Identification of Dynamically Distinct Subpopulations of T Lymphocytes That Are Differentially Affected by HIV (original) (raw)
Abstract
We examined the effects of human immunodeficiency virus infection on the turnover of CD4 and CD8 T lymphocytes in 17 HIV-infected patients by 30 min in vivo pulse labeling with bromodeoxyuridine (BrdU). The percentage of labeled CD4 and CD8 T lymphocytes was initially higher in lymph nodes than in blood. Labeled cells equilibrated between the two compartments within 24 h. Based on mathematical modeling of the dynamics of BrdU-labeled cells in the blood, we identified rapidly and slowly proliferating subpopulations of CD4 and CD8 T lymphocytes. The percentage, but not the decay rate, of labeled CD4 or CD8 cells in the rapidly proliferating pool correlated significantly with plasma HIV RNA levels for both CD4 (r = 0.77, P < 0.001) and CD8 (r = 0.81, P < 0.001) T cells. In six patients there was a geometric mean decrease of greater than 2 logs in HIV levels within 2 to 6 mo after the initiation of highly active antiretroviral therapy; this was associated with a significant decrease in the percentage (but not the decay rate) of labeled cells in the rapidly proliferating pool for both CD4 (P = 0.03) and CD8 (P < 0.001) T lymphocytes. Neither plasma viral levels nor therapy had an effect on the decay rate constants or the percentage of labeled cells in the slowly proliferating pool. Monocyte production was inversely related to viral load (r = −0.56, P = 0.003) and increased with therapy (P = 0.01). These findings demonstrate that HIV does not impair CD4 T cell production but does increase CD4 and CD8 lymphocyte proliferation and death by inducing entry into a rapidly proliferating subpopulation of cells.
Keywords: AIDS, bromodeoxyuridine, CD4 T lymphocyte proliferation, CD8 T lymphocyte proliferation, highly active antiretroviral therapy
Introduction
Infection with HIV leads to a state of chronic immune activation and a progressive deterioration in immune function, manifested most recognizably by a decline in CD4 T cell number (1, 2). While early studies suggested that there was a massive turnover of CD4 T cells that was being driven by HIV-induced cell death, recent studies have identified a more modest, ∼two- to sixfold, increase in the fraction of proliferating CD4 and CD8 T cells when compared with individuals without HIV infection (3–12). The mechanisms leading to this increase in proliferation have been ill defined. Moreover, the impact of suppression of viral replication on lymphocyte turnover has remained controversial, with some studies suggesting an initial increase in proliferation after suppression, while others have found an immediate and persistent decrease in proliferation following initiation of highly active antiretroviral therapy (HAART)* (6, 7, 9, 10, 13).
Measurement of lymphocyte turnover in humans has until recently depended on indirect markers, such as changes in cell number in the periphery following therapy, differences in telomere length, Ki67 labeling, or tracking of chromosomal abnormalities (1–10, 12, 14). Direct measures of cell replication in vivo, which can provide more reliable information regarding cell turnover and can provide longitudinal information about proliferating cells, have only recently been applied to the study of lymphocytes, using deuterium or bromodeoxyuridine (BrdU) as a marker (6, 9, 10). Labeling of newly synthesized DNA with deuterium provides a method for directly measuring turnover in a population of cells, although individual cells or minority populations cannot be easily studied. BrdU is a thymidine analogue that is incorporated into the DNA of replicating cells; incorporated BrdU can be detected using a monoclonal antibody (15, 16). BrdU has been used to measure lymphocyte proliferation in vivo in animals and ex vivo in humans (6, 13, 17–19). BrdU has also been extensively used as an in vivo labeling agent to measure proliferation of tumor cells in patients with malignancies; in this setting BrdU administration has been associated with no major toxicities (20–22). In vivo labeling with BrdU provides the opportunity not only to identify proliferating populations of cells by flow cytometry, but also to determine their decay kinetics by following proliferating cells longitudinally.
Here we show by in vivo labeling with BrdU and mathematical modeling that while there is one population of monocytes, there are at least two dynamically distinct subpopulations of CD4 and CD8 T cells, a rapidly and a slowly proliferating subpopulation. While the size of the rapidly proliferating pools of both CD4 and CD8 T cells directly correlated with plasma viral load, viral load had no impact on the size of the slowly proliferating population. HAART decreased the size of the rapidly proliferating subpopulation of both CD4 and CD8 T cells without impacting the slowly proliferating subpopulation.
Materials and Methods
Patients.
Patients with HIV infection and no major clinical or laboratory abnormalities were enrolled in the study. Patients who were pregnant or breast feeding, or receiving 5 fluorouracil were excluded from participation. Patients were selected to span the spectrum of stages of HIV infection. While BrdU has been used in cancer patients as a marker for rates of cell turnover without any observed toxicities, it has been shown to be teratogenic in animal models. For this reason the risk benefit ratio was felt by ourselves and the institutional review board to preclude it use in healthy volunteers. Participants in the study were counseled to practice effective contraception. The study was approved by the National Institute of Allergy and Infectious Diseases institutional review board, and all patients provided written informed consent.
BrdU Infusion and Flow Cytometry.
BrdU (NEOMARK-BU®) was supplied by NeoPharm through the National Cancer Institute, and infused at a dose of 200 mg/m2 over 30 min (22). For analysis of BrdU incorporation, 10 ml of EDTA treated whole blood was analyzed for surface markers and incorporated BrdU by flow cytometry as described previously (6). After staining for surface markers (23) and fixation in 1% paraformaldehyde/1% Tween 20, cells were treated with DNase 1 to expose incorporated BrdU, stained with FITC-labeled anti-BrdU monoclonal antibody (Becton Dickinson), and analyzed by flow cytometry. Lymph node biopsy samples were pushed through a mesh screen to obtain a single cell suspension and then processed as for whole blood. 20,000 to 50,000 gated events were collected for most samples; a minimum of 5,000 gated events were collected when the gated cell count was low.
Ki67 staining of cryopreserved peripheral mononuclear cells was performed as described, using a phycoerythrin-labeled anti-Ki67 antibody (clone B56; BD PharMingen; reference 2).
Immunohistochemistry.
Lymph node sections were double stained with an anti-BrdU monoclonal antibody (1:500 dilution, MO744; Dako) and an anti-CD8 monoclonal antibody (1:400 dilution, NCL-CD8–4B11, Novocastra; Vector Laboratories). Slides were pretreated with 4N hydrochloric acid for 20 min at 37°C, rinsed in pH 7.6 boric acid–borate buffer and then treated with prewarmed 0.01% trypsin for 3 min at 37°C before incubation with the anti-BrdU antibody. The Vectastain Mouse Elite ABC (avidin-biotin complex) kit (Vector Laboratories) using the rapid staining procedure was used for the secondary antibody and diaminobenzidine (DAB) was the chromagen. Subsequently slides were pretreated by microwaving in 1 mM EDTA, pH 8, then incubated with the anti-CD8 antibody. The secondary antibody was affinity purified, peroxidase labeled goat anti-mouse IgG, human serum absorbed, and the chromagen was HistoMark True Blue® (Kirkegaard and Perry Laboratories).
Modeling.
The data were fitted to the differential equations with the computer program Scientist (MicroMath). Statistical analysis was performed by using the program Statistica (Statsoft).
Results
Patient Characteristics.
17 patients (16 male; mean age, 45 yr) enrolled in the study (Table I). The mean CD4 count at enrollment was 349 cells/mm3 (range, 59 to 888 cells/mm3) and the geometric mean viral load was 3.72 log10 copies/ml (range, <1.70 to 5.55 log10 copies/ml). Six patients (five males) with a mean age of 43 yr received two infusions of BrdU. At the time of the first infusion, the mean CD4 count was 380 cells/mm3 (range, 59 to 888 cells/mm3) and the geometric mean viral load was 4.42 log10 copies/ml (range, 3.56 to 4.96 log10 copies/ml). At the first infusion four patients were receiving no antiretroviral therapy and two were receiving ineffective therapy (viral loads of 4.17 and 4.30 log10 copies/ml). At the time of the second infusion the mean CD4 count was 556 (range, 174 to 1,116) and the geometric mean viral load was 2.05 log10 copies/ml (range, <1.70 to 2.96 log10 copies/ml).
Table I.
Baseline Characteristics of the Patients
| First BrdU infusion | Second BrdU infusion | ||||||
|---|---|---|---|---|---|---|---|
| Pt. no. | Age | Antiretrovirals at thetime of infusion | CD4 no.(cells/mm3) | Plasma HIV load(Log10 copies/ml)a | Antiretrovirals at thetime of infusion | CD4 no.(cells/mm3) | Plasma HIV load(Log10 copies/ml)a |
| 1 | 43 | Rit/Ind/Sta/Lamb | 99 | 5.08 | ND | ||
| 2 | 49 | None | 134 | 5.00 | ND | ||
| 3 | 45 | None | 609 | 4.71 | ND | ||
| 4 | 57 | Saq/Nel/Sta/Lam | 247 | 3.01 | ND | ||
| 5 | 54 | None | 82 | 5.55 | ND | ||
| 6 | 41 | Nel/Sta/Lam | 99 | 3.16 | ND | ||
| 7 | 43 | Ind/Nev/Sta/Lam | 330 | 1.69 | ND | ||
| 8 | 34 | Ind/Sta/Lam | 551 | 2.62 | ND | ||
| 9 | 51 | Ind/Zid/Lam | 561 | 1.69 | ND | ||
| 10 | 59 | None | 499 | 2.51 | ND | ||
| 11 | 42 | Nel/Del/Sta/Lam | 448 | 1.75 | ND | ||
| 12 | 39 | None | 888 | 3.56 | Ind/Efa/Sta/Lam | 1,116 | 1.69 |
| 13 | 40 | None | 280 | 4.96 | Rit/Saq/Nev/Zid/Lam | 384 | 2.56 |
| 14 | 40 | Nel/Nev/Sta/Did | 169 | 4.31 | Amp/Nel/Efa/Aba | 174 | 1.69 |
| 15 | 36 | None | 442 | 4.95 | Ind/Nev/Sta/Lam | 857 | 1.69 |
| 16 | 48 | None | 443 | 4.28 | Ind/Nev/Sta/Lam | 574 | 1.69 |
| 17 | 52 | Amp/Rit/Efa/Did/Aba | 59 | 4.45 | Lop/Rit/Ind/Sta/Lam | 232 | 2.96 |
| Mean | 45 | 349 | 3.72 | 556 | 2.05 |
BrdU Incorporation Can Be Detected by Flow Cytometry.
After a 30-min infusion of BrdU, incorporation into DNA was easily detected by flow cytometry in lymphocytes as well as monocytes (Fig. 1) . The percent incorporation and rate of appearance in the periphery varied depending on the cell type. For monocytes, there was limited appearance of labeled cells in the periphery over the first 24 h, after which there was a rapid increase to a peak in labeling at day 2–4, in the range of 30 to 45% (Figs. 1 and 2 A). This was followed by a rapid decline so that no label was detectable after day 10–14 (Fig. 2 A). A previous study using in vivo labeling with tritiated thymidine to characterize monocyte kinetics in healthy men showed a very similar pattern both in the timing and the peak of labeling as seen in the current study, thus providing verification of the validity of the BrdU labeling method (24).
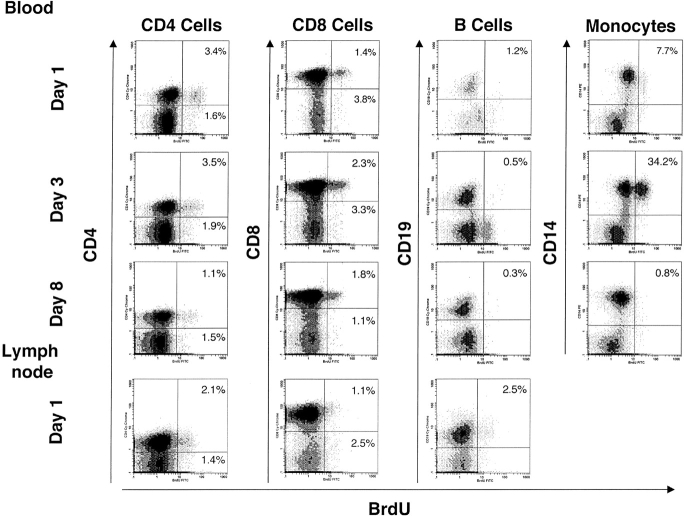
Figure 1.
Flow cytometry profiles demonstrating BrdU incorporation by lymphocytes and monocytes for a representative patient. BrdU incorporation is shown along the X axis and cell surface markers are shown along the Y axis, as indicated. The percentage in the top right quadrant indicates the percent of double-positive cells. Results are shown for days 1, 3, and 8 after the BrdU infusion for blood, and day 1 for lymph node. Gating for CD4+ and CD8+ cells was based on light scatter together with positive staining for CD3.
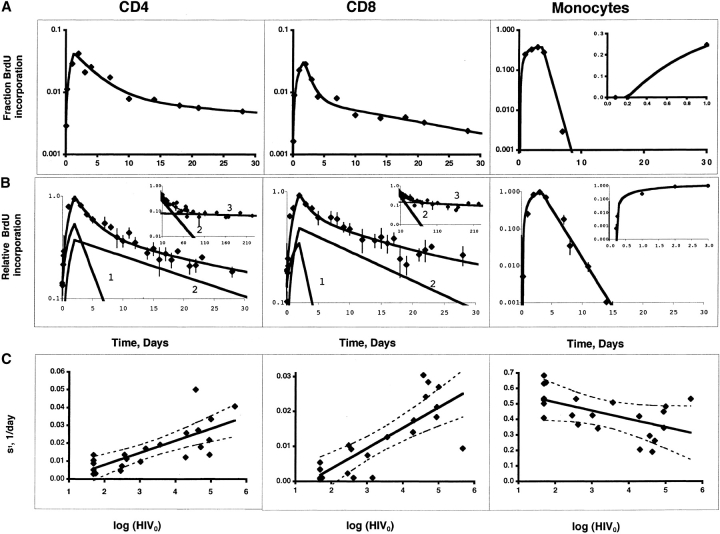
Figure 2.
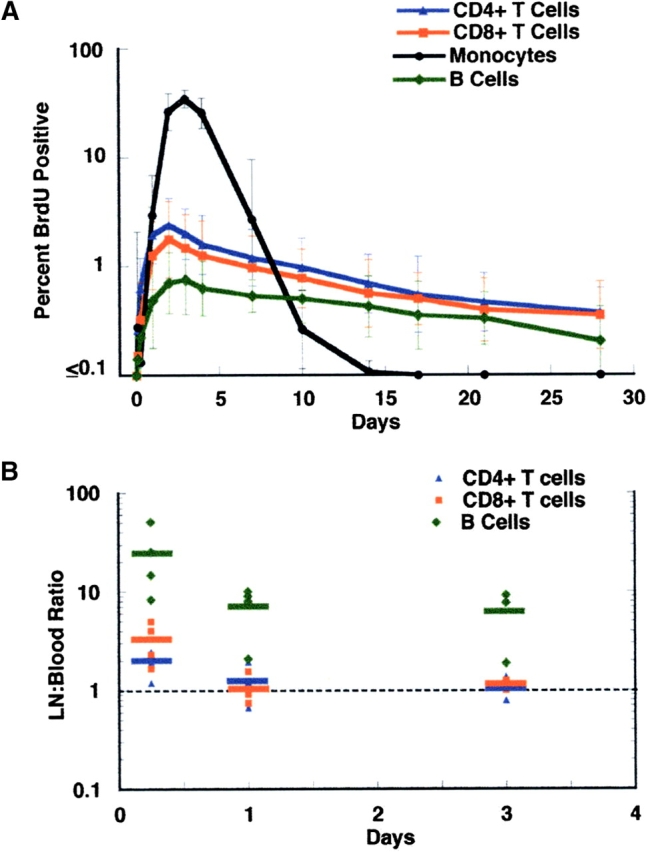
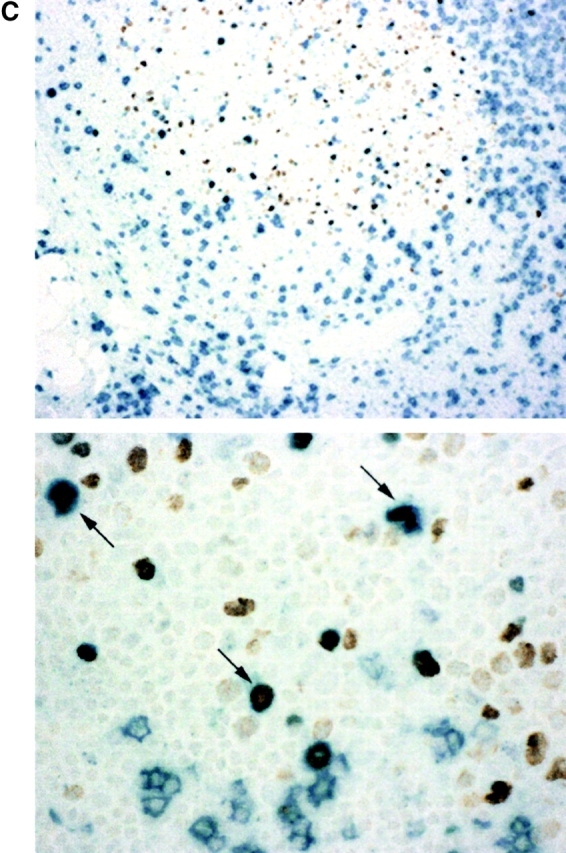
(A) Kinetics of BrdU labeled lymphocytes and monocytes in the blood. Days following the BrdU infusion are shown along the X axis, and percent BrdU-positive cells along the Y axis, which is shown in log scale to illustrate the kinetic differences between monocytes and lymphocytes. Results represent the geometric mean (± SD) for the first infusion for all 17 patients during the first 30 d. Symbols are as follows: CD4+ cells, blue triangles; CD8+ cells, red squares; B cells, green diamonds; monocytes, black circles. (B) BrdU incorporation by lymph node lymphocytes compared with blood lymphocytes. Nine patients underwent lymph node biopsy 6 h to 3 d after BrdU infusion; two patients had a second biopsy after a second BrdU infusion. Each dot represents the ratio of percent BrdU incorporation by lymph node CD4 (blue triangles), CD8 (red squares), or B (green diamonds) lymphocytes to percent BrdU incorporation by that cell in the blood. The bar represents the mean for the indicated time point. The dashed line indicates a ratio of 1. Results are shown in log scale. A single patient underwent lymph node biopsy at day 10 and showed ratios of 3.5, 1.4, and 0.3 for CD4, CD8, and B cells, respectively. (C) Immunohistochemical staining of a lymph node biopsy obtained 6 h after a BrdU infusion. CD8 cells are stained blue. Cells that incorporated BrdU are stained brown. Low power view (100×, top) demonstrates that the majority of BrdU-positive cells are localized to secondary follicles. High power view (400×, bottom) demonstrates that many of the BrdU-positive cells in the follicle are also CD8 positive (arrows).
Lymphocytes showed a distinctly different pattern of labeling. CD4 and CD8 lymphocytes with incorporated BrdU were detected in the periphery within 6 h of completion of the infusion, and gradually increased thereafter, to a peak in the range of 0.5 to 5%, generally at days 2 to 4 after the infusion, with a subsequent gradual decline (Fig. 2 A); labeled cells could still be detected for months. Peak labeling correlated with the percent of Ki67 positive cells for both CD4 cells (r = 0.63, P < 0.001) and CD8 cells (r = 0.57, P < 0.006). Peripheral blood B lymphocytes showed a similar pattern to T lymphocytes, but with greater variability to peak labeling (1 to 17 d) and a generally lower peak of incorporation (Fig. 2 A).
Rapid Equilibration of Proliferating Cells between Lymphoid Tissue and Blood.
Because proliferation of lymphocytes occurs primarily in secondary lymphoid organs, BrdU incorporation by lymphocytes residing in lymph nodes was examined for 10 patients who underwent lymph node biopsy at various time points after a BrdU infusion. At 6 h after the 30-min BrdU pulse, labeling was ∼two- to threefold greater in the lymph node compared with the blood for both CD4 and CD8 cells, but by 24 h the percent positive cells were approximately equivalent in the two compartments (Fig. 2 B). B cells, however, showed substantially higher (>6-fold) labeling in the lymph node compared with the blood at all the time points measured (Fig. 2 B). Immunohistochemical staining of lymph node biopsies showed a high proportion of proliferating cells in the lymphoid follicles; these cells included CD4 and CD8 cells, in addition to B cells (Fig. 2 C). Thus, an equilibrium between lymph nodes and blood is rapidly established (within 24 h) for CD4 and CD8 cells (but not for B cells) that have recently proliferated, and measurement of BrdU incorporation in peripheral CD4 and CD8 cells after 24 h provides an accurate reflection of the proportion of BrdU positive CD4 and CD8 cells in lymphoid tissues.
Identification of Rapidly and Slowly Proliferating Subpopulations of CD4 and CD8 T Lymphocytes.
To investigate the relationships between lymphocyte turnover and immunologic or virologic parameters, 17 patients (Table I) received a BrdU infusion; six of these patients received a second infusion 8 to 26 wk after starting HAART. Data from a total of 23 infusions were available for analysis. While HIV-negative patients were not enrolled in this study, data from ourselves and others using the technique of in vivo labeling with deuterium have demonstrated lower rates of T cell turnover in healthy volunteers compared with HIV-infected patients (9, 10). In a limited number of HIV infected patients both deuterium and BrdU labeling have been employed, with similar results (data not shown). The two methodologies complement each other: with deuterium one can label over a longer period of time and thus reach higher peak levels of incorporation, while BrdU labeling allows for more frequent and more detailed analyses of lymphocyte subpopulations.
To describe the kinetics of BrdU labeled cells we constructed a semi-empirical equation based on the following observations and considerations. The time-dependent decay in the concentration of the labeled T cells after the peak of labeling could not be represented by a single exponential function. For most of the infusions (19 for CD4 cells and 16 for CD8 cells), the decay consisted of two phases with significantly different slopes (using a logarithmic scale; Fig. 3) . For patients who were followed for longer times (longer than 30–50 d) a third phase with a slope close to zero was also observed (Fig. 3). These findings suggested the existence of at least two different subpopulations of cells each decaying with its own rate constant d. The decay of these two populations could be described by the following equations: L = L 1+L 2; d_L_ i/d_t_ = −d i*L i, where i = 1,2, represents the two different subpopulations, L is the fraction of labeled lymphocytes in the blood, and t denotes time.
Figure 3.
(A) Comparison of experimental data with modeling of the kinetics of BrdU incorporation by T lymphocytes and monocytes for a representative patient. The continuous lines represent fitting of the experimental data (black diamonds) by the model equations. For this patient, d 1 = 0.29, 0.90, 1.27 d−1; d 2 = 0.017, 0.042, 0 d−1; s 1 = 0.033, 0.027, 0.48 d−1, s 2 = 0.006, 0.004, 0.000 d−1, T = 1.3, 1.8, 3.6 d; L 01 = L 02 = 0, 0, 0; T i = 0, 0, 0.21 d, for CD4 T cells, CD8 T cells and monocytes, respectively. L 01 and L 02 are the initial values (t = 0) for L 1 and L 2, respectively. Inset shows the maturation delay of monocytes in linear scale. (B) Comparison of experimental data with modeling of the kinetics of BrdU incorporation by T lymphocytes and monocytes for all patients. The data for individual patients were normalized, assigning a value of 1 to the maximal fraction of labeled cells. The arithmetic mean of the normalized values for all patients (black diamonds) are presented in logarithmic scale as a function of time. The continuous lines represent fitting of the experimental data by the model equations with d 1 = 0.36, 0.59, 0.61 d−1; d 2 = 0.045, 0.060, 0 d−1; s 1 = 0.33, 0.26, 0.72 d−1, s 2 = 0.19, 0.25, 0 d−1, T = 2, 2, 3 d; L 01 = 0.13, 0.14, 0; L 02 = 0, 0, 0; T i = 0, 0, 0.24 d, for CD4 T cells, CD8 T cells and monocytes, respectively. Here the values of s were normalized to the maximal fraction of labeled cells L m and are approximately equal to s/L m. The lines denoted by 1 and 2 represent the model solution for the rapidly and slowly proliferating subpopulations, respectively. The insets for T cells show data for patients who were followed for longer than 30 d and exhibited a third slope (denoted by 3) in logarithmic scale (d 3 = 0.094 × 10−2, 0.19 × 10−2 day−1; s 3 = 0.040, 0.073 d−1 for CD4 T and CD8 T cells, respectively). The inset for monocytes shows their maturation delay. Each data point represents the mean for all the patients who were seen on a given day (relative to the infusion); this ranged from 21 patients for the earlier time points to one to four patients, especially for the later time points. Error bars represent 1 standard deviation. (C) Relationship between s1 and the logarithm of the HIV RNA blood plasma concentration. A positive correlation was noted for the rapidly proliferating T cell subpopulation (s 1) for CD4 and CD8 T cells while a negative correlation was noted for the rate of monocyte production (s). The HIV concentration below the limit of detection was set at 49 copies per ml. Individual results are shown by black diamonds. The regression lines and the 95% confidence intervals are shown with continuous and broken lines, respectively.
The final equation reads: d_L_ i/d_t_ = s i*_U_-d i*L i, i = 1,2, where U is a unit function equal to 1 at t < _T_ and 0 at _t_ > T, T being the time required to reach the maximum concentration of labeled cells in the blood. The rate constant d (units of inverse time) reflects cell death, delabeling and/or redistribution outside of the blood/lymphatic system; the source s (units: fraction of labeled cells entering the blood per unit time) is a complex function of cell proliferation and redistribution and is approximately proportional to cell proliferation (percentage of BrdU-labeled CD4 or CD8 cells) in the lymphoid tissue.
In developing this semi-empirical model, we assumed that the lymphoid tissue serves as an effective source of labeled cells that are distributed to the blood until equilibration is reached, at which point the effective source ceases to affect changes in the concentration of labeled cells. Because the percentage of labeled cells in the blood was approximately the same as in the lymph nodes after about one day, we have assumed that the same time dependence holds in both compartments after the peak labeling. Prior to this time, an equilibration between blood and lymphoid tissue has not yet been established, and the time dependence of the labeled cells in the two compartments will differ. The higher proportion of labeled cells in the lymph nodes immediately after labeling (Fig. 2 B) suggests an initial redistribution of cells from the lymphoid tissue to the blood. However, because of lack of longitudinal data for the lymphoid tissue in individual patients and the limited number (2–4) of time points for the blood during this early period, it is not possible to use complex functions for data fitting. Therefore, we selected a simple linear dependence on time.
The fraction of labeled cells for each subpopulation at t = T in the lymphoid tissue, N i, is approximately equal to that in the blood and proportional to s i as well as to the fraction n i at the time of labeling: n i = N i/k i. For rapidly expanding cell populations, e.g., in clonal expansions, the coefficient of proportionality k is ∼2–4 based on the experimental data for the lymphoid tissue at t = 6 h and 24 h. The fraction of the total number of cells (labeled and unlabeled) in the first subpopulation f 1 with respect to the total number of the cells in the two subpopulations (but not all cells) is approximately equal to (n 1/d 1)/(n 1/d 1 + n 2/d 2), and f 2 = 1-f 1, respectively. However, as the constant d is an effective measure of death, delabeling and redistribution to nonlymphoid tissue this estimate for the fraction of total cells should be used with caution. We also tested other relatively simple models and obtained essentially the same results; more complex models including our previous model based on the existence of distinct cell subpopulations (25) can be reduced to the simple model under some assumptions but are difficult to fit with a unique set of parameters due to the limited number of experimental points.
To analyze the changes in the monocyte population, we used an equation similar to the one described above for lymphocytes with the important difference that it takes into account the maturation stage in the bone marrow, the lack of significant recirculation of the monocytes and the relatively homogeneous nature of the monocyte population reflected in the observed one exponential decay of the fraction of labeled cells in the blood (Fig. 2): dM/dt = s*U − d*M, where M is the fraction of labeled monocytes in the blood; s is a source of labeled monocytes entering the blood; U is a function equal to zero between time 0 and the maturation delay T 1, is equal to 1 for the subsequent time interval T, and is zero at times t > T 1 + T.
Fig. 3 A illustrates the fitting of the data to these models for CD4 cells, CD8 cells, and monocytes, for one patient. The two-phase kinetics of BrdU delabeling for CD4 and CD8 cells using the entire dataset is illustrated in Fig. 3 B. For the study group as a whole, the rapidly proliferating subpopulation of cells had a half-life (0.69/d) of 1.9 d for CD4 cells and 1.7 d for CD8 cells, while for the slowly proliferating subpopulation of cells, the respective half-lives were 20 and 17 d. These values represent minimal estimates because of the possible effects of redistribution and delabeling.
Plasma Viral Load Correlates with the Size of the Rapidly Proliferating Population of CD4 and CD8 Lymphocytes.
To characterize the factors driving lymphocyte turnover, we examined the relationships between log plasma HIV RNA (log HIV), CD4 cell number, or CD8 cell number at the time of infusion, and fraction of labeled cells, N, source s, and the decay constant, d, for the rapidly and slowly proliferating subpopulation of cells. As shown in Table II and Fig. 3 C, there was a significant association between log HIV and s 1 for both CD4 (r = 0.77, P < 0.001) and CD8 cells (r = 0.81, P < 0.001) (similarly for N 1). There were no significant associations between log HIV and s 2 (similarly for N 2) or either of the decay constants. There was a negative correlation between baseline CD4 and CD4 (but not CD8) s 1 (r = −0.55, P = 0.006) and s 2 (r = −0.58, P = 0.04), and between baseline CD8 and CD8 s 2 (r = −0.45, P = 0.04).
Table II.
Differential Effect of HIV on the Dynamics of T Cell Subpopulations and Monocytes
| CD4 | CD8 | Monocytes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | r | P | Mean | SD | r | P | Mean | SD | r | P | |
| d 1 (day−1) | 0.37 | 0.26 | 0.29 | NS | 0.41 | 0.26 | −0.10 | NS | 0.80 | 0.36 | −0.21 | NS |
| d 2 (day−1) | 0.036 | 0.032 | 0.25 | NS | 0.041 | 0.039 | 0.14 | NS | NA | NA | NA | NA |
| s 1 (day−1) | 0.016 | 0.012 | 0.77 | <0.001 | 0.011 | 0.010 | 0.81 | <0.001 | 0.44 | 0.13 | −0.56 | 0.003 |
| s 2 (day−1) | 0.009 | 0.010 | 0.28 | NS | 0.005 | 0.004 | −0.05 | NS | NA | NA | NA | NA |
Decrease in the Size of the Rapidly Proliferating Population After Effective Antiretroviral Therapy.
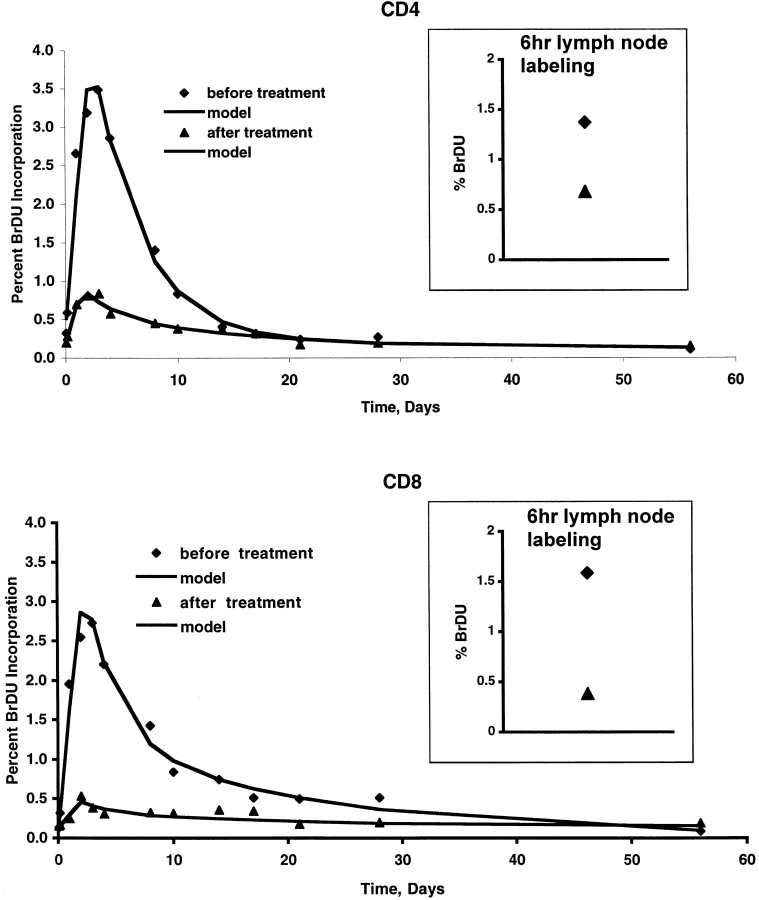
These data suggest that HIV, either directly or indirectly, is expanding the size of the pool of rapidly proliferating cells, and consequently (as these short-lived cells represent a larger proportion of the population) accelerating the overall decay (and at steady state, death) rate for CD4 and CD8 cells; however, once cells have entered this pool, their turnover or decay rate is not dependent on HIV. Based upon this one would predict that control of HIV replication by HAART should result in a decrease in the size of this rapidly proliferating subpopulation, but not in the decay rate of this subpopulation. To address this directly, we compared the various parameters for six patients after two BrdU infusions: before, and again 2 to 6 mo after successful HAART therapy (Fig. 4 , and Table III). This period between infusions was chosen to coincide with the time-frame used in two reports suggesting a HAART-related increase in CD4 cell proliferation (7, 9). Contrary to these prior reports, a statistically significant, approximately fourfold decrease in the size of the rapidly proliferating pool (s 1) was seen for both CD4 cells and CD8 cells. No significant change was seen in the size of the slowly proliferating pool (s 2) of either CD4 or CD8 cells or in the decay constants (d 1, d 2) for either subpopulation.
Figure 4.
Change in BrdU incorporation by CD4 (top) and CD8 (bottom) cells for a single patient after 12 wk of HAART. Percent of cells with incorporated BrdU before HAART are shown in diamonds, and 12 wk after therapy with HAART in triangles. CD4 counts and viral load at the time of the first infusion were 888 cells/mm3 and 3,649 HIV copies/ml, and at the time of the second infusion were 1,116 cells/mm3 and <50 copies/ml, respectively. Inset shows the percent of lymph node CD4 or CD8 cells that were BrdU positive 6 h after the first (diamond) or second (triangle) infusion. The continuous lines represent fitting of the experimental data by the model equations before/after treatment: d 1 = 0.23/0.30, 0.38/0.38 d−1; d 2 = 0.013/0.036, 0.049/0.020 d−1; s 1 = 0.019/0.0027, 0.013/0.001, _s_2 = 0.001/0.003, 0.0057/0.0016; T = 2.43/1.45, 2.36/2.0 d; L 01 = 0.0035/0.0017, 0.0/0.0012; L 02 = 0/0, 0/0; for CD4 T cells and CD8 T cells, respectively.
Table III.
Differential Effect of HAART on the Dynamics of T Cell Subpopulations and Monocytes
| CD4 | CD8 | Monocytes | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-therapy | Post-therapy | Pre-therapy | Post-therapy | Pre-therapy | Post-therapy | ||||||||||
| Mean | SD | Mean | SD | P | Mean | SD | Mean | SD | P | Mean | SD | Mean | SD | P | |
| d 1 (day−1) | 0.25 | 0.18 | 0.36 | 0.13 | NS | 0.40 | 0.19 | 0.38 | 0.13 | NS | 0.78 | 0.26 | 0.93 | 0.45 | NS |
| d 2 (day−1) | 0.028 | 0.020 | 0.034 | 0.028 | NS | 0.063 | 0.063 | 0.033 | 0.014 | NS | NA | NA | NA | NA | NA |
| s 1 (day−1) | 0.024 | 0.014 | 0.006 | 0.003 | 0.03 | 0.020 | 0.007 | 0.005 | 0.005 | <0.001 | 0.38 | 0.12 | 0.55 | 0.11 | 0.01 |
| s 2 (day−1) | 0.011 | 0.015 | 0.011 | 0.009 | NS | 0.007 | 0.006 | 0.006 | 0.004 | NS | NA | NA | NA | NA | NA |
In contrast to lymphocytes, for monocytes there was a significant inverse relationship between the production of new cells (s) and log HIV (r = −0.56, P = .003; Fig. 3 C). No correlation was seen between the decay rate (d) for the monocyte pool and log HIV (Table II). Thus, viral replication impacts monocytes and T lymphocytes differently. HIV infection leads to an increase in the rate of T lymphocyte production, but to an overall decrease in the rate of monocyte production. This may reflect the differences between a cell population maintained by peripheral expansion and one maintained by stem cell differentiation. After HAART therapy, a significant increase in s for monocytes was seen (Table III), suggesting improved progenitor cell function as a consequence of control of viral replication.
Discussion
This study provides a new framework for understanding the impact of HIV on cell turnover. The direct relationship between the size of the rapidly proliferating pool (s 1) and viral load, together with the decrease in s 1 seen after control of viral replication, for both CD4 and CD8 cells, demonstrates that a major effect of ongoing HIV replication is to drive a shift of both CD4 and CD8 cells from a slowly turning over compartment to one rapidly turning over. This shift cannot be driven exclusively by HIV-mediated cytopathicity however, as it is seen in both CD4 and CD8 T cells. A more plausible explanation is that this shift is due to immune activation of both populations, either directly in response to antigen (HIV or other pathogens) or indirectly, through induction of inflammatory mediators in lymphoid tissue that leads to bystander activation (2, 26).
Within the limits of this methodology, HIV replication has no direct overall effect on the disappearance rate (death, proliferation, and trafficking, but in steady-state ultimately death) of the individual compartments, including the rapidly decaying compartment, which must therefore be determined by other biological effects (e. g. mode of activation, expression of surface receptors, cytokine milieu). Thus, entry into the rapidly proliferating pool is driven by HIV, but once the cell enters this pool, its fate is not dependent on HIV.
What cells do the two subpopulations represent? The rapidly proliferating pool accounts for <∼1–10% of the proliferating populations of both CD4 and CD8 cells in patients with low viral loads, but increases to ∼10–40% of these populations in patients with the highest viral loads. Preliminary results suggest that this subpopulation primarily represents an expansion of activated memory or effector cells that have a high turnover rate, many of which may undergo programmed cell death after activation (25). The slowly proliferating pool likely represents naive or long-term memory cells that have a primary surveillance role and are replicating via homeostatic mechanisms. The previously reported declines in both CD4 and CD8 naive cells that occur with progressive HIV infection (27, 28) may thus result from a relative shift of these cells to the rapidly proliferating pool, without an increase in homeostatic proliferation or replenishment via the thymus. Ongoing studies using additional markers for naive and memory cells as well as activation markers should further clarify the phenotypic characteristics of the two populations.
Effective treatment of HIV infection with HAART results in a decrease in the size of the rapidly proliferating pool, but has no effect on the decay constant of this pool. Equally important, suppression of HIV replication by HAART therapy does not lead to a change in any of the kinetic parameters (s 2, n 2, or d 2) of the slowly proliferating pool. Thus there is no evidence suggesting that HIV is suppressing production of lymphocytes (either CD4 or CD8 cells), or that treatment leads to an increase in production of either the rapidly or slowly proliferating subpopulation of T lymphocytes. The data presented here regarding the relationship between HIV treatment and T cell production are the opposite of what has been previously published using deuterium labeling (9, 10). In these nonlongitudinal studies the fractional replacement of CD4 cells was found to be significantly higher in five patients after 12 wk of successful HAART when compared with a separate cohort of seven patients with uncontrolled viremia (9, 10). Our results are consistent with one study using Ki67 staining as a marker of proliferation (2), but not another that through extrapolation attempted to quantitate the total number of proliferating cells in the entire lymphocyte pool (7).
However, consistent with other studies (29), our data do suggest that HIV is suppressing bone marrow function. HIV levels are inversely correlated with rates of monocyte production (s), and HAART therapy is associated with a significant increase in monocyte production. The effects of HIV on different cell populations are presumably dependent on multiple factors related to the intrinsic biology of the cells as well as their interactions with HIV.
Most prior studies have demonstrated an ∼two- to sixfold increase of T cell proliferation in untreated HIV-infected patients compared with uninfected patients (6–10, 14). In the majority of them there was no relationship between viral load and proliferation. Methodological differences may help explain these discrepancies. Ki67 staining, for example, provides only a snapshot of proliferating cells, but does not allow the longitudinal observation of individual cells to use for development of kinetic models that is provided by BrdU labeling. Moreover, Ki67 staining is not limited to proliferating cells (30). In the current study there was a good correlation between peak labeling and Ki67 staining for both CD4 and CD8 cells, consistent with the concept that proliferation (as measured by BrdU incorporation) occurs primarily in activated (Ki67 positive) cells. Lack of an even better correlation between the two methods may be related to the observation that in HIV infection, Ki67 staining includes a high proportion of effector cells that have accumulated in G1 as well as those undergoing cell division (31). Published studies with deuterium labeling have not sampled with the frequency necessary to delineate the decay kinetics described here, and may lack the sensitivity to define the decay of the slowly proliferating subpopulation (9, 10). Pulse labeling with BrdU provides a method for longitudinally following a population of cells with a sensitivity that permits detection of labeled cells for months to years, and thus permits more accurate modeling of the kinetics of these cells.
Despite the detailed characterization of cell kinetics provided by the BrdU methodology, the mechanisms leading to the preferential decline of CD4 counts seen in patients with HIV infection remain obscure. In contrast to other studies, our data provide no evidence for decreased production as a major pathogenic mechanism in CD4 T cell depletion (7, 9, 10). Given that the loss of CD4 cells occurs gradually over a period of years, the kinetic differences in decay rates between CD4 and CD8 cells may be too subtle to measure using currently available methodologies, or may require information from a larger cohort of patients. It is clear that both cell types have increased proliferation and decay rates due to a relative increase in the size of the rapidly proliferating subpopulation of cells. Differences in the biology of the cells in response to activation, or infection of CD4 but not CD8 cells by HIV may shift the balance in CD4 cells to favor gradual depletion of this population.
Acknowledgments
We would like to thank the patients for their willingness to participate in this study, Dr. Anthony Fauci for his helpful discussions, Cathy Watkins, Cathi Pyle, and Barbara Kasprzak for technical assistance, J. Boon, E. Gee, and D. Gee for help with the initial mathematical analysis, and the nurses and support staff of the outpatient and inpatient National Institute of Allergy and Infectious Diseases-Clinical Center Intramural AIDS Program.
This project has been funded in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under contract no. NO1-C0-56000.
Footnotes
*
Abbreviations used in this paper: BrdU, bromodeoxyuridine; HAART, highly active antiretroviral therapy.
References
- 1.Fauci, A.S. 1993. Multifactorial nature of human immunodeficiency virus disease: implications for therapy. Science. 262:1011–1018. [DOI] [PubMed] [Google Scholar]
- 2.Hazenberg, M.D., J.W. Stuart, S.A. Otto, J.C. Borleffs, C.A. Boucher, R.J. de Boer, F. Miedema, and D. Hamann. 2000. T-cell division in human immunodeficiency virus (HIV)-1 infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART). Blood. 95:249–255. [PubMed] [Google Scholar]
- 3.Ho, D.D., A.U. Neumann, A.S. Perelson, W. Chen, J.M. Leonard, and M. Markowitz. 1995. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 373:123–126. [DOI] [PubMed] [Google Scholar]
- 4.Wei, X., S.K. Ghosh, M.E. Taylor, V.A. Johnson, E.A. Emini, P. Deutsch, J.D. Lifson, S. Bonhoeffer, M.A. Nowak, B.H. Hahn, et al. 1995. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 373:117–122. [DOI] [PubMed] [Google Scholar]
- 5.Wolthers, K.C., G. Bea, A. Wisman, S.A. Otto, A.M. de Roda Husman, N. Schaft, F. de Wolf, J. Goudsmit, R.A. Coutinho, A.G. van der Zee, et al. 1996. T cell telomere length in HIV-1 infection: no evidence for increased CD4+ T cell turnover. Science. 274:1543–1547. [DOI] [PubMed] [Google Scholar]
- 6.Lempicki, R.A., J.A. Kovacs, M.W. Baseler, J.W. Adelsberger, R.L. Dewar, V. Natarajan, M.C. Bosche, J.A. Metcalf, R.A. Stevens, L.A. Lambert, et al. 2000. Impact of HIV-1 infection and highly active antiretroviral therapy on the kinetics of CD4+ and CD8+ T cell turnover in HIV-infected patients. Proc. Natl. Acad. Sci. USA. 97:13778–13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleury, S., R.J. de Boer, G.P. Rizzardi, K.C. Wolthers, S.A. Otto, C.C. Welbon, C. Graziosi, C. Knabenhans, H. Soudeyns, P.A. Bart, et al. 1998. Limited CD4+ T-cell renewal in early HIV-1 infection: effect of highly active antiretroviral therapy. Nat. Med. 4:794–801. [DOI] [PubMed] [Google Scholar]
- 8.Sachsenberg, N., A.S. Perelson, S. Yerly, G.A. Schockmel, D. Leduc, B. Hirschel, and L. Perrin. 1998. Turnover of CD4+ and CD8+ T lymphocytes in HIV-1 infection as measured by Ki-67 antigen. J. Exp. Med. 187:1295–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hellerstein, M., M.B. Hanley, D. Cesar, S. Siler, C. Papageorgopoulos, E. Wieder, D. Schmidt, R. Hoh, R. Neese, D. Macallan, et al. 1999. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nat. Med. 5:83–89. [DOI] [PubMed] [Google Scholar]
- 10.McCune, J.M., M.B. Hanley, D. Cesar, R. Halvorsen, R. Hoh, D. Schmidt, E. Wieder, S. Deeks, S. Siler, R. Neese, and M. Hellerstein. 2000. Factors influencing T-cell turnover in HIV-1-seropositive patients. J. Clin. Invest. 105:R1–R8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng, Y.R., R.J. Biggar, D. Gee, D. Norwood, S.L. Zeichner, and D.S. Dimitrov. 1999. Long-term telomere dynamics: modest increase of cell turnover in HIV- infected individuals followed for up to 14 years. Pathobiology. 67:34–38. [DOI] [PubMed] [Google Scholar]
- 12.Fleury, S., G.P. Rizzardi, A. Chapuis, G. Tambussi, C. Knabenhans, E. Simeoni, J.Y. Meuwly, J.M. Corpataux, A. Lazzarin, F. Miedema, and G. Pantaleo. 2000. Long-term kinetics of T cell production in HIV-infected subjects treated with highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA. 97:5393–5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tissot, O., J.P. Viard, C. Rabian, N. Ngo, M. Burgard, C. Rouzioux, and C. Penit. 1998. No evidence for proliferation in the blood CD4+ T-cell pool during HIV- 1 infection and triple combination therapy. AIDS. 12:879–884. [DOI] [PubMed] [Google Scholar]
- 14.Paganin, C., D.S. Monos, J.D. Marshall, I. Frank, and G. Trinchieri. 1997. Frequency and cytokine profile of HPRT mutant T cells in HIV-infected and healthy donors: implications for T cell proliferation in HIV disease. J. Clin. Invest. 99:663–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolbeare, F. 1996. Bromodeoxyuridine: a diagnostic tool in biology and medicine, Part III. Proliferation in normal, injured and diseased tissue, growth factors, differentiation, DNA replication sites and in situ hybridization. Histochem. J. 28:531–575. [DOI] [PubMed] [Google Scholar]
- 16.Gratzner, H.G. 1982. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: a new reagent for detection of DNA replication. Science. 218:474–475. [DOI] [PubMed] [Google Scholar]
- 17.Sprent, J., and D.F. Tough. 1994. Lymphocyte life-span and memory. Science. 265:1395–1400. [DOI] [PubMed] [Google Scholar]
- 18.Mohri, H., S. Bonhoeffer, S. Monard, A.S. Perelson, and D.D. Ho. 1998. Rapid turnover of T lymphocytes in SIV-infected rhesus macaques. Science. 279:1223–1227. [DOI] [PubMed] [Google Scholar]
- 19.Rosenzweig, M., M.A. DeMaria, D.M. Harper, S. Friedrich, R.K. Jain, and R.P. Johnson. 1998. Increased rates of CD4(+) and CD8(+) T lymphocyte turnover in simian immunodeficiency virus-infected macaques. Proc. Natl. Acad. Sci. USA. 95:6388–6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dolbeare, F. 1995. Bromodeoxyuridine: a diagnostic tool in biology and medicine, part II: oncology, chemotherapy and carcinogenesis. Histochem. J. 27:923–964. [PubMed] [Google Scholar]
- 21.Bolger, B.S., T.G. Cooke, R.P. Symonds, A.B. MacLean, and P.D. Stanton. 1993. Measurement of cell kinetics in cervical tumours using bromodeoxyuridine. Br. J. Cancer. 68:166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodson, W.H. III, D.H. Moore ii, B.M. Ljung, K. Chew, B. Mayall, H.S. Smith, and F.M. Waldman. 2000. The prognostic value of proliferation indices: a study with in vivo bromodeoxyuridine and Ki-67. Breast Cancer Res. Treat. 59:113–123. [DOI] [PubMed] [Google Scholar]
- 23.Baseler, M.W., R.A. Stevens, L.A. Lambert, and J.A. Metcalf. 1997. Immunologic evaluation of patients with human immunodeficiency virus infection. Manual of Clinical Laboratory Immunology. N.R. Rose, E.C. De Macario, J.D. Folds, H.C. Lane, and R.M. Nakamura, editors. ASM Press, Washington, DC. 764–772.
- 24.Whitelaw, D.M. 1972. Observations on human monocyte kinetics after pulse labeling. Cell Tissue Kinet. 5:311–317. [DOI] [PubMed] [Google Scholar]
- 25.Grossman, Z., R. Herberman, and D.S. Dimitrov. 1999. T cell turnover in SIV infection. Science. 284:555a–b. [Google Scholar]
- 26.Giorgi, J.V., L.E. Hultin, J.A. McKeating, T.D. Johnson, B. Owens, L.P. Jacobson, R. Shih, J. Lewis, D.J. Wiley, J.P. Phair, et al. 1999. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J. Infect. Dis. 179:859–870. [DOI] [PubMed] [Google Scholar]
- 27.Roederer, M., J.G. Dubs, M.T. Anderson, P.A. Raju, L.A. Herzenberg, and L.A. Herzenberg. 1995. CD8 naive T cell counts decrease progressively in HIV-infected adults. J. Clin. Invest. 95:2061–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Connors, M., J.A. Kovacs, S. Krevat, J.C. Gea-Banacloche, M.C. Sneller, M. Flanigan, J.A. Metcalf, R.E. Walker, J. Falloon, M. Baseler, et al. 1997. HIV infection induces changes in CD4+ T-cell phenotype and depletions within the CD4+ T-cell repertoire that are not immediately restored by antiviral or immune-based therapies. Nat. Med. 3:533–540. [DOI] [PubMed] [Google Scholar]
- 29.Huang, S.S., J.D. Barbour, S.G. Deeks, J.S. Huang, R.M. Grant, V.L. Ng, and J.M. McCune. 2000. Reversal of human immunodeficiency virus type 1-associated hematosuppression by effective antiretroviral therapy. Clin. Infect. Dis. 30:504–510. [DOI] [PubMed] [Google Scholar]
- 30.van Oijen, M.G., R.H. Medema, P.J. Slootweg, and G. Rijksen. 1998. Positivity of the proliferation marker Ki-67 in noncycling cells. Am. J. Clin. Pathol. 110:24–31. [DOI] [PubMed] [Google Scholar]
- 31.Combadiere, B., C. Blanc, T. Li, G. Carcelain, C. Delaugerre, V. Calvez, R. Tubiana, P. Debre, C. Katlama, and B. Autran. 2000. CD4+Ki67+ lymphocytes in HIV-infected patients are effector T cells accumulated in the G1 phase of cell cycle. Eur. J. Immunol. 30:3598–3603. [DOI] [PubMed] [Google Scholar]