Cleavage by Granzyme B Is Strongly Predictive of Autoantigen Status: Implications for Initiation of Autoimmunity (original) (raw)
Abstract
Systemic autoimmune diseases are a genetically complex, heterogeneous group of disorders in which the immune system targets a diverse but highly specific group of intracellular autoantigens. The molecules targeted are not unified by common structure, function, or distribution in control cells but become clustered and concentrated in surface blebs when cells undergo apoptosis. We show here that the majority of autoantigens targeted across the spectrum of human systemic autoimmune diseases are efficiently cleaved by granzyme B in vitro and during cytotoxic lymphocyte granule–induced death, generating unique fragments not observed during any other form of apoptosis. These molecules are not cleaved by caspase-8, although this protease has a very similar specificity to granzyme B. The granzyme B cleavage sites in autoantigens contain amino acids in the P2 and P3 positions that are preferred by granzyme B but are not tolerated by caspase-8. In contrast to autoantigens, nonautoantigens are either not cleaved by granzyme B or are cleaved to generate fragments identical to those formed in other forms of apoptosis. The striking ability of granzyme B to generate unique fragments is therefore an exclusive property of autoantigens and unifies the majority of molecules targeted in this spectrum of diseases. These results focus attention on the role of the cytotoxic lymphocyte granule–induced death pathway in the initiation and propagation of systemic autoimmunity.
Keywords: apoptosis, cytotoxic T lymphocyte, protease, caspase
The highly specific autoantibody response in systemic autoimmune diseases generally predicts the biologic phenotype of the disease, making autoantibodies diagnostically useful 1 2. Although molecules targeted by the immune system in these diseases are exceptionally diverse in terms of structure, function, and subcellular distribution in healthy cells, they are strikingly redistributed in apoptotic cells, becoming clustered and concentrated in two populations of surface structures on the dying cell 3. It has been proposed that cleavage of proteins might play a role in selecting molecules as autoantigens, perhaps by cleavage-induced revelation of cryptic epitopes 4 5. In this regard, several of the clustered antigens are substrates for caspases during apoptosis. These include poly (ADP-ribose) polymerase (PARP),1 U1-70kD, the catalytic subunit of DNA-dependent protein kinase (DNA-PKcs), the nuclear mitotic apparatus protein (NuMA), nucleolus organizer region (NOR)-90, fodrin, topoisomerase I, signal recognition particle (SRP)-72, and lamin B 6 7 8 9 10. However, the autoantigen fragments produced by caspases during all forms of apoptotic death described to date (including thymocyte apoptosis) are all identical, making it likely that caspase-derived fragments have previously been generated during apoptosis and tolerized centrally and peripherally 11 12. Furthermore, there are several autoantigens (fibrillarin, centromere protein (CENP)-B, Ku, Ro, and La) that are not cleaved by caspases during apoptosis, indicating that susceptibility to cleavage by caspases is not a general feature of autoantigens (for review see reference 13).
Granzyme B is a serine protease found in the cytoplasmic granules of CTLs and NK cells that plays an important role in inducing apoptotic changes in target cells during granule exocytosis–induced cytotoxicity 14 15 16. This function is achieved partly by catalyzing the cleavage and activation of several caspases, as well as through caspase-independent pathways 17 18 19 20. We recently demonstrated that granzyme B (which shares with caspases a requirement for aspartic acid in the substrate P1 position) efficiently cleaves three caspase-3 substrates (DNA-PKcs, NuMA, and PARP), generating novel fragments not generated during any other form of cell death 21. To determine whether the generation of unique autoantigen fragments by granzyme B was a universal feature of autoantigens, we assessed whether a wide range of autoantigens was susceptible to cleavage by granzyme B in vitro and in vivo. These studies demonstrate that despite their diverse structure, distribution, and function, the majority of autoantigens in systemic autoimmune diseases are efficiently cleaved by granzyme B, generating unique fragments. In contrast, granzyme B either does not cleave or does not generate unique fragments in all of the nonautoantigen molecules that we tested. Granzyme B cleavage sites in autoantigens were defined; in all cases, the tetrapeptide sequence immediately adjacent to the cleavage site was highly conserved. Cleavage by granzyme B therefore (a) unites these otherwise unrelated molecules and (b) generates unique fragments of these antigens, strongly suggesting that this protease plays a mechanistic role in selecting the molecules against which autoimmune responses are initiated. These results highlight a potential role for the CTL granule–induced death pathway in initiation and propagation of autoimmunity.
Materials and Methods
Isolation of YT cell granule contents (GC) and purification of granzyme B was as described 21. Purified caspase-3 and -8 and CrmA (cytokine response modifier 1) were gifts from Nancy Thornberry (Merck Research Labs., Rahway, NJ). cDNAs for CENP-B, fibrillarin, topoisomerase I, and post meiotic segregation (PMS)1/PMS2 were gifts from Drs. Ann Pluta (University of Maryland, Baltimore, MD), John Aris (University of Florida, Gainesville, FL), Barbara White (University of Maryland, Baltimore, MD), and Bert Vogelstein (Johns Hopkins University), respectively. Autoantibodies to PMS1 and PMS2 are found in 3–5% of patients with autoimmune myositis 22. The patient serum recognizing ribosomal protein P was a gift from Dr. Keith Elkon (The Hospital for Special Surgery, NY, NY). All data shown represent 2–20 separate experiments.
HeLa Cell Culture and Induction of Apoptosis by UVB Irradiation.
HeLa cells were passaged in 10% heat-inactivated calf serum using standard tissue culture procedures. To induce apoptosis, cells were incubated with 1,650 J/m2 UVB and incubated overnight 3. In Fig. 1 A, the gel samples used in the lanes marked “Apoptotic Cells” consisted of pooled adherent and floating populations.
Figure 1.
Endogenous autoantigens are cleaved by granzyme B, generating unique fragments. (A) Gel samples were prepared from control and apoptotic (UVB-irradiated) HeLa cells and from HeLa lysate incubated in vitro with 0.8 nM purified caspase-3 and 5 mM DTT or 42 nM granzyme B and 2 mM IAA. 75-μg aliquots were electrophoresed in each gel lane, and autoantigens were detected by immunoblotting. Indicator lines denote intact antigens (Mi-2, 240 kD; PARP, 113 kD; SRP-72, 72 kD; Ki-67, 395 kD; U1-70kD, 70 kD; and topoisomerase I, 100 kD). Solid arrowheads mark granzyme B–specific fragments (Mi-2, 75, 72, and 48 kD; PARP, 110, 72, 62, and 55 kD; SRP-72, 62 kD; Ki-67, 167 and 148 kD; U1-70kD, 60 kD; topoisomerase I, 98, 75, and 72 kD). Open arrowheads denote fragments generated during apoptosis (Mi-2, 50 and 58 kD; PARP, 89 kD; SRP-72, 68 kD; Ki-67, 155, 140, 130, and 110 kD; U1-70kD, 40 kD; topoisomerase I, 70 kD). Note that the PARP autoradiogram shown was optimized for visualization of intact PARP and the 89-kD fragment; the 72- and 62-kD granzyme B–specific PARP fragments are clearly seen on longer autoradiogram exposures. (B) HeLa lysates were incubated for 1 h in the absence of protease (leftmost lane) or with the increasing amounts of granzyme B indicated. The samples were blotted with topoisomerase I antibodies. The data shows that the cleavage site that generates the 98-kD fragment from the 100-kD intact antigen (IEAD15-F; Table ) is exquisitely sensitive to granzyme B, with ∼70% of topoisomerase I cleaved to the 98-kD form after incubation with 0.42 nM granzyme B. Note that the 75- and 72-kD fragments are only generated after incubation with 10-fold higher concentrations of granzyme B.
In Vitro Cleavage of Endogenous Autoantigens in HeLa Lysates by Caspase-3 or Granzyme B.
HeLa lysate was prepared as described 6 and incubated at 37°C for 1 h with either 0.8 nM purified caspase-3 and 5 mM dithiothreitol (DTT) or purified granzyme B (the concentrations used are specified in the legends of Fig. 1 and Fig. 4) and 2 mM iodoacetamide (IAA). In the experiment shown in Fig. 7 B, GC were added to lysates to induce cleavage of autoantigens; the amount of GC used was based on its granzyme B activity (1 μl of GC preparation contained the same activity as 1 μl of purified granzyme B). To inhibit granzyme B activity in HeLa lysates, equimolar amounts of CrmA and granzyme B (in GC) were preincubated at 37°C for 5 min before adding to HeLa lysates. All samples were electrophoresed on 10% SDS-PAGE, with the exception of Ki-67 (7.5% SDS-PAGE). Proteins were then transferred to nitrocellulose or polyvinylidene difluoride and immunoblotted with patient sera recognizing Mi-2, PARP, SRP-72, U1-70kD, topoisomerase I, Ro-52, Ro-60, ribosomal protein P, Ku-80, or mAb to Ki-67 (Sigma Chemical Co.). Proteins were detected using horseradish peroxidase–labeled secondary antibodies and chemiluminescence (Pierce Chemical Co.).
Figure 4.
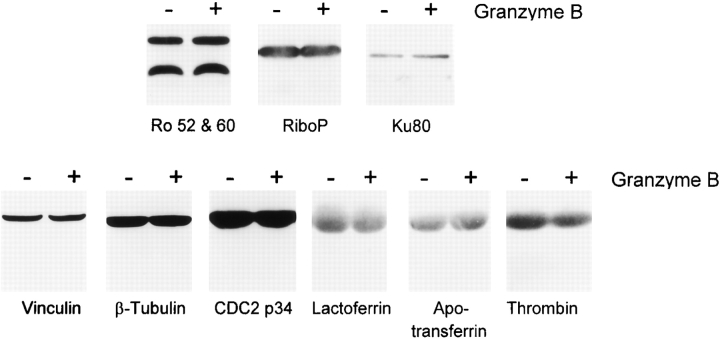
Numerous proteins are not cleaved by granzyme B. HeLa cell lysates were incubated for 60 min in the absence or presence of 42 nM granzyme B, followed by gel electrophoresis of 75-μl aliquots. The susceptibility of the autoantigens Ro (52 and 60 kD), ribosomal protein P, Ku-80, and nonautoantigens vinculin, β-tubulin, and Cdcp34 to cleavage by granzyme B was assessed by immunoblotting. Although none of these molecules was cleaved, efficient cleavage of NuMA, PARP, and U1-70kD in these lysates confirmed granzyme B activity (data not shown). The ability of granzyme B to cleave the purified nonautoantigens lactoferrin, apotransferrin, and thrombin was examined by incubating 20 μg of each substrate in the absence or presence of 30 nM granzyme B for 60 min at 37°C, followed by Coomassie blue staining of SDS-PAGE.
Figure 7.

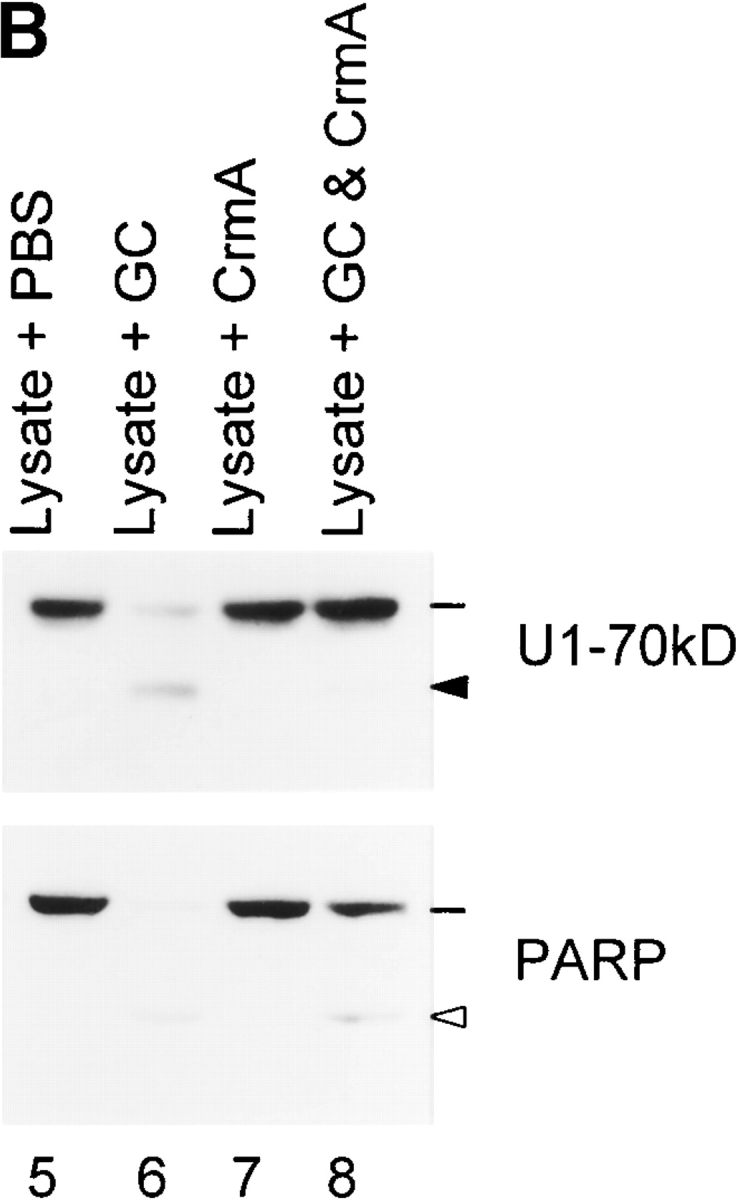
Generation of unique autoantigen fragments is abolished by Z-IETD-FMK or CrmA. Indicator lines denote the positions of intact antigens; solid and open arrowheads denote the granzyme B–specific and caspase-specific fragments, respectively. (A) LAK and K562 cells were preincubated in the absence or presence of 50 μM Z-IETD-FMK and then coincubated (E/T ratio of 3:1) for an additional 4 h 21. The samples were electrophoresed and autoantigens were blotted as described in the Fig. 6 legend. (B) GC were preincubated in the absence or presence of an equimolar amount of CrmA (amounts based on the granzyme B content of GC) for 5 min at 37°C. HeLa lysates were then incubated with these GC for a further 60 min at 37°C (0.5 μl GC per 75 μg lysate) before electrophoresis and immunoblotting.
In Vitro Cleavage of Endogenous Autoantigens in IAA-treated HeLa Cell Lysates by Caspase-8.
HeLa lysate was prepared as described 23, except that DTT was omitted from the lysis buffer. To inactivate endogenous caspase activities, 1 mM IAA was added to the lysates (see Fig. 9, all lanes except 3 and 6) and incubated for 15 min at 4°C. 5 mM DTT was then added in the absence or presence of 50 nM caspase-8, and the reactions were incubated for 1 h at 37°C. Before using the “IAA poisoning protocol” in the caspase-8 experiments, the validity of this experimental approach to inactivate endogenous caspases present in lysates while still permitting exogenously added caspases to cleave was tested. As PARP and U1-70kD are well characterized caspase-3 substrates (generating signature fragments of 89 and 40 kD, respectively [23]), their ability to be cleaved by caspase-3 in IAA-treated lysates was used in the following defined test system: (i) HeLa lysates containing 0.8 nM caspase-3 and 5 mM DTT were incubated for 1 h at 37°C; the extent of PARP (66%) and U1-70kD (25%) cleavage obtained under these conditions represented maximum possible caspase-3 activity. (ii) 1 mM IAA was added to HeLa lysates prepared in the absence of DTT. After incubating for 15 min at 4°C, 0.8 nM caspase-3 was added and the lysates were further incubated (1 h at 37°C); PARP and U1-70kD were not cleaved under these conditions. (iii) HeLa lysates were incubated as in (ii), except that 5 mM DTT was added simultaneously with caspase-3 after the IAA poisoning step. Both PARP and U1-70kD were cleaved (the amounts of cleavage were 80 and 70%, respectively, of that noted in approach [i]), confirming that exogenous caspases are active in these lysates in which endogenous caspases have previously been inactivated if subsequently added with DTT. Abolition of the contribution of endogenous caspases under these circumstances accounts for the decreased total substrate cleavage observed.
Figure 9.
Caspase-8 does not cleave endogenous autoantigens that are granzyme B substrates. HeLa lysate (in which endogenous caspases had been inactivated by 1 mM IAA) was incubated with 5 mM DTT in the absence (lanes 1, 4, 7, 9, 11, and 13) or presence (lanes 2, 5, 8, 10, 12, and 14) of 50 nM caspase-8 for 1 h at 37°C. In the lysates shown in lanes 3 and 6, omission of IAA permitted caspase-8 to activate endogenous effector caspases. After terminating the reactions, 75-μg amounts of sample were electrophoresed in each gel lane. Autoantigens were detected by blotting with monospecific patient sera. In lanes 1–6, open and solid arrowheads denote the SDS-PAGE migration positions of the intact antigens and the caspase-8–induced fragments, respectively. Radiolabeled PMS1 and PMS2 (generated by in vitro transcription/translation) were also not cleaved by purified caspase-8 (data not shown).
In Vitro Cleavage of [35S]methionine-labeled Autoantigen Substrates Generated by In Vitro Transcription/Translation.
[35S]methionine-labeled CENP-B, fibrillarin, PMS1, and PMS2 were generated by coupled in vitro transcription/translation 23. Cleavage reactions were performed in buffer A consisting of 10 mM Hepes, pH 7.4, 2 mM EDTA, and 1% NP-40 (5 mM DTT was added to the reactions containing caspases). Reactions contained the amounts of caspase-3 and granzyme B indicated in the figure legends and were incubated at 37°C for either 15 or 60 min, as specified. After terminating the reactions by adding gel buffer and boiling, samples were electrophoresed on 10% SDS-PAGE (CENP-B, PMS1, and PMS2) or 12% SDS-PAGE (fibrillarin), and their fragments were visualized by fluorography.
In Vitro Cleavage of [35S]methionine/cysteine-labeled Endogenous Autoantigen Substrates after Immunoprecipitation with Patient Sera.
HeLa cells were labeled for 2 h with [35S]methionine/cysteine, and the lysates were immunoprecipitated with patient sera recognizing histidyl tRNA synthetase, RNA polymerase II large subunit, PMScl, or alanyl tRNA synthetase, followed by protein A–agarose. The beads, containing washed, radiolabeled endogenous proteins were resuspended in buffer A in the presence or absence of granzyme B and incubated for 15 min at 37°C. The following amounts of granzyme B were used: 46 nM histidyl and alanyl tRNA synthetases and 23 nM RNA polymerase II and PMScl. Samples were electrophoresed on 10% SDS-PAGE, and radiolabeled proteins and their fragments were visualized by fluorography.
In Vitro Cleavage of Endogenous and Purified Nonautoantigen Substrates.
HeLa lysates were incubated with granzyme B for 1 h at 37°C and then electrophoresed on 8 or 10% SDS-PAGE and immunoblotted with a rabbit polyclonal antibody to Cdc2p34 (Santa Cruz Biotechnology) or mAbs to vinculin or β-tubulin (Sigma Chemical Co.). Cleavage of purified human lactoferrin, apotransferrin, and thrombin (all from Sigma Chemical Co.) was performed by incubating 20 μg of each substrate in the absence or presence of 30 nM granzyme B for 60 min at 37°C. The reactions were terminated by adding gel application buffer, and the samples were electrophoresed on 10% SDS-PAGE and visualized by Coomassie blue staining. Similar amounts of purified La were well cleaved under identical conditions (data not shown).
Calculation of Catalytic Constant (kcat/Km) Values.
k cat/K m values were determined as described 21 23 using endogenous substrates in cell lysates, radiolabeled endogenous immunoprecipitated substrates, or radiolabeled substrates generated by coupled in vitro transcription/translation. The percent cleavage of each substrate was determined by densitometry; these values were fitted to the first order rate equation,

to calculate k cat/K m. For several of the autoantigens listed in Table , k cat/K m values were obtained using two or all three of these methods, and equivalent results were obtained (reference 21; data not shown).
Table 1.
Autoantigens Are Efficiently Cleaved by Granzyme B
| No. | Autoantigen | Cleavage site | k cat/K m |
|---|---|---|---|
| M−1.s−1 | |||
| 1 | DNA-PKcs | VGPD2,698-F | 2.5 ± 0.8 × 106 |
| 2 | Topoisomerase I | IEAD15-F | 1.6 ± 0.6 × 106 |
| 3 | NuMA | VATD1,705-A | 5.4 ± 1.4 × 105 |
| 4 | Mi-2 | VDPD1,312-Y | 8.5 ± 1.9 × 104 |
| 5 | La | LEED220-A | 6.1 ± 1.7 × 104 |
| 6 | PMS1 | ISAD496-E | 6.9 ± 0.9 × 104 |
| 7 | Fibrillarin | VGPD184-G | 3.3 ± 1.9 × 104 |
| 8 | PARP | VDPD536-S | 2.3 ± 1.8 × 104 |
| 9 | U1-70kD | LGND409-S | 1.3 ± 0.4 × 104 |
| 10 | PMS2 | VEKD493-S | 1.4 ± 0.6 × 104 |
| 11 | Isoleucyl tRNA | VTPD983-Q | 7.8 ± 1.8 × 104 |
| synthetase | |||
| 12 | Histidyl tRNA | LGPD48-E | 2.3 ± 0.7 × 104 |
| synthetase | |||
| 13 | Alanyl tRNA | VAPD632-R | 1.8 × 104 |
| synthetase | |||
| 14 | RNA polymerase I | ICPD448-M | 1.3 ± 0.5 × 104 |
| 15 | Ki-67 | VCTD1481-K | 8.1 ± 2.6 × 103 |
| 16 | PMScl | VEQD252-M | 7.5 ± 1.4 × 103 |
| 17 | CENP-B | VDSD457-E | 5.9 ± 0.2 × 103 |
| 18 | RNA polymerase II | ITPD370-P | ND |
| 19 | SRP-72 | VTPD573-P | ND |
| 20 | Ku-70 | ISSD79-R | ND |
| 21 | NOR-90 | VRPD220-A | ND |
Cleavage of Endogenous Substrates after In Vivo Incubation of Fas-negative K562 Cells with YT Cell GC or Lymphokine-activated Killer Cells.
K562 cells were incubated with YT cell GC as described 21, except that the incubations were done in HBSS containing 10 mM Hepes, pH 7.4. Cells were incubated for 2 h at 37°C in the absence or presence of added GC, before terminating reactions by adding 2× SDS–gel buffer directly to cell suspensions. Similar experiments were performed using cultured HeLa cells, human umbilical vein endothelial cells, human myoblasts, and Jurkat cells with identical results (data not shown). LAK cells were prepared and incubated with K562 target cells at a 3:1 ratio in the absence or presence of 100 μM Ac-DEVD-CHO or 50 μM Z-IETD-fluoromethylketone (FMK; Calbiochem Corp.) as described 21. Incubation of either LAK or K562 cells individually with these inhibitors did not affect the autoantigens analyzed (data not shown).
Confirmation of Granzyme B Cleavage Sites by Mutagenesis of P1 Aspartic Acid Residues.
cDNA clones encoding fibrillarin, La, U1-70kD, Mi-2, PMS1, PMS2, and topoisomerase I were used as templates for mutagenesis by overlap-extension PCR to generate clones containing putative granzyme B site P1 Asp→Ala substitutions 21. [35S]methionine-labeled polypeptides were generated by coupled in vitro transcription/translation and used as substrates for granzyme B cleavage as described above.
Results
Granzyme B Specifically and Efficiently Cleaves Numerous Autoantigens Targeted in Systemic Autoimmune Diseases.
We initially determined whether and how efficiently a variety of well-defined autoantigens across the spectrum of systemic autoimmune diseases were cleaved by purified granzyme B (Table ). We used autoantibodies of known specificity to immunoblot lysates of HeLa cells that had been incubated in vitro with or without granzyme B. Lysates were pretreated with IAA (which covalently modifies the active site cysteine of caspases) to prevent endogenous caspase activity. Interestingly, several autoantigens that have previously been shown to be cleaved by caspases during apoptosis were also efficiently cleaved by granzyme B, generating unique fragments in every case. These substrates included U1-70kD, topoisomerase I, SRP-72, PARP (Fig. 1), and NOR-90 (data not shown). We also identified Mi-2, Ki-67, PMS1, PMS2, and La as additional autoantigens that are cleaved by both caspases and granzyme B; again, distinct fragments were generated by the two proteases (Fig. 1, Fig. 2, and Fig. 8). Whenever possible, efficient cleavage by granzyme B and generation of novel fragments was confirmed using both endogenous substrates in lysates (detection by immunoblotting) and in vitro–translated substrates (detection by fluorography; this was done for U1-70kD, PARP, NuMA, topoisomerase I, La, PMS1, and Mi-2). The k cat/K m values varied between 1.3 × 104 M−1.s−1 (U1-70kD) and 1.6 × 106 M−1.s−1 (topoisomerase I) (Table ). Several substrates were cleaved by granzyme B at multiple sites (e.g., Mi-2, Ki-67, PMS1, and PMS2; Fig. 1 and Fig. 2), generating a complex profile of cleavage fragments.
Figure 2.
[35S]methionine-labeled autoantigen substrates generated by in vitro transcription/translation are cleaved by purified granzyme B. [35S]methionine-labeled CENP-B, fibrillarin, PMS1, and PMS2 were incubated for 60 min at 37°C with the following amounts of caspase-3: 1 nM (PMS2) or 2.5 nM (CENP-B, fibrillarin, and PMS1). Separate incubations were performed as follows with granzyme B: 62.5 nM, 15 min (fibrillarin, PMS1); 42 nM, 60 min (CENP-B); and 28 nM, 60 min (PMS2). The samples were electrophoresed and visualized as described in Materials and Methods. Open arrowheads denote intact antigens (CENP-B, 80 kD; fibrillarin, 37 kD; PMS1, 110 kD; PMS2, 105 kD). Solid arrowheads mark the granzyme B–specific fragments (CENP-B, 58 and 40 kD; fibrillarin, 17 kD; PMS1, 50 and 60 kD; PMS2, 60, 50, and 36 kD).
Figure 8.
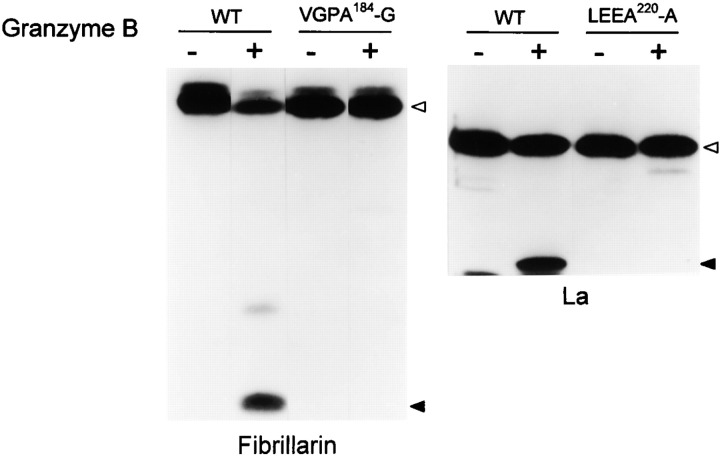
Granzyme B cleavage of fibrillarin and La is abolished by P1 D→A mutation. Site-directed mutation of aspartic acid residues in the P1 positions of fibrillarin (D184→A) and La (D220→A) was performed. Wild-type (WT) and mutated products were generated by in vitro transcription/translation before cleavage with granzyme B as described in Materials and Methods. Open and solid arrowheads mark the positions of intact molecules and granzyme B–specific fragments, respectively. Similar results were obtained when P1 aspartic acid residues in the granzyme B cleavage sites of U1-70kD, Mi-2, PMS1, PMS2, and topoisomerase I were mutated (data not shown).
Previous studies have identified several autoantigens that are not susceptible to cleavage by caspases during apoptosis 7 9. Using the immunoblotting system described above and/or cleavage assays using in vitro–translated substrates, we demonstrated that many of these autoantigens are efficiently cleaved by granzyme B. These molecules included fibrillarin, Ku-70, and RNA polymerase II large subunit. (Fig. 2 and Fig. 3; see Fig. 5). As some well-defined autoantibodies do not recognize their antigens by immunoblotting, we also addressed the susceptibility of radiolabeled endogenous substrates to cleavage by granzyme B in vitro. To perform these studies, HeLa cells were radiolabeled with [35S]methionine/cysteine, and proteins were immunoprecipitated using human autoantibodies. Protein A–agarose beads containing washed, precipitated proteins were resuspended in buffer supporting the activity of granzyme B and incubated in the absence or presence of added purified granzyme B. To confirm the validity of this approach, we tested several different autoantigens known to be cleaved by granzyme B, as well as several autoantigens and nonautoantigens that are not cleaved by granzyme B. The cleavage profiles obtained after incubating granzyme B with (a) lysates (followed by detection with immunoblotting) or (b) immunoprecipitated radiolabeled antigens (followed by detection with fluorography) were compared. Identical results were obtained using these two methods for cleaved autoantigens (topoisomerase I, Mi-2, RNA polymerase II large subunit, Ku-70, PARP, La, and NOR-90), uncleaved autoantigens (Ku-80, Ro-60kD), and control substrates (β-tubulin, vinculin). Using this immunoprecipitation approach, we demonstrated that several additional autoantigens (PMScl, RNA polymerase I and II large subunits, histidyl tRNA synthetase, isoleucyl tRNA synthetase, and alanyl tRNA synthetase) were indeed cleaved by granzyme B, generating unique fragments (Table ; Fig. 3 contains representative examples). Using identical immunoprecipitation conditions, these substrates were not cleaved by caspase-3, whereas Mi-2 cleavage by caspase-3 occurred normally (data not shown). Several autoantigens were not susceptible to cleavage by either caspases or granzyme B (Fig. 4 and references 7, 9, 24). These included Ro-52kD and -60kD, ribosomal protein P, histones, Sm proteins, threonyl tRNA synthetase, glycyl tRNA synthetase, and Ku-80.
Figure 3.
Immunoprecipitated endogenous autoantigens are cleaved by purified granzyme B. [35S]methionine/cysteine-labeled endogenous histidyl tRNA synthetase, RNA polymerase II, PMScl, and alanyl tRNA synthetase were immunoprecipitated and cleaved by granzyme B as described in Materials and Methods. Solid arrowheads denote granzyme B–specific fragments (histidyl tRNA synthetase, 40 kD; RNA polymerase II large subunit, 190, 110, and 92 kD; PMScl, 85 and 74 kD; alanyl tRNA synthetase, 58 kD).
Figure 5.
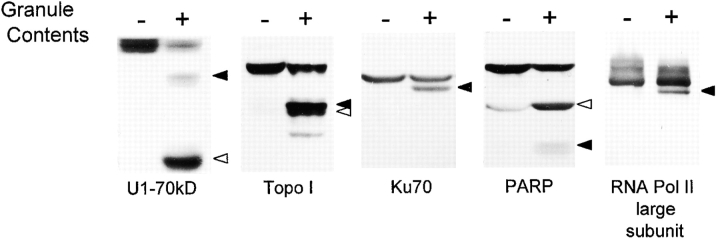
Granzyme B–specific autoantigen fragments are generated after in vivo incubation of intact K562 cells with YT cell GC. K562 cells were incubated in the absence (−) or presence (+) of YT cell GC for 2 h at 37°C. After terminating the reactions by adding gel buffer, 55 μg of protein was electrophoresed in each gel lane, and the samples were immunoblotted with monospecific patient sera. Solid and open arrowheads mark the positions of the granzyme B–specific and caspase-specific fragments, respectively.
Susceptibility to cleavage by granzyme B was a highly specific feature of autoantigens; nonautoantigens were either not cleaved by granzyme B (Fig. 4) or were cleaved but without producing novel fragments 25 26 27. The following human molecules were not cleaved by granzyme B: α1-antitrypsin, β-tubulin, apotransferrin, C3 (β chain), carbonic anhydrase, CDC2 p34, C-reactive protein, glutathione _S_-transferase, glycogen phosphorylase, hemoglobin, IgG, lactoferrin, lysozyme, orosomucoid, thrombin α chain, thrombin β chain, and vinculin. Procaspases 3 and 7, which have not been found to be autoantigens during screening of >500 autoimmune sera by immunoblotting (Casciola-Rosen, L., and A. Rosen, unpublished data), are efficiently cleaved by both granzyme B and caspase-8, generating identical fragments 25 26 27.
Thus, in addition to the three autoantigens we have previously described as being cleaved by both caspase-3 and granzyme B (DNA-PKcs, NuMA, PARP), these studies identify an additional nine autoantigens that are cleaved by both proteases but at different sites. Furthermore, another nine autoantigens are cleaved exclusively by granzyme B and not by caspases. Therefore, 21 autoantigens targeted across the spectrum of human systemic autoimmune diseases are efficiently cleaved by granzyme B, generating unique fragments not observed during other forms of cell death.
Granzyme B Cleavage Fragments Are Generated During CTL Granule–mediated Target Cell Death.
To confirm that similar autoantigen fragments are generated in intact cells during granule-induced cell death, we exposed K562 cells to YT cell GC and analyzed the biochemical status of the autoantigens by immunoblotting. In those cases where autoantigens are substrates for both caspases and granzyme B, signature fragments of both proteases were generated (U1-70kD, topoisomerase I, PARP [Fig. 5], La, Mi-2, and Ki-67 [data not shown]). The amount of granzyme B–specific fragments generated was enhanced in the presence of 100 μM Ac-DEVD-CHO, a caspase inhibitor (see below). Autoantigens known to be cleaved only by granzyme B were also cleaved in the K562/YT granule system; the granzyme B–specific fragments of Ku-70, RNA polymerase II large subunit (Fig. 5), and PMS1 (data not shown) were generated.
We next determined whether autoantigens cleaved by granzyme B in vitro are cleaved during killing of Fas-negative target cells by LAK cells. Granzyme B–specific fragments of Mi-2, topoisomerase I, U1-70kD, and SRP-72 (Fig. 6), as well as PMS1, Ku-70, and La (data not shown), are generated during this form of cell death. For the antigens shown in Fig. 6, which are directly cleaved by both caspase-3 and granzyme B, the amounts of granzyme B–specific fragments are determined by the relative efficiency of cleavage by the two proteases (Table ). Thus, granzyme B–mediated cleavage of topoisomerase I is ∼50-fold more efficient than cleavage by caspase-3, and granzyme B–specific fragments are the most prominent (Fig. 6, lane 3; note that in lanes 3 and 4, the majority of topoisomerase is in the 98-kD granzyme B–cleaved form). Where the efficiency of substrate cleavage by granzyme B and caspase-3 are similar (e.g., DNA-PKcs and Mi-2), both caspase- and granzyme B–specific fragments are generated. Inhibition of caspases abolishes the caspase-specific fragments only. In contrast, where substrates are cleaved ∼200-fold more efficiently by caspase-3 than by granzyme B (PARP, U1-70kD), no granzyme B–specific fragments were observed in the intact cell killing assay unless caspases were inhibited by adding Ac-DEVD-CHO (Fig. 6; compare lanes 3 and 4).
Figure 6.

Granzyme B–specific autoantigen fragments are generated in K562 cells attacked by LAK cells. LAK cells were incubated with K562 cells in the absence or presence of 100 μM Ac-DEVD-CHO 21. After terminating the reactions, the following numbers of cells were electrophoresed in each gel lane: 3 × 105 LAK cells (lane 1); 105 K562 cells (lane 2); 3 × 105 LAK cells plus 105 K562 cells (lanes 3 and 4). Autoantigens were detected by immunoblotting with monospecific patient sera. Solid and open arrowheads mark the positions of granzyme B–specific and caspase-specific fragments, respectively; indicator lines denote the intact antigens.
Table 2.
Relative Cleavage Efficiencies Determine Ratio of Substrate Fragments Generated In Vivo
| Autoantigen | k cat/K m (M−1.s−1) for cleavage | Prominent fragment type generated in: | |
|---|---|---|---|
| Caspase-3 | Granzyme B | LAK/K562 system | |
| Topoisomerase-I | 3.4 × 104 | 1.6 × 106 | Granzyme B |
| DNA-PKcs | 7.5 × 106 | 2.5 × 106 | Granzyme B/caspase-3 |
| Mi-2 | 2.0 × 105 | 8.5 × 104 | Granzyme B/caspase-3 |
| U1-70kD | 2.5 × 106 | 1.3 × 104 | Caspase-3 |
| PARP | 5.0 × 106 | 2.3 × 104 | Caspase-3 |
Production of unique autoantigen fragments in the LAK cell assay was decreased by inhibitors of granzyme B 28. Thus, 50 μM of Z-IETD-FMK (Fig. 7 A) greatly diminished cleavage of NuMA, Mi-2, and topoisomerase I, as evidenced by the increased amount of intact antigen detected by blotting (Fig. 7 A; compare lanes 3 and 4). This was accompanied by an abolition of the unique cleavage fragments of NuMA (175 kD) and topoisomerase I (98, 75, and 72 kD) and markedly inhibited the production of granzyme B–specific fragments of Mi-2 (48 kD). This pattern of inhibition correlated well with the efficiency of cleavage of these substrates by granzyme B (Table ). As noted above, production of granzyme B–specific fragments of autoantigens was either unchanged (Mi-2) or enhanced (PARP, U1-70kD, SRP-72) by treatment with the caspase-specific inhibitor, Ac-DEVD-CHO (Fig. 6, lane 4). Similar results were also obtained when 143 nM CrmA was used to inhibit granzyme B activity in cell lysates in which granzyme B–specific fragments of U1-70kD and PARP were abolished (Fig. 7 B), with concomitant recovery of intact antigen. Although both Z-IETD-FMK and CrmA also inhibit group III caspases, unique fragments of these autoantigens are not generated by these proteases (see Fig. 9).
Granzyme B Cleaves Autoantigens at Highly Conserved Sites.
The specificity of granzyme B has recently been defined using a positional scanning combinatorial tetrapeptide library 29 30. The protease has a preference for I, V, or L in P4, E, G, or S in P3, and P, S, N, A, Q, H, T, V, E, or D in P2, with a near absolute preference for D in P1. This specificity and the sizes of the fragments generated by granzyme B cleavage were used to predict cleavage sites. Using site-directed mutagenesis to make a series of P1 Asp→Ala substitutions in several of the granzyme B substrates, we addressed the effects of mutation on the efficiency of cleavage by the protease and thus defined the granzyme B cleavage sites in fibrillarin, Mi-2, topoisomerase I, PMS1, PMS2, La, and U1-70kD (Table ; Fig. 8 contains representative examples). The granzyme B cleavage sites in PARP and DNA-PKcs were defined previously 21 31. In every case, the cleavage site sequence is in accord with the specificity determined by the combinatorial library. The P2 and P3 residues in these cleavage sites were quite restricted, with prominent representation of P, A, and S in P2 and G, E, T, D, and S in P3. Interestingly, many of these residues are preferred by granzyme B but not by group III caspases 29. Using fragment sizes to predict likely granzyme B cleavage sites in other autoantigens, we readily identified probable cleavage sites in these proteins (Table ). Other than the consensus tetrapeptide sequence preceding the cleavage site in these autoantigens, there were no obvious similarities in primary sequence either upstream or downstream of this site. Interestingly, several granzyme B substrates also have consensus tetrapeptide sequences that are not cleaved, indicating that additional conformational information influences susceptibility to cleavage at these sites.
Autoantigens That Are Cleaved by Granzyme B Are Not Substrates for Caspase-8.
In addition to the granule exocytosis pathway, CTLs can also induce target cell proteolysis and apoptosis through ligation of target cell Fas by CTL Fas ligand 32. As caspase-8 is prominently activated when CTLs induce target cell death through the Fas pathway 33 and because group III caspases have a very similar substrate specificity to granzyme B 29, we determined if caspase-8 could generate the same proteolytic fragments of endogenous autoantigens in cell lysates that are generated by granzyme B. As caspase-8 efficiently activates precursor effector caspases in cell lysates (which in turn efficiently cleave downstream substrates), we first irreversibly inactivated these caspase precursors with IAA. Exogenous caspase-8 was then added in the presence of excess DTT, and substrate cleavage was assayed by immunoblotting. We initially confirmed that caspase-8 was active in the lysate system by using a coupled proteolysis system to demonstrate cleavage of downstream caspase-3 substrates (see Materials and Methods; Fig. 9). None of the autoantigens cleaved by granzyme B was cleaved by caspase-8; data for Mi-2, topoisomerase I, Ku-70, and RNA polymerase II large subunit are shown in Fig. 9.
Discussion
Autoantibodies Found in Human Autoimmune Diseases Recognize a Group of Substrates Efficiently Cleaved by Granzyme B During CTL-mediated Apoptosis.
We have demonstrated that components of 21 out of 29 well-defined autoantigens are susceptible to efficient cleavage by granzyme B, generating unique fragments. Although the cleaved molecules differ markedly in subcellular location, function, and extended primary sequence, they share two features: (a) all are autoantigens targeted by a high titer autoantibody response in human autoimmune diseases, including systematic lupus erythematosus, Sjogren's syndrome, diffuse and limited scleroderma, and autoimmune myositis; and (b) molecules are unified by containing a granzyme B cleavage site not susceptible to cleavage by caspase-8. The status of a molecule as an autoantigen and its unique susceptibility to cleavage by granzyme B but not by caspase-8 are therefore highly related (P < 0.0001, chi-square analysis). The positive predictive value of susceptibility to unique cleavage by granzyme B and status as an autoantigen is 100% for the 48 molecules studied, whereas the negative predictive value is 73%, indicating that additional mechanisms play a role in selection of some molecules as autoantigens. It is noteworthy that most of the uncleaved molecules are nucleoprotein complexes (e.g., components of nucleosomes and small nuclear ribonucleoproteins); we are in the process of defining whether these molecules undergo distinct structural modifications during unique forms of apoptotic cell death. Indeed, there are several other components of CTL granules that might play a role in this regard.
Interestingly, the autoantigens susceptible to cleavage by granzyme B include antigens that are targeted across the spectrum of autoimmune rheumatic diseases. The striking correlation of specific autoantibody response with unique biologic phenotype (e.g., Mi-2 in dermatomyositis and topoisomerase I in diffuse scleroderma) raises the question of how this disease specificity arises if a common mechanism (CTL granule–induced death) is responsible for targeting of this group of molecules. Although this remains unclear, the immunizing tissue and initiating stimulus may play important roles in focusing subsequent, self-sustaining injury.
Several of the cleaved molecules (PARP, U1-70kD, Ki-67, SRP-72, topoisomerase I [Fig. 1 A], DNA-PKcs, NuMA [reference 21], Mi-2, La, PMS1, PMS2, and NOR-90 [data not shown]) are also cleaved by effector caspases during apoptosis but at different sites in each case. Frequently, the granzyme B and caspase-3 cleavage sites are close to each other in the primary sequence. The striking linkage of susceptibility to cleavage by both caspases and granzyme B in these substrates (but at different sites) suggests that the two different families of apoptotic proteases recognize two distinct structural features of a common motif that have been targeted during independent evolution of the apoptotic cysteine and serine protease families. The likelihood that a functional motif has been targeted by the apoptosis-specific proteases is further underscored by the observation that the granzyme B cleavage sites in several molecules (e.g., fibrillarin, PARP, and PMS1) are highly conserved, even in drosophila and yeast. This striking conservation of sequence at granzyme B cleavage sites in organisms in which CTLs have not yet evolved implies that an important, as yet undefined function is served by these regions that is altered by proteolysis. This new, extended family of granzyme B substrates therefore provides a powerful tool with which to explore the evolution and biological functions of the aspartic acid–specific apoptotic proteases and the specific mechanisms underlying CTL granule–induced cell death.
Granzyme B Cleavage Sites in Autoantigens Contain Amino Acids in P2 and P3 Positions That Are Preferred by Granzyme B but Are Not Tolerated by Group III Caspases.
Using a combinatorial scanning tetrapeptide library, the specificity of granzyme B and caspases has recently been determined 29 30 and divides the caspases into three distinct groups. Group II caspases have a DXXD specificity and act downstream in apoptosis as effector proteases, cleaving substrates that have homeostatic and structural functions 34. In contrast, the group III caspases prefer tetrapeptide substrates with I, V, or L in P4, E, D, or Q in P3, and H, I, T, W, or V in the P2 position 29. Whereas granzyme B has a similar specificity to the group III caspases, it has a broader substrate specificity in the P3 and P2 positions. Thus, granzyme B will robustly cleave substrates containing G or S in P3 (not tolerated by group III caspases) and prefers P, A, N, and Q in P2 (none of which are tolerated by group III caspases). Interestingly, 10 of 11 proven granzyme B cleavage sites in autoantigens contain residues that are preferred by granzyme B but are poorly tolerated by group III caspases (molecules contain P [7], A [2], or S [1] in the P2 position; four cleavage sites also contain G or S in P3; Table ). Consistent with this observation is the demonstration that none of these substrates could be cleaved by caspase-8 (Fig. 9). In contrast, procaspase-3 and -7, which are not autoantigens, are efficiently cleaved by both caspase-8 and granzyme B at the same sites, generating identical fragments 33. This marked skewing of granzyme B cleavage sites in autoantigens away from P2 and P3 residues that are preferred by both granzyme B and group III caspases strongly suggests that unique cleavage by granzyme B plays a role in selection of targets in this spectrum of autoimmune disease.
Generation of Granzyme B–specific Autoantigen Fragments Is Favored when Caspases Are Inhibited.
We have previously demonstrated that DNA-PKcs, NuMA, and PARP are cleaved by both caspase-3 and granzyme B and that the most prominent fragments generated in lysates and intact cells reflect the relative cleavage efficiencies of the two proteases 21. The studies reported here extend those observations to numerous additional autoantigens (Mi-2, Ki-67, U1-70kD, topoisomerase I, and SRP-72; Fig. 1 A and Fig. 6). Where cleavage of a substrate by granzyme B is equal to or more efficient than that by caspase-3, granzyme B–specific fragments are generated in the LAK/K562 system (e.g., DNA-PKcs, Mi-2, and topoisomerase I; Table ). In contrast, where cleavage by granzyme B is less efficient than cleavage by caspases (e.g., PARP, U1-70kD, SRP-72, and topoisomerase I [72- and 74-kD fragments]), effective generation of the granzyme B–specific fragments is only seen in intact cells when caspase activity is inhibited. This observation focuses attention on defining potential immunizing microenvironments and proimmune insults in which such circumstances may arise in vivo. Relevant possibilities include conditions where viral or endogenous caspase inhibitors are expressed 35 36 37 38 39 40, as well as in long-lived cells or tumor cells that express low levels of specific effector caspases 41 42.
CTL Granule–induced Death and Autoimmunity.
Human _sy_stemic autoimmune diseases represent a highly complex disease spectrum, with numerous variables affecting individual susceptibility, initiation, and tissue targets. By demonstrating that the autoantigens targeted across the spectrum of these diseases are unified by their susceptibility to efficient cleavage by granzyme B, with the generation of unique fragments not produced during any other form of cell death, these studies focus attention on the role of the CTL granule pathway in initiation of autoimmunity. Where substrates are cleaved by both caspases and granzyme B, effective generation of unique granzyme B fragments is dependent upon relative inhibition of the caspases (Fig. 6; reference 21). We therefore propose that during proimmune 43 intracellular infections occurring in a microenvironment in which caspase activity is under relative inhibition, production of unique granzyme B fragments is favored. In susceptible individuals in whom clearance of apoptotic material might be impaired 44 45, suprathreshold amounts of these fragments accumulate and are effectively captured and presented by dendritic cells 46 47 48 49. The resulting immune response is directed against products of CTL granule–induced death, generating an autoamplifying injury characteristic of these self-sustaining diseases. The recent observation of tumor-specific CTLs in paraneoplastic cerebellar degeneration suggests that such a mechanism may be more broadly applicable to other autoimmune syndromes 50.
Acknowledgments
We thank Dr. Allan Gelber (Johns Hopkins University) for assistance with statistical analysis and Amy Cox and Steven Morris for excellent technical help.
These studies were supported by National Institutes of Health grants AR44684 (to L. Casciola-Rosen), DE12354 (to A. Rosen), and 5T32-AI07247 (to D. Ulanet and W.B. Wong), the SLE Foundation, the Scleroderma Research Foundation, and the Schauman Lupus Research Fund. A. Rosen is a Pew Scholar in the Biomedical Sciences and is supported by a Burroughs Wellcome Fund Translational Research award. F. Andrade is supported by a Fulbright/Consejo Nacional de Ciencia y Tecnologia, Mexico scholarship.
Address correspondence to Antony Rosen, Johns Hopkins University School of Medicine, 720 Rutland Ave., Ross 1059, Baltimore, MD 21205. Phone: 410-955-0139; Fax: 410-955-0964; E-mail: arosen@jhmi.edu
Footnotes
1used in this paper: CENP, centromere protein; CrmA, cytokine response modifier 1; DTT, dithiothreitol; GC, granule contents; FMK, fluoromethylketone; IAA, iodoacetamide; NuMA, nuclear mitotic apparatus protein; NOR, nucleolus organizer region; PARP, poly (ADP-ribose) polymerase; SRP, signal recognition particle
References
- Hardin J.A., Rahn D.R., Shen C., Lerner M.R., Wolin S.L., Rosa M.D., Steitz J.A. Antibodies from patients with connective tissue diseases bind specific subsets of cellular RNA–protein particles. J. Clin. Invest. 1982;70:141–147. doi: 10.1172/JCI110587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E.M. Antinuclear antibodiesdiagnostic markers for autoimmune diseases and probes for cell biology. Adv. Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- Casciola-Rosen L.A., Anhalt G., Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J. Exp. Med. 1994;179:1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamula M.J. The inability to process a self-peptide allows autoreactive T cells to escape tolerance. J. Exp. Med. 1993;177:567–571. doi: 10.1084/jem.177.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sercarz E.E., Lehmann P.V., Ametani A., Benichou G., Miller A., Moudgil K. Dominance and crypticity of T cell antigenic determinants. Annu. Rev. Immunol. 1993;11:729–766. doi: 10.1146/annurev.iy.11.040193.003501. [DOI] [PubMed] [Google Scholar]
- Casciola-Rosen L.A., Miller D.K., Anhalt G.J., Rosen A. Specific cleavage of the 70-kDa protein component of the U1 small nuclear ribonucleoprotein is a characteristic biochemical feature of apoptotic cell death. J. Biol. Chem. 1994;269:30757–30760. [PubMed] [Google Scholar]
- Casciola-Rosen L.A., Anhalt G.J., Rosen A. DNA-dependent protein kinase is one of a subset of autoantigens specifically cleaved early during apoptosis. J. Exp. Med. 1995;182:1625–1634. doi: 10.1084/jem.182.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greidinger E.L., Miller D.K., Yamin T.-T., Casciola-Rosen L., Rosen A. Sequential activation of three distinct ICE-like activities in Fas-ligated Jurkat cells. FEBS Lett. 1996;390:299–303. doi: 10.1016/0014-5793(96)00678-3. [DOI] [PubMed] [Google Scholar]
- Casiano C.A., Martin S.J., Green D.R., Tan E.M. Selective cleavage of nuclear autoantigens during CD95 (Fas/APO-1)-mediated T cell apoptosis. J. Exp. Med. 1996;184:765–770. doi: 10.1084/jem.184.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utz P.J., Hottelet M., Le T.M., Kim S.J., Geiger M.E., van Venrooij W.J., Anderson P. The 72-kDa component of signal recognition particle is cleaved during apoptosis. J. Biol. Chem. 1998;273:35362–35370. doi: 10.1074/jbc.273.52.35362. [DOI] [PubMed] [Google Scholar]
- Cryns V., Yuan J. Proteases to die for. Genes Dev. 1998;12:1551–1570. doi: 10.1101/gad.12.11.1551. [DOI] [PubMed] [Google Scholar]
- Izquierdo M., Grandien A., Criado L.M., Robles S., Leonardo E., Albar J.P., de Buitrago G.G., Martinez A.C. Blocked negative selection of developing T cells in mice expressing the baculovirus p35 caspase inhibitor. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:156–166. doi: 10.1093/emboj/18.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen A., Casciola-Rosen L. Autoantigens as substrates for apoptotic proteasesimplications for the pathogenesis of systemic autoimmune disease. Cell Death Differ. 1999;6:6–12. doi: 10.1038/sj.cdd.4400460. [DOI] [PubMed] [Google Scholar]
- Shi L., Kam C.-M., Powers J.C., Aebersold R., Greenberg A.H. Purification of three cytotoxic lymphocyte granule serine proteases that induce apoptosis through distinct substrate and target cell interactions. J. Exp. Med. 1992;176:1521–1529. doi: 10.1084/jem.176.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusel J.W., Wesselschmidt R.L., Shresta S., Russell J.H., Ley T.J. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell. 1994;76:977–987. doi: 10.1016/0092-8674(94)90376-x. [DOI] [PubMed] [Google Scholar]
- Shresta S., MacIvor D.M., Heusel J.W., Russell J.H., Ley T.J. Natural killer and lymphokine-activated killer cells require granzyme B for the rapid induction of apoptosis in susceptible target cells. Proc. Natl. Acad. Sci. USA. 1995;92:5679–5683. doi: 10.1073/pnas.92.12.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkart P.A. Lymphocyte-mediated cytotoxicitytwo pathways and multiple effector molecules. Immunity. 1994;1:343–346. doi: 10.1016/1074-7613(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Shresta S., Pham C.T., Thomas D.A., Graubert T.A., Ley T.J. How do cytotoxic lymphocytes kill their targets? Curr. Opin. Immunol. 1998;10:581–587. doi: 10.1016/s0952-7915(98)80227-6. [DOI] [PubMed] [Google Scholar]
- Sarin A., Williams M.S., Alexander-Miller M.A., Berzofsky J.A., Zacharchuk C.M., Henkart P.A. Target cell lysis by CTL granule exocytosis is independent of ICE/Ced-3 family proteases. Immunity. 1997;6:209–215. doi: 10.1016/s1074-7613(00)80427-6. [DOI] [PubMed] [Google Scholar]
- MacDonald G., Shi L., Vande Velde C., Lieberman J., Greenberg A.H. Mitochondria-dependent and -independent regulation of granzyme B–induced apoptosis. J. Exp. Med. 1999;189:131–144. doi: 10.1084/jem.189.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade F., Roy S., Nicholson D.W., Thornberry N.A., Rosen A., Casciola-Rosen L. Granzyme B directly and efficiently cleaves several downstream substratesimplications for CTL-induced apoptosis. Immunity. 1998;8:451–460. doi: 10.1016/s1074-7613(00)80550-6. [DOI] [PubMed] [Google Scholar]
- Casciola-Rosen L., Pluta A., Plotz P., Nagaraju K., Cox A., Morris S., Rosen A. hPMS1 is a novel, frequently targeted myositis autoantigen which is cleaved by granzyme B but not by caspases during apoptosis Arthritis Rheum. 41 1998. S127(Abstr.) [Google Scholar]
- Casciola-Rosen L.A., Nicholson D.W., Chong T., Rowan K.R., Thornberry N.A., Miller D.K., Rosen A. Apopain/CPP32 cleaves proteins that are essential for cellular repaira fundamental principle of apoptotic death. J. Exp. Med. 1996;183:1957–1964. doi: 10.1084/jem.183.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utz P.J., Anderson P. Posttranslational protein modifications, apoptosis, and the bypass of tolerance to autoantigens. Arthritis Rheum. 1998;41:1152–1160. doi: 10.1002/1529-0131(199807)41:7<1152::AID-ART3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Darmon A.J., Nicholson D.W., Bleackley R.C. Activation of the apoptotic protease CPP32 by cytotoxic T-cell-derived granzyme B. Nature. 1995;377:446–448. doi: 10.1038/377446a0. [DOI] [PubMed] [Google Scholar]
- Chinnaiyan A.M., Hanna W.L., Orth K., Duan H.J., Poirier G.G., Froelich C.J., Dixit V.M. Cytotoxic T-cell-derived granzyme B activates the apoptotic protease ICE-LAP3. Curr. Biol. 1996;6:897–899. doi: 10.1016/s0960-9822(02)00614-0. [DOI] [PubMed] [Google Scholar]
- Quan L.T., Tewari M., O'Rourke K., Dixit V., Snipas S.J., Poirier G.G., Ray C., Pickup D.J., Salveson G.S. Proteolytic activation of the cell death protease Yama/CPP32 by granzyme B. Proc. Natl. Acad. Sci. USA. 1996;93:1972–1976. doi: 10.1073/pnas.93.5.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S.J., Amarente-Mendes G.P., Shi L.F., Chuang T.H., Casiano C.A., O'Brien G.A., Fitzgerald P., Tan E.M., Bokoch G.M., Greenberg A.H. The cytotoxic cell protease granzyme B initiates apoptosis in a cell-free system by proteolytic processing and activation of the ICE/CED-3 family protease, CPP32, via a novel two-step mechanism. EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:2407–2416. [PMC free article] [PubMed] [Google Scholar]
- Thornberry N.A., Ranon T.A., Pieterson E.P., Rasper D.M., Timkey T., Garcia-Calvo M., Houtzager V.M., Nordstrom P.A., Roy S., Vaillancourt J.P. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J. Biol. Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- Harris J.L., Peterson E.P., Hudig D., Thornberry N.A., Craik C.S. Definition and redesign of the extended substrate specificity of granzyme B. J. Biol. Chem. 1998;273:27364–27373. doi: 10.1074/jbc.273.42.27364. [DOI] [PubMed] [Google Scholar]
- Froelich C.J., Hanna W.L., Poirier G.G., Duriez P.J., D'Amours D., Salvesen G.S., Alnemri E.S., Earnshaw W.C., Shah G.M. Granzyme B perforin-mediated apoptosis of jurkat cells results in cleavage of poly(ADP-ribose) polymerase to the 89-kDa apoptotic fragment and less abundant 64-kDa fragment. Biochem. Biophys. Res. Commun. 1996;227:658–665. doi: 10.1006/bbrc.1996.1565. [DOI] [PubMed] [Google Scholar]
- Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- Salvesen G.S., Dixit V.M. Caspasesintracellular signaling by proteolysis. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- Nicholson D.W., Thornberry N.A. Caspaseskiller proteases. TIBS (Trends Biochem. Sci.). 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- Beidler D.R., Tewari M., Friesen P.D., Poirier G., Dixit V.M. The baculovirus p35 protein inhibits Fas- and tumor necrosis factor-induced apoptosis. J. Biol. Chem. 1995;270:16526–16528. doi: 10.1074/jbc.270.28.16526. [DOI] [PubMed] [Google Scholar]
- Bump N.J., Hackett M., Hugunin M., Seshagiri S., Brady K., Chen P., Ferenz C., Franklin S., Ghayur T., Li P. Inhibition of ICE family proteases by baculovirus antiapoptotic protein p35. Science. 1995;269:1885–1888. doi: 10.1126/science.7569933. [DOI] [PubMed] [Google Scholar]
- Deveraux Q.L., Roy N., Stennicke H.R., Van Arsdale T., Zhou Q., Srinivasula S.M., Alnemri E.S., Salvesen G.S., Reed J.C. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:2215–2223. doi: 10.1093/emboj/17.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasula S.M., Ahmad M., Ottilie S., Bullrich F., Banks S., Wang Y., Fernandes-Alnemri T., Croce C.M., Litwack G., Tomaselli K.J. FLAME-1, a novel FADD-like anti-apoptotic molecule that regulates Fas/TNFR1-induced apoptosis. J. Biol. Chem. 1997;272:18542–18545. doi: 10.1074/jbc.272.30.18542. [DOI] [PubMed] [Google Scholar]
- Irmler M., Thome M., Hahne M., Schneider P., Hofmann B., Steiner V., Bodmer J.L., Schröter M., Burns K., Mattmann C. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- Thome M., Schneider P., Hofmann K., Fickenscher H., Meinl E., Neipel F., Mattmann C., Burns K., Bodmer J.L., Schröter M. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- Krajewska M., Wang H.G., Krajewski S., Zapata J.M., Shabaik A., Gascoyne R., Reed J.C. Immunohistochemical analysis of in vivo patterns of expression of CPP32 (caspase-3), a cell death protease. Cancer Res. 1997;57:1605–1613. [PubMed] [Google Scholar]
- Krajewski S., Gascoyne R.D., Zapata J.M., Krajewska M., Kitada S., Chhanabhai M., Horsman D., Berean K., Piro L.D., Fugier-Vivier I. Immunolocalization of the ICE/Ced-3-family protease, CPP32 (caspase-3), in non-Hodgkin's lymphomas, chronic lymphocytic leukemias, and reactive lymph nodes. Blood. 1997;89:3817–3825. [PubMed] [Google Scholar]
- Matzinger P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- Herrmann M., Voll R.E., Zoller O.M., Hagenhofer M., Ponner B.B., Kalden J.R. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41:1241–1250. doi: 10.1002/1529-0131(199807)41:7<1241::AID-ART15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Botto M., Dell'Agnola C., Bygrave A.E., Thompson E.M., Cook H.T., Petry F., Loos M., Pandolfi P.P., Walport M.J. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- Albert M.L., Pearce S.F.A., Francisco L.M., Sauter B., Roy P., Silverstein R.L., Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36 and cross-present antigens to cytotoxic T lymphocytes. J. Exp. Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M.L., Sauter B., Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- Inaba K., Turley S., Yamaide F., Iyoda T., Mahnke K., Inaba M., Pack M., Subklewe M., Sauter B., Sheff D. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J. Exp. Med. 1998;11:2163–2173. doi: 10.1084/jem.188.11.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovere P., Vallinoto C., Bondaza A., Crosti M.C., Rescigno M., Ricciardi-Castagnoli P., Rugarli C., Manfredi A.A. Bystander apoptosis triggers dendritic cell maturation and antigen-presenting function. J. Immunol. 1998;161:4467–4471. [PubMed] [Google Scholar]
- Albert M.L., Darnell J.C., Bender A., Francisco L.M., Bhardwaj N., Darnell R.B. Tumor-specific killer cells in paraneoplastic cerebellar degeneration. Nat. Med. 1998;11:1321–1324. doi: 10.1038/3315. [DOI] [PubMed] [Google Scholar]