Ex Vivo Isolation and Characterization of Cd4+Cd25+ T Cells with Regulatory Properties from Human Blood (original) (raw)
Abstract
It has been known for years that rodents harbor a unique population of CD4+CD25+ “professional” regulatory/suppressor T cells that is crucial for the prevention of spontaneous autoimmune diseases. Here we demonstrate that CD4+CD25+CD45RO+ T cells (mean 6% of CD4+ T cells) are present in the blood of adult healthy volunteers. In contrast to previous reports, these CD4+CD25+ T cells do not constitute conventional memory cells but rather regulatory cells exhibiting properties identical to their rodent counterparts. Cytotoxic T lymphocyte–associated antigen (CTLA)-4 (CD152), for example, which is essential for the in vivo suppressive activity of CD4+CD25+ T cells, was constitutively expressed, and remained strongly upregulated after stimulation. The cells were nonproliferative to stimulation via their T cell receptor for antigen, but the anergic state was partially reversed by interleukin (IL)-2 and IL-15. Upon stimulation with allogeneic (but not syngeneic) mature dendritic cells or platebound anti-CD3 plus anti-CD28 the CD4+CD25+ T cells released IL-10, and in coculture experiments suppressed the activation and proliferation of CD4+ and CD8+ T cells. Suppression proved IL-10 independent, yet contact dependent as in the mouse. The identification of regulatory CD4+CD25+ T cells has important implications for the study of tolerance in man, notably in the context of autoimmunity, transplantation, and cancer.
Keywords: CD4+CD25+ T cells, regulatory T cells, immune tolerance, anergy, dendritic cells
Introduction
Immunological self-tolerance is critical for the prevention of autoimmunity and maintenance of immune homeostasis. The ability of the immune system to discriminate between self and nonself is controlled by mechanisms of central and peripheral tolerance. Central tolerance involves deletion of self-reactive T lymphocytes in the thymus at an early stage of development 1 2. Several mechanisms of peripheral tolerance have been described, including T cell anergy and ignorance 1 2 3 4. Studies ongoing for more than a decade in rodents have provided firm evidence for the existence of a unique CD4+CD25+ population of “professional” regulatory/suppressor T cells that actively and dominantly prevent both the activation and the effector function of autoreactive T cells that have escaped other mechanisms of tolerance 5 6 7. The elimination or inactivation of these cells resulted in severe autoimmune disease and was also found to enhance immune responses to alloantigens and even tumors 5 7 8. Recent studies have revealed that the CD4+CD25+ regulatory T (Tr) cells constitute a rather homogenous population 9, derive from the thymus 7, and are naturally nonproliferative (i.e., anergic) to stimulation via the TCR but require activation via their TCR to become suppressive and to inhibit the proliferation of CD4+ or CD8+ T cells. However, once activated their regulatory/suppressor function was completely antigen-nonspecific and cytokine-independent, yet cell contact–dependent 10. The exact mechanisms of suppression, notably the cell surface and/or short-range soluble molecules involved in the T cell–T cell interaction, have yet to be characterized. New in vitro data suggest that the CD4+CD25+ T cells inhibit the proliferation of responders by inhibiting their IL-2 production 11. Recent in vivo studies suggest that the function of CD4+CD25+ T cells is crucially dependent on signaling via the cytotoxic T lymphocyte–associated antigen (CTLA)-4/CD152 molecule which was found to be constitutively expressed on CD4+CD25+ T cells 12 13 14.
Although it has been evident for years that the CD4+CD25+ T cell population constitutes a unique lineage of professional regulatory/suppressor T cells crucial for the prevention of spontaneous autoimmune disease 5 it is, quite surprisingly, totally unknown to date whether CD4+ T cells exhibiting similar functional properties are naturally present in man. In this study we show that this is indeed the case, and that the CD4+CD25+ T cells in the peripheral blood of adult healthy volunteers in contrast to previous reports are not conventional memory cells 15 16 17 but rather regulatory cells exhibiting functional properties identical to their rodent counterparts.
Materials and Methods
Culture Medium.
RPMI 1640 (BioWhittaker) supplemented with 1% heat-inactivated autologous plasma, 20 μg/ml gentamicin (Merck), and 2 mM glutamine (BioWhittaker) was used for the generation of dendritic cells (DCs), and X-VIVO-20 (BioWhittaker) supplemented with 1% heat-inactivated single donor human serum, 20 μg/ml gentamicin (Merck), and 2 mM glutamine (BioWhittaker) was used for T cell culture.
Cytokines.
All cytokines used in this study were recombinant human proteins. Final concentrations were: 1,000 U/ml GM-CSF (Leukomax™; Novartis); 800 U/ml IL-4 (Sandoz); and IL-2 (Proleukin; Chiron Corp.) and IL-15 (PeproTech) were used at the concentrations indicated; for DC maturation we used a cocktail consisting of 2 ng/ml IL-1β (Sigma Aldrich), 1,000 U/ml IL-6 (Sandoz), 10 ng/ml TNF-α (Bender), and 1 μg/ml prostaglandin E2 (Sigma Aldrich).
Abs.
For immunostaining PE- and FITC-conjugated Abs (all from BD PharMingen) against CD3 (UCHT1), CD4 (RPA-T4), CD5 (UCHT 2), CD8 (RPA-T8), CD14 (M5E2), CD19 (HIB 19), CD25 (M-A251), CD28 (CD28.2), CD45 RA (HI 100), CD45 RO (UCHL1), CD56 (B159), CD62L (DREG-56), CD80 (L307.4), CD83 HB15e), CD86 (FUN-1), CD95 (DX 2), CD95L (G247-4),CD122 (MiK-β2), CD152 (BNI3.1), CD154 (TRAP 1), HLA-DR (G46-6), and respective mouse and rat isotype controls were employed. Abs used for intracellular cytokine staining were FITC- and PE-conjugated anti–IL-2 (MQ1-17H12), anti–IL-4 (8D4-8), anti–IL-10 (JES3-19F1), and anti–IFN-γ (4S.B3), all from BD PharMingen. Unconjugated anti–IL-10 (JES3-19F1; BD PharMingen) and anti–TGF-β (R&D Systems) were used for neutralization experiments, and anti-CD3 (UCHT1) and anti-CD28 (CD28.2) were used for polyclonal activation of T cells.
Cytokine Assays.
T cells were stimulated with allogeneic DCs or with platebound anti-CD3 (10 μg/ml) plus soluble anti-CD28 (10 μg/ml) in X-VIVO-20 plus 1% serum. Cytokine analysis was performed at different time points by analysis of supernatants with commercially available ELISA kits for human IL-2, IL-4, IL-10, IFN-γ, and TGF-β (BD PharMingen). For analysis of intracellular cytokine production T cells were either stimulated with 20 ng/ml PMA and 500 μg/ml Ca2+ ionophore A23187 (both from Sigma Aldrich) for 6 h or with platebound anti-CD3 and soluble anti-CD28 Ab for 6 h. 2 μM monensin (Sigma Aldrich) was added for the last 5 h of culture. Cells were collected, washed, fixed and saponin permeabilized (fix/perm solution; BD PharMingen), and stained with cytokine-specific Ab or isotype.
For cytokine mRNA analysis, T cells were stimulated with platebound anti-CD3 and soluble anti-CD28 Ab. Cells were analyzed by RNase protection assay template sets (BD PharMingen).
Cell Isolation and DC Generation.
DCs were generated from buffy coats or leukapheresis products (both obtained from the Department of Transfusion Medicine, University of Erlangen-Nuremberg, from healthy donors after informed consent was given) as described previously 18 19. In brief, PBMCs were isolated by Ficoll density gradient centrifugation. Monocytes were isolated by plastic adherence and cultured in RPMI 1640 and supplemented with IL-4 and GM-CSF. At day 6 a maturation cocktail (IL-1β, IL-6, prostaglandin E2, and TNF-α) was added. At day 7 nonadherent cells were harvested and constituted mature DCs that were >90% double positive for costimulatory molecules (CD80, CD86) and CD83.
CD4+ T cells were isolated from PBMCs with a negative CD4+ T cell isolation kit (Miltenyi Biotec). CD4+CD25+ T cells were isolated from the pure, untouched CD4+ T cells using CD25 microbeads (Miltenyi Biotec). Isolation of CD8+ T cells was performed using a negative CD8+ T cell isolation kit (Miltenyi Biotec). Purity was assessed by FACS®.
Flow Cytometric Analysis.
For immunofluorescence staining, cells were washed and stained for 20 min at 4°C with optimal dilution of each Ab. Cells were washed again and analyzed by flow cytometry (FACScan™ and CELLQuest™ software; Becton Dickinson). For analysis of cell surface–CD152 expression, cells were stained with the appropriate Ab for 2 h at 37°C 14. For analysis of intracellular CD152, cells were stained for CD4 expression, fixed and saponin permeabilized (fix/perm solution; BD PharMingen), and stained with CD152-specific Ab or isotype.
Proliferation Assays.
To assess proliferation of different CD4+ subtypes, 105 sorted T cells were incubated in X-VIVO-20 with different numbers of DCs in 96-well U-bottom plates or different concentrations of platebound anti-CD3 plus soluble anti-CD28 in 96-well flat-bottomed plates. For assessment of regulatory properties 105 bulk CD4+ T cells were cultured with 5 × 103 (in some experiments also with 103 cells) DCs in 96-well U-bottom plates. Purified CD4+CD25+ or CD4+CD25− T cells were added at different concentrations. After 4–5 d of culture [3H]Tdr (37 kBq per well) was added for additional 16 h. Proliferation was measured using a liquid scintillation counter.
Transwell Experiments.
Transwell experiments were performed in 24-well plates. 106 bulk CD4+ T cells were stimulated with 5 × 104 DCs. In addition, 106 CD4+CD25+ or CD4+CD25− T cells were either added directly to the culture or were placed in transwell chambers (Millicell, 0.4 μm; Millipore). After 5 d of coculture T cells were transferred to 96-well plates (105 cells per well) in triplicates. Proliferation was measured after a 16-h pulse with [3H]Tdr using a liquid scintillation counter.
Results
CD4+CD25+ T Cells Show a Reduced Proliferative Response to Both Allogeneic and Polyclonal Stimulation.
A low proliferative potential is highly characteristic of the well-characterized regulatory CD25+CD4+ T cells in the murine system 5. To analyze the proliferative capacity of human CD4+ subpopulations we magnetically sorted CD4+ T cells for their expression of CD25. By using a MACS® CD4-negative selection kit and a positive selection for CD25 afterwards, we routinely obtained >95% pure population of CD4+CD25+ T cells (Fig. 1 A). These cells comprise ∼6% (2.8–17.2%, n = 20) of peripheral CD4+ T cells in the blood of the healthy adults we studied.
Figure 1.
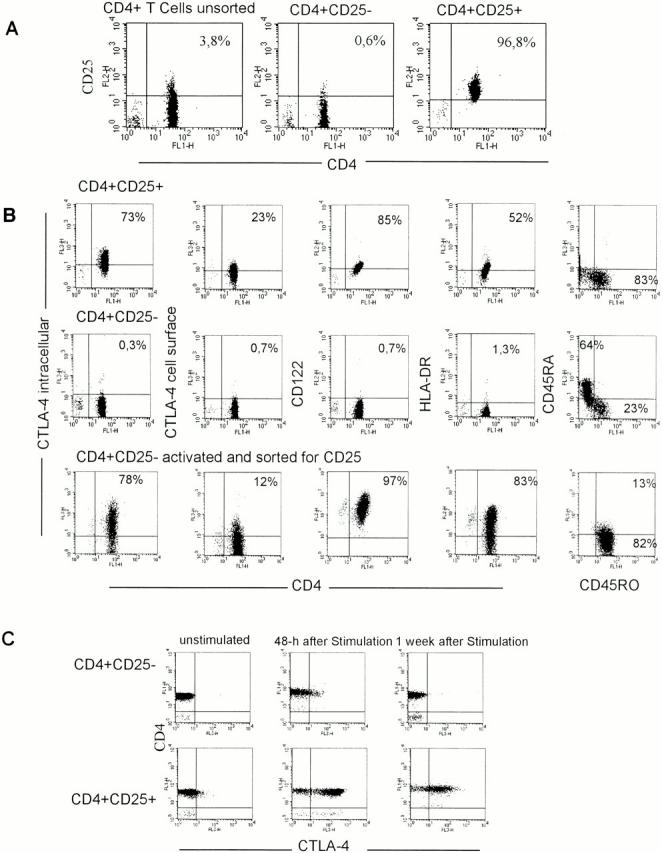
CD4+CD25+ T cells exhibit distinct phenotypical differences to CD4+CD25− T cells. CD4+ T cells were isolated from PBMCs by negative MACS® sorting yielding highly purified untouched CD4+ T cells. These cells were labeled with anti-CD25 magnetic beads and sorted. (A) Sorting resulted in virtually pure CD25+ T cells. A representative result out of 20 independent standardized experiments is shown. (B) The phenotype of CD4+CD25+, CD4+CD25−, and activated CD4+CD25− T cells was analyzed as described in Materials and Methods. In addition, CD4+CD25− T cells were activated with immobilized anti-CD3 plus soluble anti-CD28 for 48 h. After activation cells were labeled with anti-CD25 magnetic beads and sorted. Results were similar in five independent experiments. (C) CD4+CD25+ and CD4+CD25− T cells were stained with anti–CTLA-4 Ab at 37°C for 2 h. Staining was performed ex vivo and at different time points after activation with immobilized anti-CD3 plus soluble anti-CD28. One representative result of four independent experiments is shown.
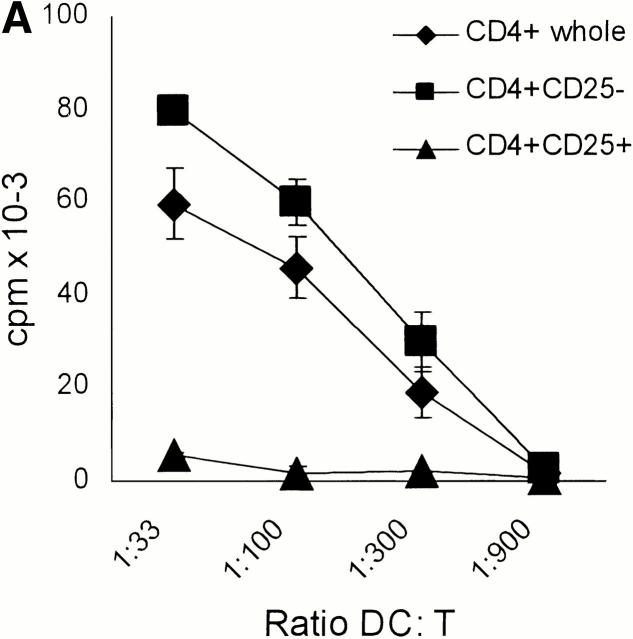
Mature DCs are known as the most powerful APCs 20. Nevertheless, the CD4+CD25+ T cells exhibited virtually no proliferative response when stimulated in vitro with fully mature allogeneic DCs in sharp contrast to the CD4+ CD25− T cells (Fig. 2 A) or the whole CD4+ population (Fig. 2 A). Interestingly, the CD4+ population depleted of CD25+ T cells showed a higher proliferation when stimulated with allogeneic DCs compared with the whole CD4+ population (Fig. 2 A).
Figure 2.
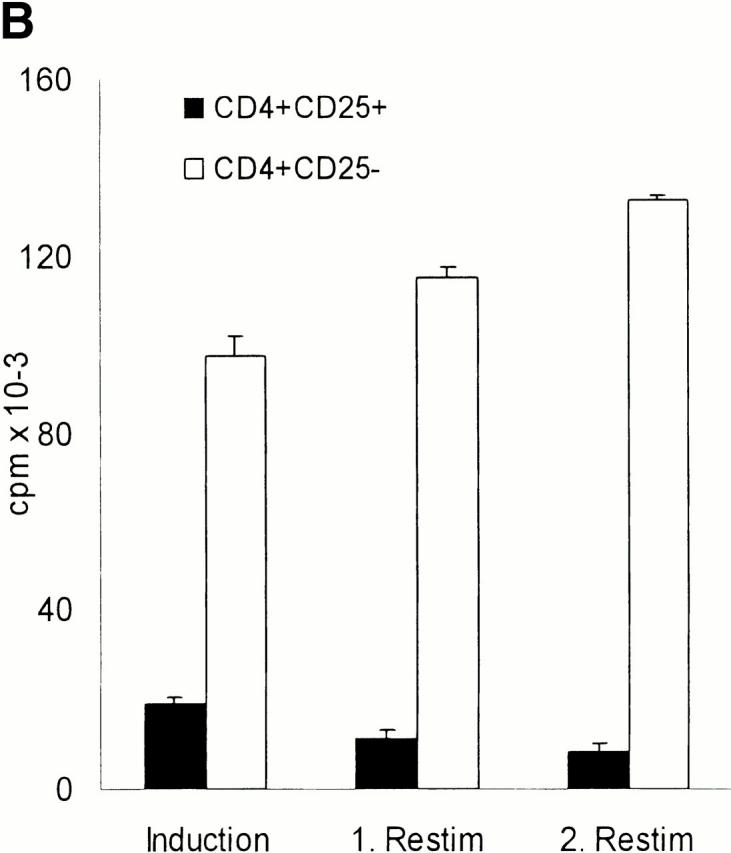
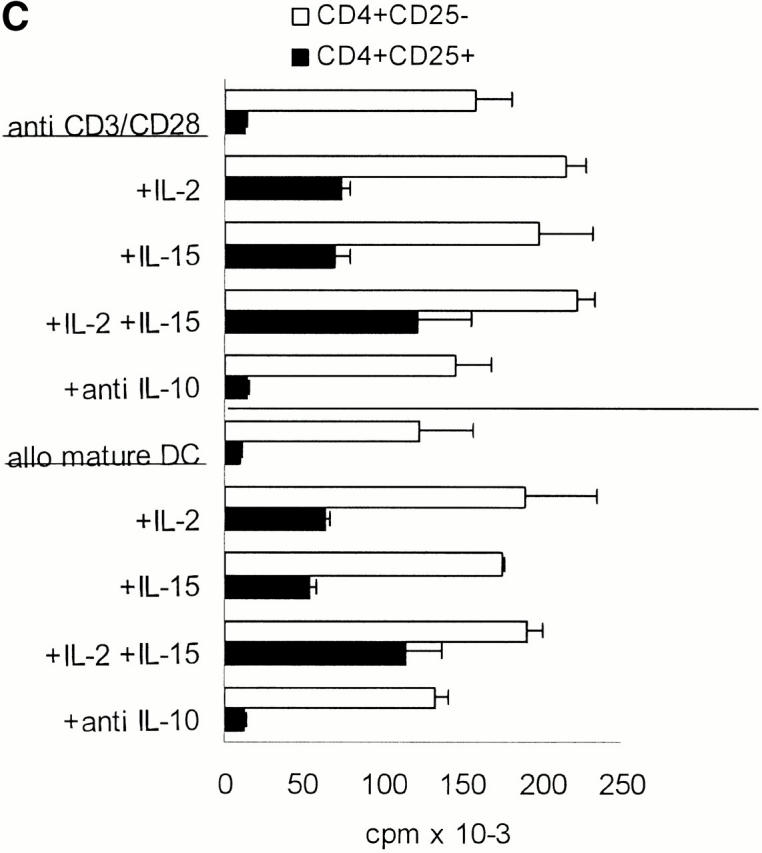
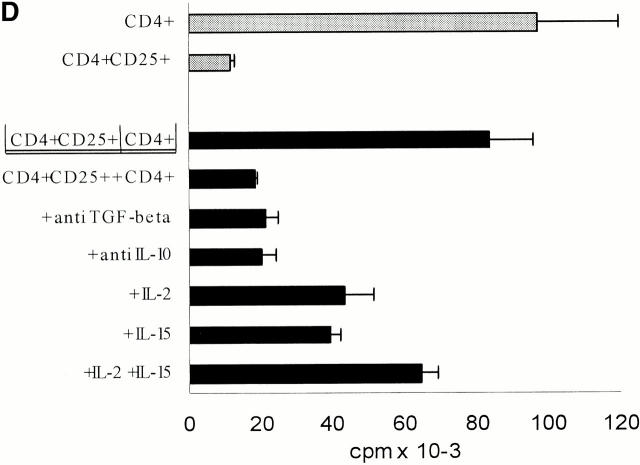
CD4+CD25+ T cells are nonproliferative/anergic to both allogeneic and polyclonal stimulation, which is partially reversed by the addition of IL-2 and/or IL-15, but not by neutralizing anti–IL-10 Abs. (A) Whole CD4+, CD4+CD25+, and CD4+CD25− T cells were isolated from adult blood by MACS® sorting as in Fig. 1. 105 T cells per 96 well were stimulated with different numbers of mature allogeneic DCs. Proliferation of T cells (triplicate cultures) was determined by [3H]Tdr incorporation. Results were similar in five independent experiments. (B) MACS®-sorted CD4+CD25+ and CD4+CD25− T cells were primed and restimulated every week with mature allogeneic DCs from the same donor (DC/T cell ratio of 1:20). Proliferation (105 T cells per 96 well) was determined by [3H]Tdr incorporation. Similar results were obtained in three independent experiments. (C) CD4+CD25+ and CD4+CD25− T cells were stimulated with 10 μg/ml immobilized anti-CD3 and 10 μg/ml soluble anti-CD28 (top) or with 5 × 103 mature allogeneic DCs (bottom) as described in (A). 500 U/ml IL-2, 100 ng/ml IL-15, a mixture of 10 U/ml IL-2 plus 1 ng/ml IL-15 or 10 μg/ml anti–IL-10 were added at the onset of culture. [3H]Tdr incorporation was measured after 5 d of culture. One of three independent experiments is shown. The addition of IL-2 and/or IL-15 in the absence of a polyclonal or allogeneic T cell stimulus did not induce significant proliferation in the CD25+ or CD25− T cell subset (data not shown).
We next sought to determine whether the CD4+CD25+ T cells would possibly only proliferate upon repetitive stimulation by mature DCs. After restimulation the proliferative response of CD25− T cells increased somewhat, whereas the response of CD25+ T cells remained very low (Fig. 2 B). Priming and restimulation by allogeneic mature DCs resulted in a 30–50-fold expansion of the CD25− population after two rounds of restimulation. In contrast, there was no significant increase of the CD25+ population (data not shown). We actually harvested a slightly (∼10%) decreased absolute number of CD4+CD25+ T cells as compared with the initial inoculum after the repetitive stimulation in the apparent absence of significant apoptosis or necrosis (data not shown).
The exceedingly low proliferative response of CD4+ CD25+ T cells was also apparent when these cell populations were polyclonally stimulated with platebound anti-CD3 plus soluble anti-CD28 (Fig. 2 C). To test whether the T cell growth factors IL-2 and IL-15 could affect the proliferative potential, various doses were added to CD4+CD25+ and CD4+CD25− T cells that were stimulated with either immobilized anti-CD3 plus soluble anti-CD28 (Fig. 2 C, top) or with mature allogeneic DCs (Fig. 2 C, bottom). A series of pilot experiments revealed that IL-2 enhanced the proliferation of CD25+ T cells only at high doses (100–1,000 U/ml). IL-15 had a similar effect, again only at very high doses of 50–100 ng/ml. When both cytokines were mixed, they had strong synergistic effects and doses of 10 U/ml IL-2 plus 10 ng/ml IL-15 were sufficient to promote the proliferation of CD4+CD25+ T cells. The addition of IL-2 and/or IL-15 in the absence of a polyclonal or allogeneic T cell stimulus did not induce significant proliferation in the CD25+ or CD25− T cell subset (data not shown).
CD4+CD25+ T Cells Exhibit Distinct Phenotypical Differences to CD4+CD25− T Cells.
To further characterize the CD25+CD4+ T cell population we compared the expression of various surface molecules on CD4+CD25+, CD4+ CD25−, and stimulated CD4+CD25− T cells (Fig. 1 B). All three populations showed homogenous expression of CD3 and CD4. No contaminating cells such as monocytes, B cells, CD8+ T cells, or NK cells could be observed by FACS® analysis (data not shown). Without prior stimulation, i.e., ex vivo, the CD25+ population already expressed high levels of intracellular and low levels of cell surface CTLA-4 (CD152). Furthermore, ex vivo–isolated CD4+CD25+ T cells constitutively expressed CD122 (IL-2 receptor [IL-2R] β chain), HLA-DR (∼50%), and consisted primarily (∼80%) of CD45RO cells resembling a memory T cell phenotype. In sharp contrast, the ex vivo–isolated CD4+CD25− T cells did not express CTLA-4 (neither intracellularly nor on the surface), CD122, or HLA-DR, and more cells expressed CD45RA rather than CD45RO. However, after activation with platebound anti-CD3 plus soluble anti-CD28 most CD4+CD25− became strongly CD25+ (the level of CD25 expression was ∼1 log higher compared with the CD4+CD25+ T cells, data not shown), and displayed high levels of HLA-DR and CD122 (again ∼1 log higher compared with CD4+CD25+ T cells) as to be expected. In addition, both intracellular and surface CTLA-4 was upregulated within 24–48 h yet quickly downregulated thereafter (Fig. 1 C, data not shown for intracellular CTLA-4) as expected. The kinetics of CTLA-4/CD152 expression proved strikingly different when CD4+CD25+ T cells were stimulated. These cells also upregulated their (constitutively already present albeit low) CD152 surface expression, yet the strong expression of CD152 remained constant for a period of >1 wk (Fig. 1 C). Staining with several other mAbs such as anti-CD28, -CD62L, -CD69, -CD95, -CD95L, and -CD154 (CD40 ligand) did not reveal reproducible and significant differences between CD4+CD25+ and CD4+CD25− T cells.
CD4+CD25+ T Cells if Stimulated via the TCR Suppress the Activation of CD4+ and CD8+ T Cells in a Cell Contact– and Dose-dependent Manner.
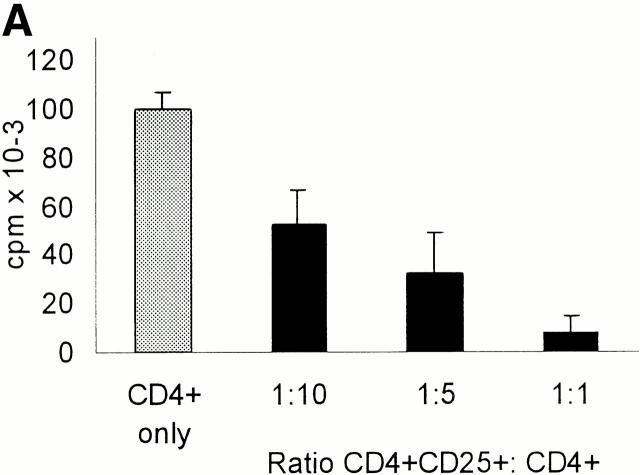
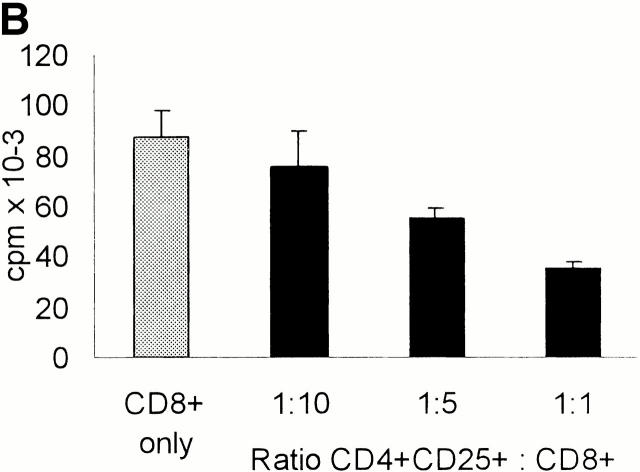
To analyze the putative regulatory properties of CD25+ T cells coculture experiments were performed. In a first series of tests we isolated from a particular donor both the total CD4+ population and the CD25+ and CD25− fractions. Whole CD4+ T cells were then mixed with CD4+CD25+ or CD4+CD25− T cell subpopulations at indicated ratios and stimulated with allogeneic mature DCs (Fig. 3 A). CD4+CD25+ T cells significantly inhibited the proliferation of whole CD4+ T cells and at a 1:1 ratio virtually blocked it (cpm then represented the background levels of CD25+ T cell proliferation, Fig. 2a Fig. b Fig. c). The addition of CD25− T cells instead of CD25+ T cells slightly enhanced proliferation (data not shown). As CD4+CD25− rapidly expressed CD25 and CD122, i.e., both chains of the IL-2R, upon polyclonal (Fig. 1 B) and stimulation by allogeneic DCs (data not shown), this finding indicated that the suppressive activity of the CD4+CD25+ T cell subset was not simply due to consumption or passive adsorption of IL-2 via their IL-2R. CD4+CD25+ T cells exerted also a suppressive activity on whole CD8+ T cells albeit downregulation was less intense (Fig. 3 B).
Figure 3.
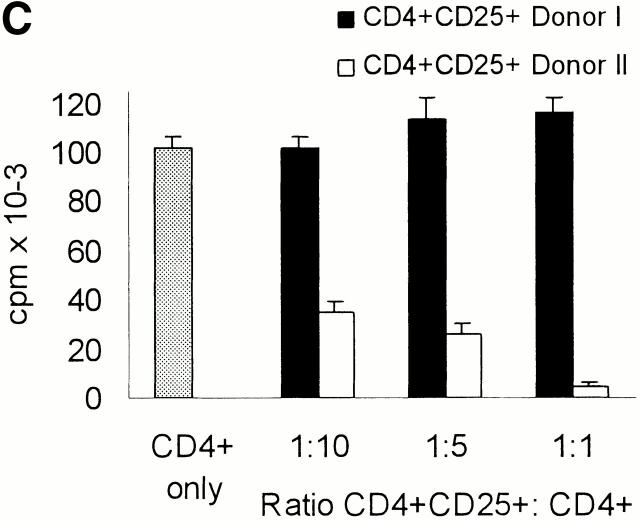
CD4+CD25+ T cells if stimulated via the TCR suppress the activation of CD4+ and CD8+ T cells in a cell contact– and dose-dependent manner. (A and B) MACS®-sorted whole CD4+ (A) and CD8+ (B) T cells (105 T cells per 96 well) were added to CD4+CD25+ T cells at the ratios indicated and stimulated with allogeneic DCs at a DC/CD4+ or CD8+ T cells ratio of 1:20. Proliferation was determined by [3H]Tdr incorporation after 5 d. One of five independent experiments is shown. (C) DCs and CD4+CD25+ T cells were generated/ isolated from the same donor (donor I). In addition, whole CD4+ T cells and CD4+ CD25+ T cells were isolated from another donor (donor II). 105 whole CD4+ T cells per 96 well were cultured with 5 × 103 DCs per well (i.e., DC/T ratio = 1:20; results were comparable at a DC/T ratio of 1:100, data not shown). CD4+CD25+ T cells from donor I and donor II were then added, respectively. Proliferation was determined by [3H]Tdr incorporation after 5 d of culture. Results are representative of three independent experiments shown as mean cpm of triplicate cultures. (D) Whole CD4+ T cells or CD4+CD25+ T cells were (105 T cells per 96 well) stimulated with 5 × 103 allogeneic mature DCs (DC/T ratio = 1:20) (upper two panels). In addition, whole CD4+ T cells were cocultured with CD4+CD25+ T cells at a ratio of 1:1 (105 T cells per 96 well each) and stimulated with allogeneic DCs again at a DC/T ratio of 1:20 in the presence or absence of 10 μg/ml anti–IL-10, 2 μg/ml anti–TGF-β, 500 U/ml IL-2, 50 ng/ml IL-15, or a mixture of 10 U/ml IL-2 and 1 ng/ml IL-15. In a parallel transwell approach the CD4+CD25+ T cells were stimulated with allogeneic DCs (DC/T cell ratio of 1:20) in a transwell chamber, and whole CD4+ T cell responders were put into the well together with allogeneic DCs again at a DC/T ratio of 1:20. Proliferation after 5 d of culture was determined by [3H]Tdr incorporation. One of four representative experiments is shown.
In a further set of experiments we wanted to determine whether activation of CD4+CD25+ T cells by syngeneic DCs was sufficient for induction of their regulatory properties. To this end mature DCs and CD4+CD25+ T cells were generated/isolated from the same donor (donor I). In addition, whole CD4+ T cells and the CD4+CD25+ T cell subset were isolated from another donor (donor II). The whole CD4+ T cells (donor II) were then stimulated with allogeneic mature DCs (donor I) in the absence (Fig. 3 C, CD4+ only) or presence of various numbers of CD4+ CD25+ T cells isolated from either donor I or donor II (Fig. 3 C). Whole CD4+ T cells from donor II proliferated vigorously as expected when stimulated with allogeneic, donor I–derived DCs (Fig. 3 C, CD4+ only). In the presence of donor I–derived CD4+CD25+ T cells (i.e., syngeneic to the DCs used) the proliferation (i.e., alloreactivity) of whole donor II–derived CD4+ T cells was not suppressed at all (Fig. 3 C). However, potent suppression occurred when donor II–derived CD4+CD25+ T cells (i.e., allogeneic to the DCs used) were added (Fig. 3 C). Suppression was also observed in experiments where DCs, whole CD4+ T cells, and CD4+CD25+ T cells were derived from three different donors (data not shown). These data indicated that TCR-mediated activation of CD4+ CD25+ T cells was required to let them exert their regulatory function and that syngeneic DCs were insufficient to induce their suppressive activity.
Next we performed transwell chamber experiments to investigate whether the regulatory function of the CD4+ CD25+ T cells was mediated primarily by soluble factors or required cell–cell contact (Fig. 3 D). As shown in Fig. 3 D the CD4+CD25+ T cells suppress proliferation of whole CD4+ T cells almost completely in the presence of allogeneic DCs. Separation of the two populations in transwell chambers virtually abolished their suppressive effect. These observations suggested that direct cell contact is essential for the inhibitory capacity of CD4+CD25+ T cells, as the semipermeable membrane of transwell chambers allows free passage of soluble factors, but excludes direct cell contact. The transwell experiments also confirmed that consumption of IL-2 by CD4+CD25+ T cells was not the mechanism responsible for suppression.
Despite the obvious requirement for close interaction between regulatory and responding cells neither a targeting of the antigen-presenting DCs or a role of soluble factors was excluded by the transwell experiments. Therefore, we also employed platebound anti-CD3 Ab in combination with soluble anti-CD28 Ab as an APC independent and polyclonal T cell stimulus. Whole CD4+ T cells alone showed strong proliferation upon this stimulation. As mentioned previously (Fig. 2 C), CD4+CD25+ T cells did not proliferate. In coculture of both populations, there was at least a 75% reduction at a 1:1 ratio compared with control (data not shown). These data suggested that regulation does not primarily occur via modulation of APC function. Neutralizing Abs to the cytokines IL-10 and TGF-β (critical for the suppressive activities of the so-called Tr1 cell and Th3, respectively; references 21, 22) did not abolish the regulatory activity of the CD4+CD25+ T cells demonstrating that these cytokines played no major suppressive role at least in the assays we looked at. The addition of IL-2 and/or IL-15 to cocultures at the high doses that promote the proliferation of CD4+CD25+ T cells (Fig. 2 C) reduced their inhibitory effects. However, the suppressive activity was likely not abolished as the significant proliferation of the CD4+CD25+ T cells has to be taken into account when interpreting the data (Fig. 3 D).
CD4_+CD25+_ T Cells Predominantly Secrete IL-10.
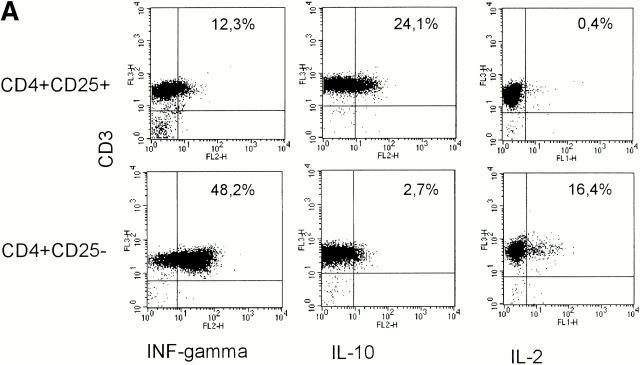
To analyze and compare the cytokine profiles, freshly sorted CD4+CD25+ and CD4+CD25− T cells were activated with platebound anti-CD3 plus anti-CD28. Supernatants were then analyzed by ELISA, and RNA expression was studied by RNase protection assays. In addition, intracellular cytokine staining was performed to determine the percentage of cells releasing a certain cytokine. As shown in Fig. 4, CD4+CD25− T cells predominantly secreted IFN-γ and IL-2 with little secretion of IL-10 and IL-4, resembling a Th1-like profile. On the other hand, CD4+CD25+ T cells predominantly produced IL-10 and only low levels of IL-2, IL-4, and IFN-γ, resembling Tr1 cells. Comparison of both subpopulations at the RNA level revealed that CD25+ T cells express more IL-10, less IFN-γ, and similar levels of IL-2 mRNA compared with CD25− T cells. IL-1 receptor antagonist mRNA was found predominantly in CD4+CD25+ T cells, whereas significant IL-1β mRNA levels were only present in CD4+CD25− T cells. TGF-β was expressed at similarly low levels in both cell types.
Figure 4.
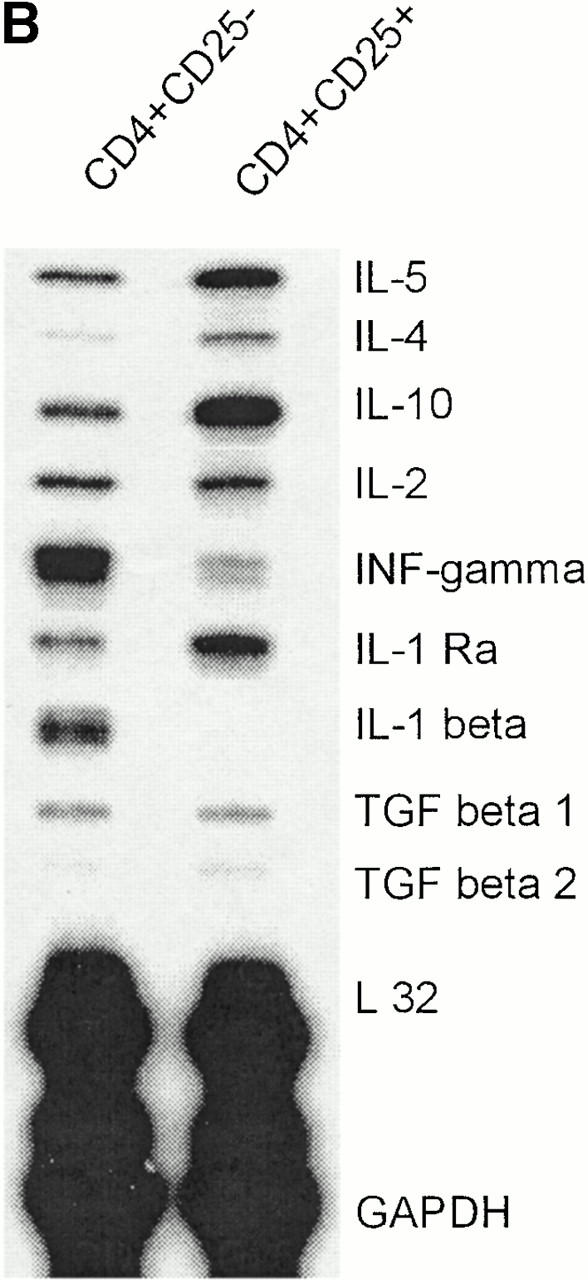
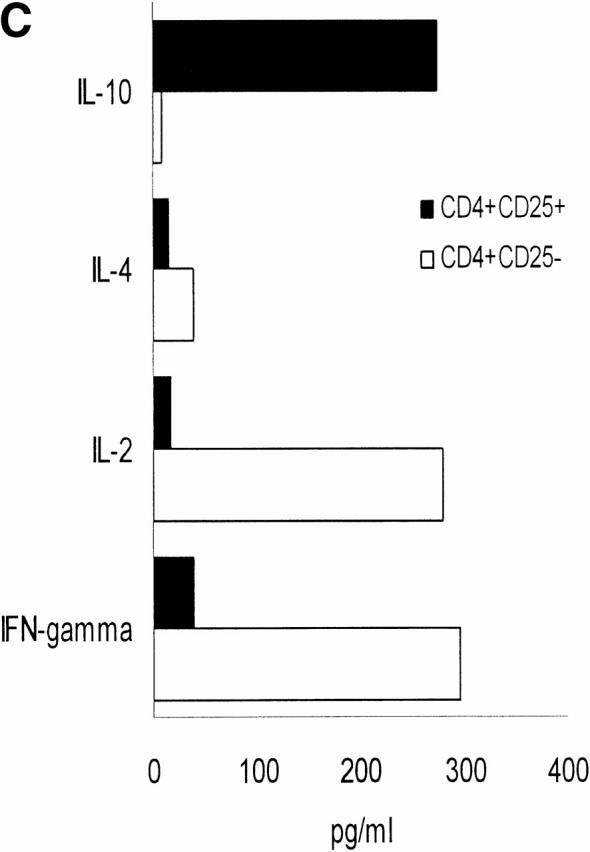
Different cytokine profiles of CD4+CD25+ and CD4+CD25− T cells. (A) MACS®-sorted CD4+CD25+ and CD4+CD25− T cells were stimulated with 20 ng/ml PMA and 500 μg/ml A23187 Ca2+ ionophore for 6 h. 2 μM monensin was added for the last 5 h. Staining of CD3 surface expression was performed. Cells were washed, fixed, permeabilized, and stained for detection of intracellular cytokines using FITC- or PE-conjugated specific Abs. One of nine independent experiments with similar results is shown. Results were identical when T cells were stimulated with platebound anti-CD3 plus soluble anti-CD28 Ab (data not shown). (B) CD4+CD25+ and CD4+CD25− T cells were activated with platebound anti-CD3 plus soluble anti-CD28. After 48 h of culture analysis of RNA expression was performed by RNase protection assay. (C) After treating cells as described in (B) cytokines in the supernatant were measured by ELISA (one of five independent experiments is shown).
Discussion
The concept of suppressor or immunoregulatory T cells has been revitalized during the past few years by the better delineation of several regulatory cell types in rodents, the mutual relationship of which is not yet finally defined. The so-called Tr1 and Th3 cells mediate bystander suppression - without need for direct cell contact - by the secretion of high levels of IL-10 and TGF-β, respectively 21 22. The best characterized and apparently most important Tr cell population identified so far are the CD4+CD25+ T cells. They occur naturally in rodents (representing ∼10% of CD4+ cells in lymphoid organs), are characterized by constitutive expression of CD25 (IL-2Rα), and are clearly of crucial importance for maintaining tolerance and preventing autoimmune disease in vivo. Surprisingly, a cell population exhibiting equivalent properties has not been described in humans to date. Here we have demonstrated that the CD4+CD25+ T cells in human blood that previously had been considered to represent conventional memory T cells 15 16 17 in fact appear to be the exact human counterpart of the unique CD4+CD25+ Tr cells that have been known and studied for many years in rodents. We were able to isolate the CD4+CD25+ T cells from adult blood in sizeable quantities (average 6% of CD4+ T cells) so that a detailed study and comparison to CD4+CD25− T cells could be undertaken. It turned out that the human cells share the key phenotypical and functional features with the murine CD4+CD25+ immunoregulatory T cells. The most interesting and previously unidentified phenotypical feature was that the CTLA-4 molecule (CD152) was already constitutively expressed (at high levels intracellularly and at low levels at the surface) by the human CD4+CD25+ T cells, was further upregulated after stimulation via the TCR, and maintained at high surface levels for at least a week after (in sharp contrast to CD4+CD25− T cells that expressed CTLA-4 de novo upon stimulation and only very transiently as described in references 23 and 24). The expression pattern of CTLA-4 by CD4+CD25− already supported their relationship to the murine CD4+CD25+ Tr cell as these cells constitutively express CTLA-4 as a molecule essential for their in vivo suppressive activity 12 13. Like their murine counterparts the human CD4+CD25+ T cells showed almost no proliferation upon stimulation, neither in response to polyclonal activation by platebound anti-CD3 plus anti-CD28 nor after (even repetitive) stimulation with the most potent natural immunostimulatory cells, i.e., mature (allogeneic) DCs. When these stimuli were combined with high doses of IL-2 (500 U/ml) anergy was partially reversed as described in the mouse 8. A novel finding was that IL-15 at high doses (50–100 ng/ml) induced comparable proliferation and that the combined action of IL-2 and IL-15 even at lower doses (10 U/ml and 10 ng/ml, respectively) had a strong synergistic action and induced vigorous proliferation. This might prove important as expansion of CD4+CD25+ T cells is vital for potential therapeutic applications and cloning of these cells for further more detailed studies (including mechanistic and molecular ones). Of interest was that neutralizing anti–IL-10 mAb failed to promote proliferation indicating that the release of IL-10 by these cells was not causing anergy in an autocrine fashion. In coculture experiments the CD4+ CD25+ T cells displayed another key feature in that they suppressed only upon activation via their own TCR the proliferation of CD4+CD25− or CD8+ T cells in a contact- and dose-dependent, yet cytokine-independent manner. Our ex vivo system has not allowed us to investigate whether the suppression is completely antigen nonspecific as has recently been shown in the mouse by taking advantage of TCR transgenic mice 8. However, respective mechanistic studies might be possible by using our IL-2 plus IL-15 approach for the expansion of these cells.
It is most remarkable that a recent report has shown that T cells with regulatory properties and a phenotype virtually identical to the CD4+CD25+ T cells we have isolated ex vivo from human blood can be generated in vitro by repetitive stimulation of human naive T cells with immature DCs 25. In the mouse CD4+CD25+ Tr cell populations are continuously generated in the thymus 7, yet the maintenance of Tr cells in the periphery requires the presence of tissue-specific antigens and IL-2 26 27. Based on the two supplementary findings (29, and this study) it is certainly tempting to speculate that immature DCs that have sampled peripheral tissues via the uptake of apoptotic cells 28 29, and present universal or tissue-specific autoantigens, are responsible for the survival and possibly slight proliferation of thymic Tr cell emigrants. We are currently testing whether the survival of the ex vivo–isolated CD4+CD25+ Tr cells can be promoted by interaction with immature DCs and whether the recently reported “generation” of CD4+CD25+ T cells from naive T cells by immature DCs in vitro 25 possibly rather represents maintenance of survival of preexisting CD4+CD25+ in the initial inoculum. It is also of note that we found that interaction of ex vivo–isolated human CD4+CD25+ T cells with syngeneic mature DCs was insufficient to activate their suppressive properties while allogeneic mature DCs were potent inducers of regulation. This observation again suggests testable hypotheses. For example, will immature in contrast to mature syngeneic DCs activate CD4+CD25+ T cells (suggesting that they carry some specific ligand for interaction), or will they do so only after ingestion of apoptotic bodies (suggesting that presentation of autoantigens is required)? Furthermore, can mature DCs that present nominal recall antigens (e.g., influenza proteins/peptides) stimulate T cells both in the CD4+CD25− T cell population and the CD4+CD25+ T cells suggesting that besides autoantigens also the recognition of foreign antigens could trigger regulation at inflammatory sites?
In summary, we demonstrate that a sizeable population (∼6%) of CD4+CD25+ T cells exists in the peripheral blood of normal human adults that in contrast to previous belief do not represent conventional memory but rather Tr cells equivalent to the unique population of CD4+CD25+ professional regulatory/suppressor T cells that have been studied for years in rodents. The identification and characterization of the human CD4+CD25+ Tr cells will now allow for their monitoring in various disease states and has important implications for understanding and treating autoimmunity, graft rejection, and cancer.
Acknowledgments
This work was supported by the German Science Foundation (SFB 263, C13).
References
- Rocha B., von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991;251:1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Bluthmann H., Staerz U.D., Steinmetz M., von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+CD8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Schwartz R.H. A cell culture model for T lymphocyte clonal anergy. Science. 1990;248:1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- Miller J.F.P., Heath W.R. Self-ignorance in the peripheral T-cell pool. Immunol. Rev. 1993;133:131–150. doi: 10.1111/j.1600-065x.1993.tb01514.x. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S., Sakaguchi N., Asano M., Itoh M., Toda M. Immunologic self-tolerance by activated T cells expressing IL-2 receptor α chains (CD25)breakdown of a single mechanism of self-tolerance causes various autoimmune disease. J. Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- Takahashi T., Kuniyasu Y., Toda M., Sakaguchi N., Itoh M., Itawata J M., Shimizu, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cellsinduction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- Itoh M., Takahashi T., Sakaguchi N., Kuniyasu Y., Shimizu J., Otsuka F., Sakaguchi S. Thymus and autoimmunityproduction of CD4+CD25+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J. Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- Shimizu J., Yamazaki S., Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cellsa common basis between tumor immunity and autoimmunity. J. Immunol. 1999;163:5211–5218. [PubMed] [Google Scholar]
- Thornton A.M., Shevach E.M. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J. Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- Shevach E.M. Regulatory T cells in autoimmunity. Annu. Rev. Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- Thornton A.M., Shevach E.M. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S., Malmstrom V., Powrie F. Cytotoxic T lymphocyte–associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon B., Lentschow D.J., Rhee L., Ashourian N., Singh B., Sharpe A., Bluestone J.A. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Tagami T., Yamazaki S., Uede T., Shimizu J., Sakaguchi N., Mak T.W., Sakaguchi S. Immunologic self-tolerance is maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte–associated antigen 4. J. Exp. Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanegane H., Miyawaki T., Kato K., Yokoi T., Uehara T., Yachie A., Taniguchi N. A novel subpopulation of CD45RA+CD4+ T cells expressing IL-2 receptor α-chain (CD25) and having a functionally transitional nature into memory cells. Int. Immunol. 1991;3:1349–1356. doi: 10.1093/intimm/3.12.1349. [DOI] [PubMed] [Google Scholar]
- Taga K., Kasahara Y., Yachie A., Miyawaki T., Taniguchi N. Preferential expression of IL-2 receptor subunits on memory population within CD4+ and CD8+ T cells. Immunol. 1990;72:15–19. [PMC free article] [PubMed] [Google Scholar]
- Jackson A.L., Matsumo H., Janszen M., Maino V., Blidy A., Shye S. Restricted expression of p55 interleukin 2 receptor (CD25) on normal T cells. Clin. Immunol. Immunopathol. 1990;54:126–133. doi: 10.1016/0090-1229(90)90012-f. [DOI] [PubMed] [Google Scholar]
- Romani N., Reider D., Heuer M., Ebner S., Kampgen E., Eibl B., Niederwieser D., Schuler G. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J. Immunol. Methods. 1996;196:137–151. doi: 10.1016/0022-1759(96)00078-6. [DOI] [PubMed] [Google Scholar]
- Thurner B., Roder C., Dieckmann D., Heuer M., Kruse M., Glaser A., Keikavoussi P., Kampgen E., Bender A., Schuler G. Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. J. Immunol. Methods. 1999;223:1–15. doi: 10.1016/s0022-1759(98)00208-7. [DOI] [PubMed] [Google Scholar]
- Bancherau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Groux H., O'Garra A., Bigler M., Rouleau M., Antonenko S., de Vries J.E., Roncarolo M.G. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevent colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- Fukaura H., Kent S.C., Pietrusewicz M.J., Khoury S.J., Weiner H.L., Hafler D.A. Induction of circulating myelin basic protein and proteolipid protein-specific transforming growth factor-β1-secreting Th3 T cells by oral administration of myelin in multiple sclerosis patients. J. Clin. Invest. 1996;98:70–77. doi: 10.1172/JCI118779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C.B., Allison J.P. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7:445–450. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- Chambers C.A., Krummel M.F., Boitel B., Hurwitz A., Sullivan T.J., Fourneir S., Cassell D., Brunner M., Allison J.P. The role of CTLA-4 in the regulation and initiation of T-cell responses. Immunol. Rev. 1996;153:27–46. doi: 10.1111/j.1600-065x.1996.tb00919.x. [DOI] [PubMed] [Google Scholar]
- Jonuleit H., Schmitt E., Schuler G., Knop J., Enk A.H. Induction of interleukin 10–producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J. Exp. Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papiernik M., de Moraes M.L., Pontoux C., Vasseur F., Penit C. Regulatory CD4 T cellsexpression of IL-2R α chain, resistance to clonal deletion and IL-2 dependency. Int. Immunol. 1998;10:5317–5321. doi: 10.1093/intimm/10.4.371. [DOI] [PubMed] [Google Scholar]
- Seddon B., Mason D. Peripheral autoantigen induces regulatory T cells that prevent autoimmunity. J. Exp. Med. 1999;189:877–890. doi: 10.1084/jem.189.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R.M., Turley S., Mellman I., Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J. Exp. Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncarolo M.G., Levings M.K., Traversari C. Differentiation of T regulatory cells by immature dendritic cells. J. Exp. Med. 2001;193:F5–F9. doi: 10.1084/jem.193.2.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]