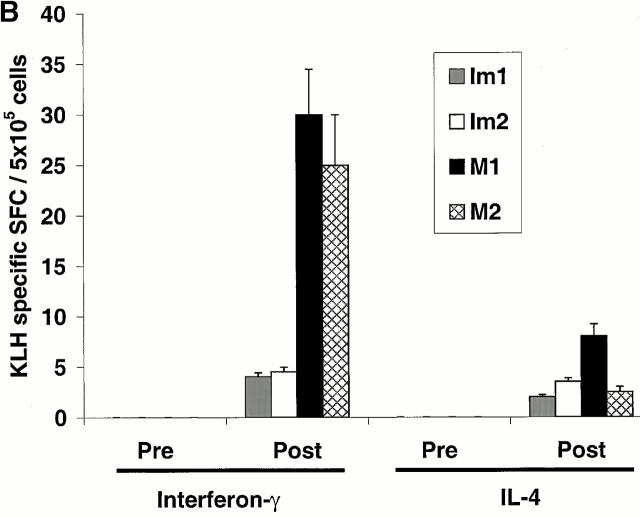
The two volunteers immunized with mature DCs (M1, M2) were not HLA A2.1+ and therefore could not be studied for their response to the influenza MP peptide. However, cryopreserved samples of PBMCs from all volunteers were assayed together for KLH-specific proliferative responses. All samples showed strong responses to a superantigen used as a positive control, but the KLH priming was much greater when mature DCs had been used (Fig. 3 A). Small proliferative responses to KLH were noted in freshly thawed specimens after immature DC injections (not shown), but no KLH-specific IFN-γ–secreting cells were evident, in contrast to clear Th1 priming with mature DCs (Fig. 3 B). Therefore, the primary CD4 T cell responses were weaker when immature DCs were used to immunize to KLH.
Figure 3.
Priming of KLH-specific T cells in vivo. (A) Antigen-dependent proliferation. Pre- and postimmunization PBMCs were thawed together and cultured in the absence or presence of KLH (10 μg/ml). Data shown are KLH-specific proliferation after subtracting [3H]TdR incorporation in control wells. SEM for all measurements is <30%. (B) KLH-specific IFN-γ– and IL-4–producing cells from before and after DC immunization were quantified in freshly isolated uncultured PBMCs using an ELISPOT assay. KLH-specific SFCs were calculated after subtracting data from control wells without antigen.
Discussion
In this study, we have shown that injection of antigen-bearing immature DCs in humans is not immunologically silent but can lead to antigen-specific inhibition of preexisting effector T cell function. Moreover, T cells secreting an immunosuppressive cytokine, IL-10, can be induced by immature DCs 7. Immature DCs are thus not simply weaker adjuvants and they do activate the immune system, as indicated by the expansion of MP-specific tetramer–binding CD8+ T cells in vivo. However, while these antigen-binding T cells did retain in vitro proliferative potential typical of memory T cells, they were defective in IFN-γ secretion and lacked killer function.
To our knowledge, this is first report describing the induction of antigen-specific IL-10–producing CD8+ T cells in vivo in humans. CD4+ T cells producing IL-10 have been proposed to act as regulatory T cells in vivo 7. Interestingly, there is a study of IL-10 function in culture showing that IL-10 allows T cell proliferation but dampens their effector function 8, a situation that occurred here. Neutralization of IL-10 in culture in this study led to some improvement but <50% reversal of the T cell inhibition (data not shown), suggesting that both IL-10–dependent and –independent mechanisms may play a role in the inhibition of CD8+ T cell function observed here.
Induction of IL-10–producing CD4+ T cells with regulatory properties by repetitive stimulation with immature DCs was recently observed by Jonuleit et al., another group studying mixed lymphocyte reaction in vitro, in the November 6 issue (reference 9). Although the immature DCs that we injected were also pulsed with KLH, we were unable to detect KLH-specific IL-10–producing CD4+ T cells in this study. We cannot exclude the possibility that this may relate to the low frequency of such cells after a single DC injection or the fact that we were measuring priming of a CD4+ T cell response.
It has been proposed that immature DCs carrying cellular antigens (e.g., phagocytosed apoptotic cells) might elicit tolerance to naive peripheral T cells in vivo 10 11. Our findings surprisingly reveal that immature DCs can dampen preexisting antigen-specific effector T cell function. Responses to immature DCs bearing tumor/viral antigens in vivo can therefore help explain the occasional observation of dysfunctional T cells in humans and mice 12 13 14, particularly in the setting of deficient CD4+ T cell function or malignancy, which may affect DC maturation in vivo 15 16.
DCs are currently under active clinical investigation, mostly for their immunostimulatory properties in cancer and viral infections 17. The optimal DC maturation stage for these studies has yet to be fully established. Our data urge caution with the use of immature DCs when trying to enhance tumor and microbial immunity. On the other hand, the suppressive properties of immature DCs observed here should be pursued for antigen-specific inhibition of T cell function in the setting of autoimmune diseases and organ transplantation in humans. Indeed, immature DCs have been shown to prolong allograft survival in several preclinical models 18. DCs have been injected without ex vivo maturation stimulus in other clinical studies, however this has been done mostly in the context of tumor antigens, wherein the preexisting effector function is low or absent 17.
We were unable to continue injection of MP-pulsed immature DCs in this study due to the potential for a reduction in influenza-specific effectors in our study subjects. While we are now pursuing similar experiments in mice, it is notable that there are significant differences in the degree of spontaneous maturation in human and mouse DC cultures, making the mouse a more difficult system to exploit 1. Further studies in clinically appropriate settings are being designed to assess if injection of immature DCs can silence autoreactive T cells in vivo.
Acknowledgments
The authors thank all study subjects for their interest and participation in this study, Maria Baston and Rockefeller University nursing staff for help with patient care, Dr. Knut Wittkowski for guidance with study design, and Casper Paludan for help with MHC tetramer synthesis.
This work was supported in part by an investigator award from the Cancer Research Institute (to M.V. Dhodapkar) and grants from the National Institutes of Health (CA 81138 to M.V. Dhodapkar and MO-RR00102 to the Rockefeller General Clinical Research Center). N. Bhardwaj is supported in part by the Burroughs Wellcome Fund.
References
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Cella M., Sallusto F., Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr. Opin. Immunol. 1997;9:10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- Dhodapkar M.V., Krasovsky J., Steinman R., Bhardwaj N. Mature dendritic cells boost functionally superior T cell in humans without foreign helper epitopes. J. Clin. Invest. 2000;107:R9–R14. doi: 10.1172/JCI9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhodapkar M.V., Steinman R.M., Sapp M., Desai H., Fossella C., Krasovsky J., Donahoe S.M., Dunbar P.R., Cerundolo V., Nixon D.F. Rapid generation of broad T-cell immunity in humans after a single injection of mature dendritic cells. J. Clin. Invest. 1999;104:173–180. doi: 10.1172/JCI6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurner B., Haendle I., Roder C., Dieckmann D., Keikavoussi P., Jonuleit H., Bender A., Maczek C., Schreiner D., von den Driesch P. Vaccination with mage-3A1 peptide–pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J. Exp. Med. 1999;190:1669–1678. doi: 10.1084/jem.190.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch D.H., Pilip I.M., Vijh S., Pamer E.G. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity. 1998;8:353–362. doi: 10.1016/s1074-7613(00)80540-3. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Regulatory T cellskey controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- Macatonia S.E., Doherty T.M., Knight S.C., O'Garra A. Differential effect of IL-10 on dendritic cell-induced T cell proliferation and IFN-gamma production. J. Immunol. 1993;150:3755–3765. [PubMed] [Google Scholar]
- Jonuleit H., Schmitt E., Schuler G., Knop J., Enk A. Induction of IL-10–producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature dendritic cells. J. Exp. Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F.P., Platt N., Wykes M., Major J.R., Powell T.J., Jenkins C.D., MacPherson G.G. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med. 2000;191:435–444. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R.M., Turley S., Mellman I., Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J. Exp. Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appay V., Nixon D.F., Donahoe S.M., Gillespie G.M., Dong T., King A., Ogg G.S., Spiegel H.M., Conlon C., Spina C.A. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 2000;192:63–76. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac A.J., Blattman J.N., Murali-Krishna K., Sourdive D.J., Suresh M., Altman J.D., Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P.P., Yee C., Savage P.A., Fong L., Brockstedt D., Weber J.S., Johnson D., Swetter S., Thompson J., Greenberg P.D. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat. Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- Caux C., Massacrier C., Vanbervliet B., Dubois B., Van Kooten C., Durand I., Banchereau J. Activation of human dendritic cells through CD40 cross-linking. J. Exp. Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell D., Chomarat P., Broyles D., Netto G., Harb G.M., Lebecque S., Valladeau J., Davoust J., Palucka K.A., Banchereau J. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J. Exp. Med. 1999;190:1417–1426. doi: 10.1084/jem.190.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhodapkar M., Bhardwaj N. Active immunization of humans with dendritic cells. J. Clin. Immunol. 2000;20:167–174. doi: 10.1023/a:1006681312249. [DOI] [PubMed] [Google Scholar]
- Thomson A.W., Lu L. Are dendritic cells the key to liver transplant tolerance? Immunol. Today. 1999;20:27–32. doi: 10.1016/s0167-5699(98)01378-4. [DOI] [PubMed] [Google Scholar]