Early Activation of Caspases during T Lymphocyte Stimulation Results in Selective Substrate Cleavage in Nonapoptotic Cells (original) (raw)
Abstract
Apoptosis induced by T cell receptor (TCR) triggering in T lymphocytes involves activation of cysteine proteases of the caspase family through their proteolytic processing. Caspase-3 cleavage was also reported during T cell stimulation in the absence of apoptosis, although the physiological relevance of this response remains unclear. We show here that the caspase inhibitor benzyloxycarbonyl (Cbz)-Val-Ala-Asp(OMe)-fluoromethylketone (zVAD) blocks proliferation, major histocompatibility complex class II expression, and blastic transformation during stimulation of peripheral blood lymphocytes. Moreover, T cell activation triggers the selective processing and activation of downstream caspases (caspase-3, -6, and -7), but not caspase-1, -2, or -4, as demonstrated even in intact cells using a cell-permeable fluorescent substrate. Caspase-3 processing occurs in different T cell subsets (CD4+, CD8+, CD45RA+, and CD45RO+), and in activated B lymphocytes. The pathway leading to caspase activation involves death receptors and caspase-8, which is also processed after TCR triggering, but not caspase-9, which remains as a proenzyme. Most importantly, caspase activity results in a selective substrate specificity, since poly(ADP-ribose) polymerase (PARP), lamin B, and Wee1 kinase, but not DNA fragmentation factor (DFF45) or replication factor C (RFC140), are processed. Caspase and substrate processing occur in nonapoptotic lymphocytes. Thus, caspase activation is an early and physiological response in viable, stimulated lymphocytes, and appears to be involved in early steps of lymphocyte activation.
Keywords: T cell receptor, proliferation, apoptosis, protease, inhibitor
Cysteine proteases of the IL-1β–converting enzyme (ICE) family (caspases) are critical executioners of apoptosis in several mammalian cell death pathways 1. At present, 14 human and murine caspases have been isolated, and the exact role of each one during apoptosis has yet to be fully characterized. Caspases are synthesized as inactive precursors that require proteolytic conversion to become active proteases. For instance, during apoptosis triggered by TCR cross-linking in T lymphocytes, the 32-kD caspase-3 proenzyme is first cleaved to release fragments of 12 and 20 kD. Removal of the 3-kD propeptide from the p20 generates the p17 form associated with caspase-3 activity and apoptosis 2 3. Caspase-3 cleavage also occurs in vivo during negative selection of thymocytes through TCR signaling, where only the p17 is detected 4 5. This mechanism prevails in a wide variety of cellular systems, and is triggered upon induction of apoptosis by several exogenous stimuli 1.
The current model suggests that after apoptotic stimulation, activation of “upstream” caspases containing a large prodomain such as caspase-8, -2, or -9 leads to the proteolytic cleavage of “downstream” caspases (caspase-3, -6, and -7), which mediate the apoptotic response 1 6. Another caspase subfamily including caspase-1, -5, -11, -12, and -13 is essentially involved in cytokine maturation, and does not seem to play a critical role during apoptosis. Caspase activity results in the cleavage of several cellular proteins, which leads to the apoptotic response (for a review, see reference 7). These substrates include structural proteins such as gelsolin 8, proteins involved in DNA repair, such as poly(ADP-ribose) polymerase (PARP) 2 9 or DNA-dependent protein kinase (DNA-PK 10), and proteins involved in cell cycle regulation, such as the 140-kD subunit of replication factor C (RFC140 [11]), mouse double minute 2 (MDM2 12), or the cell division control 2 (Cdc2) kinase Wee1 13. A critical substrate for caspase-3 during apoptosis is the 45-kD subunit of DNA fragmentation factor (DFF45) that once cleaved by caspase-3 activates DNA fragmentation by releasing the caspase-activated DNase 14 15 16.
Surprisingly, caspase-3 processing and activity have been observed in the absence of apoptosis after polyclonal activation of PBMCs, and in the absence of DNA fragmentation 17 18. However, there is no evidence yet for a role of caspases during T cell activation, and a recent report suggested that the observed caspase processing was due to the production of granzyme B by CD8+ activated cells and artifactual release during cell lysis of PBMCs 19. Interestingly, such a role for caspases in T cell activation is indirectly supported by several recent observations. Indeed, interference with pathways leading to caspase processing, as in Fas-associated death domain protein (FADD) knockout, FADD dominant negative, or Bcl-2 transgenic mice, results in impaired mature T cell proliferation 20 21 22 23. Moreover, overexpression of Bcl-2 also delays the reentry of resting NIH 3T3 cells into cell cycle 24. These data suggest that inactivation of pathways leading to caspase activity results in reduced cell cycle progression in primary T lymphocytes and in cell lines.
In this study, we have investigated whether caspase processing was a physiological response after TCR triggering. We show that a caspase inhibitor blocks T cell activation, that caspase-3 and other downstream caspases are processed in stimulated lymphocytes, and that a selective cleavage of caspase substrates occurs in viable and proliferating cells. These results show that caspase activation is a physiological response to TCR triggering, and indicate that this enzymatic cascade is not only involved in induction of cell death, but might also be involved in the early steps leading to lymphocyte proliferation.
Materials and Methods
Reagents and Antibodies.
The broad spectrum benzyloxycarbonyl (Cbz)-Val-Ala-Asp(OMe)-fluoromethylketone (zVAD), the caspase-3–like Cbz-Asp-Glu-Val-Asp(OMe)-fluoromethylketone (zDEVD) or caspase-8–like Cbz-Ileu-Glu-Thr-Asp(OMe)-fluoromethylketone (zIETD) caspase inhibitors, and the biotinylated zVAD were purchased from Enzyme Systems Products. IL-2 was obtained from the National Institutes of Health AIDS Research and Reference Reagent Program, and PHA from Murex Diagnostics. Staphylococcus aureus Cowan fixed cells (SAC) were purchased from Calbiochem. Rabbit antiserum against caspase-3 was generated as described previously 5. The anti–caspase-8 and anti-DFF45 antisera are rabbit polyclonal antibodies generated in the laboratory against the p18 of caspase-8 and the full-length DFF45, respectively. The anti-PARP antiserum, a second anti–caspase-8 serum, the anti-lamin B, and anti-Fas (M3) mAbs were gifts from Dr. G. Poirier (Centre Hospitalier de l'Université Laval, Québec, Canada), Dr. M.E. Peter (German Cancer Research Center, Heidelberg, Germany), Dr. R. Bertrand (Centre de Recherche du Centre Hospitalier de l'Université de Montréal), and Dr. D. Lynch (Immunex Corp., Seattle, WA), respectively. Dr. D.W. Nicholson (Merck Frosst, Kirkland, Canada) provided antisera against caspase-1, -6, -7, and -9. The mAbs against caspase-2, caspase-4, and Wee1 were purchased from Transduction Laboratories, Santa Cruz Biotechnology, and StressGen Biotechnologies Corp., respectively. The anti-CD3 was produced and purified from the OKT3 clone (American Type Culture Collection).
PBMC Isolation and Proliferation Assay.
PBMCs were purified by Ficoll-Hypaque and resuspended in 10% FCS-RPMI medium (GIBCO BRL). To monitor proliferation, 105 cells were cultured for 1–4 d at 37°C in 96-well plates in the absence or presence of 1 μg/ml anti-CD3, anti-TCR (BMA031; Immunotech), or PHA and 20 U/ml recombinant human IL-2. For B cell stimulation, PBMCs were cultured in the presence of SAC (1:104 vol/vol). 3H-labeled thymidine (1 μCi/well) was added during the last 6 h for each time point. Cells were harvested, and DNA-associated radioactivity was counted by liquid scintillation (Betaplate™; Wallac) and expressed as a mean cpm ± SEM of triplicate cultures.
Flow Cytometry.
Cell staining for T cell activation markers was performed on PBMCs activated as described for 4 d, and using anti-CD69, anti-CD25 (Becton Dickinson), or anti–HLA-DR (Caltag Laboratories) antibodies. Cell sorting was performed after 4 d of stimulation with 1 μg/ml anti-CD3 and 20 U/ml IL-2, using PBMCs stained with annexin V (AV)-FITC (BIODESIGN International). Living (AV−) and dying or dead cells (AV+) were sorted using a FACStar™ (Becton Dickinson) after gating on lymphocytes on the basis of forward/side scatter. Purified lymphocyte subsets from treated PBMCs were obtained by cell sorting after staining with anti-CD4, anti-CD8, anti-CD19 (Becton Dickinson), or anti-CD45RO and anti-CD45RA (Serotec Ltd.) antibodies. For T cell subset sorting, dead cells were gated out on the basis of forward/side scatter, and the purity of sorted T cell subsets was >99%. For each sample, 104 events were collected using the FACScan™ flow cytometer.
Staining for in vivo caspase activity was performed using the cell-permeable substrate Phiphilux-G1D2 (OncoImmunin, Inc.) containing the consensus sequence DEVDG. In brief, resting or activated cells were washed, then incubated with the substrate (10 μM final) for 1 h at 37°C, followed by another wash with the dilution buffer, according to the manufacturer's instructions. Fluorescence was monitored using the FL1 detector of an EPICS® XL flow cytometer (Coulter Corp.), and gates were set on living and either resting or blastic cells before analysis. For each sample, 3 × 104 events were collected.
Western Blotting.
Treated cells were washed with PBS, and pellets were resuspended in sample buffer (62.5 mM Tris-HCl, pH 6.8, 6 M urea, 10% glycerol, 2% SDS, 0.00125% bromophenol, 5% 2-ME) and boiled for 3 min. Proteins from 0.5–1 × 106 cells were separated on SDS-PAGE and transferred to nitrocellulose membrane (Hybond C Super; Amersham). Blots were blocked for 1 h at room temperature in PBS, 0.05% Tween, containing 5% nonfat dried milk, washed, and incubated with the different antibodies. Detection was achieved with the appropriate secondary antibodies coupled to horseradish peroxidase (HRP), followed by enhanced chemiluminescence ECL Western blotting kit (NEN), and visualized by autoradiography.
Immunoprecipitation and Affinity Blot.
Proteins from PBMCs (5 × 106 cells), stimulated with 1 μg/ml PHA and 20 U/ml IL-2 for 4 d, were extracted in lysis buffer (2% NP-40, 0.5% deoxycholic acid, 50 mM Tris, pH 7.5, 150 mM NaCl, 50 mM NaF, 10 mM NaH2PO4, 2 mM EGTA, 10 mM EDTA, 0.5 mM Na3VO4). Cell lysates were incubated with 5 μM of biotinylated zVAD-fmk and subjected or not to immunoprecipitation using the caspase-3 antiserum. Protein G–Sepharose-coupled beads (Amersham Pharmacia Biotech) were preincubated for 30 min at 4°C with 10 μl of anti–caspase-3 antiserum. After three washes with lysis buffer, the beads were added to total cell lysates for a 2-h incubation at 4°C with gentle agitation. The beads were then washed three times with lysis buffer and boiled for 5 min in 50 μl of Laemmli buffer. Whole cell lysates or immunoprecipitated proteins were loaded on polyacrylamide gels for electrophoresis and transferred to Hybond C membranes. Blots were blocked overnight at 4°C with PBS, Tween 0.05% supplemented with BSA (3%) and nonfat dry milk (2%), and revealed after incubation with HRP-conjugated extravidin (Extra-HRP, 1:2,000; Sigma Chemical Co.) for 1 h at room temperature for affinity blot, or with anti–caspase-3 antiserum for Western blot as described above.
Fas Treatment.
Jurkat cells or PBMCs stimulated for 4 d with anti-CD3 antibody and IL-2 were incubated at 106 cells/well in 24-well plates coated or not with anti-Fas (M3) mAb. Coating was performed for 2 h at 37°C using 20 μg/ml of antibody in 0.05 M Tris, pH 9.3. After 4 h (Jurkat cells) or 6 h (PBMCs) of incubation on coated plates, cells were washed with PBS, and the pellet was kept at −80°C until Western blot analysis. An aliquot of stimulated cells was used for AV staining as described previously 11.
Results
The Caspase Inhibitor zVAD Blocks Anti-CD3–induced T Cell Activation.
Several reports have demonstrated a costimulatory function of TNF receptor (TNFR) family members, such as Fas and TNFR, which are also associated with apoptosis through activation of the caspase cascade 25 26 27. Whether caspases play a role in the costimulatory function of TNFR family members has yet to be demonstrated, although caspase-3 processing has been reported after T cell stimulation 17. To determine whether caspase-3 activation was involved in the response to TCR triggering, we used the broad spectrum caspase inhibitor zVAD 4 5 11 28. Stimulation of PBMCs with anti-CD3 antibody and IL-2 in the presence of zVAD resulted in a dose-dependent and reproducible inhibition of T cell proliferation (76.3 ± 7.7% inhibition at day 4 with 100 μM of zVAD, n = 8 [Fig. 1 A]) and a 2.6-fold decrease in the percentage of cells in S phase after 4 d of stimulation, as assessed by DNA content analysis using propidium iodide (PI) staining (data not shown). Similar results were obtained using zIETD, an inhibitor of caspase-8–like members, or the caspase-3–like protease inhibitor zDEVD (inhibition of >50% of the proliferation with 100 μM in three independent experiments; data not shown).
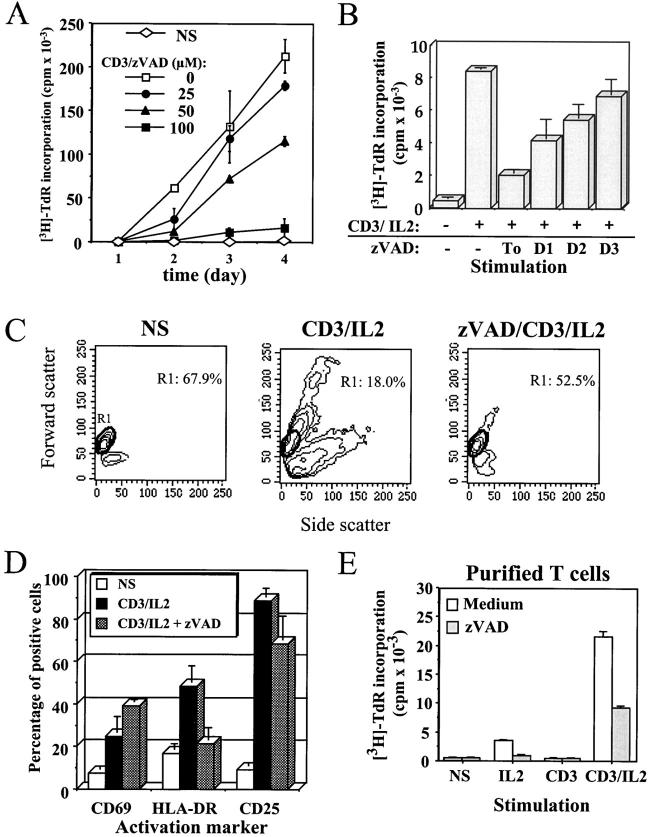
Figure 1.
The general caspase inhibitor zVAD blocks anti-CD3–induced T cell activation. (A) Inhibition of PBMC proliferation by zVAD. Purified PBMCs were cultured in medium alone (NS) or stimulated with 1 μg/ml anti-CD3 and 20 U/ml IL-2 (CD3) in the presence of the indicated concentration of zVAD. At each time point, 1 μCi/well of [3H]thymidine was added for the last 6 h of culture, cells were harvested, and incorporated thymidine was counted by liquid scintillation. [3H]Thymidine ([3H]-TdR) incorporation is expressed as mean cpm ± SEM of triplicate cultures. Results are representative of three experiments where full kinetics was carried out. (B) zVAD does not inhibit cell cycle machinery. Fresh PBMCs were placed in RPMI medium, and 100 μM of zVAD was added 1 h before or 1, 2, or 3 d after stimulation in the presence of anti-CD3 antibody and IL-2. Cells were cultured for 4 d at 37°C, and [3H]thymidine was added for the last 6 h of incubation. Thymidine incorporation at day 4 is expressed as cpm ± SEM from triplicate cultures. Two independent experiments gave similar results. (C) Inhibition of blastic transformation by zVAD. Change in PBMCs morphology was monitored by flow cytometric analysis of cellular forward (y-axis) and side (x-axis) scatter. Percentage of resting PBMCs (region R1) is indicated inside dot plots. One representative experiment out of five is shown. (D) zVAD affects the expression of MHC class II. Surface staining was performed for CD69, HLA-DR, and CD25 on PBMCs activated for 4 d with anti-CD3/IL-2, in the presence or absence of 100 μM of zVAD. One representative experiment of three is shown. (E) The effect of zVAD is independent of accessory cells. Purified T lymphocytes isolated from PBMCs by negative selection were preincubated for 1 h in medium alone or with 100 μM zVAD, and cultured for 4 d without stimulation (NS) or in the presence of IL-2, anti-CD3, or both. Proliferation was assessed by [3H]thymidine incorporation as described in A. Results are representative of two independent experiments.
To exclude the possibility that zVAD exerts a general cytostatic effect, the caspase inhibitor was added at 1–3 d after stimulation of PBMCs, and DNA synthesis was assessed after 4 d of culture. Inhibition of T cell proliferation by zVAD was much less significant when added 2 or 3 d after T cell activation (Fig. 1 B), indicating that it did not inhibit a component of the cell cycle machinery, and was not endowed with a nonspecific cytostatic activity. A toxic effect of zVAD was also excluded, since after 4 d of stimulation, zVAD could block up to 73% of the proliferation without affecting cell viability, as assessed at day 2 or 4 by AV/PI staining (% AV− cells was 56.7% with zVAD vs. 57.3% in the absence of zVAD). On the contrary, in the same experiment the serine protease inhibitor _N_-_p_-tosyl-l-lysine chloromethyl ketone (TLCK) also blocked DNA synthesis, but induced a high level of cell death (up to 90% AV+ cells; data not shown). Anti-CD3–induced blastic transformation was also abolished by zVAD, as assessed by light scatter properties in flow cytometry (Fig. 1 C), confirming that inhibition of caspase activity blocked an early and critical step of T cell activation. Addition of zVAD also resulted in the accumulation of cells expressing the early and transient activation marker CD69, whereas surface expression of the late activation marker HLA-DR was inhibited (Fig. 1 D). Induction of CD25 (the α chain of the high-affinity IL-2 receptor) and CD95 upregulation remained comparable in the presence or absence of zVAD (Fig. 1 D, and data not shown). Since an excess of IL-2 is added at the onset of T cell activation, and since the expression of CD25 is maintained in the presence of zVAD, it appears that the caspase inhibitor does not block T cell proliferation by interfering with the IL-2–IL-2R autocrine loop. As illustrated in Fig. 1 D, the lack of effect of zVAD on the de novo expression of CD69 and CD25, and the fact that Jurkat cell proliferation was not affected by addition of zVAD (data not shown) further confirm that zVAD effect on T cell proliferation cannot be attributed to a general cytostatic effect.
Engagement of CD3 on PBMCs induces the production of cytokines by monocytes such as IL-1β and IFN-γ inducible factor (IGIF)/IL-18, which in turn will enhance T cell activation 29 30. Since production of these cytokines is dependent on caspase-1 (ICE) activity 31 32, inhibition of T cell proliferation by zVAD could be due to a lower level of costimulatory cytokines produced by monocytes. To exclude this possibility, purified T cells (>95% TCR+) were stimulated with anti-CD3 and IL-2 in the presence of zVAD. Proliferation of these T cells triggered by anti-CD3 and IL-2 was also blocked by the caspase inhibitor (Fig. 1 E), confirming that zVAD was directly inhibiting T cell activation, and not accessory cell function. Taken together, these results show that inhibition of caspase activity resulted in defective T cell activation and impaired proliferation after TCR triggering of resting T cells.
Early Processing of Caspase-3 during Activation of Resting Lymphocytes.
To rule out the possibility that caspase-3 processing induced by TCR triggering resulted from a lysis artifact 19, anti-CD3–activated PBMCs were boiled directly in Laemmli buffer containing 2% SDS, 5% mercaptoethanol, and 8 M urea, and cell lysates were subjected to Western blot analysis. Even under these denaturing conditions, caspase-3 proenzyme was reproducibly found (n = 10) completely processed into protein species ranging from 24 to 17 kD after TCR triggering of PBMCs (Fig. 2). These species included the 17-kD form previously associated to the induction of apoptosis 2 3 4 5 and a doublet migrating at ∼20 kD. Processing of caspase-3 occurred as early as day 1 after TCR stimulation and before the detection of DNA synthesis (Fig. 2 A). None of these bands was observed in resting T cells. The same caspase-3 pattern was also obtained after anti-TCR or PHA/IL-2 stimulation (data not shown), confirming previous results 17. A role for granzyme B in the processing of caspase-3 during cell lysis was excluded by using the same denaturing lysis buffer in all experiments.
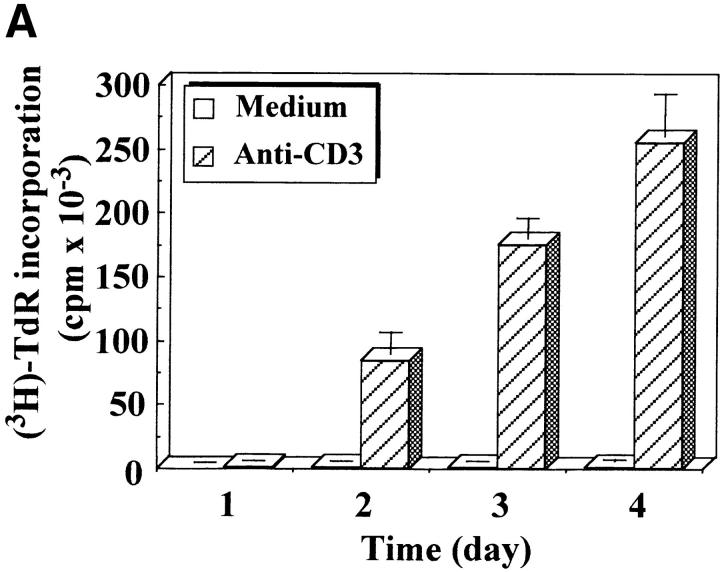
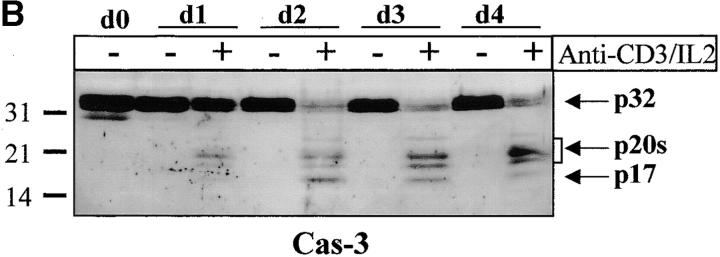
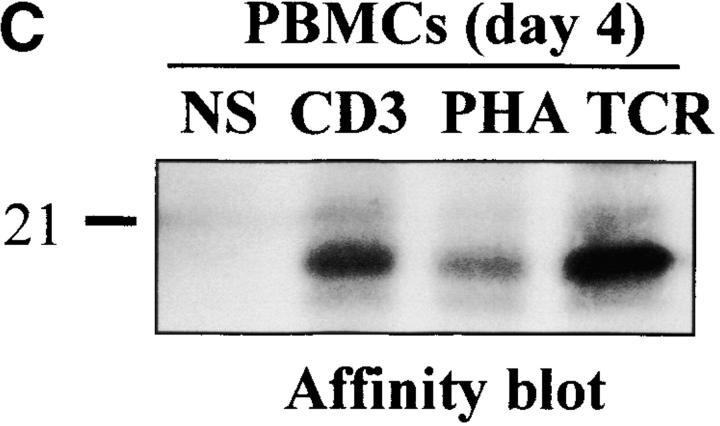
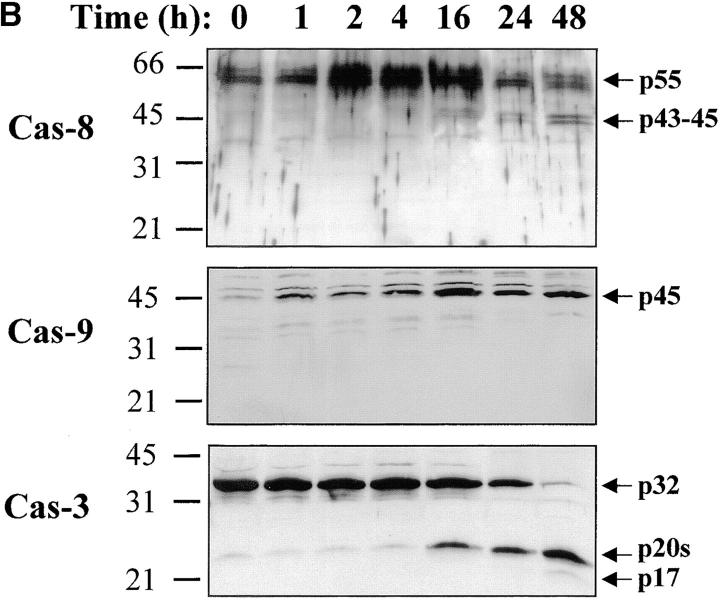
Figure 2.
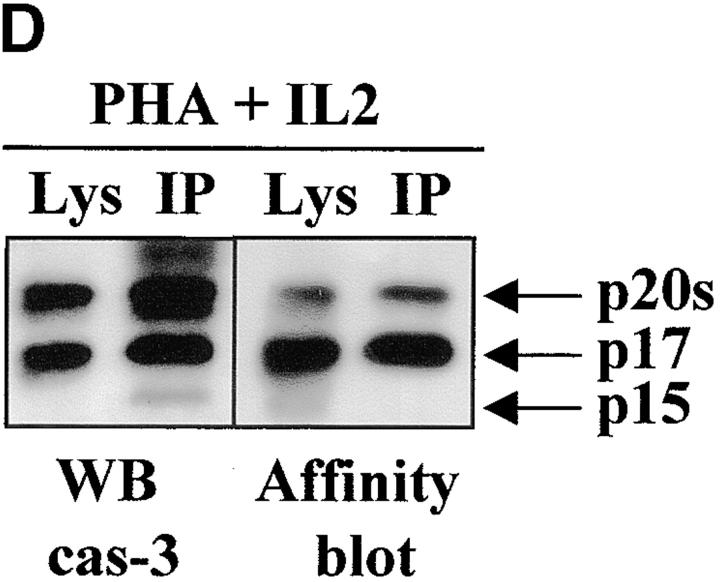
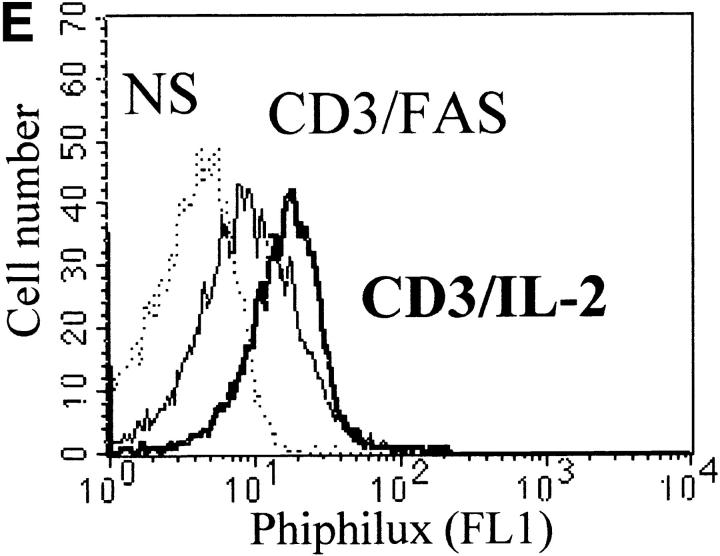
Caspase-3 is cleaved into multiple fragments in stimulated lymphocytes. (A) Proliferation. Purified PBMCs were obtained through Ficoll gradient separation and stimulated with 1 μg/ml of anti-CD3 and 20 U/ml of IL-2 (Anti-CD3) or not (Medium). After 1–4 d at 37°C, thymidine incorporation was determined as in the legend to Fig. 1. (B) Caspase-3 Western blot. Proteins were extracted from the same PBMCs treated in A by cell lysis using a denaturing Laemmli buffer containing 8 M urea. Total proteins extracted from 106 fresh (d0), unstimulated (−), or stimulated cells (+) were separated on SDS-PAGE, and Western blot analysis was performed using a polyclonal rabbit anti–caspase-3 (reference 5). (C) Detection of active caspases by affinity blot. PBMCs stimulated or not for 4 d with anti-CD3, anti-TCR, or PHA in the presence of IL-2 were lysed using a nondenaturing lysis buffer (see Materials and Methods), and lysates were incubated with 5 μM of a biotinylated zVAD peptide, followed by a separation by SDS-PAGE and Western blot analysis using Extra-HRP. (D) Identification of active subunits of caspase-3. PBMCs were activated for 4 d with PHA and IL-2, and cell lysates were incubated with the substrate. Caspase-3 immunoprecipitation was then performed on half of the lysate. Total extract (Lys) or immunoprecipitated caspase-3 (IP) was subjected to Western blot analysis using the anti–caspase-3 antibody (left, WB cas-3) or with Extra-HRP (right, Affinity blot). Results are from one of at least three independent experiments giving similar results. (E) Detection of caspase activity in intact cells. Fresh PBMCs were left untreated (NS) or stimulated (CD3/IL-2) with anti-CD3 antibody and IL-2 for 4 d. A fraction of these activated cells was then incubated for 6 h in anti-Fas–coated plates (CD3/Fas). After a 1-h labeling with the cell-permeable substrate Phiphilux, the cells were analyzed by flow cytometry. Results are from one representative experiment out of three.
To determine which of these bands constituted the active enzyme, their ability to bind to a peptidic substrate was assessed by affinity blot. The affinity blot technique takes advantage of the ability of peptides mimicking caspase substrates to bind irreversibly to the active caspases, but not to their precursors 33. Fresh PBMCs were stimulated for 4 d with anti-CD3, anti-TCR antibody, or PHA in the presence of IL-2, and cell lysates were incubated with a biotinylated zVAD peptide. Proteins were separated by SDS-PAGE, and the blot was probed using Extra-HRP. Three forms ranging from 17 to 21 kD were detected in lysates of activated, but not resting cells (Fig. 2 C). To verify whether these proteins originated from caspase-3, the caspase was immunoprecipitated from lysates of PHA-stimulated PBMCs using the antiserum, and whole lysates or immunoprecipitated proteins were separated on SDS-PAGE. Western blot analysis (Fig. 2 D, left) shows that the immunoprecipitate contained a doublet at 20 kD, as well as the 17-kD subunit and a 15-kD band sometimes observed in late apoptotic Jurkat cells using this antiserum (data not shown). When the membrane was probed with Extra-HRP, only the 17-kD band and the upper band from the doublet were detected (Fig. 2 D, right), showing that both caspase-3 forms were able to bind to the synthetic substrate.
Activation of caspase-3 was also assessed in intact cells using the cell-permeable caspase substrate Phiphilux, containing the caspase-3 consensus sequence GDEVDGI 34. Fluorescence of resting or stimulated PBMCs was compared by flow cytometry after staining in the presence of the substrate. Fig. 2 E shows that Phiphilux was cleaved in T cells 4 d after anti-CD3 stimulation. Similar results were also obtained as early as 2 d of stimulation, in which a 4.7-fold increase in mean fluorescence intensity was observed (mean fluorescence intensity of 27.3 vs. 5.8 for resting cells). Interestingly, induction of apoptosis by anti-Fas antibody did not result in increased Phiphilux cleavage in activated PBMCs (Fig. 2 E), suggesting that caspase activity was already elevated in proliferating cells. Therefore, caspase-3 cleavage in stimulated lymphocytes corresponded to activation of the proenzyme.
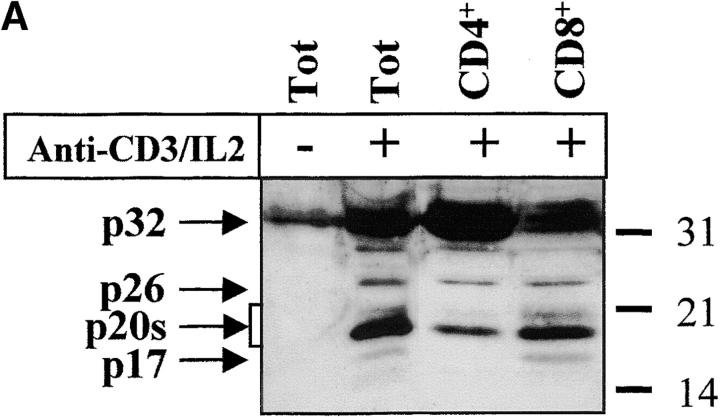
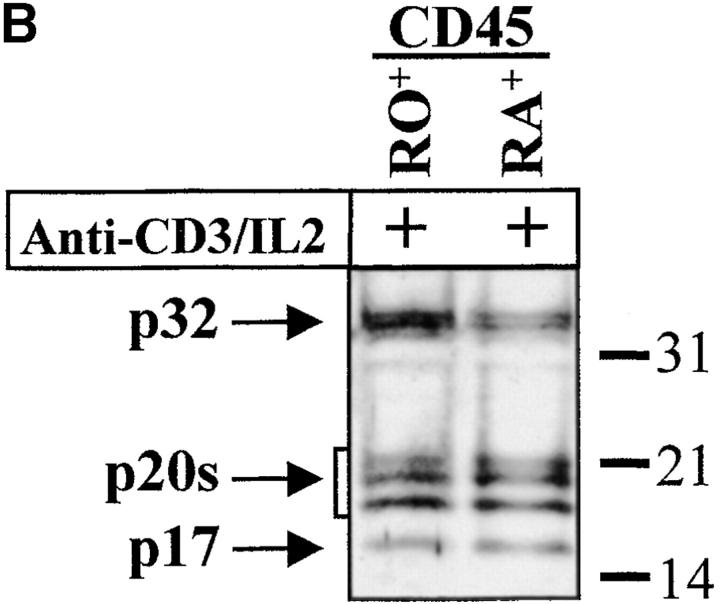
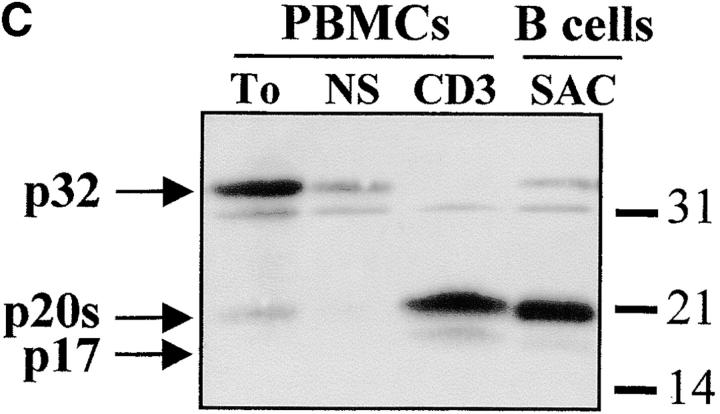
The complex pattern of caspase-3 subunits suggested that they could originate from several proenzyme species observed in nonstimulated cells (Fig. 2 B), or from different cell subsets. To distinguish between these two possibilities, sorting of CD4+, CD8+, CD45RO+, and CD45RA+ cells was performed on viable, blastic cells. Processing of caspase-3 was similar in both CD4 and CD8 T cell subsets and occurred not only in CD45RO+, but also in the remaining CD45RA+/CD25+ cells (Fig. 3a and Fig. b), further confirming that caspase activation was an early event after TCR triggering. When PBMCs were stimulated for 4 d with the B cell mitogen SAC, and B lymphocytes were purified by CD19+ cell sorting, caspase-3 cleavage was observed in sorted, viable cells and resulted in the production of the 20-kD doublet and the 17-kD large subunit as in activated T lymphocytes (Fig. 3 C). These results indicate that caspase-3 processing occurs in several T cell subsets and in activated B cells.
Figure 3.
Caspase-3 activation occurs in various lymphocyte subsets. (A and B) Caspase cleavage is detected in CD4+, CD8+, CD45RO+, or CD45RA+ lymphocytes. PBMCs were stimulated (+) or not (−) with anti-CD3 and IL-2 for 4 d at 37°C, and stained for CD4, CD8, CD45RO, or CD45RA expression. Each subset was sorted by flow cytometry after gating on living cells on the basis of forward/side scatter. The purity of sorted cells was >99%. Total population (Tot) or sorted cells were then lysed as in the legend to Fig. 3, and subjected to Western blot analysis for caspase-3 processing. (C) Caspase-3 is cleaved after B cell receptor triggering. Fresh PBMCs (To) were cultured with medium alone (NS), anti-CD3 and IL-2 (CD3), or SAC for 4 d, and whole PBMCs (lane 1–3) or sorted CD19+ B lymphocytes (lane 4) were lysed in Laemmli buffer and subjected to Western blot analysis using the anti–caspase-3 antibody. After cell sorting, viability was >95% as assessed by trypan blue exclusion. These results are representative of four (A and B) and two (C) independent experiments with PBMCs from different individuals.
Selective Activation of the Caspase Cascade after TCR Triggering.
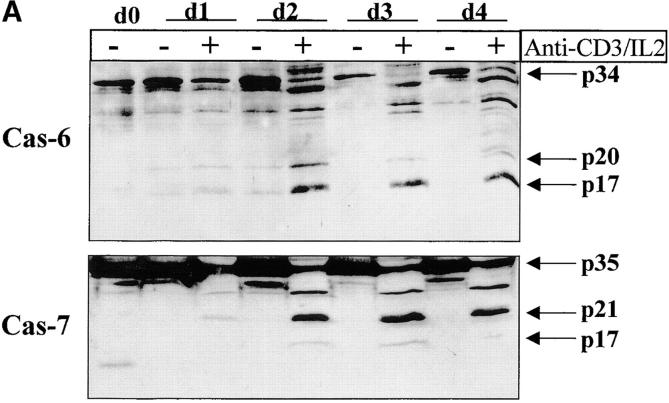
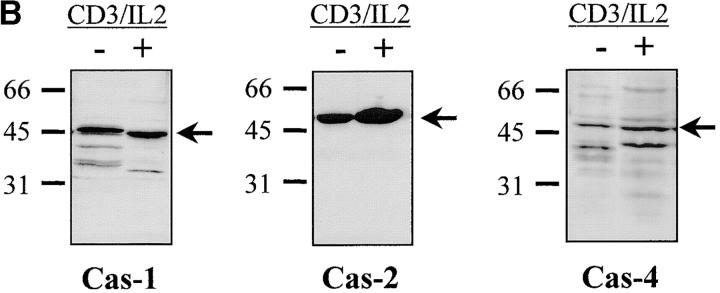
Having confirmed caspase-3 activation in stimulated T lymphocytes, we next considered whether other caspases could also be activated under the same conditions. Western blot analysis was performed on lysates of unstimulated or activated PBMCs, using a panel of antibodies recognizing several caspases. Fig. 4 A shows that caspase-6 and caspase-7 were also cleaved into their active subunits after TCR triggering. A decrease in levels of both proenzymes (p34 for caspase-6 and p35 for caspase-7) already occurred after 24 h of stimulation, and their cleaved subunits (p20 and p17) were clearly detected 24 h later. However, caspase processing was selective since caspase-1, caspase-2, and caspase-4 remained as proenzymes under these same conditions (Fig. 4 B). These results further exclude a role for granzyme B since one of its substrates, caspase-2 35, remained as a proenzyme in activated cell lysates.
Figure 4.
Selective downstream caspase processing in activated lymphocytes. (A) Caspase-6 and -7 are also processed after TCR triggering. PBMCs were stimulated (+) or not (−) with anti-CD3 antibody and IL-2 for 1–4 d, and total proteins from 106 cells were separated by SDS-PAGE and analyzed by Western blot using anti–caspase-6 or -7 antisera. The proenzymes and cleaved subunits are indicated on the right by arrows. (B) All caspases are not processed in activated cells. Day 4 unstimulated (−) or anti-CD3/IL-2 stimulated (+) PBMCs were analyzed for caspase-1, -2, or -4 processing by Western blot, as in A.
Processing of Effector Caspases Results from the Activation of Caspase-8.
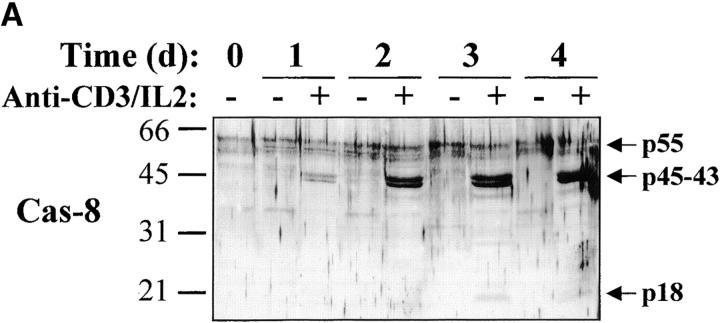
Activation of the downstream caspases during apoptosis is usually triggered by either caspase-8 (associated to the cytoplasmic tail of death receptors) or caspase-9 (activated through cytochrome c release from mitochondria), depending on the apoptotic stimulus 36 37 38 39. To identify the pathway involved in caspase-3, -6, and -7 activation during T lymphocyte stimulation, Western blot analysis was performed using antibodies against caspase-8 or caspase-9. Fig. 5 shows that after stimulation of PBMCs, caspase-8 was fragmented into a 43–45-kD doublet corresponding to the large subunit of caspase-8 linked to the propeptide 40, whereas the 18-kD large subunit was detected only at day 3 (observed in at least three independent experiments), when levels of DNA synthesis were already high (Fig. 1 A and Fig. 2). This result was confirmed using another anti–caspase-8 serum also specific for the p18 (data not shown). In the same extracts, caspase-9, which was upregulated after TCR triggering, remained as a proenzyme (Fig. 5 B), whereas caspase-8 and -3 were processed as early as 16 h after T cell activation. Therefore, these results suggest that caspase-8–mediated processing of the downstream caspases was triggered through upregulation of death receptor ligands and FADD recruitment after T cell activation.
Figure 5.
Caspase-8 is cleaved in activated lymphocytes, whereas caspase-9 remains as a proenzyme. (A) Caspase-8 is processed in proliferating cells. PBMCs were stimulated (+) or not (−) with anti-CD3 antibody and IL-2 for 1–4 d, and total proteins from 106 cells were analyzed by Western blot using an anti–caspase-8 antiserum. The proenzyme and cleaved subunits are indicated on the right by arrows. (B) Caspase-8, but not caspase-9, is activated early after T cell stimulation. Fresh (0) or anti-CD3–stimulated PBMCs (1–48 h) were lysed, and 10 μg of proteins from each sample was separated by SDS-PAGE and analyzed for caspase-3, -8, and -9 processing by Western blot, as described above. Results are representative of two independent experiments.
Caspase Substrate Specificity in Activated Lymphocytes.
Since several downstream caspases were activated after TCR triggering, we next determined whether caspase substrates were processed in stimulated lymphocytes. PARP was the first caspase-3 substrate identified during apoptosis 2 9. Stimulation with anti-CD3 and IL-2 increased PARP protein levels within 24 h (Fig. 6), confirming previous reports showing an upregulation of PARP message and activity after PHA stimulation of primary T lymphocytes 41 42. Concomitant with its induction, >90% of PARP protein was found cleaved in activated cell lysates (Fig. 6, and see Fig. 8). PARP fragment comigrated in the same gel with the 85-kD form found in apoptotic Jurkat cell extracts, further confirming that caspase activity was induced after primary T cell activation. Another recently identified caspase substrate, the Cdc2 tyrosine kinase Wee1 13, was also upregulated in stimulated PBMCs (Fig. 6). Interestingly, the 97-kD full-length Wee1 that appeared 48 h after stimulation was always detected along with 65-, 34-, and 32-kD bands that result from caspase-mediated cleavage during Fas-mediated apoptosis in Jurkat cells (Fig. 6; reference 13). Therefore, two caspase substrates, PARP and Wee1, were processed in activated T cells in a pattern expected from their cleavage by caspases.
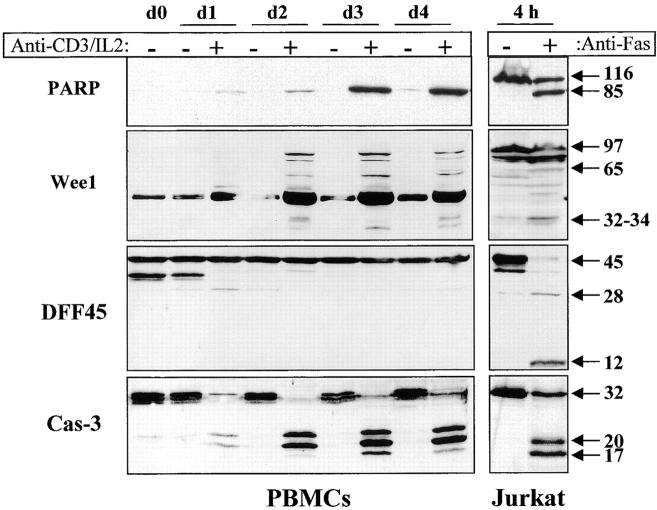
Figure 6.
Caspase activation results in selective substrate cleavage. Total proteins from 106 unstimulated (−) or anti-CD3–activated (+) PBMCs were obtained at different time points (day 1 to day 4), separated by SDS-PAGE, and analyzed by Western blot using an anti-PARP, DFF45, or caspase-3 antiserum, or an anti-Wee1 mAb. As a control for caspase-mediated cleavage, total proteins from 106 Jurkat cells cultured on uncoated or anti-Fas–coated plates (M3: 20 μg/ml) for 4 h were subjected to the same treatment as PBMCs, and results are shown on the right. The 45-kD strong band observed with the anti-Wee1 antibody throughout the kinetic results from a cross-reactivity observed in some tissues with this antibody, according to the manufacturer. These experiments were performed three times, and gave similar results with different PBMC donors.
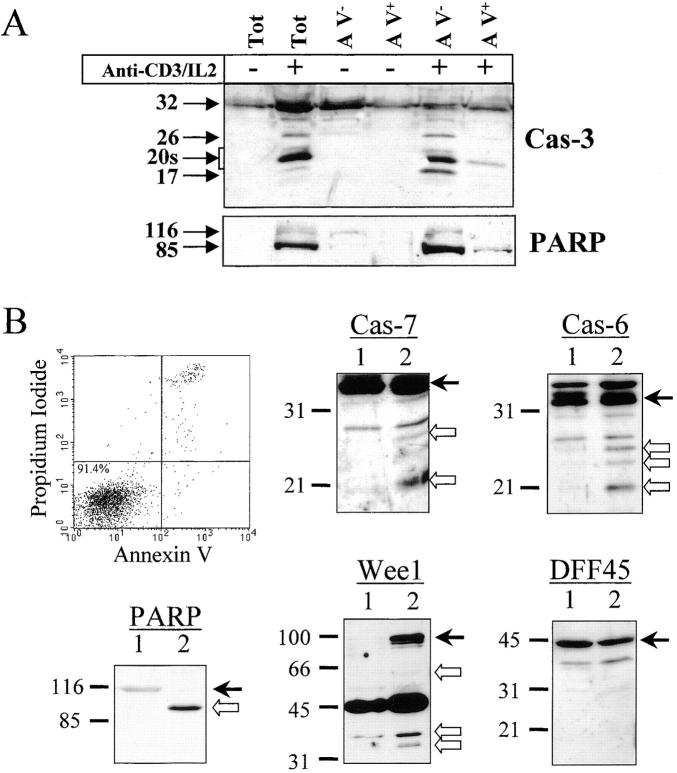
Figure 8.
Caspase and substrate cleavage occur in nonapoptotic cells. (A) Caspase-3 and PARP processing are detected in AV− cells. PBMCs were stimulated (+) or not (−) with anti-CD3 antibody and IL-2 for 4 d, and stained for 10 min with FITC-conjugated AV. Living (AV−) and dying (AV+) activated lymphocytes were purified by flow cytometric cell sorting. Proteins from sorted cells were subjected to Western blot analysis for caspase-3 and PARP. Both antibodies were used on the same membrane. One representative experiment of three is shown. (B) Caspase-6 and -7 and the caspase substrates are processed in viable sorted cells. Unstimulated and unsorted PBMCs (lane 1) or activated and AV− sorted cells (lane 2) were lysed in Laemmli buffer. Proteins from 5 × 105 cells were analyzed by Western blot for PARP, DFF45, Wee1, caspase-6, or caspase-7 processing using the appropriate antisera. Arrows on the right indicate the full-length form of each protein. Results are representative of two independent experiments.
Caspase-mediated cleavage of substrates such as DFF45 or RFC140 in activated T lymphocytes would not be compatible with the maintenance of DNA integrity and the proliferation of T cells, since they play a critical role in DNA fragmentation and DNA synthesis, respectively. Thus, it was of interest to determine whether caspase activation in stimulated lymphocytes resulted in cleavage of these substrates. The same extracts analyzed for PARP and Wee1 cleavage were subjected to Western blot analysis using an anti-DFF45 antiserum generated against the full-length DFF45. Results shown in Fig. 6 demonstrate that DFF45 was not cleaved in these lysates, although caspase-3 was almost completely processed (Fig. 6). The cleavage of RFC140 was analyzed by Western blot, and results confirmed that this substrate was also resistant to caspase-mediated cleavage in activated lymphocytes, as we have observed previously (11; data not shown).
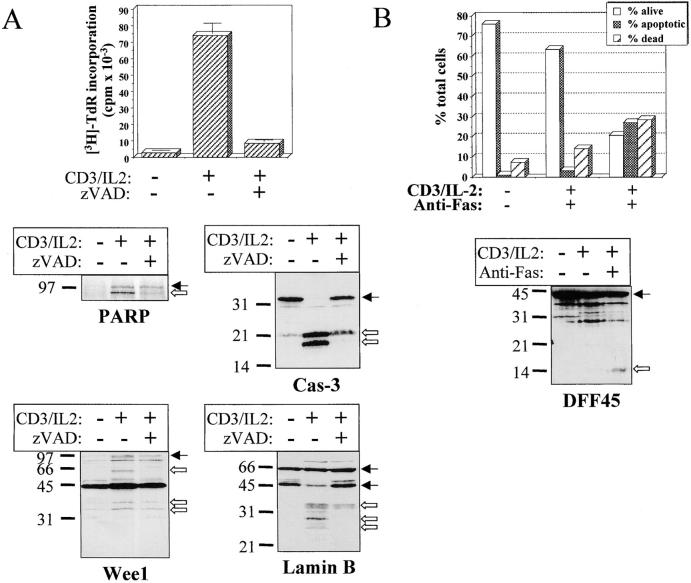
To ensure that Wee1 and PARP cleavage resulted from caspase activity, fresh PBMCs were stimulated with anti-CD3 antibody and IL-2 in the absence or presence of 100 μM of zVAD. Western blot analysis of cell lysates shows that Wee1 cleavage to a 60-kD fragment and the appearance of the 85-kD form of PARP were both inhibited by zVAD (Fig. 7 A). Interestingly, addition of zVAD, which inhibited T cell proliferation, also reduced caspase-3 processing (Fig. 7 A), further supporting a correlation between caspase-3 activation and cell cycle entry. The caspase-6 substrate lamin B 7 was also analyzed by Western blot in lysates of activated lymphocytes, and a zVAD-sensitive cleavage generating a 28- and a 35-kD fragment was observed in stimulated cells (Fig. 7 A), further confirming that caspase-6 was active in these cells. As published previously, cleavage of RFC140 was detectable in activated PBMCs only after anti-Fas treatment 11. To ensure that this was also the case for DFF45, anti-CD3–stimulated PBMCs were incubated for 6 h on anti-Fas–coated plates at 37°C and subjected to Western blot analysis using anti-DFF45 antiserum. This treatment resulted in a decrease of living, AV− cells from 65 to 20% (Fig. 7 B). Western blot analysis demonstrated that the 14-kD fragment of DFF45 was only detectable in lysates of apoptotic cells. Therefore, caspase activity observed in proliferating T cells resulted in selective substrate specificity, different from the one observed during T cell apoptosis.
Figure 7.
Substrate processing during T cell activation is caspase dependent and is different than after apoptosis. (A) Substrate cleavage is caspase dependent. Fresh PBMCs were stimulated for 48 h with anti-CD3 and IL-2 in the presence or absence of 100 μM of zVAD. An aliquot was conserved for quantitation of DNA synthesis by thymidine incorporation, and cells were lysed in denaturing buffer for Western blot analysis of PARP, Wee1, lamin B, and caspase-3 cleavage. Black arrows indicate the full-length proteins, and white arrows designate the cleaved forms. (B) DFF45 cleavage is detected only after apoptosis induction. PBMCs activated for 4 d with anti-CD3 and IL-2 were placed in anti-Fas–coated plates and cultured for 6 h at 37°C. Apoptosis was assessed by AV/PI staining, and the cells were subjected to Western blot analysis using the DFF45 antiserum. Results are from one representative experiment out of three.
Caspase and Substrate Cleavage Occur in Proliferating and Nonapoptotic Cells.
To rule out a potential contribution of dying cells for cleaved forms of caspases or for their substrates, Western blot analysis was performed on highly purified living cells. PBMCs were stimulated for 4 d using anti-CD3 antibody in the presence of IL-2, and stained with AV-FITC. Purified living (AV_−_) or dying (AV+) cells were obtained by flow cytometric cell sorting. The sorted populations were restained with AV to ensure that sorting did not affect cell viability, and >92% of the cells were found to be AV−. Lysates of sorted cells were subjected to caspase-3 or PARP Western blot analysis. Results show that all of the processed forms of caspase-3, as well as the 85-kD fragment of PARP, were present in AV_−_ cells (Fig. 8 A), further confirming that the presence of caspase-3 cleavage products was not due to contamination with dead cells. In nonapoptotic sorted cells, the caspase-3 substrate PARP was almost completely cleaved, showing that caspase-3 processing correlated with caspase activity. Strikingly, caspase-3–processed forms were almost absent in lysates of apoptotic (AV+) cells, suggesting that their half-life could be very short. Confirming this hypothesis, we have observed that caspase-3–cleaved forms progressively disappear from activated lymphocytes after IL-2 withdrawal, concomitant with an increase in mortality (up to 70%; data not shown).
To ensure that cleavage of other caspases or substrates also occurred in nonapoptotic cells, PBMCs stimulated for 2 d (to further limit the proportion of apoptotic cells) with anti-CD3 antibody were stained with AV-FITC, and after gating on blasts, AV− cells were sorted by flow cytometry. Extracts from 5 × 105 cells were prepared from resting, unsorted PBMCs or activated, AV− sorted cells, and proteins were analyzed by Western blot using antibodies against caspase-6, caspase-7, PARP, and Wee1. As a control for dead cell contamination, these extracts were also analyzed for DFF45 cleavage. Fig. 8 B shows that both caspases, as well as PARP and DFF45, were not cleaved in resting cells (lane 1), whereas Wee1 was not detected. After TCR cross-linking, caspase-6– and caspase-7–cleaved subunits were detected in AV− sorted cells (lane 2), as well as the PARP 85-kD cleaved form and the 66-, 32-, and 34-kD fragments of Wee1. In these extracts, DFF45 was not processed, excluding a potential contamination by dead cells. Altogether, these results clearly indicate that a selective caspase substrate cleavage occurs in viable T lymphocytes activated through the TCR.
Discussion
Results presented in this report show that caspase activation after TCR triggering is a physiological, tightly regulated, and early response that appears to be required for efficient T cell activation. Indeed, the selective processing of caspase-3, -6, -7, and -8 was detected within 24 h after anti-CD3 stimulation of peripheral blood lymphocytes. Caspase processing occurred in various T and B cell subsets, and was found in proliferating and nonapoptotic lymphocytes. Activation of caspases was confirmed through binding of caspase-3–processed forms to a specific substrate, and by showing that a cell-permeable substrate was cleaved in intact, activated lymphocytes. Importantly, activation of the caspase cascade was associated to restricted substrate specificity, with cleavage of PARP and Wee1 being observed while two other substrates, DFF45 and RFC140, remained unaffected. Caspase processing after T cell stimulation correlated with a defective lymphocyte activation in the presence of the caspase inhibitor zVAD, suggesting that caspase activity could be involved in some early steps of lymphocyte activation.
Death receptors such as TNFR and Fas can act as costimulatory molecules and enhance T cell proliferation 25 26 27. We have also observed that a soluble form of TNFR significantly inhibited T cell proliferation induced by anti-CD3 antibody (our unpublished results). Cross-linking of these death receptors triggers the FADD-dependent recruitment of caspase-8, which is processed and can directly mediate the activation of caspase-3, -6, and -7 by proteolytic cleavage 36 37. Our results show that caspase-8 is processed after T cell stimulation (as early as 16 h after TCR cross-linking), and activates caspase-3, -6, and –7, leading to a selective cleavage of their substrates. Interestingly, addition of the caspase-8 inhibitor zIETD during T cell stimulation inhibited >60% of the proliferation (data not shown), which further confirms that caspase-8 activity is involved in cell cycle entry of activated lymphocytes. The fact that activation of the whole caspase cascade occurs in proliferating, viable (AV−) cells indicates that caspases could be the executioners in the costimulatory function of members of the TNFR family. In support of this hypothesis, impaired T cell proliferation is also observed in FADD-deficient and in FADD dominant negative transgenic mice 20 21 22, suggesting a potential role for caspases in the early events leading to cell division. Caspase processing in stimulated cells appears to be mediated mostly through the activation of caspase-8 associated to death receptor. The other pathway of downstream caspase activation involves caspase-9, which is triggered after the release in the cytosol of mitochondrial cytochrome c 38 39. However, we have found that in stimulated T cells this caspase remains as a proenzyme.
Bcl-2 and other antiapoptotic members of the family have been shown to block caspase activation after several apoptotic stimuli 43. However, Bcl-2 is also endowed with the ability to influence cell cycle progression. Indeed, Bcl-2 overexpression in transgenic mice was reported to decrease cell cycle entry of primary lymphocytes 23. Transfection of Bcl-2 into NIH 3T3 cells also delayed their reentry into cell cycle after serum withdrawal, and a tyrosine 28 mutant in the NH2-terminal BH4 domain exhibited dominant negative effects over the wild-type Bcl-2 24. Whether the effect of Bcl-2 on cell cycle progression also involves regulation of caspase activity remains to be addressed. However, these data associated with our results showing the inhibitory effect of zVAD on DNA synthesis suggest that caspases (along with their upstream regulators FADD and Bcl-2) may be involved in cell cycle entry during lymphocyte activation.
Results presented in this study clearly demonstrate a selective processing of caspase substrates. PARP, which is involved in DNA repair 44, is probably processed in apoptotic cells to allow for DNA fragmentation after DFF45 cleavage. Of note, PARP expression and activity increase after T cell stimulation 41. Therefore, the 85-kD fragment of PARP probably conserves an enzymatic activity that mediates an unknown function in proliferating cells. Our results also demonstrate the selective processing of Wee1. This tyrosine kinase is a negative regulator of Cdc2 45, a cyclin-dependent kinase required for the G2/M transition during cell cycle, as well as for Fas-mediated apoptosis in immortalized cell lines 13. Wee1 is a critical component of the G2/M cell cycle checkpoint machinery, and mediates cell cycle arrest after DNA damage by phosphorylation of Cdc2 45. Therefore, cleavage of Wee1 in proliferating lymphocytes could lead to its inactivation, thus allowing cell cycle progression. Of note, Wee1 processing by caspases during apoptosis in Jurkat cells correlates with a 20-fold decrease in Wee1 activity and an increase in Cdc2 activity 13. The nuclear protein lamin B is considered as a caspase-6 substrate 7. Our results show that in fresh PBMCs, two forms of 66 and 45 kD can be identified by Western blot using an anti–lamin B antibody, and at least two fragments of 35 and 28 kD are produced after T cell activation. Appearance of these products correlates with a decrease in the amount of the 45-kD form of lamin B, and is inhibited by addition of zVAD (Fig. 7 A). Although the significance of this cleavage in activated lymphocytes is unclear, it confirms the activation of caspase-6, which generates the 28-kD fragment after a cleavage at the consensus sequence VEID 7.
In our experiments, DFF45 is present in resting as well as in proliferating cells, whereas RFC140 is upregulated after T cell stimulation. Since caspase-mediated DFF45 processing leads to DNA fragmentation, one could easily predict that it would not be cleaved in activated living cells. Indeed, we were unable to detect DFF45 cleavage in AV− cells after T cell activation, although the 14-kD fragment generated by caspase-3 cleavage at the second site was observed in lysate from activated lymphocytes after anti-Fas treatment. The lack of RFC140 cleavage in proliferating T cells was also critical, since processing of RFC140 by caspases leads to inactivation of the DNA replication machinery and cell cycle arrest at the G2/M boundary 11. This selective substrate processing could explain why T lymphocytes survive and proliferate although the caspase cascade including caspase-8, -3, -6, and -7 is activated after TCR triggering. To explain this selective caspase specificity in stimulated lymphocytes, several hypotheses can be proposed. Endogenous caspase inhibitors, such as the inhibitor of apoptosis (IAP) family members, could selectively inhibit the cleavage of specific substrates in stimulated cells 46. Supporting this hypothesis, the expression of thymic IAP, a murine homologue of survivin, is upregulated within 24 h of stimulation in splenic T cells 47, and this member of the IAP family was shown to inhibit already processed caspase-3, -7, and -9 46. Association of thymic IAP to caspases could have a differential effect on the cleavage of their substrates, depending on the affinity of the caspase for these substrates or their accessibility. Alternatively, some caspase substrates involved in cell survival could be protected against cleavage by phosphorylation near the caspase cleavage site. This mechanism was recently demonstrated for presenilin-2. Caspase-mediated cleavage of this molecule was abrogated by Ser327 and Ser330 phosphorylation inside the consensus cleavage site DSYD↓S 48.
In summary, activation of the caspase cascade in nonapoptotic lymphocytes, together with the impairment of T cell activation by caspase inhibitors, indicates that these proteases may play an important role during T cell stimulation, and caspases may be added to the growing list of molecules that are involved in cell death and proliferation. However, elucidation of the function of caspase processing in activated lymphocytes will require further experiments and will provide new insights into the biological function of caspases, as well as the regulation of T cell activation.
Acknowledgments
We are very grateful to Dr. G. Poirier and Dr. M.E. Peter for kindly providing us with anti-PARP and anti–caspase-8 antisera, respectively. We are indebted to Drs. D.W. Nicholson and S. Roy (Merck Frosst, Quebec, Canada) for the gift of several caspase-specific antisera. Our gratitude is addressed to Drs. M. Bourbonnière, F. Denis, and F. Erard for critical review of the manuscript, to N. Tessier for excellent technical assistance in cell sorting, and to D. Dubreuil for very helpful assistance.
This work was supported by grants from the Medical Research Council of Canada to R.-P. Sékaly (MRC Industry grant UI12373), and R.P. Sékaly holds an MRC Scientist Award.
Footnotes
A. Alam's current address is INSERM U395, CHU Purpan, BP3028, 31024 Toulouse, Cedex 3, France.
Abbreviations used in this paper: AV, annexin V; Cdc2, cell division control 2; DFF45, the 45-kd subunit of DNA fragmentation factor; Extra-HRP, HRP–conjugated extravidin; FADD, Fas-associated death domain protein; HRP, horseradish peroxidase; IAP, inhibitor of apoptosis; PARP, poly(ADP-ribose) polymerase; PI, propidium iodide; RFC140, the 140-kD subunit of replication factor C; SAC, Staphylococcus aureus Cowan cell extracts; zVAD, benzyloxycarbonyl (Cbz)-Val-Ala-Asp(OMe)-fluoromethylketone.
A. Alam and L.Y. Cohen contributed equally to this work.
References
- Thornberry N.A., Lazebnik Y. Caspasesenemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- Nicholson D.W., Ali A., Thornberry N.A., Vaillancourt J.P., Ding C.K., Gallant M., Gareau Y., Griffin P.R., Labelle M., Lazebnick Y.A. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- Han Z., Hendrickson E.A., Bremner T.A., Wyche J.H. A sequential two-step mechanism for the production of the mature P17:P12 form of caspase-3 in vitro . J. Biol. Chem. 1997;272:13432–13436. doi: 10.1074/jbc.272.20.13432. [DOI] [PubMed] [Google Scholar]
- Clayton L.K., Ghendler Y., Mizoguchi E., Patch R.J., Ocain T.D., Orth K., Bhan A.K., Dixit V.M., Reinherz E.L. T-cell receptor ligation by peptide/MHC induces activation of a caspase in immature thymocytesthe molecular basis of negative selection. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:2282–2293. doi: 10.1093/emboj/16.9.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam A., Braun M.Y., Hartgers F., Lesage S., Cohen L., Hugo P., Denis F., Sékaly R.P. Specific activation of the cysteine protease CPP32 during the negative selection of T cells in the thymus. J. Exp. Med. 1997;186:1503–1512. doi: 10.1084/jem.186.9.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornberry N.A., Rano T.A., Peterson E.P., Rasper D.M., Timkey T., Garcia-Calvo M., Houtzager V.M., Nordstrom P.A., Roy S., Vaillancourt J.P. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J. Biol. Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- Denis F., Rheaume E., Aouad S.M., Alam A., Sékaly R.P., Cohen L.Y. The role of caspases in T cell development and the control of immune responses. Cell. Mol. Life Sci. 1998;54:1005–1019. doi: 10.1007/s000180050228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothakota S., Azuma T., Reinhard C., Klippel A., Tang J., Chu K., McGarry T.J., Kirschner M.W., Koths K., Kwiatkowski D.J., Williams L.T. Caspase-3-generated fragments of gelsolineffector of morphological change in apoptosis. Science. 1997;278:294–298. doi: 10.1126/science.278.5336.294. [DOI] [PubMed] [Google Scholar]
- Tewari M., Quan L.T., O'Rourke K., Desnoyers S., Zheng Z., Beidler D.R., Poirier G.G., Salvesen G.S., Dixit V.M. YAMA/CPP32b, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- Song Q., Lees-Miller S.P., Kumar S., Zhang Z., Chan D.W., Smith G.C., Jackson S.P., Alnemri E.S., Litwack G., Khanna K.K., Lavin M.F. DNA-dependent protein kinase catalytic subunita target for an ICE-like protease in apoptosis. EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:3238–3246. [PMC free article] [PubMed] [Google Scholar]
- Rhéaume E., Cohen L.Y., Uhlmann F., Lazure C., Alam A., Hurwitz J., Sékaly R.P., Denis F. The large subunit of replication factor C is a substrate for caspase-3-like protease during Fas-mediated apoptosis. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:6346–6354. doi: 10.1093/emboj/16.21.6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt P., Tomaselli K.J., Cooper G.M. Identification of the MDM2 oncoprotein as a substrate for CPP32-like apoptotic proteases. J. Biol. Chem. 1997;272:15049–15052. doi: 10.1074/jbc.272.24.15049. [DOI] [PubMed] [Google Scholar]
- Zhou B.B., Li H., Yuan J., Kirschner M.W. Caspase-dependent activation of cyclin-dependent kinases during Fas-induced apoptosis in Jurkat cells. Proc. Natl. Acad. Sci. USA. 1998;95:6785–6790. doi: 10.1073/pnas.95.12.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Zou H., Slaughter C., Wang X. DFF45, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell. 1997;89:175–184. doi: 10.1016/s0092-8674(00)80197-x. [DOI] [PubMed] [Google Scholar]
- Sakahira H., Enari M., Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 1998;391:96–99. doi: 10.1038/34214. [DOI] [PubMed] [Google Scholar]
- Enari M., Sakahira H., Yokoyama H., Okawa K., Iwamatsu A., Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- Miossec C., Dutilleul V., Fassy F., Diu-Hercend A. Evidence for CPP32 activation in the absence of apoptosis during T lymphocyte stimulation. J. Biol. Chem. 1997;272:13459–13462. doi: 10.1074/jbc.272.21.13459. [DOI] [PubMed] [Google Scholar]
- Wilhelm S., Wagner H., Hacker G. Activation of caspase-3-like enzymes in non-apoptotic T cells. Eur. J. Immunol. 1998;28:891–900. doi: 10.1002/(SICI)1521-4141(199803)28:03<891::AID-IMMU891>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Zapata J.M., Takahashi R., Salvesen G.S., Reed J.C. Granzyme release and caspase activation in activated human T-lymphocytes. J. Biol. Chem. 1998;273:6916–6920. doi: 10.1074/jbc.273.12.6916. [DOI] [PubMed] [Google Scholar]
- Zhang J., Cado D., Chen A., Kabra N.H., Winoto A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature. 1998;392:296–300. doi: 10.1038/32681. [DOI] [PubMed] [Google Scholar]
- Newton K., Harris A.W., Bath M.L., Smith K.G.C., Strasser A. A dominant interfering mutant of FADD/MORT1 enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:706–718. doi: 10.1093/emboj/17.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zornig M., Hueber A.O., Evan G. p53-dependent impairment of T-cell proliferation in FADD dominant-negative transgenic mice. Curr. Biol. 1998;8:467–470. doi: 10.1016/s0960-9822(98)70182-4. [DOI] [PubMed] [Google Scholar]
- O'Reilly L.A., Huang D.C., Strasser A. The cell death inhibitor Bcl-2 and its homologues influence control of cell cycle entry. EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:6979–6990. [PMC free article] [PubMed] [Google Scholar]
- Huang D.C., O'Reilly L.A., Strasser A., Cory S. The anti-apoptotic function of Bcl-2 can be genetically separated from its inhibitory effect on cell cycle entry. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:4628–4638. doi: 10.1093/emboj/16.15.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderson M.R., Armitage R.J., Maraskovsky E., Tough T.W., Roux E., Schooley K., Ramsdell F., Lynch D.H. Fas transduces activation signals in normal human T lymphocytes. J. Exp. Med. 1993;178:2231–2235. doi: 10.1084/jem.178.6.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderson M.R., Tough T.W., Braddy S., Davis-Smith T., Roux E., Schooley K., Miller R.E., Lynch D.H. Regulation of apoptosis and T cell activation by Fas-specific mAb. Int. Immunol. 1994;6:1799–1806. doi: 10.1093/intimm/6.11.1799. [DOI] [PubMed] [Google Scholar]
- Yuan J. Transducing signals of life and death. Curr. Opin. Cell Biol. 1997;9:247–251. doi: 10.1016/s0955-0674(97)80069-5. [DOI] [PubMed] [Google Scholar]
- Jurgensmeier J.M., Xie Z., Deveraux Q., Ellerby L., Bredesen D., Reed J.C. Bax directly induces release of cytochrome c from isolated mitochondria. Proc. Natl. Acad. Sci. USA. 1998;95:4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C.A. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- Kohno K., Kataoka J., Ohtsuki T., Suemoto Y., Okamoto I., Usui M., Ikeda M., Kurimoto M. IFN-gamma-inducing factor (IGIF) is a costimulatory factor on the activation of Th1 but not Th2 cells and exerts its effect independently of IL-12. J. Immunol. 1997;158:1541–1550. [PubMed] [Google Scholar]
- Thornberry N.A., Bull H.G., Calaycay J.R., Chapman K.T., Howard A.D., Kostura M.J., Miller D.K., Molineaux S.M., Weidner J.R., Aunins J. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- Ghayur T., Banerjee S., Hugunin M., Butler D., Herzog L., Carter A., Quintal L., Sekut L., Talanian R., Paskind M. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature. 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- Faleiro L., Kobayashi R., Fearnhead H., Lazebnik Y. Multiple species of CPP32 and Mch2 are the major active caspases present in apoptotic cells. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:2271–2281. doi: 10.1093/emboj/16.9.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finucane D.M., Bossy-Wetzel E., Waterhouse N.J., Cotter T.G., Green D.R. Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondria is inhibitable by Bcl-xL. J. Biol. Chem. 1999;274:2225–2233. doi: 10.1074/jbc.274.4.2225. [DOI] [PubMed] [Google Scholar]
- Harvey N.L., Trapani J.A., Fernandes-Alnemri T., Litwack G., Alnemri E.S., Kumar S. Processing of the Nedd2 precursor by ICE-like proteases and granzyme B. Genes Cells. 1996;1:673–685. doi: 10.1046/j.1365-2443.1996.00255.x. [DOI] [PubMed] [Google Scholar]
- Nunez G., Benedict M.A., Hu Y., Inohara N. Caspasesthe proteases of the apoptotic pathway. Oncogene. 1998;17:3237–3245. doi: 10.1038/sj.onc.1202581. [DOI] [PubMed] [Google Scholar]
- Van de Craen M., Van Loo G., Declercq W., Schotte P., Van den brande I., Mandruzzato S., Van der Bruggen P., Fiers W., Vandenabeele P. Molecular cloning and identification of murine caspase-8. J. Mol. Biol. 1998;284:1017–1026. doi: 10.1006/jmbi.1998.2226. [DOI] [PubMed] [Google Scholar]
- Li P., Nijhawan D., Budihardjo I., Srinivasula S.M., Ahmad M., Alnemri E.S., Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Slee E.A., Harte M.T., Kluck R.M., Wolf B.B., Casiano C.A., Newmeyer D.D., Wang H.G., Reed J.C., Nicholson D.W., Alnemri E.S. Ordering the cytochrome c-initiated caspase cascadehierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J. Cell Biol. 1999;144:281–292. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema J.P., Scaffidi C., Kischkel F.C., Shevchenko A., Mann M., Krammer P.H., Peter M.E. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC) EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:2794–2804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger N.A., Adams J.W., Sikorski G.W., Petzold S.J., Shearer W.T. Synthesis of DNA and poly(adenosine diphosphate ribose) in normal and chronic lymphocytic leukemia lymphocytes. J. Clin. Invest. 1978;62:111–118. doi: 10.1172/JCI109094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegazzi M., Gerosa F., Tommasi M., Uchida K., Miwa M., Sugimura T., Suzuki H. Induction of poly(ADP-ribose) polymerase gene expression in lectin-stimulated human T lymphocytes is dependent on protein synthesis. Biochem. Biophys. Res. Commun. 1988;156:995–999. doi: 10.1016/s0006-291x(88)80942-2. [DOI] [PubMed] [Google Scholar]
- Reed J.C. Bcl-2 family proteins. Oncogene. 1998;17:3225–3236. doi: 10.1038/sj.onc.1202591. [DOI] [PubMed] [Google Scholar]
- De Murcia J.M., Niedergang C., Trucco C., Ricoul M., Dutrillaux B., Mark M., Oliver F.J., Masson M., Dierich A., LeMeur M. Requirement of poly(ADP-ribose) polymerase in recovery from DNA damage in mice and in cells. Proc. Natl. Acad. Sci. USA. 1997;94:7303–7307. doi: 10.1073/pnas.94.14.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan C.H., Russell P. Human Wee1 kinase inhibits cell division by phosphorylating p34cdc2 exclusively on Tyr15. EMBO (Eur. Mol. Biol. Organ.) J. 1993;12:75–85. doi: 10.1002/j.1460-2075.1993.tb05633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereaux Q.L., Reed J.C. IAP family proteins—suppressors of apoptosis. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Hatano M., Otaki M., Ogasawara T., Tokuhisa T. Expression of a murine homologue of the inhibitor of apoptosis protein is related to cell proliferation. Proc. Natl. Acad. Sci. USA. 1999;96:1457–1462. doi: 10.1073/pnas.96.4.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J., Schindzielorz A., Grunberg J., Haass C. Phosphorylation of presenilin-2 regulates its cleavage by caspases and retards progression of apoptosis. Proc. Natl. Acad. Sci. USA. 1999;96:1391–1396. doi: 10.1073/pnas.96.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]