The Extracellular Domain of CD83 Inhibits Dendritic Cell–mediated T Cell Stimulation and Binds to a Ligand on Dendritic Cells (original) (raw)
Abstract
CD83 is an immunoglobulin (Ig) superfamily member that is upregulated during the maturation of dendritic cells (DCs). It has been widely used as a marker for mature DCs, but its function is still unknown. To approach its potential functional role, we have expressed the extracellular Ig domain of human CD83 (hCD83ext) as a soluble protein. Using this tool we could show that immature as well as mature DCs bind to CD83. Since CD83 binds a ligand also expressed on immature DCs, which do not express CD83, indicates that binding is not a homophilic interaction. In addition we demonstrate that hCD83ext interferes with DC maturation downmodulating the expression of CD80 and CD83, while no phenotypical effects were observed on T cells. Finally, we show that hCD83ext inhibits DC-dependent allogeneic and peptide-specific T cell proliferation in a concentration dependent manner in vitro. This is the first report regarding functional aspects of CD83 and the binding of CD83 to DCs.
Keywords: CD83, dendritic cells, MLR, T cell inhibition, recombinant expression
Introduction
Dendritic cells (DCs)* are the most potent APCs of the immune system. DC maturation, with its associated functional and phenotypic changes is crucial to this role. In their immature state, DCs reside in peripheral tissues. Upon antigen uptake and inflammatory and microbial stimuli, they start to migrate to the T cell areas of the peripheral lymph nodes, where as mature DCs, they express additional molecules that will lead to binding and stimulation of T cells, while losing their ability for antigen-capturing (1–3). In this respect, CD83 is the best known marker for mature DCs. Human CD83 (hCD83) is a 45-kD glycoprotein and member of the Ig superfamily (4, 5). Recently, also the cloning and biochemical characterization of the murine CD83 (mCD83) has been reported (6, 7). mCD83 shares an amino acid identity of 63% with hCD83 suggesting a possibly conserved function.
The selective expression and upregulation together with costimulatory molecules like CD80 and CD86 suggests an important role of CD83 in the immune response (4, 8–10). Although the precise function of CD83 is still unknown (no inhibitory antibody exists), we have recently shown that by interfering with the translocation of mRNA encoding CD83 and thus inhibiting CD83 protein synthesis, the T cell stimulation capacity of DCs was significantly reduced (11). In addition, we demonstrated a selective downregulation of the surface expression of CD83 after the infection of mature DCs with Herpes simplex virus type 1 and a reduced ability to stimulate allogeneic T cells in mixed leukocyte reactions (MLRs; reference 12). These observations indicated, but did not prove a critical role for CD83.
Here we report the recombinant expression and purification of the extracellular domain of the hCD83 (hCD83ext). Interestingly, hCD83ext completely abrogates the DC-mediated primary allostimulation of T cells in vitro. Furthermore, also the capacity to stimulate antigen specific T cells was inhibited by this soluble molecule. This is the first report describing a functional role for CD83 which will contribute to a better understanding of the DC biology in general and will hopefully lead to the development of new therapeutic strategies. Finally, we show that CD83 binds to immature as well as mature DCs.
Materials and Methods
Cloning of hCD83ext.
The extracellular domain of human CD83 (amino acids 23–128) was PCR-amplified using the following primers: sense- pGEX2ThCD83: 5′-TCCCCCGGGAACGCCGGAGGTGAAGGTGGCT-3′ and antisense-CD83 extra: 5′-AATTAGAATTCTCAAATCTCCGCTCTGTATT-3′. The amplified fragment was subcloned into the SmaI and EcoRI sites of the expression vector pGEX2T (Amersham Pharmacia Biotech) resulting in the plasmid pGEX2ThCD83ext and transformed into the E.coli strain TOP10FÄ (Invitrogen). The correct insert was verified by sequencing.
Expression and Purification of hCD83ext.
The expression of hCD83ext was induced in Escherichia coli as described previously (7). Briefly, the cells were then pelleted and resuspended in 10 ml native buffer (140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2 PO4, 2.6 mM MnCl, 26 mM MgCl2, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 1 μg/ml DNaseI, pH 7.6) per 500 ml culture. 50 μg/ml lysozyme were also added. After 15-min incubation on ice the lysate was spun at 20,000 g. Protein purification: capture step: 40 ml supernatant were added to a GSTrap 5 ml column on an ÄKTA Explorer 10 system (Amersham Pharmacia Biotech). Binding buffer: PBS (140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2 PO4, pH 7.6). Elution buffer: 50 mM Tris-HCl, pH 8.0, with 5 mM reduced glutathione. Flow rate: 5 ml/min. Chromatographic procedure: 4CV (column volumes) binding buffer, 40 ml supernatant, 12CV binding buffer, 5CV elution buffer, 5CV 2N NaCl/PBS, pH 7.6, 5CV binding buffer. Intermediate purification steps: The GST-hCD83ext containing elutions were dialysed against 50 mM 1-methyl-piperazine, 50 mM Bis-Tris, 25 mM Tris, pH 9.5 (buffer A), and loaded onto source 15Q PE 4.6/100 anion exchange column on a ÄKTA Explorer 10 system. Proteins were separated by three different linear salt gradients: 16CV to target concentration 10% buffer B (buffer A/1N NaCl). 20CV to target concentration 50% buffer B. 10CV to target concentration 100% buffer B. The GST-hCD83ext containing elutions were dialysed against PBS, pH 7.6. Then the GST-hCD83ext. fusion protein was incubated with Thrombin 20 U/ml at 22°C for 16 h. To separate the hCD83ext protein from GST the elution was loaded onto prepacked glutathione sepharose 4B columns using the capture step buffer conditions. Under binding buffer conditions the flow through containing recombinant human CD83ext protein was collected. Polishing step: finally a preparative gel filtration separation was performed loading the flow through onto a Superdex 75 (26/16) prep grade column on a ÄKTA Explorer 10 system, running buffer PBS, pH 7.6, flow rate 3 ml/min. The GST protein was used as a negative control in functional analyses.
Expression and Purification of Recombinant CD83-Fc-Fusion Protein.
CD83-Fc was constructed by fusing the extracellular domain of CD83 with the Fc part of human IgG1. A PCR product of the extracellular domain of CD83 was amplified using Pfu polymerase (Promega). The CD83 sense primer contains a XhoI restriction site (5′-GCGGGGCTCGAGGCCACCATGTCGCGCGGCCTCCAGCTTCTGC) and the antisense primer a BglII restriction site (5′-CCCCGGAGATCTGCAGGGCATCCTGTCACTCTCA). PCR conditions were as follows: 2 min 94°C; 35× (1 min 94°C, 1 min 56°C, 1 min 72°C); 10 min 72°C. The PCR product was purified using a PCR purification kit (QIAGEN). After digestion with XhoI and BglII the PCR product was cloned into the XhoI and BamHI sites of the pCDM7 vector (a gift from Kolanus, Genecenter, Munich, Germany) containing the Fc part of IgG1 and transformed into MC1061P3 bacteria. The construct was sequenced in order to exclude possible mutations (Sequiserve). 293-T cells, cultured in DMEM medium (Life Technologies) supplemented with 2 mM l-glutamine (Life Technologies), 100 U/ml penicillin/streptomycin (Life Technologies), 1 mM sodium-pyruvat (Life Technologies), and 10% FBS (Dynacyte), were transiently transfected with CDM7/CD83-Fc using LipofectAMINE™ reagent and OPTIMEM 1 medium (Life Technologies). 293-T cells were cultured 10 d in serum free medium (293 SFMII; Life Technologies). Secretion of recombinant CD83-Fc protein into the supernatant was verified by Western blot analysis. The CD83-Fc fusion protein was purified by protein A-Sepharose affinity chromatography (Amersham Pharmacia Biotech).
Production of mAbs Against Human CD83.
∼50 μg of the hCD83ext-GST fusion protein were injected intraperitoneally and subcutaneously into LOU/C rats. After a 2-mo interval a final boost with the antigen was given intraperitoneally and subcutaneously 3 d before fusion. Fusion of the myeloma cell line P3X63-Ag8.653 with the rat immune spleen cells was performed according to standard procedure. Hybridoma supernatants were tested in a solid-phase immuno assay using the hCD83ext-GST protein adsorbed to polystirene microtiter plates. After incubation with culture supernatants for 1 h, bound mAbs were detected with peroxidase-labeled goat anti–rat IgG plus IgM antibodies (Dianova) and _O_-phenylenediamine as chromogen in the peroxidase reaction. An irrelevant GST-fusion protein served as a negative control. Ig type of the monoclonal antibodies was determined using biotinylated anti–rat IgG subclass-specific mAbs (American Type Culture Collection). CD83–1G11 (rat IgG1) and CD83–4B5 (rat IgG2a) were used for Western blot analysis and FACS® analysis. Both detected specifically human CD83. Functional studies revealed, that, like the commercially available anti-CD83 antibodies, these antibodies had no inhibitory activity.
Immunoblotting Analyses.
Western blot analyses of purified protein was performed as described previously (12) using monoclonal anti-CD83 antibodies (CD83–1G11; CD83–4B5 both described in this paper; and anti-CD83 from Immunotech).
1-D NMR Studies.
1-D NMR studies were recorded with a sample of the hCD83ext using a Bruker AM 400 spectrometer at a temperature of 300K as described previously (7).
Generation of DCs.
DCs were generated from PBMCs in the presence of GM-CSF and IL-4 as described previously (12). For the final DC maturation the medium containing 1% human plasma, GM-CSF and IL-4 was supplemented with IL-1β (1 ng/ml), TNF-α (1.25 ng/ml), and prostaglandin E2 (0.5 μg/ml) (maturation mix).
Plate Adhesion Assay.
A 96-well flat-bottomed plat (Maxi Sorp; Nunc) was precoated with 50 μl goat anti–human Fc-specific F(ab′)2 (4 μg/ml, Jackson ImmunoResearch Laboratories) or with 50 μl rat anti–human GST (1 mg/ml) for 1 h at 37°C and blocked with 1% BSA in Tris-sodium buffer (20 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1 mM CaCl2, 2 mM MgCl2) for 30 min at 37°C. Subsequently, the plate was incubated with 500 ng/ml intercellular adhesion molecule (ICAM)-I-Fc (13), 500 ng/ml CD83-Fc or 500 ng/ml GST. 40,000 cells per well were labeled in PBS with Calcein-AM (25 μg/107 cells per milliliter; Molecular Probes) for 30 min at 37°C. Labeled cells were washed and preincubated for 10 min with different concentrations of recombinant hCD83ext or medium at RT. Cells were allowed to adhere for 45 min at 37°C. Adherent cells were lysed with 100 μl lysis buffer (50 mM Tris, 0.1% SDS), and the fluorescence was quantified using a Cytofluor II (Perseptive Biosystems) or a Victor (Wallac) fluorometer. Results are expressed as the mean percentage of cells binding from triplicate wells.
Coating of Beads with CD83-Fc.
Streptavidin-coated TransFluorSpheres (488/645 nm; 1.8 μm; Molecular Probes) were generated as described previously (13). The streptavidin-coated beads (15 μl) were incubated with biotinylated goat anti–human anti-Fc F(ab′)2 fragments (33 μg/ml) in 0.3 ml PBS, 0.5% BSA for 2 h at 37°C. The beads were washed once with PBS, 0.5% BSA, and incubated with CD83-Fc (500 ng/ml) in 500 μl PBS, 0.5% BSA overnight at 4°C. The binding efficiency was determined by FACS® analysis.
FACS® Analysis.
Phenotypic analysis of cells or beads were performed by flow cytometry using saturating concentrations of the following mAbs: CD83 (CD83–1G11; CD83–4B5 both described in this paper and anti-CD83 from Immunotech); CD80 (Immunotech); CD14 and CD86, MHC-I (Dianova); CD3 (Bio Research), CD4, CD8, CD45RO, and CD45RA (Dako). The isotype controls IgG1a, IgG2a, and IgG2b (Becton Dickinson) were run in parallel. Cells were analyzed on a FACScan™ (Becton Dickinson). Nonviable cells were gated out on the basis of their light scatter properties.
Allogeneic T Cell Proliferation.
Human PBMCs were isolated from buffy coats and the T cell fraction was purified by rosetting with neuramidase-treated sheep RBCs as described by Bender et al. (14). The CD4- and CD8-positive T cells were stimulated at different ratios with mature allogeneic DCs. DCs were treated with different concentrations of hCD83ext. GST or BSA was used as negative control (Bio-Rad Laboratories). CD83-Fc coated beads were also used for inhibitory studies. In these experiments uncoated beads were used as negative control. T cell proliferation was determined as described previously (12).
Enzyme-linked Immunospot Assay.
Assessment of the IFN-γ production by the influenza matrix peptide M1 specific CTL clone (C9) was performed as described previously (15). The HLA-A2.1 restricted M1 peptide (GILGFVFTL, amino acids 58–66) was used at a concentration of 10 μM. The number of spots forming cells were counted with a video imaging analysis system (Carl Zeiss Vision), using the KS enzyme-linked immunospot assay (ELISPOT) software version 4.1.143.
Results
Recombinant Protein Expression.
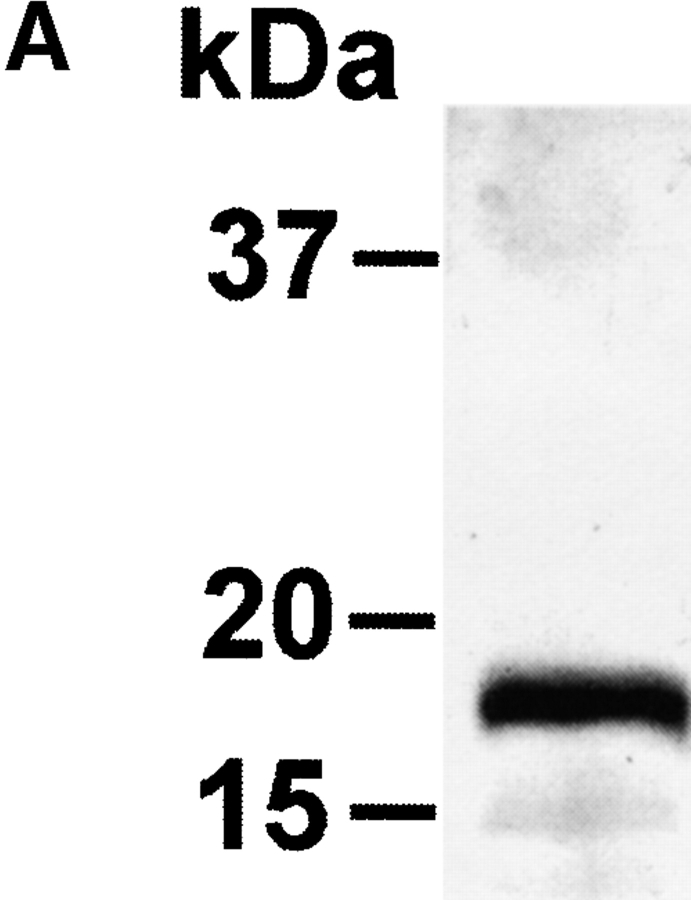
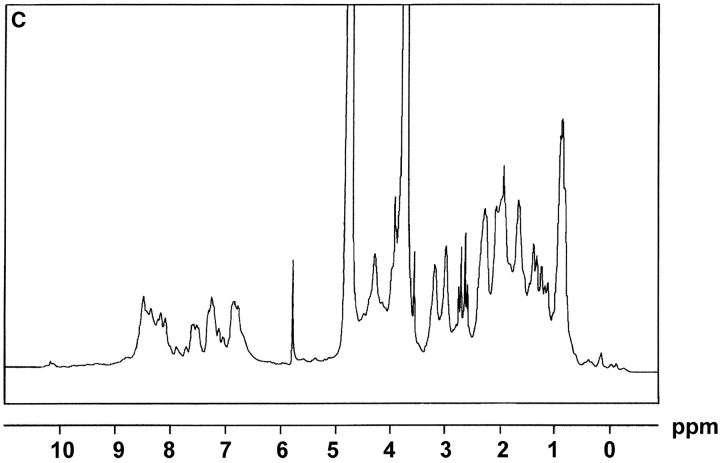
The extracellular domain of hCD83 (hCD83ext) was expressed in E.coli as a gluthatione S-transferase (GST) fusion protein in sufficient quantities. For functional studies the fusion protein was cleaved by thrombin and only the hCD83ext was used, whereas GST served as a negative control. The correct expression of the protein was analyzed by silver staining and Western blot analysis (Fig. 1 A and B). Amino-terminal amino acid sequencing analyses further confirmed the correct identity of the purified protein (data not shown). To determine whether or not the recombinant protein was correctly folded, one-dimensional NMR studies were performed. The 1-D NMR spectrum of hCD83ext at 300K showed chemical dispersion typical of a structured protein (Fig. 1 C). The presence of slowly exchanging amide resonances (∼7–9 ppm) indicates that certain parts of the protein backbone are protected from solvent. Downfield-shifted α-CH resonances (∼4.5–5.7 ppm) are indicative of β-structures. Upfield-shifted methyl resonances (σ < 0.9 ppm) provide further evidence of the protein being folded. These NMR data strongly support that the recombinant expressed hCD83ext. is structurally folded and relevant functional studies can be performed using this protein.
Figure 1.
Biophysical analyses of recombinant hCD83ext. (A) hCD83ext was separated by SDS-PAGE and silver stained. (B) Western blot analysis of the blotted hCD83ext using monoclonal anti-CD83 antibodies and (C) one-dimensional NMR spectrum, showing that hCD83ext is folded.
Human CD83 Binds to Immature and Mature DCs.
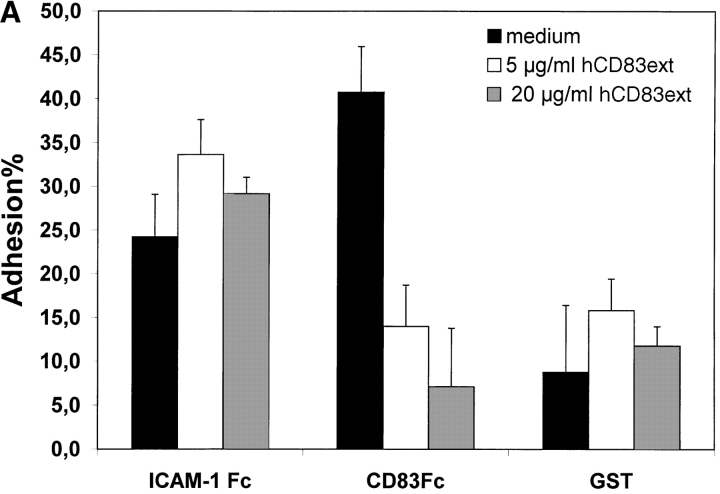
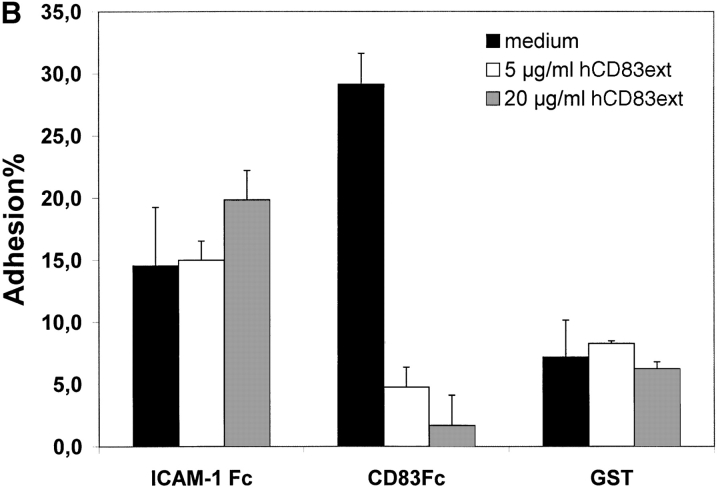
To establish the cell surface binding of human CD83 a standard plate adhesion assay was employed. As shown in Fig. 2 immature day 4 DCs as well as mature day 7–8 DCs (Fig. 2 A and B, respectively) bind to human CD83. This binding could be inhibited in a concentration-dependent manner by the recombinant expressed extracellular domain of CD83 (hCD83ext). Binding to ICAM-1-Fc as a control could not be inhibited by hCD83ext. Uncoupled GST served as a negative control. The binding of ICAM-1 and beads was not specifically influenced by hCD83ext. In addition, as shown in Fig. 2 C, binding of DCs to CD83 is concentration dependent. The specific binding was lost when decreasing amounts of CD83-Fc were used (Fig. 2 C).
Figure 2.
Plate adhesion assay. ICAM-Fc and hCD83-Fc were bound to goat anti–human Fc-specific F(ab′)2 precoated plates. Calcein-AM labeled DCs (40,000) were allowed to adhere for 45 min at 37°C. Fluorescence of adherent cells was quantified using fluorometric analyses. Clearly, immature (A) as well as mature (B) DCs bind to CD83. Preincubation of labeled DCs with recombinant hCD83ext inhibited the binding of the immature and mature DCs to plate-bound CD83-Fc. The binding could be inhibited in a concentration-dependent manner (5 and 20 μg/ml hCD83ext). Binding to ICAM-1-Fc as a control could not be inhibited by hCD83ext, while GST was used as a negative control. Both were not specifically influenced by the addition of hCD83ext. In addition the binding of DCs to CD83 was concentration dependent (C). The binding was lost when decreasing amounts of CD83-Fc were used. In these experiments, Ig coated via goat anti–human-Fc, was used as negative control. The Ig binding was not influenced by hCD83ext. These experiments were performed five times using different donors for DC generation. This represents a typical experiment.
Interestingly, we could not demonstrate binding of naive CD4+or CD8+ T cells to CD83 (data not shown). From these data we conclude that immature and mature DCs bind specifically to CD83 and that the recombinant prokaryotic expressed hCD83ext could be used for further functional studies, indicating that glycosylation is not necessary. Furthermore, the fact that immature DCs, which do not express CD83, also bind to CD83 implies that there is no homophilic interaction between CD83 molecules. Therefore, it is most likely a heterophilic binding reaction.
hCD83ext Induces Downmodulation of Specific Molecules on DCs.
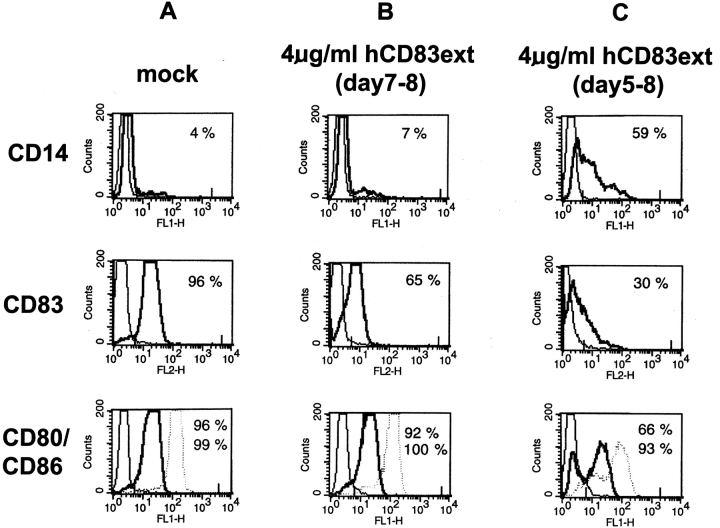
To analyze the influence of hCD83ext on the phenotype of DCs, FACS® analyses were performed on day 8 (Fig. 3). DCs can be fully matured with the use of a specific maturation cocktail composed of IL-1β, TNF-α, and PGE2. Interestingly, when this maturation cocktail was administered to immature DCs on day 5 together with hCD83ext (4 μg/ml) and left until the final FACS® analyses on day 8, these cells revealed a clear reduction in CD80 (from 96 to 66%) and CD83 cell surface expression (96–30%), when compared with normally matured DCs (compare Fig. 3 A with C). Thus, hCD83ext induces a reduction in DC maturation (see also increase of CD14-positive cells). In contrast, mature DCs which where incubated with hCD83 for 24 h on day 7 and analyzed on day 8, showed only a minimal influence on CD80 expression (96–92%), while CD83 expression was also reduced (96–66%) (compare Fig. 3 A with B). Interestingly, CD86 expression was not influenced at any time point by the administration of hCD83ext. Also MHC class I and II expression was not affected, neither in immature nor in mature DCs. Noteworthy, no significant phenotypical changes could be observed within T cell populations by the addition of hCD83ext (data not shown), indicating that CD83 affects primarily DCs.
Figure 3.
FACS® analyses of DCs. (A) Immature DCs were matured in the presence of the maturation cocktail from day 5–8 (= mock control for mature DCs). (B) Immature DCs where matured in the presence of the maturation cocktail (day 5–8) and on day 7 hCD83ext was added for 24 h. (C) Immature DCs where incubated in the presence of the maturation cocktail in combination with hCD83 from day 5–8. On day 8 cells where washed and stained with the indicated antibodies and analyzed by FACS®.
hCD83ext Inhibits Allogeneic T Cell Proliferation.
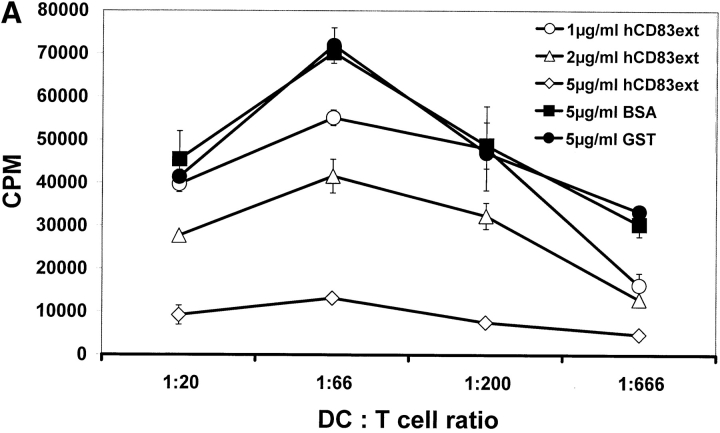
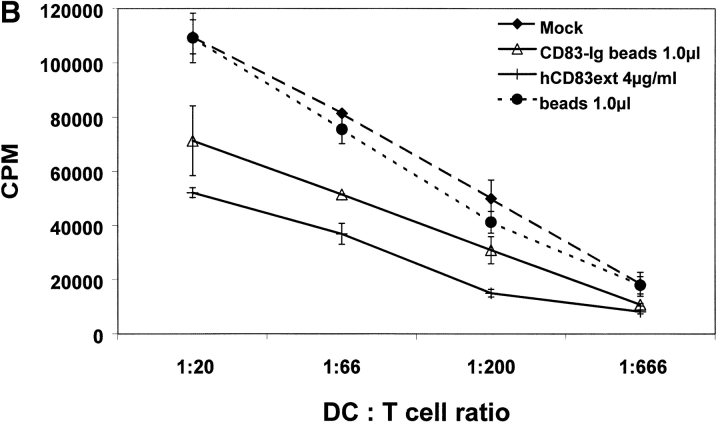
MLRs are a simple, yet very powerful tool in order to analyze the T cell stimulatory capacity of DCs. A typical feature of these MLR-assays is the formation of large DC–T cell clusters. Addition of hCD83ext at day 1 strongly inhibited the typical cell cluster formation of DCs and proliferating T cells, whereas GST had no influence (data not shown). As shown in Fig. 4 A hCD83ext strongly inhibited [3H]thymidine incorporation in a dose–dependent manner. GST, which was expressed and purified in the same way as hCD83ext, was used as negative control and had, like BSA, no influence on T cell proliferation. Also the CD83-Fc molecule (expressed in an eukaryotic system) coupled to beads inhibited T cell proliferation (Fig. 4 B). These findings indicate again that CD83-glycosylation is not a prerequisite, since the prokaryotic expressed hCD83ext also inhibits T cell proliferation.
Figure 4.
hCD83ext inhibits allogeneic T cell proliferation. (A) MLR analyses: the prokaryotic expressed hCD83ext reduced T cell proliferation in a dose-dependent manner. GST, which was purified in the same way as hCD83ext and BSA (each 5 μg/ml) were used as controls. (B) Also the eukaryotic expressed CD83–Fc fusion protein couplet to beads inhibits T cell proliferation. Uncoupled beads alone were used as a negative control and hCD83ext as a positive control. (C) The inhibition is not due to a contamination: to prove that the purified hCD83ext protein was responsible for the inhibitory activity and not a possible contamination present in the protein preparation, purified hCD83ext was filtered using a 10-kD Microcon system. Filtered and unfiltered hCD83ext exhibit comparable inhibitory effects. Whereas, cells which were treated with the filtrate alone showed no inhibition and the stimulation was comparable to mock-treated cells. All experiments were performed at least three times. Data presented here represent a typical experiment.
To prove that the inhibitory effects of hCD83ext are not due to cytotoxic effects, day 5 DCs were incubated for 24 h with 5 μg/ml hCD83ext together with the final maturation mix. Then, hCD83ext was washed out, new maturation mix was added and the allostimulatory capacity of these cells was determined. Interestingly, T cell proliferation was not inhibited when hCD83ext has been washed out (data not shown). These data clearly show that hCD83ext is not cytotoxic and indicate that CD83ext must be present during the experiment to exhibit its inhibitory function.
To further demonstrate that the purified protein itself was responsible for the inhibitory activity and not a possible contamination present in the protein preparation, purified hCD83ext was filtered using a 10-kD Microcon system. Then, the filtered protein as well as the filtrate were tested (Fig. 4 C). These experiments clearly show that the filtered hCD83ext protein was responsible for the inhibitory effects, while the filtrate had no influence on the T cell proliferation.
To demonstrate that hCD83ext had no toxic effects on T cell level, cell viability was determined using the trypan blue exclusion method. T cells were incubated with hCD83ext (4 μg/ml) for 4 d and compared with control cells (incubated with 4 μg/ml BSA). No significant decrease in T cell viability, after incubation with inhibitory concentrations of hCD83ext were detected (data not shown). Therefore, T cell death seems not responsible for the reduced proliferation.
hCD83 Inhibits Proliferation of Influenza Virus Specific CTLs.
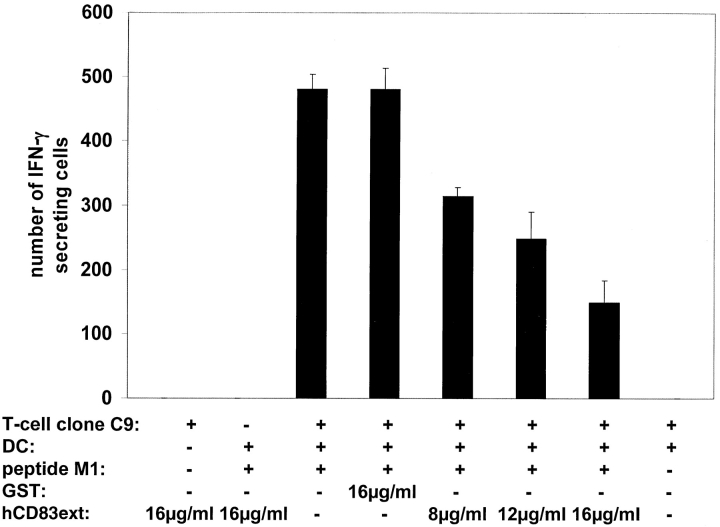
The ELISPOT assay is a very useful tool to analyze DC-mediated proliferation of specific CTLs, assessing the cytokine release at a single cell level. Furthermore, it can also be used for direct ex vivo quantification of peptide-reactive T cell responses. In this study we used the influenza matrix peptide M1 specific CTL clone C9 (16). HLA-A2.1 positive DCs were incubated with the M1-peptide and then cocultivated with different numbers of CTLs. A computer-assisted video image analysis system was used for the evaluation of the experiments (17). Again, increasing amounts of hCD83ext resulted in a clear reduction of CTL specific responses (Fig. 5), indicating that the interaction between DCs and peptide specific T cells is inhibited.
Figure 5.
hCD83ext inhibits proliferation of influenza matrix peptide M1-specific CTL clone. DCs were loaded with influenza matrix peptide M1 and used to assess the stimulation (IFN-γ production) of the M1-specific CTL clone C9. Number of IFN-γ–producing spot are shown on the y-axis. Increasing concentrations of hCD83ext clearly reduced the number of spot forming cells. GST, which was purified in the same way as hCD83ext was used a negative control. Three independent experiment were performed. The data presented represent a typical experiment.
Secondary Stimulation of hCD83ext Treated T Cells.
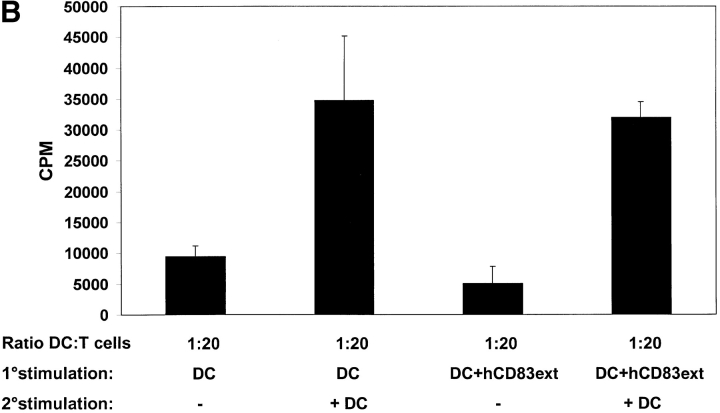
To investigate whether or not the proliferation arrest of hCD83ext treated T cells can be overcome, cells from the primary MLRs, which have been either incubated with or without hCD83ext, were stimulated for a second time period of 4 d without the addition of hCD83ext. For this, half of the medium was discharged and new medium containing IL-2 at a final concentration of 500 U/ml or without IL-2 was added to the cultures. After an incubation of 3 d, cells were pulsed and T cell proliferation was determined (Fig. 6 A). Interestingly, T cells which were strongly inhibited by hCD83ext in the primary MLRs could be restimulated with IL-2. The proliferation was comparable to that obtained by untreated cells in the primary MLRs. These data clearly show that the proliferation arrest in T cells, caused by hCD83ext, is not due to an irreversible toxic effect. In fact proliferation can be restored using IL-2. Furthermore, to investigate this phenomenon in a more physiological setting, T cells which were inhibited with hCD83ext in the primary MLRs, were restimulated with allogeneic DCs, instead of IL-2 (Fig. 6 B). Again, arrested T cells could be restimulated with DCs, reaching stimulation levels comparable to those obtained with untreated cells in primary MLRs. Thus the inhibitory effect of hCD83ext is reversible and is not mediated via T cell anergy.
Figure 6.
Proliferation arrest of hCD83ext-treated T cells can be restored. (A) hCD83ext-treated T cells derived from the primary MLRs, which have been either treated with or without hCD83ext, were stimulated for a second time period of 4 d without the addition of hCD83ext. Medium containing IL-2 or medium without IL-2 was added to the cultures. After a 3-d incubation period proliferation was determined. T cells which were strongly inhibited by hCD83ext in the primary MLRs could be restimulated with IL-2, clearly showing that the proliferation arrest, caused by hCD83ext in the primary MLRs, is not due to an irreversible toxic effect. (B) T cells which were inhibited with hCD83ext (4 μg/ml) in the primary MLRs (ratio DC:T cell = 1:20), were restimulated with allogeneic DCs (ratio 1:20). Arrested T cells could be restimulated with DCs, reaching stimulation levels comparable to those obtained with untreated cells in primary MLRs. Thus, the inhibitory function of hCD83ext is reversible. Experiments were performed at least three times. The data presented here represent a typical experiment.
Discussion
DCs act as nature's adjuvant in inducing T cell–mediated immunity. To understand the biological properties of DCs it is vital to gain further knowledge regarding the role of DCs specific proteins on a molecular level. Here, we provide for the first time evidence that CD83, the best known cell surface marker for mature DCs, has also a biological function. During DC maturation CD83 is rapidly upregulated together with the costimulatory molecules CD80 and CD86. These molecules provide the so-called second signal for the T cell activation (18). Multiple studies have shown that allostimulation can be prevented by blocking the CD28-mediated costimulatory signals using either anti–CD80/CD86 mAbs or the fusion protein CTLA4Ig (19–23). As a result this blockage of signal two leads to T cell anergy.
Although CD83 is strongly upregulated on mature DCs the function of this molecule has been unknown to date. To elucidate the mode of action of CD83 the extracellular Ig domain of human CD83 was expressed as a recombinant protein and used for functional studies in vitro. 1D-NMR data strongly support that the recombinant hCD83ext is correctly folded and therefore the suppressive effects observed in the T cell assays are due to a functionally active molecule. In addition, a CD83-Fc molecule, expressed in an eukaryotic system was used.
Using plate adhesion analyses we could show for the first time that immature and mature DCs bind to CD83. The specificity of the CD83-binding to DCs could be demonstrated by the fact that the soluble extracellular hCD83ext domain inhibited the binding in a concentration-dependent manner (Fig. 2 A–C). Furthermore, the binding of DCs to CD83 itself is also concentration dependent. The binding was lost when decreasing amounts of CD83 were used (Fig. 2 C). These findings are highly relevant for several aspects: (i) since hCD83ext, which was expressed in a prokaryotic system, inhibits the binding of the eukaryotic expressed CD83-Fc underlines the fact that glycosilation is not necessary for CD83 binding to its ligand; (ii) the prokaryotic expressed hCD83ext protein is functionally active; (iii) the fact that CD83 binds also to immature DCs, which do not express CD83, indicates that there is no homophilic interaction between CD83 molecules. These findings rather suggest the existence of a heterophilic binding.
Interestingly, immature DCs which were incubated with soluble hCD83ext could not be fully matured anymore. Even in the presence of the potent maturation cocktail composed of IL-1β, TNF-α, and PGE2 the maturation was blocked. hCD83ext treatment lead to the specific downmodulation of CD80, a crucial costimulatory molecule and of CD83 itself. This suggests, that soluble hCD83ext induces a maturation block, which could be a regulatory mechanism in order to control immune responses. In this respect it is very interesting to know that a soluble form of CD83, like described for CD86 (24), has been characterized in vivo and is detectable in normal human sera and is released from activated DCs and B cells (25), underlying a possible important role in the regulation of immune responses. In addition, also mature DCs downmodulate CD83 from their cell surface when incubated with hCD83ext, indicating a regulatory mechanism also on mature DCs. The soluble molecule may either work by delivering a negative signal or blocking a positive signal that might be generated by interactions of maturing DCs. However, this hypothesis has to be proven in an experimental setting.
Furthermore, when mature DCs were incubated with soluble hCD83ext they completely lost their ability to stimulate allogeneic T cell responses. In addition, also CTL specific T cell responses were inhibited by hCD83ext. Again, the existence of a soluble form of CD83 in vivo (25) is an additional support of our data regarding the inhibitory effects of hCD83ext.
Noteworthy, these effects are not due to cytotoxic effects on T cells. We demonstrate that T cells can be restimulated either with IL-2 or with allogeneic DCs. This represents a novel mechanism which is not connected to T cell anergy, induced by the inhibition of the B7-CD28 costimulatory pathway (23). Since the inhibition mediated by hCD83ext is a competitive inhibition, hCD83ext has to be present throughout the experiment. Dilution of the hCD83ext concentration with medium alone allowed already the restimulation of T cells. Also the number of APCs is crucial. High numbers of DCs can only be blocked with correspondingly higher hCD83ext concentrations.
Considering recent results, it becomes clear that CD83 plays an important role in DC biology. Previously, we could show that inhibition of CD83 expression, by interfering with a specific RNA export pathway, leads to a clear reduction of DC-mediated T cell stimulation (11). An additional indication, pointing toward the biological importance of CD83 is the fact that several viruses including herpes simplex virus type 1 and pox viruses downregulate CD83 (12, 26). Interestingly, these virus infected DCs are also inhibited in their allostimulatory capacity (12, 26–29). Nevertheless, regarding the biological function of CD83, these reports provided only circumstantial evidence.
In contrast, here we report the identification of CD83 binding to DCs and the characterization of a reversible inhibitor of DC-mediated T cell stimulation, representing a new tool to explore DC biology and new therapeutic strategies. Future studies including the use of k.o. mice will help to elucidate the biological role of CD83 even in more detail. Further characterizations are also needed regarding the CD83 structure and the characterization of the ligand(s) present on DCs.
Acknowledgments
We would like to thank Ralph Steinman and Manfred Lutz for suggestions and critical reading of the manuscript. We are grateful to Kristian Schweimer for performing the MNR analyses.
This work was supported by the Deutsche Forschungsgemeinschaft (SFB466-Grant B5). M. Lechmann is supported by the Else Kröner-Fresenius-Stiftung. D.J.E.B. Krooshoop is supported by The Netherlands Heart Foundation (NHS grant 96-150).
Footnotes
*
Abbreviations used in this paper: DC, dendritic cell; ICAM, intercellular adhesion molecule; MLR, mixed leukocyte reaction.
References
- 1.Steinman, R.M. 1991. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 9:271–296. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. [DOI] [PubMed] [Google Scholar]
- 3.Dieu, M.C., B. Vanbervliet, A. Vicari, J.M. Bridon, E. Oldham, S. Ait-Yahia, F. Briere, A. Zlotnik, S. Lebecque, and C. Caux. 1998. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J. Exp. Med. 188:373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou, L.J., R. Schwarting, H.M. Smith, and T.F. Tedder. 1992. A novel cell-surface molecule expressed by human interdigitating reticulum cells, Langerhans cells, and activated lymphocytes is a new member of the Ig superfamily. J. Immunol. 149:735–742. [PubMed] [Google Scholar]
- 5.Kozlow, E.J., G.L. Wilson, C.H. Fox, and J.H. Kehrl. 1993. Subtractive cDNA cloning of a novel member of the Ig gene superfamily expressed at high levels in activated B lymphocytes. Blood. 81:454–461. [PubMed] [Google Scholar]
- 6.Twist, C.J., D.R. Beier, C.M. Disteche, S. Edelhoff, and T.F. Tedder. 1998. The mouse Cd83 gene: structure, domain organization, and chromosome localization. Immunogenetics. 48:383–393. [DOI] [PubMed] [Google Scholar]
- 7.Berchtold, S., P. Mühl-Zürbes, C. Heufler, P. Winklehner, G. Schuler, and A. Steinkasserer. 1999. Cloning, recombinant expression and biochemical characterization of the murine CD83 molecule which is specifically upregulated during dendritic cell maturation. FEBS Letters. 461:211–216. [DOI] [PubMed] [Google Scholar]
- 8.Weissman, D., Y. Li, J. Ananworanich, L.J. Zhou, J. Adelsberger, T.F. Tedder, M. Baseler, and A.S. Fauci. 1995. Three populations of cells with dendritic morphology exist in peripheral blood, only one of which is infectable with human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA. 92:826–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou, L.J., and T.F. Tedder. 1995. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J. Immunol. 154:3821–3835. [PubMed] [Google Scholar]
- 10.Zhou, L.J., and T.F. Tedder. 1996. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc. Natl. Acad. Sci. USA. 93:2588–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kruse, M., O. Rosorius, F. Kratzer, D. Bevec, C. Kuhnt, A. Steinkasserer, G. Schuler, and J. Hauber. 2000. Inhibition of CD83 cell surface expression during dendritic cell maturation by interference with nuclear export of CD83mRNA. J. Exp. Med. 191:1581–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kruse, M., O. Rosorius, F. Kratzer, G. Stelz, C. Kuhnt, G. Schuler, J. Hauber, and A. Steinkasserer. 2000. Mature dendritic cells infected with Herpes Simplex virus type 1 exhibit inhibited T-cell stimulatory capacity. J. Virol. 74:7127–7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geijtenbeek, T.B.H., Y. van Kooyk, S.J. van Vliet, M.H. Renes, R.A.P. Raymakers, and C. Figdor. 1999. High frequency of adhesion defects in B-lineage acute lymphoblastic leukemia. Blood. 94:754–764. [PubMed] [Google Scholar]
- 14.Bender, A., M. Sapp, G. Schuler, R.M. Steinman, and N. Bhardwaj. 1996. Improved methods for the generation of dendritic cells from nonproliferating progenitors in human blood. J. Immunol. Methods. 196:121–135. [DOI] [PubMed] [Google Scholar]
- 15.Strobel, I., S. Berchtold, A. Götze, U. Schulze, G. Schuler, and A. Steinkasserer. 2000. Human dendritic cells transfected with either RNA or DNA encoding influenza matrix protein M1 differ in their ability to stimulate cytotoxic T lymphocytes. Gene Ther. 7:2028–2035. [DOI] [PubMed] [Google Scholar]
- 16.Bednarek, M.A., S.Y. Sauma, M.C. Gammon, G. Porter, S. Tamhankar, A.R. Williamson, and H.J. Zweerink. 1991. The minimum peptide epitope from the influenza virus matrix protein. Extra and intracellular loading of HLA-A2. J. Immunol. 147:4047–4053. [PubMed] [Google Scholar]
- 17.Herr, W., B. Linn, N. Leister, E. Wandel, K.H. Meyer zum Büschenfelde, and T. Wolfel. 1997. The use of computer-assisted video image analysis for the quantification of CD8+ T lymphocytes producing tumor necrosis factor α spots in response to peptide antigens. J. Immunol. Methods. 203:141–152. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz, R.H. 1992. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 71:1065–1068. [DOI] [PubMed] [Google Scholar]
- 19.Lenschow, D.J., Y. Zeng, K.S. Hathcock, L.A. Zuckerman, G. Freeman, J.R. Thistlethwaite, G.S. Gray, R.J. Hodes, and J.A. Bluestone. 1995. Inhibition of transplant rejection following treatment with anti-B7-2 and anti-B7-1 antibodies. Transplantation. 60:1171–1178. [DOI] [PubMed] [Google Scholar]
- 20.Larsen, C.P. 1996. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 381:434–438. [DOI] [PubMed] [Google Scholar]
- 21.Akalin, E., A. Chandraker, M.E. Russell, L.A. Turka, W.W. Hancock, and M.H. Sayegh. 1996. CD28-B7 T cell costimulatory blockade by CTLA4Ig in the rat renal allograft model: inhibition of cell-mediated and humoral immune responses in vivo. Transplantation. 62:1942–1945. [DOI] [PubMed] [Google Scholar]
- 22.Newell, K.A., G. He, Z. Guo, O. Kim, G.L. Szot, I. Rulifson, P. Zhou, J. Hart, J.R. Thistlethwaite, and J.A. Bluestone. 1999. Cutting edge: blockade of the CD28/B7 costimulatory pathway inhibits intestinal allograft rejection mediated by CD4+ but not CD8+ T cells. J. Immunol. 163:2358–2362. [PubMed] [Google Scholar]
- 23.Boussiotis, V.A., J.G. Gribben, G.J. Freeman, and L.M. Nadler. 1994. Blockade of the CD28 co-stimulatory pathway: a means to induce tolerance. Curr. Opin. Immunol. 6:797–807. [DOI] [PubMed] [Google Scholar]
- 24.Jeannin, P., G. Magistrelli, J.P. Aubry, G. Caron, J.F. Gauchat, T. Renno, N. Herbault, L. Goetsch, A. Blaecke, P.Y. Dietrich, et al. 2000. Soluble CD86 is a costimulatory molecule for human T lymphocytes. Immunity. 13:303–312. [DOI] [PubMed] [Google Scholar]
- 25.Hock, B.D., M. Kato, J.L. McKenzie, and D.N.J. Hart. 2001. A soluble form of CD83 is released from activated dendritic cells and B lymphocytes, and is detectable in normal human sera. International Immunology. 13:959–967. [DOI] [PubMed] [Google Scholar]
- 26.Jenne, L., C. Hauser, J.F. Arrighi, J.H. Saurat, and A.W. Hügin. 2000. Poxvirus as a vector to transduce human dendritic cells for immunotherapy: abortive infection but reduced APC function. Gene Ther. 7:1575–1583. [DOI] [PubMed] [Google Scholar]
- 27.Salio, M., M. Cella, M. Suter, and A. Lanzavecchia. 1999. Inhibition of dendritic cell maturation by herpes simplex virus. Eur. J. Immunol. 29:3245–3253. [DOI] [PubMed] [Google Scholar]
- 28.Drillien, R., D. Spehner, A. Bohbot, and D. Hanau. 2000. Vaccinia virus-related events and phenotypic changes after infection of dendritic cells derived from human monocytes. Virology. 268:471–481. [DOI] [PubMed] [Google Scholar]
- 29.Engelmayer, J., M. Larsson, M. Subklewe, A. Chahroudi, W.I. Cox, R.M. Steinman, and N. Bhardwaj. 1999. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J. Immunol. 163:6762–6768. [PubMed] [Google Scholar]