Cd11c+B220+Gr-1+ Cells in Mouse Lymph Nodes and Spleen Display Characteristics of Plasmacytoid Dendritic Cells (original) (raw)
Abstract
Human plasmacytoid dendritic cells (pDCs) are major producers of IFNα, are activated by CpG motifs, and are believed to enter lymph nodes (LNs) via L-selectin dependent extravasation across high endothelial venules. To identify a similar murine DC type, CD11c+ cells in the LNs of L-selectin–deficient and control BALB/c mice were compared, revealing a population of CD11c+CD11b− cells that is reduced 85% in the LNs of L-selectin–deficient mice. These cells are Gr-1+B220+CD19−, either CD4+ or CD8+, and localize within T cell zones of LNs. Freshly isolated CD11c+Gr-1+ cells express major histocompatibility complex class II at low levels, display a plasmacytoid morphology, and survive poorly in culture. Their survival is increased and they develop a DC-like morphology in interleukin 3 and CpG. Like human pDCs, CD11c+Gr-1+ cells stimulate T cell proliferation after activation with CpG and produce IFNα after stimulation with influenza virus. These cells also display a strain-specific variation in frequency, being fivefold increased in the LNs of BALB/c relative to C57BL/6 mice. These CD11c+CD11b−B220+Gr-1+ cells appear to be the murine equivalent of human pDCs.
Keywords: dendritic cells, lymph nodes, B220 antigens, L-selectin, interferon type 1
Introduction
Human and murine dendritic cell (DC) subsets can be distinguished by their morphology, expression of cell surface markers and cytokines, characteristic localization, requirements for growth factors, and response to activating stimuli 1. The exact relationship between DC subsets remains unclear. However, increasing evidence suggests that each subset has unique characteristics that contribute the development specific immune responses 2. In mice, three major DC subsets have been described that function in the activation of T cells: CD8− DCs, CD8+ DCs, and Langerhans-derived cells (LCs). CD8− DCs represent the classic “myeloid” tissue DCs originally isolated from spleen but found in almost all tissues 3. LCs appear to be a variant of CD8− DCs specialized for the epidermis but functionally similar. CD8− DCs and LCs are found as immature cells in peripheral tissues that mature and migrate to LNs via lymphatics upon activation 4. CD8+ DCs were identified as a major splenic population that are similar to thymic DCs in their expression of CD8α and therefore proposed to be of lymphoid origin 5. Although studies have suggested that this is not the case 6, their designation as “lymphoid” has continued. CD8+ DCs have not been identified in peripheral tissues and the origin of these cells remains unclear, in part because expression of their characteristic surface marker, CD8α, can be induced on other DC subsets 7.
CD8− DCs and LCs are found in humans and appear to function similarly to their murine counterparts 2. Human DCs do not express CD8 so a human equivalent of murine CD8+ DCs has not been obvious. Instead, studies have identified a circulating immature DC type known as “plasmacytoid” DCs (pDCs). pDCs were first identified in pathological specimens of reactive or neoplastic LNs where they display a plasma cell–like appearance and are associated with high endothelial venules (HEVs) 8. It has since been recognized that these cells correspond to circulating immature CD11c− DCs that can be induced to develop a DC morphology and activate T cells when cultured in the presence of IL-3 and CD40L 9 10 11. Unlike other human DC types, pDCs produce high levels of IFNα in response to viral stimulation 12 and can be activated by immunostimulatory DNA (CpG) sequences 13. Circulating pDCs express L-selectin and stimulation appears to promote their entry into LNs and some peripheral tissues 14 15. Even without stimulation, pDCs have been identified as resident immature cells in LNs, tonsils, and thymus 11 16. pDCs have been shown to stimulate both Th1 and Th2 T cell polarization in vitro 17 18 19. Their function in vivo remains to be demonstrated.
We now describe the identification of a novel CD11c+ CD11b−Gr-1+B220+ DC type present within mouse LN and spleen. These cells appear as immature plasmacytoid cells when freshly isolated but develop a dendritic morphology and the capacity to stimulate T cell proliferation after activation with CpG. They produce IFNα after viral stimulation. Numbers of CD11c+Gr-1+ DCs are reduced in the LNs of L-selectin–deficient mice, suggesting their extravasation across HEV, and they display a marked strain-specific variation in frequency in both LN and spleen. These CD11c+Gr-1+ DCs appear to be the murine equivalent of human pDCs.
Materials and Methods
Mice.
BALB/c, C57BL/6, DBA/2, 129/Sv, CD-1, and CD-1-nu/nu mice were purchased from Charles River Laboratories. B10.BR, C57BL/6-Rag-1−/−, and BALB/c-Rag-1−/− mice were purchased from The Jackson Laboratory. BALB/c-L-selectin−/− mice were kindly provided from Dr. T. Tedder (Duke University, Durham, NC). All mice were kept under specific pathogen-free conditions and used at 7 to 12 wk of age according to institutional guidelines.
Isolation of DCs.
DCs were prepared as previously reported with some modifications 20. Briefly, peripheral LNs (cervical, axillary, brachial, and inguinal) or spleens were minced in Mg2+- and Ca2+-free HBSS/5% FCS and digested with 1mg/ml of collagenase A (Roche Biochemicals) and 0.2 mg/ml of DNase I (Sigma-Aldrich) for 35 min at 37°C. EDTA was added (20 mM final concentration), the cells incubated for 5 min at room temperature, passed through 70-μm nylon mesh, layered over RPMI 1640/10% FCS/14.5% Metrizamide (Accurate Chemical and Scientific), and centrifuged at 450 g for 20 min at room temperature. Low-density cells at interface were collected and washed.
Flow Cytometric Analysis.
Cells were stained with the following FITC, PE, allophycocyanin (APC), or biotin-conjugated mAbs in PBS containing 0.1% NaN3, 10 mM EDTA, 3% FCS, 5% normal mouse serum, 5% normal rat serum, and 5 μg/ml Fc block (BD PharMingen): CD3 (145–2C11), CD4 (RM4–5), CD8 (53–6.7), CD11b (M1/70), CD11c (HL3), CD40 (3/23), CD45R/B220 (RA3–6B2), CD56 (3E2), CD62L (MEL-14), CD80 (16–10A1), CD86 (GL1), I-Ab, (25–9-17), I-Ad (AMS-32–1), I-A/I-E (2G9), Gr-1 (RB6–8C5), isotype controls for mouse IgG (G155–178), rat IgG1 (R3–34), rat IgG2a (R35–95), rat IgG2b (A95–1) and hamster IgG (G235–2356) (BD PharMingen), and F4/80 (Serotec). Biotinylated mAbs were followed by streptavidin-conjugated FITC or APC (BD PharMingen). Stained cells were analyzed on a FACSVantage™ (Becton Dickinson) flow cytometer and CELLQuest™ software. Dead cells positive for 7-aminoactinomycin D (7-AAD; BD PharMingen) staining and auto-fluorescent cells were excluded using the FL3 channel.
Sorting and Culture of DCs.
Low-density 129/Sv spleen cells were stained as above with anti–CD11c-PE and anti–Gr-1-APC for 30 min on ice. After washing twice, cells were sorted and auto-fluorescencelo 7-AAD−CD11cintGr-1+ and CD11chiGr-1− cells collected. Cell purity after sorting was >96%. 105 sorted DCs were cultured in 200 μl complete RPMI-10 (RPMI-1640 medium, 10% FCS, 20 mM Hepes, 2-ME, penicillin, and streptomycin) in U-bottom 96-well plates with or without the following supplements: recombinant mouse (rm) IL-3 (10 ng/ml), rmIL-4 (25 ng/ml), rmGM-CSF (5 ng/ml), rmFlt-3L (20 ng/ml; R&D Systems), phosphorothiolated CpG oligonucleotide (TCCATGACGTTCCTGATGCT, 1 μM), and GpC (TCCATGAGCTTCCTGATGCT, 1 μM, synthesized and purified in the Duke DNA Core Facility). For CD40 stimulation, sorted DCs were incubated with anti-CD40 (clone HM40–3; BD PharMingen; 5 μg/ml) for 30 min on ice, washed twice, and incubated with goat anti–hamster IgG (10 μg/ml; Jackson ImmunoResearch Laboratories). Viability of the cells was assessed by trypan-blue exclusion in triplicate after 48 h.
T Cell Stimulation and DC Cytokine Production.
Freshly isolated DCs or DCs cultured for 2 d under conditions described above were cocultured with 105 naive CD4 T cells isolated from B10.BR mice. CD4 T cells were prepared as single cell suspensions from LNs and spleens and purified by negative selection on a MACS column (Miltenyi Biotec) using mAbs to CD8, CD11b, CD11c, CD16/CD32 (2.4G2), B220, Gr-1, CD25 (7D4), mouse IgM (R6–602), TER-119 (TER-119) (BD PharMingen), and F4/80. Naive high-density cells were collected by centrifugation on a 70% percoll gradient (Amersham Pharmacia Biotech). Over 97% of the purified cells were CD4+CD62L+CD45RB+ CD44int/low. 105 purified T cells were cultured with graded doses of 30 Gy gamma ray-irradiated or nonirradiated purified DCs in 200 μl complete RPMI-10 for 5 d. T cell proliferation cells was measured using the WST-1 system (Roche) according to the manufacturer's instructions and the OD450/650 measured after 4 h on a micro plate reader. To assess IFNα production, 105 DCs were cultured with 1.3 HAU/ml influenza virus A/PR/8/34 (provided from Melissa Beck, UNC-Chapel Hill, Chapel Hill, NC) in 200 μl complete RPMI-10 for 24 h. Supernatants were assayed using an IFNα ELISA kit (Performance Biomedical Laboratories).
Histological Analysis.
Cytospins of freshly isolated or cultured FACS®-purified CD11c+Gr-1+ cells were prepared (500 g, 5 min) and stained with Giemsa solution. Cryostat sections (6 μm) of LNs or spleen were stained as previously reported with some modification 21. After preincubation (PBS, 5% rat serum, 5% mouse serum, 1% BSA), sections were stained with anti–B220-FITC and anti–Gr-1-PE and analyzed on a LSM-410 confocal laser microscope (ZEISS).
Results
While most DC types enter LNs by migrating from peripheral tissues, pDCs are thought to enter LNs directly from the blood by crossing HEVs. To determine if there are murine DC types that enter LNs via HEVs, we examined LN DC populations in L-selectin–deficient (Sell −/−) mice. Splenic DC populations were examined simultaneously to assist in the identification of individual DC types. DCs were isolated from spleens and LNs of wild-type BALB/c and BALB/c-Sell −/− mice by collagenase/DNase digestion and partial purification on metrizimide gradients. Low density cells were stained anti-CD11c, CD11b, and CD8 to discriminate individual DC types. Similar to previous reports, we find that DCs isolated from the spleens of BALB/c mice express CD11c at high levels (Fig. 1 A) and fall into two clear subsets: CD11bhiCD8− DCs and CD11bintCD8+ DCs (Fig. 1 B). The CD11bhiCD8− DC subset can be further subdivided into CD4+ and CD4− populations (not shown).
Figure 1.
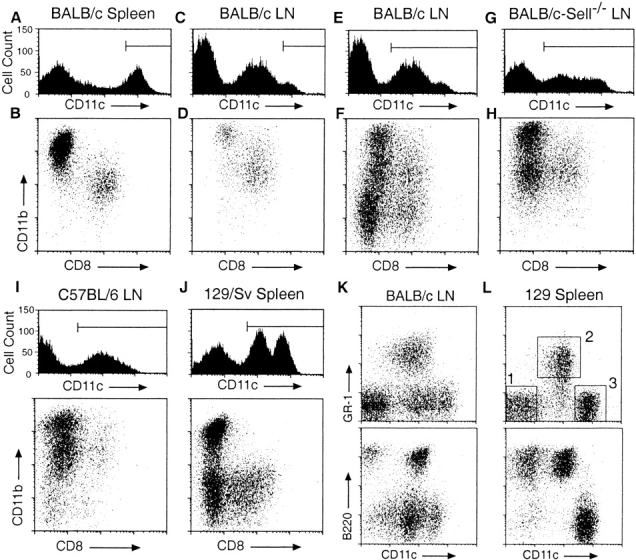
Flow cytometric analysis of DC populations in mouse lymph nodes and spleens. Low density cells, prepared from the indicated organs and mouse strains by enzymatic digestion and purification on Metrizimide gradients, were incubated with blocking reagents, the indicated fluorochrome-conjugated Abs, and 7-AAD before analysis. All panels are gated on forward and side scatter to exclude debris and on low autofluorescent, 7-AAD− cells. Horizontal lines in the CD11c histograms indicate gates used for dot plots immediately below. Numbered squares in L indicate gates used for subsequent subset analyses and FACS® purification. All panels are representative of at least three separate analyses.
In BALB/c LNs, only a small proportion of low density cells express CD11c at levels comparable to that seen in spleen while the majority of CD11c expression occurs at lower levels (Fig. 1C and Fig. E). Gating on CD11chi cells demonstrates CD11bhiCD8− and CD11bintCD8+ populations that appear similar to the splenic DC subsets (Fig. 1 D). When all LN CD11c+ cells are examined, three populations are apparent that are not seen in spleen (Fig. 1 F). One of these, a CD11bintCD8− population, has been shown to be derived from migrating LCs 22. Two other CD11c+ populations are present which are low or negative for CD11b: a minor CD8+ population and a predominant CD8− population.
In BALB/c-Sell −/− LNs, CD11c expression levels are similar to that seen in BALB/c mice (Fig. 1 G) and the CD11bhiCD8−, CD11bintCD8+, and CD11bintCD8− DC populations are easily identified. However, there is a striking decrease in the number of CD11c+CD11b− cells (Fig. 1 H). LN cells from heterozygous BALB/c-Sell+/− littermates display staining profiles similar to that of wild-type BALB/c mice (data not shown), demonstrating that the paucity of CD11c+CD11b− cells is due to the expected recessive defect at the Sell locus. This result suggests that low density CD11c+CD11b− cells enter LNs by crossing HEVs in an L-selectin–dependent manner. The fact that these cells express CD11c suggests that they represent a DC population; however, these cells do not correspond to any previously defined DC subset.
Once identified, the presence of CD11c+CD11b− cells was evaluated in other mouse strains. Interestingly, when compared with BALB/c mice, the number of CD11c+ CD11b− cells is markedly reduced in the LNs of C57BL/6, DBA/2, and 129/Sv mice while the number of both CD11bhiCD8− DCs and LCs are increased in these animals (Fig. 1 I, and data not shown). An examination of low density cells in spleen reveals that 129/Sv mice, but no other strains, posses a large number of splenic CD11cint cells (Fig. 1 J). When segregated by CD11b and CD8 expression, these cells display a CD11b− phenotype. CD11c+CD11b− cells from BALB/c LN and 129/Sv spleen were analyzed for the expression of other surface markers. Strikingly, these cells are uniformly B220+ and the majority (80–90%) express Gr-1 (Fig. 1K and Fig. L). A small portion of CD11c+ B220+Gr-1_−_ cells were found to be CD19+ (data not shown), thus further analysis was limited to CD11cintGr-1+ cells (gate 2 in Fig. 1 L).
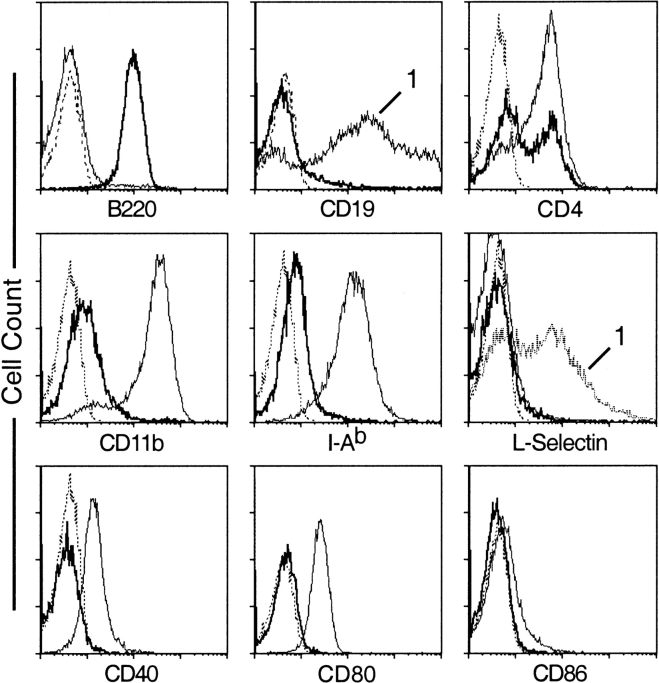
Splenic CD11c+Gr-1+ cells are uniformly B220+ but do not express CD19, CD79b, or IgM (Fig. 2, and data not shown), suggesting that they do not represent a B cell lineage. CD11c+Gr-1+ cells can be separated into CD4+ and CD4− populations. They express CD11b and MHC class II at very low levels and do not express CD40, CD80, CD86, or L-selectin. CD11c+Gr-1+ cells from BALB/c LNs display a similar pattern of surface marker expression (data not shown).
Figure 2.
Surface marker expression on splenic CD11c+Gr-1+ cells. Low density 129/Sv spleen cells were stained with anti-CD11c, Gr-1, and the indicated FITC-conjugated mAbs. In each panel, staining of CD11c+Gr-1+ cells (gate 2 in Fig. 1 L) is shown in bold. Unless otherwise indicated, thin solid lines indicated staining profiles of CD11chiGr-1− cells (gate 3 in Fig. 1 L). Staining profiles of CD11c− cells (gate 1 in Fig. 1 L) are indicate by the number “1.” Dotted lines represent staining of isotype controls. All panels are representative of at least three separate analyses.
Taking advantage of the unusual combination of B220 and Gr-1 expression, the possible presence of CD11c+ Gr-1+ cells in the spleens of mice other than 129/Sv was reexamined. As shown in Fig. 3A and Fig. B, B220+Gr-1+ cells are present in the spleens of both BALB/c and C57BL/6 mice. However, their level of CD11c expression is lower than occurs in LN or in 129/Sv spleen cells, making their identification on this basis alone difficult (data not shown). To ensure that CD11c+B220+Gr-1+ cells are not derived from a mature lymphocyte population, these cells were examined in BALB/c-Rag−/− mice. B220+Gr-1+ cells are present in normal proportions (relative to other DC types) in both the LNs and spleens of BALB/c-Rag−/− mice (Fig. 3C and Fig. D, and data not shown). The proportion of these cells was also found to be normal in the spleens and LNs of nude mice (data not shown).
Figure 3.
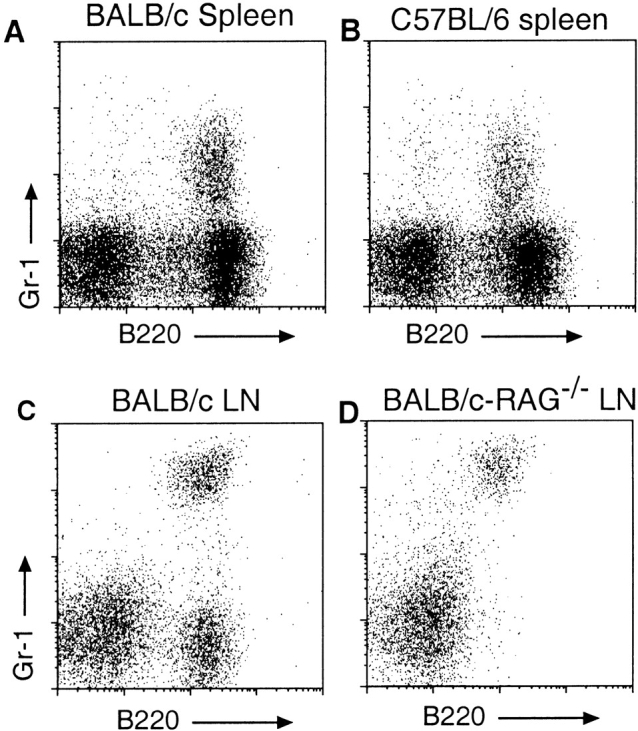
Presence of low density B220+Gr-1+ cells in BALB/c, C57BL/6, and Rag− _/_− mice. Low density cells were prepared from the indicated organs of mice as described in the legend to Fig. 1, stained with the indicated mAbs, and gated on low autofluorescent, 7-AAD− cells. (A and B) Presence of B220+Gr-1+ cells in the spleens of BALB/c and C57BL/6 mice, respectively. (C and D) Frequency of B220+Gr-1+ cells in the LNs of BALB/c and BALB/c-Rag−/− mice. The intensity of Gr-1 staining in top and bottom panels are not directly comparable due to differences methodology.
The above findings suggest that CD11c+CD11b− B220+Gr-1+ cells represent a novel murine DC subset. The fact that these cells are reduced in the LN of L-selectin–deficient mice and therefore may cross HEVs suggests they are similar to human pDCs. Human pDCs display several characteristics that are not shared with other DC types. These include their plasmacytoid morphology, their dependence on IL-3, their sensitivity to activation by CpG, and their production of IFNα. These characteristics were examined in CD11c+Gr-1+ cells purified by FACS® from the spleens of 129/Sv mice (gate 2 in Fig. 1 L).
In cytospins, freshly prepared CD11c+Gr-1+ cells appear as large round cells with diffuse nuclei and very rare dendritic-like processes (Fig. 4A and Fig. B). This appearance is similar to that of freshly isolated human pDCs 10 11. After overnight culture in IL-3 and GM-CSF, these cells mature, developing elongated processes typical of DCs (Fig. 4C and Fig. D). After culture in IL-3 and CpG, CD11c+Gr-1+ cells develop numerous dendritic processes and display a clear DC morphology (Fig. 4 E). CD11c+Gr-1+ cells were also localized within BALB/c LNs by staining with anti-B220 and Gr-1 and examined by confocal microscopy. B220+Gr-1+ cells appear predominately within the LN paracortex in the region of the outer T cell zones (Fig. 4 F). A higher power view demonstrates clear colocalization of B220 and Gr-1 on individual cells in this region (Fig. 4 G).
Figure 4.
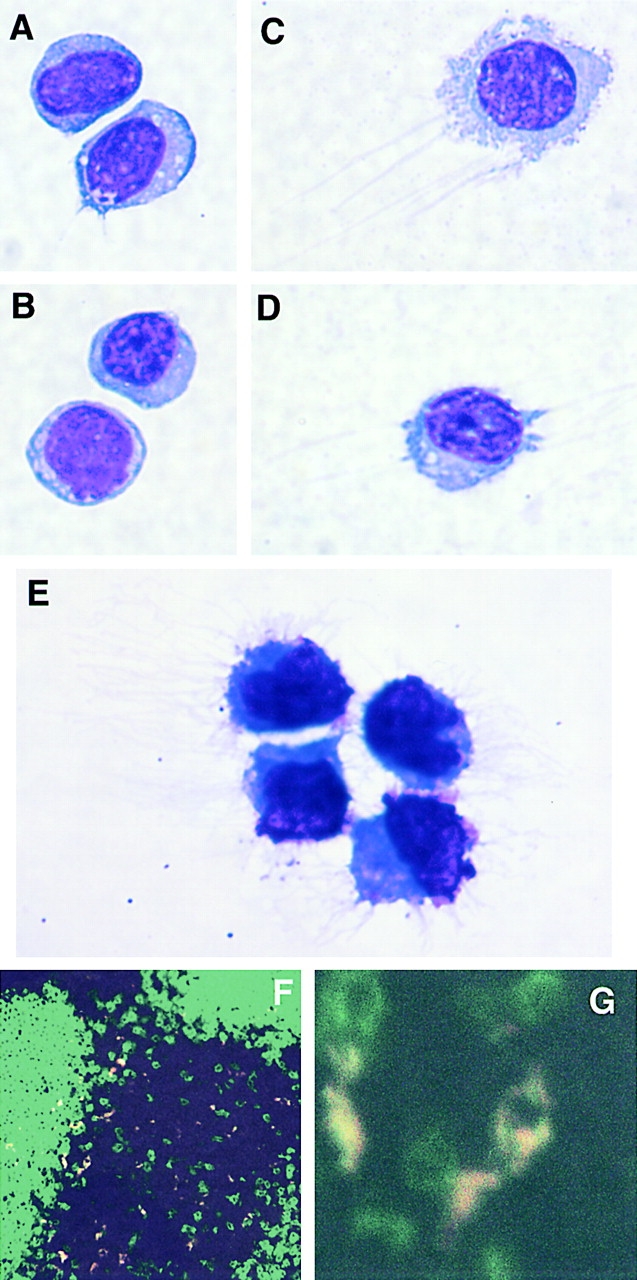
Morphology and localization of CD11c+Gr-1+ cells. (A–E) Cytospins of FACS®-purified CD11c+Gr-1+ cells were Giemsa-stained and photographed at 100×. (A and B) Appearance of freshly isolated CD11c+Gr-1+ cells. (C and D) CD11c+Gr-1+ cells after overnight culture in IL-3 and GM-CSF. (E) CD11c+Gr-1+ cells after activation in IL-3 and CpG for 40 h. (F) Confocal microscopy of BALB/c LN frozen section stained with anti–B220-FITC (green) and anti–Gr-1-PE (red). Colocalization of B220 and Gr-1 signals appears as yellow. (G) Higher magnification demonstrates individual B220+Gr-1+ cells within T cell zone.
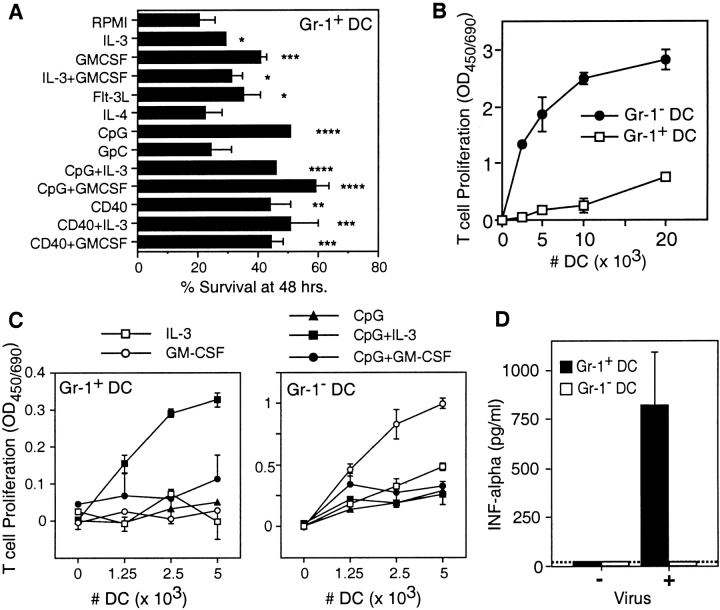
When cultured in media alone (RPMI/10% FCS), the majority (∼80%) of CD11c+Gr-1+ cells die within 48 h. Survival of these cells is increased in the presence of IL-3, GM-CSF, or flt-3L or by activation with CD40 cross-linking or CpG (Fig. 5 A). The combination of a growth factor and an activating agent further increases survival only marginally.
Figure 5.
Survival and activities of CD11c+Gr-1+ cells. CD11c+Gr-1+ (Gr-1+ DC) and CD11chiGr-1− (Gr-1- DC) cells were purified by FACS® from the spleens of 129/Sv mice. (A) Survival of CD11c+Gr-1+ cells after 48 h of culture in the indicated agents. (B) Proliferation of naive allogenic T cells incubated with the indicated numbers of freshly isolated CD11c+Gr-1+ or CD11chiGr-1− cells. (C) Proliferation of naive allogenic T cells incubated with cytokine and CpG activated CD11c+Gr-1+ or CD11chiGr-1− cells. A–C are representative of three independent experiments, each preformed in triplicate (mean ± SD; *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.001). (D) IFNα production by CD11c+Gr-1+ or CD11chiGr-1− cells incubated 24 h in the presence of absence of 1.3 HAU/ml influenza virus (mean ± SD, n = 2). Dashed line, detection limit of the assay.
The responsiveness of CD11c+Gr-1+ cells to CpG and viral stimulation was compared with that of CD11chiGr-1− cells (gate 3 in Fig. 1 L), which were purified simultaneously and comprise all other defined DC subsets in the spleens of 129/Sv mice. When freshly isolated, CD11cintGr-1+ cells fail to stimulate significant proliferation of naive B10.BR allogenic T cells while similarly prepared CD11chiGr-1− cells stimulate robust levels of T cell proliferation (Fig. 5 B). However, after 40 h of culture in the presence of IL-3 and CpG, CD11cintGr-1+ cells gain the capacity to stimulate T cell proliferation (Fig. 5 C). Culture in the presence of CpG and GM-CSF also leads to the development of T cell stimulatory capacity, though to a lesser extent. In contrast, CD11chiGr-1− DCs stimulate T cell proliferation when cultured in GM-CSF alone and this is somewhat inhibited in the presence of CpG.
One of the most characteristic features of human pDC is their expression of type I interferons after viral stimulation. When cultured in the presence of influenza virus, CD11cintGr-1+ cells produce high levels of IFNα (Fig. 5 D). CD11chiGr-1− DCs demonstrate no detectable IFNα production after viral stimulation at any concentration.
Discussion
By examining cell populations that are reduced in the LN of L-selectin–deficient mice, we identified a novel murine cell type, CD11c+CD11b−B220+Gr-1+ DCs, that displays several characteristics of human pDCs. Similarities between CD11c+Gr-1+ cells and human pDCs include their apparent L-selectin–dependent migration across HEVs 14, their plasmacytoid morphology 11, their immature phenotype when freshly isolated from lymphoid organs 11, their localization within T cell zones 10, their activation by CpG 13, and their production of IFNα after viral stimulation 12. Taken together, these similarities strongly suggest that the CD11c+Gr-1+ cells we describe are the murine counterpart of human pDCs.
Several differences between the cells we describe and human pDCs should be noted. First, the cells we describe are CD11c+, while human pDCs in blood and tissues lack CD11c expression. The role played by CD11c on DCs is not known, so the functional significance of this expression difference is not clear. Second, the survival of human pDCs is markedly increased by IL-3. While the survival of CD11c+Gr-1+ cells is increased by IL-3, this effect is no greater than that seen with GM-CSF. IL-3 does appear to support greater levels of activation than GM-CSF or standard media alone, so some selective effect of this cytokine is evident.
It has been suggested that splenic CD8+ DCs are the murine counterparts of human pDCs 23. Like human pDCs, CD8+ DCs have been proposed to be of lymphoid origin, are localized within T cell zones 4, and express IFNα after stimulation with CpG and poly I:C 23. However, murine CD8+ DCs differ from human pDCs in several important respects. Human pDC stimulate Th2 polarization and the production of IL-4, IL-5, and IL-10 by allogenic T cells 17 18. Stimulation of Th1 polarization by pDCs has also been demonstrated 19, suggesting that pDC T cell polarizing activity is dependent on the methods and stimuli used to activate these cells. In contrast, murine lymphoid DCs have been found to be uniformly Th1 polarizing 24 25. We have just begun to examine the capacity of CD11c+Gr-1+ cells to stimulate T cell polarization, but preliminary results suggest that, as for human pDCs, the regulation of this activity will be complex.
Murine CD8+ DCs also differ from human pDCs in their maturational status. Freshly isolated murine CD8+ DCs express MHC class II, CD40, and CD80, and can stimulate T cell proliferation 20. Human pDCs, like CD11c+Gr-1+ cells, express MHC class II at very low levels, do not express CD40 or CD80, and fail to stimulate T cell proliferation 10. Finally, as shown in Fig. 1, the frequency of CD11b+CD8+ DCs in peripheral LNs is only slightly decreased by the absence of L-selectin expression. Based on their expression of L-selectin and association with HEVs in pathologic sections, human pDCs are believed to enter LNs by extravasation across HEVs 14. The marked paucity of CD11c+Gr-1+ cells in the LNs of L-selectin–deficient mice suggests that these cells also enter LNs by L-selectin–mediated extravasation across HEV. We were not able to test this hypothesis directly because CD11c+Gr-1+ cells, like human pDCs 11, are L-selectin− when freshly isolated from secondary lymphoid organs. Such studies will likely require the identification and purification of circulating CD11c+Gr-1+ cell precursors from blood or bone marrow. The above findings suggest that CD11c+Gr-1+ cells, rather than CD8+ DCs, are the true murine pDC equivalent.
Given the high frequency of CD11c+Gr-1+ cells in some mouse strains, it is somewhat surprising that this population has not been described previously. Several reasons may account for this. First, murine DC populations have been studied predominately in spleen and predominately in C57BL/6 or closely related strains of mice. As we show, CD11c+Gr-1+ cells represent only a minor population in the spleens of C57BL/6 mice and express very low levels of CD11c. Second, most murine DC purification protocols have included the depletion of B220+ or Gr-1+ cells. Third, it is likely that some investigators have identified CD11c+Gr-1+ cells, but believed them to represent a contaminating cell type. In one case, CD11c+B220+ cells were purified from Peyer's patches, but found to express CD19 mRNA and therefore not further examined 26. We have found that a proportion of Peyer's patch CD11c+B220+ cells also express Gr-1 (data not shown), suggesting that they are similar to the cells we find in LNs and spleen.
To our knowledge, the finding of Gr-1 expression by a DC population is novel. However, B220+ DC types have been reported on several occasions. A CD11c+B220+ DEC-205+ DC population has been shown to arise from cultures of purified CD19+ pro-B cells in the presence of multiple cytokines 27. Unlike the cells we describe, these do not express CD4, CD8, or Gr-1 but they do appear to lose CD19 expression upon maturation. More recently, a DEC205+B220+CD19− DC population has been described that can be propagated from mouse liver cells cultured in IL-3 and anti-CD40 28. These cells appear to stimulate the apoptosis of activated T cells and prolong cardiac allograft survival. They differ from the population we describe in that they are predominately CD11c−, CD4−, and CD8− and display a mature DC phenotype. A CD8α+CD11c+B220+CD19− cell population has been identified in murine bone marrow that facilitates the engraftment of allogenic stem cells 29. These cells display a plasmacytoid morphology and appear similar to the CD8+ subset of our CD11c+Gr-1+ cells. These findings suggest that CD11c+Gr-1+ DCs may be part of a broader DC subset whose specific phenotypes and activities are dependent on their state of maturation and activation.
Several roles for pDCs have been proposed. These cells may respond to viral infection by migrating across HEV and presenting viral Ag in the context of IFNα, perhaps via the stimulation of CCR7 14 16. pDCs have also been shown to enter peripheral tissues 15 and may subsequently migrate to LNs via lymphatics. pDCs may also act to regulate T cell polarization. Stimulation by CpG motifs or viral products may lead to IFNα production and Th1 polarization while, under most other conditions, pDCs are Th2 polarizing. Consistent with this, CD11c+Gr-1+ DCs are increased in the LNs of BALB/c mice, a strain with an increased propensity to develop Th2 polarized immune responses. Finally, the similarity of CD11c+Gr-1+ cells to DC types in liver and bone marrow that facilitate transplant survival suggests that these cells may have some immune inhibitory function. This may explain their substantial presence within secondary lymphoid organs as an immature DC population. Immature DCs have been shown to reduce Ag-specific immune responses, probably by stimulating the production of regulatory T cells 30. At present, the contribution of pDCs to any or all of these immune functions remains purely speculative. Hopefully, the identification of these cells in mice will lead to a better understanding of their function during immune response.
Acknowledgments
We thank Dr. Thomas Tedder for providing L-selectin deficient mice, Dr. Melissa Beck (University of North Carolina, Chapel Hill) for providing influenza virus, John Whitesides and Patti McDermott of the Duke University Department of Medicine, Human Vaccine Institute Flow Cytometry Facility, and Kaiko Nakano for their excellent technical assistance.
H. Nakano was supported by a Fellowship Training Award from the Cancer Research Institute. M. Yanagita is a research fellow of the Japanese Society for the Promotion of Science for Young Scientists.
References
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Pulendran B., Banchereau J., Maraskovsky E., Maliszewski C. Modulating the immune response with dendritic cells and their growth factors. Trends Immunol. 2001;22:41–47. doi: 10.1016/s1471-4906(00)01794-4. [DOI] [PubMed] [Google Scholar]
- Steinman R.M., Cohn Z.A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J. Exp. Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R.M., Pack M., Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol. Rev. 1997;156:25–37. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]
- Vremec D., Zorbas M., Scollay R., Saunders D.J., Ardavin C.F., Wu L., Shortman K. The surface phenotype of dendritic cells purified from mouse thymus and spleeninvestigation of the CD8 expression by a subpopulation of dendritic cells. J. Exp. Med. 1992;176:47–58. doi: 10.1084/jem.176.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traver D., Akashi K., Manz M., Merad M., Miyamoto T., Engleman E.G., Weissman I.L. Development of CD8alpha-positive dendritic cells from a common myeloid progenitor. Science. 2000;290:2152–2154. doi: 10.1126/science.290.5499.2152. [DOI] [PubMed] [Google Scholar]
- Merad M., Fong L., Bogenberger J., Engleman E.G. Differentiation of myeloid dendritic cells into CD8alpha-positive dendritic cells in vivo. Blood. 2000;96:1865–1872. [PubMed] [Google Scholar]
- Facchetti F., de Wolf-Peeters C., Mason D.Y., Pulford K., van den Oord J.J., Desmet V.J. Plasmacytoid T cells. Immunohistochemical evidence for their monocyte/macrophage origin. Am. J. Pathol. 1988;133:15–21. [PMC free article] [PubMed] [Google Scholar]
- O'Doherty U., Peng M., Gezelter S., Swiggard W.J., Betjes M., Bhardwaj N., Steinman R.M. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology. 1994;82:487–493. [PMC free article] [PubMed] [Google Scholar]
- Grouard G., Rissoan M.C., Filgueira L., Durand I., Banchereau J., Liu Y.J. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J. Exp. Med. 1997;185:1101–1111. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olweus J., BitMansour A., Warnke R., Thompson P.A., Carballido J., Picker L.J., Lund-Johansen F. Dendritic cell ontogenya human dendritic cell lineage of myeloid origin. Proc. Natl. Acad. Sci. USA. 1997;94:12551–12556. doi: 10.1073/pnas.94.23.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal F.P., Kadowaki N., Shodell M., Fitzgerald-Bocarsly P.A., Shah K., Ho S., Antonenko S., Liu Y.J. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- Bauer M., Redecke V., Ellwart J.W., Scherer B., Kremer J.P., Wagner H., Lipford G.B. Bacterial CpG-DNA triggers activation and maturation of human CD11c(−), CD123(+) dendritic cells. J. Immunol. 2001;166:5000–5007. doi: 10.4049/jimmunol.166.8.5000. [DOI] [PubMed] [Google Scholar]
- Cella M., Jarrossay D., Facchetti F., Alebardi O., Nakajima H., Lanzavecchia A., Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- Jahnsen F.L., Lund-Johansen F., Dunne J.F., Farkas L., Haye R., Brandtzaeg P. Experimentally induced recruitment of plasmacytoid (CD123high) dendritic cells in human nasal allergy. J. Immunol. 2000;165:4062–4068. doi: 10.4049/jimmunol.165.7.4062. [DOI] [PubMed] [Google Scholar]
- Bendriss-Vermare N., Barthelemy C., Durand I., Bruand C., Dezutter-Dambuyant C., Moulian N., Berrih-Aknin S., Caux C., Trinchieri G., Briere F. Human thymus contains IFN-alpha-producing CD11c(−), myeloid CD11c(+), and mature interdigitating dendritic cells. J. Clin. Invest. 2001;107:835–844. doi: 10.1172/JCI11734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissoan M.C., Soumelis V., Kadowaki N., Grouard G., Briere F., de Waal Malefyt R., Liu Y.J. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–1186. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- Ito T., Amakawa R., Inaba M., Ikehara S., Inaba K., Fukuhara S. Differential regulation of human blood dendritic cell subsets by IFNs. J. Immunol. 2001;166:2961–2969. doi: 10.4049/jimmunol.166.5.2961. [DOI] [PubMed] [Google Scholar]
- Cella M., Facchetti F., Lanzavecchia A., Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent Th1 polarization. Nat. Immunol. 2000;1:305–310. doi: 10.1038/79747. [DOI] [PubMed] [Google Scholar]
- Vremec D., Pooley J., Hochrein H., Wu L., Shortman K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J. Immunol. 2000;164:2978–2986. doi: 10.4049/jimmunol.164.6.2978. [DOI] [PubMed] [Google Scholar]
- Nakano H., Mori S., Yonekawa H., Nariuchi H., Matsuzawa A., Kakiuchi T. A novel mutant gene involved in T-lymphocyte-specific homing into peripheral lymphoid organs on mouse chromosome 4. Blood. 1998;91:2886–2895. [PubMed] [Google Scholar]
- Henri S., Vremec D., Kamath A., Waithman J., Williams S., Benoist C., Burnham K., Saeland S., Handman E., Shortman K. The dendritic cell populations of mouse lymph nodes. J. Immunol. 2001;167:741–748. doi: 10.4049/jimmunol.167.2.741. [DOI] [PubMed] [Google Scholar]
- Hochrein H., Shortman K., Vremec D., Scott B., Hertzog P., O'Keeffe M. Differential production of IL-12, IFN-alpha, and IFN-gamma by mouse dendritic cell subsets. J. Immunol. 2001;166:5448–5455. doi: 10.4049/jimmunol.166.9.5448. [DOI] [PubMed] [Google Scholar]
- Maldonado-Lopez R., De Smedt T., Michel P., Godfroid J., Pajak B., Heirman C., Thielemans K., Leo O., Urbain J., Moser M. CD8alpha+ and CD8alpha− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B., Smith J.L., Caspary G., Brasel K., Pettit D., Maraskovsky E., Maliszewski C.R. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc. Natl. Acad. Sci. USA. 1999;96:1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A., Kelsall B.L. Freshly isolated Peyer's patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J. Exp. Med. 1999;190:229–239. doi: 10.1084/jem.190.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorck P., Kincade P.W. CD19+ pro-B cells can give rise to dendritic cells in vitro. J. Immunol. 1998;161:5795–5799. [PubMed] [Google Scholar]
- Lu L., Bonham C.A., Liang X., Chen Z., Li W., Wang L., Watkins S.C., Nalesnik M.A., Schlissel M.S., Demestris A.J. Liver-derived DEC205+B220+CD19− dendritic cells regulate T cell responses. J. Immunol. 2001;166:7042–7052. doi: 10.4049/jimmunol.166.12.7042. [DOI] [PubMed] [Google Scholar]
- Gandy K.L., Domen J., Aguila H., Weissman I.L. CD8+TCR+ and CD8+TCR− cells in whole bone marrow facilitate the engraftment of hematopoietic stem cells across allogeneic barriers. Immunity. 1999;11:579–590. doi: 10.1016/s1074-7613(00)80133-8. [DOI] [PubMed] [Google Scholar]
- Dhodapkar M.V., Steinman R.M., Krasovsky J., Munz C., Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J. Exp. Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]