Antigenic Cancer Cells Grow Progressively in Immune Hosts without Evidence for T Cell Exhaustion or Systemic Anergy (original) (raw)
Abstract
One enigma in tumor immunology is why animals bearing malignant grafts can reject normal grafts that express the same nonself-antigen. An explanation for this phenomenon could be that different T cell clones react to the normal graft and the malignant cells, respectively, and only the tumor-reactive clonotypes may be affected by the growing tumor. To test this hypothesis, we used a T cell receptor transgenic mouse in which essentially all CD8+ T cells are specific for a closely related set of self-peptides presented on the MHC class I molecule Ld. We find that the tumor expressed Ld in the T cell receptor transgenic mice but grew, while the Ld-positive skin was rejected. Thus, despite an abundance of antigen-specific T cells, the malignant tissue grew while normal tissue expressing the same epitopes was rejected. Therefore, systemic T cell exhaustion or anergy was not responsible for the growth of the antigenic cancer cells. Expression of costimulatory molecules on the tumor cells after transfection and preimmunization by full-thickness skin grafts was required for rejection of a subsequent tumor challenge, but there was no detectable effect of active immunization once the tumor was established. Thus, the failure of established tumors to attract and activate tumor-specific T cells at the tumor site may be a major obstacle for preventive or therapeutic vaccination against antigenic cancer.
One of the most important questions in tumor immunology is why the immune system often fails to eliminate antigenic cancers. A variety of reasons could account for the lack of efficient immune responses to antigenic tumor cells. For example, deficiencies in quantity, processing, presentation, or affinity may reduce the antigenicity of certain tumor antigens. Lack of expression of costimulatory molecules on the tumor cells can lead to anergy of tumor-reactive T cells (for review see reference 1). Expression of Fas ligand on tumor cells can induce apoptosis of T cells entering the site of tumor growth (2–4). In addition, tumors and/or their surrounding stroma may produce immunosuppressive factors such as TGF-β that oppose effective stimulation, particularly of naive T cells (5–7). Another critical factor may be that cancer cells, like infectious agents, are proliferating antigens, and the precursor frequency of specifically reactive T cells may be too low to expand to the number of T cells needed to eradicate the growing tumor. Thus, the failure of the immune system to reject tumors could be in part the result of the expansion of the tumor cell population outpacing that of the specific T cells. Finally, the stroma of established tumors consists of nonmalignant, nonantigenic host cells that may prevent immune destruction (8).
Numerous studies have shown that preventive vaccinations or vaccinations early after tumor cell inoculation can be effective in inducing rejection of inoculated tumor cells. However, therapeutic vaccinations at later stages of tumor growth usually fail (for review see reference 9). While late vaccination of a tumor-bearing host can induce specific immune responses against antigens expressed by the tumor and can even lead to rejection of a second fresh tumor cell challenge, the original tumor is usually not rejected. This phenomenon is often referred to as concomitant immunity and is taken as evidence that the established tumor is somehow protected from immune attack. In at least some tumor models nonimmunological mechanisms, like the secretion of the plasminogen fragment angiostatin that results from the growth of the primary tumor, may cause resistance of the tumor-bearing host to a second tumor challenge (10, 11). However, other experiments clearly show that antigen-specific immunity can exist in mice carrying long-term tumors. A striking example of this is the observation that tumor-bearing mice can reject allogeneic skin grafts, but fail to reject the established tumor that expresses the same alloantigen (12).
Though specific evidence is lacking, it is possible that the set of self-peptides presented on allogeneic MHC class I molecules may be different on normal and malignant cells; therefore, the ability of the tumor-bearing host to reject normal tissue may result from different T cell clonotypes responding to the normal allograft. To bypass this possibility, we used 2C TCR transgenic mice in which all the CD8+ T cells carry the same TCR specific for an alloantigen expressed by the tumor (13). The 2C TCR recognizes several closely related natural peptides, all of which are derived from the murine enzyme α-ketoglutarate dehydrogenase (α-KGDH)1, presented by the MHC class I molecule Ld (14, 15). As a housekeeping protein, α-KGDH is expressed by normal and malignant tissues. The use of this defined transgenic mouse model offers several advantages. (a) The supply of tumor-specific CD8+ T cells in the transgenic mice should be virtually unlimited; thus, we can test the hypothesis that antigenic cancers grow due to insufficient numbers of tumor-specific precursor T cells. (b) As far as we know, the transgenic CD8+ T cells recognize the same epitopes present on both Ld-positive tumors as well as Ld-positive normal tissues; thus, a comparison of the immune responses against malignant and normal tissue should be possible. In this study, we show that hosts with well-established tumor burdens showed no evidence of T cell exhaustion, peripheral anergy, or generalized immune suppression. However, expression of a strong nonself-antigen on tumors was not sufficient to cause rejection of established tumors. Rejection of a tumor cell challenge was observed only after powerful preimmunization of the mice. In addition, expression of costimulatory molecules by the tumor cells was needed for rejection in 100% of the mice.
Materials and Methods
Mice and Tumor Lines.
Female C3H/HeN (MTV−), BALB/c, DBA/2, C57BL/6, and athymic nude mice, 4–6 wk old, were purchased from the National Cancer Institute, Frederick Cancer Research Facility, (Frederick, MD). The 2C TCR transgenic mouse strain (13), bearing a TCR recognizing the MHC class I molecule H-2Ld in association with a set of closely related peptides, was provided by Dr. D. Loh (Washington University, St. Louis, MO), and was bred and maintained in a specific pathogen-free barrier facility at the University of Chicago. After back-crossing the 2C TCR transgenic mouse strain to the C57BL/6 strain for >10 generations, the mice were crossbred with C3H mice and the C3H × 2C TCR F1 mice were used for all experiments. Expression of the transgenic TCR on CD8+ T cells was determined by flow cytometry analyses using the clonotype-specific mAb 1B2 (16). The AG104A fibrosarcoma grew out spontaneously in an aging C3H mouse (2 yr and 1 mo old) and was adapted to culture as described (17). The B7+CD48+AG104A transfectant of AG104A cells (renamed here AG104ABC) has been described previously (18). The tumor cell lines were tested regularly for mycoplasma contamination by staining with HOECHST 33258 and examination by immunofluorescence.
Transfection of Murine H-2Ld.
AG104A cells were transfected by N,N-bis[2-hydroxyethyl]-2-aminoethansulfonic acid (BES) precipitation with the vector LK444 containing the entire open reading frame of murine H-2Ld (19), provided by Dr. A. Sant (University of Chicago), and the transfectants were selected in DMEM containing 10% FCS (CDMEM) and 500 μg/ml G418. AG104ABC cells were transfected by electroporation with the same vector and the pUHD10-3-puro vector containing a puromycin resistance gene, provided by Dr. D. Mumberg (University of Chicago), and were selected in DMEM containing 10% FCS and 8.5 μg/ml puromycin. Individual resistant clones were obtained by limiting dilution cloning and analyzed for Ld expression by FACS® analysis.
Tumor Growth In Vivo and Readaptation to Culture.
Tumor cells were injected subcutaneously into the flanks of the mice. We injected 5 × 105 untransfected AG104A wild-type tumor cells (AG104A–wt) and 2.5 × 106 transfected AG104A–Ld, AG104ABC, and AG104ABC–Ld tumor cells because these doses yielded tumors with similar rates of growth. Tumor growth was measured every 3 to 4 d with a caliper. Size in cubic centimeters was calculated by the formula V = π_abc_/6, where a, b, and c are three orthogonal diameters. At the end of the experiment, animals were killed and tumor tissue from two or three different locations of the tumor was reisolated. Tumor fragments were incubated in CDMEM containing 10% FCS, gentamycin, and penicillin/streptomycin for a few days before analysis.
Full-Thickness Skin Grafting.
Donor skin was obtained from the ventral surface of the donor mice (BALB/c or DBA/2) and applied to the dorsal thoracic wall according to an adaption of the method of Billingham and Medawar (20). Bandages were removed on day 8 and grafts were monitored daily until rejection (defined as loss of at least 80% of grafted tissue) or the end point of the experiment.
Immunization Studies.
Mice, in groups of six, were injected intraperitoneally with 5 × 106 and subcutaneously with 5 × 106 γ-irradiated (10,000 rads) AG104A–Ld or AG104ABC–Ld tumor cells. After 14 d, they were challenged subcutaneously, in groups of three, with either AG104A–Ld or AG104ABC–Ld cells and tumor growth was measured as described above. As indicated in the text, Ld-negative AG104A–wt tumor cells or AG104ABC tumor cells were injected on the opposite flank as a specificity control.
Generation of Cytolytic Ld-specific CTL and an Ld-specific T Cell Clone.
Splenocytes were harvested from 2C TCR transgenic mice and washed once in CDMEM containing 10% FCS. Erythrocytes were removed by centrifugation over Ficoll–Hypaque. The remaining cells were washed twice and resuspended in CDMEM. Cells were then distributed into round-bottomed, 96-well plates at 0.5 cells/well to be stimulated with mitomycin C–treated (100 μg/ml for 90 min) P815 mastocytoma cells expressing murine CD80 (mB7-1; provided by Dr. L. Lanier, DNAX Research Institute, Palo Alto, CA) in the presence of 20 U/ml recombinant human IL-2 (rhIL-2; Cetus Corporation, Emeryville, CA) and 10 ng/ml recombinant murine IL-4 (rmIL-4; Immunex Corporation, Seattle, WA). After 7 d in culture, wells containing growing T cells were harvested and washed twice in CDMEM. The T cell clone 900-2 was selected from among these positive clones and was restimulated in 24-well tissue culture plates (Linbro Corporation, ICN Biomedicals, Inc., Aurora, OH) with 3 × 105 P815–B7-1 cells/well in the presence of 20 U/ml rhIL-2. After 3 wk of passage on P815–B7-1 cells, the clone was restimulated for weekly passage with irradiated (2,000 rads) DBA/2J splenocytes in 24-well plates (6 × 106 splenocytes/well, 1 × 105 T cells/well, 20 U/ml rhIL-2). To compare the cytolytic activity of T cells from naive and tumor-bearing mice, spleen cells were incubated with mitomycin C–treated tumor cells in a mixed lymphocyte–tumor cell culture (MLTC) as previously described (17). Cytotoxicity of T cell clones or MLTC effector cells was determined in a 4.5-h 51Cr release assay at different E/T ratios as described (21). The percentage-specific lysis was calculated by the formula: percent lysis = ([experimental release − spontaneous release] / [maximum release − spontaneous release]) × 100. Spontaneous release was ⩽15% of maximum. Maximum release was determined by detergent lysis of targets.
Antibodies, Immunostaining, and Flow Cytometry Analyses.
Purified and FITC- or PE-conjugated mAbs to B7-1 (CD80), CD48, CD8a, and CD4 were purchased from _PharMingen Corp_oration (San Diego, CA). The mAbs 30-5-7S (anti-H-2Ld; reference 22) and 237 (anti-AG104A; reference 17) were generated as culture supernatant of the respective hybridomas. The purified mAb 1B2 (anti-2C TCR; reference 16) was a gift of T. Walunas (University of Chicago). Mice were depleted of CD8+ or CD4+ cells by intraperitoneal injection of 0.2 ml of ascites fluid from nude mice bearing the rat anti–mouse Lyt-2 hybridoma YTS169.4.2 (anti-CD8; reference 23) or rat anti–mouse L3T4 hybridoma GK1.5 (anti-CD4; reference 24), respectively. The mice received prophylactic antibiotic in the drinking water (sulfamethoxazole/trimethoprin pediatric suspension at 5 ml per 200 ml of water; Geneva Pharmaceuticals, Broomfield, CO). To measure the lymphocyte depletion, peripheral blood from the orbital plexus or splenic cell suspensions were treated with Tris ammonium chloride (0.83%) to eliminate erythrocytes and then stained for FACS® analysis. Greater than 99% depletion of the specific subset was observed. For immunofluorescence staining, single cell suspensions of tumor cells, peripheral blood, or spleen were incubated on ice for 30 min with the indicated reagents in FACS® buffer (PBS + 0.2% BSA + 0.02% NaN3). After washing three times with FACS® buffer, cells were either immediately analyzed or, if unconjugated antibodies were used for the primary staining, incubated with FITC-conjugated goat anti–mouse IgG (American Qualex, San Clemente, CA) or PE-coupled streptavidin (Calbiochem–Novabiochem Corporation, La Jolla, CA) at 4°C for 20 min. Following three washes with FACS® buffer 10,000 to 25,000 cells were analyzed on a FACScan® (Becton Dickinson) using LYSIS II software.
Preparation of Total RNA and cDNA.
Total RNA from 5 × 106 cells grown in culture or 30 mg tumor tissue was isolated using the RNeasy Kit (Qiagen, Hilden, Germany) as prescribed by the manufacturer. For cDNA synthesis, 4 μg of total RNA were reverse transcribed in the presence of 1.25 μg oligo(dt)15 primer (Boehringer Mannheim, Indianapolis, IN) and 300 U M-MLV reverse transcriptase (GIBCO BRL, Gaithersburg, MD) in a total volume of 40 μl. No reverse transcriptase was added to the control samples. Reactions were incubated at 37°C for 60 min and then heat-inactivated at 95°C for 15 min.
PCR Amplification.
PCR reactions were performed in a total volume of 50 μl containing 200 mM dNTPs (Boehringer Mannheim), 50 pM oligonucleotide primers, and 0.25 μl Taq polymerase (Promega, Madison, WI; 5U/ml). PCR reactions were subjected to 1-min denaturation at 94°C, 1-min annealing at 60°C, and 1.5-min extension at 72°C. The number of cycles was determined individually for each primer pair. Primers: β-actin: forward, 5′-GGATGACGATATCGCTGCGCTG-3′, reverse, 5′-GTACTTCAGGGTCAGGATACCTC-3′, Fas ligand: forward, 5′-CACTCAAGGTCCATCCCTCTG-3′, reverse, 5′-TAGCTGACCTGTTGGACCTTGC-3′.
Results
AG104A Tumor Cells Transfected to Express Ld Grow Progressively in Anti-Ld TCR Transgenic Mice as Antigen-positive Tumors.
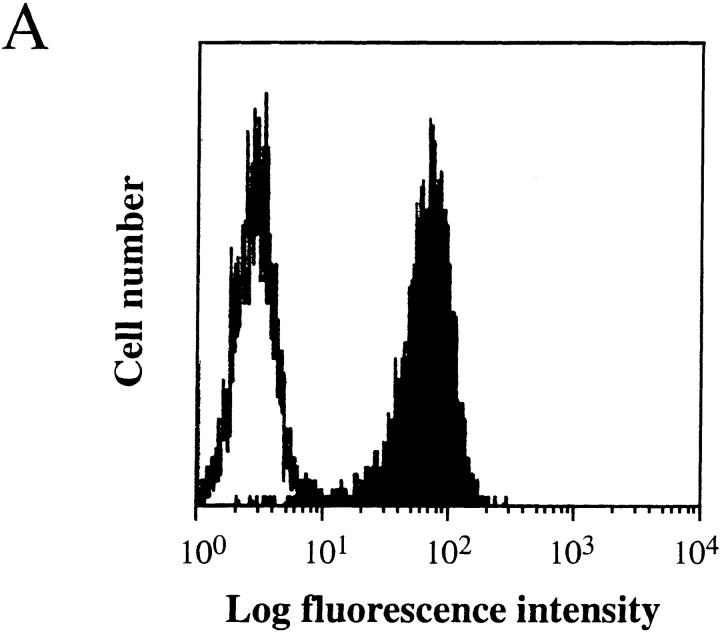
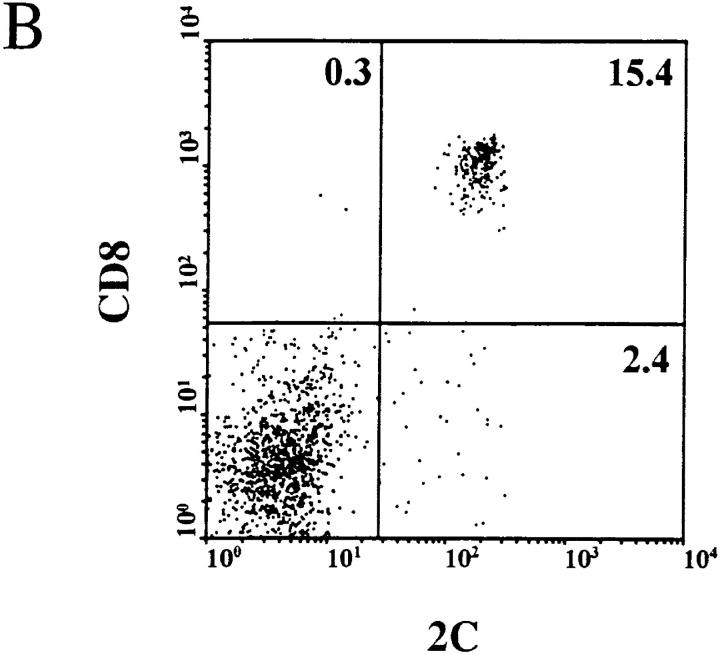
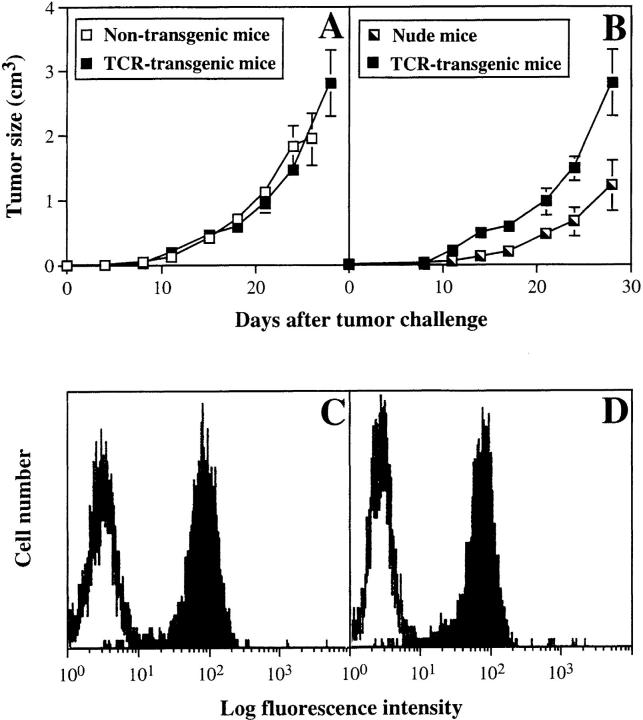
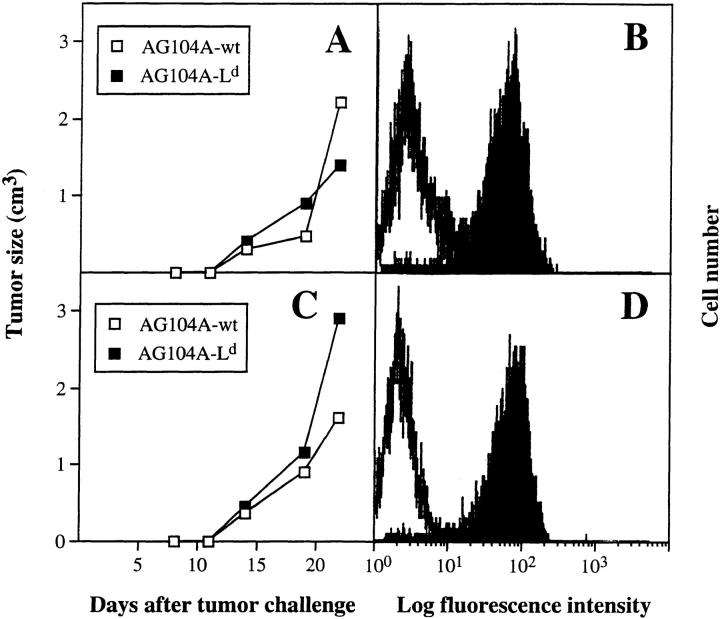
We have shown previously that PRO4L fibrosarcoma cells transfected to express certain alloantigens can grow progressively in immunocompetent mice, while normal skin transplants expressing the same alloantigens are rejected (12, 25). To determine whether this characteristic is shared by other tumor cell lines, we transfected a poorly immunogenic C3H fibrosarcoma that arose spontaneously in an aging mouse with the MHC class I alloantigen Ld. The transfected AG104A tumor cells (AG104A–Ld) expressed Ld at a level ∼40-fold above background as measured by flow cytometry (Fig. 1 A). Nevertheless all of the C3H × C57BL/6 F1 mice developed progressively growing lethal, antigen-positive tumors when injected subcutaneously with AG104A–Ld tumor cells. (Fig. 2 A). The tumor also grew progressively in C3H/HeN mice (data not shown). We reasoned that the precursor frequency of Ld-specific T cells in these mice may be too low to match the rapid growth of the Ld-expressing tumor cells. Therefore, AG104A–Ld tumor cells were injected into C3H × 2C anti-Ld TCR transgenic mice (13) in which all CD8+ T cells express the TCR 2C (see Fig. 1 B) that recognizes the Ld antigen in association with a set of closely related natural self-peptides (14, 15). Surprisingly, all of the transgenic mice developed lethal AG104A–Ld tumors despite the predominance of the anti-Ld T cells. In addition, the rate of tumor growth in transgenic mice was unaffected by the presence of the anti-Ld T cells because similar rates of growth were also observed in nontransgenic mice (Fig. 2 A) and nude mice (Fig. 2 B). Reisolation of the AG104A–Ld tumors showed that the expression of the Ld antigen was retained in vivo in the nontransgenic (Fig. 2 C) as well as in the transgenic mice (Fig. 2 D).
Figure 1.
Homogenous expression of the Ld molecule on the transfected tumor cells and of the anti-Ld TCR 2C on the transgenic CD8+ T cells. (A) Stable transfection of AG104A tumor cells with an Ld cDNA expression vector resulted in Ld expression 40-fold above background as shown by FACS® analysis. Shaded curve, anti-Ld staining; unshaded curve, staining with goat anti–mouse FITC secondary antibody alone. (B) Two-color staining of peripheral blood cells from the C3H × 2C transgenic mice with biotinylated anti-CD8a, and 1B2–FITC (anti–2C) mAbs showed that all CD8+ T cells express the 2C TCR.
Figure 2.
AG104A–Ld tumor cells grow progressively and retain Ld expression in normal C3H × C57BL/6 mice and anti-Ld TCR transgenic mice. AG104A–Ld cells were injected subcutaneously into the flanks of four normal C3H × C57BL/6 mice and five anti-Ld C3H × 2C TCR transgenic mice (A) or two nude mice and three anti-Ld C3H × 2C TCR transgenic mice (B). Tumors grew out in all types of mice. Bars indicate the SEM. FACS® analysis of AG104A–Ld tumor cells reisolated at day 27 from a nontransgenic mouse (C) or at day 33 from a TCR transgenic mouse (D) showed that antigen expression was retained in the course of tumor growth. Shaded curve, anti-Ld staining; unshaded curve, staining with goat anti–mouse FITC secondary antibody alone.
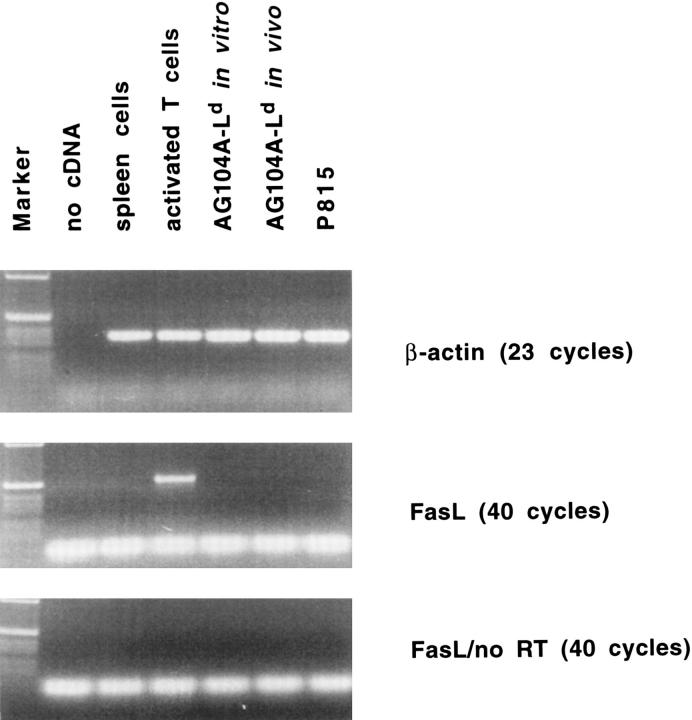
Because it has been described that antigen-specific T cells can undergo apoptotic death due to Fas ligand expression on the target tumor cells (2–4), we analysed in vitro and in vivo grown AG104A–Ld cells for the expression of Fas ligand by PCR analysis. Neither AG104A–Ld cells grown in culture nor AG104A–Ld cells reisolated from a tumor grown in vivo expressed Fas ligand (Fig. 3). Activated T cells, which expressed Fas ligand, were used as positive control and analysis of Fas ligand expression in the absence of reverse transcriptase showed that this signal was not due to genomic DNA contamination.
Figure 3.
AG104A–Ld tumor cells do not express Fas ligand. Total RNA was isolated from AG104A–Ld tumor cells grown in culture or as tumors in vivo, P815 tumor cells, and spleen cells. Total RNA from the T cell clone pGL10 (37), activated for 7 d on syngeneic feeder cells in the presence of 0.2 mg/ml chicken ovalbumin and 12 IU/ml rhuIL-2, was provided by Dr. U. Korthäuer (University of Chicago). Expression of Fas ligand and β-actin was analyzed by RT-PCR. To test for genomic DNA contamination, RT-PCR was also performed without the addition of reverse transcriptase for the cDNA synthesis (no RT).
Ld-positive Normal Skin Is Rejected by CD8+ Lymphocytes in the Anti-Ld TCR Transgenic Mice.
Allogeneic full-thickness skingrafts are rejected by normal, immunocompetent mice about 12–13 d after transplantation even when grafts are antigenically disparate from the recipient in only one MHC locus (12). Table 1 shows that the anti-Ld TCR transgenic mice could reject Ld-positive full-thickness skin allografts as effectively as nontransgenic littermates. Furthermore, treatment of the mice with anti-CD8 antibody, but not anti-CD4 antibody, prevented this rejection. Flow cytometric analysis of the lymphocytes from the antibody-treated mice revealed specific and complete elimination of the T cell subsets (data not shown). These results showed that the CD8+ T cell compartment of the transgenic mice is necessary for skin graft rejection and does not require CD4+ T cells.
Table 1.
Anti-Ld TCR Transgenic Mice Reject Ld-positive Full-Thickness Skin Allografts as Effectively as Nontransgenic Littermates
| Recipient | Strain of origin of Ld-positive full-thickness skin | Take of graft | Survival of graft | |
|---|---|---|---|---|
| Strain | Antibody treatment | |||
| d ± SD* | ||||
| C3H × 2C (TCR transgenic) | None | BALB/c | 0/34 | 13 ± 1 |
| None | DBA/2 | 0/12 | 13 ± 1 | |
| Anti-CD4 | BALB/c | 0/2 | 13 ± 1 | |
| Anti-CD8 | BALB/c | 3/3 | >24 | |
| C3H × C57BL/6 (nontransgenic) | None | BALB/c | 0/2 | 12 |
| None | DBA/2 | 0/4 | 12 ± 1 | |
| BALB/c | None | BALB/c | 8/8 | >24 |
| DBA/2 | None | DBA/2 | 3/3 | >24 |
AG104A–Ld Tumor Cells Are Sensitive In Vitro to Preactivated CD8+ Cytolytic T Cells.
Because the anti-Ld TCR transgenic mice failed to reject Ld-positive tumor cells, but rejected Ld-positive skin grafts, we determined whether the tumor cells could be lysed in vitro by Ld-specific T cells expressing the 2C clonotype. Fig. 4 shows that the Ld-transfected AG104A tumor cells were lysed specifically in a 4.5-h 51Cr release assay by the anti-Ld CD8+ cytolytic T cell clone 900-2 derived from the transgenic mice. This result showed that the failure of the AG104A–Ld tumor cells to be rejected by the C3H × 2C mice was not because the tumor cells were resistant to lysis by the transgenic T cells.
Figure 4.
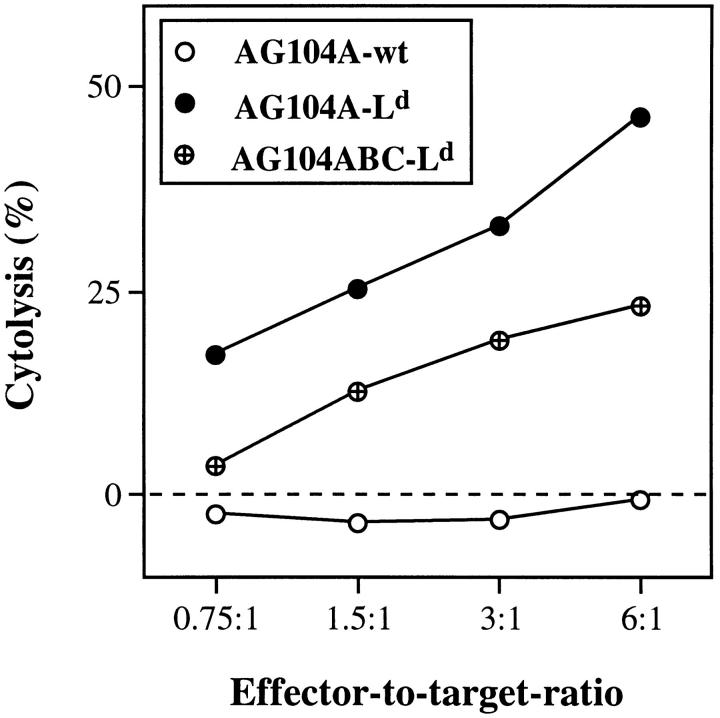
An anti-Ld–specific CD8+ T cell clone from TCR transgenic mice has cytolytic activity against AG104A–Ld and AG104ABC–Ld tumor cells in vitro. The CD8+ T cell clone 900-2 derived from the 2C TCR transgenic mice specifically recognizes and lyses AG104A–Ld and AG104ABC–Ld tumor cells, but not AG104A–wt tumor cells in a 4.5-h 51Cr release assay in vitro.
Failure of the Anti-Ld TCR Transgenic Mice to Reject Ld-positive Tumor Cells Is Not Due to Clonal Exhaustion or Systemic Anergy.
Whereas AG104A–Ld tumor cells can be lysed by preactivated cytolytic T cells in vitro, the activation of the anti-Ld T cells by the tumor in vivo may be inefficient. Rejection of full-thickness skin grafts is a powerful immunization procedure, probably owing to the abundance of dendritic cells (Langerhans cells) in the skin. Therefore, to determine whether activation of the T cells by skin graft rejection would also lead to rejection of the tumor, we inoculated TCR transgenic mice with Ld-positive tumor cells at the same time that we transplanted Ld-positive skin onto these mice. Mice rejected the skin grafts at day 13 after transplantation; however, there was no effect on the growth of the Ld-positive tumors that were already well established at this time (Fig. 5, A and C). The outgrowth of the Ld-positive tumors was not due to antigen loss, because FACS® analysis of the tumors reisolated at day 24 showed no loss or decrease in Ld expression (Fig. 5, B and D).
Figure 5.
Anti-Ld TCR transgenic mice reject Ld-positive skin, but do not reject a simultaneous challenge with Ld-expressing tumor cells. Two TCR transgenic mice were transplanted with full-thickness Ld-positive skin from a BALB/c mouse and concurrently received subcutaneous injections of AG104A–wt cells and AG104A–Ld cells on opposite flanks. Whereas both mice rejected the skin graft at day 13 after transplantation (see Table 1), both wt and Ld-expressing tumors grew progressively (A and C). Bars indicate the SEM. The outgrowth of AG104A–Ld was not due to antigen loss, because tumor cells reisolated on day 24 still stained positive for Ld in a FACS® analysis (B and D). Shaded curve, anti-Ld staining, unshaded curve, staining with goat anti–mouse FITC secondary antibody alone.
The tumors described in the previous experiment were still in earlier stages of growth (day 13) at the time the skin graft was rejected by the mice. Therefore, it could be argued that at that early time sufficient T cells were still available to reject the skin graft, whereas at later stages the CD8+ Ld-specific T cells were functionally or physically exhausted from the tumor-bearing host. Thus, we placed full-thickness BALB/c skin grafts on five anti-Ld TCR transgenic mice bearing AG104A–Ld tumors for 2, 3, or 4 wk. We found that even these late tumor-bearing mice completely rejected the skin grafts, although with a 2–3 d delay when compared with nontumor-bearing mice (data not shown). This result indicates that even at a late stage of tumor growth, the relevant T cells were still present to reject the skin graft. However, there was no detectable effect of the graft rejection on the growth of the Ld-positive tumor (data not shown).
Expression of Costimulatory Molecules by the Ld-positive Tumor Cells Leads to Slower Tumor Growth in Anti-Ld TCR Transgenic Mice.
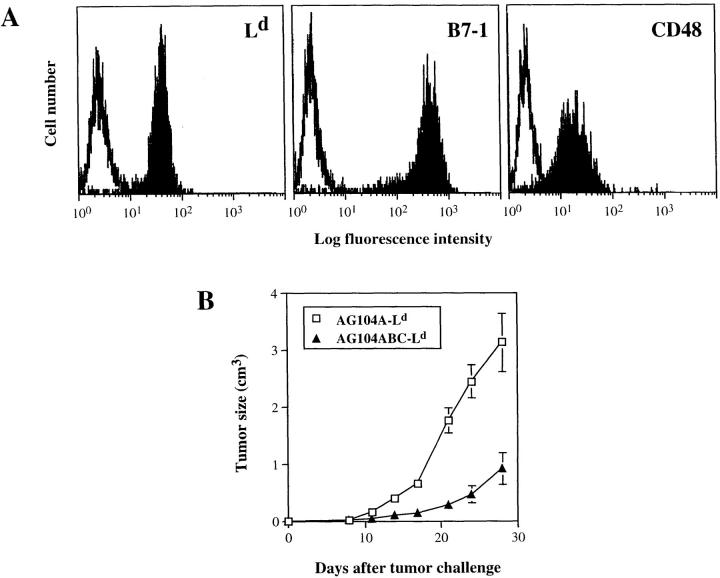
The above experiments showed that mice bearing early or late Ld-positive tumors can show specific immunity against Ld-positive skin tissue without detectable effects on tumor growth. Because skin tissue differs from tumor tissue in that it contains a high number of potent antigen-presenting cells (Langerhans cells) that express costimulatory molecules, we determined whether expression of costimulatory molecules by the growing tumor would suffice to activate the CD8+ T cells and result in tumor rejection. We introduced an Ld expression vector into AG104A cells already transfected to express B7-1 and CD48 (18; renamed AG104ABC cells). The expression level of Ld, B7-1 and CD48 in these triple transfectants (AG104ABC–Ld), also shown in Fig. 6 A, is 15-fold, 200-fold, and 11-fold above background, respectively. AG104ABC–Ld cells were sensitive to lysis in vitro by the anti-Ld CD8+ T cell clone 900-2 in a 4.5-h 51Cr release assay (see Fig. 4). However, 10 of the 11 anti-Ld transgenic mice failed to reject the challenge of AG104ABC-Ld tumor cells (tumor incidence 91%; Table 2). In nine of the mice, the outgrowth of AG104ABC–Ld was significantly slower as compared with tumor cells expressing only the Ld antigen, but no costimulatory molecules (Fig. 6 B). Nevertheless, the AG104ABC–Ld tumors grew out to kill the animals. An additional mouse showed a persistent nongrowing 1-mm3 firm nodule that contained AG104ABC–Ld tumor cells upon reisolation and FACS® staining with AG104A-specific and Ld-specific mAbs. Only one mouse rejected the tumor cell challenge completely. The untransfected tumor cells or those expressing only Ld or B7 and CD48 grew progressively in all of the mice (Table 2).
Figure 6.
Expression of the costimulatory molecules B7-1 and CD48 by the Ld-positive AG104A tumor cells leads to slower tumor outgrowth in naive TCR transgenic mice. AG104A cells expressing the costimulatory molecules B7-1 and CD48 (18) were transfected with an Ld cDNA expression vector. The expression level of Ld, B7-1, and CD48 in these triple transfected cells, named AG104ABC–Ld, is shown in A. To determine the effect of expression of B7-1 and CD48 on tumor cell outgrowth (B), naive TCR transgenic mice were injected with either AG104A–Ld cells (3 mice) or AG104ABC–Ld cells (11 mice). AG104ABC–Ld tumor cells grew slower than AG104A–Ld tumor cells. Bars indicate the SEM.
Table 2.
Incidence (%) of AG104A Tumors in Anti-Ld TCR Transgenic Mice
| Treatment of TCR transgenic mice | Tumor incidence (%) in mice challenged with | |||
|---|---|---|---|---|
| AG104A–wt and AG104A–Ld * | or | AG104ABC and AG104ABC–Ld‡ | ||
| None | 13/13 (100) | 13/13 (100) | 11/11 (100) | 10/11 (91) |
| Preimmunization with irradiated AG104A–Ld cells§ | 3/3 (100) | 3/3 (100) | 3/3 (100) | 3/3 (100) |
| Preimmunization with irradiated AG104ABC–Ld cells§ | 3/3 (100) | 3/3 (100) | 3/3 (100) | 3/3 (100) |
| Preimmunization by rejection of Ld-positive skin‖ | 15/15 (100) | 8/18 (44) | 6/6 (100) | 0/6 (0) |
Only Ld-positive Tumor Cells that Provide Costimulation Are Rejected after Preimmunization.
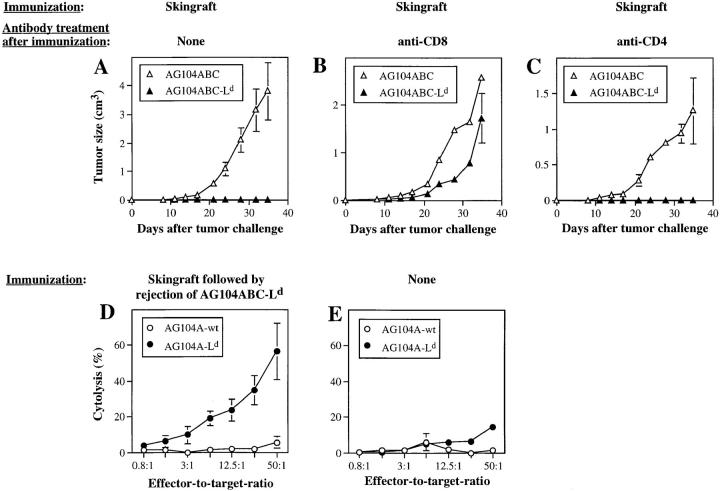
To test whether CD8+ T cells from anti-Ld TCR transgenic mice may be more effective in rejecting the Ld-positive tumor cells after preimmunization, the anti-Ld TCR transgenic C3H × 2C mice were immunized either with Ld-positive (BALB/c) full-thickness skin grafts or with lethally irradiated AG104A tumor cells expressing either Ld alone or in combination with B7-1 and CD48. Immunization with the transfected tumor cells caused only minimal reduction in the rate of growth of a subsequent challenge with AG104ABC–Ld or AG104A–Ld tumor cells (Table 2); each of the four groups showed progressive tumor growth in all mice. By contrast, immunization with the Ld-positive skin caused full protection against a subsequent challenge with AG104ABC–Ld tumor cells (Fig. 7 A), but only a partial protection against AG104A–Ld tumor cells (Table 2). Protective immunization by Ld-positive skin grafts was antigen specific, because there was no protection against Ld-negative AG104A tumor cells whether or not transfected with B7-1 and CD48 (Fig. 7 A; Table 2). The rejection of the tumor challenge was dependent upon CD8+ T cells, because the tumor cells grew progressively in two mice treated with anti-CD8 antibody, while the rejection of the tumor cells was unaffected in the two mice treated with anti-CD4 antibody (Fig. 7, B and C). Furthermore, the mice that had rejected the AG104ABC–Ld tumor cells after immunization with the Ld-positive skin grafts mounted stronger Ld-specific responses in vitro (Fig. 7 D) than naive mice (Fig. 7 E) or mice that only rejected Ld-positive skin (data not shown).
Figure 7.
Antigen-positive tumor cells providing costimulation are effectively rejected after preimmunization of TCR transgenic mice with antigen-positive skin. Six TCR transgenic mice were challenged subcutaneously with AG104ABC–Ld tumor cells and AG104ABC tumor cells on opposite flanks 3–5 d after rejection of a full-thickness BALB/c skin graft. All six mice rejected the AG104ABC–Ld tumor cells, but not the simultaneous AG104ABC tumor cell challenge, showing that the protective effect of Ld-positive skin was antigen specific (A). Four mice, each of which rejected the skin graft, were treated with anti-CD8 antibody (B) or anti-CD4 antibody (C) 3 d before challenge with AG104ABC–Ld tumor cells. The tumors grew in the anti-CD8–treated mice, but not in the anti-CD4–treated mice, showing that tumor rejection is mediated by CD8+ T cells. Spleen cells from the six mice that rejected the AG104ABC–Ld tumor cells (D) and six naive TCR transgenic mice (E) were stimulated in vitro with AG104A–Ld tumor cells in a MLTC. Specific cytolytic T cells were detected only in the culture from mice that had rejected the AG104ABC–Ld tumor challenge. Bars indicate the SEM.
Discussion
Our initial experiments in this study showed that normal C3H or C3H × C57BL/6 F1 mice were able to reject skin grafts expressing the MHC class I alloantigen Ld, but failed to reject Ld-transfected tumor cells. We reasoned that an alternate set of predominant self-peptides may be presented in the context of this MHC class I molecule in normal and malignant tissue. Therefore, the two types of transplants may affect various Ld-specific T cell clonotypes differently. Furthermore, it is possible that the precursor frequency of Ld-specific T cells in these mice may simply not be sufficient to prevent the tumor cells from growing. Therefore, we examined how mice transgenic for the anti-Ld TCR 2C (13) would respond to skin grafts and tumor cell challenges expressing the Ld antigen. The 2C TCR is expressed on all CD8+ T cells of these mice (13) and specifically recognizes Ld in combination with a closely related set of peptides derived from the ubiquitously expressed housekeeping enzyme α-KGDH (14, 15). Surprisingly, we found that TCR transgenic mice rejected Ld-positive full-thickness skin grafts, but at the same time failed to reject Ld-positive tumor cells. Even transgenic mice bearing an Ld-expressing tumor for two to four weeks were still able to reject Ld-positive skin without any detectable effect on the further outgrowth of the tumor. Thus, antigenic cancer cells can still grow progressively when all of the CD8+ T cells in the host are specific for an antigen on the tumor. Only after prior activation of the CD8+ T cells by the rejection of antigen-positive skin could about half of the transgenic mice eliminate a subsequent antigen-positive tumor cell challenge.
One reason for the failure to reject antigen-positive tumor cells could be that the Ld-specific CD8+ T cells in the TCR transgenic mice were not fully functional or were lacking sufficient help for activation due to a reduced number of CD4+ T cells in these mice (26). However, the rejection of Ld-positive skin shows that the transgenic mice were able to mount an Ld-specific immune response as well as nontransgenic mice. Using subset specific antibodies, we showed that the Ld-specific CD8+ T cells were necessary for the rejection of the skin and that CD4+ T cells were not required for this effect. In addition, rejection of AG104ABC–Ld by preimmunized mice also did not require CD4+ T cells, indicating that CD4+ T cell help is not required for CD8+ T cell activation in this model. An alternative explanation could be that the tumor cells express insufficient numbers of the relevant peptide–MHC complexes on the cell surface to trigger lysis by the transgenic T cells. It has been reported that different closely related peptides derived from the α-KGDH enzyme can be presented on Ld and are recognized by the 2C TCR with different affinities (14, 15, 27). We do not know whether the tumor cells produce all of these peptides. In addition, the abundance of these peptides in different tissues varies profoundly (28). However, the Ld-transfected AG104A tumor cells represented sensitive targets in vitro for an Ld-specific CD8+ cytolytic T cell clone derived from the transgenic mice, indicating that there is sufficient quantity of the Ld–peptide complexes on the AG104A–Ld tumor cells to promote lysis in vitro. Also, AG104A–Ld cells appeared to remain sensitive to lysis in vivo, because intravenous injection of those tumor cells along with the in vitro–activated transgenic CTL clone significantly reduced the number of lung metastases (data not shown). Finally, the tumor cells that grew out were not selected for antigen loss, because analysis of cells reisolated from AG104A–Ld tumors showed that progressively growing tumor cells still expressed Ld.
An adverse local environment generated by tumor cells could account for the escape from immune destruction. In some tumor models, the established tumor graft inhibits the outgrowth of the other grafts by preventing vascularization (10, 11). However, in our model the antigenic skin grafts were fully vascularized and healed-in before being rejected; thus, vascular establishment per se did not allow the antigenic skin to avoid immune destruction in the tumor-bearing host. Another effective way for antigenic tumors to escape immunological rejection is the expression of Fas ligand, which induces apoptosis of the tumor-infiltrating lymphocytes by the engagement of Fas on the T cells (2–4). Such a mechanism may ultimately lead to the exhaustion of the antigen-specific T cells in hosts with large tumor burdens, comparable to the T cell exhaustion observed as a result of persistent viral infections (29, 30). Furthermore, systemic T cell signaling defects have been described, particularly in late tumor-bearing hosts (31–33). However, we showed that AG104A–Ld tumor cells do not express Fas ligand in vitro or in vivo. Also, systemic defects in T cell signaling per se most likely do not account for the failure of tumor rejection, because mice had 13-d-old established tumors when they rejected the skin grafts and even late tumor-bearing hosts (>5 wk) with large tumors could still reject skin grafts with a 2–3-d delay when compared with control mice. These data also indicated that there was no general exhaustion of the antigen-specific T cells by the growing tumor.
In addition to the interaction between the MHC class I–peptide complex and the respective TCR, costimulatory signals are necessary for efficient activation of CD8+ T cells. Absence of costimulatory molecules on tumor cells can result in an unresponsive state or apoptotic cell death of specifically reactive T cells (for an overview see references 1, 34). Consistent with this concept, tumor cells transfected to express costimulatory molecules can induce more effective immunity than untransfected tumor cells (18, 35, 36). Therefore, one attractive explanation for the differential response of the transgenic mice to the two tissues, i.e., rejection of the antigen-positive skin and the persistence of the tumor, could be the differences in local T cell activation by these two types of tissues. In contrast with the tumor, skin is rich in potent antigen-presenting dendritic cells, the Langerhans cells, which express costimulatory molecules as well as the target antigen. However, the lack of costimulation may not be sufficient to explain inefficient tumor rejection, because Ld-positive AG104A tumor cells expressing the costimulatory molecules B7-1 and CD48 still grow progressively, though at a reduced rate. Only after preimmunization by rejection of antigen-positive skin, were AG104ABC–Ld cells completely eliminated. Preimmunization with Ld-positive skin was much more effective in inducing an anti-Ld tumor response than preimmunization with lethally irradiated AG104A–Ld cells transfected to express the costimulatory molecules B7-1 and CD48. Possibly, the large dose of x ray we used may have shortened the survival of the immunizing cells and lessened the effectiveness of the immunization. However, the amount of irradiation we used was needed to prevent in vitro and in vivo growth of the tumor cells used for vaccination.
Taken together, our results point out critical problems for inducing anti-tumor immune responses. Using a TCR transgenic mouse model we could show that, even in the absence of systemic anergy or clonal exhaustion, a very high number of tumor-specific precursor CD8+ T cells per se was not sufficient for tumor rejection in vivo. Tumor cell challenges were only rejected effectively after prior activation of the CD8+ T cells by preimmunization and when the cells used for tumor challenge were manipulated so that they also expressed costimulatory molecules. Only the most potent immunization, rejection of antigen-positive full-thickness skin, was successful in inducing immunity that could lead to the rejection of a subsequent antigen-positive tumor challenge. Although we are not certain why preimmunization was needed, it may be that these T cells are activated more effectively and recirculate to enter the tumor site, and/or are more resistant to an adverse intratumor environment. Immunization started at the time of tumor inoculation or thereafter did not influence the tumor outgrowth, indicating that the establishment of the solid tumor prevented therapy. During tumor establishment, cancer cells become surrounded by nonantigenic stroma (interstitial cells and vessels), which can clearly counteract effective immunological rejection (8). By contrast, antigenicity of the stroma of normal tissue allografts is a major reason for effective rejection of such grafts. Therefore, it is possible that the nonantigenic stroma of cancers may prevent tumor-specific T cells from being attracted to and penetrating into the tumor. This is consistent with our finding that the Ld-positive tumors, once established in the TCR transgenic mice, are not infiltrated by T cells (data not shown). In summary, our results show that even an abundance of tumor-specific T cells and vigorous active immunization procedures such as rejection of a full-thickness skin graft are insufficient to cause rejection of established tumors. Thus, a critical hurdle for the immunotherapy of cancer may be to find effective ways to direct tumor-specific T cells into established tumors and to activate them at the tumor site.
Acknowledgments
We thank Drs. F.W. Fitch, D. Mumberg, and D.A. Rowley for critical reading of the manuscript. We also thank T. Walunas and R. Dick for providing the transgenic males for breeding and S. Wanderling for assistance with the breeding of the F1 TCR transgenic mice. We are also very grateful to Drs. U. Korthäuer, L. Lanier, D. Loh, D. Mumberg, A. Sant, and T. Walunas for the gift of reagents.
This work was supported by National Institutes of Health grants R01-CA22677, R01-CA37156, Cancer Center grant CA-14599 (Core Facility), Corinne Kreissl Memorial Foundation Grant of the American Cancer Society (IM-773), Deutsche Forschungsgemeinschaft (M. Wick), and a gift from the Passis family.
Footnotes
M. Wick and P. Dubey contributed equally to this paper.
Part of this work was presented in abstract form at the Keystone Symposia “Cellular Immunology and the Immunotherapy of Cancer III,” Copper Mountain, February 1997.
1
Abbreviations used in this paper: α-KGDH, α-ketoglutarate dehydrogenase; BES, N,N-bis[2-hydroxyethyl]-2-aminoethanesulfonic acid; CDMEM, DMEM containing 10% FCS; CTL, cytolytic T lymphocyte(s); MLTC, mixed lymphocyte–tumor cell culture; rhIL-2, recombinant human IL-2; rmIL-2, recombinant murine IL-2.
References
- 1.Chen L, Linsley PS, Hellström KE. Costimulation of T cells for tumor immunity. Immunol Today. 1993;14:483–486. doi: 10.1016/0167-5699(93)90262-J. [DOI] [PubMed] [Google Scholar]
- 2.O'Connell J, O'Sullivan GC, Collins JK, Shanahan F. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med. 1996;184:1075–1082. doi: 10.1084/jem.184.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strand S, Hofmann WJ, Hug H, Müller M, Otto G, Strand D, Mariani SM, Stremmel W, Krammer PH, Galle PR. Lymphocyte apoptosis induced by CD95 (APO-1/FAS) ligand-expressing tumor cells—a mechanism of immune evasion? . Nature Med. 1996;2:1361–1370. doi: 10.1038/nm1296-1361. [DOI] [PubMed] [Google Scholar]
- 4.Hahne M, Rimoldi D, Schröter M, Romero P, Schreier M, French LE, Schneider P, Bornand T, Fontana A, Lienard D, Cerottini J-C, Tschopp J. Melanoma cell expression of Fas(Apo-1/CD95) ligand: implications for tumor immune escape. Science (Wash DC) 1996;274:1363–1366. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 5.Ranges GE, Figari IS, Espevik T, Palladino MA. Inhibition of cytotoxic T cell development by transforming growth factor β and reversal by recombinant tumor necrosis factor α. J Exp Med. 1987;166:991–998. doi: 10.1084/jem.166.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torre-Amione G, Beauchamp RD, Koeppen H, Park BH, Schreiber H, Moses HL, Rowley DA. A highly immunogenic tumor transfected with a murine TGF-β cDNA escapes immune surveillance. Proc Natl Acad Sci USA. 1990;87:1468–1490. doi: 10.1073/pnas.87.4.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cousins SW, McCabe MM, Danielpour D, Streilein JW. Identification of transforming growth factor-beta as an immunosuppressive factor in aqueous humor. Invest Opthalmol Vis Sci. 1991;32:2201–2211. [PubMed] [Google Scholar]
- 8.Singh S, Ross SR, Acena M, Rowley DA, Schreiber H. Stroma is critical for preventing or permitting immunological destruction of antigenic cancer cells. J Exp Med. 1992;175:139–146. doi: 10.1084/jem.175.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schreiber, H. 1993. Tumor immunology. In Fundamental Immunology. Third edition. W.E. Paul, editor. Raven Press, Ltd., New York. 1143–1178.
- 10.O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 11.O'Reilly MS, Holmgrem L, Chen C, Folkman J. Angiostatin induces and sustains dormancy of human primary tumors in mice. Nature Med. 1996;2:689–692. doi: 10.1038/nm0696-689. [DOI] [PubMed] [Google Scholar]
- 12.Perdrizet GA, Ross SR, Stauss HJ, Singh S, Koeppen H, Schreiber H. Animals bearing malignant grafts reject normal grafts that express through gene transfer the same antigen. J Exp Med. 1990;171:1205–1220. doi: 10.1084/jem.171.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sha WC, Nelson CA, Newberry RD, Kranz DM, Russell JH, Loh DY. Selective expression of an antigen receptor on CD8-bearing T lymphocytes in transgenic mice. Nature (Lond) 1988;335:271–274. doi: 10.1038/335271a0. [DOI] [PubMed] [Google Scholar]
- 14.Udaka K, Tsomides TJ, Eisen HN. A naturally occurring peptide recognized by alloreactive CD8+cytotoxic T lymphocytes in association with a class I MHC protein. Cell. 1992;69:989–998. doi: 10.1016/0092-8674(92)90617-l. [DOI] [PubMed] [Google Scholar]
- 15.Udaka K, Tsomides TJ, Walden P, Fukusen N, Eisen HN. A ubiquitous protein is the source of naturally occurring peptides that are recognized by a CD8+T-cell clone. Proc Natl Acad Sci USA. 1993;90:11272–11276. doi: 10.1073/pnas.90.23.11272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kranz DM, Tonegawa S, Eisen HN. Attachment of an anti-receptor antibody to non-target cells renders them susceptible to lysis by a clone of cytotoxic T lymphocytes. Proc Natl Acad Sci USA. 1984;81:7922–7926. doi: 10.1073/pnas.81.24.7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward PL, Koeppen H, Hurteau T, Schreiber H. Tumor antigens defined by cloned immunological probes are highly polymorphic and are not detected on autologous normal cells. J Exp Med. 1989;170:217–232. doi: 10.1084/jem.170.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Hellström KE, Newby SA, Chen L. Costimulation by CD48 and B7-1 induces immunity against poorly immunogenic tumors. J Exp Med. 1996;183:639–644. doi: 10.1084/jem.183.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loss GE, Jr, Elias CG, Fields PE, Ribaudo RK, McKisic M, Sant AJ. Major histocompatibility complex class II–restricted presentation of an internally synthesized antigen displays cell-type variability and segregates from the exogenous class II and endogenous class I presentation pathways. J Exp Med. 1993;178:73–85. doi: 10.1084/jem.178.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Billingham RE, Medawar PB. The technique of free skin grafting in mammals. J Exp Biol. 1951;28:385–402. [Google Scholar]
- 21.Seung LP, Rowley DA, Dubey P, Schreiber H. Synergy between T-cell immunity and inhibition of paracrine stimulation causes tumor rejection. Proc Natl Acad Sci USA. 1995;92:6254–6258. doi: 10.1073/pnas.92.14.6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozato K, Hansen TH, Sachs DH. Monoclonal antibodies to mouse MHC antigens. II. Antibodies to the H-2Ldantigen, the products of a third polymorphic locus of the mouse major histocompatibility complex. J Immunol. 1980;125:2473–2477. [PubMed] [Google Scholar]
- 23.Cobbold SP, Jayasuriya A, Nash A, Prospero TD, Waldmann H. Therapy with monoclonal antibodies by elimination of T cell subsets in vivo. Nature (Lond) 1984;312:548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- 24.Dialynas DP, Wilde DB, Marrack P, Pierres A, Wall KA, Havran W, Otten G, Loken MR, Pierres M, Kappler J, Fitch FW. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 25.Koeppen H, Acena M, Drolet A, Rowley DA, Schreiber H. Tumors with reduced expression of a cytotoxic T lymphocyte recognized antigen lack immunogenicity but retain sensitivity to lysis by cytotoxic T lymphocytes. Eur J Immunol. 1993;23:2770–2776. doi: 10.1002/eji.1830231108. [DOI] [PubMed] [Google Scholar]
- 26.Chen FL, Kung JT. Deficient CD4+T cell proliferation in the class I MHC-restricted 2C TCR-transgenic mouse. J Immunol. 1996;156:2036–2044. [PubMed] [Google Scholar]
- 27.Sykulev Y, Brunmark A, Tsomides TJ, Kageyama S, Jackson M, Peterson PA, Eisen HN. High-affinity reactions between antigen-specific T-cell receptors and peptides associated with allogeneic and syngeneic major histocompatibility complex class I proteins. Proc Natl Acad Sci USA. 1994;91:11487–11491. doi: 10.1073/pnas.91.24.11487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu MX, Tsomides TJ, Eisen HN. Tissue distribution of natural peptides derived from a ubiquitous dehydrogenase, including a novel liver-specific peptide that demonstrates the pronounced specificity of low affinity T cell reactions. J Immunol. 1995;154:4495–4502. [PubMed] [Google Scholar]
- 29.Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature (Lond) 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 30.Moskophidis D, Laine E, Zinkernagel RM. Peripheral clonal deletion of antiviral memory CD8+T cells. Eur J Immunol. 1993;23:3306–3311. doi: 10.1002/eji.1830231237. [DOI] [PubMed] [Google Scholar]
- 31.Mizoguchi H, O'Shea JJ, Longo DL, Loeffler CM, McVicar DW, Ochoa AC. Alterations in signal transduction molecules in T lymphocytes from tumor-bearing mice. Science (Wash DC) 1992;258:1795–1798. doi: 10.1126/science.1465616. [DOI] [PubMed] [Google Scholar]
- 32.Nakagomi H, Petersson M, Magnusson I, Juhlin C, Matsuda M, Mellstedt H, Taupin JL, Vivier E, Anderson P, Kiessling R. Decreased expression of the signal-transducing zeta chains in tumor-infiltrating T-cells and NK cells of patients with colorectal carcinoma. Cancer Res. 1993;53:5610–5612. [PubMed] [Google Scholar]
- 33.Matsuda M, Petersson M, Lenkei R, Taupin JL, Magnusson I, Mellstedt H, Anderson P, Kiessling R. Alterations in the signal-transducing molecules of T cells and NK cells in colorectal tumor-infiltrating, gut mucosal and peripheral lymphocytes: correlation with the stage of the disease. Int J Cancer. 1995;61:765–772. doi: 10.1002/ijc.2910610605. [DOI] [PubMed] [Google Scholar]
- 34.Sperling AI, Auger JA, Ehst BD, Rulifson IC, Thompson CB, Bluestone JA. CD28/B7 interactions deliver a unique signal to naive T cells that regulates cell survival but not early proliferation. J Immunol. 1996;157:3909–3917. [PubMed] [Google Scholar]
- 35.Chen L, Ashe S, Brady WA, Hellström I, Hellström KE, Ledbetter JA, McGowan P, Linsley PS. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell. 1992;71:1093–1102. doi: 10.1016/s0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- 36.Townsend SE, Allison JP. Tumor rejection after direct costimulation of CD8+T cells by B7-transfected melanoma cells. Science (Wash DC) 1993;259:368–370. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- 37.Gajewski TF, Fitch FW. Anti-proliferative effect of IFN-gamma in immune regulation. IV. Murine CTL clones produce IL-3 and GM-CSF, the activity of which is masked by the inhibitory action of secreted IFN-gamma. J Immunol. 1990;144:548–556. [PubMed] [Google Scholar]