TGF-β1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells (original) (raw)
Abstract
Transforming growth factor (TGF)-β1 is a major pluripotential cytokine with a pronounced immunosuppressive effect and its deficiency results in lethal autoimmunity in mice. However, mechanisms of its immunosuppressive action are not completely understood. Here, we report that TGF-β1 supports the maintenance of Foxp3 expression, regulatory function, and homeostasis in peripheral CD4+CD25+ regulatory T (T reg) cells, but is not required for their thymic development. We found that in 8–10-d-old TGF-β1–deficient mice, peripheral, but not thymic, T reg cells are significantly reduced in numbers. Moreover, our experiments suggest that a defect in TGF-β–mediated signaling in T reg cells is associated with a decrease in Foxp3 expression and suppressor activity. Thus, our results establish an essential link between TGF-β1 signaling in peripheral T reg cells and T reg cell maintenance in vivo.
CD4+CD25+ regulatory T (T reg) cells play a major role in the maintenance of immune tolerance to self and in the control of autoimmunity (1, 2). T reg cells have been shown to inhibit autoimmunity in a number of experimental models, including diabetes and inflammatory bowel disease in rodents (3, 4). They are also involved in regulating T cell homeostasis (1, 5). Additional studies have demonstrated their role in organ transplant tolerance and in modulation of immune responses to pathogens (6, 7). This CD4+ T cell subset constitutes ∼5–10% of peripheral CD4+ T cells and is capable of inhibiting the responses of CD4+CD25− and CD8 T cells in vitro and in vivo (1).
Recently, Foxp3, a member of the forkhead winged helix protein family of transcription factors, has been identified as a specific molecular marker for T reg cells and its expression is essential for programming T reg cell development and function (8–11). The Foxp3 gene is highly conserved, and the function of Foxp3 appears to be similar in both humans and mice, as Foxp3 mutations result in a fatal autoimmune pathologies affecting multiple organs in both species (9, 10, 12, 13).
The phenotype of Foxp3 knockout mice closely resembles that of animals deficient in TGF-β 1 (_Tgf-β_1 − / −), with both mutants developing a lethal lymphoproliferative autoimmune syndrome and succumbing to disease at 3–4 wk of age (10, 14). TGF-β1 is a widely distributed immunomodulatory cytokine. TGF-β1 signals through a heterodimeric receptor complex formed by TGF-βRI and TGF-βRII subunits. Binding of TGF-β1 to the receptor complex activates the intracellular kinase domain, which leads to the phosphorylation and activation of members of the Smad protein family and subsequent regulation of TGF-β–dependent gene expression (15).
An important role of TGF-β1 in down-modulation of T cell–mediated immune responses and in controlling autoimmunity has been clearly established (15). Mice deficient in TGF-βRII receptor or expressing a dominant negative form of TGF-βRII receptor (DN–TGF-βRII) encoded by a transgene under a T cell–specific promoter exhibit inflammatory infiltration in multiple organs and uncontrolled T cell proliferation (16, 17) with features similar to that of the _Tgf-β_1 − / − mice. Notably, several recent reports suggested that TGF-β1 produced by T reg cells, and possibly decorating their plasma membrane via binding to T reg cell TGF-β receptors, may serve as an effector mechanism of suppression (18, 19). However, a subsequent in vitro study failed to reproduce this finding (20).
Therefore, we revisited the role that TGF-β1 plays in T reg cell biology by directly examining potential effect of TGF-β1 deficiency on T reg cells. This was achieved by analyzing the T reg cell subsets in young Tgf_-β_1 − / − mice. We have found that TGF-β1 produced by T reg cells is unlikely to play a role as a nonredundant effector molecule mediating T reg cell suppressor function. However, Foxp3 expression, the size of peripheral T reg cell compartment, and suppressive activity are dependent on signals induced by TGF-β in T reg cells.
Results and Discussion
TGF-β1 is required for peripheral T reg cell homeostasis
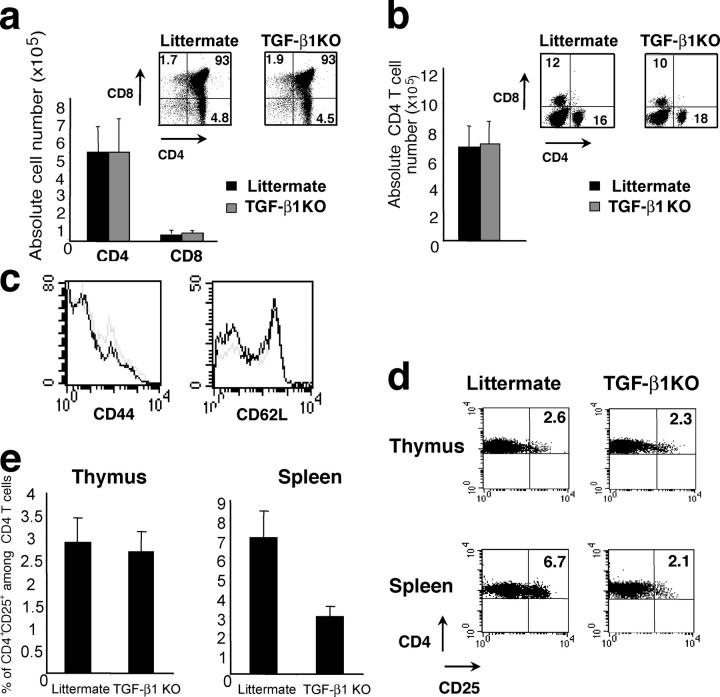
Although a role of TGF-β–dependent signals has been well documented in the control of T cell activation and inflammation in vivo (16), a connection between TGF-β1 and T reg cell function in vivo and in vitro has been controversial. Two recent in vitro studies have shown no demonstrable role for TGF-β1 produced by T reg cells in the suppressor function (20, 21). However, another set of studies suggested that TGF-β1 produced by T reg cells is an essential effector molecule because, in these experiments, either anti–TGF-β1–neutralizing antibodies or a soluble form of TGF-βRII abrogated T reg cell–mediated suppression in vitro and in vivo (18, 19). To reexamine a role for TGF-β1 in T reg cell development and function, we used mice deficient for _Tgf-β_1 expression, which are known to develop an early onset lethal lymphoproliferative autoimmune syndrome (14). To avoid potential artifacts due to pathology observed in affected _Tgf-_β_1_–deficient mice, we examined the T cell compartment in 8–10-d-old _Tgf-β_1 − / − mice before the onset of lymphoproliferation and clinical symptoms. In these young mice, the analysis of thymus and spleen did not reveal any difference in the proportion or absolute number of single positive or double positive thymocytes, and the peripheral CD4 and CD8 T cell compartments were similar in _Tgf-β_1 − / − mice and littermate controls (Fig. 1, a and b). In addition, peripheral _Tgf-β_1 − / − CD4 T cells expressed the same level of the activation markers CD44 and CD62L as cells from WT littermate controls (Fig. 1 c). However, in 12–14-d-old mice, the number of peripheral CD4 T cells was increased and some of these cells exhibited an activated phenotype (i.e., increased CD44 and diminished CD62L expression as compared with T cells in WT littermate controls; unpublished data). Thus, we have chosen to study the T reg cell subset in 8–10-d-old Tgf_-β_1 − / − mice. Flow cytometric analysis revealed the peripheral CD4+CD25+ T cell compartment reduced by 2.5–3-fold in Tgf_-β_1 − / − mice as compared with the WT littermate controls (Fig. 1, d and e). However, the CD4+CD25+ thymocyte subset in these animals was similar in size to that of littermate controls (Fig. 1, d and e). Thus, these results indicate that TGF-β1 plays a role in the peripheral T reg cell maintenance. In contrast with these data, no difference was previously found in the CD25+CD4+ T cell subsets not only in the thymus but also in the periphery in 5–7-d-old TGF-β1–deficient mice (20). This apparent discrepancy is most likely due to the fact that the numbers of peripheral T reg cells in 5–7-d-old mice are very small (unpublished data) and the vast majority of these cells are recent arrivals from the thymus exhibiting normal levels of Foxp3 characteristic of thymic T reg cells.
Figure 1.
Decrease in peripheral CD4+CD25+ T cells in _Tgf-β_1 −/− mice. Thymocytes (a) and splenocytes (b and c) from 8–10-d-old _Tgf-β_1 − / − mice or littermate controls were counted and stained for CD4 and CD8 and analyzed by flow cytometry. Peripheral CD4 T cells from _Tgf-β_1 −/− mice (gray line) or littermate controls (black line) were stained for CD44 and CD62L. Thymocytes and splenocytes from 8–10-d-old _Tgf-β_1 − / − mice or littermate controls were stained for CD4 and CD25 and analyzed by flow cytometry (d). Proportion of CD4+CD25+ cells among CD4 T cells (e, n = 20).
TGF-β1 is required to maintain Foxp3 expression in T reg cells
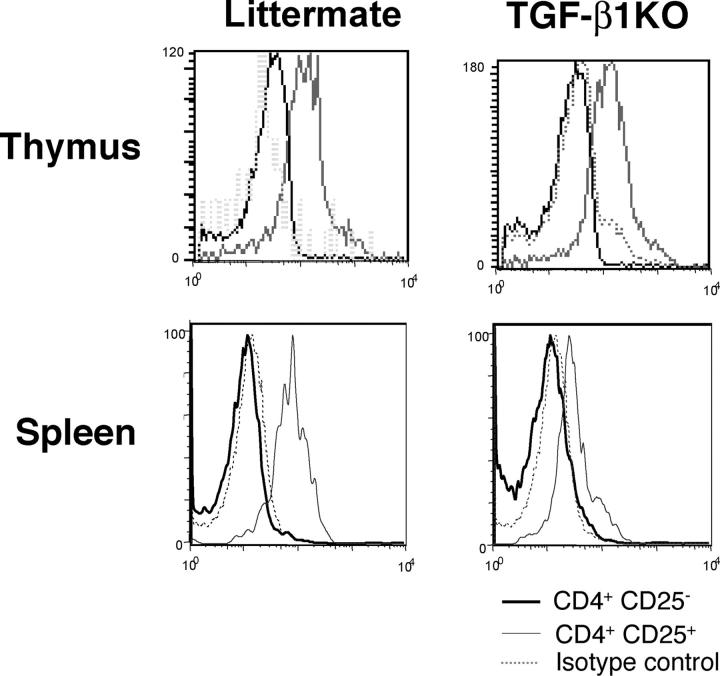
Next, we investigated whether TGF-β1 can regulate Foxp3 expression in T reg cells. First, we analyzed Foxp3 expression by intracellular staining of thymic and splenic CD4+CD25+ T cells isolated from Tgf_-β_1 − / − mice or WT littermate controls (Fig. 2). No significant difference in Foxp3 expression was observed in CD4+CD25+ thymocytes in mutant versus WT mice, whereas peripheral Tgf_-β_1 − / − CD4+CD25+ T cells expressed significantly diminished level of Foxp3 compared with the control. Thus, the absence of TGF-β1 results in diminished Foxp3 expression in peripheral CD4+CD25+ T cells in addition to a substantial decrease in size of this T cell compartment. Nevertheless, some peripheral regulatory T cells in TGF-β1 null mice still maintain Foxp3 expression. The latter is likely due to normal level of Foxp3 expression in thymocytes being preserved in recent thymic emigrants. Provision of small amounts of TGF-β1 by mother via breastfeeding and potential compensatory role of TGF-β2 and TGF-β3 can also contribute to maintaining Foxp3 expression in some regulatory T cells in the knockout mice. This result strongly suggests that TGF-β1 is required for the maintenance of Foxp3 levels in peripheral T reg cells.
Figure 2.
Decrease in Foxp3 level in _Tgf-β_1 −/− CD4+CD25+ T cells. Thymocytes and splenocytes from 8–10-d-old _Tgf-β_1 − / − mice or littermate controls were stained for CD4 and CD25 followed by anti-Foxp3 intracellular staining and analyzed by flow cytometry. Foxp3 staining in CD4+CD25− T cells (black line) and in CD4+CD25+ (gray line) are shown. Isotype control staining is shown (dashed line). These results are representative of three different experiments.
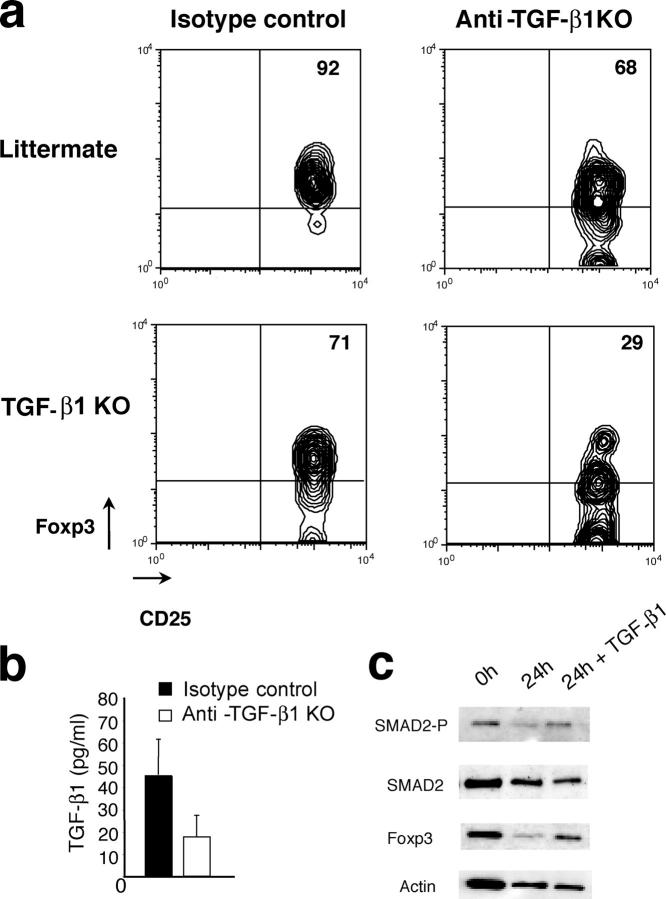
To test this hypothesis, CD4+CD25+ T cells from either 8–10-d-old Tgf_-β_1 − / − mice or littermate control mice were adoptively transferred into lymphopenic TCRβ/δ-deficient host treated with the neutralizing anti–TGF-β antibody or isotype control IgG and analyzed 4 d later by flow cytometry (Fig. 3 a). As expected, the majority of Tgf_-β_1 − / − CD4+CD25+ T cells transferred into anti–TGF-β–treated recipient mice continued to exhibit decreased Foxp3 levels, whereas few Foxp3high cells were observed. The latter may be due to incomplete antibody-mediated TGF-β1 deletion in addition to the aforementioned compensatory role of TGF-β2 and TGF-β3. However, transfer of these cells into control IgG1-treated recipients, expressing normal amounts of TGF-β1 (Fig. 3 b), led to an increase in Foxp3 expression in T reg cells comparable to that of WT littermate control T reg cells. Furthermore, transfer of CD4+CD25+ T cells from littermate control mice into recipients treated with the anti–TGF-β antibody resulted in somewhat diminished Foxp3 expression in those cells after 4 d. Altogether, these results, involving both genetic modifications and in vivo adoptive transfer approaches, show that TGF-β1 maintains Foxp3 expression in peripheral T reg cells. Furthermore, the engagement of TGF-β signaling pathway in T reg cells cultured in the presence of TGF-β1 resulted in induction of Smad2 phosphorylation and concomitant increase in Foxp3 expression (Fig. 3 c). This observation further confirms that TGF-β maintains Foxp3 high expression in T reg cells. By inference from recent observations of acquisition of T reg cell phenotype and suppressor activity by CD4+CD25− T cells upon retroviral transduction with Foxp3 (9, 10), the loss of peripheral T reg cells and their diminished suppressor activity in _Tgf-β_1 − / − mice can be a direct consequence of decreasing levels of Foxp3.
Figure 3.
TGF-β1 is required to maintain Foxp3 in CD4+CD25+ T cells. T reg cells purified from spleen of 8–10-d-old _Tgf-β_1 − / − mice or littermate controls were transferred into TCRβ/δ-deficient mice injected every other day with anti–TGF-β1 antibody or isotype control IgG. 4 d after transfer, cells from spleen and lymph nodes were stained for CD4 and CD25 followed by anti-Foxp3 intracellular staining and analyzed by flow cytometry (a). Results are representative of three different experiments. Serum TGF-β1 in treated animals was measured by ELISA (b). Western blot analysis of Smad2 phosphorylation and Foxp3 expression in purified wild-type T reg cells either freshly isolated or cultured for 24 h in the presence or absence of 100 pg/ml TGF-β1 (c).
TGF-β1 is required to maintain T reg cell suppressive function
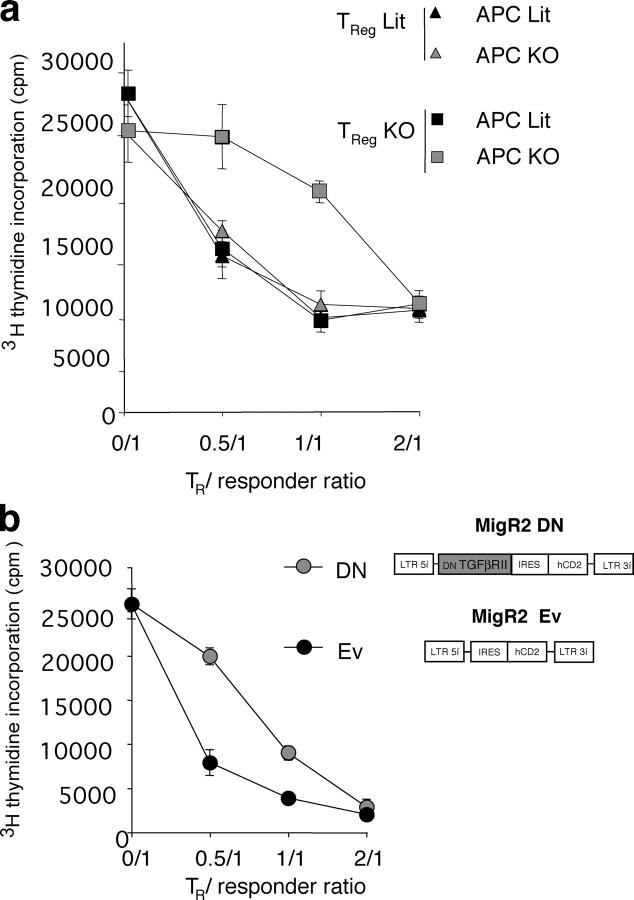
After establishing a decreased level of Foxp3 expression in T reg cells in the absence of TGF-β1, we investigated whether TGF-β1 is also involved in the maintenance of their regulatory function. Purified T reg cells from _Tgf-β_1 −/− mice or littermate control mice were cocultured with responder CD4+CD25− T cells from control mice in the presence of Con A and T cell–depleted splenic APCs isolated from either _Tgf-β_1 −/− or littermate control mice (Fig. 4 a). All combinations of WT and mutant T cells and APCs resulted in a comparable suppression except for a significantly diminished suppression by _Tgf-β_1 −/− T reg cells observed only in the presence of _Tgf-β_1 −/− APCs. Thus, these data demonstrate that TGF-β1 production by T reg cells is dispensable for their suppressor function because APCs can produce sufficient amounts of TGF-β1 to support T reg cell–mediated suppression.
Figure 4.
TGF-β signaling is required to maintain regulatory function T reg cells. (a) Analysis of suppressor activity of T reg cells isolated from _Tgf-β_1 − / − (gray symbols) or WT littermates (Lit, black symbols) in cocultures with freshly isolated B6 CD4+CD25− responder T cells in the presence of either _Tgf-β_1 − / − or littermate control APCs and Con A. Proliferation of CD4+CD25− T cells was determined after 72 h of culture by [3H]thymidine incorporation. Proliferation of CD4+CD25+ from either control or _Tgf-β_1 − / − mice in response to Con A was ∼300–500 cpm. The results are shown as mean cpm of [3H]thymidine incorporation in triplicate cultures ± SD. (b) T reg cells transduced with DN–TGF-βRII–MigR2 (MigR2 DN) or empty vector control (MigR2 Ev) were purified and cocultured with freshly isolated B6 CD4+CD25− responder T cells in the presence of irradiated T cell–depleted splenic APCs and Con A. Proliferation was measured as described before. Results are representative of two or three different experiments.
Although relative contribution of TGF-β1 produced by T reg cells versus other cellular sources to T reg cell function in vivo remains to be studied further, our results suggest a requirement for paracrine TGF-β1 to maintain T reg cell function in the periphery. This observation is in agreement with recent experiments by Powrie et al., who showed that an adoptive transfer of T reg cells isolated from TGF-β1–deficient DO11.10 TCR transgenic mice can prevent colitis mediated by WT CD4+CD25− T cells transferred into SCID recipient mice. However, this suppression was abrogated in the presence of neutralizing antibodies specific for TGF-β1 leading to disease progression (22). We also observed that T reg cells from _Tgf-β_1 −/− mice were able to suppress autoimmunity developing in _Tgf-β_1 +/+ RAG −/− mice upon transfer of _Tgf-β_1 −/− CD4+CD25− T cells (23). These findings are consistent with our notion that _Tgf-β_1 −/− T reg cells require TGF-β1 provided by other cells to preserve their functionality. Although the types of accessory cells supplying TGF-β1 for T reg cells in vivo remains to be determined, our analysis of in vitro TGF-β1 production using ELISA implicated APCs, such as dendritic cells, as the likely candidates (unpublished data).
TGF-β1–mediated signaling in T reg cells is required to maintain suppressor function
However, these results do not address the question as to whether T reg cells require TGF-β1 signaling to maintain their functionality or whether T reg cells are “armed” with the receptor-bound TGF-β1 produced either endogenously or secreted by APCs. The latter possibility was raised by two reports suggesting that receptor-bound TGF-β1 displayed on the surface of T reg cells may function as an effector molecule in T reg cell–mediated suppression as assessed in an in vitro assay (19, 24). To address these two possibilities, we transduced T reg cells isolated from B6 mice with the Mig-R2 retroviral vector encoding a dominant negative form of the TGF-β1 receptor (DN–TGF-βRII) and IRES-driven tail-less human CD2 as a reporter. DN–TGF-βRII was generated upon deletion of the TGF-βRII kinase domain. As a control, T reg cells were transduced with the MIG-R2 vector without insert. Transduced T reg cells were purified by magnetic bead sorting using anti–human CD2 antibody. Suppressor activity of DN–TGF-βRII– and empty vector–transduced T reg cells was tested in an in vitro suppression assay. The regulatory capacity of T reg cells expressing DN–TGF-βRII was diminished, but not completely abolished, as compared with the control T reg cells (Fig. 4 b). This is likely due to the fact that T reg cells expressing DN–TGF-βRII remain still sensitive to TGF-β, albeit require an ∼10-fold higher dose to induce Smad2 phosphorylation at a level comparable to that of control MigR2-transduced cells (unpublished data). Importantly, although expression of DN–TGF-βRII inhibits TGF-β–mediated signaling, it is expected to increase ability of T reg cells to capture TGF-β on the plasma membrane. The analysis of suppressor function of T reg cells transduced with DN–TGFβ-RII suggests that impairment in suppressor function in the absence of TGF-β1 is due to a signaling defect and not due to lack of receptor-mediated display of TGF-β on the T reg cell surface. Therefore, our results are consistent with the role of TGF-β not as an effector molecule of suppression, but as a mediator of signaling in T reg cells required to maintain their suppressor function. Thus, our findings provide novel important insights into the role of TGF-β1 in T reg cell function reconciling previous contradictory observations.
Recently, several in vitro studies have reported acquisition of suppressor activity and Foxp3 expression in the bulk populations of cultured murine and human CD4+CD25− T cells upon activation in the presence of nanomolar amounts of recombinant TGF-β1 (25, 26). Although these studies opened up an important avenue for pharmacologic in vitro manipulation of T cell function for potential therapeutic purposes, there is no experimental evidence so far that such high concentrations of TGF-β1 are ever attainable in vivo and that such a conversion of CD4+CD25− into CD4+ CD25+ T cells expressing Foxp3 can occur under physiologic conditions or in the course of immune inflammation. In our earlier experiments, we failed to detect any measurable conversion of CD4+CD25− T cells into CD4+CD25+ upon their transfer into newborn Foxp3-deficient mice (10). Ramsdell et al. have recently reported high levels of TGF- β1 in Foxp3 mutant mice in addition to highly elevated levels of a number of other cytokines (11). Nevertheless, it will be of interest to experimentally test whether substantial amounts of TGF-β1 displayed by some immunoprivileged tissues and organs, like the ocular chamber of the eye, can facilitate maintenance, expansion, or de novo generation of antigen-specific T reg cells (27). This idea is supported by the recent observation of significant expansion and/or generation of protective T reg cells in pancreatic lymph nodes and islets in NOD mice upon temporal TGF-β1 induction in the β-cells (28).
In conclusion, our study demonstrates an essential in vivo role for the TGF-β1–mediated signaling in the maintenance of naturally arising regulatory CD4+CD25+ T cell numbers and function, and in the maintenance of Foxp3 expression in these cells in the periphery. These results demonstrate an important role of TGF-β1 in T reg cell biology and suggest additional applications for targeting of TGF-β1 signaling pathway for therapeutic immunomodulation.
Materials and Methods
Mice.
C57BL/6 (B6) and TCRβ/δ-deficient mice were purchased from Charles River Breeding Laboratories and The Jackson Laboratory, respectively. Tgf_-β_1 − / − mice were previously described (29). All mice were maintained in a specific pathogen-free animal facility at the University of Washington and National Institutes of Health and handled in accordance with the institutional guidelines.
Antibodies_._
Anti-Foxp3 IgG was purified on a protein G–Sepharose 4 Fast Flow column from the rabbit antiserum raised against purified His-tagged recombinant Foxp3 protein. Biotinylated (bio), FITC-, PE-, cy-chrome (CyC)-, peridinin chlorophyl protein (PerCP)-, and allophycocyanin-conjugated monoclonal antibodies to CD4 (L3T4), CD8α (53–6.7), CD25 (7D4), CD25 (PC-61), CD62L (MEL-14), CD69 (H1.2F3), and CD44 (IM7) were purchased from BD Biosciences and eBioscience. For Western blotting, rabbit anti-Smad2, anti–Smad2-P (Chemicon), and anti-Foxp3 antibodies were used in combination the donkey anti–rabbit IgG–horseradish peroxidase secondary antibody (Amersham Biosciences). Intracellular staining for Foxp3 was performed upon cell fixation and permeabilization with rabbit anti-Foxp3 IgG followed by biotinylated–goat anti–rabbit IgG antibody (Jackson ImmunoResearch Laboratories) and streptavidin-allophycocyanin (BD Biosciences; unpublished data).
TGF-β depletion_._
Mouse monoclonal anti–TGF-β1 (2G7; IgG1) antibody (30) and mouse IgG1 (Sigma-Aldrich) used as isotype control were injected intraperitoneally (150 μg/injection/animal) every other day, 4 d before and after adoptive cell transfer. TGF-β1–specific ELISA kit (Quantikine; R&D Systems) was used to measure TGF-β1 according to the manufacturer's protocol.
Cell purification.
Cell populations were purified using an AutoMACS magnetic cell sorter and magnetic beads (Miltenyi Biotec) as described (10) and/or using a FACSVantage cell sorter (BD Biosciences). Routinely, purity of all cell preparations was >90%. Flow cytometric analysis was performed using a FACSCalibur flow cytometer.
Cell culture and in vitro suppression assay.
Cells were cultured in RPMI 1640 supplemented with 10% FCS, 200 mM l-glutamine, 1 mM sodium pyruvate, 10 mM Hepes, 100 U/ml penicillin/streptomycin, and 5 × 10−5 M 2-mercaptoethanol (RP-10).
Purified T reg cells were cultured for 24 h in the presence or absence of 100 pg/ml of TGF-β1 (R&D Systems). Western blot analysis was performed after cell lysate separation in 12% SDS-PAGE gels (3 × 106 cell equivalents/lane) as described elsewhere (10).
Suppression assays were performed as described (10). In brief, CD4+ CD25− T cells (4 × 104 cells/well) were stimulated for 72 h with 1 μg/ml of Con A in the presence of irradiated (2,000 rad) T cell–depleted splenocytes (APCs) (2 × 105/well) in 96-well round-bottom plates, with indicated numbers of CD4+CD25+ T cells and pulsed with 1 μCi/well of [3H]thymidine for the final 16 h of culture. Data are shown as mean [3H]thymidine incorporation in triplicate cultures.
Retroviral infection.
The first 656 bp of the coding sequence of TGF-βRII were subcloned from pcDNA3.1-myc-HisB DN-TGF-βRII (+) into MSCV MigR2 retroviral vector. The vector DNA with or without insert was transfected into the ΦNX-E packaging cell line using Fugene 6 reagent (Roche) according to the manufacturer's protocol. Retrovirus-containing supernatant was collected after a 36-h culture of the transfected packaging cells at 32°C. Freshly isolated MACS-purified CD4+CD25+ T cells were activated using plate-bound anti-TCRβ chain and anti-CD28 antibodies, in the presence of 100 U/ml recombinant human IL-2 (Hoffmann–La Roche). After 24 h of activation, CD4+CD25+ T cells were infected by resuspending cells in retrovirus-containing supernatants supplemented with 8 μg/ml polybrene and 50 U/ml of recombinant IL-2, followed by centrifugation for 90 min at 2,500 revolutions/min. Cells were cultured for 48 h at 37°C in the presence of 200 U/ml IL-2 and 10 pg/ml TGF-β1 to maintain CD25 and Foxp3 expression.
Acknowledgments
We thank the members of the Rudensky laboratory for discussions and for reading the text.
This work was supported by grants from International Human Frontier Science Program (to J.C. Marie) and National Institutes of Health nos. AI34206 and AI061816 (to A.Y. Rudensky). A.Y. Rudensky is a Howard Hughes Medical Institute investigator.
The authors have no conflicting financial interests.
References
- 1.Sakaguchi, S., N. Sakaguchi, J. Shimizu, S. Yamazaki, T. Sakihama, M. Itoh, Y. Kuniyasu, T. Nomura, M. Toda, and T. Takahashi. 2001. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol. Rev. 182:18–32. [DOI] [PubMed] [Google Scholar]
- 2.Shevach, E.M. 2002. CD4+ CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2:389–400. [DOI] [PubMed] [Google Scholar]
- 3.Salomon, B., D.J. Lenschow, L. Rhee, N. Ashourian, B. Singh, A. Sharpe, and J.A. Bluestone. 2000. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 12:431–440. [DOI] [PubMed] [Google Scholar]
- 4.Stephens, L.A., and D. Mason. 2000. CD25 is a marker for CD4+ thymocytes that prevent autoimmune diabetes in rats, but peripheral T cells with this function are found in both CD25+ and CD25− subpopulations. J. Immunol. 165:3105–3110. [DOI] [PubMed] [Google Scholar]
- 5.Annacker, O., R. Pimenta-Araujo, O. Burlen-Defranoux, and A. Bandeira. 2001. On the ontogeny and physiology of regulatory T cells. Immunol. Rev. 182:5–17. [DOI] [PubMed] [Google Scholar]
- 6.Taylor, P.A., R.J. Noelle, and B.R. Blazar. 2001. CD4+CD25+ immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J. Exp. Med. 193:1311–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belkaid, Y., C.A. Piccirillo, S. Mendez, E.M. Shevach, and D.L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 420:502–507. [DOI] [PubMed] [Google Scholar]
- 8.Brunkow, M.E., E.W. Jeffery, K.A. Hjerrild, B. Paeper, L.B. Clark, S.A. Yasayko, J.E. Wilkinson, D. Galas, S.F. Ziegler, and F. Ramsdell. 2001. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 27:68–73. [DOI] [PubMed] [Google Scholar]
- 9.Hori, S., T. Nomura, and S. Sakaguchi. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science. 299:1057–1061. [DOI] [PubMed] [Google Scholar]
- 10.Fontenot, J.D., M.A. Gavin, and A.Y. Rudensky. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4:330–336. [DOI] [PubMed] [Google Scholar]
- 11.Khattri, R., T. Cox, S.A. Yasayko, and F. Ramsdell. 2003. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4:337–342. [DOI] [PubMed] [Google Scholar]
- 12.Bennett, C.L., J. Christie, F. Ramsdell, M.E. Brunkow, P.J. Ferguson, L. Whitesell, T.E. Kelly, F.T. Saulsbury, P.F. Chance, and H.D. Ochs. 2001. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 27:20–21. [DOI] [PubMed] [Google Scholar]
- 13.Wildin, R.S., F. Ramsdell, J. Peake, F. Faravelli, J.L. Casanova, N. Buist, E. Levy-Lahad, M. Mazzella, O. Goulet, L. Perroni, et al. 2001. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 27:18–20. [DOI] [PubMed] [Google Scholar]
- 14.Shull, M.M., I. Ormsby, A.B. Kier, S. Pawlowski, R.J. Diebold, M. Yin, R. Allen, C. Sidman, G. Proetzel, D. Calvin, et al. 1992. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 359:693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Letterio, J.J., and A.B. Roberts. 1998. Regulation of immune responses by TGF-beta. Annu. Rev. Immunol. 16:137–161. [DOI] [PubMed] [Google Scholar]
- 16.Gorelik, L., and R.A. Flavell. 2000. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 12:171–181. [DOI] [PubMed] [Google Scholar]
- 17.Leveen, P., J. Larsson, M. Ehinger, C.M. Cilio, M. Sundler, L.J. Sjostrand, R. Holmdahl, and S. Karlsson. 2002. Induced disruption of the transforming growth factor beta type II receptor gene in mice causes a lethal inflammatory disorder that is transplantable. Blood. 100:560–568. [DOI] [PubMed] [Google Scholar]
- 18.Zhang, X., L. Izikson, L. Liu, and H.L. Weiner. 2001. Activation of CD25(+)CD4(+) regulatory T cells by oral antigen administration. J. Immunol. 167:4245–4253. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura, K., A. Kitani, and W. Strober. 2001. Cell contact-dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface-bound transforming growth factor β. J. Exp. Med. 194:629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piccirillo, C.A., J.J. Letterio, A.M. Thornton, R.S. McHugh, M. Mamura, H. Mizuhara, and E.M. Shevach. 2002. CD4+CD25+ regulatory T cells can mediate suppressor function in the absence of transforming growth factor β1 production and responsiveness. J. Exp. Med. 196:237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi, T., Y. Kuniyasu, M. Toda, N. Sakaguchi, M. Itoh, M. Iwata, J. Shimizu, and S. Sakaguchi. 1998. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 10:1969–1980. [DOI] [PubMed] [Google Scholar]
- 22.Fahlen, L., S. Read, L. Gorelik, S.D. Hurst, R.I. Coffman, R.A. Flavell, and F. Powrie. 2005. T cells that cannot respond to TGF-β escape control by CD4+CD25+ regulatory T cells. J. Exp. Med. 201:737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mamura, M., W. Lee, T.J. Sullivan, A. Felici, A.L. Sowers, J.P. Allison, and J.J. Letterio. 2004. CD28 disruption exacerbates inflammation in Tgf-{beta}1−/− mice: in vivo suppression by CD4+ CD25+ regulatory T cells independent of autocrine TGF-beta1. Blood. 103:4594–4601. [DOI] [PubMed] [Google Scholar]
- 24.Annunziato, F., L. Cosmi, F. Liotta, E. Lazzeri, R. Manetti, V. Vanini, P. Romagnani, E. Maggi, and S. Romagnani. 2002. Phenotype, localization, and mechanism of suppression of CD4+CD25+ human thymocytes. J. Exp. Med. 196:379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen, W., W. Jin, N. Hardegen, K.J. Lei, L. Li, N. Marinos, G. McGrady, and S.M. Wahl. 2003. Conversion of peripheral CD4+ CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 198:1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng, S.G., J.H. Wang, J.D. Gray, H. Soucier, and D.A. Horwitz. 2004. Natural and induced CD4+CD25+ cells educate CD4+ CD25− cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J. Immunol. 172:5213–5221. [DOI] [PubMed] [Google Scholar]
- 27.Stein-Streilein, J., and J.W. Streilein. 2002. Anterior chamber associated immune deviation (ACAID): regulation, biological relevance, and implications for therapy. Int. Rev. Immunol. 21:123–152. [DOI] [PubMed] [Google Scholar]
- 28.Peng, Y., Y. Laouar, M.O. Li, E.A. Green, and R.A. Flavell. 2004. TGF-{beta} regulates in vivo expansion of Foxp3-expressing CD4+ CD25+ regulatory T cells responsible for protection against diabetes. Proc. Natl. Acad. Sci. USA. 101:4572-4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kulkarni, A.B., C.G. Huh, D. Becker, A. Geiser, M. Lyght, K.C. Flanders, A.B. Roberts, M.B. Sporn, J.M. Ward, and S. Karlsson. 1993. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc. Natl. Acad. Sci. USA. 90:770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucas, C., L.N. Bald, B.M. Fendly, M. Mora-Worms, I.S. Figari, E.J. Patzer, and M.A. Palladino. 1990. The autocrine production of transforming growth factor-beta 1 during lymphocyte activation. A study with a monoclonal antibody-based ELISA. J. Immunol. 145:1415–1422. [PubMed] [Google Scholar]