Cytokine Requirements for Acute and Basal Homeostatic Proliferation of Naive and Memory CD8+ T Cells (original) (raw)
Abstract
Both naive and memory T cells undergo antigen-independent proliferation after transfer into a T cell–depleted environment (acute homeostatic proliferation), whereas only memory T cells slowly divide in a full T cell compartment (basal proliferation). We show, first, that naive and memory CD8+ T cells have different cytokine requirements for acute homeostatic proliferation. Interleukin (IL)-7 receptor(R)α–mediated signals were obligatory for proliferation of naive T cells in lymphopenic hosts, whereas IL-15 did not influence their division. Memory T cells, on the other hand, could use either IL-7Rα– or IL-15–mediated signals for acute homeostatic proliferation: their proliferation was delayed when either IL-7Rα was blocked or IL-15 removed, but only when both signals were absent was proliferation ablated. Second, the cytokine requirements for basal and acute homeostatic proliferation of CD8+ memory T cells differ, as basal division of memory T cells was blocked completely in IL-15–deficient hosts. These data suggest a possible mechanism for the dearth of memory CD8+ T cells in IL-15– and IL-15Rα–deficient mice is their impaired basal proliferation. Our results show that naive and memory T lymphocytes differ in their cytokine dependence for acute homeostatic proliferation and that memory T lymphocytes have distinct requirements for proliferation in full versus empty compartments.
Keywords: homeostasis, IL-7, IL-15, T cell numbers, memory
Introduction
Peripheral T lymphocyte numbers are maintained at remarkably stable levels throughout adulthood in spite of the continuing addition of cells, due to emigration from the thymus and proliferation in response to antigen encounter, and loss of cells owing to the removal of antigen-specific effectors after antigen clearance (1–3). The size of the peripheral T cell compartment is likely regulated by multiple factors that influence both proliferation and survival. For example, T lymphocytes are known to divide independently of cognate antigen in lymphopenic environments. This “acute homeostatic proliferation” is thought to reflect a distinct mechanism for maintaining homeostasis of peripheral T cell numbers.
In the absence of overt antigen stimulation, naive T lymphocytes divide minimally in a full compartment. However, they require signals delivered by MHC molecules for their survival, as evidenced by the abbreviated life-span of both naive CD4+ and CD8+ T cells in the absence of MHC molecules (for reviews, see references 2 and 3). Similarly, naive T cells also require specific interactions with self-peptide/MHC complexes in order to undergo efficient homeostatic proliferation in lymphopenic conditions (for a review, see reference 2). In contrast to naive T lymphocytes, memory T cells are continually undergoing “basal proliferation,” the low level of antigen-independent division observed in a full lymphocyte compartment (4, 5). Also, in contrast to naive T cells, memory cells appear to be largely free of requirements for TCR–MHC interactions to survive in the lymphoid periphery (for a review, see reference 3). The proliferation of memory cells in response to lymphopenia is also MHC independent (5, 6). Thus, for both naive and memory T cells, the need for TCR:self-peptide–MHC complex interactions to stimulate acute homeostatic proliferation mirrors the requirements for such interactions for survival.
How the same signal (TCR:self-peptide–MHC) can be interpreted by naive T cells as a cue for survival in one context and for proliferation in another is not understood (3, 6). Given that TCR recognition of self-peptide–MHC complexes stimulates the proliferation of naive T cells only in a lymphopenic environment, but supports survival in a full compartment, additional signals that are peculiar to lymphopenic environments must be necessary for their homeostatic proliferation. Likewise, the signal for acute homeostatic proliferation of memory T cells is unknown. Cytokines are known to regulate T lymphocyte differentiation, survival, and proliferation at many stages of their maturation. Cytokines could provide a signal for acute homeostatic proliferation of lymphocytes either due to increased cytokine production in response to lymphopenia or due to increased availability in a less competitive environment. Two cytokines, IL-7 and IL-15, are good candidates for eliciting homeostatic proliferation of CD8+ T cells as both are known to mediate antigen-independent proliferation of T cells (7–9).
IL-7 plays a key role in the survival and proliferation of thymocytes during early stages of T cell development. Both IL-7– and IL-7Rα–deficient mice show severe defects in T cell differentiation (10–13); treatment of wild-type mice with a neutralizing antibody to IL-7 or IL-7Rα leads to an analogous defect (14). In vitro, IL-7 enhances proliferation and survival of mature T cells, and the administration of exogenous IL-7 in vivo results in proliferation and an increase in numbers of both CD4+ and CD8+ T cells without apparent activation (8, 13, 15, 16). As mice deficient in IL-7 and its receptor are impaired in T cell differentiation, the role of this cytokine in peripheral T cell homeostasis has been difficult to address. However, several groups have recently shown that IL-7 contributes to peripheral T lymphocyte survival (17–19) and that it is a crucial factor in promoting acute homeostatic proliferation of naive T cells (18, 19).
IL-15 is a T and NK cell stimulatory factor, similar in structure and function to IL-2. Both cytokines induce proliferation of T cells; their shared functions are thought to result from both receptors using the IL-2/IL-15Rβ and common γ chains. However, IL-2 and IL-15 also have contrasting functions on similar cell types. For example, IL-2 has been implicated in promoting activation-induced cell death of T cells, whereas IL-15 inhibits death (20–23). Such differences have been attributed to the expression of the unique receptor α chains as well as to the competition between IL-2 and IL-15 to bind IL-2/IL-15Rβ and γ chains. Evidence supporting a role for IL-15 in homeostasis of memory CD8+ T cells is substantial. Administration of IL-15 to mice results in a selective and potent stimulation of CD8+ T cells of a memory phenotype (9). Additionally, blocking the IL-2/IL-15Rβ chain, but not IL-2, resulted in inhibition of memory CD8+ T cell proliferation, implicating IL-15 as an important regulator of basal proliferation of memory CD8+ T cells (24). Finally, both IL-15– and IL-15Rα–deficient mice have reduced numbers of CD8+ T cells of memory phenotype in spleen and LNs (25, 26). While these data highlight a role for IL-15 in maintaining homeostasis of CD8+ memory T cell numbers, the mechanisms by which IL-15 exerts its effects upon the memory compartment (i.e., on formation, survival, or proliferation) and the necessity of IL-15 for maintenance of memory T lymphocytes have yet to be determined. Furthermore, naive CD8+ T cells upregulate the IL-2/IL-15Rβ chain early during homeostatic proliferation (27–29), raising the possibility that IL-15 might also mediate proliferation of naive T cells.
Here, we report the cytokine requirements for both acute and basal homeostatic proliferation of CD8+ T lymphocytes. We find that naive and memory T cells differ in their cytokine dependence for acute homeostatic proliferation and that memory T lymphocytes have distinct requirements for proliferation in full versus empty compartments.
Materials and Methods
Mice and Cell Transfers.
Wild-type female C57BL/6 (B6) mice were obtained from Taconic Farms or The Jackson Laboratory. IL-15–deficient (IL-15°/°, B6 background) mice (26) were bred at Immunex or at Harvard Medical School. All mice were housed under specific pathogen-free conditions. Recipient B6 or IL-15°/° mice were either irradiated with 650 rads or left untreated. 1 or 2 d later, donor cells were labeled with the intracellular fluorescent CFSE dye (Molecular Probes), and were transferred to hosts by intravenous injection. Beginning at time of the transfer of donor T cells, indicated hosts were given 1 mg of anti–IL-7Rα (A7R34; reference 14) intraperitoneally every other day for the duration of the experiment.
Polyclonal donor T cells were obtained from pooled LNs and/or spleen from C57BL/6-Ly5.2 mice (CD45.1 congenic) (The Jackson Laboratory or Fredrick Cancer Research Center). Antigen-specific donor cells were from OT-I mice (transgenic for a MHC class I–restricted TCR [Vα2Vβ5] recognizing chicken egg ovalbumin 357–364 [OVAp]/Kb complexes on the B6 background; reference 30) crossed to the recombination activation gene (RAG)*-deficient (RAG°/°, B6 background) or B6.PL_Thy1_ a/Cy (Thy1.1 congenic) backgrounds (The Jackson Laboratory). Polyclonal CD8+ naive (CD8+CD44loCD122lo) and memory (CD8+CD44hiCD122hi) T cells were obtained by CD8+ T cell enrichment by MACS® (Miltenyi Biotec) magnetic depletion using a murine CD8+ enrichment cocktail (Stem Cell Technologies) and sorting according to expression of CD8, CD44, and CD122 (IL-2Rβ) using a FACSVantage™ (Becton Dickinson). Naive OT-I RAG° CD8+ T cells were enriched by magnetic depletion of CD44hi and MHC class II positive cells; the remaining cells were CD8+Vα2+CD44loCD122lo.
CD8+ memory cells specific for OVAp were generated by intravenous transfer of 2 × 106 OT-I Thy1.1+ pooled spleen and LN cells (B6.PL_Thy1_ a/Cy background) into B6 (Thy1.2+) or B6 by B6.PL_Thy1_ a/Cy F1 (Thy1.1+Thy1.2+) hosts followed by intravenous infection 1 d later with 5 × 106 PFU of Vaccinia virus carrying OVA cDNA (27). Memory cells were isolated >70 d after infection from spleen by enrichment over MACS® columns (Miltenyi Biotec). Briefly, cell suspensions were incubated with biotinylated anti–I-Ab, B220, HSA, and Thy1.2 antibodies followed by streptavidin-conjugated beads and magnetic purification.
Donor cells were labeled with CFSE before transfer (30). Cells were washed twice in PBS 0.1% BSA, resuspended to 107 cells/ml in PBS 0.1% BSA with 10 μM CFSE, and incubated for 10 min at 37°C. Cells were washed twice with cold RPMI 1640/20% FCS, and then washed twice in PBS or RPMI 1640. 1–2 × 106 CFSE-labeled CD8+ T cells were transferred in 200 μl by intravenous injection into host mice.
Flow Cytometry.
The following antibodies were used for flow cytometry (BD PharMingen): CD8-APC (53–6.7); Vα2-PE and biotin (B20.1); CD44-biotin (IM7); CD122 (IL2Rβ)-PE (TMβ1); Ly6C-FITC (AL-21); Thy1.2-biotin (30-H12); Thy1.1 PE (OX-7); CD45.1-biotin (A20) CD45.2-PE (104); and IL-7R-biotin (A7R34, gift of C. Kieper and C. Surh, Scripps Research Institute, La Jolla, CA). PE-Texas Red (Caltag) or Red-670-conjugated streptavidin (Invitrogen) were used to detect biotinylated antibodies. Samples were run on a FACSCalibur™ (Becton Dickinson) or MoFlo (Cytomation) and data analyzed with WinMDI software (a gift from Joseph Trotter, Scripps Clinic, La Jolla, CA).
Realtime PCR Analysis.
DNase-treated RNA was used as a template to synthesize first-strand cDNA. 104 cell equivalents of cDNA were used as template to set up PCR reactions as specified by the manufacturer (Applied Biosystems). The primers used in the reactions were: IL-15Rα forward primer 5′ gct act gtt gct ccc gct ga 3′ and reverse primer 5′ cat gct caa tag ata cgg gag gt 3′ at 5 μM each with the 6-FAM probe 5′ aca cgt ggt gcc cgg cgt cac 3′ at 20 μM final concentration; IL-7Rα forward primer 5′ ggg aca cag agc cgc tgt a 3′ and reverse primer 5′ taa ctg ttt ctg gtg ggc tga c 3′ at 5 μM each with the 6-FAM probe, 5′ agt gca aac cgc tcg cct gag act 3′ 20 μM final concentration. Rodent GAPDH (20 μM final VIC probe, 10 μM each primers final (Applied Biosystems) was used as the reference house-keeping gene for both IL-7Rα and IL-15Rα. Reactions were run on an Applied Biosystems ABI Prism 7700 using the following PCR conditions: 50°C, 2′ 95°C, 10′ followed by 45 cycles of 95°C, 15′′ 60°C, 1′. The data shown have been normalized to GAPDH and CD8α gene expression, and plotted relative to expression in naive cells.
Results and Discussion
The Role of IL-15 and IL-7 in Acute Homeostatic Proliferation of Naive CD8+ T Cells.
The necessity of IL-15– and IL-7Rα–mediated signals in the acute proliferation of naive CD8+ T cells in lymphopenic hosts was addressed first. Naive OT-I TCR transgenic (OT-I RAG°/°, Fig. 1 A) or naive polyclonal CD8+ (CD44loCD122lo, Fig. 1 B) T cells were CFSE-labeled and transferred into irradiated B6 or IL-15°/° hosts. In some host mice, IL-7Rα–mediated signals were blocked by treatment with anti–IL-7Rα mAb (Fig. 1 A and B, top verus bottom panels). Under this treatment protocol, IL-7Rα–dependent survival and maturation of T cells are blocked (reference 17 and data not shown). The proliferation of donor cells was assessed by monitoring CFSE dilution by FACS® analysis 5–7 d after transfer.
Figure 1.
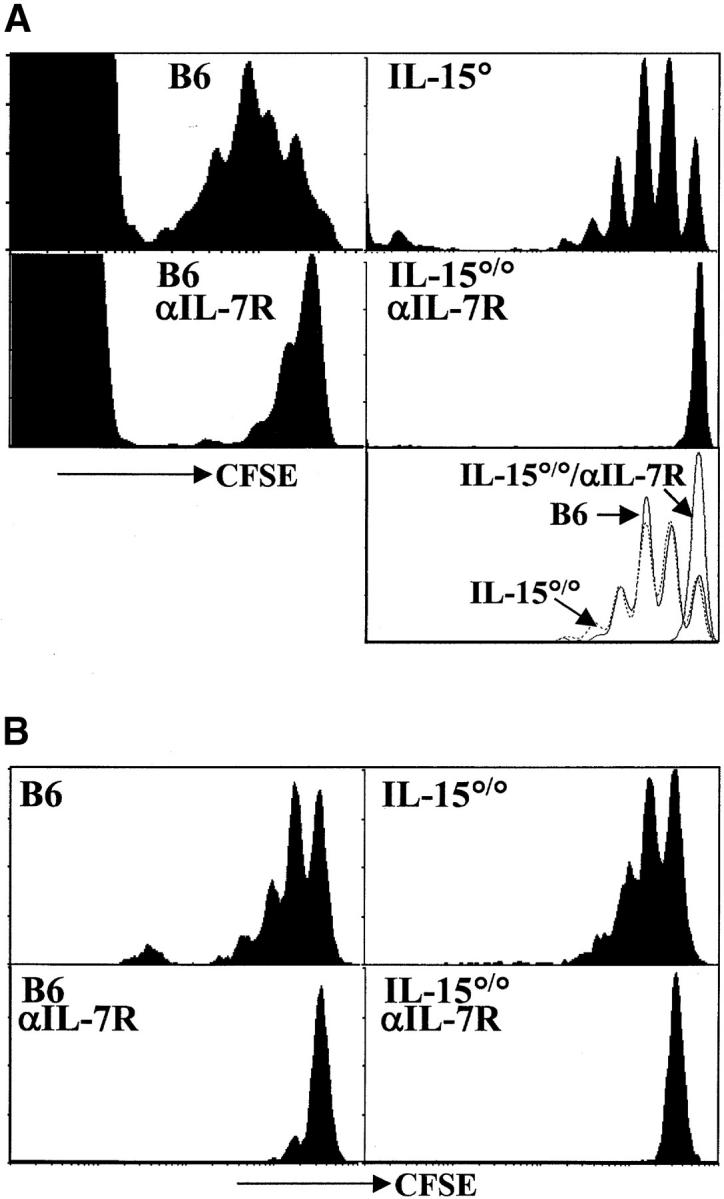
Acute homeostatic proliferation of naive CD8+ T cells requires IL-7Rα–, but not IL-15–, mediated signals. (A) Cells pooled from LN and spleen of OT-I RAG°/° mice were labeled with CFSE and transferred intravenously into B6 or IL-15°/° hosts irradiated the previous day. Indicated recipients were given 1 mg anti–IL-7Rα mAb intraperitoneally every other day beginning at the time of transfer. Data are from two experiments. Histogram plots of CFSE intensity in Vβ5+CD8+ cells (left panels from one transfer) or Vα2+CD8+ gated cells (right panels from a second transfer) are shown for representative recipients 6 d after transfer. The bottom right panel is an overlay of CFSE intensity in CD8+Vα2+ gated cells from B6 (solid line), IL-15°/° (broken line), and anti–IL-7Rα–treated IL-15°/°hosts (gray line) all from the same experiment. (B) Naive polyclonal CD44loCD122lo CD8+ T cells were sorted from LN and spleen cells from CD45.1 congenic mice, labeled with CFSE, and transferred into recipients as above. Histogram plots of CFSE intensity in CD8+CD45.1+ gated cells on day 6 after transfer are shown. Data are representative of three or more experiments.
We found no significant delay in the proliferation of either OT-I (Fig. 1 A) or polyclonal (Fig. 1 B) CD8+ T cells transferred into the IL-15°/° versus the wild-type B6 hosts, indicating that IL-15 is not required for acute proliferation of naive CD8+ T cells. This observation is in agreement with recent reported results (19). Interestingly, however, in experiments in which cytokines were provided exogenously, IL-15, IL-4, and IL-12, were shown to enhance homeostatic proliferation of naive T cells (19, 31). Thus, although naive T cells upregulate a functional IL-15 receptor during homeostatic proliferation, IL-15 is not necessary for this division.
In contrast to the minimal effect of IL-15 deficiency, blocking of IL-7Rα led to a drastic decrease in proliferation of naive CD8+ T cells transferred into either irradiated B6 or IL-15°/° hosts (Fig. 1). In both untreated hosts, nearly all donor cells divided in the week after transfer, and many underwent more than one division. When the host mice were treated with anti–IL-7Rα mAb, minimal division of the transferred cells was observed indicating that IL-7Rα–mediated signals are obligatory for the acute homeostatic proliferation of naive CD8+ T cells.
Both IL-7 and thymic stromal–derived lymphopoietin (TSLP) bind and mediate signals through the IL-7Rα chain (32). Although we cannot rule out a role for TSLP, it is likely that the inhibition of homeostatic proliferation described herein was due to blocking of IL-7/IL-7Rα interactions as these results are in accord with those obtained when T cells were transferred into IL-7°/° hosts (18, 19). Furthermore, in preliminary experiments, using an antibody specific for IL-7, we have been able to confirm the data obtained with the anti–IL-7Rα antibody (data not shown). We believe this effect is due to the blocking of IL-7R function and not toxicity of the antibody as: (i) the kinetics of decay are not indicative of antibody depletion; (ii) IL-7R–dependent thymocyte development is blocked faster than peripheral T cell survival (even though both populations bear IL-7R at similar levels, reference 14); (iii) naive T cells decline more rapidly than memory cells (which have higher levels of the surface receptor, Fig. 3) (reference 17); (iv) after transfer into irradiated mice, we recover cells from the anti–IL-7R–treated IL-15°/° hosts which are completely within the undivided peak (Figs. 1 and 2) . If antibody depletion were a factor, it would be occurring selectively within the dividing population (which has similar levels of IL-7Rα expression as the input population, data not shown). This seems unlikely further indicating antibody toxicity is not a major factor. Thus, in agreement with other reports, we find that IL-7 is a critical mediator of homeostatic proliferation of naive T cells. Importantly, these observations were true for both naive OT-I TCR transgenic T cells (Fig. 1 A) and naive polyclonal CD8+ T cells (Fig. 1 B).
Figure 3.
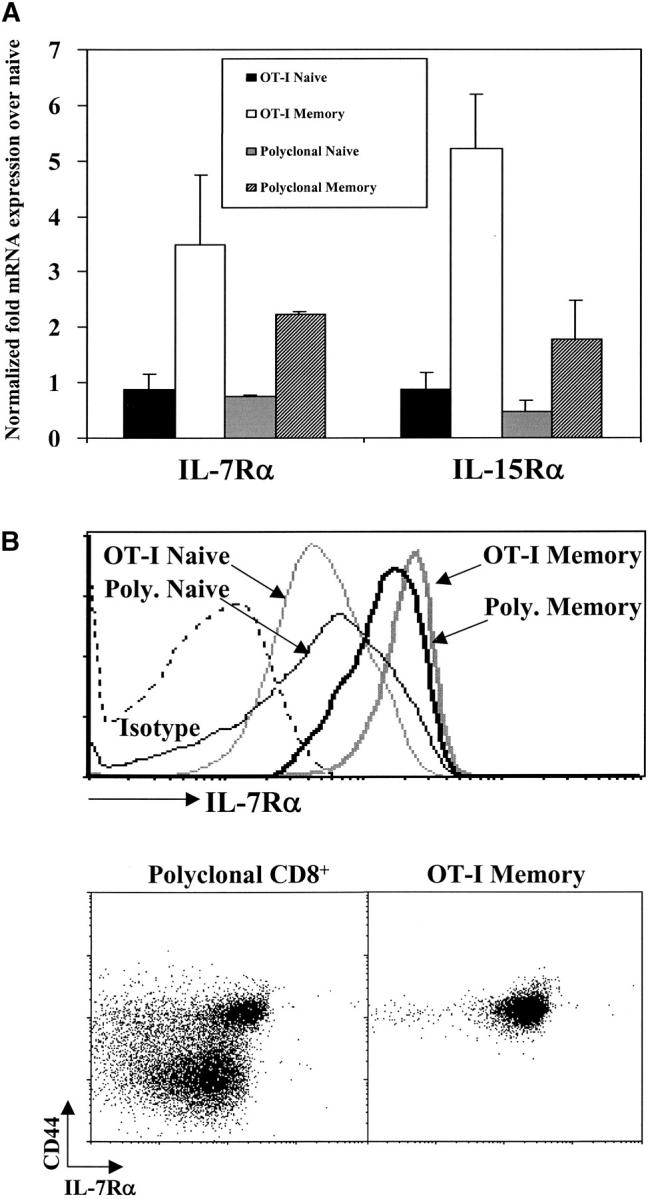
CD8+ memory T cells express higher levels of IL-7Rα and IL-15Rα than naive CD8+ T cells. (A) Relative expression of IL-7Rα and IL-15Rα mRNA by naive and memory OT-I and polyclonal CD8+ T cells. Data shown are representative of duplicate of RNA samples for each condition that were each analyzed three times. Data were normalized to respective housekeeping and CD8α gene expression and plotted relative to OT-I naive cells. (B) IL-7Rα surface expression for OT-I and polyclonal naive and memory cells. Histogram overlays (left panel) represent staining for IL-7Rα for polyclonal naive (CD8+CD44lo, thin gray line), polyclonal memory (CD8+CD44hi, thick gray line), OT-I naive (OT-I RAG°/°, thin black line), or OT-I memory (CD8+ Thy1.1+, thick black line) cells or isotype staining (dashed line). Dot plots show CD44 versus IL-7Rα surface expression on gated polyclonal CD8+ and CD8+ Thy1.1+ OT-I memory T cells from the same mouse >6 mo after transfer of OT-I cells and infection with recombinant vaccinia virus.
Figure 2.
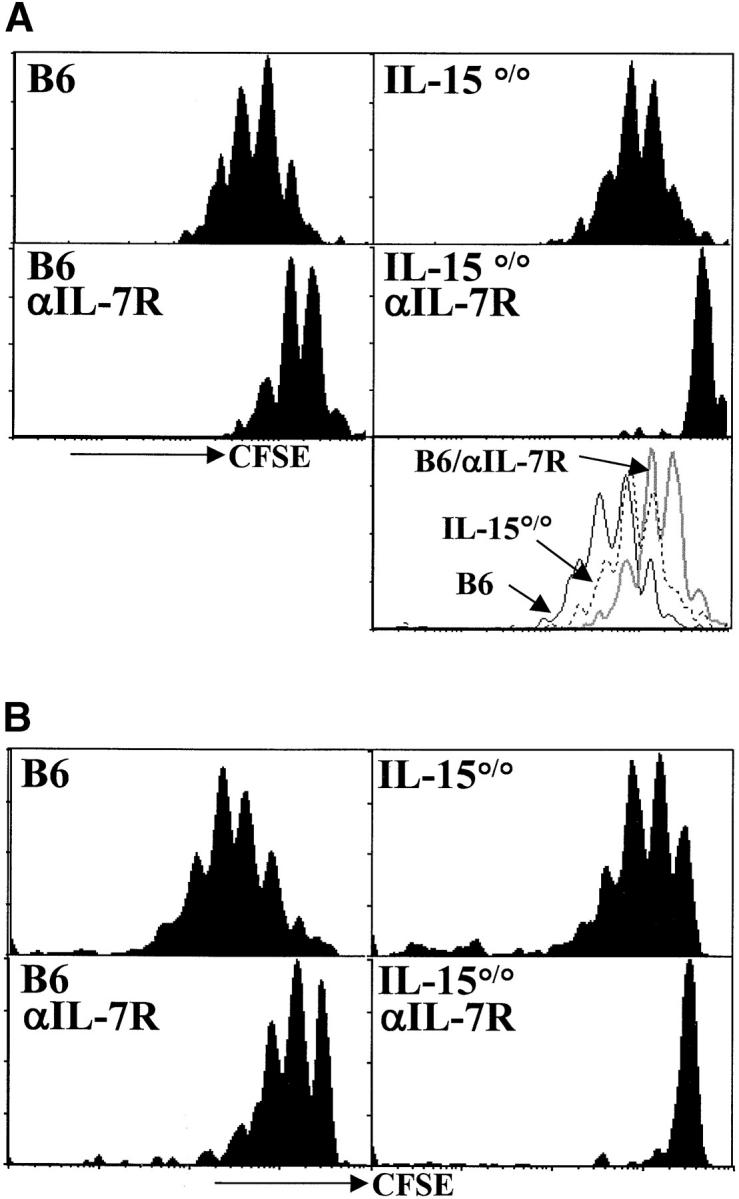
Both IL-7Rα– and IL-15R–mediated signals contribute to the acute homeostatic proliferation of CD8+ memory T cells in irradiated hosts. (A) OT-I memory T cells (CD8+ Thy1.1+) were enriched, labeled with CFSE, and transferred to irradiated recipients as described for Fig. 1. Histograms of CFSE intensity for CD8+Vα2+Thy1.1+ gated cells are shown 6 d after transfer. The bottom right panel is an overlay of CFSE intensity in CD8+Thy1.1+ gated cells from B6 (solid line), IL-15°/° (broken line) and anti–IL-7Rα–treated B6 hosts (gray line). (B) Memory phenotype (CD44hiCD122hi) polyclonal CD8+ T cells were sorted from LN and spleen of CD45.1 congenic mice, CFSE labeled and transferred into recipients as above. Histogram plots of CFSE intensity of CD8+CD45.1+ gated cells are shown on day 6 after transfer. Data are representative of three experiments.
The requirement by naive T cells for IL-7 to undergo homeostatic proliferation mirrors their survival requirements, as has also been observed for TCR–MHC interactions. However, this finding does not address the issue of how a set of signals that mediate survival in a full T lymphocyte compartment is translated differently in an empty compartment to promote proliferation. In the future it will be important to differentiate the influence of a unique signal present in the lymphopenic environment from a purely quantitative difference in availability of factors (for instance, IL-7) that promote acute homeostatic proliferation.
Acute Homeostatic Proliferation of Memory CD8+ T Cells.
We next studied the role of IL-7Rα– and IL-15–generated signals in the proliferation of CD8+ memory cells in a lymphopenic environment. OT-I memory T cells (Fig. 2 A) or polyclonal (Fig. 2 B) CD8+ T cells of memory phenotype (CD8+CD44hiCD122hi) were transferred into irradiated B6 or IL-15°/° hosts (left verus right panels) which were untreated or injected with anti–IL-7Rα (Fig. 2 A and B top versus bottom panels). Proliferation was monitored by loss of CFSE signal 6 d after transfer. OT-I and polyclonal CD8+ memory T cells divided in the absence of cognate antigen in irradiated wild-type hosts. The extent of proliferation was similar for both memory populations and the rate of proliferation was generally faster than for their naive counterparts (compare Figs. 1 and 2).
We found a delay in homeostatic proliferation of both OT-I and polyclonal memory T cells when either IL-7Rα– or IL-15–mediated signals were absent (Fig. 2). Impaired proliferation of transferred memory cells in anti–IL-7Rα-treated hosts was generally more severe in B6 than IL-15°/° hosts, but cells transferred into both hosts consistently demonstrated less proliferation than the B6 controls. This is in contrast to naive T cells which were entirely dependent on IL-7Rα–mediated signals and did not show any delay of proliferation in IL-15°/° hosts (Fig. 1). Acute homeostatic proliferation of both OT-I memory (Fig. 2 A) and polyclonal memory CD8+ T cells (Fig. 2 B) was blocked completely when IL-15°/° hosts were treated with anti–IL-7Rα (Fig. 2 A and B bottom right panels). Thus, in agreement with a recent report (18), our data show that IL-7–/IL-7R–mediated signals are not absolutely required for acute homeostatic proliferation of memory T cells. In fact, IL-15 seems to provide a very similar signal. Both cytokines are capable of promoting acute homeostatic proliferation of CD8+ memory T cells in empty lymphoid compartments and, together, they appear to account for all of the acute homeostatic proliferative activity in our system.
To better understand how memory T cells can use both IL-7– and IL-15–induced signals, whereas naive T cells utilize only IL-7, we compared expression of the relevant receptors by the two populations. Using real-time PCR, we found that both OT-I and polyclonal memory CD8+ T cells had two- to fivefold more IL-7Rα and IL-15Rα mRNA compared with their naive counterparts (Fig. 3 A). Similar results were obtained using RNA from naive and memory OT-I T cells to probe microarrays: memory cells expressed about fourfold more IL-7Rα mRNA than did naive cells (data not shown). Analysis of surface expression by cytofluorimetry revealed an increase (approximately twofold) in IL-7Rα on both OT-I and polyclonal memory T cells compared with naive T cells (Fig. 3 B). Currently, no antibody is available for cytofluorimetric analysis of IL-15Rα expression; however, memory T cells are known to express higher levels of CD122, the IL-2/IL-15Rβ chain (27–29). Thus, the ability of CD8+ memory cells to respond more rapidly to both cytokines might be a reflection of higher expression of their receptors.
Basal Proliferation of Memory CD8+ T Cells in a Full Compartment.
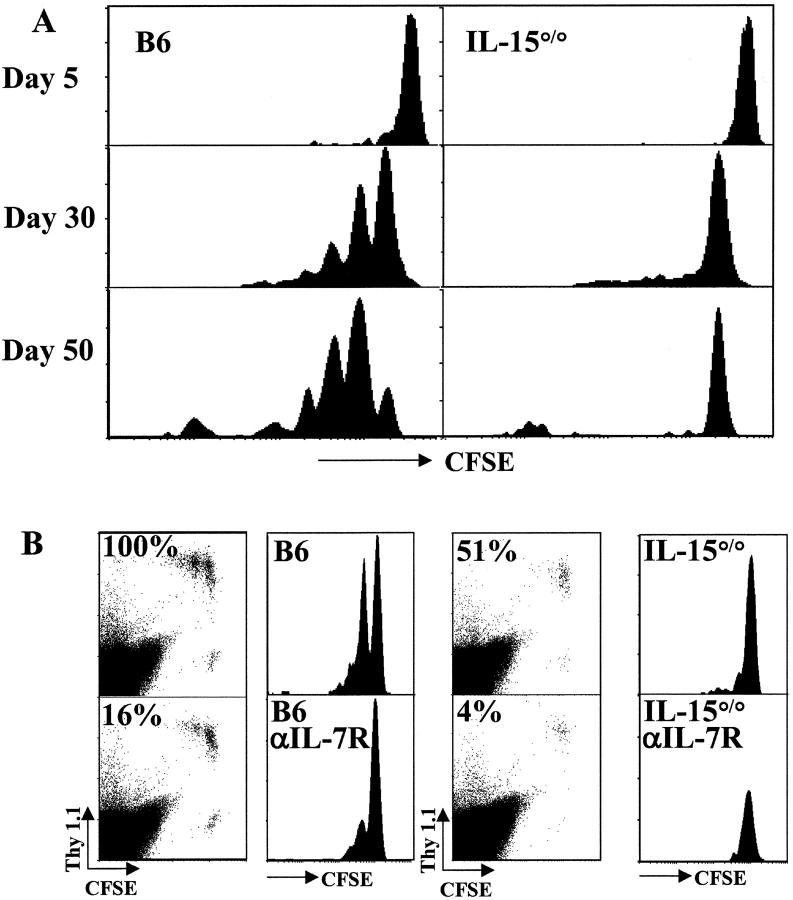
As CD8+ memory T cells also divide in a full lymphoid compartment, we asked whether this basal level of proliferation shared similar cytokine requirements with the acute homeostatic proliferation that occurs in lymphopenic hosts. OT-I memory T cells were CFSE labeled and transferred to unirradiated B6 or IL-15°/° recipients, and proliferation was monitored over time (Fig. 4 A). By day 30, about half of the transferred memory T cells had divided in the wild-type hosts, and by 50 d, nearly all had undergone at least one division. In sharp contrast, almost no memory cell division was seen at any time point in the IL-15°/° recipients. Thus, IL-15 is essential for the basal proliferation of CD8+ memory T cells in a full compartment, whereas either IL-15 or IL-7 will suffice during acute homeostatic proliferation. These data are in agreement with, and extend, previous experiments where antibody blocking of the IL-2/IL-15Rβ chain and IL-2 served to implicate IL-15 in the division of memory T cells (24).
Figure 4.
Basal proliferation of memory CD8+ T cells requires IL-15. (A) OT-I memory T cells (CD8+ Thy1.1+) were enriched, labeled with CFSE, and transferred to unirradiated B6 (left panels) or IL-15°/° (right panels) recipients. Proliferation of transferred memory T cells was followed as CFSE staining intensity of CD8+Thy1.1+ cells at 5, 30, and 50 d after transfer. (B) OT-I memory T cells were transferred into B6 (left panels) or IL-15°/° (right panels) hosts and untreated (top panels) or treated with anti–IL-7Rα mAb for 30 d (bottom panels). Histograms of CFSE intensity in CD8+Thy1.1+ gated cells and dot plots of live gated pooled spleen and LN cells are shown. The percentages indicated on each dot plot represent the relative recovery of transferred memory cells from spleen (mean number of recovered OT-I memory cells from each condition divided by the number recovered from the B6 control hosts). Data are representative of multiple experiments.
The role of IL-7Rα–mediated signals in basal proliferation of OT-I memory T cells was also studied (Fig. 4 B). When unirradiated B6 hosts were treated with anti–IL-7Rα mAb, basal proliferation of OT-I memory cells was apparent at 30 d, although it was reduced compared with that observed in the untreated B6 host (Fig. 4 B). These results suggest that IL-7Rα–mediated signals are not necessary for basal proliferation of CD8+ memory T cells in a full lymphoid compartment, whereas IL-15 is obligatory. Competition may explain the differential requirements for IL-7 by memory cells for proliferation in a full versus empty compartment. In an empty compartment, high levels of available IL-7 (due to decreased competition and/or production) would promote proliferation whereas in a full compartment competition among T cells would limit IL-7 to levels sufficient for survival but not proliferation of memory cells. This conclusion may be problematic as long-term treatment with anti–IL-7Rα inhibits T cell survival and production, rendering the hosts lymphopenic (reference 17, and data not shown). Thus, the IL-7Rα–independent proliferation seen in the treated B6 hosts may be due to acute homeostatic proliferation of memory cells as the lymphoid compartment is gradually depleted. As acute division of CD8+ T cells in lymphopenic hosts can be driven by both IL-7Rα– and IL-15–mediated signals (Fig. 2), it is not possible at this time to address this issue directly. Nonetheless, the difference in acute and basal proliferation of CD8+ memory T cells is clear: IL-15 is not obligatory for acute proliferation in an empty T cell compartment yet is required for basal division in a full compartment.
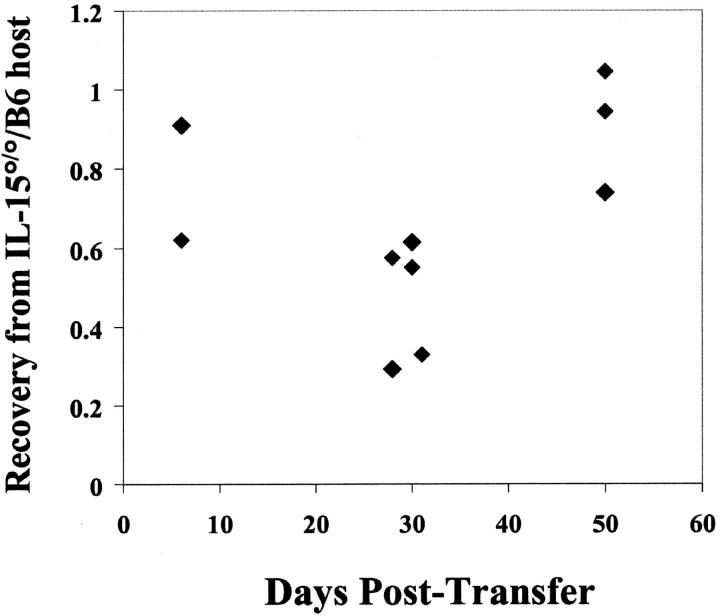
These experiments also permitted us to address the contribution of IL-15 to the survival of CD8+ memory T cells. The recovery of OT-I memory cells from IL-15°/° hosts was generally less than that from B6 hosts (Fig. 5) . However, a substantial number of memory T cells remained at each time point compared with the number of cells recovered from B6 recipients indicating that IL-15 alone is not essential for CD8+ memory survival (27–70% of B6 control at 26–30 d and 74–105% at 50 d after transfer; Fig. 5). We did note a relative increase of recovered memory T cells in the IL-15°/° compared with B6 hosts at day 50. This is, in fact, a reflection of a loss of absolute numbers of memory T cells in the B6 host during this time (61% of the day 30 time point) compared with the IL-15°/° hosts (99% of the day 30 time point) (data not shown). This could be explained by a higher level of competition among memory cells in the full memory compartment of the B6 hosts compared with the smaller IL-15°/° memory compartment (25, 26). Preliminary data indicated that when IL-7Rα was blocked in vivo, both B6 and IL-15°/° hosts yielded 2–27% of the number of OT-I memory cells recovered from untreated B6 controls (Fig. 4 B and data not shown). Similarly, a recent report showed that OT-I memory T cells lacking IL-7Rα survived very poorly compared with OT-I memory cells bearing the IL-7R (where ∼10% of IL-7Rα–deficient compared with wild-type memory T cells remained at day 40; reference 18). This previous report together with our data indicate that IL-7 may be the more prominent player in supporting CD8+ memory T cell survival, whereas IL-15 drives basal proliferation and contributes less to survival.
Figure 5.
Recovery of OT-I memory T cells from B6 and IL-15°/° hosts. Results are expressed as the relative number of recovered Thy1.1+ OT-I memory T cells recovered from the spleen of IL-15°/° hosts normalized to the number of recovered memory T cells from B6 controls. Data are from multiple experiments.
These data highlight a role for basal proliferation in maintaining the memory compartment. In mice deficient in either IL-15 or IL-15Rα, there is a selective deficiency in the CD8+ memory pool, suggesting that the formation and/or maintenance of these cells requires IL-15 (25, 26). Initial experiments addressing the generation of memory T cells in IL-15°/° mice suggested that the formation of antigen-specific memory CD8+ T cells is not IL-15–dependent but that their long-term survival (>18 mo) is impaired in the absence of IL-15 (data not shown). Thus, the deficiency in the memory compartment of IL-15°/° and IL-15Rα°/° mice may reflect the fact that IL-15–driven basal proliferation is crucial to the long-term maintenance of the CD8+ memory compartment.
Homeostasis of T lymphocytes appears to operate on many levels. We have investigated the cytokine requirements for antigen-independent proliferation observed within the CD8+ T cell compartment. Naive CD8+ T cells use IL-7Rα– but not IL-15–mediated signals for proliferation in a lymphopenic host. In contrast, memory CD8+ cells use both IL-7Rα– and IL-15–mediated signals to divide in a lymphopenic environment. Thus, IL-7 levels may set the size of T cell compartment as both naive and memory T cells are responsive and should compete for this cytokine. IL-15, in turn, would influence only the stability of the memory compartment. Specifically, IL-15 appears to be critical for the turnover of memory T cells in a full compartment, whereas IL-7Rα seems to provide signals for survival. This study has carefully compared within one experimental system the requirements for IL-7– and IL-15–mediated signals during homeostatic proliferation for both polyclonal and TCR transgenic CD8+ lymphocytes residing in the naive and memory compartments. These data provide a foundation for the beneficial intervention in both full and lymphopenic CD8+ T cell compartments.
Acknowledgments
We would like to thank Drs. Shannon Turley, Jacques Peschon, and Mike Widmer for fruitful discussion and for critically reviewing the manuscript. We thank Daniel Hirschstein and Julie Hill for assistance with flow cytometry and David Fitzpatrick for help with real time PCR analysis.
These studies are supported by National Institutes of Health grant AI51530-01, and Ananda Goldrath is supported by the Irvington Institute-Juvenile Diabetes Research Fellowship.
A.W. Goldrath and P.V. Sivakumar contributed equally to this work.
Footnotes
*
Abbreviations used in this paper: RAG, recombination activation gene; TSLP, thymic stromal–derived lymphopoietin.
References
- 1.Marrack, P., J. Bender, D. Hildeman, M. Jordan, T. Mitchell, M. Murakami, A. Sakamoto, B.C. Schaefer, B. Swanson, and J. Kappler. 2000. Homeostasis of αβ TCR+ T cells. Nat. Immunol. 1:107–111. [DOI] [PubMed] [Google Scholar]
- 2.Freitas, A.A., and B. Rocha. 2000. Population biology of lymphocytes: the fight for survival. Annu. Rev. Immunol. 18:83–111. [DOI] [PubMed] [Google Scholar]
- 3.Goldrath, A.W., and M.J. Bevan. 1999. Selecting and maintaining a diverse T-cell repertoire. Nature. 402:255–262. [DOI] [PubMed] [Google Scholar]
- 4.Tough, D.F., and J. Sprent. 1994. Turnover of naive- and memory-phenotype T cells. J. Exp. Med. 179:1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murali-Krishna, K., L.L. Ming, S. Sambhara, F. Lemonnier, J. Altman, and R. Ahmed. 1999. Persistence of memory CD8 T cells in MHC class I deficient mice. Science. 286:1377–1381. [DOI] [PubMed] [Google Scholar]
- 6.Ernst, B., D. Lee, J.M. Chang, J. Sprent, and C.D. Surh. 1999. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 11:173–181. [DOI] [PubMed] [Google Scholar]
- 7.Komschlies, K.L., T.A. Gregorio, M.E. Gruys, T.C. Back, C.R. Faltynek, and R.H. Wiltrout. 1994. Administration of recombinant human IL-7 to mice alters the composition of B-lineage cells and T cell subsets, enhances T cell function, and induces regression of established metastases. J. Immunol. 152:5776–5784. [PubMed] [Google Scholar]
- 8.Geiselhart, L.A., C.A. Humphries, T.A. Gregorio, S. Mou, J. Subleski, and K.L. Komschlies. 2001. IL-7 administration alters the CD4:CD8 ratio, increases T cell numbers, and increases T cell function in the absences of activation. J. Immunol. 166:3019–3027. [DOI] [PubMed] [Google Scholar]
- 9.Zhang, X., S. Sun, I. Hwang, D.F. Tough, and J. Sprent. 1998. Potent and selective stimulation of memory-phenotype CD8+ cells in vivo by IL-15. Immunity. 8:591–599. [DOI] [PubMed] [Google Scholar]
- 10.Peschon, J.J. 1994. Early lymphocyte expansion is severely impaired in interleukin 7 receptor deficient mice. J. Exp. Med. 180:1955–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Freeden-Jeffry, U., P. Viera, L.A. Lucian, T. McNiel, S.E. Burdach, and R. Murray. 1995. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 181:1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Freeden-Jeffry, U., N. Solvason, M. Howard, and R. Murray. 1997. The earliest T lineage-committed cells depend on IL-7 for Bcl-2 expression and normal cell cycle progression. Immunity. 7:147–154. [DOI] [PubMed] [Google Scholar]
- 13.Maraskovsky, E., M. Teepe, P.J. Morrissey, S. Braddy, R.E. Miller, D.H. Lynch, and J.J. Peschon. 1996. Impaired survival and proliferation in IL-7 receptor-deficient peripheral T cells. J. Immunol. 157:5315–5323. [PubMed] [Google Scholar]
- 14.Sudo, T., S. Nishikawa, N. Ohno, M. Akiyama, M. Tamakoshi, H. Yoshida, and S. Nishikawa. 1993. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc. Natl. Acad. Sci. USA. 90:9125–9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hassan, J., and D.J. Reen. 1998. IL-7 promotes the survival and maturation but not differentiation of human post-thymic CD4+ T cells. Eur. J. Immunol. 28:3057–3065. [DOI] [PubMed] [Google Scholar]
- 16.Porter, B.O., P. Scibelli, and T.R. Malek. 2001. Control of T cell development in vivo by subdomains within the IL-7 receptor α-chain cytoplasmic tail. J. Immunol. 166:262–269. [DOI] [PubMed] [Google Scholar]
- 17.Vivien, L., C. Benoist, and D. Mathis. 2001. T lymphocytes need IL-7 but not IL-4 or IL-6 to survive in vivo. Int. Immunol. 13:763–768. [DOI] [PubMed] [Google Scholar]
- 18.Schluns, K.S., W.C. Kieper, S.C. Jameson, and L. Lefrancois. 2000. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 1:426–432. [DOI] [PubMed] [Google Scholar]
- 19.Tan, J.T., E. Dudl, E. LeRoy, R. Murray, J. Sprent, K.I. Weinberg, and C.D. Surh. 2001. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc. Natl. Acad. Sci. USA. 98:8732–8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lenardo, M.J. 1991. Interleukin-2 programs mouse αβ T lymphocytes for apoptosis. Nature. 353:858–861. [DOI] [PubMed] [Google Scholar]
- 21.Refaeli, Y., L. Van Parijs, C.A. London, J. Tschopp, and A.K. Abbas. 1998. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity. 8:615–623. [DOI] [PubMed] [Google Scholar]
- 22.Vella, A.T., S. Dow, T.A. Potter, J. Kappler, and P. Marrack. 1998. Cytokine-induced survival of activated T cells in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 95:3810–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marks-Konczalik, J., S. Dubois, J.M. Losi, H. Sabzevari, N. Yamada, L. Feigenbaum, T.A. Waldmann, and Y. Tagaya. 2000. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc. Natl. Acad. Sci. USA. 97:11445–11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ku, C.C., M. Marakami, A. Sakamoto, J. Kappler, and P. Marrack. 2000. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 288:675–678. [DOI] [PubMed] [Google Scholar]
- 25.Lodolce, J.P., D.L. Boone, S. Chai, R.E. Swain, T. Dassopoulos, S. Trettin, and A. Ma. 1998. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 9:669–676. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy, M.K., M. Glaccum, S.N. Brown, E.A. Butz, J.L. Viney, M. Embers, N. Matsuki, K. Charrier, L. Sedger, C.R. Willis, et al. 2000. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 191:771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldrath, A.W., L.Y. Bogatzki, and M.J. Bevan. 2000. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J. Exp. Med. 192:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho, B.K., P.R. Varada, Q. Ge, H.N. Eisen, and J. Chen. 2000. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory cells. J. Exp. Med. 192:549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murali-Krishna, K., and R. Ahmed. 2000. Naive T cells masquerading as memory cells. J. Immunol. 165:1733–1737. [DOI] [PubMed] [Google Scholar]
- 30.Goldrath, A.W., and M.J. Bevan. 1999. Low affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 11:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kieper, W.C., M. Prlic, C.S. Schmidt, M.F. Mescher, and S.C. Jameson. 2001. IL-12 enhances CD8 T cell homeostatic proliferation. J. Immunol. 166:5515–5521. [DOI] [PubMed] [Google Scholar]
- 32.Park, L.S., U. Martin, K. Garka, B. Gliniak, J.P. Di Santo, W. Muller, L. Largaespada, N.G. Copeland, N.A. Jenkins, A.G. Farr, et al. 2000. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: formation of a functional heteromeric complex requires interleukin 7 receptor. J. Exp. Med. 192:659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]