Flexibility of Mouse Classical and Plasmacytoid-derived Dendritic Cells in Directing T Helper Type 1 and 2 Cell Development: Dependency on Antigen Dose and Differential Toll-like Receptor Ligation (original) (raw)
Abstract
Distinct dendritic cell (DC) subsets have been suggested to be preprogrammed to direct either T helper cell (Th) type 1 or Th2 development, although more recently different pathogen products or stimuli have been shown to render these DCs more flexible. It is still unclear how distinct mouse DC subsets cultured from bone marrow precursors, blood, or their lymphoid tissue counterparts direct Th differentiation. We show that mouse myeloid and plasmacytoid precursor DCs (pDCs) cultured from bone marrow precursors and ex vivo splenic DC subsets can induce the development of both Th1 and Th2 effector cells depending on the dose of antigen. In general, high antigen doses induced Th1 cell development whereas low antigen doses induced Th2 cell development. Both cultured and ex vivo splenic plasmacytoid-derived DCs enhanced CD4+ T cell proliferation and induced strong Th1 cell development when activated with the Toll-like receptor (TLR)9 ligand CpG, and not with the TLR4 ligand lipopolysaccharide (LPS). The responsiveness of plasmacytoid pDCs to CpG correlated with high TLR9 expression similarly to human plasmacytoid pDCs. Conversely, myeloid DCs generated with granulocyte/macrophage colony-stimulating factor enhanced Th1 cell development when stimulated with LPS as a result of their high level of TLR4 expression. Polarized Th1 responses resulting from high antigen dose were not additionally enhanced by stimulation of DCs by TLR ligands. Thus, the net effect of antigen dose, the state of maturation of the DCs together with the stimulation of DCs by pathogen-derived products, will determine whether a Th1 or Th2 response develops.
Keywords: dendritic cell, Th1, Th2, TLR, cytokines
Introduction
Effective eradication of pathogens requires the intricate interplay between the innate and adaptive immune responses. In this, dendritic cells (DCs)*are crucial because they are a unique group of bone marrow–derived leukocytes that are specialized for the uptake, transport, processing, and presentation of antigen to T cells (1–4). At an immature stage DCs act as sentinels, continuously take up antigen, and become activated by microbial products to secrete proinflammatory cytokines. This results in up-regulation of costimulatory molecules required for the effective interaction with T cells upon migration of the DCs to the lymph nodes. Triggering of T cells into cell cycle progression resulting in massive clonal expansion, is a central function of DCs.
DC subsets have been suggested to be preprogrammed to direct the differentiation of CD4+ T cells into either IFN-γ–producing Th1 cells or IL-4–producing Th2 cells (5–9; for review see 2). Human monocyte–derived DCs activated with CD40 ligand were found to induce Th1 differentiation (5) whereas DCs derived from CD4+ CD3− CD11c− plasmacytoid cells activated with CD40 ligand induced Th2 differentiation (5). In vivo, mouse antigen–pulsed splenic CD11c+ CD8α+ CD11b− DCs induced the development of Th1 cells whereas CD11c+ CD8α− CD11b+ DCs induced the development of Th2 cells when injected into mice (6, 8). However, these DCs had received maturation signals either in vitro (overnight culture in GM-CSF; reference 8) or in vivo (Flt3 ligand administration; reference 6). Conversely, both subsets of DCs when freshly isolated directed Th1 cell development in vitro (7). Taken together, these studies suggest that these DC subsets may not have an intrinsic capacity to direct either Th1 or Th2 cell development, but rather might be modified by external factors. Indeed, DC subsets can be influenced by a range of stimuli including pathogen-derived products and inflammatory mediators like cytokines to impact the subsequent Th cell response (5, 10–26).
A wide range of DCs, when appropriately stimulated, direct the development of Th1 cells largely by their production of IL-12 and/or IFN-α (5, 12, 14, 17, 18, 22, 23, 27). IL-12 production by DCs can be stimulated by a large range of microbial products and augmented by CD40 ligation or cytokines (10–12, 15, 16, 18–24). However, DCs may produce IL-12 temporarily and become refractory to additional stimuli for the production of IL-12, and this has been referred to as “exhaustion” (23) or “paralysis” (24). Thus, previously stimulated DCs (23, 24) could help skew the balance toward a Th0 or Th2 response. In addition, although certain signals stimulate DCs to induce the development of Th1 cells, others, such as prostaglandin E2 (28), cholera toxin, nematode worm, or yeast products will trigger these DCs to direct Th2 development (13, 25, 26, 29). Thus, pathogen-derived products and/or inflammatory mediators may clearly affect the ability of DCs to direct Th1 or Th2 cell development.
Recognition of some pathogen-derived products involves specific receptors, such as the Toll-like receptors (TLRs), which were initially identified in Drosophila but are also expressed in vertebrates (30–32). In human, distinct DC subsets have been shown to express different TLRs and consequently to respond to distinct microbial products (33–35). For example, monocyte-derived DCs express TLR2 and TLR4 whereas plasmacytoid precursor DCs (pDCs) express TLR7 and TLR9 (33–35). An inability to produce IL-12 or IFN-α upon stimulation with specific microbial products may therefore reflect the lack of expression of the corresponding TLR and hence their ability to direct Th1 development (30, 31, 33–36).
In addition to pathogen-derived products, the strength of signal and/or the antigen dose can affect the development of Th1 or Th2 cells (23, 37–40). Thus, it is possible that distinct DC subsets, by their expression of different levels of MHC class II and/or costimulatory molecules, may change the effective dose of antigen presented by the APC to the T cell. It is therefore important that a range of antigen doses be examined when assessing the ability of DC subsets to direct Th cell development.
In this study we show that both mouse classical and plasmacytoid pDCs generated from bone marrow as well as lymphoid tissue DCs can direct Th1 or Th2 responses depending on the dose of antigen presented. Moreover, LPS and CpG showed differential effects on the different DC populations to direct Th1 development as a result of differential expression of TLR4 and TLR9.
Materials and Methods
Mice.
DO11.10 mice transgenic for an OVA323–339-specific αβTCR were used as a source of antigen-specific T cells (41). BALB/c mice (Taconics or NIMR) were used to provide DCs. All mice were housed under specific pathogen-free conditions. Female mice were used between 8–12-wk of age.
Reagents.
mAbs used for T cell preparation were anti-B220, anti-CD8α, anti-CD11b, anti–CD4-FITC, and anti–CD62L-PE (all mouse specific; BD Biosciences). mAbs used for the preparation of DC subsets were anti-erythrocyte (Ter119), anti-CD3 (17A2), anti-CD19 (1D3), B220-FITC, anti–CD11c-PE, anti–CD11b-APC, anti–CD8α-APC, B220-FITC, and GR1-APC. Anti–IL-12p40 (C17.8.20) mAbs were used in culture. mAbs used for intracellular staining were anti–IFN-γ–APC (XMG1.2), anti–IL-4–PE (11B11), and isotype controls (all from BD Biosciences). Culture medium was RPMI 1640 with 10% heat-inactivated FCS, 0.05 mM 2-mercaptoethanol, 10mM Hepes buffer, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, and 1mM sodium pyruvate or culture medium specifically made for NIMR by GIBCO BRL. Endotoxin-free OVA peptide (chicken OVA323–339) was from Biosynthesis. DCs were stimulated with LPS Salmonella minnesota serotype Re595 (Sigma-Aldrich) or phosphorothioate CpG DNA (CpG1668: TCCATGACGTTCCTGATGCT; Invitrogen). Mouse Flt3 ligand was produced at DNAX and mouse GM-CSF was obtained from Schering-Plough.
Generation of Bone Marrow–derived DCs.
Bone marrow cells were isolated by flushing femurs and tibia with culture medium. Red blood cells were lysed using 0.83% ammonium chloride. CD11c+ CD11b+ DCs were generated using GM-CSF as described by Inaba et al. (42). In brief, bone marrow cells were plated at 106 cells/ml in medium supplemented with 10 ng/ml GM-CSF in 12-well plates in a volume of 2 ml. At days 2 and 4, supernatant containing nonadherent cells were removed, the wells were washed gently, and fresh medium containing GM-CSF was added. At day 6, nonadherent cells were collected, centrifuged, resuspended in fresh medium with GM-CSF, and cultured for an additional 24 h in Petri dishes. Cells were sorted as CD11c+ cells. Plasmacytoid pDCs were generated by culturing bone marrow cells in culture medium containing 100 ng/ml Flt3 ligand for 10 d at 106 cells/ml in 12-well plates in a volume of 2 ml. At day 5, 1 ml medium was replaced by 1 ml fresh medium containing Flt3 ligand (43). Plasmacytoid pDCs generated with Flt3 ligand were sorted as CD11c+ CD11b− B220+. The purity was always ≥98%.
Preparation of Splenic DC Subsets.
For the purification of splenic DCs, spleens were treated for 30 min at 37°C with 0.4 mg/ml Liberase Cl (Boehringer), followed by red blood cell lysis using 0.83% ammonium chloride. Cells were maintained throughout the procedure in cold PBS, 5% FCS, and 0.5 mM EDTA. The spleen cells were incubated with anti-CD3, anti-CD19, and anti-Ter119 mAb, followed by goat anti–rat Ig–coated beads (BioMag Laboratory and Polysciences). The enriched DCs were either stained with CD11c-PE and CD8α-APC, or B220-FITC, CD11c-PE, and GR1-APC, and sorted using a FACSVantage™ (Becton Dickinson) or MoFlo® cytometer (DakoCytomation). The purity was always ≥98%.
Preparation of T Cells.
CD4+ T cells were enriched from DO11.10 spleen cell preparations by negative depletion using a cocktail of anti-CD8α, anti-B220, and anti-CD11b mAb, followed by goat anti–rat Ig–coated beads. Enriched CD4+ T cells were then additionally purified using a FACSVantage™ (Becton Dickinson) or MoFlo® cytometer (DakoCytomation) to achieve >99% naive CD4+ T cells on the basis of bright CD62L and CD4 staining (44).
Stimulation of Transgenic CD4+ T Cells for Cytokine Production.
Naive CD4+ T cells (105 cells/well) were cultured with purified DC subsets (2 × 104 cells/well) in a total volume of 1 ml in 48-well plates. CD4+ T cells were obtained from DO11.10 mice whereas DCs were from BALB/c mice. Cultures were performed with 10, 1, 0.1, or 0.01 μM OVA peptide. To some cultures 10 μg/ml anti–IL-12p40 mAb, 25 μg/ml LPS, or 1 μM CpG DNA was added. Cells were split on day 4. On days 6–8, cells were harvested, washed, counted, and restimulated with 50 ng/ml PMA and 500 ng/ml ionomycin (both from Sigma-Aldrich; reference 45). After 2 h of incubation, 10 μg/ml brefeldin A (Epicentre Technologies) was added and the cells were incubated for another 2 h followed by fixation with 2% formaldehyde and permeabilization with 0.5% saponin. The cells were stained as previously described (45). The samples were measured on a FACSCalibur® flow cytometer (Becton Dickinson) and analyzed using CellQuest software (Becton Dickinson).
T Cell Proliferation Assay.
For OVA-specific CD4+ Mel14+ T cell proliferation, DC subsets (BALB/c) were γ irradiated (1,500 rad), washed, and cocultured with CD4+ Mel14+ T cells (DO11.10), which were added at 104 cells/well in a volume of 200 μl in round-bottom plates. After 60 h, cells were pulsed for 18 h with 1 μCi [3H]thymidine before they were collected and counted. Titration of the OVA peptide dose was with 103 DCs/well and 104 T cells/well. Titration of the DC numbers was with 2.5 × 104 T cells/well and 1 μM OVA peptide.
In Vitro Stimulation of DCs and Quantitation of Cytokine Production.
7 × 104 sorted DCs were cultured in 200 μl in 96-well flat-bottom culture plates and stimulated with medium alone, 25 μg/ml LPS, or 1 μM CpG DNA. After 24 h, supernatants of the cell cultures were collected and IL-12p70 levels were determined by commercially available ELISA kits (BD Biosciences).
Quantitative mRNA Analysis for TLR4 and TLR9.
RNA from different DC subsets was extracted using an RNeasy Kit (QIAGEN) according to the manufacturer's protocol and reverse transcribed with oligo dT14-18 (Life Technologies) and random hexamer primers (Promega). cDNA was analyzed for the expression of TLR4, TLR9, and 18s by PCR assay using the PerkinElmer 5700 Sequence Detection System (PerkinElmer). Quantification of the target gene expression was by comparison with 18s rRNA expression using a VIC-labeled probe. The primer sequences were as follows: forward TLR4 primer TGA CAG GAA ACC CTA TCC AGA GTT, reverse TLR4 primer TCT CCA CAG CCA CCA GAT TCT, probe TLR4 TTC CCC AGG AAG TTT CTC TGG ACT AAC AAG TTTA; forward TLR9 primer AGC TTC CTG CTG GCT CAGC, reverse TLR9 primer GGA CGC AGG ATC ACC AAC AC, probe TLR9 TGT TGG AAG ACC GCA AGG ACG TGG.
Results and Discussion
Bone Marrow–derived Myeloid DCs, but not Plasmacytoid pDCs, Induce Significant T Cell Proliferation: both DC Subsets Induce Th1 or Th2 Cell Development Depending on the Antigen Dose.
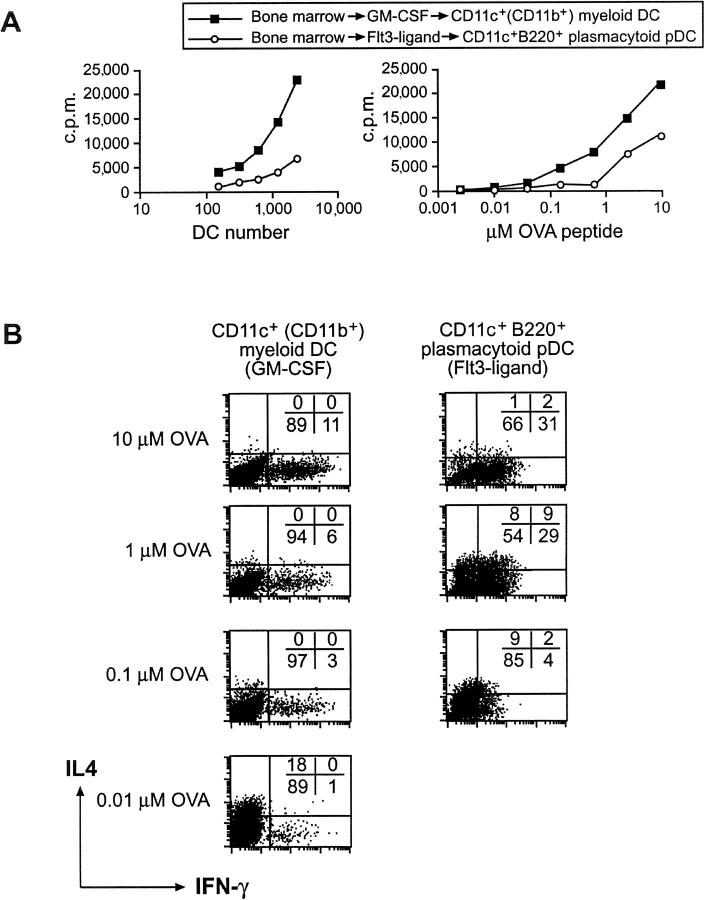
Distinct DC subsets have been suggested to be preprogrammed to direct Th1 or Th2 cell development. Most studies addressing whether DCs have an intrinsic capacity to direct Th1 or Th2 development, or do so as a result of stimulation by microbial products or other environmental signals (12, 14, 17, 19, 23), have been performed with cultured human DC populations including monocyte- or plasmacytoid-derived DCs (5, 17, 23). In this, mouse bone marrow–derived DCs have been studied to a limited extent (13) and no studies have been reported on plasmacytoid-derived DCs with respect to Th cell development. Mouse CD11c+ CD11b+ myeloid DCs were obtained from bone marrow precursors by culturing in GM-CSF (42) and were purified by flow cytometry on the basis of CD11c+ (all were CD11b+). Additionally, plasmacytoid pDCs expressing CD11c+ CD11b− B220+ were purified by flow cytometry after culture of bone marrow cells in the presence of Flt3 ligand (43). Myeloid DCs induced higher proliferation of OVA-specific TCR transgenic CD4+ T cells than plasmacytoid pDCs (Fig. 1 A). This could be explained by the low expression of MHC class II and costimulatory molecules by plasmacytoid pDCs (43 and unpublished data), and may reflect differences in the maturation state of the distinct DC subsets after in vitro culture.
Figure 1.
The differential development of naive CD4+ T cells by bone marrow–derived DC subsets into a Th1 or Th2 phenotype depends on the antigen dose. Bone marrow cells were cultured for 7 d with GM-CSF to generate CD11c+ CD11b+ B220− myeloid DCs or for 10 d with Flt3 ligand to generate CD11c+ CD11b− B220+ plasmacytoid pDCs. (A) Proliferation of CD4+ CD62L+ T cells obtained from DO11.10 mice stimulated by FACS®-purified CD11c+ myeloid DCs or CD11c+ B220+ plasmacytoid pDCs was assessed after 60 h in the presence of 1 μM OVA peptide and varying DC numbers (left) or different OVA peptide doses (right). (B) To assess Th cell development, GM-CSF and Flt3 ligand–generated bone marrow–derived DC subsets were cocultured with naive DO11.10 CD4+ T cells in the presence of 10, 1, 0.1, or 0.01 μM OVA peptide. After 9 d, cells were washed, counted, and restimulated with PMA and ionomycin. The cytokine profile was determined by intracellular cytokine staining for IL-4 and IFN-γ using flow cytometry.
To investigate the ability of myeloid or plasmacytoid pDCs isolated from bone marrow to induce Th cell development, we stimulated naive OVA-specific TCR transgenic CD4+ T cells using the distinct DC populations at different doses of OVA peptide. Myeloid DCs generated from bone marrow with GM-CSF induced Th1 cell development (11% IFN-γ+ and 0% IL-4+ cells; Fig. 1 B) in the presence of a high antigen dose (10 μM OVA peptide). Lower OVA peptide concentrations resulted in reduced Th1 cell development and eventually a weak Th2 response (18% IL-4+ and 1% IFN-γ+ cells) at 0.01 μM OVA peptide. Similarly, plasmacytoid pDCs generated from bone marrow with Flt3 ligand induced the most pronounced Th1 phenotype in cultures containing high doses of OVA peptide (31% IFN-γ+ and 1% IL-4+ cells). Activation of Th cells with plasmacytoid pDCs at 1 μM OVA peptide led to a mixed Th1/Th2 phenotype (29% IFN-γ+, 9% IFN-γ+/IL-4+, and 8% IL-4+). At 0.1 μM OVA peptide, a weak Th2 development was observed (9% IL-4+ and 4% IFN-γ+). Plasmacytoid pDCs induced poor proliferation of Th cells at 0.01 μM OVA and consequently did not induce Th cell polarization. The absolute dose of antigen directing Th1 or Th2 cell development could vary slightly between experiments, but the trend was always the same. The ability of the antigen dose to affect Th cell development (23, 37–40) could provide an explanation as to why different DC subsets have been suggested to intrinsically direct Th1 or Th2 development (5–8), particularly when measured in a mixed lymphocyte response where the exact dose of antigen cannot be established. Distinct DC subsets at different stages in maturation may express different levels of MHC class II and costimulatory molecules, or different amounts of MHC class II peptide on their surface as a result of differential processing, resulting in a different effective antigen dose leading to altered Th cell development.
Th1 Cell Development Is Enhanced at Medium to Low Antigen Doses Using Bone Marrow–derived Myeloid DCs Stimulated with LPS or Bone Marrow–derived Plasmacytoid pDCs Stimulated with CpG.
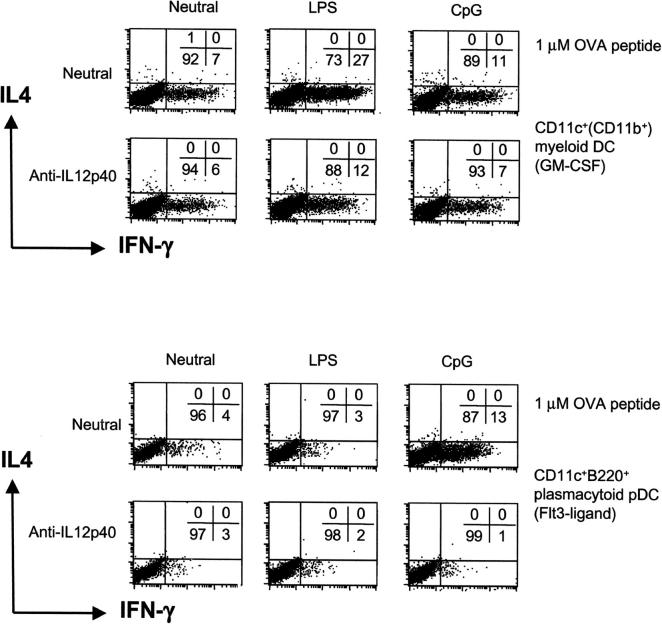
To determine whether any pathogen-derived products could affect the ability of bone marrow–derived myeloid or plasmacytoid pDCs to direct Th cell development at medium to low antigen doses, we performed experiments in the presence of the TLR4 ligand LPS (46, 47) and the TLR9 ligand CpG (48). Here, we show that myeloid DC stimulated with LPS induced the development of Th1 cells (from 7 to 27% IFN-γ+ and 0% IL-4+; Fig. 2 , top) at 1 μM OVA, as previously shown (13). This is partly blocked by an mAb to IL-12p40 (Fig. 2). CpG stimulation of myeloid DCs weakly enhanced Th1 cell development. On the other hand, plasmacytoid pDCs generated from bone marrow with Flt3 ligand stimulated with CpG greatly enhanced T cell proliferation at 1 μM OVA peptide and induced strong Th1 cell development (from 4% IFN-γ+ to 13%; Fig. 2, bottom), but were unresponsive to LPS to induce T cell proliferation (not depicted) or Th1 development (Fig. 2). The CpG-induced Th1 cell development was completely inhibited by anti–IL-12p40 mAb. At high antigen dose, myeloid and plasmacytoid pDC–directed Th1 development was not additionally enhanced by LPS nor CpG (not depicted). However, low dose antigen–induced Th2 development was overridden by LPS and CpG, respectively, resulting in Th1 development (not depicted). Thus, myeloid DCs can respond to the TLR4 ligand LPS and to a small extent the TLR9 ligand CpG, whereas bone marrow–derived plasmacytoid pDCs can respond to CpG but not LPS to direct Th1 cell development. It is possible that this differential responsiveness to LPS and CpG reflects differential expression of TLR4 and TLR9.
Figure 2.
Myeloid and plasmacytoid bone marrow–derived DCs induce Th1 development with CpG whereas only myeloid DCs are responsive to LPS. FACS®-purified bone marrow–derived DCs were cultured with 1 μM OVA and CD4+ Mel14+ T cells for 9 d under neutral conditions with LPS or CpG. To some cultures, anti–IL-12p40 mAbs were added. At day 9, the percentage of IL-4 and IFN-γ+ T cells was determined by flow cytometry after restimulation with PMA and ionomycin.
Splenic DC Subsets Induce Distinct Th Cell Phenotypes Depending on the Antigen Dose.
The classical splenic CD11c+ CD8α+ CD11b− and CD11c+ CD8α− CD11b+ DC subsets have been shown to direct Th1 or Th2 responses in vivo, respectively (6, 8), but these DCs had been subjected to maturation in vitro (overnight culture in GM-CSF; reference 8) or in vivo (Flt3 ligand administration; reference 6). Furthermore, both subsets of splenic DC, when freshly isolated in vitro, could direct Th1 development (49). The ability of the more recently described CD11cdull Gr1+ B220+ spleen plasmacytoid pDC (50–52) to direct Th cell development has not yet been assessed. In addition, the functional relationship between cultured and lymphoid tissue DCs is unknown and functional similarities to human DCs have not been considered. To this end, we determined the effects of antigen dose and microbial products on the ability of freshly isolated spleen DC subsets to induce Th cell development.
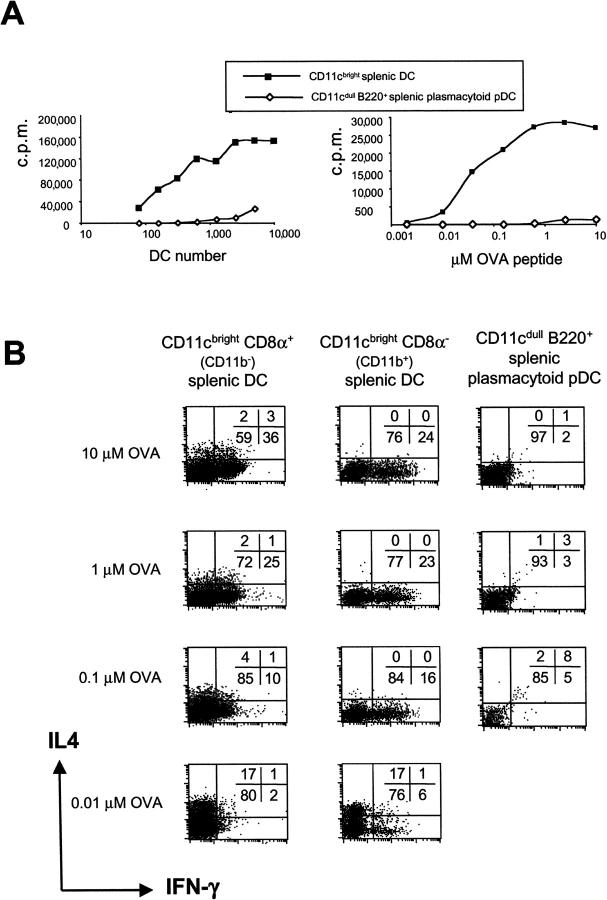
As previously described, freshly isolated and FACS®-purified splenic classical CD11c+ DCs stimulated very significant T cell proliferation. In contrast, splenic plasmacytoid pDCs stimulated very weak to no proliferation of CD4+ T cells (Fig. 3 A), which could be explained by their low expression of costimulatory (CD40, CD80, CD86, and intercellular adhesion molecule 1) and MHC II molecules (50). Again, this could reflect different relative levels of maturation of classical CD11cbright and plasmacytoid pDCs.
Figure 3.
Splenic DC subsets direct Th1 or Th2 cell development of naive CD4+ T cells depending on the antigen dose. (A) Proliferation of CD4+ CD62L+ T cells from DO11.10 mice stimulated by FACS®-purified CD11c+ CD8α+, CD11c+ CD8α− DCs, or splenic B220+ plasmacytoid pDCs was assessed after 60 h in the presence of 1 μM OVA peptide and varying numbers of DCs (left) or titration of the dose of OVA peptide (right). (B) To assess Th cell development, splenic DC subsets were cocultured with naive CD4+ T cells from DO11.10 mice in the presence of the 10, 1, 0.1, or 0.01 μM OVA peptide. After 9 d, cells were washed, counted, and restimulated with PMA and ionomycin. The cytokine profile was determined by intracellular cytokine staining for IL-4 and IFN-γ.
Titration of the antigen had a similar effect on the ability of CD11c+ CD8α+ CD11b− and CD11c+ CD8α− CD11b+ DCs to induce Th cell development (Fig. 3 B). At high OVA peptide doses, both CD8α+ CD11b− and CD8α− CD11b+ DCs induced Th1 cell development. Gradual lowering of the antigen dose from 10 to 0.01 μM OVA peptide resulted in reduced Th1 cell development and the induction of a Th2 phenotype at a low antigen dose (Fig. 3 B). CD8α− CD11b+ DCs generally induced a weaker Th1 phenotype than CD8α+ CD11b− DCs (24 vs. 36% IFN-γ+ cells, respectively). Again, the absolute level and number of IFN-γ–producing cells varied between experiments although the trend always remained the same. The strong Th1 cell development observed at high antigen doses may result from higher up-regulation of CD40 ligand on T cells (15, 16, 53), leading to higher levels of IL-12 or other Th1-inducing factors. In this respect, anti–IL-12p40 mAb only partially blocked Th1 cell development seen at high antigen doses (not depicted), which suggests the involvement of other Th1-inducing factors (54).
Splenic plasmacytoid pDCs could not trigger Th cell development at any antigen dose used, probably because they did not induce CD4+ T cell proliferation and led to very low T cell recoveries in culture as a result of low expression of MHC class II, CD40, and other costimulatory molecules. The proliferation of T cells induced by splenic B220+ plasmacytoid pDCs (freshly isolated at 4°C) was lower than that of their B220+ counterpart cultured from bone marrow with Flt3 ligand (Fig. 1 A), which perhaps received some maturation signals during culture at 37°C, e.g., stromal cell–derived signals. This suggests that the freshly isolated splenic plasmacytoid pDCs are less mature and therefore unable to induce T cell proliferation and drive Th cell development even at high antigen doses.
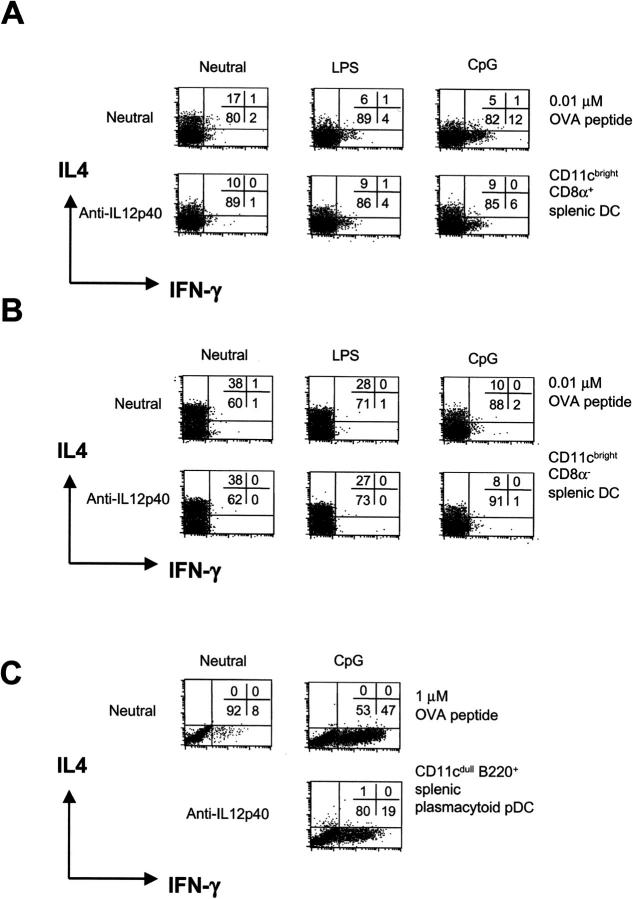
CpG, but not LPS, Enhanced Th1 Cell Development upon Stimulation of Splenic CD8α+ CD11b− DCs and Plasmacytoid pDCs.
Microbial products were examined for their effect on Th cell development driven by splenic DC subsets. CpG could induce some Th1 cell development with CD8α+ CD11b− DCs at 0.01 μM OVA peptide (increase from 2 to 12% IFN-γ+ cells), which could partly be blocked by anti–IL-12p40 mAb (Fig. 4) . This was not observed with CD8α− CD11b+ DCs. However, stimulation with LPS or CpG of both CD8α+ CD11b− and CD8α− CD11b+ DCs resulted in reduced Th2 cell development and some T cell proliferation (not depicted).
Figure 4.
Plasmacytoid pDCs and splenic CD8α+, but not CD8α−, DCs direct Th1 cell development with CpG. Classical splenic CD11c+ CD8α+ and CD11c+ CD8α− DCs were cultured with 0.01 μM OVA peptide (A and B) or CD11cdull B220+ plasmacytoid pDCs with 1 μM OVA peptide (C) in cocultures with CD4+ Mel14+ T cells for 9 d under neutral conditions with LPS or CpG DNA. To neutralize endogenous IL-12, anti–IL-12p40 mAb was added to some cultures. After 9 d, the cytokine profile was determined by flow cytometry.
Splenic B220+ plasmacytoid pDCs cultured in the presence of CpG induced expression of MHC class II, costimulators, and CD8α (50), and an 18-fold enhancement of CD4+ T cell proliferation (not depicted), suggesting their development into mature DCs. CpG-stimulated plasmacytoid-derived DCs induced strong Th1 cell development at 1 μM OVA peptide (and 10 μM; not depicted), which could be partly blocked with anti–IL-12p40 mAb. The partial block of Th1 cell development by anti–IL-12p40 mAb, which neutralizes both IL-12 and IL-23 activity (55), suggests the possible involvement of other cytokines in Th1 cell development (for review see 54). Again, similarly to plasmacytoid pDCs isolated from bone marrow, LPS had no effect on spleen plasmacytoid pDCs to induce CD4+ T cell proliferation or Th1 cell development (not depicted).
Differential Expression of TLR on Mouse DC Subsets.
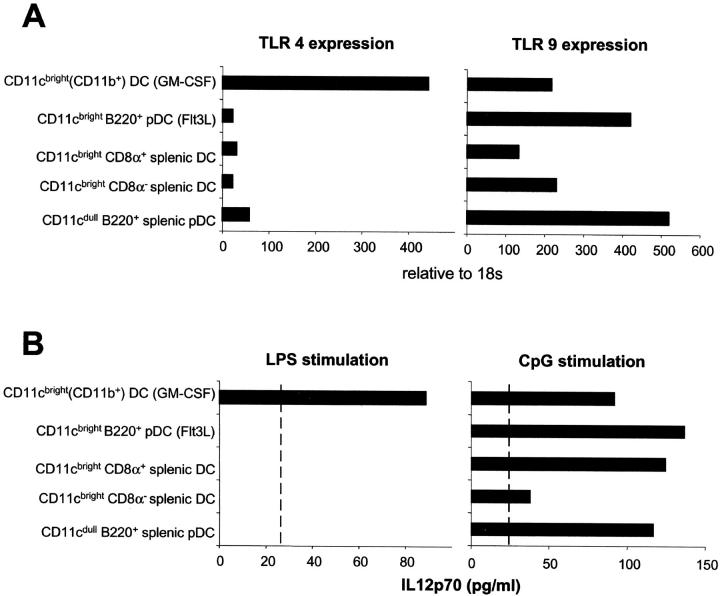
The differential responses to LPS and CpG of the bone marrow–derived myeloid and plasmacytoid pDCs as well as of spleen DC subsets prompted us to examine the expression of their respective TLRs, TLR4 and TLR9. In keeping with the functional data, TLR4 is expressed at high levels on bone marrow–derived myeloid DCs but at low levels on plasmacytoid pDCs and classical splenic DC subsets (Fig. 5 A). In contrast, TLR9 is highly expressed on bone marrow–derived and spleen plasmacytoid pDCs and also to a lesser extent on classical spleen DCs and GM-CSF–cultured myeloid DCs. This differential expression of TLR4 and TLR9 was reflected by their ability to produce IL-12p70 in response to their respective ligands (Fig. 5 B). LPS stimulated bone marrow–derived myeloid DCs expressing TLR4, but not the other subsets, to produce IL-12p70. In contrast, CpG stimulated IL-12p70 production from plasmacytoid pDCs but also by myeloid DCs and spleen CD8α+ DCs, which all expressed TLR9.
Figure 5.
Quantitative real time PCR analysis of TLR4 and TLR9 expression on bone marrow–derived and splenic DC subsets. (A) Expression is relative to 18s. (B) 7 × 104 sorted DCs were stimulated in a volume of 200 μl with medium, LPS, or CpG for 24 h. IL-12p70 levels were determined by immunoassay. The sensitivity of the ELISA was 25 pg/ml.
Concluding Remarks.
We have shown that cultured bone marrow–derived DCs or lymphoid tissue–derived spleen DCs are remarkably flexible in their ability to direct the development of Th cells with distinct cytokine profiles. One major factor in determining this plasticity is the dose of antigen. High dose of antigen generally induced the development of Th1 responses whereas low dose favored Th2 cell development, regardless of the DC subset. Th1 cell development induced with a high antigen dose may reflect a high level of CD40 ligand expression on the T cell that may result in enhanced CD40 signaling (53), leading to higher levels of IL-12 induction.
Mouse plasmacytoid pDCs enhanced CD4+ T cell proliferation and induced strong Th1 cell development when activated with the TLR9 ligand CpG, but not with the TLR4 ligand LPS. Myeloid DCs cultured with GM-CSF, on the other hand, drive Th1 cell development after LPS stimulation. This is explained by our observations that plasmacytoid pDCs express high levels of TLR9 and low levels of TLR4 whereas only GM-CSF–cultured myeloid DCs express TLR4. These results are in good agreement with published reports of human DC subsets with respect to TLR expression and DC function. Specifically, human monocyte–derived DCs express TLR4 and plasmacytoid pDCs express TLR9 (33–35). However, it should be noted that mouse TLR9 expression is not restricted to plasmacytoid pDCs but is expressed more broadly on other DC subsets. The differential expression of TLR4 and TLR9 on distinct DC subsets will determine whether they will be triggered by specific pathogen products to make IL-12 or other appropriate factors that direct Th1 cell development. Interestingly, at high antigen dose, Th1 cell development was not enhanced by LPS nor CpG-stimulated DCs, possibly because the DCs may already be producing maximal levels of Th1-inducing cytokines as a result of their strong interaction with T cells. At lower antigen dose, when Th1 development was less pronounced, and even when Th2 development was observed, LPS or CpG-stimulated DCs did direct Th1 development.
In conclusion, the net effect of the dose of antigen, the state of maturation of the DC, and of environmental signals will determine whether naive CD4+ T cells develop into Th1 or Th2 cells. These “plastic” properties of the DC are in keeping with its role as a sensor to direct appropriate responses in order to eradicate pathogens with minimum pathology to the host.
Acknowledgments
We would like to thank Drs. Paulo Vieira and Joao Pedro Pereira for helpful comments during this study, and Drs. B. (Gitta) Stockinger and Pedro L. Vieira for suggestions on the manuscript. We are grateful to Dr. Jim Cupp, John Shoemaker, Chris Atkins, and Mary Holman for their excellent assistance in obtaining sorted DC subsets and T cells.
This study was supported by the Schering-Plough Corporation and the Medical Research Council, United Kingdom.
A. Boonstra and A. O'Garra were previously at DNAX Research Institute.
Y.-J. Liu's present address is Department of Immunology, The University of Texas, MD Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030.
Footnotes
*
Abbreviations used in this paper: DC, dendritic cell; pDC, precursor DC; TLR, Toll-like receptor.
References
- 1.Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. [DOI] [PubMed] [Google Scholar]
- 2.Shortman, K., and Y.J. Liu. 2002. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2:151–161. [DOI] [PubMed] [Google Scholar]
- 3.Steinman, R.M. 1991. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 9:271–296. [DOI] [PubMed] [Google Scholar]
- 4.Lanzavecchia, A., and F. Sallusto. 2001. Regulation of T cell immunity by dendritic cells. Cell. 106:263–266. [DOI] [PubMed] [Google Scholar]
- 5.Rissoan, M.-C., V. Soumelis, N. Kadowaki, G. Grouard, F. Briere, R. de Waal Malefyt, and Y.-J. Liu. 1999. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 283:1183–1186. [DOI] [PubMed] [Google Scholar]
- 6.Pulendran, B., J.L. Smith, G. Caspary, K. Brassel, D. Pettit, E. Maraskovsky, and C.R. Maliszewski. 1999. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc. Natl. Acad. Sci. USA. 96:1036–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwasaki, A., and B.L. Kelsall. 2001. Unique functions of CD11b+, CD8 alpha+, and double-negative Peyer's patch dendritic cells. J. Immunol. 166:4884–4890. [DOI] [PubMed] [Google Scholar]
- 8.Maldonado-Lopez, R., T. De Smedt, P. Michel, J. Godfroid, B. Pajak, C. Heirman, K. Thielemans, O. Leo, J. Urbain, and M. Moser. 1999. CD8alpha+ and CD8alpha− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 189:587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pulendran, B., P. Kumar, C.W. Cutler, M. Mohamadzadeh, T. Van Dyke, and J. Banchereau. 2001. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J. Immunol. 167:5067–5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulz, O., A.D. Edwards, M. Schito, J. Aliberti, S. Manickasingham, A. Sher, and C. Reis e Sousa. 2000. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 13:453–462. [DOI] [PubMed] [Google Scholar]
- 11.Hochrein, H., M. O'Keeffe, T. Luft, S. Vandenabeele, R.J. Grumont, E. Maraskovsky, and K. Shortman. 2000. Interleukin (IL)-4 is a major regulatory cytokine governing bioactive IL-12 production by mouse and human dendritic cells. J. Exp. Med. 192:823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vieira, P.L., E.C. de Jong, E.A. Wierenga, M.L. Kapsenberg, and P. Kalinski. 2000. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J. Immunol. 164:4507–4512. [DOI] [PubMed] [Google Scholar]
- 13.Whelan, M., M.M. Harnett, K.M. Houston, V. Patel, W. Harnett, and K.P. Rigley. 2000. A filarial nematode-secreted product signals dendritic cells to acquire a phenotype that drives development of Th2 cells. J. Immunol. 164:6453–6460. [DOI] [PubMed] [Google Scholar]
- 14.Macatonia, S.E., N.A. Hosken, M. Litton, P. Vieira, C.-S. Hsieh, J. Culpepper, M. Wysocka, G. Trinchieri, K.M. Murphy, and A. O'Garra. 1995. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J. Immunol. 154:5071–5079. [PubMed] [Google Scholar]
- 15.Cella, M., D. Scheidegger, K. Palmer-Lehmann, P. Lane, A. Lanzavecchia, and G. Alber. 1996. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T–T help via APC activation. J. Exp. Med. 184:747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koch, F., U. Stanzl, P. Jennewein, K. Janke, C. Heufler, E. Kampgen, N. Romani, and G. Schuler. 1996. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J. Exp. Med. 184:741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalinski, P., C.M. Hilkens, E.A. Wierenga, and M.L. Kapsenberg. 1999. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol. Today. 20:561–567. [DOI] [PubMed] [Google Scholar]
- 18.de Jong, E.C., P.L. Vieira, P. Kalinski, J.H. Schuitemaker, Y. Tanaka, E.A. Wierenga, M. Yazdanbakhsh, and M.L. Kapsenberg. 2002. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse Th cell-polarizing signals. J. Immunol. 168:1704–1709. [DOI] [PubMed] [Google Scholar]
- 19.Hochrein, H., K. Shortman, D. Vremec, B. Scott, P. Hertzog, and M. O'Keeffe. 2001. Differential production of IL-12, IFN-alpha, and IFN-gamma by mouse dendritic cell subsets. J. Immunol. 166:5448–5455. [DOI] [PubMed] [Google Scholar]
- 20.Aliberti, J., C. Reis e Sousa, M. Schito, S. Hieny, T. Wells, G.B. Huffnagle, and A. Sher. 2000. CCR5 provides a signal for microbial induced production of IL-12 by CD8 alpha+ dendritic cells. Nat. Immunol. 1:83–87. [DOI] [PubMed] [Google Scholar]
- 21.Edwards, A.D., S.P. Manickasingham, R. Sporri, S.S. Diebold, O. Schulz, A. Sher, T. Kaisho, S. Akira, and C. Reis e Sousa. 2002. Microbial recognition via Toll-like receptor-dependent and -independent pathways determines the cytokine response of murine dendritic cell subsets to CD40 triggering. J. Immunol. 169:3652–3660. [DOI] [PubMed] [Google Scholar]
- 22.Cella, M., F. Facchetti, A. Lanzavecchia, and M. Colonna. 2000. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat. Immunol. 1:305–310. [DOI] [PubMed] [Google Scholar]
- 23.Langenkamp, A., M. Messi, A. Lanzavecchia, and F. Sallusto. 2000. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat. Immunol. 1:311–316. [DOI] [PubMed] [Google Scholar]
- 24.Reis e Sousa, C., G. Yap, O. Schulz, N. Rogers, M. Schito, J. Aliberti, S. Hieny, and A. Sher. 1999. Paralysis of dendritic cell IL-12 production by microbial products prevents infection-induced immunopathology. Immunity. 11:637–647. [DOI] [PubMed] [Google Scholar]
- 25.d'Ostiani, C.F., G. Del Sero, A. Bacci, C. Montagnoli, A. Spreca, A. Mencacci, P. Ricciardi-Castagnoli, and L. Romani. 2000. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J. Exp. Med. 191:1661–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bozza, S., R. Gaziano, A. Spreca, A. Bacci, C. Montagnoli, P. di Francesco, and L. Romani. 2002. Dendritic cells transport conidia and hyphae of Aspergillus fumigatus from the airways to the draining lymph nodes and initiate disparate Th responses to the fungus. J. Immunol. 168:1362–1371. [DOI] [PubMed] [Google Scholar]
- 27.Maldonado-Lopez, R., C. Maliszewski, J. Urbain, and M. Moser. 2001. Cytokines regulate the capacity of CD8alpha(+) and CD8alpha(−) dendritic cells to prime Th1/Th2 cells in vivo. J. Immunol. 167:4345–4350. [DOI] [PubMed] [Google Scholar]
- 28.Kalinski, P., J.H. Schuitemaker, C.M. Hilkens, and M.L. Kapsenberg. 1998. Prostaglandin E2 induces the final maturation of IL-12-deficient CD1a+CD83+ dendritic cells: the levels of IL-12 are determined during the final dendritic cell maturation and are resistant to further modulation. J. Immunol. 161:2804–2809. [PubMed] [Google Scholar]
- 29.Gagliardi, M.C., F. Sallusto, M. Marinaro, A. Langenkamp, A. Lanzavecchia, and M.T. De Magistris. 2000. Cholera toxin induces maturation of human dendritic cells and licences them for Th2 priming. Eur. J. Immunol. 30:2394–2403. [DOI] [PubMed] [Google Scholar]
- 30.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675–680. [DOI] [PubMed] [Google Scholar]
- 31.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135–145. [DOI] [PubMed] [Google Scholar]
- 32.Rock, F.L., G. Hardiman, J.C. Timans, R.A. Kastelein, and J.F. Bazan. 1998. A family of human receptors structurally related to Drosophila Toll. Proc. Natl. Acad. Sci. USA. 95:588–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kadowaki, N., S. Ho, S. Antonenko, R.W. Malefyt, R.A. Kastelein, F. Bazan, and Y.J. Liu. 2001. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194:863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hornung, V., S. Rothenfusser, S. Britsch, A. Krug, B. Jahrsdorfer, T. Giese, S. Endres, and G. Hartmann. 2002. Quantitative expression of Toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 168:4531–4537. [DOI] [PubMed] [Google Scholar]
- 35.Jarrossay, D., G. Napolitani, M. Colonna, F. Sallusto, and A. Lanzavecchia. 2001. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur. J. Immunol. 31:3388–3393. [DOI] [PubMed] [Google Scholar]
- 36.Wagner, H. 2001. Toll meets bacterial CpG-DNA. Immunity. 14:499–502. [DOI] [PubMed] [Google Scholar]
- 37.Constant, S., C. Pfeiffer, A. Woodard, T. Pasqualini, and K. Bottomly. 1995. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J. Exp. Med. 182:1591–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hosken, N.A., K. Shibuya, A.W. Heath, K.M. Murphy, and A. O'Garra. 1995. The effect of antigen dose on CD4+ T cell phenotype development in an αβ-TCR–transgenic mouse model. J. Exp. Med. 182:1579–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruedl, C., P. Koebel, M. Bachmann, M. Hess, and K. Karjalainen. 2000. Anatomical origin of dendritic cells determines their life span in peripheral lymph nodes. J. Immunol. 165:4910–4916. [DOI] [PubMed] [Google Scholar]
- 40.Constant, S.L., and K. Bottomly. 1997. Induction of the Th1 and Th2 CD4+ T cell responses: alternative approaches. Annu. Rev. Immunol. 15:297–322. [DOI] [PubMed] [Google Scholar]
- 41.Murphy, K.M., A.B. Heimberger, and D.Y. Loh. 1990. Induction by antigen of intrathymic apoptosis of CD4+ CD8+TCRlo thymocytes in vivo. Science. 250:1720–1723. [DOI] [PubMed] [Google Scholar]
- 42.Inaba, K., M. Inaba, N. Romani, H. Aya, M. Deguchi, S. Ikehara, S. Muramatsu, and R.M. Steinman. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176:1693–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gilliet, M., A. Boonstra, C. Paturel, S. Antonenko, X.L. Xu, G. Trinchieri, A. O'Garra, and Y.J. Liu. 2002. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 195:953–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bradley, L.M., D.D. Duncan, S. Tonkonogy, and S.L. Swain. 1991. Characterization of antigen-specific CD4+ effector T cells in vivo: immunization results in a transient population of MEL-14-, CD45RB-helper cells that secretes interleukin 2 (IL-2), IL-3, IL-4, and interferon gamma. J. Exp. Med. 174:547–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Openshaw, P., E. Murphy, N.A. Hosken, V. Maino, K. Davis, K. Murphy, and A. O'Garra. 1995. Heterogeneity of intracellular cytokine synthesis at the single cell level in polarized T helper 1 and T helper 2 populations. J. Exp. Med. 182:1357–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poltorak, A., X. He, I. Smirnova, M.Y. Liu, C.V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, et al. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 282:2085–2088. [DOI] [PubMed] [Google Scholar]
- 47.Qureshi, S.T., L. Lariviere, G. Leveque, S. Clermont, K.J. Moore, P. Gros, and D. Malo. 1999. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J. Exp. Med. 189:615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, et al. 2000. A Toll-like receptor recognizes bacterial DNA. Nature. 408:740–745. [DOI] [PubMed] [Google Scholar]
- 49.Iwasaki, A., and B.L. Kelsall. 1999. Freshly isolated Peyer's patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J. Exp. Med. 190:229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Asselin-Paturel, C., A. Boonstra, M. Dalod, I. Durand, N. Yessaad, C. Dezutter-Dambuyant, A. Vicari, A. O'Garra, C. Biron, F. Briere, et al. 2001. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2:1144–1150. [DOI] [PubMed] [Google Scholar]
- 51.Bjorck, P. 2001. Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte-macrophage colony-stimulating factor-treated mice. Blood. 98:3520–3526. [DOI] [PubMed] [Google Scholar]
- 52.Nakano, H., M. Yanagita, and M.D. Gunn. 2001. CD11c(+)B220(+)Gr-1(+) cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J. Exp. Med. 194:1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruedl, C., M.F. Bachmann, and M. Kopf. 2000. The antigen dose determines T helper subset development by regulation of CD40 ligand. Eur. J. Immunol. 30:2056–2064. [DOI] [PubMed] [Google Scholar]
- 54.Robinson, D.S., and A. O'Garra. 2002. Further checkpoints in Th1 development. Immunity. 16:755–758. [DOI] [PubMed] [Google Scholar]
- 55.Oppmann, B., R. Lesley, B. Blom, J.C. Timans, Y. Xu, B. Hunte, F. Vega, N. Yu, J. Wang, K. Singh, et al. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 13:715–725. [DOI] [PubMed] [Google Scholar]