Local Delivery of Interleukin 4 by Retrovirus-Transduced T Lymphocytes Ameliorates Experimental Autoimmune Encephalomyelitis (original) (raw)
Abstract
Experimental autoimmune encephalomyelitis (EAE) is an inflammatory autoimmune disease of the central nervous system which serves as a model for the human disease multiple sclerosis. We demonstrate here that encephalitogenic T cells, transduced with a retroviral gene, construct to express interleukin 4, and can delay the onset and reduce the severity of EAE when adoptively transferred to myelin basic protein–immunized mice. Thus, T lymphocytes transduced with retroviral vectors can deliver “regulatory cytokines” in a site-specific manner and may represent a viable therapeutic strategy for the treatment of autoimmune disease.
Inflammatory T cell responses to self antigens are implicated in a number of autoimmune diseases (1–3). Modulation of these responses by the systemic administration of antiinflammatory cytokines such as IL-4 or transforming growth factor β has shown therapeutic potential in animal models of autoimmunity (4–6). However, side effects inherent in the systemic administration of cytokines necessitate their local delivery (7). The therapeutic efficacy of tissue-specific expression of IL-4 has recently been demonstrated by transgenic expression in the nonobese diabetic mouse (8), but thus far, no practical methods have been developed to affect the local delivery of cytokines to the site of pathology.
Techniques for gene transfer in vivo, such as retrovirusmediated gene transduction, have the potential to deliver immunosuppressive molecules in a site-specific manner, thus limiting systemic effects. The feasibility of this approach has been recently demonstrated (9). In this study, gene expression in vivo, required injection of high titer virus stocks directly to the site of inflammation, an impractical method for treating multifocal autoimmune diseases. An alternative approach would be to use retrovirally transduced T lymphocytes as delivery vehicles to target the modulatory cytokines. This technique was originally described in a model of demyelination experimental autoimmune neuritis in which a T cell line with specificity for the P2 protein was transduced for the expression of nerve growth factor; subsequent adoptive transfer ameliorated experimental autoimmune neuritis by mechanisms which are not well understood (10). The studies described below report our results of tissue-specific delivery of IL-4 to the central nervous system (CNS) of mice with experimental autoimmune encephalomyelitis (EAE).
Materials and Methods
Retroviral Constructs and Ecotropic Viral Production.
The retroviral plasmid MSCV-(SD-)-IL-4-neo (11) contains the poliovirus type 2 internal ribosome entry site sequence (12) between the bacterial neomycin phosphotransferase gene (neo), conferring neomycin resistance to eukaryotic cells, and the mouse IL-4 gene. The proviral DNA is transcribed as a single polycistronic mRNA. The internal ribosome entry site sequence allows for subsequent translation of the two proteins. Ecotropic retrovirus was produced in the PHEONIX packaging cell line (G.P. Nolan, manuscript in preparation). In brief, PHEONIX cells were plated at 2 × 106 cells/well in 6-cm plates and allowed to adhere overnight. The cells were transfected with the IL-4-neo plasmid (10 μg/plate) by CaCl2 transfection. Replication-defective retrovirus was harvested 48 hr after transfection, sterile filtered to remove nonadherent producer cells, and used to infect T cell hybrids.
T Cell Infections.
Cells were grown to log phase, harvested, and washed two times with PBS. Cells were resuspended to 2 × 106 cells/ml using retrovirus containing supernatant stocks. Polybrene was added to a final concentration of 8 μg/ml. The cells were placed in 6-well tissue culture plates (2 ml/plate) and centrifuged at 2,500 rpm for 90 min at 32°C in a tabletop centrifuge. The cells were then incubated for a further 8 h at 37°C in a CO2 incubator before the cells were washed and replated in fresh media without polybrene. After 48 h of culture to allow gene expression, transduced cells were selected by growth in the presence of the neomycin analogue G418 (2 μg/ml) for 5–7 d.
Induction of EAE and Adoptive Transfer of Transduced T Cells.
EAE was induced by active immunization with mylein basic protein (MBP). In brief, MBP (200 μg/mouse final concentration) in PBS was emulsified in an equal volume of CFA. (PL/J × SJL/ J)F1 mice (5 mice/group) were immunized subcutaneously at four sites in the flanks, draining the axial and inguinal lymph nodes (50 μl/site). Animals were also given an injection of the coadjuvant pertussis toxin (200 ng/mouse) intravenously on the day of immunization and 48 h later. Mice were scored daily for clinical signs of EAE according to the following scale: 0, no clinical disease; 1, flaccid tail; 2, single hind leg paralysis; 3, dual hind leg paralysis; 4, fore limb paralysis; 5, moribund; 6, death. On day 9 or 10, mice were transferred 106 of each cell type intravenously, and were observed daily for clinical signs of EAE. Mice were housed in the Stanford Department of Laboratory Animal Medicine (Stanford, CA) under National Institutes of Health (NIH) approved conditions.
Quantitation of IL-4 and IL-10 by ELISA.
IL-4 and IL-10 were quantitated using a sandwich ELISA. In brief, microtiter plates were coated with primary antiinterleukin antibody overnight, washed with PBS–Tween 20 and blocked with PBS–0.1% BSA. Plates were then washed and samples were added to the wells. An IL-4 or IL-10 standard was included in each assay for quantification purposes. After a 2-h incubation, plates were washed and a biotin-conjugated secondary antiinterleukin antibody was added. The plates were then washed and an avidin–horseradish peroxidase conjugate was added. A standard colormetric assay was then performed by the addition of the peroxidase substrate 2,2′-azinobis (3′ethylbenzthiazoline-6-sulfonic acid). Color change was measured in a microplate reader equipped with a 405-nm filter.
Results and Discussion
To test the concept of site-specific delivery of cytokines by transduced T cells, the murine model of EAE was used. An encephalitogenic, MBP-specific T cell hybridoma, G1.15H, was used in these preliminary studies due to its transduction efficiency. Adoptive transfer of the unmodified G1.15H to naive (SJL/J × PL/J)F1 mice verified its EAE-inducing, and thus CNS-homing, capability (Fig. 1). It was decided to transduce this T cell line for expression of the IL-4 gene since this cytokine has been demonstrated to be a mediator in EAE regulation as evidenced by its role in the natural recovery from disease (13), experimental protection from disease after oral tolerance induction (14), and after immunization with altered peptide ligands of MBP (15).
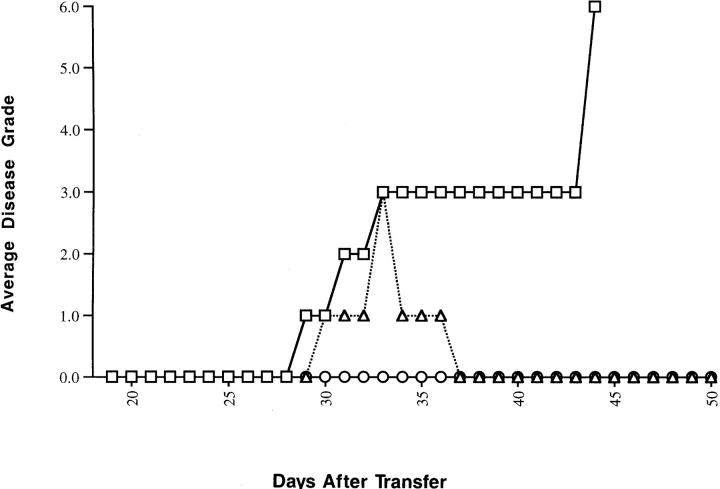
Figure 1.
Adoptive transfer of EAE using the MBP-specific T cell hybridoma G1.15H. Three naive (PL/J × SJL/J)F1 mice were given 106 G1.15H cells intravenously on day 0. Each mouse (circles, squares, or triangles, respectively) was scored daily for clinical signs of EAE as described in Materials and Methods.
After transduction of G1.15H with an IL-4–encoding retrovirus and drug selection, the line was confirmed to secrete IL-4 by ELISA (Table 1). Subsequent limiting dilution cloning of these transduced hybrids yielded individual lines secreting between 1 and 8 pg/ml IL-4/105 cells (Table 1).
Table 1.
IL-4 and IL-10 Secreted by Cell Lines Used in this Study
| Cell line | Description | IL-4 secreted | IL-10 secreted |
|---|---|---|---|
| ng/ml/105 cells | |||
| G1.15H | MBP-specific, H-2s restricted T cell hybridoma | 0 | – |
| Bw.Z.4 | T cell hybridoma fusion partner, IL-4 transduced | 2.3 | – |
| G1.15H.4.9 | IL-4 transduced variant of G1.15H | 2.5 | – |
| G1.15H.N5 | IL-4 transduced variant of G1.15H | 7.9 | – |
| G1.15H.marv1 | IL-10–transduced variant of G1.15H | – | 255 |
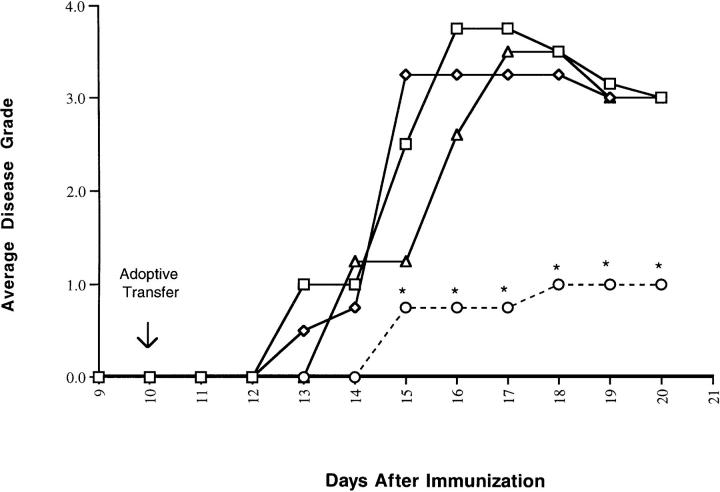
10 d after MBP immunization to induce EAE, (SJL/J × PL/J)F1 mice were given transduced or control cells. Clinical EAE was ameliorated by adoptive transfer of 106 transduced T cells secreting high levels of IL-4 (line N5). Disease onset for the IL-4 treatment group was delayed by 2 d (P <0.01 Student's t test) and the average disease score was significantly lower (P <0.05) than an experimental group receiving cells transduced to express IL-10, or control mice receiving untransduced cells or PBS (Fig. 2).
Figure 2.
Adoptive transfer of T cells transduced to express IL-4 ameliorates MBP-induced EAE. Mice immunized to induce EAE were treated with T cells transduced to express IL-4 (circles) or IL-10 (triangles), untransduced T cells (diamonds), or PBS (squares), and observed for clinical signs of EAE. Data are given as mean disease scores, and are representative of the results of at least two independent experiments. Disease incidence in all groups was 100%. *The mean disease score was statistically different from all other groups, Student's t test, P <0.05.
That amelioration of disease was due to local delivery of IL-4 was supported by several experimental approaches. In the first, mice that received T cells transduced to express IL-4 were bled at various time points after cell transfer, and serum IL-4 was determined by ELISA. No cytokine was detected in the serum until day 24 after transfer, well after initial recovery from disease. Serum IL-4 levels at this time were low, ranging from 1.19 pg/ml to 2.53 pg/ml (Table 2). This finding indicates that disease remission was not due to high systemic levels of IL-4. In a second experimental approach, we verified the presence of transduced T cells in the CNS at the time of disease amelioration by testing spinal cord tissue from treated animals for retroviral-specific IL-4 expression by reverse transcriptase PCR analysis. Retroviral IL-4 transcription could be detected in the CNS of treated animals 15 d after transfer of transduced T cells (data not shown). The third experimental approach demonstrated that amelioration of disease was dependent on T cell homing to the CNS.
Table 2.
Serum Levels of IL-4 at Various Times after Transfer of T Cell Hybrids Transduced to Express IL-4
| Serum IL-4 levels | |||
|---|---|---|---|
| Day 6 | Day 13 | Day 24 | |
| pg/ml | |||
| Mouse 1 | n.d. | n.d. | 1.26 |
| Mouse 2 | n.d. | n.d. | 1.19 |
| Mouse 3 | n.d. | n.d. | 2.11 |
| Mouse 4 | n.d. | n.d. | 2.53 |
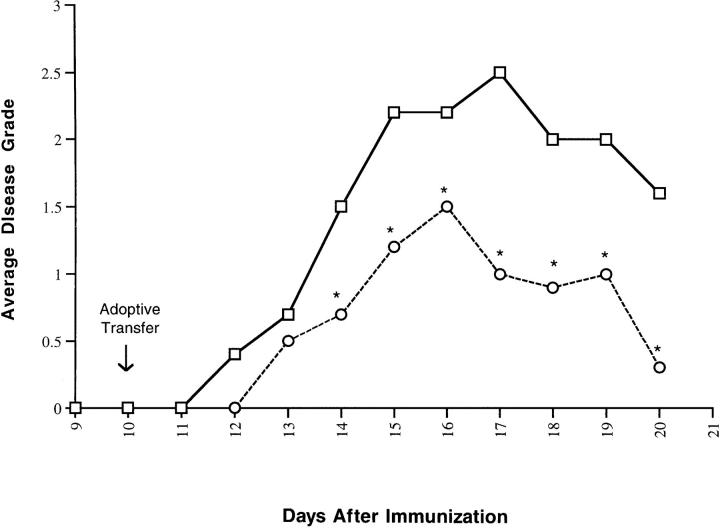
We reasoned that T cells transduced to express IL-4 that could not recognize CNS antigens would be ineffective at delivering IL-4 to the CNS. To test this hypothesis, additional transfer experiments were performed, using as controls, transduced T cells expressing IL-4 but lacking antigen specific TCR expression. In the first experiment, an IL-4– expressing transductant of the hybridoma fusion partner BW5147 was used as a control. Transfer of this cell line to MBP-immunized mice had no effect on the disease course, whereas transfer of an MBP-specific line, clone 4.9, secreting low levels of IL-4 (matched to the fusion partner control) had a significant therapeutic effect (P <0.05; data not presented). In the second experimental approach, a TCR negative variant of the IL-4–secreting disease ameliorating N5 clone was used as a control. This line had significantly less effect on the disease course (P <0.05) when compared to its TCR-expressing counterpart (Fig. 3).
Figure 3.
Amelioration of EAE is dependent upon TCR expression by IL-4–secreting T cells. MBP-immunized (PL/J × SJL/J)F1 mice (10 mice/group) were given 106 transduced TCR-positive, IL-4–secreting N5 cells (circles) or TCR-negative, IL-4–secreting N5 cells (squares). Mice were scored daily for clinical signs of EAE. Data are given as the mean disease scores of those mice developing disease, and are representative of the results of at least two independent experiments. Disease incidence was 90% in each group. *The mean disease score was statistically different from all other groups, Student's t test, P <0.05.
The majority of approaches taken to control autoimmune disease result in deleterious side effects due to the systemic administration of antiinflammatory agents. Our findings indicate that the disease processes can be modulated by the transfer of MBP-specific T cells which have been retrovirally modified to express the antiinflammatory cytokine IL-4. Disease amelioration was due to local, rather than systemic, delivery of IL-4 as evidenced by the following points. (a) Serum levels of IL-4 were undetectable at the time of disease remission. (b) Retroviral-encoded IL-4 expression could be detected in the CNS of treated mice. (c) Disease amelioration was dependent upon antigen-specific T cell receptor expression on transduced T cells, indicating that T cell antigen recognition and presumably “trafficking” were necessary for delivery of cytokines.
The use of antigen-specific T cells, transduced to express regulatory cytokines, selectively target antiinflammatory molecules to the site of pathology represents a unique therapeutic approach to the treatment of autoimmune disease. T cells are advantageous since they are easily manipulated and expanded in tissue culture before reintroduction into the host. More importantly, the antigen specificity of T cells allows them to home to depots of antigen in the body, such as at inflammatory sites of autoimmune disease. This has been demonstrated in the murine model of EAE where MBP-specific T cells have been shown to traffic to the CNS, both during the induction phase of disease as well as during relapses in a relapsing-remitting model of EAE (16, 17). Results presented here demonstrate that a statistically significant benefit can be observed when mice, immunized to develop EAE, are given MBP-specific T cells retrovirally transduced to express IL-4.
Acknowledgments
The authors would like to thank Ms. Robyn Kizer and Ms. Kathy Sturgis for their excellent secretarial assistance in the preparation of this manuscript.
Footnotes
M.K. Shaw is the recipient of a fellowship from the National Multiple Sclerosis Society. J. B. Lorens is the recipient of a fellowship from the Norwegian Cancer Society. This work was supported by National Institutes of Health grants AI36535 and NO1-AR-6-2227.
References
- 1.Steinman L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell. 1996;85:299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- 2.Tisch R, McDevitt H. Insulin-dependent diabetes mellitus. Cell. 1996;85:291–297. doi: 10.1016/s0092-8674(00)81106-x. [DOI] [PubMed] [Google Scholar]
- 3.Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307–310. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 4.Inobe JI, Chen Y, Weiner HL. In vivo administration of IL-4 induces TGF-beta–producing cells and protects animals from experimental autoimmune encephalomyelitis. Ann NY Acad Sci. 1996;778:390–392. doi: 10.1111/j.1749-6632.1996.tb21153.x. [DOI] [PubMed] [Google Scholar]
- 5.Racke MK, Dhib-Jalbut A, Cannella B, Albert PS, Raine CS, McFarlin DE. Prevention and treatment of chronic relapsing experimental allergic encephalomyelitis by transforming growth factor–β 1. J Immunol. 1991;146:3012–3017. [PubMed] [Google Scholar]
- 6.Johns LD, Flanders KC, Ranges GE, Sriram S. Successful treatment of experimental allergic encephalomyelitis with transforming growth factor–β 1. J Immunol. 1991;147:1792–1796. [PubMed] [Google Scholar]
- 7.Steinman L. A few autoreactive cells in an autoimmune infiltrate control a vast population of nonspecific cells: a tale of smart bombs and the infantry. Proc Natl Acad Sci USA. 1996;93:2253–2256. doi: 10.1073/pnas.93.6.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meuller R, Krahl T, Sarvetnick N. Pancreatic expression of interleukin-4 abrogates insulitis and autoimmune diabetes in nonobese diabetic (NOD) mice. J Ex Med. 1996;184:1093–1100. doi: 10.1084/jem.184.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin L, Chavin KD, Ding Y, Tahara H, Favaro JP, Woodward JE, Suzuki T, Robbins PD, Lotze MT, Bromberg JS. Retrovirus-mediated transfer of viral IL-10 gene prolongs murine cardiac allograft survival. J Immunol. 1996;156:2316–2323. [PubMed] [Google Scholar]
- 10.Kramer R, Zhang Y, Gehrmann J, Gold R, Thoenen H, Wekerle H. Gene transfer through the blood– brain barrier: NGF-engineered neuritogenic T lymphocytes attenuate experimental autoimmune neuritis. Nat Med. 1995;1:1162–1166. doi: 10.1038/nm1195-1162. [DOI] [PubMed] [Google Scholar]
- 11.Hawley RG, Lieu FH, Fong AZ, Hawley TS. Versatile retroviral vectors for potential use in gene therapy. Gene Ther. 1994;1:136–138. [PubMed] [Google Scholar]
- 12.Dirks W, Wirth M, Hauser H. Dicistronic transcription units for gene expression in mammalian cells. Gene (Amst) 1993;128:247–249. doi: 10.1016/0378-1119(93)90569-o. [DOI] [PubMed] [Google Scholar]
- 13.Karpus WJ, Gould KE, Swanborg RH. CD4+suppressor cells of autoimmune encephalomyelitis respond to T cell receptor-associated determinants on effector cells by interleukin-4 secretion. Eur J Immunol. 1993;22:1757–1763. doi: 10.1002/eji.1830220714. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science (Wash DC) 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 15.Brocke S, Gijbels K, Allegretta M, Ferber I, Piercy C, Blankenstein T, Martin R, Utz U, Karin N, Mitchell D, et al. Treatment of experimental encephalomyelitis with a peptide analogue of myelin basic protein. Nature (Lond) 1996;379:343–346. doi: 10.1038/379343a0. [DOI] [PubMed] [Google Scholar]
- 16.Cross AH, Cannella B, Brosnan CF, Raine CS. Homing to central nervous system vasculature by antigen-specific lymphocytes. I. Localization of 14C labeled cells during acute, chronic and relapsing experimental autoimmune encephalomyelitis. Lab Invest. 1990;63:162–170. [PubMed] [Google Scholar]
- 17.Skundric DS, Kim C, Tse HY, Raine CS. Homing of T cells to the central nervous system throughout the course of relapsing experimental autoimmune encephalomyelitis in Thy-1 congenic mice. J Neuroimmunol. 1993;46:113–122. doi: 10.1016/0165-5728(93)90240-y. [DOI] [PubMed] [Google Scholar]