Differentiation of T Regulatory Cells by Immature Dendritic Cells (original) (raw)
The induction of antigen-specific tolerance is critical for the prevention of autoimmunity and maintenance of immune homeostasis. The immune system's ability to distinguish between self and non-self and between innocuous and harmful foreign antigens is controlled by mechanisms of central and peripheral tolerance. Central tolerance is a well established mechanism that involves deletion of self-reactive T cells upon interaction with bone marrow–derived dendritic cells (DCs) in the thymus 1 2. Well characterized mechanisms of peripheral tolerance include the induction of cell death or the development of a state of nonresponsiveness (anergy) of T cells 3 4. In addition, active suppression by T regulatory (Tr) cells is also key for peripheral tolerance 5. However, the mechanisms by which Tr cells arise in vivo and exert their immunoregulatory effects remain to be defined and are the subject of intensive investigation.
A role for DCs in the induction of peripheral tolerance has been supported by several studies 6 7. At present, the mechanisms responsible for this process are not clear 8. However, it is widely assumed that, in the presence of self/harmless antigens, control of the maturation/activation state of DCs 7, and/or the subtype of DCs 9 10 is fundamental in the induction of peripheral tolerance. Two papers, one by Jonuleit et al. in the November 6 issue 11 and one by Dhodapkar et al. in this issue 12, strongly suggest that immature DCs (iDCs) may control peripheral tolerance by inducing the differentiation of human Tr cells.
Tr Subsets and Their Role in Peripheral Tolerance.
At present, the term “T regulatory” cell is used to describe a variety of cells that display regulatory function in vitro or in vivo and that can be subdivided into a number of subsets based on expression of cell surface markers, production of cytokines, and mechanisms of action 13. One of the best characterized subsets of CD4+ Tr cells is defined by its constitutive expression of the α chain of IL-2R (CD25). Much evidence indicates that CD4+CD25+ cells arise in the thymus, perhaps via an altered negative selection by self-antigens 5, but that they may need to reencounter the antigen in the periphery to become fully mature 14. After TCR-mediated activation, CD4+CD25+ T cells suppress immune responses in vitro and in vivo via an antigen-nonspecific mechanism that in some models seems to be independent of the production of immunosuppressive cytokines 15 and related to their constitutive expression of CTLA-4 16 17.
Another subset of CD4+ Tr cells, isolated after cloning of human T cells activated with alloantigens in the presence of IL-10, was termed type 1 Tr (Tr1) cells 18. Tr1 cells specific for recall antigens such as tetanus toxoid can also be isolated, suggesting that these cells are present in both the naive and memory pools (unpublished data). Tr1 cells are distinct from classical Th1 or Th2 cells in that they produce high levels of IL-10, moderate amounts of TGF-β, IFN-γ, and IL-5, low IL-2, and no IL-4 18. Tr1 cells proliferate poorly when stimulated via the TCR, and recent evidence suggests that cytokines such as IL-15 are critical for stimulating their proliferation in vitro (unpublished data). Importantly, Tr1 cells suppress immune responses in vitro and in vivo via a mechanism that is partially dependent on the production of the immunoregulatory cytokines IL-10 and TGF-β 18 19.
The CD4+ Tr cells induced by iDCs in vitro 11 or the CD8+ Tr cells induced in vivo 12 appear to share a key property with Tr1 cells in that they both produce high levels of IL-10, but no IL-4 or IL-2. However, in contrast to Tr1 cells, IL-10 appears to be dispensable for the in vitro suppressive activity of iDC-induced CD4+ Tr cells. Rather, CD4+ Tr cells primed in vitro by iDCs directly suppress the proliferative responses of mature Th1 cells via a mechanism that is antigen independent, requires cell–cell contact, and can be inhibited by addition of exogenous IL-2. Thus, functionally these Tr cells appear to be more similar to the CD4+CD25+ Tr cells described above.
Overall, the relationship between CD4+CD25+ Tr and Tr1 cells is currently unclear. It is possible that they are in fact the same subset of Tr cells in different stages of differentiation. CD4+CD25+ Tr cells may emerge from the thymus in a partially differentiated state and terminally differentiate into IL-10– and TGF-β–producing Tr1 cells only upon encountering antigen in the periphery. On the other hand, the observation that murine CD4+CD25+ Tr cells are comprised by the memory CD45RBlow population 17, whereas Tr1 cells can be differentiated from CD45RA+ naive cells in vitro 18, favors the hypothesis that CD4+CD25+ Tr cells and Tr1 cells are two distinct Tr subsets with similar functions.
There is also evidence for the existence of regulatory cells within the CD8+ T cell subset. Similar to CD4+ Tr cells, CD8+ Tr cells can be isolated in vitro, after multiple stimulations, and suppress antigen-specific responses by inhibiting IL-2 production and upregulation of CD40L on T cells and downregulating CD80/86 expression on APCs 20 21.
DC Maturation as a Control Point for the Induction of Tr Cells.
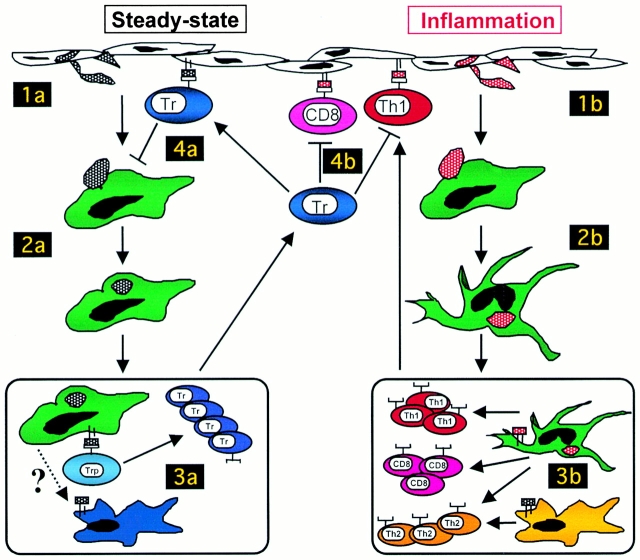
It is an attractive notion that by controlling the maturation state of DCs, the activation state of T cells can be altered. In the absence of inflammation, i.e., under homeostatic conditions, iDCs circulate and take up inhaled or ingested proteins and dying cells derived from normal cell turnover 22. Upon phagocytosis in the absence of inflammation, DCs remain immature but may still migrate to regional lymph nodes, a process that could be facilitated by expression of CCR7 after interaction with apoptotic cells (Fig. 1; references 22 and 23). In regional nodes, naive T cells may encounter antigen on iDCs, receive a suboptimal signal, and, via a process similar to that described in an allogeneic system by Jonuleit et al. 11, differentiate into Tr cells rather than effector T cells (Fig. 1). However, in contrast to alloantigens, endocytosed self-antigens cannot be processed by iDCs to form cell surface–expressed MHC–peptide complexes 24. Thus, we must hypothesize that there are stimuli in the lymph node environment that can allow iDCs to present endocytosed antigens and prime naive T cells to become regulatory cells. Alternatively, iDCs loaded with apoptotic cells may transfer tissue-derived peptides to a specialized subset of DCs that reside in lymph nodes and are dedicated to priming CD4+ or CD8+ Tr cells (reference 25 and Fig. 1).
Figure 1.
Control of peripheral tolerance to self-antigens. In the steady state, iDCs take up proteins from cells undergoing normal cell turnover (1a). In the absence of inflammatory signals, DCs remain immature but may still enter regional lymph nodes (2a). Precursors of Tr cells (Trp) encounter self-antigen on iDCs and are primed to become regulatory rather than effector T cells (3a). This step may require that antigen is “handed off” to specialized DCs (in blue), which are dedicated to priming Tr cells. Primed Tr cells home to the tissue and have suppressive effects, mediated by inhibitory cytokines and cell surface molecules, that ensure that DCs remain in an immature state in the absence of inflammation (4a). During an infection, iDCs take up self-antigens in the context of maturation signals (1b). mDCs migrate to regional lymph nodes (2b) and prime naive CD4+ or CD8+ T cells to become effector T cells (3b). Here, DC1 cells (in green) may polarize CD4+ cells toward a Th1 phenotype, whereas DC2 cells (in yellow) would polarize toward a Th2 phenotype. T effector cells migrate to the site of inflammation, where they are regulated by Tr cells (4b). CD4+ or CD8+ Tr cells specific for self-antigens regulate immune responses by producing immunoregulatory cytokines such as IL-10 and TGF-β or by directly suppressing activated Th1 cells via a mechanism that requires cell–cell contact.
The primary function of iDCs in vivo would be to prime Tr cells and generate tolerance to self-antigens, but iDCs may also induce Tr cells specific for foreign peptides, as described for CD8+ Tr cells specific for the influenza matrix peptide 12 or CD4+ Tr cells specific for alloantigens 11. This model is consistent with many data indicating that the generation of Tr cells depends on the environmental context in which a T cell encounters its antigen (self or foreign), and that altered expression of costimulatory molecules on APCs and/or exposure to immunoregulatory cytokines 26 can direct DCs toward tolerogenic rather than stimulatory cells 27.
In contrast, in the context of an inflammatory environment when the immune system senses “danger,” DCs encounter maturation stimuli (e.g., viral and microbial components such as LPS and dsRNA) that cause them to undergo morphological and functional modifications that guide mature DCs (mDCs) into regional nodes 28. Here, mDCs express high levels of co-stimulatory molecules and stimulatory cytokines that together result in the priming of antigen-specific immune effectors 28 rather than Tr cells (Fig. 1). Depending on the ratio of mDCs to naive T cells 29 or on the presence of different subsets of mDCs (i.e., DC1 versus DC2; reference 30), naive T cells may be polarized toward distinct effector phenotypes (i.e., Th1 versus Th2).
Is There a Unique DC Subset Dedicated to Priming Tr Cells?
The notion that iDCs prime naive T cells to become Tr is attractive. However, the body of literature supporting a role for specialized subsets of “tolerogenic” DCs cannot be ignored 31 32. Indeed, in humans, lymphoid-derived DCs (the so-called DC2 cells; reference 33) have been shown to polarize naive T cells toward Th1 cells 34 and Th2 cells or IL-10–producing T cells when infected by viruses that induce high levels of IFN-α production 35. Interestingly, we have recently shown that IFN-α synergizes with IL-10 to induce the differentiation of Tr1 cells (unpublished data), suggesting that via the production of large amounts of IFN-α, DC2 cells may be critical for the induction of Tr1 cells in vivo. Thus, upon viral infection, DC2 cells may simultaneously alert the innate and adaptive immune responses by producing type I IFNs. In support of a role for both IL-10 and IFN-α in regulating responses to viral infections, it has been shown that in the absence of IL-10 36 or signals from IFN-α 37, the host suffers from detrimental effects of excessive cellular immune responses elicited during acute infection.
The primary function of Tr cells that differentiate upon priming by DC2 cells during viral infections would not be to maintain tolerance to self, but rather to downregulate T cell responses to foreign peptides. These Tr cells would become fully mature after repeated antigen stimulation so that their regulatory effects would only be operational after the viral infection is cleared. This hypothesis correlates with many data indicating that multiple stimulations are required for the induction of functional Tr1 cells 11 13. To allow the immune system to control a second infection with the same agent, we may also speculate that virus-specific Tr cells are relatively short-lived cells. Indeed, Dhodapkar et al. 12 report in this issue that the number of matrix protein–specific Tr cells decreases as quickly as 30 d after in vivo immunization with iDCs.
Mechanisms of Action of Tr Cells.
Important issues that must be addressed are how Tr cells exert their powerful regulatory effects and on which target cells. After priming, antigen-specific Tr cells exit the regional nodes and circulate in peripheral tissues. We hypothesize that Tr cells can control/regulate immune responses at the level of both DC maturation and antigen-specific T cell expansion (Fig. 1).
In the steady state, the major function of Tr cells would be to maintain DCs in an immature state via cell–cell contact and secretion of cytokines such as IL-10 and TGF-β (Fig. 1). IL-10 downregulates the antigen presenting capacity of many APCs, including bone marrow–derived DCs and Langerhans cells, by downregulating MHC class II and a number of costimulatory molecules, including CD80, CD86, and CD154 38. Similarly, TGF-β downregulates MHC class II and prevents upregulation of CD80 and CD86 39 40.
During an inflammatory response, the major role of Tr cells resident in the tissue would be to control self-reactive effector T cells that “accidentally” arise during an immune response to pathogens. In this situation, cell death occurs with the consequent uptake of cellular debris and presentation of both pathogen-derived and self/harmless antigens by mDCs. Therefore, effector T cells are primed in the lymph node by mDCs that present both MHC–self-peptide and MHC–foreign peptide complexes. These effector T cells recirculate, reach the original tissue, and ultimately mount an effector response against both foreign and self-antigens. We can hypothesize that Tr cells specific for many self/harmless antigens already exist at the tissue site before foreign antigen exposure, and at the onset of inflammation they are rapidly recruited to suppress the effector function of self-reactive mature T cells. Thus, during an inflammatory immune response, the major function of Tr cells would be to block the effector function of self-reactive mature T cells. Jonuleit et al. 11 elegantly demonstrate that IL-10–producing Tr cells can act directly on in vitro–activated Th1 cells and inhibit antigen-specific proliferative responses (Fig. 1). Despite the fact that Tr cells differentiated by iDCs produce high levels of IL-10, this cytokine appeared to be dispensable for their ability to suppress Th1 cell lines activated by mDCs, and Tr cells must make direct contact with Th1 cells to exert their regulatory effects. This observation is consistent with previous reports that IL-10 does not have suppressive effects on either mDCs or activated Th1 cells 26 38 41 but does not exclude a role for IL-10–mediated suppression of iDC and/or activation of resting T cells, as previously described 18 26.
Despite the fact that regulation of mature T cell responses by Tr cells requires cell–cell contact and is not mediated by suppressive cytokines, Jonuleit et al. 11 claim that Tr cell–mediated suppression is not antigen specific. Therefore, it remains to be determined how Tr cells present at the tissue site would suppress responses of mature T cells specific for self-peptides but not the effector function of T cells specific for foreign pathogenic peptides. One possibility is that the number of T cells specific for the harmful antigen is so high that any bystander suppression mediated by Tr cells is ineffective. Alternatively, it is possible that some level of specificity in the suppressive effect mediated by Tr cells does exist. For example, it is possible that Tr cells deliver a negative signal by recognizing a specific molecule present only on self-reactive T cells.
Finally, we should consider that tissue-resident Tr cells may have restricted specificity and therefore it is unlikely that their TCR repertoire will cover all possible self or harmless antigens. Thus, as discussed above, during an effector immune response, de novo Tr cells may be primed by specialized DCs and migrate to the site of infection to ensure that both the ongoing antipathogen and antiself/harmless immune responses are self limiting.
Whatever the real mechanism for this ability to distinguish between self/harmless and foreign antigens in an inflammatory environment may be, evidence that many autoimmune diseases are triggered by infectious agents 42 suggests that it may not be as robust as other responses of the immune system.
Clinical Implications.
The observation by Dhodapkar et al. 12 that immunization with iDCs results in downregulation of CD8+ T cell responses to recall antigens and induction of IL-10–producing T cells, together with the demonstration by Jonuleit et al. 11 that iDC-induced Tr1 cells can suppress responses of activated T cells, opens new therapeutic perspectives for the use of iDCs in autoimmune diseases and allogenic transplantation. Using a protocol similar to what is described in these two studies, in vitro pulsing of iDCs with self- (e.g., glutamic acid decarboxylase in diabetes or myelin basic protein in multiple sclerosis) or alloantigens and subsequent injection could lead to the in vivo generation of a Tr cell population able to downregulate self/alloreactivity mediated by both Th1 and CD8+ T cells. A major caveat to this experimental approach is that iDCs are not likely to remain in the immature state in vivo after recirculation and homing to the damaged tissues where chronic inflammation is always present. Alternatively, vaccination with self/allopeptides together with cytokines able to expand the number of iDCs (e.g., FLT-3 ligand 43 and to preserve their immaturity (e.g., IL-10 or TGF-β) could be used to induce the Tr cells in vivo. Preclinical studies in animal models of autoimmunity and organ transplantation are warrant to test both approaches.
Another important conclusion that can be drawn from these two studies is that the use of iDCs for vaccination with tumor antigens must be avoided. Indeed, this approach would result in downregulation of immune responses against the tumor rather than the desired opposite effect. Therefore, the maturation stage of DCs used for in vitro loading with tumor antigens should be carefully monitored.
Finally, these studies may provide useful information for in vitro differentiation and expansion of Tr cells for cellular therapy of immune-mediated pathologies such as acute organ rejection.
Acknowledgments
We are grateful to V. Russo for helpful discussion.
The authors' work is partially supported by grants from the Italian Telethon Foundation, the Italian Association for Cancer Research (AIRC), and the Italian Ministry of Health. M.K. Levings is a postdoctoral fellow of the Canadian Institutes for Health Research.
References
- Vandekerckhove B.A., Namikawa R., Bacchetta R., Roncarolo M.G. Human hematopoietic cells and thymic epithelial cells induce tolerance via different mechanisms in the SCID-hu mouse thymus. J. Exp. Med. 1992;175:1033–1043. doi: 10.1084/jem.175.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisielow P., Bluthmann H., Staerz U.D., Steinmetz M., von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Rocha B., von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991;251:1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- Jenkins M.K., Schwartz R.H. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J. Exp. Med. 1987;165:302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach E.M. Regulatory T cells in autoimmmunity. Annu. Rev. Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- Fairchild P.J., Waldmann H. Dendritic cells and prospects for transplantation tolerance. Curr. Opin. Immunol. 2000;12:528–535. doi: 10.1016/s0952-7915(00)00134-5. [DOI] [PubMed] [Google Scholar]
- Steinman R.M., Turley S., Mellman I., Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J. Exp. Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabbe S., Kampgen E., Schuler G. Dendritic cellsmulti-lineal and multi-functional. Immunol. Today. 2000;21:431–433. doi: 10.1016/s0167-5699(00)01694-7. [DOI] [PubMed] [Google Scholar]
- Reid S.D., Penna G., Adorini L. The control of T cell responses by dendritic cell subsets. Curr. Opin. Immunol. 2000;12:114–121. doi: 10.1016/s0952-7915(99)00059-x. [DOI] [PubMed] [Google Scholar]
- Chang C.C., Wright A., Punnonen J. Monocyte-derived CD1a+ and CD1a- dendritic cell subsets differ in their cytokine production profiles, susceptibilities to transfection, and capacities to direct Th cell differentiation. J. Immunol. 2000;165:3584–3591. doi: 10.4049/jimmunol.165.7.3584. [DOI] [PubMed] [Google Scholar]
- Jonuleit H., Schmitt E., Schuler G., Knop J., Enk A.H. Induction of interleukin 10–producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogenic immature human dendritic cells. J. Exp. Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhodapkar M.V., Steinman R.M., Krasovsky J., Munz C., Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J. Exp. Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncarolo M.G., Levings M.K. The role of different subsets of T regulatory cells in controlling autoimmunity. Curr. Opinion. Immunol. 2000;12:676–683. doi: 10.1016/s0952-7915(00)00162-x. [DOI] [PubMed] [Google Scholar]
- Seddon B., Mason D. The third function of the thymus. Immunol. Today. 2000;21:95–99. doi: 10.1016/s0167-5699(99)01559-5. [DOI] [PubMed] [Google Scholar]
- Suri-Payer E., Amar A.Z., Thornton A.M., Shevach E.M. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J. Immunol. 1998;160:1212–1218. [PubMed] [Google Scholar]
- Takahashi T., Tagami T., Yamazaki S., Uede T., Shimizu J., Sakaguchi N., Mak T.W., Sakaguchi S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte–associated antigen 4. J. Exp. Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S., Malmstrom V., Powrie F. Cytotoxic T Lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J. Exp. Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux H., O'Garra A., Bigler M., Rouleau M., Antonenko S., de Vries J.E., Roncarolo M.G. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- Cottrez F., Hurst S.D., Coffman R.L., Groux H. T regulatory cells 1 inhibit a Th2-specific response In vivo. J. Immunol. 2000;165:4848–4853. doi: 10.4049/jimmunol.165.9.4848. [DOI] [PubMed] [Google Scholar]
- Colovai A.I., Liu Z., Ciubotariu R., Lederman S., Cortesini R., Suciu-Foca N. Induction of xenoreactive CD4+ T-cell anergy by suppressor CD8+CD28- T cells. Transplantation. 2000;69:1304–1310. doi: 10.1097/00007890-200004150-00016. [DOI] [PubMed] [Google Scholar]
- Li J., Liu Z., Jiang S., Cortesini R., Lederman S., Suciu-Foca N. T suppressor lymphocytes inhibit NF-kappa B-mediated transcription of CD86 gene in APC. J. Immunol. 1999;163:6386–6392. [PubMed] [Google Scholar]
- Huang F.P., Platt N., Wykes M., Major J.R., Powell T.J., Jenkins C.D., MacPherson G.G. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med. 2000;191:435–444. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao M., Onai N., Hiroishi K., Watkins S.C., Matsushima K., Robbins P.D., Lotze M.T., Tahara H. CC chemokine receptor-7 on dendritic cells is induced after interaction with apoptotic tumor cellscritical role in migration from the tumor site to draining lymph nodes. Cancer Res. 2000;60:2209–2217. [PubMed] [Google Scholar]
- Turley S.J., Inaba K., Garrett W.S., Ebersold M., Unternaehrer J., Steinman R.M., Mellman I. Transport of peptide-MHC class II complexes in developing dendritic cells. Science. 2000;288:522–527. doi: 10.1126/science.288.5465.522. [DOI] [PubMed] [Google Scholar]
- Inaba K., Turley S., Yamaide F., Iyoda T., Mahnke K., Inaba M., Pack M., Subklewe M., Sauter B., Sheff D. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J. Exp. Med. 1998;188:2163–2173. doi: 10.1084/jem.188.11.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrink K., Wolfl M., Jonuleit H., Knop J., Enk A.H. Induction of tolerance by IL-10-treated dendritic cells. J. Immunol. 1997;159:4772–4780. [PubMed] [Google Scholar]
- Banchereau J., Briere F., Caux C., Davoust J., Lebecque S., Liu Y.J., Pulendran B., Palucka K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Demeure C.E., Rubio M., Delespesse G., Sarfati M. Human monocyte-derived dendritic cells induce naive T cell differentiation into T helper cell type 2 (Th2) or Th1/Th2 effectorsrole of stimulator/responder ration. J. Exp. Med. 2000;192:405–411. doi: 10.1084/jem.192.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissoan M.C., Soumelis V., Kadowaki N., Grouard G., Briere F., de Waal Malefyt R., Liu Y.J. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–1186. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- Suss G., Shortman K. A subclass of dendritic cells kills CD4 T cells via Fas/Fas-ligand–induced apoptosis. J. Exp. Med. 1996;183:1789–1796. doi: 10.1084/jem.183.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronin V., Winkel K., Suss G., Kelso A., Heath W., Kirberg J., von Boehmer H., Shortman K. A subclass of dendritic cells regulates the response of naive CD8 T cells by limiting their IL-2 production. J. Immunol. 1996;157:3819–3827. [PubMed] [Google Scholar]
- Siegal F.P., Kadowaki N., Shodell M., Fitzgerald-Bocarsly P.A., Shah K., Ho S., Antonenko S., Liu Y.J. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- Cella M., Facchetti F., Lanzavecchia A., Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent Th1 polarization. Nat. Immunol. 2000;1:305–310. doi: 10.1038/79747. [DOI] [PubMed] [Google Scholar]
- Kadowaki N., Antonenko S., Lau J.Y., Liu Y.J. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J. Exp. Med. 2000;192:219–226. doi: 10.1084/jem.192.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzinelli R.T., Wysocka M., Hieny S., Scharton-Kersten T., Cheever A., Kuhn R., Muller W., Trinchieri G., Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma, and TNF-alpha. J. Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- Durbin J.E., Fernandez-Sesma A., Lee C.K., Rao T.D., Frey A.B., Moran T.M., Vukmanovic S., Garcia-Sastre A., Levy D.E. Type I IFN modulates innate and specific antiviral immunity. J. Immunol. 2000;164:4220–4228. doi: 10.4049/jimmunol.164.8.4220. [DOI] [PubMed] [Google Scholar]
- Moore K.W., O'Garra A., de Waal Malefyt R., Vieira P., Mosmann T.R. Interleukin-10. Annu. Rev. Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- Geissmann F., Revy P., Regnault A., Lepelletier Y., Dy M., Brousse N., Amigorena S., Hermine O., Durandy A. TGF-beta 1 prevents the noncognate maturation of human dendritic Langerhans cells. J. Immunol. 1999;162:4567–4575. [PubMed] [Google Scholar]
- Strobl H., Knapp W. TGF-beta1 regulation of dendritic cells. Microbes Infect. 1999;15:1283–1290. doi: 10.1016/s1286-4579(99)00256-7. [DOI] [PubMed] [Google Scholar]
- Steinbrink K., Jonuleit H., Muller G., Schuler G., Knop J., Enk A.H. Interleukin-10-treated human dendritic cells induce a melanoma-antigen-specific anergy in CD8(+) T cells resulting in a failure to lyse tumor cells. Blood. 1999;93:1634–1642. [PubMed] [Google Scholar]
- Horwitz M.S., Sarvetnick N. Viruses, host responses, and autoimmunity. Immunol. Rev. 1999;169:241–253. doi: 10.1111/j.1600-065X.1999.tb01319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson E., Westrich G.M., Viney J.L. Modulating dendritic cells to optimize mucosal immunization protocols. J. Immunol. 1999;163:3668–3675. [PubMed] [Google Scholar]