Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues (original) (raw)
Abstract
T cell activation by intestinal dendritic cells (DC) induces gut-tropism. We show that, reciprocally, DC from peripheral lymph nodes (PLN-DC) induce homing receptors promoting CD8 T cell accumulation in inflamed skin, particularly ligands for P- and E-selectin. Differential imprinting of tissue-tropism was independent of Th1/Th2 cytokines and not restricted to particular DC subsets. Fixed PLN-DC retained the capacity to induce selectin ligands on T cells, which was suppressed by addition of live intestinal DC. By contrast, fixed intestinal DC failed to promote gut-tropism and instead induced skin-homing receptors. Moreover, the induction of selectin ligands driven by antigen-pulsed PLN-DC could be suppressed “in trans” by adding live intestinal DC, but PLN-DC did not suppress gut-homing receptors induced by intestinal DC. Reactivation of tissue-committed memory cells modified their tissue-tropism according to the last activating DC's origin. Thus, CD8 T cells activated by DC acquire selectin ligands by default unless they encounter fixation-sensitive signal(s) for gut-tropism from intestinal DC. Memory T cells remain responsive to these signals, allowing for dynamic migratory reprogramming by skin- and gut-associated DC.
To function in immune protection, T cells must be able to leave the blood and reach virtually every tissue in the body. A critical step in this process is the adhesion and subsequent transmigration of lymphocytes through the endothelial barrier. This strictly regulated multistep process has been partially characterized, at least for some tissues and T cell subsets (1, 2). Whereas naïve T cells express homing receptors that allow them to migrate to lymphoid organs, like LN, Peyer's patches (PP), and the spleen, they are normally excluded from nonlymphoid peripheral tissues (2, 3). However, once T cells have become activated by their cognate antigen (Ag), they change their pattern of adhesion receptors and acquire the capacity to migrate to extralymphoid sites (3, 4).
T cells localizing to the small intestine lamina propria selectively express the integrin α4β7 and the chemokine receptor CCR9 (5, 6), homing molecules that are essential for efficient T cell migration into the small bowel (7–9). On the other hand, cutaneous effector–memory T cells express E- and P-selectin ligands and the chemokine receptors CCR4 and/or CCR10 (10), receptors critical for T cell homing into the skin (10–14). Therefore, the expression of gut- and skin-homing receptors establishes a dichotomy in the distribution of effector–memory T cells, compartmentalizing the immune responses into two major surface areas exposed to largely distinct sets of Ag.
Several reports have shown that the route of Ag entry determines the differential acquisition of tissue-specific homing molecules on lymphocytes (15, 16). We and others have shown recently that DC from PP or mesenteric LN specifically induced the expression of the gut-homing molecules α4β7 and CCR9 on CD8 T cells as well as the capacity to migrate to the small bowel (9, 17–19). However, the factors that induce T cell acquisition of skin-migratory potential remain unknown.
It has also not been determined if effector–memory T cells with gut- or skin-homing potential represent a stable, irreversible differentiation stage or if, alternatively, tissue-tropic memory cells can adapt their migratory commitment when they are reactivated in a different anatomic context.
Here we show that naïve CD8 T cells activated by DC from skin-draining LN express significantly higher levels of E- and P-selectin ligands, adhere better to skin venules in vivo, and home significantly more efficiently to inflamed skin as compared with CD8 T cells activated with DC from gut-derived lymphoid tissues. Furthermore, the induction of selectin ligands driven by antigen-pulsed peripheral lymph nodes (PLN)-DC could be suppressed “in trans” by adding live intestinal DC. Moreover, we show that effector–memory CD8 T cells with an already committed skin- or gut-homing phenotype can be reeducated to adopt new migratory preferences in accordance with the anatomic source of the most recently encountered DC.
Results
Reciprocal control of CD8 T cell homing by DC
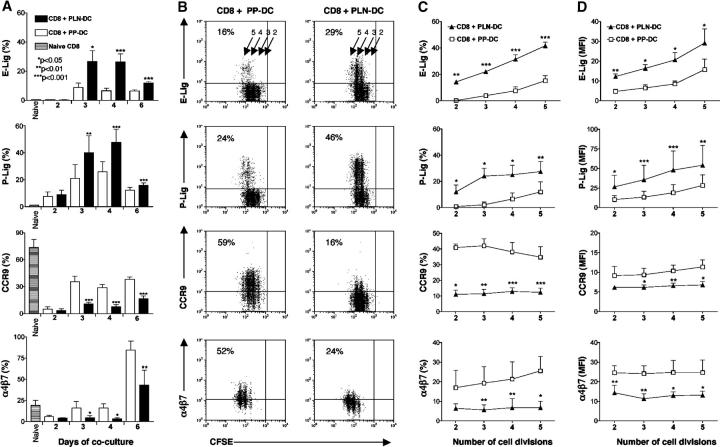
To study the effect of DC from skin- and gut-associated lymphoid tissues, naïve CD8 T cells from P14xT-GFP TCR-transgenic mice were cocultured for different time intervals with Ag-pulsed DCs from skin-draining LN (PLN-DC) or PP (PP-DC). A kinetic analysis showed that at day 2 of coculture, the expression level of skin- and gut-homing molecules was in general low, and there were no significant differences between CD8 T cells activated with PLN-DC and those activated with PP-DC (Fig. 1 A). However, as early as day 3, CD8 T cells activated with PLN-DC expressed significantly higher levels of E- and P-selectin ligands, as compared with those activated with PP-DC. The levels of E- and P-selectin ligands decreased after day 4, although the differences between CD8 T cells activated with PLN-DC and those activated with PP-DC remained statistically significant until day 6 of coculture.
Figure 1.
Reciprocal control of T cell homing potential by DC from skin-draining LN and PP. Naïve P14 CD8 T cells were cultured with peptide-pulsed PLN-DC or PP-DC and analyzed after different time intervals for the expression of skin- (E- and P-selectin ligands) and gut-homing molecules (α4β7 and CCR9). (A) Kinetics of the induction of skin- and gut-homing molecules on CD8 T cells activated with PLN-DC or PP-DC. (B) Representative experiment showing the expression of skin- or gut-homing molecules on CFSE-labeled CD8 T cells activated with PLN-DC or PP-DC after 3 d of coculture. Vertical quadrant markers were set so that undivided CFSE-labeled input cells fall in the right quadrants. Arrows and numbers indicate cell divisions. Percent of gated cells in upper left quadrants is also shown. Relationship between the frequency (C) and MFI (D) of T cells expressing skin- and gut-homing molecules and the number of cells divisions of CD8 T cells activated with PLN-DC or PP-DC after 3 d of coculture. Gates were set on viable CD8+ T cells. Statistical analyses were performed using a paired Student's t test comparing CD8 T cells activated with PLN-DC vs. those activated with PP-DC (mean ± SEM, n = 5–16).
CD8 T cells activated with PP-DC expressed significantly higher levels of α4β7 and CCR9 than those activated with PLN-DC starting from day 3, but CD8 T cells activated with PP-DC expressing high levels of α4β7 were observed only after 4–5 d of coculture (17). Although naïve CD8 T cells expressed α4β7 detectably (Fig. 1 A), their mean fluorescence intensity (MFI) for this marker was uniformly low (unpublished data). Since 3 d of coculture was the minimal period required to observe significant differences in both skin- and gut-homing molecules between CD8 T cells activated with PLN-DC and those activated with PP-DC, we chose this time point to perform CFSE (carboxyfluorescein diacetate succinimidyl ester) dilution assays in order to correlate the expression of homing molecules to the number of cell divisions. As shown in Fig. 1 (B–D), differential expression of selectin ligands as well as α4β7 and CCR9 was observed on CD8 T cells that had experienced a similar number of cell divisions. Comparable results for all traffic molecules examined were obtained when we used CD8 T cells from P14xTCRα−/− mice, indicating that the observed differences were not due to expansion of an initially rare nonnaïve T cell population (unpublished data).
mRNA expression and chemotactic responsiveness of CD8 T cells activated with PLN-DC or PP-DC
Selectin ligands are formed by post-translational modification of glycoproteins like P-selectin glycoprotein ligand-1 (PSGL-1) (20). PSGL-1 is constitutively present on T cells, and we have shown previously that it remains equivalently expressed on CD8 T cells activated with PLN-DC or PP-DC (17). To acquire the capacity to bind selectins, PSGL-1 must undergo post-translational modifications, including α(1,3)-fucosylation (20). Since fucosyltransferase-VII (FucT-VII) is a key enzyme in this process (21), we compared FucT-VII mRNA expression on CD8 T cells activated with PLN-DC and those activated with PP-DC using real-time PCR (Fig. 2 A). CD8 T cells activated with PLN-DC expressed significantly higher levels of FucT-VII mRNA as early as 2 d after coculture and maintained these elevated levels throughout the observation period. There was no significant difference in mRNA levels for FucT-IV (a second enzyme that can generate fucosylated selectin ligands) or core-2 β1,6-_N_-acetylglucosaminyltransferase (C2GlcNAcT-I), an enzyme that generates O-linked glycans that are required substrates for α(1,3)-fucosylation of selectin ligands (20) (see Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20041645/DC1).
Figure 2.
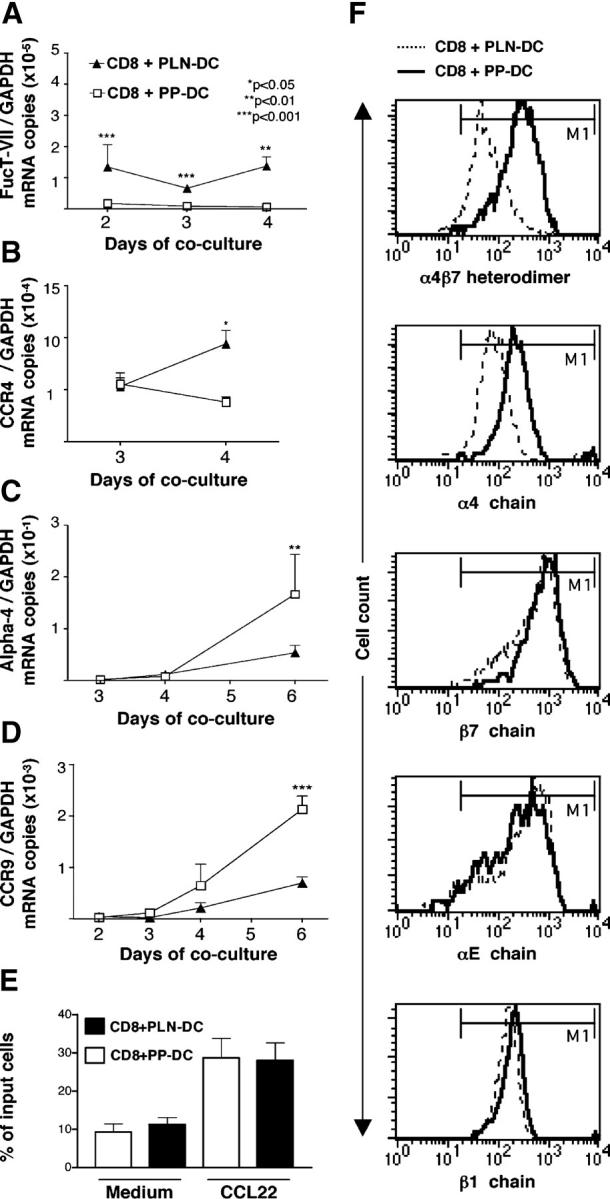
mRNA expression, chemotactic responsiveness, and integrin levels of CD8 T cells activated with PLN-DC or PP-DC. After different time intervals, CD8 T cells activated with PLN-DC or PP-DC were analyzed by real-time PCR for their relative mRNA expression of (A) fucosyltransferase-VII (FucT-VII), (B) the skin-associated chemokine receptor CCR4, as well as the gut homing molecules α4 integrin chain (C) and CCR9 (D). All mRNA levels were normalized with respect to GAPDH. Statistical analyses were performed using a paired Student's t test comparing CD8 T cells activated with PLN-DC vs. those activated with PP-DC (mean ± SEM, n = 3). (E) Chemotactic response toward the CCR4-ligand CCL22. Results are expressed as the percentage of total cells migrating toward a chemokine or toward medium alone. For chemotaxis experiments, cells were used after 4–6 d of coculture (mean ± SEM, n = 11). (F) Flow cytometry histograms showing expression of α4β7 heterodimer, α4, β7, αE, and β1 integrin chains on CD8 T cells activated with PLN-DC or PP-DC after 6 d of culture. Results show one representative experiment out of five performed with similar results. The marker (M1; indicating specific labeling) was set according to appropriate isotype controls.
The murine chemokine receptors CCR4 and CCR10 have been implicated in T cell homing to the skin (14). CD8 T cells activated with PLN-DC expressed significantly higher levels of CCR4 mRNA at day 4 as compared with those activated with PP-DC (Fig. 2 B), whereas CCR10 mRNA was virtually undetectable in all samples analyzed. Accordingly, neither CD8 T cells activated with PLN-DC nor those activated with PP-DC migrated above the background to the CCR10 agonists CCL27 or CCL28 (Fig. S1; unpublished data). Similarly, although CCR8 was recently shown to be expressed by the majority of T cells in normal human skin (22), neither CD8 T cells activated with PLN-DC nor those activated with PP-DC migrated toward the CCR8 ligand CCL1. However, both sets of cells migrated equally efficiently toward gradients of the CCR4 ligands CCL22 or CCL17 (Fig. 2 E; unpublished data).
The integrin α4β7 is composed of two chains. We have shown previously that α4, αE, β1, and β7 integrin chains are up-regulated on CD8 T cells activated with either PP-DC or PLN-DC, but the α4 chain is significantly higher on CD8 T cells activated with PP-DC (Fig. 2 F; Fig. S1; [17]). Concurrent with these observations, the mRNA levels for α4 were significantly higher in CD8 T cells activated with PP-DC than in those activated with PLN-DC (Fig. 2 C), but there was no difference in αE, β1, or β7 mRNA expression (Fig. S1). In agreement with Fig. 1, CCR9 mRNA levels were also significantly higher on CD8 T cells activated with PP-DC, starting at day 4 of coculture (Fig. 2 D).
CD8 T cells activated with PLN-DC adhere to cutaneous venules and migrate better to inflamed skin than those activated with PP-DC
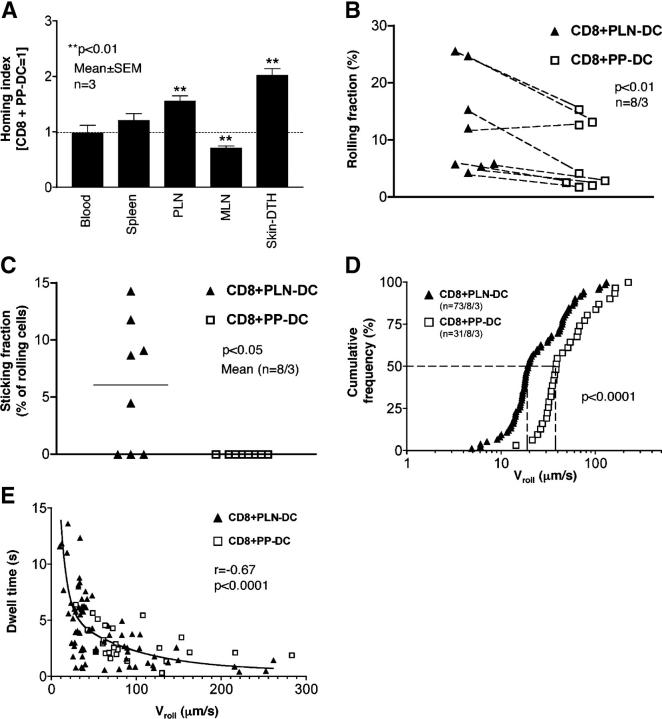
Since CD8 T cells activated with PLN-DC expressed both CCR4 and higher levels of E- and P-selectin ligands than those activated with PP-DC, we postulated that they should be better equipped to migrate to the skin than CD8 T cells activated with PP-DC. However, pilot experiments failed to detect homing of either CD8 T cells activated with PLN-DC or PP-DC to normal skin (unpublished data). This was not unexpected because T cell migration to noninflamed murine skin is known to be very low, even upon adoptive transfer of endogenous cutaneous LN-derived T cells that express P- and E-selectin ligands (13). Therefore, homing of effector T cells into cutaneous sites has mostly been examined in inflamed skin (13, 14, 19). Thus, we compared the migration of CD8 T cells activated with PLN-DC or PP-DC into the ear skin after induction of a delayed-type hypersensitivity response (13). After adoptive transfer of a 1:1 mixture of differentially labeled CD8 T cells activated with PLN-DC or PP-DC, both subsets were equivalently represented in the blood and spleen (homing index ≈ 1.0; Fig. 3 A). Consistent with earlier experiments (17), CD8 T cells activated with PP-DC localized significantly better to mesenteric LN, whereas CD8 T cells activated with PLN-DC were more frequently found in PLN. Most importantly, CD8 T cells activated with PLN-DC homed significantly more efficiently into inflamed skin than CD8 T cells activated with PP-DC, thus confirming that the surface phenotype of CD8 T cells activated with PLN-DC or PP-DC is meaningful to predict in vivo migratory behavior.
Figure 3.
CD8 T cells activated with PLN-DC migrate more efficiently into inflamed skin and interact significantly better with cutaneous venules than those activated with PP-DC. CD8 T cells activated with PLN-DC or PP-DC were used at day 4–5 for homing experiments and IVM in mouse ear venules. (A) Competitive homing experiments between CD8 T cells activated with PLN-DC or PP-DC. Both populations were differentially labeled, mixed at a 1:1 ratio, and injected into naïve recipient mice. 18 h later, tissues were collected and the HI was calculated for each organ. IVM of T cell interactions in ear skin venules was used to determine (B) the rolling fraction and (C) the sticking fraction (n = venules/mice). (D) A cumulative rolling velocity histogram was generated by measuring rolling velocities (Vroll) of individual CD8 T cells activated with PLN-DC (median Vroll = 19 μm/s) and CD8 T cells activated with PP-DC (median Vroll = 38 μm/s) in ear venules by plotting the percentage of all cells that rolled at or below a given Vroll (n = cells/venules/mice). (E) Correlation between Vroll and the dwell time of individual rolling cells in each venule. The dwell time was measured as the time a T cell spent rolling within the venular segment that was used to measure Vroll. To compare homing experiments, results were analyzed using a one-sample t test (comparing experimental HI vs. HI = 1). To compare rolling and sticking fractions, a paired Student's t test was used comparing CD8 T cells activated with PLN-DC vs. those activated with PP-DC. The Mann-Whitney U-test was employed to compare Vroll of CD8 T cells activated with PLN-DC vs. those activated with PP-DC, and the Spearman rank correlation test was used for analysis of Vroll vs. dwell time.
To determine if the preferential accumulation of CD8 T cells activated with PLN-DC in skin correlated with their ability to adhere within cutaneous microvessels, we performed intravital microscopy (IVM) to analyze interactions between fluorescently tagged CD8 T cells activated with PLN-DC or PP-DC and the venular endothelium in mouse ears. Since ear skin venules express E- and P-selectin constitutively and support abundant leukocyte rolling under steady-state conditions (23), it was not necessary to induce inflammation for these experiments. Both CD8 T cells activated with PLN-DC or PP-DC interacted with cutaneous venules, but those activated with PLN-DC rolled at a significantly higher frequency than CD8 T cells activated with PP-DC (Fig. 3 B). Interestingly, although we did not detect homing of CD8 T cells activated with PLN-DC into normal skin, we consistently observed a low number of rolling CD8 T cells activated with PLN-DC that adhered firmly to noninflamed venules, whereas those activated with PP-DC did not arrest under these conditions (Fig. 3 C).
It has been shown that P- and E-selectin fulfill different functional roles in leukocyte rolling, with P-selectin being mainly responsible for the initial tethering, while E-selectin reduces the rolling velocity (Vroll) (23, 24). Consistent with the higher expression of P- and E-selectin ligands, we found that the median Vroll of CD8 T cells activated with PLN-DC was about half that of those activated with PP-DC (Fig. 3 D). Moreover, the dwell time during which CD8 T cells activated with PLN-DC continued to roll in cutaneous microvessels was significantly longer than for those activated with PP-DC and it was inversely correlated to the Vroll (Fig. 3 E). This difference in dwell time might selectively allow CD8 T cells activated with PLN-DC to activate integrins necessary for firm arrest in response to presumably low abundance chemoattractants.
Role of DC subsets in the expression of homing molecules
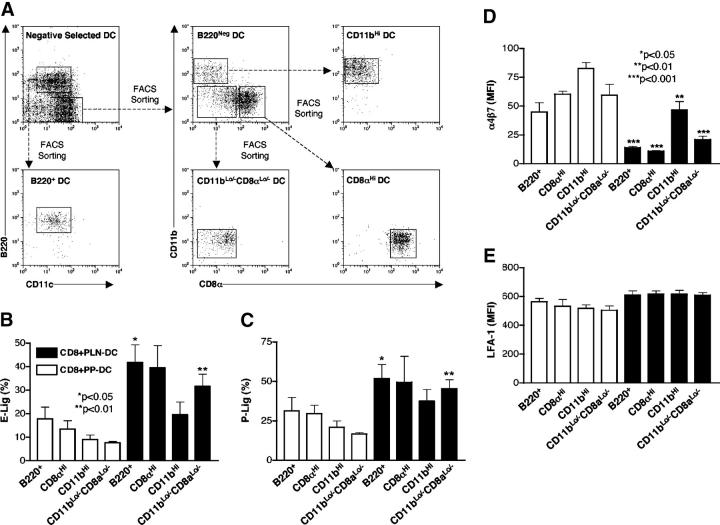
Several DC subpopulations have been described in lymphoid tissues (25), and the composition and magnitude of each subset varies between different lymphoid organs (26). For example, we have reported previously that PLN-DC contain a higher proportion of CD8α+ DC than PP-DC (17). Moreover, in agreement with a previous report (27), PP-DC have a higher proportion of cells that are negative/low for the classic DC subset markers B220, CD11b, CD8α (Fig. 4 A; unpublished data). Since different DC subsets may induce distinct effector activities in T cells (25), it was important to explore whether the differences between PP-DC and PLN-DC in the induction of skin- and gut-homing molecules on CD8 T cells were related to their subset composition. However, when naïve T cells were separately cocultured with each FACS-sorted constituent subset, all PLN-DC subsets induced more selectin ligands than their respective PP-derived counterparts, although CD11b+ (myeloid) PLN-DC tended to induce somewhat fewer selectin ligands than other PLN-DC (Fig. 4, B and C).
Figure 4.
Effect of sorted DC subsets on the acquisition of selectin ligands and α4β7 on CD8 T cells. (A) DC from skin-draining LN or PP were isolated by negative selection and then subdivided by FACS sorting into CD11cLoB220+ (‘plasmacytoid’ DC) and CD11cHiB220Neg DC. The latter were further sorted into CD8αNegCD11bHi (“myeloid” DC), CD8αHiCD11bLo (“lymphoid” DC), and CD8αLo/-CD11bLo subsets. Each DC subpopulation was pulsed with peptide Ag and cocultured with naïve CD8 T cells for 5–6 d. Expression of (B) E- and (C) P-selectin ligands, (D) α4β7, and (E) LFA-1 on CD8 T cells activated with DC subsets from PLN or PP. Gates were set on viable CD8+ T cells. Statistical analyses were performed using a paired Student's t test comparing CD8 T cells activated with each PLN-DC subset vs. those activated with its PP-DC counterpart (mean ± SEM; n = 2–4).
Conversely, all PP-DC fractions induced higher levels of α4β7 on CD8 T cells than the corresponding PLN-DC subset (Fig. 4 D). Interestingly, CD11b+ DC from both PP and PLN induced higher levels of α4β7 on CD8 T cells than other DC (Fig. 4 D; P < 0.05 for CD11b+ PLN-DC versus all other PLN-DC subsets). We also analyzed the expression of LFA-1/CD11a, which is required for T cell adhesion in a multitude of tissues, and found that it was equivalently up-regulated on CD8 T cells, regardless of the activating DC subset (Fig. 4 E). In two additional experiments, we included sorted subsets of splenic DC in our analysis. Spleen-DC, like PLN-DC, induced higher levels of selectin ligands and lower expression of α4β7 on CD8 T cells as compared with PP-DC (see Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20041645/DC1). Of note, splenic CD11bhigh DC, unlike those from PLN, were not different from other splenic subsets in their imprinting capacity.
Effect of Th1/Th2 cytokines on skin- and gut-homing receptor expression
Having established that all DC subsets from intestinal and cutaneous lymphoid tissues possess the reciprocal capacity to imprint in T cells a gut- or skin-homing phenotype, respectively, we set out to examine possible communication pathways that might contribute to these effects. T cells that are polarized under Th1, but not Th2, conditions acquire the capacity to bind E- and P-selectin and migrate efficiently into inflamed skin (28). In addition, IL-12 (Th1 cytokine produced by DC) up-regulates, whereas IL-4 (Th2 cytokine) down-regulates the expression of FucT-VII in lymphocytes (29). Therefore, we asked whether the differential induction of E- and P-selectin ligands on CD8 T cells activated with PLN-DC versus PP-DC was dependent on these cytokines.
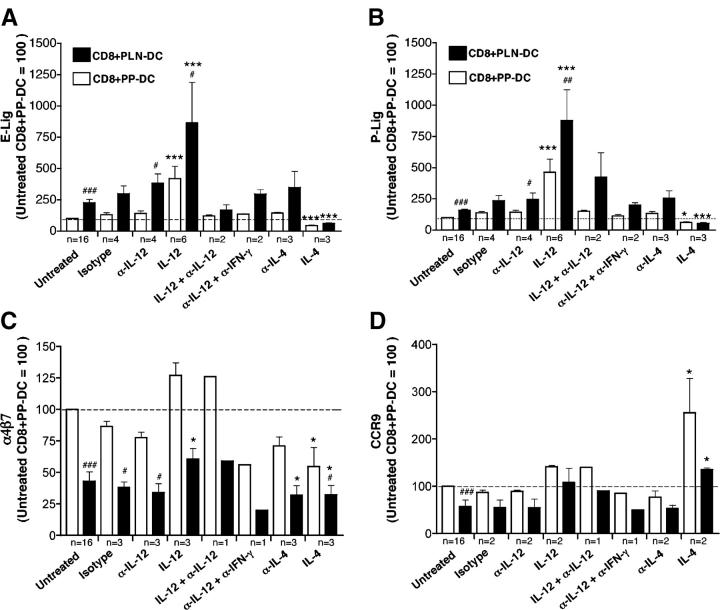
To this end, CD8 T cells were cocultured with either PLN-DC or PP-DC in the presence or absence of blocking antibodies against IL-12 or IL-4. As shown in Fig. 5 (A and B), addition of blocking anti-IL-12 (or anti-IL-12 plus anti-IFN-γ) did not affect the generation of E- and P-selectin ligands on CD8 T cells activated with PLN-DC. However, the presence of exogenous IL-12 in culture media significantly increased the expression of selectin ligands on CD8 T cells activated with PLN-DC or PP-DC, and this additional effect was blocked by the concomitant addition of anti–IL-12. Although the inclusion of IL-4 in culture media decreased the expression of E-selectin ligands in all cocultures, a blocking antibody against this cytokine did not increase the expression of these ligands on CD8 T cells activated with PLN-DC or PP-DC.
Figure 5.
Effect of Th1/Th2 cytokines on the expression of selectin ligands, α4β7, and CCR9 on CD8 T cells activated with PLN-DC and PP-DC. (A) Naïve CD8 T cells were cultured with peptide-pulsed PLN-DC or PP-DC, either in the presence of blocking antibodies against IL-12, IFN-γ, or IL-4, and/or the cytokines themselves. CD8 T cells were analyzed after 4–5 d of coculture for expression of (A) E- and (B) P-selectin ligands, (C) α4β7, and (D) CCR9. Cells were gated on viable (FSC/SSC) CD8+ T cells. Results were normalized in each experiment to values from a parallel culture of untreated CD8 T cells activated with PP-DC (assigned a value of 100). To compare CD8 T cells activated with PP-DC or PLN-DC in the presence of cytokines and/or mAbs vs. the respective untreated CD8 T cells activated with the same DC, an ANOVA with Dunnett's post-test was used (*, P < 0.05; ***, P < 0.001). To compare CD8 T cells activated with PLN-DC vs. CD8 T cells activated with PP-DC in each group, a two-tailed paired Student's t test was used (#, P < 0.05; ###, P < 0.001). Only groups with n ≥ 3 independent experiments were used for statistical comparison. Bars reflect mean ± SEM.
Although α4β7 expression on CD4 T cells has been associated with a Th1 phenotype (30), the differential induction of α4β7 by PLN-DC and PP-DC was not significantly affected by inhibition of IL-12 or IL-4 (Fig. 5 C). Nonetheless, addition of exogenous IL-12 tended to increase and IL-4 decreased α4β7 on both sets of activated T cells. On the other hand, CCR9 was not appreciably affected by anti–IL-12 or anti–IL-4, whereas exogenous IL-12 slightly increased its expression (Fig. 5 D). Interestingly, addition of IL-4 increased significantly the percentage and MFI of CCR9+ in all cocultures, although this effect of IL-4 was not observed on anti-CD3 plus anti-CD28 activated T cells (Fig. 5 D; unpublished data). Taken together, these results confirm and expand previous observations that Th1 and Th2 cytokines can influence the expression of T cell traffic molecules (28), but neither IL-4 nor IL-12 appear to be responsible for the DC-induced imprinting of skin- or gut-tropism.
Effect of DC fixation on T cell imprinting
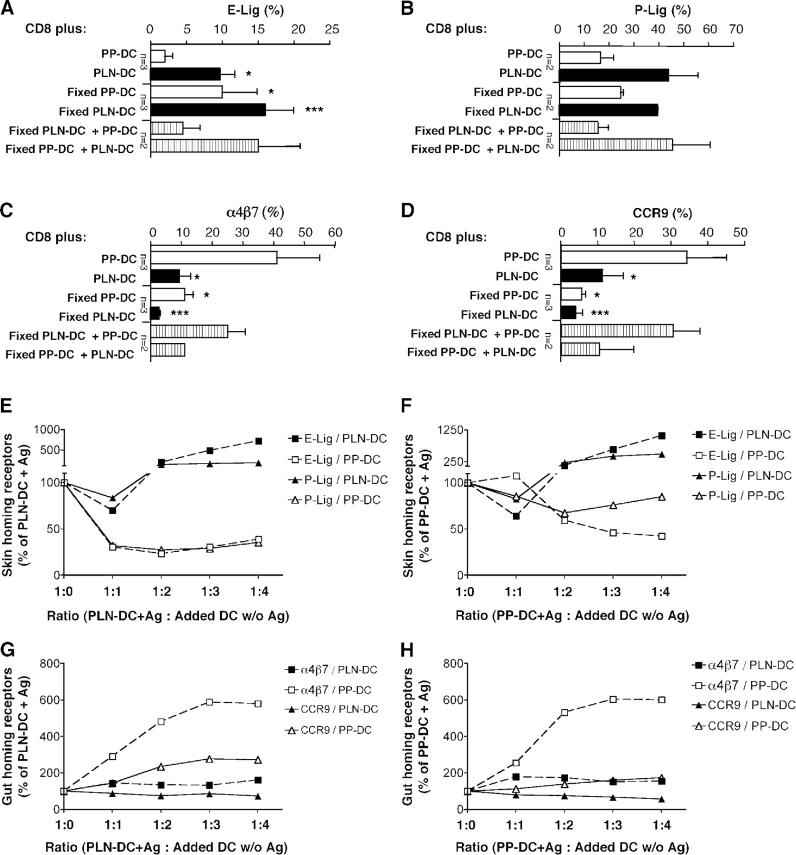
Next, we asked whether DC require an intact metabolism to imprint tissue-tropism in T cells. Ag-pulsed PP-DC and PLN-DC were lightly fixed with glutaraldehyde before coculture with naïve T cells. Consistent with earlier reports (31), fixed DC retained their normal capacity to activate T cells, evidenced by the degree of proliferation and viability of the resulting effector T cells. However, fixed PP-DC induced more ligands for E- (Fig. 6 A) and P-selectin (Fig. 6 B) on CD8 T cells than unfixed PP-DC, and fixed PLN-DC induced selectin ligands comparably to their unfixed counterparts. On the other hand, the ability to induce α4β7 (Fig. 6 C) and CCR9 (Fig. 6 D) expression on T cells was severely impaired when DC were fixed before coculture. Interestingly, the presence of Ag-pulsed unfixed but not fixed PP-DC antagonized the induction of both selectin ligands by fixed PLN-DC. Indeed, addition of unfixed PP-DC to cocultures with fixed PLN-DC induced higher levels of α4β7 and CCR9 on CD8 T cells.
Figure 6.
Effect of DC fixation and mixed DC cultures on homing molecule expression by T cells. (A–D) Naïve CD8 T cells were cultured with peptide-pulsed PLN-DC or PP-DC, which were either left untreated or fixed with 0.05% glutaraldehyde. CD8 T cells were analyzed after 4 d of coculture for expression of (A) E- and (B) P-selectin ligands, (C) α4β7, and (D) CCR9. Cells were gated on viable (FSC/SSC) CD8+ T cells. Results in A–D are expressed as percentage of marker-positive CD8 T cells. Bars represent mean ± SEM (mean ± range when n < 3). To compare CD8 T cells activated with unfixed PP-DC alone to cells activated under any other condition, an ANOVA with Dunnett's post-test was employed. (E–H) Naïve CD8 T cells (105/well) were cocultured with an equivalent number of Ag-pulsed PLN-DC (E and G) or PP-DC (F and H), with or without addition of unpulsed PLN-DC or PP-DC. Cells were analyzed after 4 d for the expression of selectin ligands (E and F) or α4β7 heterodimer and CCR9 (G and H). Cells were gated on viable CD8+ T cells. Results were normalized in each experiment to values from parallel cultures of CD8 T cells with Ag-pulsed PLN-DC (E and G) or PP-DC (F and H), which were arbitrarily assigned a value of 100. Symbols represent the average of two experiments with comparable results.
In summary, these results reveal fundamental differences in the cellular mechanisms of DC-induced imprinting of skin- versus gut-tropism; DC do not appear to require an active metabolism (and/or surface molecules whose function is destroyed by fixation) to induce selectin ligands on T cells. By contrast, the ability of PP-DC to induce a gut-homing phenotype and to suppress the expression of skin-homing receptors is exquisitely sensitive to fixation.
The presence of PP-DC overrides PLN-DC induction of selectin ligands on T cells
Intestinal DC can imprint gut homing even when T cells are stimulated with antibodies and the DC are not presenting an activating Ag (17, 18). Having observed that Ag-pulsed PP-DC can inhibit the induction of selectin ligands by fixed-PLN-DC (Fig. 6, A and B), we asked if the mere presence of PP-DC without a specific Ag could modulate “in trans” the acquisition of skin traffic molecules on CD8 T cell activated with Ag-pulsed (and unfixed) PLN-DC, and vice versa. As shown in Fig. 6 E, PP-DC markedly suppressed the induction of E- and P-selectin ligands by peptide-pulsed PLN-DC. Interestingly, high concentrations of unpulsed PLN-DC had a potentiating effect on the induction of selectin ligands during Ag stimulation by a standard number of PLN-DC or PP-DC (Fig. 6, E and F). On the other hand, excess numbers of unpulsed PLN-DC did not affect the induction of α4β7 by Ag-pulsed PP-DC, but tended to decrease somewhat the induction of CCR9 (Fig. 6, G and H). Furthermore, unpulsed PP-DC increased the induction of α4β7 by peptide-pulsed PLN-DC or PP-DC in a dose-dependent manner, and they increased the expression of CCR9 on CD8 T cells activated with Ag-pulsed PLN-DC. Importantly, when live PP-DC and PLN-DC were present in cocultures at equivalent concentrations, the resulting homing receptor repertoire on effector T cells was always skewed toward a gut-homing phenotype, irrespective of which DC population presented the cognate Ag.
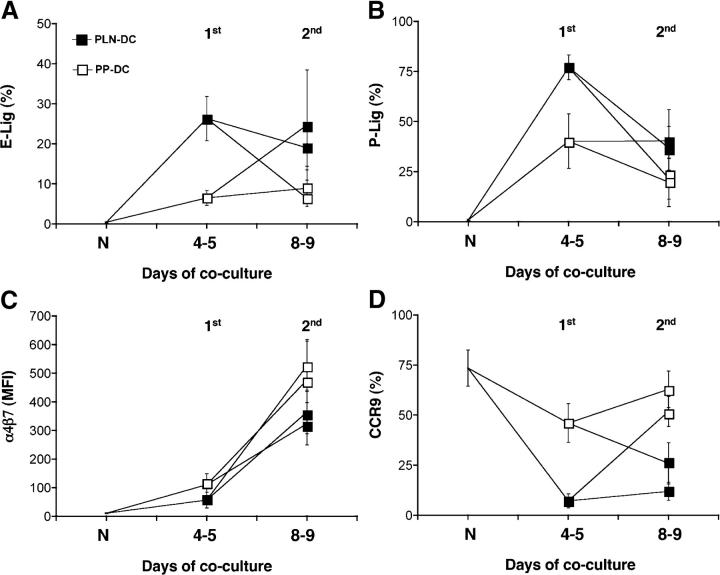
Tissue-tropic CD8 T cells preserve their susceptibility to further imprinting signals
An important question is whether the tissue-specific homing potential of effector–memory T cells is a stable property or if it can be modulated when T cells are reactivated in a different tissue context. We addressed this question by reactivating either CD8 T cells activated with PLN-DC or PP-DC (4–5 d after their primary activation) with the same or the opposite DC, and then analyzing the reactivated cells 4–5 d later. When CD8 T cells activated with PLN-DC were reactivated with PLN-DC, they maintained high levels of selectin ligands, whereas restimulation with PP-DC induced a dramatic loss of selectin ligands to levels equivalent to those found on CD8 T cells activated exclusively with PP-DC (Fig. 7, A and B). Conversely, CD8 T cells activated with PP-DC and reactivated with PLN-DC rapidly gained high levels of selectin ligands similar to those found on CD8 T cells activated with PLN-DC. Restimulation had essentially the same effect on T cell expression of α4β7 (Fig. 7 C) and CCR9 (Fig. 7 D). In each case, the last DC population encountered by CD8 T cells determined the homing phenotype, irrespective of the first imprinting event. As reported previously (17), prolonged stimulation with PLN-DC or PP-DC increased the levels of α4β7 on both CD8 T cells activated with PLN-DC or PP-DC above those on naïve T cells (Fig. 7 C). Nevertheless, the increase was always significantly higher when cells were stimulated by PP-DC as compared with PLN-DC.
Figure 7.
Preservation of plasticity of ex vivo–generated tissue-tropic effector CD8 T cells. Naïve CD8 T cells were activated with either PLN-DC or PP-DC (1st activation) and the resulting effector T cells were restimulated 4–5 d later with either PLN-DC or PP-DC (2nd activation). 4–5 d after the second activation, effector cells were compared with naïve T cells (N) for expression of ligands for (A) E-selectin and (B) P-selectin or (C) α4β7 and (D) CCR9. Statistically significant (P < 0.05) differences were found for all markers analyzed between CD8 T cells activated with PLN-DC vs. those activated with PP-DC irrespective of the starting population. Statistical analyses were performed using ANOVA with Newman-Keuls post-test, comparing all groups of effector CD8 T cells at day 8–9 (mean ± SEM, n = 3-4).
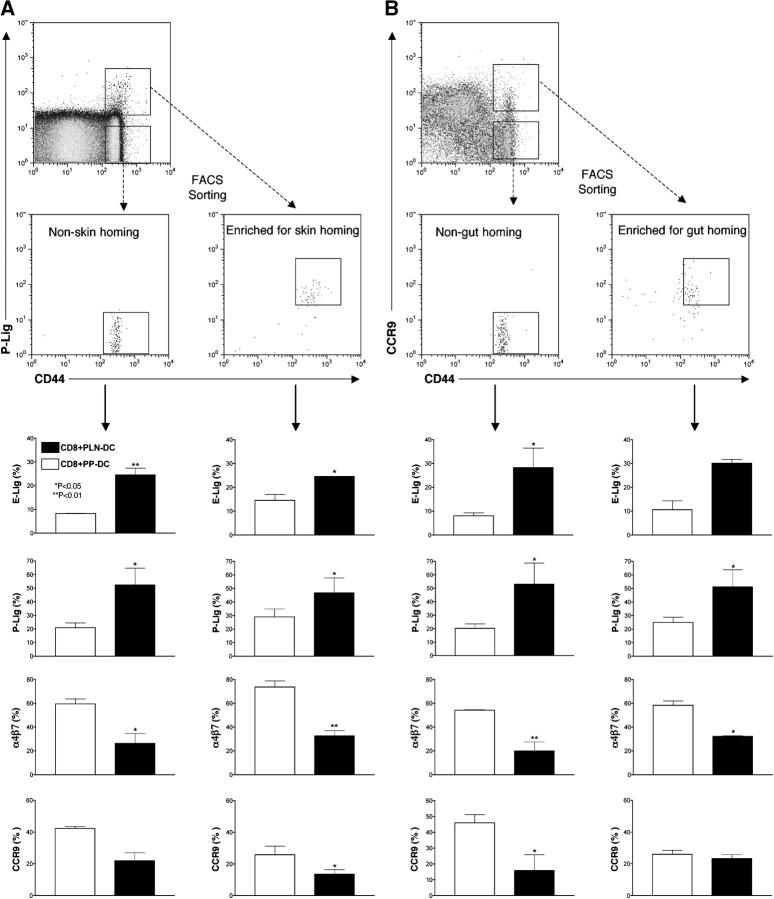
Having discovered that recently activated ex vivo–generated CD8 T cells can change their homing phenotype when they are reactivated in a new context, we investigated whether endogenously generated effector–memory T cells retain a similar plasticity. To address this question, we isolated CD44High effector–memory CD8+ T cells from pooled lymphoid organs of adult donors and sorted them based on their ability to bind P-selectin (Fig. 8 A) or to express CCR9 (Fig. 8 B), which served as surrogate markers for skin- and gut-homing potential, respectively. These markers were chosen because control experiments showed that 50% of CD8+CD44HighCCR9+ cells expressed α4β7 but few cells expressed selectin ligands. On the other hand, a minority of α4β7+ memory cells coexpressed CCR9 (see Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20041645/DC1), which is necessary for homing to the small intestine (6, 8, 9). Therefore, sorting of CD8+CD44HighCCR9+ T cells generated the most enriched memory population with the potential to home to the small bowel. Conversely, CD8+ CD44HighP-lig+ cells were mostly CCR9Negα4β7Neg and thus likely to contain a sizeable fraction of skin-tropic memory cells (11). Consistent with our in vitro–generated effector CD8 T cells, FACS-sorted endogenous effector–memory CD8 T cells that were activated with anti-CD3 plus anti-CD28 expressed more selectin ligands and less α4β7 when cocultured with PLN-DC, whereas PP-DC enhanced the expression of α4β7 and suppressed selectin ligands irrespective of the phenotype of the starting effector–memory T cells. The same trend was also observed with CCR9, although the differential effects of DC restimulation on this molecule did not always reach statistical significance.
Figure 8.
Plasticity of endogenous tissue-tropic effector–memory CD8 T cells. CD8 T cells were isolated by negative selection from pooled cells from spleen, PLN, MLN, and PP from 4–6-mo-old C57BL/6 wild-type mice, and the CD8+CD44High fraction was sorted by FACS into (A) P-Lig+ and P-LigNeg subsets or (B) CCR9+ and CCR9Neg subsets. The sorted cells were plated on immobilized anti-CD3+anti-CD28 and supplemented with either PLN-DC or PP-DC. The resulting effector CD8 T cells were analyzed 4 d later for their capacity to bind E- and P-selectin and their expression of α4β7 and CCR9. Statistical analyses were performed using a paired Student's t test comparing effector–memory CD8 T cells activated with PLN-DC vs. those activated with PP-DC (mean ± SEM, n = 3).
Discussion
DC from gut-associated lymphoid tissues induce on activated CD8 T cells the expression of the gut-homing molecules α4β7 (9, 17–19) and CCR9 (9, 17), as well as the capacity to migrate to the small bowel (17). We now provide evidence that naïve CD8 T cells activated by DC from skin-draining LN assume a skin-homing phenotype, i.e., they express significantly more selectin ligands and mRNA for CCR4, undergo more frequent and prolonged adhesive interactions with skin venules, and home more efficiently to inflamed skin as compared with CD8 T cells activated with intestinal DC.
IVM observations strongly suggest that the preferential migration of CD8 T cells activated with PLN-DC to inflamed skin in competitive homing experiments was due to their superior ability to adhere to dermal microvessels compared with CD8 T cells activated with PP-DC. Not only did CD8 T cells activated with PLN-DC roll more frequently and at a lower velocity, but also, unlike those activated with PP-DC, CD8 T cells activated with PLN-DC underwent spontaneous arrest in noninflamed skin-venules. In the mouse ear, postcapillary venules constitutively express both P- and E-selectin, which mediate exclusively the tethering and rolling step (23). Thus, T cells must express selectin ligands at a sufficient density to marginate and to scan the endothelial surface for chemoattractants (10, 13). α(1,3)-fucosylation of sialomucins by FucT-VII is essential for selectin ligand biosynthesis in leukocytes (20, 21, 23). Consistent with this concept, CD8 T cells activated with PLN-DC contained more FucT-VII mRNA than those activated with PP-DC as early as 2 d after stimulation, i.e., immediately preceding the up-regulation and ultimately high surface expression of selectin ligands. This time course is in good agreement with in vivo studies of selectin ligand induction on CD4 T cells (16) and faster than the kinetics of selective α4β7 up-regulation on CD8 T cells activated with PP-DC, which was already apparent at day 3, but α4β7High T cells arose only after day 4–5 of coculture (17).
Once a skin-homing T cell has engaged in rolling, the integrin-activating stimulus must be provided by chemokines that act either on CCR4 or CCR10 (11, 12). Agonists for both receptors are probably expressed at a low level in normal skin and either one is sufficient for T cell recruitment (14). CCL1, the agonist for CCR8 was recently detected in human skin (22), but the role of this pathway in T cell traffic to murine skin has not yet been determined. We could not detect evidence for CCR8 or CCR10 expression in CD8 T cells activated with PLN-DC or PP-DC, but those activated with PLN-DC contained higher mRNA levels for CCR4, although both populations responded similarly to CCR4 ligands in chemotaxis assays. These seemingly conflicting data could either indicate that CCR4 protein expression and/or function are not regulated at the mRNA level or that the lower levels of CCR4 on CD8 T cells activated with PP-DC might still be sufficient to allow chemotaxis but not firm arrest in venules, as has been shown for CXCR4 on naïve CD8 T cells (32).
In this context, it is interesting to note that only CD8 T cells activated with PLN-DC, but not those activated with PP-DC, underwent spontaneous firm arrest in ear venules. While it remains to be determined if this adhesion event was mediated by CCR4, it is likely that the higher selectin ligand expression on CD8 T cells activated with PLN-DC made an indirect contribution. P-selectin primarily mediates the initial tethering and determines the frequency of rolling interactions, whereas E-selectin decreases the rolling velocity (23, 24). Consistent with the high expression of E- and P-selectin ligands, CD8 T cells activated with PLN-DC rolled not only at a higher frequency than those activated with PP-DC, but also at a lower speed, which resulted in longer intravenular dwell times. The increased duration of rolling contacts might have allowed CD8 T cells activated with PLN-DC to sense and respond to chemokine(s), presumably CCR4 agonists, that may have been presented at low densities in skin microvessels, thus increasing the likelihood for triggering integrin-dependent firm arrest (24, 33).
A tissue-specific multistep adhesion cascade is also responsible for T cell homing to the small intestine (2). The critical receptor–ligand pairs in this setting are α4β7–MAdCAM and CCR9–CCL25, although recent reports indicate that P-selectin may also play a role in gut homing, at least for Th1-polarized CD4+ cells (34). The constituent chains of the α4β7 heterodimer are also components of two other integrins, α4β1 and αEβ7. Thus, α4β7 surface expression levels could be regulated by a variety of mechanisms that affect the equilibrium between these three integrins. However, the only consistent difference observed by real-time PCR between CD8 T cells activated with PLN-DC and those activated with PP-DC was in mRNA levels for α4, but not for β7, β1, or αE. These observations are consistent with previous observations that activation by PP-DC or PLN-DC up-regulates several integrin chains on the surface of CD8 T cells, including α4, αE, αL, β1, and β7, but only the surface expression of α4 (and the α4β7 heterodimer), but not the β7 chain is selectively increased on CD8 T cells activated with PP-DC (17). Therefore, it seems plausible that the differential induction of α4β7 on CD8 T cells activated with PLN-DC versus those activated with PP-DC is at least in part controlled at the level of the α4 chain. However, this does not exclude the possibility that α4β7 expression is subject to additional posttranslational regulation that might affect the stoichiometry of integrin chain pairing.
Our current observations suggest that the regulation of CCR9 is similarly complex. CCR9 is highly expressed on naïve CD8+ T cells (35). Our present kinetic analysis indicates that at day 2 of coculture, activated T cells uniformly down-regulate CCR9 mRNA and surface protein levels irrespective of the activating DC, but CCR9 was selectively reexpressed in CD8 T cells activated with PP-DC starting on day 3. Thus, imprinting signal(s) provided by PP-DC promote reexpression of this receptor after initial activation-induced down-regulation.
It remains to be determined how PP-DC and PLN-DC imprint gut- and skin-tropism in T cells, respectively. Compared with PLN-DC, PP-DC express higher levels of several surface markers, including the integrin αEβ7 and the “nonclassical” costimulatory molecules B7-H1/PDL1 and B7-DC/PDL2, but blocking mAbs to these molecules did not affect their imprinting capacity (unpublished data). Likewise, a survey of several other candidate pathways (OX-40L, Qa2, MAdCAM, IL-10R, TGFβ, CCL25, and pertussis toxin treatment) was unrevealing. However, the induction of α4β7 on CD8 T cells activated with PP-DC was very sensitive to inhibitors of T cell activation, such as anti-CD18, anti-CD11a, anti-CD80, or loading of DC with a low peptide concentration (unpublished data). Therefore, efficient T cell activation seems to be necessary but not sufficient for the induction of gut-homing T cells.
Since PLN and PP differ in DC subset composition (17, 26, 27), and different DC subsets induce distinct T cell responses (25), it seemed reasonable to explore the role of individual DC subsets in tissue imprinting. Irrespective of the DC population examined and despite some subtle differences between subsets (particularly with respect to CD11bhigh PLN DC), spleen- and PLN-derived DC induced preferentially skin-homing receptors, while their PP-derived counterparts always induced more gut-homing molecules, indicating that the ability to acquire tissue-specific imprinting mechanism(s) is a common property of all DC in the same lymphoid tissue. Of note, a recent report found that Langerhans cells from skin explants induce higher E-selectin ligands on CD8 T cells than mesenteric LN DC (19). This suggests that the ability of DC to induce a skin-homing phenotype in T cells is not only acquired in PLN. Indeed, we observed that T cell activation by Ag-presenting DC from spleen or by anti-CD3 plus anti-CD28 also induced selectin ligands (but not α4β7 or CCR9) on CD8 T cells, although at somewhat lower levels than in response to PLN-DC (Fig. S2; unpublished data). These findings are in good agreement with reports showing that signaling through the TCR is sufficient for the induction of FucT-VII and E-selectin ligands on T cells independent of IL-12/STAT-4 signaling (36). This is consistent with our observation that anti–IL-12 did not interfere with the induction of selectin ligands by PLN-DC.
Together, these results raise the possibility that the up-regulation of selectin ligands on CD8 T cells reflects a hard-wired response to activation in general, and that this default differentiation pathway is actively suppressed by gut-derived DC. Several experimental observations are consistent with this notion. First, fixed PP-DC were incapable of inducing gut-homing receptors. Second, CD8 T cells that were activated by fixed PP-DC or unfixed PLN-DC equivalently up-regulated E- and P-selectin ligands, and fixed PLN-DC tended to super-induce these traffic molecules. Third, the presence of unfixed, but not fixed, PP-DC in cocultures with fixed PLN-DC drove T cells toward a gut-homing phenotype rather than allowing the acquisition of skin-homing receptors, indicating that the fixation procedure destroyed one or more pathways in PP-DC that are not only critical for intestinal imprinting per se, but also for their ability to suppress the induction of cutaneous homing receptors. Fourth, unfixed PP-DC did not need to present Ag to suppress the induction of selectin ligands by Ag-presenting fixed or unfixed PLN-DC; the mere presence of PP-DC was sufficient to confer “in trans” the preferential expression of gut-homing molecules. Fifth, both the suppression of skin-homing receptors and the induction of gut tropism by PP-DC were clearly apparent in cocultures with a 1:1 ratio of PLN-DC:PP-DC. Thus, T cells assume a gut-homing phenotype when afforded with equal opportunity to interact with PP-DC and PLN-DC, indicating that the former override putative imprinting signals from the latter. Finally, sixth, T cells in cocultures with high ratios of unpulsed PLN-DC to antigen-pulsed PP-DC expressed increased levels of selectin ligands, but did not lose the expression of gut-homing receptors. Thus, while PP-DC can efficiently suppress the induction of skin tropism by cutaneous DC, PLN-DC appear incapable of antagonizing intestinal imprinting signals generated by gut-derived DC.
While selectin ligands may be more readily induced in CD8 T cells than gut-homing receptors, it is likely that the magnitude of homing receptor expression is subject to regulation. For example, it has been reported that the cutaneous lymphocyte Ag (the principal E-selectin ligand on human skin-homing T cells) is reversibly up- or down-regulated on activated CD4 T cells upon exposure to different cytokines (37). Moreover, we have observed that a large fraction of TCRαβ+CD8αβ+ T cells in the small intestine express little or no α4β7, even though α4β7 is presumably required for their homing to the intestine (reference 17; unpublished data). These observations suggested that tissue-tropism remains malleable, even after a T cell has been imprinted in a specific tissue context.
Consistent with this idea, we observed remarkable plasticity in the response of tissue-committed CD8 T cells activated with PLN-DC or PP-DC to restimulation by PP-DC and PLN-DC, respectively. However, it could be argued that short-term differentiation, as performed in our ex vivo assay, is insufficient to establish an irreversible homing phenotype. A case in point is the differentiation of polarized Th1 cells, which can be reprogrammed to produce Th2 cytokines after short-term differentiation, but lose this plasticity once they become fully committed (38). To address this possibility, we also tested whether PP-DC and PLN-DC induce differential tissue-tropism upon restimulation of endogenously generated effector–memory CD8 T cells from adult donor mice that expressed either P-selectin ligands or CCR9, presumably in response to prior encounters with environmental Ag in skin- or gut-associated lymphoid tissues, respectively. The results from these experiments were consistent with those obtained with in vitro–differentiated effector cells and further support the intriguing possibility that tissue specificity is a dynamic property of effector–memory T cells, which can be modified if T cells are reactivated in a different anatomic context.
Under what circumstances might tissue-committed effector–memory cells be exposed to DC from another organ system? One mechanism may be the progressive reexpression of homing receptors for LN on long-lived effector memory cells (39). Thus, when effector memory cells in the skin or gut reexpress CCR7, they should be able to enter draining lymphatics and return to the blood stream (40). Indeed, it has been shown that the majority of α4β7High and CLA+ memory T cells in human blood coexpress the essential LN homing receptors CCR7 and L-selectin (41). These cells may then be able to home to both MLN and PLN where they could encounter a recall Ag that arises in the gut or skin, respectively. Thus, the acquisition and maintenance of migratory specificity in memory T cells might be a much more dynamic process than has been assumed. This question also has practical implications, because if memory T cells can be readily reeducated with regard to their tissue specificity, then it should be feasible to modulate effector–memory cell trafficking for therapeutic purposes.
MATERIALS AND Methods
Mice and reagents.
C57BL/6 and C57BL/6 Thy-1.1 mice were obtained from Jackson ImmunoResearch Laboratories. P14xT-GFP double transgenic mice have been described (42). P14xTCRα−/− mice were obtained from Taconic Farms (43). Mice were housed in an SPF/VAF facility and used in accordance with CBR1 and Harvard Medical School animal committees' guidelines. mAbs to the murine α4β7 heterodimer (DATK32), α4 (R1-2), β7 (M293), αE (M290), and β1 (HMβ1-1) integrin chains, as well as P-selectin-Fc and all mAbs for T cell and DC purification were from BD Biosciences. E-selectin-Fc chimera and cytokines were from R&D Systems. Anti-mouse CCR9 (clone 5F2) was generated at Millennium Pharmaceuticals, Inc. LCMVgp33-41 peptide was from Biosource International.
Cell isolation and cocultures.
C57BL/6 mice were injected s.c. with B16 cells secreting Flt3 ligand as previously described (17). After 12–14 d, mice were killed and PLN and PP were digested using 250 μg/ml Liberase CI plus 50 μg/ml DNase-I (Roche) in IMDM for 40 min at 37°C with mild agitation. Cell separation was performed at 4°C in PBS containing 2 mM EDTA and 2% FBS (GIBCO BRL). For negative selection, cells were incubated with mAbs to CD3, CD19, Pan-NK, Ter-119, and Thy-1, incubated with anti-rat IgG microbeads and purified on BS columns (Miltenyi Biotec). DC (85–90% CD11c+) were resuspended to 107/ml, pulsed for 2 h at 37°C with 10 μg/ml LCMVgp33-41 in IMDM plus 10% FBS, 50 μg/ml gentamycin, 0.5 μg/ml fungizone, 50 μM 2-ME, and standard supplements, washed twice, and used immediately in cocultures. In some experiments, peptide-pulsed DC were fixed with 0.05% glutaraldehyde (Sigma-Aldrich). For some experiments, negatively selected DC were sorted into different DC subpopulations (FACS Vantage; BD Biosciences).
Naïve CD8+ T cells were purified from splenocytes by negative selection as previously described (17), and in some experiments effector–memory T cells were isolated by negative selection and FACS sorting. For cocultures, 106 naïve T cells were added at a 1:1 ratio to peptide-pulsed DC in complete IMDM using 12-well plates, or for some experiments, in flat bottom 96-well plates (105 naïve CD8 T cells and DC). In some experiments, cocultures were supplemented from day 0 with 10 ng/ml rmIL-12, 25 ng/ml rmIL-4, and/or 20 μg/ml of blocking mAbs (from BD Biosciences) to IL-12p40/p70 (C17.8), IFN-γ (XMG1.2), or IL-4 (11B11).
For fluorescent labeling, T cells were stained with 5 μM CFSE (Molecular Probes) in DMEM + 1% FBS + 20 mM Hepes. For homing experiments (see below), T cells were also labeled with TRITC (Molecular Probes). Cells were resuspended at 2 × 107/ml, incubated with 2.5 μg/ml TRITC for 20 min at 37°C, and washed with an FBS gradient.
Reverse transcription and real-time PCR.
Real-time PCR was performed as previously described (17) on CD8 T cells activated with PLN-DC or PP-DC, using the following primers: FucT-VII forward, ACTGATGTTGAAACCAAAGAGC, and reverse, GCCCAGTCTTCTCCTTATATCC; CCR4 forward, GGTACCTAGACTACGCCATCC, and reverse, ATGTACTTGCGGAATTTCTCC; α4 integrin chain forward, AAACACTGGGATTAGCATGG, and reverse, ATTGCCCTGTAGTTGTCTGG; CCR9 forward, AGGTTAGTCAGCCAATGTACAGC, and reverse, ATCCTTTCCTAGTTTGTGCTTGC; GAPDH forward, CAACTTTGTCAAGCTCATTTCC and reverse, GGTCCAGGGTTTCTTACTCC. mRNA levels were expressed relative to GAPDH mRNA for each sample.
Homing assays.
Thy1.2+ CD8 T cells activated with PLN-DC or PP-DC were differentially labeled with TRITC and CFSE (Molecular Probes) as previously described (17). 2–3 × 107 cells from each population were mixed and injected into recipient C57BL/6 Thy1.1+ mice with preexisting cutaneous inflammation in both ears induced by a standard delayed-type hypersensitivity protocol (13). In brief, shaved abdomens of Thy-1.1+ mice were painted with 25 μl 0.1% 2,4-dinitro-1-fluorobenzene (DNFB; Sigma-Aldrich) in acetone on days 0 and 2. On day 5, mice were challenged with 25 μl 0.25% DNFB on both ears, and used on the next day as recipients for competitive homing experiments. Recipients were killed after 18 h, and single cell suspensions were generated from blood, spleen, PLN, MLN, and both ears after digestion with collagenase-D (Roche). Cell samples were incubated with anti-CD8 and anti–Thy-1.2 and analyzed on a FACScalibur (BD Biosciences) by gating on viable CD8+Thy-1.2+ cells. The homing index (HI) was calculated as HI = [CD8 T cells activated with PLN-DC]tissue/[CD8 T cells activated with PP-DC]tissue: [CD8 T cells activated with PLN-DC]input/[CD8 T cells activated with PP-DC]input.
Intravital microscopy (IVM).
C57BL/6 mice were anesthetized by i.p. injection of physiologic saline (10 ml/kg) containing ketamine HCL (5 mg/ml) and xylazine (1 mg/ml). The right carotid artery was catheterized, T cells were labeled with Calcein AM (Molecular Probes), administered intra-arterially in small boluses and visualized by video-triggered stroboscopic epi-illumination. Samples of CD8 T cells activated with PLN-DC or PP-DC were injected consecutively and analyzed in the same venules as previously described (44). Rolling (cells interacting visibly with venules and traveling at a slower velocity than the blood stream) and noninteracting T cells were counted in each venule. The rolling fraction was calculated as percentage of rolling cells among the total number of T cells that entered a venule. The sticking fraction was determined as percentage of T cells becoming firmly adherent for ≥30 s among all T cells that rolled in a venule during the same time interval. Velocity analysis was performed using a customized image analysis system as previously described (44).
Online supplemental material.
The supplemental material (Figs. S1–S3) is available at http://www.jem.org/cgi/content/full/jem.20041645/DC1. Fig. S1 shows mRNA expression, integrin chain expression, and chemotactic responsiveness of CD8 T cells activated with PLN-DC or PP-DC. Fig. S2 depicts the effect of sorted DC subsets in the acquisition of selectin ligands and α4β7 on CD8 T cells. Fig. S3 shows the correlation betwen the expression of the homing molecules α4β7, CCR9, and ligands for E- and P- selectin on endogenous effector–memory T cells used for FACS sorting.
Acknowledgments
We are grateful to N. Barteneva for assistance with the FACS sorting. We thank I. Mazo for technical suggestions and J. Moore for editorial assistance. J.R. Mora is indebted to Ingrid Ramos for constant support.
Supported by a Pew Fellowship to J.R. Mora and National Institutes of Health grants HL62524, HL54936, HL56949, and AI-061663 to U.H. von Andrian.
The authors have no conflicting financial interests.
Note added in proof: While this paper was under review, Iwata et al. (Iwata, M., A. Hirakiyama, Y. Eshima, H. Kagechika, C. Kato, and S.Y. Song. 2004. Immunity. 21:527–538) reported that intestinal DC, but not DC from other tissues, produce retinoic acid, which was necessary and sufficient to imprint gut-tropism and suppress skin-homing molecules on T cells.
Abbreviations used: Ag, antigen; CFSE, carboxyfluorescein diacetate succinimidyl ester; FucT, fucosyltransferase; HI, homing index; IVM, intravital microscopy; MFI, mean fluorescence intensity; PLN, peripheral lymph nodes; PP, Peyer's patches; PSGL-1, P-selectin glycoprotein ligand-1.
The online version of this article contains supplemental material.
References
- 1.Butcher, E.C. 1991. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 67:1033–1036. [DOI] [PubMed] [Google Scholar]
- 2.von Andrian, U.H., and C.R. Mackay. 2000. T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 343:1020–1034. [DOI] [PubMed] [Google Scholar]
- 3.Mackay, C.R., W.L. Marston, and L. Dudler. 1990. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J. Exp. Med. 171:801–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masopust, D., V. Vezys, A.L. Marzo, and L. Lefrancois. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 291:2413–2417. [DOI] [PubMed] [Google Scholar]
- 5.Berlin, C., E.L. Berg, M.J. Briskin, D.P. Andrew, P.J. Kilshaw, B. Holzmann, I.L. Weissman, A. Hamann, and E.C. Butcher. 1993. α4 β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 74:185–195. [DOI] [PubMed] [Google Scholar]
- 6.Zabel, B.A., W.W. Agace, J.J. Campbell, H.M. Heath, D. Parent, A.I. Roberts, E.C. Ebert, N. Kassam, S. Qin, M. Zovko, et al. 1999. Human G protein-coupled receptor GPR-9-6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J. Exp. Med. 190:1241–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamann, A., D.P. Andrew, D. Jablonski-Westrich, B. Holzmann, and E.C. Butcher. 1994. Role of α4-integrins in lymphocyte homing to mucosal tissues in vivo. J. Immunol. 152:3282–3293. [PubMed] [Google Scholar]
- 8.Svensson, M., J. Marsal, A. Ericsson, L. Carramolino, T. Broden, G. Marquez, and W.W. Agace. 2002. CCL25 mediates the localization of recently activated CD8alphabeta(+) lymphocytes to the small-intestinal mucosa. J. Clin. Invest. 110:1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansson-Lindbom, B., M. Svensson, M.A. Wurbel, B. Malissen, G. Marquez, and W. Agace. 2003. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J. Exp. Med. 198:963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Picker, L.J., T.K. Kishimoto, C.W. Smith, R.A. Warnock, and E.C. Butcher. 1991. ELAM-1 is an adhesion molecule for skin-homing T cells. Nature. 349:796–798. [DOI] [PubMed] [Google Scholar]
- 11.Campbell, J., G. Haraldsen, J. Pan, J. Rottman, S. Qin, P. Ponath, D.P. Andrew, R. Warnke, N. Ruffing, N. Kassam, et al. 1999. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 400:776–780. [DOI] [PubMed] [Google Scholar]
- 12.Morales, J., B. Homey, A.P. Vicari, S. Hudak, E. Oldham, J. Hedrick, R. Orozco, N.G. Copeland, N.A. Jenkins, L.M. McEvoy, and A. Zlotnik. 1999. CTACK, a skin-associated chemokine that preferentially attracts skin-homing memory T cells. Proc. Natl. Acad. Sci. USA. 96:14470–14475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tietz, W., Y. Allemand, E. Borges, D. von Laer, R. Hallmann, D. Vestweber, and A. Hamann. 1998. CD4+ T cells migrate into inflamed skin only if they express ligands for E- and P-selectin. J. Immunol. 161:963–970. [PubMed] [Google Scholar]
- 14.Reiss, Y., A.E. Proudfoot, C.A. Power, J.J. Campbell, and E.C. Butcher. 2001. CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J. Exp. Med. 194:1541–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kantele, A., J. Zivny, M. Hakkinen, C.O. Elson, and J. Mestecky. 1999. Differential homing commitments of antigen-specific T cells after oral or parenteral immunization in humans. J. Immunol. 162:5173–5177. [PubMed] [Google Scholar]
- 16.Campbell, D.J., and E.C. Butcher. 2002. Rapid acquisition of tissue-specific homing phenotypes by CD4+ T cells activated in cutaneous or mucosal lymphoid tissues. J. Exp. Med. 195:135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mora, J.R., M.R. Bono, N. Manjunath, W. Weninger, L.L. Cavanagh, M. Rosemblatt, and U.H. von Andrian. 2003. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 424:88–93. [DOI] [PubMed] [Google Scholar]
- 18.Stagg, A.J., M.A. Kamm, and S.C. Knight. 2002. Intestinal dendritic cells increase T cell expression of alpha4beta7 integrin. Eur. J. Immunol. 32:1445–1454. [DOI] [PubMed] [Google Scholar]
- 19.Dudda, J.C., J.C. Simon, and S. Martin. 2004. Dendritic cell immunization route determines CD8+ T cell trafficking to inflamed skin: role for tissue microenvironment and dendritic cells in establishment of T cell-homing subsets. J. Immunol. 172:857–863. [DOI] [PubMed] [Google Scholar]
- 20.Ley, K., and G.S. Kansas. 2004. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat. Rev. Immunol. 4:325–335. [DOI] [PubMed] [Google Scholar]
- 21.Maly, P., A.D. Thall, B. Petryniak, C.E. Rogers, P.L. Smith, R.M. Marks, R.J. Kelly, K.M. Gersten, G. Cheng, T.L. Saunders, et al. 1996. The α(1,3) fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell. 86:643–653. [DOI] [PubMed] [Google Scholar]
- 22.Schaerli, P., L. Ebert, K. Willimann, A. Blaser, R.S. Roos, P. Loetscher, and B. Moser. 2004. A skin-selective homing mechanism for human immune surveillance T cells. J. Exp. Med. 199:1265–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weninger, W., L.H. Ulfman, G. Cheng, N. Souchkova, E.J. Quackenbush, J.B. Lowe, and U.H. von Andrian. 2000. Specialized contributions by alpha(1,3)-fucosyltransferase-IV and FucT-VII during leukocyte rolling in dermal microvessels. Immunity. 12:665–676. [DOI] [PubMed] [Google Scholar]
- 24.Ley, K., M. Allietta, D.C. Bullard, and S. Morgan. 1998. Importance of E-selectin for firm leukocyte adhesion in vivo. Circ. Res. 83:287–294. [DOI] [PubMed] [Google Scholar]
- 25.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y.J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767–811. [DOI] [PubMed] [Google Scholar]
- 26.Anjuere, F., P. Martin, I. Ferrero, M.L. Fraga, G.M. del Hoyo, N. Wright, and C. Ardavin. 1999. Definition of dendritic cell subpopulations present in the spleen, Peyer's patches, lymph nodes, and skin of the mouse. Blood. 93:590–598. [PubMed] [Google Scholar]
- 27.Iwasaki, A., and B.L. Kelsall. 2000. Localization of distinct Peyer's patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3alpha, MIP-3beta, and secondary lymphoid organ chemokine. J. Exp. Med. 191:1381–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Austrup, F., D. Vestweber, E. Borges, M. Lohning, R. Brauer, U. Herz, H. Renz, R. Hallman, A. Scheffold, A. Radbruch, and A. Hamann. 1997. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflammed tissues. Nature. 385:81–83. [DOI] [PubMed] [Google Scholar]
- 29.Wagers, A.J., C.M. Waters, L.M. Stoolman, and G.S. Kansas. 1998. Interleukin 12 and interleukin 4 control T cell adhesion to endothelial selectins through opposite effects on alpha1, 3-fucosyltransferase VII gene expression. J. Exp. Med. 188:2225–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abramson, O., S. Qiu, and D.J. Erle. 2001. Preferential production of interferon-gamma by CD4+ T cells expressing the homing receptor integrin alpha4/beta7. Immunology. 103:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warren, T.L., S.K. Bhatia, A.M. Acosta, C.E. Dahle, T.L. Ratliff, A.M. Krieg, and G.J. Weiner. 2000. APC stimulated by CpG oligodeoxynucleotide enhance activation of MHC class I-restricted T cells. J. Immunol. 165:6244–6251. [DOI] [PubMed] [Google Scholar]
- 32.Weninger, W., H.S. Carlsen, M. Goodarzi, F. Moazed, M.A. Crowley, E.S. Baekkevold, L.L. Cavanagh, and U.H. von Andrian. 2003. Naïve T cell recruitment to non-lymphoid tissues: a role for endothelium-expressed CCL21 in autoimmune disease and lymphoid neogenesis. J. Immunol. 170:4638–4648. [DOI] [PubMed] [Google Scholar]
- 33.Jung, U., K.E. Norman, K. Scharffetter-Kochanek, A.L. Beaudet, and K. Ley. 1998. Transit time of leukocytes rolling through venules controls cytokine-induced inflammatory cell recruitment in vivo. J. Clin. Invest. 102:1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haddad, W., C.J. Cooper, Z. Zhang, J.B. Brown, Y. Zhu, A. Issekutz, I. Fuss, H.O. Lee, G.S. Kansas, and T.A. Barrett. 2003. P-selectin and P-selectin glycoprotein ligand 1 are major determinants for Th1 cell recruitment to nonlymphoid effector sites in the intestinal lamina propria. J. Exp. Med. 198:369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carramolino, L., A. Zaballos, L. Kremer, R. Villares, P. Martin, C. Ardavin, A.C. Martinez, and G. Marquez. 2001. Expression of CCR9 beta-chemokine receptor is modulated in thymocyte differentiation and is selectively maintained in CD8+ T cells from secondary lymphoid organs. Blood. 97:850–857. [DOI] [PubMed] [Google Scholar]
- 36.White, S.J., G.H. Underhill, M.H. Kaplan, and G.S. Kansas. 2001. Cutting edge: differential requirements for Stat4 in expression of glycosyltransferases responsible for selectin ligand formation in Th1 cells. J. Immunol. 167:628–631. [DOI] [PubMed] [Google Scholar]
- 37.Teraki, Y., and L.J. Picker. 1997. Independent regulation of cutaneous lymphocyte-associated antigen expression and cytokine synthesis phenotype during human CD4+ memory T cell differentiation. J. Immunol. 159:6018–6029. [PubMed] [Google Scholar]
- 38.Grogan, J.L., M. Mohrs, B. Harmon, D.A. Lacy, J.W. Sedat, and R.M. Locksley. 2001. Early transcription and silencing of cytokine genes underlie polarization of T helper cell subsets. Immunity. 14:205–215. [DOI] [PubMed] [Google Scholar]
- 39.Wherry, E.J., V. Teichgraber, T.C. Becker, D. Masopust, S.M. Kaech, R. Antia, U.H. von Andrian, and R. Ahmed. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4:225–234. [DOI] [PubMed] [Google Scholar]
- 40.von Andrian, U.H., and T.R. Mempel. 2003. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 3:867–878. [DOI] [PubMed] [Google Scholar]
- 41.Campbell, J.J., K.E. Murphy, E.J. Kunkel, C.E. Brightling, D. Soler, Z. Shen, J. Boisvert, H.B. Greenberg, M.A. Vierra, S.B. Goodman, et al. 2001. CCR7 expression and memory T cell diversity in humans. J. Immunol. 166:877–884. [DOI] [PubMed] [Google Scholar]
- 42.Manjunath, N., P. Shankar, J. Wan, W. Weninger, M.A. Crowley, K. Hieshima, T.A. Springer, X. Fan, H. Shen, J. Lieberman, and U.H. von Andrian. 2001. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J. Clin. Invest. 108:871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mombaerts, P., A.R. Clarke, M.A. Rudnicki, J. Iacomini, S. Itohara, J.J. Lafaille, L. Wang, Y. Ichikawa, R. Jaenisch, M.L. Hooper, and S. Tonegawa. 1992. Mutations in T-cell antigen receptor genes α and β block thymocyte development at different stages. Nature. 360:225–231. [DOI] [PubMed] [Google Scholar]
- 44.Stein, J.V., G. Cheng, B.M. Stockton, B.P. Fors, E.C. Butcher, and U.H. von Andrian. 1999. L-selectin-mediated leukocyte adhesion in vivo: microvillous distribution determines tethering efficiency, but not rolling velocity. J. Exp. Med. 189:37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]