Complementary Signaling through flt3 and Interleukin-7 Receptor α Is Indispensable for Fetal and Adult B Cell Genesis (original) (raw)
Abstract
Extensive studies of mice deficient in one or several cytokine receptors have failed to support an indispensable role of cytokines in development of multiple blood cell lineages. Whereas B1 B cells and Igs are sustained at normal levels throughout life of mice deficient in IL-7, IL-7Rα, common cytokine receptor gamma chain, or flt3 ligand (FL), we report here that adult mice double deficient in IL-7Rα and FL completely lack visible LNs, conventional IgM+ B cells, IgA+ plasma cells, and B1 cells, and consequently produce no Igs. All stages of committed B cell progenitors are undetectable in FL−/− × IL-7Rα−/− BM that also lacks expression of the B cell commitment factor Pax5 and its direct target genes. Furthermore, in contrast to IL-7Rα−/− mice, FL−/− × IL-7Rα−/− mice also lack mature B cells and detectable committed B cell progenitors during fetal development. Thus, signaling through the cytokine tyrosine kinase receptor flt3 and IL-7Rα are indispensable for fetal and adult B cell development.
Keywords: lymphopoiesis, IL-7 receptor, Flt3 ligand, Pax5, B1 cells
Introduction
Hematopoiesis is a tightly regulated process in which multipotent hematopoietic stem cells commit and differentiate along a number of specific pathways to continuously replenish cells of all blood lineages (1). Several lineage restricted hematopoietic growth factors (cytokines) and their corresponding receptors promote the development of specific blood cell lineages in vitro and in vivo (2–5). However, targeted deletions of most of these cytokine receptors or their ligands have resulted in surprisingly mild phenotypes and rarely in complete losses of specific blood cell lineages (3, 6–12). Furthermore, since the physiological functions of most blood lineages remain intact at reduced cell numbers, such mutations frequently have limited or no functional consequences. For instance, although thrombopoietin-deficient mice have an 85% reduction in platelets, they have no bleeding disorder (13).
As a result of these observations, the concept and controversy of cytokine redundancy has emerged, implicating that many lineage-restricted cytokines may play a limited role in the regulation of hematopoiesis. This latter point also raises the possibility that combinatorial interactions between two or more cytokines may frequently be required for development of specific blood cell lineages (3). The last possibility has already provoked extensive research but so far provided limited evidence for the requirement of such in vivo synergy between lineage-restricted cytokines and, for instance, in myeloid development failed to support an absolute combinatorial cytokine requirement for the formation of both platelets and granulocytes (14–24).
Several cytokines have been implicated to be involved in the regulation of B cell development (25). Of these, IL-7 has emerged as a key cytokine involved in B cell genesis (9, 26, 27). However, recent studies have demonstrated that although B cell development is almost completely arrested at a very early stage in the BM of adult IL-7 and IL-7Rα–deficient mice, a pool of mature B cells, primarily of fetal and perinatal origin, is sustained and stable throughout adult life, capable of producing normal levels of Igs (28–30). Furthermore, the compartment of self-replenishing B1 cells is normal in IL-7–deficient mice and has therefore been suggested to be IL-7 independent (28).
Whether IL-7 in combination with other cytokines might be indispensable for B cell genesis has been thoroughly investigated in mice with targeted deletions of the common γ receptor chain (γc), essential for signaling by six lymphokines (IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21). However, γc−/− mice have a B cell phenotype corresponding closely to that of IL-7Rα−/− mice (31, 32), suggesting that among known lymphokines, only IL-7 is critically involved in B cell genesis and that the other lymphokines using γc may not be required for B cell development.
Unlike lineage-restricted cytokine receptors belonging to the hematopoietin receptor superfamily (33), the fms-like tyrosine kinase-3 (flt3 or flk2) receptor is expressed primarily at early stages of hematopoiesis and in particular early lymphoid development (34). Although flt3 expression is lost at the pre-B cell stage (35) and flt3 ligand (FL)–deficient mice only have marginally reduced mature B cell numbers and normal Ig levels (36, 37), there is ample evidence that flt3 and its ligand indeed play an important role in B cell development. Most importantly, the BM of flk2 and FL-deficient mice have reduced levels of B cell progenitors (36, 38), including the common lymphoid progenitor (CLP) (37). Furthermore, the combined action of FL and IL-7 is both sufficient and strictly required for stroma-independent in vitro B cell development from adult multipotent hematopoietic progenitors (39).
Thus, we hypothesized that rather than a combination of lymphokines, the concerted action of two cytokine receptors—flt3, an early acting tyrosine kinase receptor and the later acting hematopoietin receptor IL-7Rα—might be indispensable for B cell genesis in vivo. Herein we present compelling evidence supporting this hypothesis. During both fetal and adult hematopoiesis, mice deficient in the expression of FL and IL-7Rα completely lack mature conventional IgM+ B cells, IgA+ plasma cells, and B1 B cells, and in striking contrast to FL or IL-7Rα single-deficient mice, fail to produce circulating Ig.
Materials and Methods
Generation of Double Knockout Mice Deficient in FL and IL-7α Expression.
Mice deficient in FL (36) or IL-7Rα (26) expression were generated as described previously. FL−/− mice were on a pure C57BL/6 background (36), whereas IL-7Rα−/− mice had been backcrossed for five generations with C57BL/6 mice. Mice double deficient for FL and IL-7Rα expression were obtained by crossbreeding of single FL−/− and IL-7Rα−/− mice and subsequent interbreeding of heterozygous FL+/− IL-7Rα+/− mice. Mice used for experiments were obtained through breeding of FL−/− × IL-7Rα−/− mice, and C57BL/6 mice were used as WT controls. All mice used for the study were genotyped using PCR with specific primers to verify the absence of FL and IL-7Rα genes (26, 36). All studies of adult B lymphopoiesis were performed on age-matched mice, 8–12 wk old, kept under specific pathogen-free conditions.
For analysis of embryonic development, timed pregnancies were determined as described previously (40); the day the vaginal plug was observed was considered as day 0. All performed experiments were approved by the Ethical Committee at Lund University.
Tissues.
Peripheral blood (PB) was collected from the retro-orbital sinus venous plexus or vena cava into heparinized tubes. White blood cells were isolated using 1% dextran (Amersham Biosciences) solution, and the remaining red cells were lysed using ammonium chloride. BM cell suspensions were prepared by flushing isolated tibiae and femora with PBS (Sigma-Aldrich) containing 5% FCS (BioWhittaker). Cell suspensions were prepared from spleens, mesenteric LNs, and Peyer's patches by gently breaking up the tissues in PBS (Sigma-Aldrich) containing 5% FCS. Lamina propria lymphocytes were isolated as described before (41). Spleens and livers were collected from embryos at day 17–18 of gestation, and cell suspensions were prepared by gently breaking up the tissues in PBS (Sigma-Aldrich) containing 5% FCS.
mAbs and Flow Cytometry Analysis.
The following antibodies were used for flow cytometry: anti-B220 (RA3–6B2), anti-CD19 (1D3), anti-CD5 (53–7.3), anti-CD43 (S7), anti-NK1.1 (PK136), anti-IgM (R6–60.2), anti-CD3e (145–2C11), anti-CD11b (M1/70) (all from Amersham Biosciences), and anti-IgA (11–44–2) (Southern Biotechnology Associates, Inc.). Anti-AA4.1 antibody was a gift from Dr. Ihor Lemischka (Princeton University, Princeton, NJ). Isotype-matched controls used included mouse IgG1, mouse IgG2a, rat IgG2a, rat IgG2b, and hamster IgG labeled with appropriate fluorochromes (BD Biosciences). Up to 106 cells per sample were first incubated in PBS (Sigma-Aldrich) containing 5% FCS (BioWhittaker) and Fc block (CD16/CD32; BD Biosciences) to minimize nonspecific binding. Cells were then labeled with the appropriate antibodies for 15 min at 4°C in PBS containing 5% FCS. Finally, 7AAD was added to exclude dead cells from the analysis. Samples were analyzed on FACSCalibur (Becton Dickinson). Between 50,000 and 400,000 events were collected, and analysis was performed using CellQuest (Becton Dickinson) and FlowJo (Tree Star Inc.) software. All FACS® data were displayed using a log 10 scale.
Ig ELISA.
ELISA analysis was performed according to Engvall and Perlman (42). Briefly, microtiter ELISA plates (Costar Corning 3590) were plated with 100 μl/well of 10 μg/ml catcher antibody in PBS and incubated overnight at 4°C. For blocking of nonspecific binding, the solutions were replaced with 200 μl/well 1% BSA in PBS and incubated overnight at 4°C. The plates were washed three times with 200 μl/well washing buffer (0.05% Tween 20, 0.02% NaN3 in PBS) and then incubated overnight at 4°C with different dilutions of the serum samples (IgM, IgA: 1:50,00 and 1:20,000; IgG: 1:20,000 and 1:80,000). Serum from FL−/− × IL-7Rα−/− mice was in addition diluted at 1:100 and 1:400 for all analysis. The plates were washed three times with 200 μl/well washing buffer and further incubated with 100 μl/well alkaline phosphatase (AP) coupled detecting antibody (IgM: 1.2 μg/ml; IgG: 1.2 μg/ml), or biotinylated anti-IgA (2.5 μg/ml), overnight at 4°C. Only for detection of IgA, the plates were washed three times with 200 μl/well washing buffer and incubated overnight at 4°C with 100 μl/well AP conjugated with streptavidin (1 μg/ml). After washing three times with 200 μl/well washing buffer, 100 μl/well AP developing solution was added (1 M diethanolamine, 4 mM MgCl2, pH 9.8 with freshly added 0.5 mg/ml pNPP [no. O-104; Sigma-Aldrich]). The reaction was developed at room temperature and measured at several time points on an ELISA reader at 415 nm. The following antibodies were used: donkey anti–mouse IgM (low cross reactivity to rat) (Jackson ImmunoResearch Laboratories), donkey anti–mouse IgM AP (low cross reactivity to rat) (Jackson ImmunoResearch Laboratories), donkey anti–mouse IgG (low cross reactivity to rat) (Jackson ImmunoResearch Laboratories), donkey anti–mouse IgG AP (low cross reactivity to rat) (Jackson ImmunoResearch Laboratories), rat anti–mouse IgA (C10–3; BD Biosciences), biotinylated rat anti–mouse IgA (C10–1; BD Biosciences), and AP-conjugated streptavidin (Jackson ImmunoResearch Laboratories).
D-J Rearrangement Analysis.
BM cells were suspended into single cells by gentle mechanic stress and filtering to remove debris. Genomic DNA was prepared using Trizol (GIBCO BRL) according to the manufacturers instructions. D-J rearrangements were then assayed by PCR using a protocol based on that by Schlissel et al. (43). The D-J–rearranged and germline-configurated DNAs were amplified in the same reaction by 30 cycles (94°C for 30 s, 60°C for 45 s, and 72°C for 1 min) using the DH and J3 primer at 1 μM and the Mu0 primer at 0.1 μM final concentration. The Mu0 amplifies together with the J3 primer germline DNA and the aim of using reduced amounts of Mu0 was to reduce the signal from the germline amplification. The PCR products were blotted onto Hybond N+ nylon membranes (Amersham Biosciences) using capillary blotting. Membranes were prehybridized in 5× Denhardt's, 6 × SSPE, 0.1% SDS, and 100 μg/ml Salmon Sperm DNA at 45°C for 60 min and hybridized with a γ-32P–labeled oligonucleotide complementary to the J3 region (JH3) for 12 h at 45°C in the same solution. Membranes were washed at room temperature in 2 × SSC supplemented with 0.1% SDS for 15 min and 0.1 × SSC with 0.1% SDS for 5–10 min. The hybridized membrane was then subject to autoradiography.
The following oligonucleotides were used for PCR: DH, 5′-GGAATTCG(A/C)TTTTTGT(C/G)AAGGGATCTACTACTGTG-3′; J3, 5′-GTCTAGATTCTCACAAGAGTCCGATAGACCCTGG-3′; Mu0, 5′-CCGCATGCCAAGGCTAGCCTGAAAGATTACC-3′. The following oligonucleotides was used for hybridization: JH3, 5′-AGACAGTGACCAGAGTCCCTTGG-3′.
Expression of B Cell Genes.
Total RNA was prepared using RNAzol (Tel-Test) from 10 million BM cells and dissolved in 50 μl. 10 μl was used for cDNA preparation in a total volume of 20 μl using Superscript II and random primers (Life Technologies). 1 μl cDNA was used per PCR reaction, except serial fivefold dilutions for HGPRT starting with 1 μl. The following conditions were used for PCR reactions: HGPRT, 30 cycles at 55°C annealing temperature; all other reactions, 35 cycles at 55–58°C annealing temperature.
The following primer sequences were used: pax-5, sense, 5′-TCCTCGGACCATCAGGACAG-3′ and antisense, 5′-CCTGTTGATGGAGCTGACGC-3′; for mb-1 sense, 5′-GGGGTCTAGAAGCCCTCAGAGCCCTGCCTC-3′ and antisense, 5′-TGCCGAATTCCTGGTAGGTGCCCTGGAGTC-3′; and CD19, sense, 5′-TACCTTAGTAGGAGGCAGGCC-3′ and antisense, 5′-CCGGAACATCTCCCCACTAT-3′.
Statistics.
All results were expressed as means (± SD). The statistical significances between groups were determined using the Student's t test.
Results
Mice Double Deficient in flt3 Ligand and IL-7Rα Expression Are Severely Immune Compromised and Lack Peyer's Patches and Identifiable LNs.
Initially, FL−/− × IL-7Rα−/− mice showed reduced survival compared with either FL−/− or IL-7Rα−/− mice. The reduced survival was most likely due to enhanced susceptibility to opportunistic infections, since breeding and housing of FL−/− × IL-7Rα−/− mice in specific pathogen-free conditions resulted in mice with a normal life span.
No significant differences in BM cellularity were observed between WT, IL-7Rα−/−, FL−/−, or FL−/− × IL-7Rα−/− mice (Table I). In contrast, spleen cellularity in FL−/−, IL-7Rα−/−, and FL−/− × IL-7Rα−/− mice was reduced to 60, 22, and 16%, respectively, of WT controls. The total peripheral white blood cell counts in FL−/− × IL-7Rα−/− mice were more significantly reduced (to 10% of WT mice) compared with either single FL−/− or IL-7Rα−/− mice (Table I). Previous studies have reported reduced cellularity in the LNs and Peyer's Patches of IL-7Rα−/− mice (26, 44). In contrast to IL-7Rα−/− mice in which LNs were present but with reduced cellularity, no peripheral (e.g., para-aortic [Fig. 1] , axial, and inguinal [not depicted]) or mesenteric LNs were identifiable in FL−/− × IL-7Rα−/− mice (Table I and Fig. 1). Similarly, unlike IL-7Rα−/− mice where Peyer's Patches were present at reduced numbers (and cellularity), these could not be detected in FL−/− × IL-7Rα−/− mice (Table I). Collectively, the severe lymphoid hypoplasia detected in FL−/− × IL-7Rα−/− mice suggests a critical and complimentary role of flt3 and IL-7Rα signaling in lymphoid development.
Table I.
Mice Double Deficient in flt3 Ligand and IL-7Rα Expression Have Impaired Development of Lymphoid Tissues
| Tissue | WTCellularity ×106 | FL−/− % WT control | IL-7Rα2/− % WT control | FL−/− × IL-7Rα2/− % WT control |
|---|---|---|---|---|
| BM | 80 (11) | 77 (16) | 99 (7) | 102 (8) |
| Spleen | 187 (39) | 60 (14) | 22 (6) | 16 (3) |
| PB | 6.8 (1.7) (per ml) | 73 (24) | 25 (3) | 10 (3)a |
| cMesenteric LNs | 17 (3) | 78 (19) | 6.3 (1.2) | Lackingb |
| dPeyer's Patches number | 7 (1) | 4 (2) | 2 (1) | Lackingb |
| Peyer's Patches total cellularity | 1.7 (0.6) | 53 (16) | 5.5 (1.5) |
Figure 1.
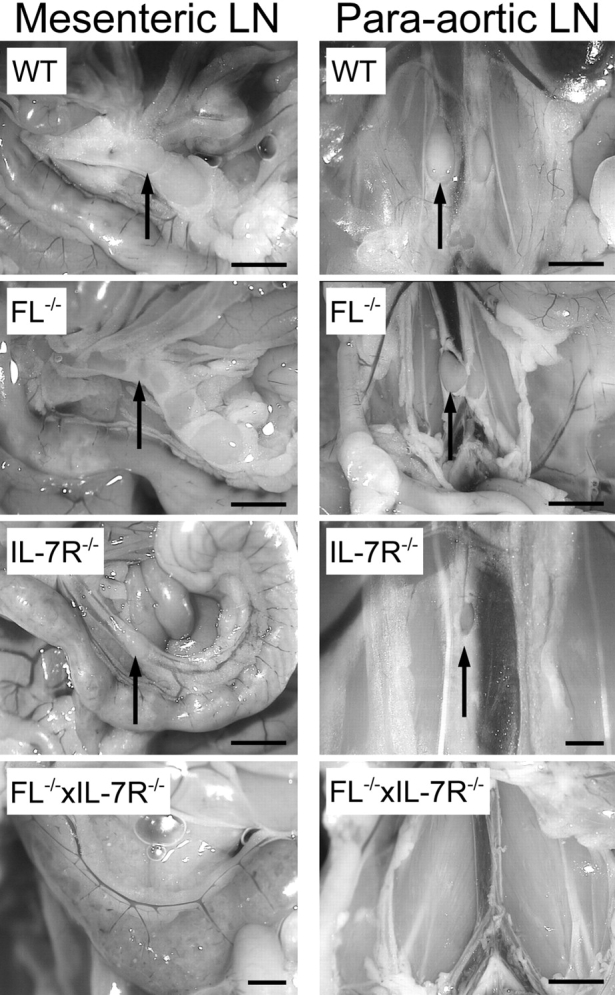
Mice double deficient in flt3 ligand and IL-7Rα expression show impairment in the development of lymphoid tissue. Photographs of mesenteric and para-aortic LNs taken with 2.2 to 3.45 magnification; arrows point to location of LNs. Bar, 2.2 cm.
Absence of Mature IgM+ B Cells in the Blood, Hematopoietic, and Lymphoid Tissues of Adult FL−/− × IL-7Rα−/− Mice.
The absence of identifiable LNs in FL−/− × IL-7Rα−/− mice suggested that the generation of mature B cells may be more severely affected in FL−/− × IL-7Rα−/− than either FL−/− or IL-7Rα−/− mice. Thus, the presence of mature B220+IgM+ B cells in the PB, spleen, and BM of 8–12-wk-old FL−/− × IL-7Rα−/− mice was next investigated (Fig. 2, A and B) . As reported previously (36), B220+IgM+ cells were reduced by 53–75% in FL−/− mice (Fig. 2, A and B), whereas IL-7Rα−/− mice had clearly reduced but detectable B220+IgM+ cells in PB, BM, and spleen in agreement with previous studies (26) (Fig. 2, A and B). In striking contrast, FL−/− × IL-7Rα−/− mice completely lacked mature B220+IgM+ B cells in the PB, spleen, and BM (Fig. 2, A and B).
Figure 2.
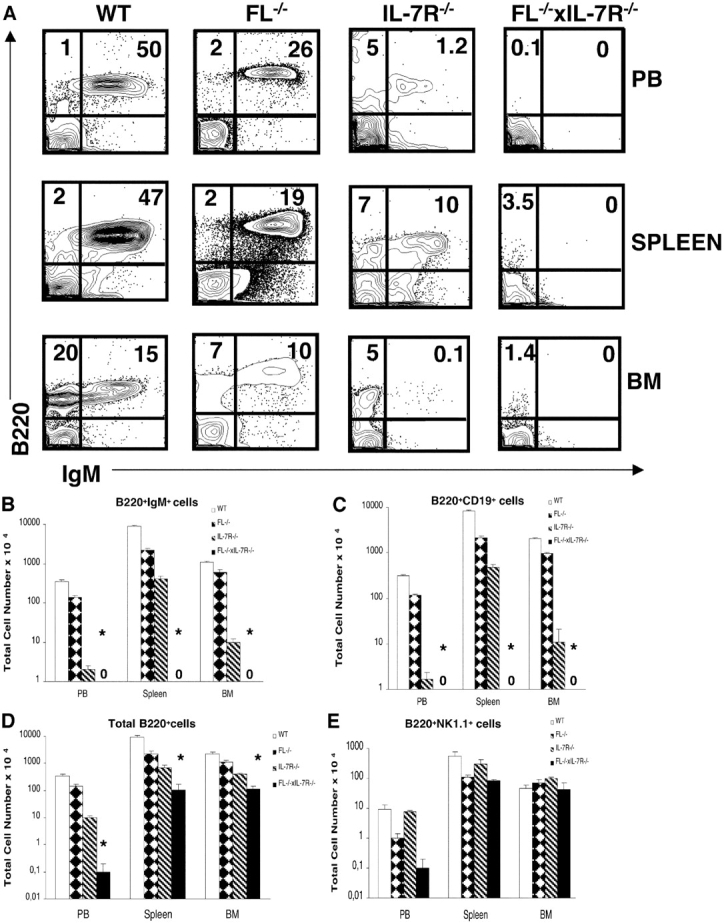
Adult mice double deficient in Flt3 ligand and IL-7Rα expression lack B cells in the PB, spleen, and BM. PB, spleen, and BM cells from 8–12-wk-old mice were stained with mAbs against B220, IgM, CD19, and NK1.1 (as described in Materials and Methods). (A) Plots of B220 and IgM expression in WT, FL-deficient, IL-7Rα–deficient and FL−/− × IL-7Rα−/− double deficient mice, respectively. Numbers represent mean values from 6–12 mice. (B) Total number of B220+ cells coexpressing IgM, B220+ cells expressing CD19 (C), B220+ cells (D), and B220+ cells expressing NK1.1 (E). BM cellularity represents cell counts from both tibiae and femora, whereas total number of white blood cells in PB was calculated per 1 ml. Data in B–E are expressed as mean values (SD) from 6–12 age-matched mice in each group. *Statistically significant differences (P < 0.01) between IL-7Rα−/− and FL−/− × IL-7Rα−/− mice. 0, no B220+IgM+ or B220+CD19+ cells were detected in FL−/− × IL-7Rα−/− mice.
The absence of B cells in FL−/− × IL-7Rα−/− mice was further confirmed by a complete lack of B220+CD19+ cells in PB, spleens, and BM, whereas these were present in IL-7Rα−/− mice (Fig. 2 C). Although present at dramatically reduced levels compared with FL−/− and IL-7Rα−/− mice, B220+ cells were detectable in the PB, spleen, and BM of FL−/− × IL-7Rα−/− mice (Fig. 2 D). Noteworthy, however, most of the rare B220+ cells detected in FL−/− × IL-7Rα−/− mice coexpressed NK1.1 (Fig. 2 E), consistent with the phenotype of NK cell progenitors rather than bona fide B cells (45). Thus, in contrast to either FL−/− or IL-7Rα−/− mice, adult FL−/− × IL-7Rα−/− mice completely lack B cells.
In contrast to the complete absence of mature B cells, the number of PB T (CD3+) cells was only slightly reduced in FL−/− × IL-7Rα−/− mice compared with IL-7Rα−/− mice (Table II), whereas the number of myeloid (CD11b+) cells was comparable to that of FL−/− mice (Table II).
Table II.
Presence of T and Myeloid Cells in Mice Double Deficient in flt3 Ligand and IL-7Rα Expression
| Mouse | Total number of T (CD3+) cellsper 1 ml × 104 | Total number of myeloid (CD11b+) cellsper 1 ml × 104 |
|---|---|---|
| WT | 163 (47) | 100 (30) |
| FL−/− | 220 (13) | 73 (21) |
| IL-7Rα2/− | 21 (8) | 120 (29) |
| FL−/− × IL-7Rα2/− | 12 (6) | 70 (16) |
Absence of IgA+ Plasma Cells in Lamina Propria and Peritoneal B1 Cells in Mice Double Deficient in flt3 Ligand and IL-7Rα Expression.
Since production of IgA has been demonstrated to be regulated by mechanisms distinct from those of IgM (46), FL−/− × IL-7Rα−/− mice were next examined for the presence of IgA+ plasma cells in the lamina propria of the gut (Fig. 3 A).
Figure 3.
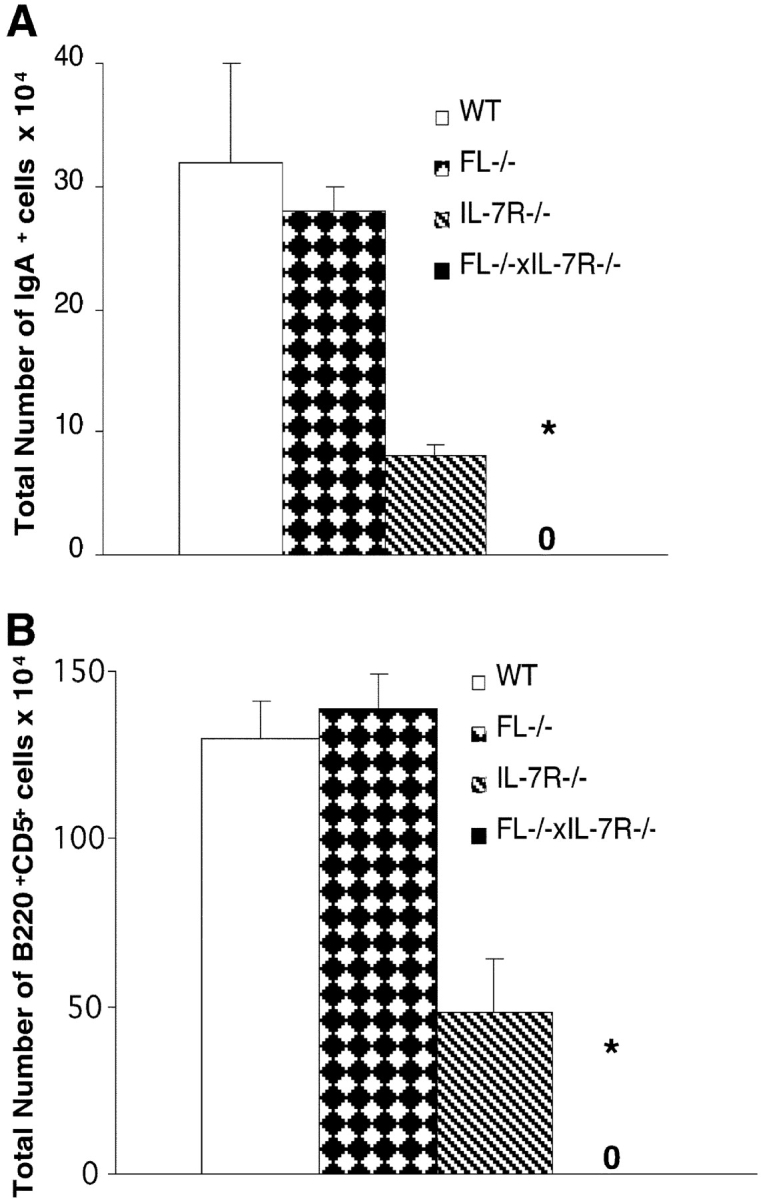
Mice double deficient in Flt3 ligand and IL-7Rα expression have reduced numbers of IgA+ plasma cells and B1 cells. (A). Cells from lamina propria of 8–12-wk-old WT, FL−/−, IL-7Rα−/−, and FL−/− × IL-7Rα−/− mice were stained with mAbs against IgA (as described in Materials and Methods). 0, no IgA+ cells were detected in FL−/− × IL-7Rα−/− mice. (B) Cells from peritoneal lavage of 8–12-wk-old WT, FL−/−, IL-7Rα−/−, and FL−/− × IL-7Rα−/− mice were stained with mAbs against B220 and CD5 (described in Materials and Methods). All data are expressed as mean values (SD) from three to six mice. *Indicates statistically significant differences (P < 0.01) between IL-7Rα−/− and FL−/− × IL-7Rα−/− mice. 0, no B220+CD5+ cells were detected in FL−/− × IL-7Rα−/− mice.
The number of lymphocytes in the lamina propria was reduced by 79 ± 4% in FL−/− × IL-7Rα−/− mice, whereas only a slight reduction in lymphocyte numbers was detected in the lamina propria of FL−/− and IL-7Rα−/− mice (14 ± 12% and 34 ± 4% reduction, respectively). Strikingly, whereas IgA+ plasma cells were present in normal numbers in FL−/− mice and reduced by 75% in IL-7Rα−/− mice, IgA+ plasma cells were completely absent in FL−/− × IL-7Rα−/− mice (Fig. 3 A).
B1a cells, characterized by expression of low levels of B220 and intermediate levels of CD5, represent a subset of self-replenishing B cells that arise early during fetal development before the appearance of conventional B cells (47, 48). Several studies suggest that B1 cells are regulated by mechanisms distinct from those of conventional B cells. This is supported by studies of γc−/− and IL-7−/− mice in which conventional B cells are dramatically reduced, whereas B1 cells are present at near normal levels (28, 31). Whereas the number of peritoneal leukocytes was normal (106 ± 4%) in FL−/− mice and present at almost normal levels (70 ± 10%) in IL-7Rα−/− mice, they were significantly reduced in FL−/− × IL-7Rα−/− mice (27 ± 4%; P < 0.01 compared with IL-7Rα−/−). Whereas the total number of B1a cells was normal in FL−/− mice and reduced by 63% compared to control levels in IL-7Rα−/− mice, they were completely absent in FL−/− × IL-7Rα−/− mice (Fig. 3 B). Thus, signaling through flt3 and IL-7Rα is also critical for generation and/or maintenance of B1a cells.
Mice Double Deficient in flt3 ligand and IL-7Rα Expression Fail To Produce Igs.
Although γc−/− mice and IL-7Rα−/− mice have reduced numbers of conventional B cells, these mice have normal levels of circulating Ig (30, 31). This could reflect that residual numbers of conventional B cells and/or normal numbers of B1 cells (known to produce all Ig types) (47) are capable of sustaining Ig production in these mice. Thus, we next examined the levels of production of IgM, IgG, and IgA in the serum of either FL−/−, IL-7Rα−/−, or FL−/− × IL-7Rα−/− mice by a sensitive ELISA assay. In contrast to FL−/− or IL-7Rα−/− mice, which produce normal levels of serum IgM and IgG, absolutely no IgM or IgG was detectable in the serum of FL−/− × IL-7Rα−/− mice (Table III). Similarly, IgA, which was present at slightly reduced levels in IL-7Rα−/− mice, was undetectable in FL−/− × IL-7Rα−/− mice (Table III). Similar findings were observed for IgD (unpublished data), further confirming the complete absence of mature functional B cells in adult FL−/− × IL-7Rα−/− mice.
Table III.
Mice Double Deficient in flt3 Ligand and IL-7Rα Expression Do Not Produce Igs
| Mouse | IgM levels | IgG levels | IgA levels |
|---|---|---|---|
| μg/ml | μg/ml | μg/ml | |
| WT | 198 ± 95 | 625 ± 117 | 186 ± 84 |
| FL−/− | Normala | Normala | Normala |
| IL-7Rα2/− | 237 ± 53 | 501 ± 314 | 39 ± 21 |
| FL−/− × IL-7Rα2/− | 0b (bd) | 0b (bd) | 0b (bd) |
Absence of Detectable Committed B Cell Progenitors in FL−/− × IL-7Rα−/− Adult BM and Fetal Liver.
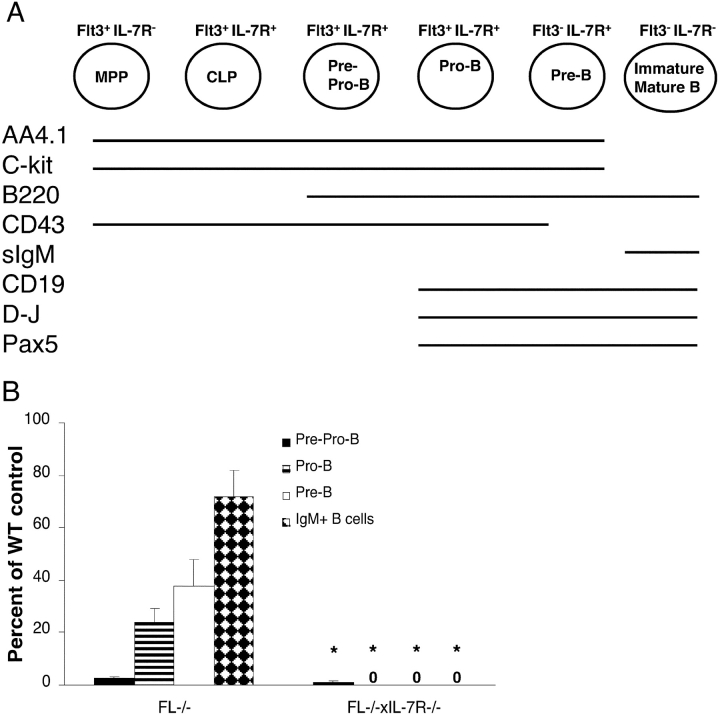
Since no mature B cells were detected in adult FL−/− × IL-7Rα−/− mice, we next investigated whether committed B cell progenitors could be detected in FL−/− × IL-7Rα−/− BM. Recent studies have demonstrated that little or no B cell development takes place in the BM of IL-7−/− and IL-7Rα−/− mice after 7 wk of age, reflected in virtually no detectable B cell progenitors (28, 29). In agreement with this, using a similar staging (29, 49, 50) we found that B220+CD43+AA4.1+CD19− pre-pro-B cells were reduced by >99% in FL−/− × IL-7Rα−/− BM, whereas B220+CD43+AA4.1+CD19+ pro-B and pre-B cells were nondetectable (Fig. 4 B). Interestingly, FL−/− mice also showed a dramatic (97.5%) reduction in B220+CD43+ AA4.1+CD19− pre-pro-B cells and, as previously shown (37), also reduced levels of pro-B (76%) and pre-B (62%) cells (Fig. 4 B).
Figure 4.
Mice double deficient in flt3 ligand and IL-7Rα expression show dramatic reductions in early and late B cell progenitors in adult BM. (A) Schematic illustration of cell surface phenotype, B cell gene expression, and D-J rearrangement during normal B cell development. Also shown are expression patterns for flt3 and IL-7R. MPP, multipotent progenitor, Lin−Sca-1+c-kit+flt3+ IL-7R− (63). CLP, common lymphoid progenitor; sIgM, surface IgM. (B) BM cells from WT, FL−/−, and FL−/− × IL-7Rα−/− mice were investigated for the presence of B cell progenitors as described previously (29, 50); pre-pro-B cells (B220+CD43+AA4.1+CD19−), pro-B cells (B220+CD43+AA4.1+CD19+), pre-B cells (B220+CD43−CD19+), and immature/mature B cells (B220+IgM+). Results are mean values (SD) from six to twelve mice for each group and are expressed as percentages of WT controls (no significant differences in BM cellularity between different genotypes). *Indicates statistically significant differences (P < 0.01) between FL−/− and FL−/− × IL-7Rα−/− mice. 0, no pro-B, pre-B, or B220+IgM+ cells were detected in FL−/− × IL-7Rα−/− mice.
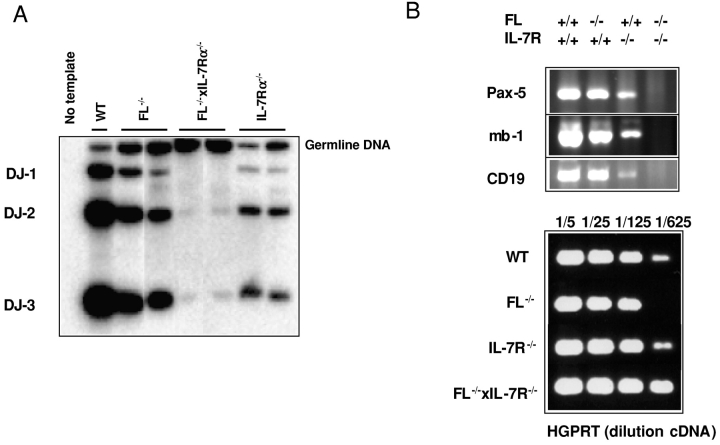
Compared with FL−/− and IL-7Rα−/− mice, only very low levels of D-J rearrangement could be detected in the BM of FL−/− × IL-7Rα−/− mice (Fig. 5 A). The development of mature B cells is dependent on the expression of several transcription factors (25, 51). The B cell lineage–specific activator protein encoded by the Pax5 gene (52) appears to be crucial for B cell development, which in _Pax5_-deficient mice is blocked at the pro-B cell stage (53, 54). Pax5 RNA was highly expressed in the BM of FL−/− mice, reduced in IL-7Rα−/− mice, and was completely undetectable in FL−/− × IL-7Rα−/− BM, in agreement with the complete absence of pro-B cells and all subsequent stages of B cell development (Fig. 5 B). Similarly, mb-1 and CD19, two direct target genes of Pax5 (55–58), were expressed in FL−/− BM cells, reduced in IL-7Rα−/− BM cells, but completely absent in FL−/− × IL-7Rα−/− mice (Fig. 5 B). Thus, flt3 and IL-7Rα signaling appears to be essential for commitment to a _Pax5_-dependent B cell stage.
Figure 5.
Absence of expression of Pax5 and Pax5 target genes in BM of adult FL−/− × IL-7Rα−/− mice. (A) DNA was isolated from BM of WT, FL−/−, IL-7Rα−/−, and FL−/− × IL-7Rα−/− mice and D-J rearrangement assessed by PCR (described in Materials and Methods). Representative results from two out of seven analyzed mice. (B) RNA was isolated from BM of WT, FL−/−, IL-7Rα−/−, and FL−/− × IL-7Rα−/− mice (described in Material and Methods) and expression of Pax5, mb-1, and CD19 (top) or HGPRT (bottom) analyzed by RT-PCR. Data are from one of three experiments with similar results.
Due to the complete absence of not only conventional B cells but also B1 cells in adult FL−/− × IL-7Rα−/− mice, we next compared fetal B lymphopoiesis in IL-7Rα−/− and FL−/− × IL-7Rα−/− mice. B220+ IgM+ B cells were present, although at reduced levels, in the fetal spleen and liver of IL-7Rα−/− mice, in striking contrast to FL−/− × IL-7Rα−/− mice in which no B220+IgM+ B cells were detectable (Fig. 6 A). Furthermore, in contrast to IL-7Rα−/−–deficient mice, FL−/− × IL-7Rα−/− fetal livers lacked detectable B220+CD19+ and B220+AA4.1+ cells, suggesting that generation of committed B cell progenitors is strictly dependent on IL-7Rα and flt3 signaling also during fetal development (Fig. 6 B).
Figure 6.
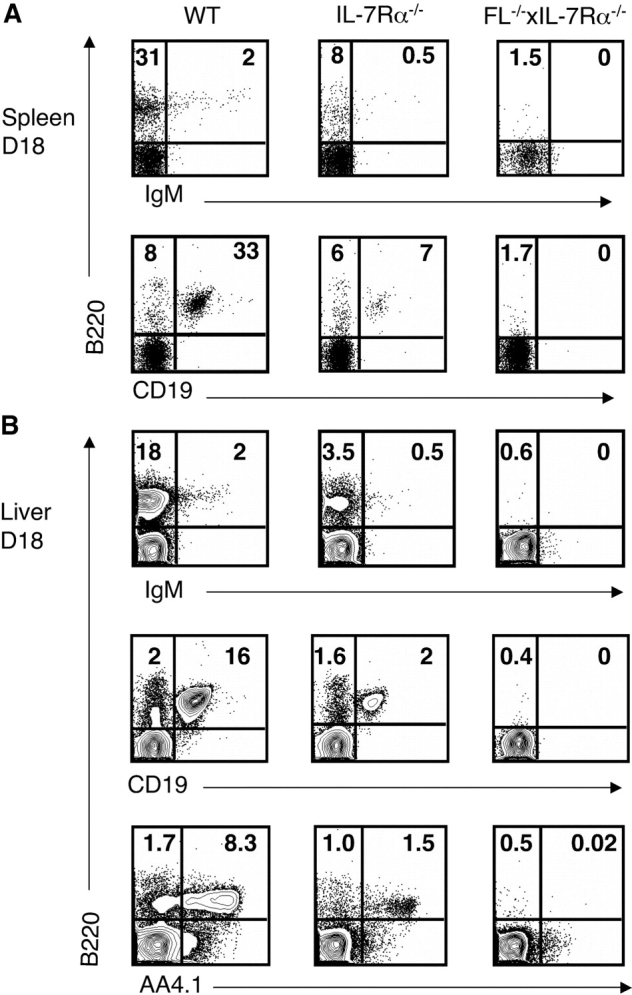
Lack of detectable mature B cells and B cell progenitors during fetal development of mice double deficient in flt3 ligand and IL-7Rα expression. Spleen and liver cells isolated from embryos at day 17–18 of gestation were stained with mAbs against B220, CD19, IgM, and AA4.1 (Materials and Methods). (A) Plots of B220, CD19, and IgM expression in the spleens of WT, IL-7Rα−/−, and FL−/− × IL-7Rα−/− mice. Numbers represent mean values from three to eight mice. (B) Plots of B220, CD19, and IgM expression in the livers of WT, IL-7Rα−/−, and FL−/− × IL-7Rα−/− mice. Numbers in A and B represent mean values (percentage of total cells in lymphoid gate) from three to eight mice.
Discussion
Several cytokines have been shown to promote B cell development in vitro and in vivo (25, 59), but extensive studies of mice deficient in different cytokine ligands and receptors have so far failed to support an indispensable role for cytokines in B cell development. Although the production of mature B cells in the BM of IL-7 and IL-7Rα–deficient mice takes place almost exclusively during fetal and postnatal development (9, 26, 27, 30), a stable pool of mature B cells are maintained throughout adult life in peripheral organs, sustaining normal levels of circulating Ig (9, 26, 27, 30). These B cells might largely be offspring of fetally derived B1 cells, and since present at normal levels in IL-7–deficient mice they have been suggested to represent an important IL-7–independent pathway for B cell development (28). Alternatively, fetal B cell development and maintenance of B cells and Ig production during adult life might be dependent on the concerted action of IL-7 with one or multiple other cytokines. However, mice deficient in the γc chain receptor, required for signaling of six different lymphokines (including IL-7), express a similar phenotype to that of IL-7Rα−/− mice (31, 32), suggesting that among known lymphokines only IL-7 plays a nonredundant function in B cell development. As the result of these observations, an alternative hypothesis emerged, namely that signaling through cytokine receptors might not be strictly required for the development of mature B cells. Through similar findings, the fundamental importance of cytokine signaling has also been questioned in the development of a number of other blood cell lineages (6–8, 10–12, 14–17, 20–24).
In the present study, we unequivocally demonstrate that signaling through two separate families of cytokine receptors (i.e., an “early acting” receptor tyrosine kinase and a “later acting” hematopoietin receptor) are indispensable for fetal and adult B cell development. This conclusion is based on multiple lines of evidence obtained using methods capable of detecting minute levels of B cells. In contrast to either FL−/− or IL-7Rα−/− mice, peripheral and mesenteric LNs and Peyer's Patches were not identifiable in FL−/− × IL-7Rα−/− mice. Furthermore, IgM+ B cells were found to be completely lacking in the blood, spleen, and BM of adult FL−/− × IL-7Rα−/− mice, whereas, consistent with previous studies, B cells were present (at reduced levels) at all sites in IL-7Rα−/− mice. Similarly, whereas IgA+ plasma cells were found at normal or near normal levels in either FL−/− or IL-7Rα−/− mice, they were undetectable in FL−/− × IL-7Rα−/− mice.
Flt3 and IL-7Rα signaling proved essential not only for development of conventional B cells but also for B1 cells and fetal B lymphopoiesis. B1 cells, arising early during fetal development before the appearance of conventional B cells, are believed to be regulated via distinct pathways to those of conventional B cells (28, 47, 48). In fact, before the present study a crucial role for cytokines in B1 cell genesis had not been identified. In this regard, both γc−/− and IL-7–deficient mice have been shown to have normal levels of B1 cells but reduced conventional B cells (31, 28), and herein we show that single FL−/− mice have near normal levels of peritoneal B1 cells, whereas IL-7Rα2/− mice have a 63% reduction. In contrast, B1 cells are completely absent in FL−/− × IL-7Rα−/− mice, demonstrating that IL-7Rα and flt3 signaling are indispensable for B1 cell development and/or maintenance. It is noteworthy that in IL-7Rα−/− mice we found a significant reduction in B1 cells, whereas B1 cells are normal in IL-7−/− mice (28). Since another cytokine, thymic stromal lymphopoietin, has been shown to utilize the IL-7Rα in its signaling (60), this might suggest that thymic stromal lymphopoietin together with FL and IL-7 might be critically involved in generation of B1 cells.
A critical role for flt3 and IL-7R signaling in fetal B cell development was further substantiated by a complete absence of mature B220+IgM+ B cells, in striking contrast to IL-7Rα−/− mice. Furthermore, whereas the fetal liver of IL-7Rα−/− mice contained B220+CD19+ and B220+ AA4.1+ B cell progenitors, no B220+ cells coexpressing CD19 or AA4.1 were detected in the fetal liver of FL−/− × IL-7Rα−/− mice, suggesting a critical role of FL and IL-7 in the earliest stages of fetal B cell commitment and development. Finally, and most strikingly, whereas a highly sensitive ELISA assay confirmed that the residual levels of B cells in adult IL-7Rα−/− mice can produce normal Ig levels, no Ig production (IgM, IgG, IgA and IgD) was observed in FL−/− × IL-7Rα−/− mice.
Although certain transcription factors have been demonstrated to be critically involved in B cell commitment and development (25, 51), and our present studies demonstrate that B cell genesis is also strictly dependent on specific cytokine signaling, the potential cross talk between the two remain poorly understood. It is well known, however, that transcription factors can regulate cytokine receptor expression (4), but there is less evidence for a role of cytokine signaling in the regulation of transcription factor expression. It is striking that expression of Pax5, the proposed master regulator of B cell commitment and development (53, 54), was completely absent in the BM of FL−/− × IL-7Rα−/− mice, as were the direct Pax5 target genes mb-1 and CD19 (55–58). This could potentially reflect an indispensable role of flt3 and IL-7Rα in regulation of Pax5 expression, but since pro-B cells and all downstream stages of B cell development were completely lacking in the BM of FL−/− × IL-7Rα−/− mice, we rather favor that flt3 and IL-7Rα are absolutely required to promote B cell development to a _Pax5-_dependent stage. This is interesting in the context that Pax5 is thought to act as a “B cell lineage locker,” restricting the lineage potentials of otherwise multipotent progenitors to a committed B cell fate (54, 61), implicating that B cell commitment might be dependent on signaling through flt3 and IL-7Rα. Although FL−/− × IL-7Rα−/− BM showed low levels D-J rearrangement, this could potentially derive from cells not committed to the B cell lineage, since D-J rearrangement can be found in T cell progenitors (62).
Despite flt3 and c-kit being coexpressed at early stages of B cell development, including CLP (37, 63), the present findings demonstrate a distinct role of flt3 but not c-kit in early B cell development, since γc−/− × c-kit−/− mice produce B cells at levels comparable to that of single γc−/− mice (64). Although the mechanisms by which flt3 and IL-7Rα interact to maintain B cell production remains to be established, the function of the two cytokine receptors are evident at distinct and overlapping stages of B cell development (34, 65). Flt3 is expressed (34, 63) at earlier stages than IL-7Rα, and FL-deficient mice have a defect primarily in early B cell progenitors, including the CLP (37). In contrast, IL-7Rα is primarily expressed at later stages of B cell development, and IL-7–deficient mice have normal levels of CLPs (29) but reduced levels of B cell progenitors and mature B cells (26, 27). However, the complete absence of conventional and B1 cells in fetal and adult FL−/− × IL-7Rα−/− mice most likely also reflects important synergistic interactions and potential cross-talk between these two cytokine receptor signaling pathways during multiple stages of B cell development (from CLP to pre-B cells) where both receptors are coexpressed. This is supported by the potent in vitro synergy between FL and IL-7 in promoting B cell development and expansion from adult uncommitted BM progenitors in which the combined action of FL and IL-7 proved both sufficient and absolutely essential (39).
The potent synergy between one of the cytokine tyrosine kinase receptors flt3 and/or c-kit and multiple members of the hematopoietic receptor superfamily in promoting growth and development of different blood cell lineages is a well-described in vitro phenomenon (2, 3, 34). The present findings propose an important physiological synergy between an early acting tyrosine kinase receptor and late acting hematopoietin receptor during B cell development, rather than between intensively explored combinations of hematopoietic lineage factors. This apparent synergy may not only be critical for B cell development but may also prove essential for the development of other blood cell lineages. In support of this hypothesis, a combined deficiency in both c-kit and γc chain receptor expression has been shown to result in a profound block in early thymocyte development (64). Although c-kit−/− mice die 2–6 d after birth (due to anemia) complicating further analysis and it remains unclear which (one or multiple) of the 6 cytokines acting through the γc chain receptor are involved in thymocyte development, this finding lends further support toward an essential role of interactions between early acting cytokine tyrosine kinase receptors and later acting hematopoietin receptors in blood lineage development.
Acknowledgments
We thank Dr. Stewart Lyman for facilitating these studies and Dr. Ihor Lemischka for AA4.1 antibody. The expert technical assistance of Lilian Wittmann, Gunilla Gärdebring, Eva Gynnstam, and Irene Persson is highly appreciated.
These studies were supported by grants from ALF (Governmental Public Health Grant) (to C.M. Cilio and S.E.W. Jacobsen), Alfred Österlund Foundation, Funds of Lunds Sjukvårdsdistrikt, the Swedish Medical Research Council (to C.M. Cilio, S.E.W. Jacobsen, and W.W. Agace grant 13131), Swedish Foundation for Strategic Research, Swedish Cancer Society, the Swedish Society of Pediatric Cancer, and the Tobias Foundation. E. Sitnicka is a Senior Scientist supported by the Swedish Gene Therapy Program. W.W. Agace is an Assistant Professor with the Swedish Science Research Council. I.-L. Martensson is supported by the Biology and Biotechnology Science Research Council (UK). S.E.W. Jacobsen has a Senior Scientist position from the Swedish Cancer Society.
Abbreviations used in this paper: AP, alkaline phosphatase; CLP, common lymphoid progenitor; FL, flt3 ligand; PB, peripheral blood; γc, γ receptor chain.
References
- 1.Morrison, S.J., N. Uchida, and I.L. Weissman. 1995. The biology of hematopoietic stem cells. Annu. Rev. Cell Dev. Biol. 11:35–71. [DOI] [PubMed] [Google Scholar]
- 2.Ogawa, M. 1993. Differentiation and proliferation of hematopoietic stem cells. Blood. 81:2844–2853. [PubMed] [Google Scholar]
- 3.Metcalf, D. 1993. Hematopoietic regulators: redundancy or subtlety? Blood. 82:3515–3523. [PubMed] [Google Scholar]
- 4.Zhu, J., and S.G. Emerson. 2002. Hematopoietic cytokines, transcription factors and lineage commitment. Oncogene. 21:3295–3313. [DOI] [PubMed] [Google Scholar]
- 5.Lotem, J., and L. Sachs. 2002. Cytokine control of developmental programs in normal hematopoiesis and leukemia. Oncogene. 21:3284–3294. [DOI] [PubMed] [Google Scholar]
- 6.Schorle, H., T. Holtschke, T. Hunig, A. Schimpl, and I. Horak. 1991. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 352:621–624. [DOI] [PubMed] [Google Scholar]
- 7.Lantz, C.S., J. Boesiger, C.H. Song, N. Mach, T. Kobayashi, R.C. Mulligan, Y. Nawa, G. Dranoff, and S.J. Galli. 1998. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature. 392:90–93. [DOI] [PubMed] [Google Scholar]
- 8.Kuhn, R., K. Rajewsky, and W. Muller. 1991. Generation and analysis of interleukin-4 deficient mice. Science. 254:707–710. [DOI] [PubMed] [Google Scholar]
- 9.von Freeden-Jeffry, U., P. Vieira, L.A. Lucian, T. McNeil, S.E. Burdach, and R. Murray. 1995. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 181:1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanley, E., G.J. Lieschke, D. Grail, D. Metcalf, G. Hodgson, J.A. Gall, D.W. Maher, J. Cebon, V. Sinickas, and A.R. Dunn. 1994. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc. Natl. Acad. Sci. USA. 91:5592–5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lieschke, G.J., D. Grail, G. Hodgson, D. Metcalf, E. Stanley, C. Cheers, K.J. Fowler, S. Basu, Y.F. Zhan, and A.R. Dunn. 1994. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood. 84:1737–1746. [PubMed] [Google Scholar]
- 12.Bunting, S., R. Widmer, T. Lipari, L. Rangell, H. Steinmetz, K. Carver-Moore, M.W. Moore, G.A. Keller, and F.J. de Sauvage. 1997. Normal platelets and megakaryocytes are produced in vivo in the absence of thrombopoietin. Blood. 90:3423–3429. [PubMed] [Google Scholar]
- 13.Gurney, A.L., K. Carver-Moore, F.J. de Sauvage, and M.W. Moore. 1994. Thrombocytopenia in c-mpl-deficient mice. Science. 265:1445–1447. [DOI] [PubMed] [Google Scholar]
- 14.Kaushansky, K., N. Fox, N.L. Lin, and W.C. Liles. 2002. Lineage-specific growth factors can compensate for stem and progenitor cell deficiencies at the postprogenitor cell level: an analysis of doubly TPO- and G-CSF receptor-deficient mice. Blood. 99:3573–3578. [DOI] [PubMed] [Google Scholar]
- 15.Gainsford, T., A.W. Roberts, S. Kimura, D. Metcalf, G. Dranoff, R.C. Mulligan, C.G. Begley, L. Robb, and W.S. Alexander. 1998. Cytokine production and function in c-mpl-deficient mice: no physiologic role for interleukin-3 in residual megakaryocyte and platelet production. Blood. 91:2745–2752. [PubMed] [Google Scholar]
- 16.Gainsford, T., H. Nandurkar, D. Metcalf, L. Robb, C.G. Begley, and W.S. Alexander. 2000. The residual megakaryocyte and platelet production in c-mpl-deficient mice is not dependent on the actions of interleukin-6, interleukin-11, or leukemia inhibitory factor. Blood. 95:528–534. [PubMed] [Google Scholar]
- 17.Chen, Q., G. Solar, D.L. Eaton, and F.J. de Sauvage. 1998. IL-3 does not contribute to platelet production in c-Mpl-deficient mice. Stem Cells. 16:31–36. [DOI] [PubMed] [Google Scholar]
- 18.Scott, C.L., L. Robb, R. Mansfield, W.S. Alexander, and C.G. Begley. 2000. Granulocyte-macrophage colony-stimulating factor is not responsible for residual thrombopoiesis in mpl null mice. Exp. Hematol. 28:1001–1007. [DOI] [PubMed] [Google Scholar]
- 19.Liu, F., J. Poursine-Laurent, H.Y. Wu, and D.C. Link. 1997. Interleukin-6 and the granulocyte colony-stimulating factor receptor are major independent regulators of granulopoiesis in vivo but are not required for lineage commitment or terminal differentiation. Blood. 90:2583–2590. [PubMed] [Google Scholar]
- 20.Seymour, J.F., G.J. Lieschke, D. Grail, C. Quilici, G. Hodgson, and A.R. Dunn. 1997. Mice lacking both granulocyte colony-stimulating factor (CSF) and granulocyte-macrophage CSF have impaired reproductive capacity, perturbed neonatal granulopoiesis, lung disease, amyloidosis, and reduced long-term survival. Blood. 90:3037–3049. [PubMed] [Google Scholar]
- 21.Gillessen, S., N. Mach, C. Small, M. Mihm, and G. Dranoff. 2001. Overlapping roles for granulocyte-macrophage colony-stimulating factor and interleukin-3 in eosinophil homeostasis and contact hypersensitivity. Blood. 97:922–928. [DOI] [PubMed] [Google Scholar]
- 22.Nishinakamura, R., N. Nakayama, Y. Hirabayashi, T. Inoue, D. Aud, T. McNeil, S. Azuma, S. Yoshida, Y. Toyoda, K. Arai, et al. 1995. Mice deficient for the IL-3/GM-CSF/IL-5 beta c receptor exhibit lung pathology and impaired immune response, while beta IL3 receptor-deficient mice are normal. Immunity. 2:211–222. [DOI] [PubMed] [Google Scholar]
- 23.Robb, L., C.C. Drinkwater, D. Metcalf, R. Li, F. Kontgen, N.A. Nicola, and C.G. Begley. 1995. Hematopoietic and lung abnormalities in mice with a null mutation of the common beta subunit of the receptors for granulocyte-macrophage colony-stimulating factor and interleukins 3 and 5. Proc. Natl. Acad. Sci. USA. 92:9565–9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishinakamura, R., A. Miyajima, P.J. Mee, V.L. Tybulewicz, and R. Murray. 1996. Hematopoiesis in mice lacking the entire granulocyte-macrophage colony-stimulating factor/interleukin-3/interleukin-5 functions. Blood. 88:2458–2464. [PubMed] [Google Scholar]
- 25.Hardy, R.R. 2003. B-cell commitment: deciding on the players. Curr. Opin. Immunol. 15:158–165. [DOI] [PubMed] [Google Scholar]
- 26.Peschon, J.J., P.J. Morrissey, K.H. Grabstein, F.J. Ramsdell, E. Maraskovsky, B.C. Gliniak, L.S. Park, S.F. Ziegler, D.E. Williams, C.B. Ware, et al. 1994. Early lymphocyte expansion is severely impaired in interleukin 7 receptor–deficient mice. J. Exp. Med. 180:1955–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maki, K., S. Sunaga, Y. Komagata, Y. Kodaira, A. Mabuchi, H. Karasuyama, K. Yokomuro, J.I. Miyazaki, and K. Ikuta. 1996. Interleukin 7 receptor-deficient mice lack gammadelta T cells. Proc. Natl. Acad. Sci. USA. 93:7172–7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carvalho, T.L., T. Mota-Santos, A. Cumano, J. Demengeot, and P. Vieira. 2001. Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7(−/)− mice. J. Exp. Med. 194:1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller, J.P., D. Izon, W. DeMuth, R. Gerstein, A. Bhandoola, and D. Allman. 2002. The earliest step in B lineage differentiation from common lymphoid progenitors is critically dependent upon interleukin 7. J. Exp. Med. 196:705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maraskovsky, E., J.J. Peschon, H. McKenna, M. Teepe, and A. Strasser. 1998. Overexpression of Bcl-2 does not rescue impaired B lymphopoiesis in IL- 7 receptor-deficient mice but can enhance survival of mature B cells. Int. Immunol. 10:1367–1375. [DOI] [PubMed] [Google Scholar]
- 31.Cao, X., E.W. Shores, J. Hu-Li, M.R. Anver, B.L. Kelsall, S.M. Russell, J. Drago, M. Noguchi, A. Grinberg, E.T. Bloom, et al. 1995. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 2:223–238. [DOI] [PubMed] [Google Scholar]
- 32.DiSanto, J.P., W. Muller, D. Guy-Grand, A. Fischer, and K. Rajewsky. 1995. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc. Natl. Acad. Sci. USA. 92:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kishimoto, T., T. Taga, and S. Akira. 1994. Cytokine signal transduction. Cell. 76:253–262. [DOI] [PubMed] [Google Scholar]
- 34.Lyman, S.D., and S.E. Jacobsen. 1998. c-kit ligand and Flt3 ligand: stem/progenitor cell factors with overlapping yet distinct activities. Blood. 91:1101–1134. [PubMed] [Google Scholar]
- 35.Wasserman, R., Y.S. Li, and R.R. Hardy. 1995. Differential expression of the blk and ret tyrosine kinases during B lineage development is dependent on Ig rearrangement. J. Immunol. 155:644–651. [PubMed] [Google Scholar]
- 36.McKenna, H.J., K.L. Stocking, R.E. Miller, K. Brasel, T. De Smedt, E. Maraskovsky, C.R. Maliszewski, D.H. Lynch, J. Smith, B. Pulendran, et al. 2000. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 95:3489–3497. [PubMed] [Google Scholar]
- 37.Sitnicka, E., D. Bryder, K. Theilgaard-Monch, N. Buza-Vidas, J. Adolfsson, and S.E. Jacobsen. 2002. Key role of flt3 ligand in regulation of the common lymphoid progenitor but not in maintenance of the hematopoietic stem cell pool. Immunity. 17:463–472. [DOI] [PubMed] [Google Scholar]
- 38.Mackarehtschian, K., J.D. Hardin, K.A. Moore, S. Boast, S.P. Goff, and I.R. Lemischka. 1995. Targeted disruption of the flk2/flt3 gene leads to deficiencies in primitive hematopoietic progenitors. Immunity. 3:147–161. [DOI] [PubMed] [Google Scholar]
- 39.Veiby, O.P., S.D. Lyman, and S.E. Jacobsen. 1996. Combined signaling through interleukin-7 receptors and flt3 but not c-kit potently and selectively promotes B-cell commitment and differentiation from uncommitted murine bone marrow progenitor cells. Blood. 88:1256–1265. [PubMed] [Google Scholar]
- 40.Cumano, A., K. Dorshkind, S. Gillis, and C.J. Paige. 1990. The influence of S17 stromal cells and interleukin 7 on B cell development. Eur. J. Immunol. 20:2183–2189. [DOI] [PubMed] [Google Scholar]
- 41.Svensson, M., J. Marsal, A. Ericsson, L. Carramolino, T. Broden, G. Marquez, and W.W. Agace. 2002. CCL25 mediates the localization of recently activated CD8alphabeta(+) lymphocytes to the small-intestinal mucosa. J. Clin. Invest. 110:1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Engvall, E., and P. Perlman. 1971. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 8:871–874. [DOI] [PubMed] [Google Scholar]
- 43.Schlissel, M.S., L.M. Corcoran, and D. Baltimore. 1991. Virus-transformed pre-B cells show ordered activation but not inactivation of immunoglobulin gene rearrangement and transcription. J. Exp. Med. 173:711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adachi, S., H. Yoshida, K. Honda, K. Maki, K. Saijo, K. Ikuta, T. Saito, and S.I. Nishikawa. 1998. Essential role of IL-7 receptor alpha in the formation of Peyer's patch anlage. Int. Immunol. 10:1–6. [DOI] [PubMed] [Google Scholar]
- 45.Rolink, A., E. ten Boekel, F. Melchers, D.T. Fearon, I. Krop, and J. Andersson. 1996. A subpopulation of B220+ cells in murine bone marrow does not express CD19 and contains natural killer cell progenitors. J. Exp. Med. 183:187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Macpherson, A.J., L. Hunziker, K. McCoy, and A. Lamarre. 2001. IgA responses in the intestinal mucosa against pathogenic and non- pathogenic microorganisms. Microbes Infect. 3:1021–1035. [DOI] [PubMed] [Google Scholar]
- 47.Rothstein, T.L. 2002. Cutting edge commentary: two B-1 or not to be one. J. Immunol. 168:4257–4261. [DOI] [PubMed] [Google Scholar]
- 48.Martin, F., and J.F. Kearney. 2001. B1 cells: similarities and differences with other B cell subsets. Curr. Opin. Immunol. 13:195–201. [DOI] [PubMed] [Google Scholar]
- 49.Hardy, R.R., C.E. Carmack, S.A. Shinton, J.D. Kemp, and K. Hayakawa. 1991. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J. Exp. Med. 173:1213–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li, Y.S., R. Wasserman, K. Hayakawa, and R.R. Hardy. 1996. Identification of the earliest B lineage stage in mouse bone marrow. Immunity. 5:527–535. [DOI] [PubMed] [Google Scholar]
- 51.Schebesta, M., B. Heavey, and M. Busslinger. 2002. Transcriptional control of B-cell development. Curr. Opin. Immunol. 14:216–223. [DOI] [PubMed] [Google Scholar]
- 52.Adams, B., P. Dorfler, A. Aguzzi, Z. Kozmik, P. Urbanek, I. Maurer-Fogy, and M. Busslinger. 1992. Pax-5 encodes the transcription factor BSAP and is expressed in B lymphocytes, the developing CNS, and adult testis. Genes Dev. 6:1589–1607. [DOI] [PubMed] [Google Scholar]
- 53.Urbanek, P., Z.Q. Wang, I. Fetka, E.F. Wagner, and M. Busslinger. 1994. Complete block of early B cell differentiation and altered patterning of the posterior midbrain in mice lacking Pax5/BSAP. Cell. 79:901–912. [DOI] [PubMed] [Google Scholar]
- 54.Nutt, S.L., B. Heavey, A.G. Rolink, and M. Busslinger. 1999. Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature. 401:556–562. [DOI] [PubMed] [Google Scholar]
- 55.Kozmik, Z., S. Wang, P. Dorfler, B. Adams, and M. Busslinger. 1992. The promoter of the CD19 gene is a target for the B-cell-specific transcription factor BSAP. Mol. Cell. Biol. 12:2662–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fitzsimmons, D., W. Hodsdon, W. Wheat, S.M. Maira, B. Wasylyk, and J. Hagman. 1996. Pax-5 (BSAP) recruits Ets proto-oncogene family proteins to form functional ternary complexes on a B-cell-specific promoter. Genes Dev. 10:2198–2211. [DOI] [PubMed] [Google Scholar]
- 57.Nutt, S.L., P. Urbanek, A. Rolink, and M. Busslinger. 1997. Essential functions of Pax5 (BSAP) in pro-B cell development: difference between fetal and adult B lymphopoiesis and reduced V-to-DJ recombination at the IgH locus. Genes Dev. 11:476–491. [DOI] [PubMed] [Google Scholar]
- 58.Nutt, S.L., A.M. Morrison, P. Dorfler, A. Rolink, and M. Busslinger. 1998. Identification of BSAP (Pax-5) target genes in early B-cell development by loss- and gain-of-function experiments. EMBO J. 17:2319–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hirayama, F., and M. Ogawa. 1996. Cytokine regulation of early lymphohematopoietic development. Stem Cells. 14:369–375. [DOI] [PubMed] [Google Scholar]
- 60.Park, L.S., U. Martin, K. Garka, B. Gliniak, J.P. Di Santo, W. Muller, D.A. Largaespada, N.G. Copeland, N.A. Jenkins, A.G. Farr, et al. 2000. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: formation of a functional heteromeric complex requires interleukin 7 receptor. J. Exp. Med. 192:659–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mikkola, I., B. Heavey, M. Horcher, and M. Busslinger. 2002. Reversion of B cell commitment upon loss of Pax5 expression. Science. 297:110–113. [DOI] [PubMed] [Google Scholar]
- 62.Corcoran, L., I. Ferrero, D. Vremec, K. Lucas, J. Waithman, M. O'Keeffe, L. Wu, A. Wilson, and K. Shortman. 2003. The lymphoid past of mouse plasmacytoid cells and thymic dendritic cells. J. Immunol. 170:4926–4932. [DOI] [PubMed] [Google Scholar]
- 63.Adolfsson, J., O.J. Borge, D. Bryder, K. Theilgaard-Monch, I. Astrand-Grundstrom, E. Sitnicka, Y. Sasaki, and S.E. Jacobsen. 2001. Upregulation of Flt3 expression within the bone marrow Lin(−)Sca1(+)c- kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 15:659–669. [DOI] [PubMed] [Google Scholar]
- 64.Rodewald, H.R., M. Ogawa, C. Haller, C. Waskow, and J.P. DiSanto. 1997. Pro-thymocyte expansion by c-kit and the common cytokine receptor gamma chain is essential for repertoire formation. Immunity. 6:265–272. [DOI] [PubMed] [Google Scholar]
- 65.Fry, T.J., and C.L. Mackall. 2002. Interleukin-7: from bench to clinic. Blood. 99:3892–3904. [DOI] [PubMed] [Google Scholar]
- 66.Lansford, R., J.P. Manis, E. Sonoda, K. Rajewsky, and F.W. Alt. 1998. Ig heavy chain class switching in Rag-deficient mice. Int. Immunol. 10:325–332. [DOI] [PubMed] [Google Scholar]