Adjuvants of Immunity: Harnessing Innate Immunity to Promote Adaptive Immunity (original) (raw)
Since Jules Freund reported that crude mycobacterial extracts greatly promoted immune responses to antigens (1), the use of adjuvants has become a widespread, but poorly understood practice to promote T and B cell responses (2). Recent studies have begun to identify the chemical nature of several adjuvants and the cellular and molecular mechanisms of their long-elusive immunological effects. For example, conserved microbial structures are recognized by innate immunity receptors such as Toll-like receptors (TLRs) and the complement system, eliciting specific signaling cascades which, ultimately, result in enhancing and guiding T and B cell responses (for reviews, see references 3 and 4).
Despite these considerable advances, the task of enhancing CD8 T cell priming to nonliving antigens, a major goal of vaccines against a range of infectious and cancer diseases, has eluded immunologists. In this issue, Gonzalez-Aseguinolaza and colleagues report that α-galactosylceramide (α-GalCer), a glycolipid originally extracted from marine sponges on the basis of its antitumor properties, promotes anti-malarial CD8 T cell immunity when coinjected with irradiated sporozoites (5). Unlike common microbial adjuvants, which signal through TLRs, α-GalCer functions as an antigen presented by CD1d to NKT cells expressing a conserved semi-invariant αβ TCR (for a review, see reference 6). Like microbial adjuvants, however, α-GalCer activates dendritic cells (DCs), though it does so indirectly through the cognate interaction with CD1d-restricted α-GalCer–specific NKT cells. DCs are the antigen-presenting cell type that is central to adaptive immunity (7), and it is likely that the efficacy of different adjuvants can be explained by differences in signaling DCs to undergo the complex and coordinate maturation events that are required for efficient T cell priming. Here, we will review the emerging families of adjuvant-specific receptors, highlighting the recent recognition that specialized subsets lymphocytes, such as NKT cells, can function at the innate phase of immunity to promote and regulate adaptive immunity through interactions with DCs.
Toll Receptors.
Purified and synthetic components of microbial extracts exert potent adjuvant effects (for recent reviews, see references 8–10). These include a variety of lipids and glycolipids, such as mycolic acid, lipoarabinomannan (LAM), LPS, lipoteichoic acid and microbial GPI, polynucleotides, such as bacterial DNA (i.e., with unmethylated CpG sequences) and double stranded RNA (produced upon viral infection), lipoproteins, and even a conserved protein, flagellin, which enables bacterial motility. Surprisingly, most of their effects now appear to be mediated by receptors belonging to a single gene family, the TLRs. Although Toll was originally discovered for its contribution to dorsoventral patterning in Drosophila embryos through an intracellular cascade homologous to the nuclear factor (NF)-κB signaling pathway, later studies identified the critical role of Toll in the fly innate immune response to microbial infection (11). Remarkably, there appeared to be some specificity in both the input and output of the Toll pathways. Thus, whereas fungi and G+ bacteria activated the antifungal peptide gene drosomycin through Toll, G− bacteria activated the anti-G− peptide diptericin through a distinct Toll-like pathway. These observations have suggested a simple and powerful scenario whereby innate immunity receptors not only recognize conserved microbial structures directly, as signatures of class of pathogen, but also trigger an effector response that is tailored to the pathogen. The discovery of TLRs in vertebrates and their connection to costimulatory ligand and cytokine induction by antigen-presenting cells have been a prelude to an explosion of knowledge, which has assigned most of the effects of microbial adjuvants to one of ten distinct TLR family members (see Fig. 1) . Moreover, triggering the TLRs by microbial adjuvants, such as CFA, is not only sufficient for the activation of the adaptive immune responses, but it appears to be required for the induction of Th1 effector responses (12). Interestingly, adjuvants that do not trigger TLRs, such as alum, which is devoid of microbial components, tend to potentiate Th2 responses.
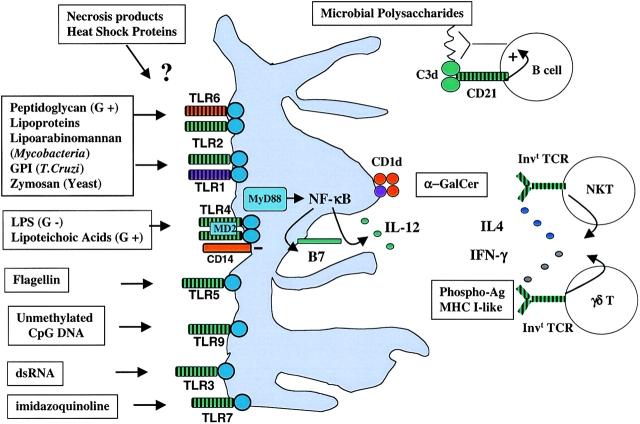
Figure 1.
Adjuvant pathways. Various classes of microbial or nonmicrobial adjuvants are specifically recognized by invariant receptors such as TLRs expressed by DCs, soluble complement factors, or even invariant TCRs expressed by specialized subsets of CD1d-restricted NKT αβ T cells and γδ T cells. The resulting adjuvant cascades generally lower the antigen concentration required to trigger the adaptive B or T cell responses, and/or provide cytokines which favor Th1 or Th2 differentiation.
Although it is tempting to speculate that, as in Drosophila, specificity upstream and downstream of individual TLRs will connect different classes of pathogens to different classes of immune responses, there are important differences in the mechanisms of Toll function between humans and flies. For example, several lines of evidence suggest that mammalian TLRs may recognize their ligands directly, in some cases with the help of accessory proteins (for a review, see reference 8). In contrast, the Drosophila Toll is induced by a self-protein Spatzle, which is processed and activated by a proteolytic cascade triggered by infection. Thus, the microbial recognition step in flies occurs upstream of Toll and was recently shown to be mediated by a soluble peptidoglycan recognition protein (PGRP) (13). Another unresolved issue is how signal transduction differ between different TLRs, given the similarity of their intracytoplasmic domains. The recent discovery of additional signaling pathways connected to different TLRs is likely to shed light on this question (14–16).
Complement Receptors.
Microbial carbohydrates activating the complement cascade elicit a distinct adjuvant pathway which is mediated by the complement receptor CD21 expressed on B cells in association with the signaling molecule CD19. Linked recognition of antigen by the BCR and covalently attached C3d by CD21 amplifies, through Vav-mediated activation of lipid and protein kinases, BCR signaling, allowing detection of minute concentrations of antigen that would otherwise fail to stimulate B cells (17, 18). Thus, C3d-antigen fusion proteins may be of great interest for B cell vaccine strategies.
Invariant TCRs.
NKT cells are a specialized subset of CD1d-restricted autoreactive T cells which is reported to regulate various conditions ranging from type I diabetes to tumor rejection in vivo through the release of Th1 or Th2 cytokines (for a review, see reference 6). α-GalCer, which was originally extracted from marine sponges based on its anti-tumor properties (19), binds CD1d and is recognized at picomolar concentrations by the conserved semi-invariant, CD1d-restricted αβ TCR of mouse (Vα14-Jα18/Vβ8) and human (Vα24-Jα18/Vβ11) NKT cells (20). It is thought therefore that αGalCer acts as a surrogate antigen mimicking an as yet unidentified self-antigen which is loaded onto CD1d in the endosomal compartment. In vivo, as little as 1 ng of α-GalCer injected systemically into mice induces activation of NKT cells, which explosively release both Th1 and Th2 cytokines and elicit a downstream cascade of activation spreading to DCs (B7 and IL-12 induction), NK cells (IFN-γ secretion and killer function), and B cells (B7 induction) (21–24). In this issue, Gonzalez-Aseguinolaza and colleagues (5) report that α-GalCer should be added to the arsenal of vaccine adjuvants, as was predicted from the range of its biological effects, but also that it exhibits the rare property of enhancing CD8 T cell–mediated immunity against malaria infection when coinjected with irradiated sporozoites. Priming anti-malarial CD8 T cells with a nonliving antigen likely requires the coordinate induction of several fundamental steps in the maturation program of DCs, including the expression of costimulatory molecules and IL-12, MHC class I–mediated antigen processing and presentation through the exogenous pathway, and homing to the T cell areas of the spleen. In that regard, one potential advantage of α-GalCer over the TLR-mediated adjuvants may be that NKT cells engage in rich, cognate interaction with DCs, which include CD40L/CD40 signaling, an essential step for CD8 T cell priming (25–27) as well as exposure to a range of cytokines and chemokines (Fig. 1).
α-GalCer is a promising immunomodulatory agent which is currently being explored for its potential benefits in antitumor and antiinfectious therapies as well as in the prevention of type I diabetes and autoimmune encephalomyelitis (28–36). A unique property of the NKT/α-GalCer adjuvant pathway is the ability to induce both Th1 and Th2 immunity. However, it is not yet fully understood how NKT cells can promote Th2 immunity in some cases and Th1 in others, and future research is warranted to better control and exploit this versatility of α-GalCer (21, 33, 37).
Interestingly, there are similar subsets of specialized lymphocytes straddling innate and adaptive immunity which express invariant receptors for which surrogate ligands are available (for a review, see reference 6). For example, a large proportion of circulating γδ T cells in humans express Vγ9 TCR which can uniformly be activated by synthetic phosphoantigens. Other invariant γδ TCR expressed by specialized populations in the skin and mucosal tissues are actively studied and it is envisioned that in the near future, they could be exploited as well for site-specific vaccine strategies.
The discovery of surrogate synthetic antigens which uniformly activate whole subsets of innate lymphocytes such as CD1d-restricted NKT cells and Vγ9 γδ T cells not only lends support to the view that these cells belong to the innate rather than the adaptive arm of the immune system, more resembling NK cells than conventional T cells, but also considerably extends the scope and promise of adjuvant research.
Other Adjuvants.
Products released by necrotic cell death and stressed or damaged tissues can act as powerful adjuvants as well (38–40). Interestingly, physiological apoptosis does not activate, and may even in some cases suppress, immunity. These endogenous adjuvant effects indicate that cell injury and tissue damage alone, in the absence of foreign microbial elements, activate immunity. Thus, they may account for the various forms of anti–self-immune responses which do not seem to be associated with microbial infection, including many chronic autoimmune diseases and some acute, transient response to damaged tissues, for example after mycocardial infarction. They may also account for transplant rejection and allergy (7, 41).
The coexistence of endogenous, sterile, and exogenous, microbial adjuvants supports the idea that evolution selected diverse mechanisms enabling the detection of both indirect (damage) and direct (pathogen structures) signatures of infection. It has been suggested that some microbial adjuvants might in fact mimic endogenous signs of damage (42) or, to the contrary, that endogenous adjuvants might mimic microbial products (4). Elucidating the nature of these endogenous adjuvants associated with stress and damage will not only shed light on these theoretical issues, but also will undoubtedly bring novel insights into the inner workings of the immune response to stress and damage and its general involvement in immunity and autoimmunity.
DCs.
Ultimately, it is striking that all microbial and nonmicrobial T cell adjuvant pathways intersect at the level of the DC, the antigen-presenting cell type which is central to the priming of adaptive T cell responses. Adjuvant cascades are complex, involving a number of molecular and cellular activation events, but their effects on distinct steps of the maturation program of DCs provide an attractive framework to envision the future dissection of adjuvanticity. For example, activation of DCs regulates in a coordinate manner the induction of costimulation and cytokines such as IL-12, the antigen processing and presentation pathways, including the exogenous pathway of MHC class I presentation, and the tissue migration pattern. A detailed molecular understanding of the impact of adjuvant signaling on these processes will be required to elucidate the basis for the qualitative and quantitative differences between adjuvants.
Conclusion.
Underlying the emerging diversity of adjuvants and the expanding universe of corresponding receptors is the simple concept that, whether self or foreign, conserved structures exposed during tissue damage and microbial invasion constitute important cues for several families of receptors of innate immunity. These innate immunity receptors in turn activate, in a variety of cell types, specialized effector pathways designed by evolution to meet the threat of individual pathogens. Of importance to future strategies of T cell vaccination against intracellular infections and tumors is the convergence of all T cell adjuvants to one cell type, the DC, and specifically the effects of adjuvants on the coordinate maturation program that underlies efficient antigen presentation by DCs for priming CD4 and CD8 T cell responses.
Undoubtedly, many more natural adjuvants remain to be discovered. The paper by Gonzalez-Aseguinolaza in this issue (5) illustrates the unusual story and the potentially important role of a glycolipidic adjuvant which was originally extracted from marine sponges. It also draws attention to subsets of lymphocytes expressing rearranged, but conserved, TCR or BCR, which may function at the innate rather than adaptive phase of immunity and could be harnessed to promote and guide adaptive immunity, particularly against intracellular infections and tumors.
Understanding the structural details of the recognition of adjuvants by innate immunity receptors, the intracellular signaling and the intercellular communication network that they trigger is a major challenge of future research. In particular, it is envisioned that such research will generate the knowledge required for the rational design of a new generation of adjuvants tailored to elicit specific effector functions.
Acknowledgments
The authors thank A.M. Silverstein for discussion.
References
- 1.Freund, J., and K. McDermott. 1942. Sensitization to horse serum by means of adjuvants. Proc. Soc. Exp. Biol. Med. 49:548–553. [Google Scholar]
- 2.Janeway, C.A. 1989. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 54:1–13. [DOI] [PubMed] [Google Scholar]
- 3.Fearon, D.T., and R.M. Locksley. 1996. The instructive role of innate immunity in the acquired immune response. Science. 272:50–54. [DOI] [PubMed] [Google Scholar]
- 4.Medzhitov, R., and C.A. Janeway, Jr. 1997. Innate immunity: the virtues of a nonclonal system of recognition. Cell. 91:295–298. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Aseguinolaza, G., L.V. Kaer, C.G. Bergmann, J.M. Wison, J. Schmieg, M. Kronenberg, T. Nakayama, M. Taniguchi, Y. Koezuka, and M. Tsuji. 2002. Natural killer T cell ligand α-galactosylceramide enhances protective immunity induced by malaria vaccines. J. Exp. Med. 195:617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bendelac, A., M. Bonneville, and J.F. Kearney. 2001. Autoreactivity by design: innate B and T lymphocytes. Nat. Rev. Immunol. 1:177–186. [DOI] [PubMed] [Google Scholar]
- 7.Steinman, R.M., and M.C. Nussenzweig. 2002. Inaugural Article: Avoiding horror autotoxicus: The importance of dendritic cells in peripheral T cell tolerance. Proc. Natl. Acad. Sci. USA. 99:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675–680. [DOI] [PubMed] [Google Scholar]
- 9.Kimbrell, D.A., and B. Beutler. 2001. The evolution and genetics of innate immunity. Nat. Rev. Genet. 2:256–267. [DOI] [PubMed] [Google Scholar]
- 10.Krutzik, S.R., P.A. Sieling, and R.L. Modlin. 2001. The role of Toll-like receptors in host defense against microbial infection. Curr. Opin. Immunol. 13:104–108. [DOI] [PubMed] [Google Scholar]
- 11.Lemaitre, B., E. Nicolas, L. Michaut, J.-M. Reichhart, and J.A. Hoffmann. 1996. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in drosophila adults. Cell. 86:973–983. [DOI] [PubMed] [Google Scholar]
- 12.Schnare, M., G.M. Barton, A.C. Holt, K. Takeda, S. Akira, and R. Medzhitov. 2001. Toll-like receptors control activation of adaptive immune responses. Nat. Immunol. 2:947–950. [DOI] [PubMed] [Google Scholar]
- 13.Michel, T., J.M. Reichhart, J.A. Hoffmann, and J. Royet. 2001. Drosophila Toll is activated by Gram-positive bacteria through a circulating peptidoglycan recognition protein. Nature. 414:756–759. [DOI] [PubMed] [Google Scholar]
- 14.Horng, T., G.M. Barton, and R. Medzhitov. 2001. TIRAP: an adapter molecule in the Toll signaling pathway. Nat. Immunol. 2:835–841. [DOI] [PubMed] [Google Scholar]
- 15.Fitzgerald, K.A., E.M. Palsson-McDermott, A.G. Bowie, C.A. Jefferies, A.S. Mansell, G. Brady, E. Brint, A. Dunne, P. Gray, M.T. Harte, et al. 2001. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 413:78–83. [DOI] [PubMed] [Google Scholar]
- 16.Kawai, T., O. Takeuchi, T. Fujita, J. Inoue, P.F. Muhlradt, S. Sato, K. Hoshino, and S. Akira. 2001. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J. Immunol. 167:5887–5894. [DOI] [PubMed] [Google Scholar]
- 17.O'Rourke, L.M., R. Tooze, M. Turner, D.M. Sandoval, R.H. Carter, V.L. Tybulewicz, and D.T. Fearon. 1998. CD19 as a membrane-anchored adaptor protein of B lymphocytes: costimulation of lipid and protein kinases by recruitment of Vav. Immunity. 8:635–645. [DOI] [PubMed] [Google Scholar]
- 18.Dempsey, P.W., M.E. Allison, S. Akkaraju, C.C. Goodnow, and D.T. Fearon. 1996. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 271:348–350. [DOI] [PubMed] [Google Scholar]
- 19.Natori, T., M. Morita, K. Akimoto, and Y. Koezuka. 1994. Agelasphins, novel antitumor and immunostimulatory cerebrosides from the sponge _Agelas mauritianus_Tetrahedron. 50:2771–2784. [Google Scholar]
- 20.Kawano, T., J. Cui, Y. Koezuka, I. Toura, Y. Kaneko, K. Motoki, H. Ueno, R. Nakagawa, H. Sato, E. Kondo, et al. 1997. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 278:1626–1629. [DOI] [PubMed] [Google Scholar]
- 21.Singh, N., S. Hong, D.C. Scherer, I. Serizawa, N. Burdin, M. Kronenberg, Y. Koezuka, and L. Van Kaer. 1999. Cutting edge: activation of NK T cells by CD1d and alpha-galactosylceramide directs conventional T cells to the acquisition of a Th2 phenotype. J. Immunol. 163:2373–2377. [PubMed] [Google Scholar]
- 22.Carnaud, C., D. Lee, O. Donnars, S.-H. Park, A. Beavis, Y. Koezuka, and A. Bendelac. 1999. Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J. Immunol. 163:4647–4650. [PubMed] [Google Scholar]
- 23.Kitamura, H., K. Iwakabe, T. Yahata, S. Nishimura, A. Ohta, Y. Ohmi, M. Sato, K. Takeda, K. Okumura, L. Van Kaer, et al. 1999. The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)- 12 production by dendritic cells and IL-12 receptor expression on NKT cells. J. Exp. Med. 189:1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eberl, G., and H.R. MacDonald. 2000. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur. J. Immunol. 30:985–992. [DOI] [PubMed] [Google Scholar]
- 25.Ridge, J.P., F. Di Rosa, and P. Matzinger. 1998. A conditioned dendritic cell can be a temporal bridge between a CD4+ T- helper and a T-killer cell. Nature. 393:474–478. [DOI] [PubMed] [Google Scholar]
- 26.Schoenberger, S.P., R.E. Toes, E.I. van der Voort, R. Offringa, and C.J. Melief. 1998. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 393:480–483. [DOI] [PubMed] [Google Scholar]
- 27.Bennett, S.R., F.R. Carbone, F. Karamalis, R.A. Flavell, J.F. Miller, and W.R. Heath. 1998. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 393:478–480. [DOI] [PubMed] [Google Scholar]
- 28.Kakimi, K., L.G. Guidotti, Y. Koezuka, and F.V. Chisari. 2000. Natural killer T cell activation inhibits hepatitis B virus replication In vivo. J. Exp. Med. 192:921–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez-Aseguinolaza, G., C. de Oliveira, M. Tomaska, S. Hong, O. Bruna-Romero, T. Nakayama, M. Taniguchi, A. Bendelac, L. Van Kaer, Y. Koezuka, and M. Tsuji. 2000. alpha-galactosylceramide-activated Valpha 14 natural killer T cells mediate protection against murine malaria. Proc. Natl. Acad. Sci. USA. 97:8461–8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toura, I., T. Kawano, Y. Akutsu, T. Nakayama, T. Ochiai, and M. Taniguchi. 1999. Cutting edge: inhibition of experimental tumor metastasis by dendritic cells pulsed with alpha-galactosylceramide. J. Immunol. 163:2387–2391. [PubMed] [Google Scholar]
- 31.Hong, S., M.T. Wilson, I. Serizawa, L. Wu, N. Singh, O.V. Naidenko, T. Miura, T. Haba, D.C. Scherer, J. Wei, et al. 2001. The natural killer T-cell ligand alpha-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat. Med. 7:1052–1056. [DOI] [PubMed] [Google Scholar]
- 32.Sharif, S., G.A. Arreaza, P. Zucker, Q.S. Mi, J. Sondhi, O.V. Naidenko, M. Kronenberg, Y. Koezuka, T.L. Delovitch, J.M. Gombert, et al. 2001. Activation of natural killer T cells by alpha-galactosylceramide treatment prevents the onset and recurrence of autoimmune type 1 diabetes. Nat. Med. 7:1057–1062. [DOI] [PubMed] [Google Scholar]
- 33.Miyamoto, K., S. Miyake, and T. Yamamura. 2001. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 413:531–534. [DOI] [PubMed] [Google Scholar]
- 34.Singh, A.K., M.T. Wilson, S. Hong, D. Olivares-Villagomez, C. Du, A.K. Stanic, S. Joyce, S. Sriram, Y. Koezuka, and L. Van Kaer. 2001. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J. Exp. Med. 194:1801–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang, B., Y.B. Geng, and C.R. Wang. 2001. CD1-restricted NK T cells protect nonobese diabetic mice from developing diabetes. J. Exp. Med. 194:313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jahng, A.W., I. Maricic, B. Pedersen, N. Burdin, O. Naidenko, M. Kronenberg, Y. Koezuka, and V. Kumar. 2001. Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. J. Exp. Med. 194:1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cui, J., N. Watanabe, T. Kawano, M. Yamashita, T. Kamata, C. Shimizu, M. Kimura, E. Shimizu, J. Koike, H. Koseki, et al. 1999. Inhibition of T helper cell type 2 cell differentiation and immunoglobulin E response by ligand-activated Valpha14 natural killer T cells. J. Exp. Med. 190:783–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallucci, S., M. Lolkema, and P. Matzinger. 1999. Natural adjuvants: endogenous activators of dendritic cells. Nat. Med. 5:1249–1255. [DOI] [PubMed] [Google Scholar]
- 39.Sauter, B., M.L. Albert, L. Francisco, M. Larsson, S. Somersan, and N. Bhardwaj. 2000. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J. Exp. Med. 191:423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Basu, S., R.J. Binder, R. Suto, K.M. Anderson, and P.K. Srivastava. 2000. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int. Immunol. 12:1539–1546. [DOI] [PubMed] [Google Scholar]
- 41.Matzinger, P. 1998. An innate sense of danger. Semin. Immunol. 10:399–415. [DOI] [PubMed] [Google Scholar]
- 42.Matzinger, P. 1994. Tolerance, danger and the extended family. Annu. Rev. Immunol. 12:991–1045. [DOI] [PubMed] [Google Scholar]